Abstract
Most malignant bone tumors are treated with surgical excision, adhering to oncologic principles, followed by reconstruction to preserve form and function whenever feasible. Primary bone tumors around the elbow are rare accounting for <1% of all skeletal tumors. They pose a reconstructive challenge, due to the complex interplay between the osseous & capsulo-ligamentous structures which is essential for elbow stability and function. Tumors affecting the proximal ulna are rare and reconstruction of the defects following these tumors is extremely challenging. Various reconstruction options like arthrodesis, autogenous bone grafts, allografts, re-implantation of sterilized tumor bone, pseudoarthrosis, and endoprosthesis have been tried with variable success. However, due to lack of standardization and the rarity of the site, surgeons are often in a dilemma to choose the correct option. This can lead to suboptimal functional outcomes and long-term failures. In this article, we reviewed the published literature on proximal ulnar tumors and noted the pros and cons of various reconstructive procedures. We have also attempted to formulate reconstruction recommendations based on the level of resection of proximal ulna.
Keywords: Single bone forearm, Biological reconstruction, Allograft, Autograft, Endoprosthetic reconstruction, Elbow function
1. Introduction
Primary bone tumors around the elbow joint are extremely rare, accounting for <1% of all skeletal tumors.1,2 The elbow is a complex hinge joint owing to the anatomy of articulating bone, ligaments and muscular attachments traversing the joint.1,3 Because of this, tumors around the elbow pose an immense challenge both in terms of resection and reconstruction.
Surgical management of malignant and locally aggressive benign tumors with significant bone loss usually requires resection of the proximal ulna. This leads to loss of ulno-humeral and proximal radio-ulnar articulations. Proximal ulna contributes majorly in the elbow joint hence its reconstruction is essential to provide a stable elbow and optimize elbow and hand function. The reconstruction is challenging owing to the complex anatomy of the joint (humero-ulnar and proximal radio-ulnar articulations), thin soft tissue cover, and the need for extensor apparatus reconstruction. Various reconstruction techniques have been described in the literature. These include arthrodesis, radius transposition, recycling of tumor bone, mega prosthesis, free fibula transfer, etc.4, 5, 6, 7, 8
Given the scarcity of data on primary bone tumors involving the proximal ulna, there is no gold standard reconstruction option advocated in the literature. The published studies are only case reports or studies with a very small sample size. In this review, we have attempted to compare all the studies and analyze the pros and cons of each reconstruction option in terms of stability of the joint, functional outcome, and complications post-reconstruction. We have also described a new classification for the management of elbow defects after complete/partial excision of the olecranon.
2. Material and methods
We performed a comprehensive literature search and tried to answer the following research questions –
-
1)
What are the various options available for reconstruction of defects after excision for proximal ulna tumors?
-
2)
What are the advantages/disadvantages of these described techniques?
-
3)
What is the best reconstruction option following resection of proximal ulna based on the level of resection?
A detailed Pubmed search was performed with the keywords “proximal ulna, tumors” in March 2021. The selection process for studies included in the present review is described in Fig. 1. We included the studies on proximal ulnar tumors, treated with excision and had followed up of more than 6 months. Studies on-proximal ulnar tumors treated with curettage, percutaneous treatments or observation with less than 6 months follow-up were excluded. We only included studies published in English literature. The full text and cited references were reviewed. As the papers/reports used different evaluation systems a meta-analysis was not feasible, hence a qualitative analysis was undertaken.
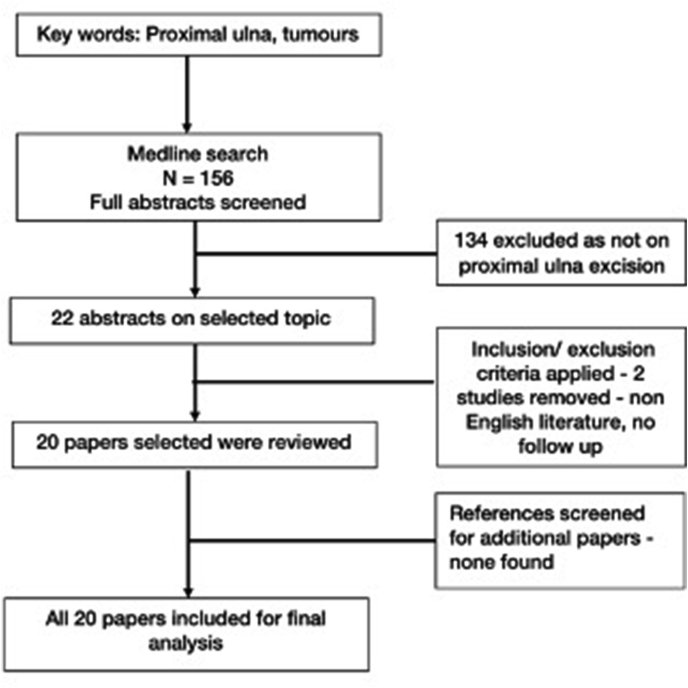
Fig. 1.
Flowchart depicting the selection process methodology.
A total of 156 abstracts were available for review following the primary search. Twenty-two abstracts were found on the current topic. However, two abstracts were excluded due to no follow-up information in 1 case and non-English literature in the other (Fig. 1). The rest of the 20 studies/articles were included in this review.
The summary of results is available in Table 1.
Table 1.
Outcomes of all studies identified in literature.
| Study | Reconstruction technique | Sample size | Follow up in months | Functional outcome & scores (TESS, DASH, MSTS, MEPS, wherever available) | Complications |
|---|---|---|---|---|---|
| Rydholm, 19873 | Radius neck to humerus trochlea articulation | 1 | 9 | ROM: 35°–135° Pronosupination: 40° | Muscle weakness nearly 50% of normal |
| Gianoutsos et al., 199411 | Osteocutaneous fibular free flap | 1 | 36 | ROM: 10°–100° Pronation: 45° Supination: 35° Power: 4/5 | Instability of the reconstructed joint, Donor site: leg edema |
| Kimura et al., 20028 | Vascularized fibular graft | 1 | 48 | MSTS 100% (30/30) | Nil |
| Weber et al., 200316 | Total elbow replacement | 11 elbows (1 proximal ulna tumor) | 12–124 (mean – 47), Median – 37) | Mean MSTS: 83% (25/30) | Periprosthetic lysis |
| Duncan et al., 20087 | Radial neck to humerus trochlea transposition | 2 | 96 12 |
MSTS (Mean): 88.3% (26.5/30) | Joint instability and muscle weakness |
| Guo et al., 200817 | Total elbow arthroplasty | 19 elbows (5 proximal ulna tumors) | 24 | MEPS: Good in 14/19: 77.8% Poor in 4/19: 22.2% | Stem loosening, periprosthetic lysis and revision surgeries |
| Ogose et al., 201020 | Combined vascularized fibula + osteochondral extracorporeal irradiated graft | 1 | 120 | ROM: −20°–120° Pronation: 80° Supination: 10° | Proximal osteotomy site nonunion: iliac crest bone grafting at 16 months after surgery |
| Minhas et al., 201023 | Arthrodesis with fibula | 1 | 60 | Elbow fusion | Nil |
| Chen et al., 20129 | Radius neck to humerus trochlea transposition | 1 | 12 | MSTS: 83% (25/30) ROM: 10°–90°. Slight restriction in prono-supination. | Joint instability Muscle strength weakness |
| Sewell et al., 201218 | Custom proximal ulna endoprosthetic replacement | 4 | 85 | Mean MSTS: 90% (27/30) Mean TESS: 81 (73–88) | Triceps weakness |
| Goyal et al., 201315 | Non-vascularized fibula to remaining olecranon | 1 | 24 | ROM - 40°–130, 70° prono-supination | Nil |
| Wang et al., 201514 | Non-vascularized fibula to remaining olecranon | 1 | 24 | ROM - 0 ° to 135 °, Pronation: 30 °, Supination: 85 °. The grip power of the left hand was 36 kg, which was 86% of the contralateral side (42 kg) | Nil |
| Kalaiah et al., 201513 | Non-vascularized fibula with trans-osseous sutures | 1 | 24 | Not mentioned | Not mentioned |
| Sulko, 201310 | Radial head transposition with inverted V-plasty of triceps | 1 | 27 | MSTS: 96.67% (29/30) DASH: zero Power: 5/5 | Restricted Pronation. Limb length discrepancy: 2 cm |
| Puri et al., 201619 | Medialization of radius to a preserved proximal articular segment of ulna | 1 | 60 | ROM: 10°–130° MSTS: 90% (27/30) | Restricted prono-supination, limb length discrepancy. Implant prominence over elbow |
| Goyal P et al., 201625 | Partial excision with soft tissue reattachment | 1 | 60 | Full ROM of elbow | Nil |
| Sboui et al., 20171 | Medialization of radius to a preserved proximal articular segment of ulna | 1 | 36 | ROM: 20°–125°, no prono-supination | No prono-supination |
| Megas et al., 201712 | Non-vascularized fibula with trans-osseous sutures | 1 | 25 | ROM: 20°–110°, supination - 30°, pronation - 40°, Mayo Elbow Score - 75 points | Nil |
| Gundavda et al., 20196 | Osteoarticular extracorporeal irradiation and reimplantation of proximal ulna resected segment | 3 | 28–42 | ROM: 0°–130° Pronosupination: full Power: 5/5 MSTS: 100% (30/30) DASH: zero MEPS: 100 | Implant prominence over the elbow in 1 case |
| Houdek et al., 20195 | Transposition of the radial neck to the trochlea of the humerus | 2 | 24 | TESS: 87, 91.4; MSTS: 87, 83; DASH: 20.8, 24.2 ROM: 30–110° flexion & 45° of prono-supination to neutral ROM: 30–130 & 45 pronosupination to neutral |
Nil |
Histology of lesions, reconstruction option, follow-up, and functional evaluation were noted. Complications of each of the reconstruction options were also analyzed. Survival of the construct wherever provided was noted.
3. Results
3.1. Description of studies
Most of the articles retrieved were either case reports or small case series. There were 15 case reports and 5 case series. The largest number of cases in a series was 5 by Guo et al.,9 followed by 4 cases in series by Sewell et al.10 Follow-up ranged from 9 to 120 months with a median of 27 months. The main procedures described are reconstruction with vascularized/non-vascularized fibular grafts, radial neck to trochlea transposition, total elbow arthroplasty, recycled tumor bone and re-implantation, arthrodesis. We retrieved 31 cases for final analysis.
3.2. Levels of resection
Complete proximal ulna resection was performed in 25/31 cases while partial excision, preserving either a sliver of triceps attachment to 1–2 cm of the proximal ulna was done in 6 cases.
3.3. Outcome measures
Information regarding elbow range of movements and forearm prono-supination as outcome measures were available in eighteen reports. Along with the functional movements, MSTS scores were mentioned in 8 reports, DASH & MEPS score in 3 reports each and TESS score in 2 reports. Radial neck to humerus articulation (5 reports, 7 cases)3,5,7,9,10 usually resulted in a limited range of movements (ROM) of the elbow and forearm. Houdek et al.5 reported elbow ROM of 30-1100 with 450 of prono-supination with this procedure. Rydholm et al.3 also reported similar outcomes with elbow ROM of 35-1350 and prono-supination of 400 with this procedure.
Four reports (1 case each) described reconstruction with free fibula/non-vascularized fibula.8,11, 12, 13 Cases with complete resection of the proximal ulna and reconstruction with fibular graft had functional outcomes similar to radius transfers. Gianoutsos et al.11 reported elbow ROM of 10-1000 with 45-300 of prono-supination in a case of adamantinoma. Megas et al.12 reported 20-1100 of elbow ROM with 40-300 of prono-supination at more than 2 years follow-up with non-vascularized fibula in a case of metastatic carcinoma. However, the outcomes were superior when part of the olecranon was saved, and fibular graft was used as intercalary grafts.14,15 Wang et al.14 reported elbow ROM of 0-1350 with 30-850 of prono-supination after intercalary fibular autograft.
Recycled host tumor bone and re-implantation has been used sparingly but with good outcomes. Gundavda et al.6 reported 0-1300 of elbow ROM and full prono-supination in 3 cases (2 complete proximal ulna, 1 intercalary) of reconstruction with extracorporeal radiation therapy. A megaprosthesis was used after complete proximal ulna resection in 10 patients distributed among 3 studies.16, 17, 18 Sewell et al.18 used proximal ulnar megaprosthesis and had near normal prono-supination with elbow extension ranging from 0-150 and flexion ranging from 90-1250.
3.4. Complications
Complete loss of prono-supination is seen in 2 cases of single bone forearm.19,1 Radial neck to humerus articulation as well as fibula transfer resulted in restricted prono-supination and elbow ROM in 9/11 cases. Instability of elbow joint was not seen in most of the reports with fibular/radius transfers. Periprosthetic lysis and stem loosening were seen in few cases reconstructed with megaprosthesis,15,16 though exact numbers are not mentioned. Non-union of osteotomy site was seen in 1 case of combined recycling of bone with fibular graft which required bone grafting.20 Implant prominence over the proximal ulna was also reported in 2 cases.
Outcomes of all the studies identified are summarized in Table 1.
4. Discussion
Reconstruction is challenging after proximal ulna excision. Due to the rarity of occurrence of tumor necessitating proximal ulna excision (<1%), there is limited literature available on this subject. Though there are various reconstruction techniques described which aim to provide a stable and functional elbow, there is a lack of consensus regarding the method of choice. Anatomical reconstructions like the use of osteo-articular graft and recycled tumor bone seem to provide good initial function but are associated with availability issues and higher delayed complications.2,21 Non-anatomical reconstruction like radial transposition to humeral trochlea, though durable are plagued with restricted function.3,5,7,9,10 Non-biological options like megaprosthesis and intercalary implant cement spacer, provide an excellent early function with good cosmesis but are associated with a high rate of late complications like loosening and implant failure enforcing revision surgeries.16, 17, 18,22
The level of resection of the proximal ulna is an important factor guiding the choice of reconstruction option. Complete proximal ulna resection defects are reconstructed with radio-humeral transfers, recycled tumor bone, fibula transfers, arthrodesis and megaprosthesis. Each of these techniques has its own merits and demerits. The transposition of the radial neck to the humeral trochlea has been used widely with moderate success. This technique does not restore the complex anatomy of the elbow joint resulting in restricted movements of the elbow and the forearm but provides a stable elbow for good hand function.3,5,7,9,10 This durable biological technique avoids donor site morbidity and seldom requires revision surgeries. Non-vascularized fibula with trans-osseous sutures has also been used with good functional outcomes. This technique provides stable elbow but with a mild loss of elbow ROM as well as forearm rotatory movements.8,11, 12, 13
Size and side matched frozen allografts and recycled tumor bone (Extra-corporeal radiation therapy or cryotherapy) give an anatomical reconstruction, hence elbow and forearm movements are preserved.2,4,6 Reconstruction using extracorporeal irradiation has been shown to have promising results.6 It has the advantages of being size-matched, cost-effective but can be associated with complications such as infection and long-term arthritis. Both these options require a unique infrastructure for graft processing and are associated with complications like osteotomy healing, wound healing issues, graft resorption, late graft fractures and instability.4,6,21 These techniques may not be suitable in cases that require adjuvant radiation for adequate oncological control.
Few studies have supported the use of total elbow megaprosthesis to achieve an anatomical reconstruction. Megaprosthesis provides a hinge joint reconstruction with encouraging early functional results with excellent cosmesis. Lack of soft tissue cover and thin intramedullary canal of ulna pose a unique challenge, limiting the use of standardized cementing technique and robust intramedullary stem. Complications like infection, periprosthetic loosening, and implant failures are a big concern with the use of megaprostheses. Elbow arthrodesis is a permanent procedure resulting in a fixed joint & major functional restrictions.23
Partial proximal ulna resections can be reconstructed with techniques like radius transfer, intercalary fibular grafts and recycling of the host bone and non-biological options like customized prosthesis and implant cement spacers. Radius transfer creates a single bone forearm resulting in loss of rotatory movements hence has inferior function as compared to intercalary fibular grafts. Osteotomy site non-union and risk of graft fracture may be seen, especially with non-vascularized fibula transfers. Recycled tumor bone used as an intercalary graft is an effective alternative. Annular ligament reconstruction is also required if the olecranon osteotomy is proximal to the proximal radio-ulnar joint.
Based on the reviewed literature, we propose a resection classification (based on the level of proximal ulna resection and the status of proximal radio-ulnar articulation) and highlight reconstruction options for optimization of the functional outcomes (Table 2).
Table 2.
Proposed ulna resection classification.
| Type of resection | Reconstruction | Advantages | Disadvantages |
|---|---|---|---|
| Type I | Fibular graft | Biological, Ease | Healing issues if adjuvant radiation is needed |
| Implant cement spacer | Ease, Can be used in infection, cases requiring adjuvant radiation | Non-biological, long term failure | |
| Type II | Fibular graft + soft tissue reconstruction | Biological, ease | Difficult to achieve fixation, prono-supination limitation, prolonged immobilisation |
| Radius transposition - single bone forearm | Biological, no donor site morbidity | Loss of prono-supination, limited elbow ROM | |
| Type III | Fibular graft + soft tissue reconstruction | Biological, ease | Instability, healing, fracture, limitation of movements, donor site morbidity |
| Radius transfer + soft tissue reconstruction | Biological, ease, no donor site morbidity | Instability, limitation of movements | |
| Megaprosthesis | Easily available | Infection, Implant loosening, late implant failure | |
| Recycling of tumor bone + soft tissue reconstruction | Biological, restores anatomy | resorption, non union, difficult to give local adjuvant therapy if needed, arthritis, Need special infrastructure | |
| Arthrodesis | Biological | Loss of movements | |
| Osteo-articular allografts | Biological, restores anatomy | Resorption, failure, arthritis, Non union |
4.1. Type I resection
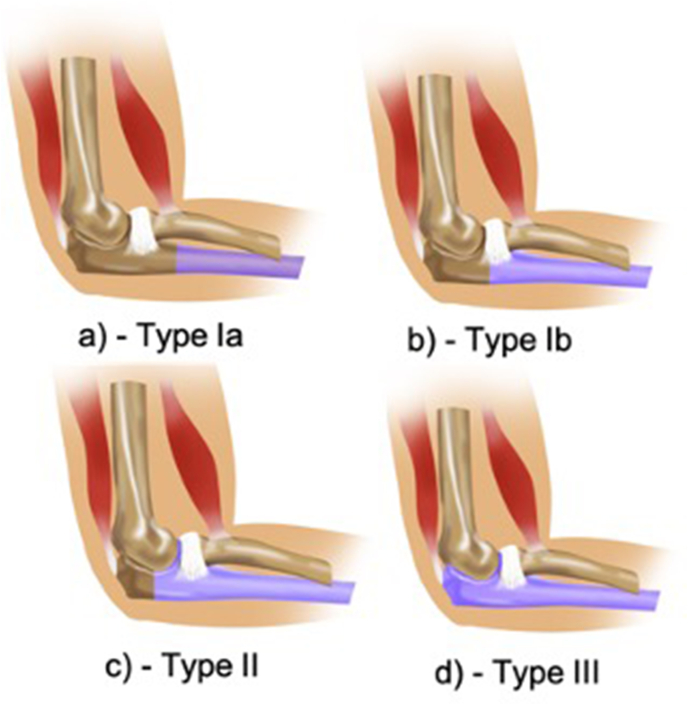
Resection through the meta-diaphyseal junction of the ulna (Fig. 2a and b). This is further subdivided into 1a & 1b depending upon whether radial notch is saved or not.
Type Ia - Proximal ulna resection distal to radial notch
Type Ib - Proximal ulna resection through the radial notch
Fig. 2.
Schematic diagram showing levels of proximal ulnar resections.
a) Type Ia, b) Type Ib, c) Type II, d) Type III.
Type Ia resections result in a meta diaphyseal defect in the ulna with an anatomically intact ulno-humeral and proximal radioulnar joint (Fig. 3). Both biological (non-vascularized/vascularized bone graft) and non-biological (Implant-cement spacer construct) options of reconstructions can be utilized for reconstruction. Vascularized bone grafts like vascularized free fibula may be preferred in defects more than 8 cm. Type Ib resections involve loss of radial notch resulting in loss of superior radio-ulnar joint anatomy (Fig. 4). These defects can also be reconstructed with options like Type Ia but in addition, annular ligament reconstruction is advisable to preserve rotatory movements. Various options like triceps tendon, fascia lata graft with suture anchors have been used to reconstruct annular ligament to enhance superior radio-ulnar joint stability. The choice of option is guided by histopathology, loss of surrounding soft tissue, availability of reconstruction material, need for adjuvant treatment and availability of expertise.
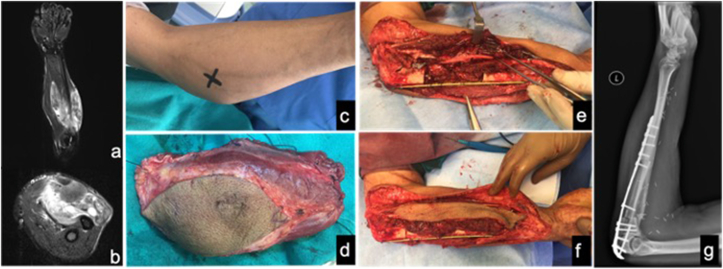
Fig. 3.
Synovial sarcoma of forearm with ulnar involvement treated with wide excision with intercalary ulnar resection (type Ia) and reconstruction with vascularized fibular graft. a&b) pre-operative MRI showing large soft tissue sarcoma abutting ulnar cortex c) clinical picture d) specimen excised e&f) intra-operative pics before and after intercalary vascularized fibular graft g) follow-up radiograph at 1 year.
Fig. 4.
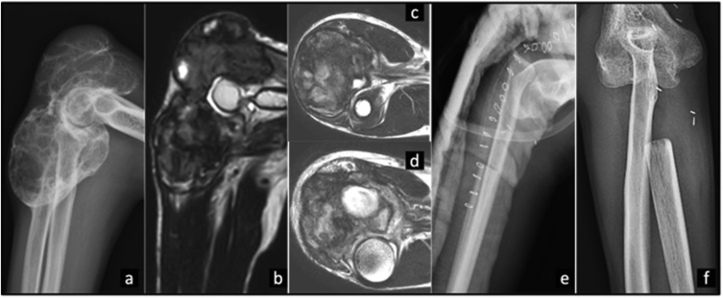
Right proximal ulna Ewing's sarcoma treated with Intercalary resection (type Ib) and extra corporeal irradiation and reimplantation of tumor bone. a) Radiograph showing an aggressive lesion with periosteal reaction involving diaphysis and proximal metaphysis of ulna. b) MRI showing a lesion in Coronal STIR and c-e) T1 Axial sequences with soft tissue component. f) Post operative radiograph. g) radiograph at 18 months follow-up.
4.2. Type II resection
Type II resection plane passes through the olecranon. This leads to meta-diaphyseal defect with partial to near-complete loss of articular surface with an intact triceps attachment to olecranon (Fig. 2c). Type II resection causes instability of ulno-humeral and radio-ulnar articulations. It is imperative to create an articular surface to preserve the range of motion and provide stability to the elbow joint. Various reconstruction options like the use of recycled tumor bone and reimplantation, transposition of radius to the remaining olecranon, or an intercalary fibular graft may be utilized to achieve the aforementioned goal. Non-biological options like implant cement spacer are not preferred as these are not suitable for articulation. Custom-made 3D implants may be utilized for anatomical reconstruction of these defects.24
4.3. Type III resection
Type III resections involve excision of the full proximal ulna (Fig. 2d). The defect needs bony as well as soft tissue reconstruction to provide a stable and functional elbow. Reconstruction options available to these defects include recycled tumor bone, radial neck transfer to humeral trochlea (Fig. 5), megaprosthesis, osteoarticular allografts, elbow arthrodesis, and free vascularized fibula graft. All options (except arthrodesis) require reconstruction of the extensor mechanism to achieve an active range of elbow movements.
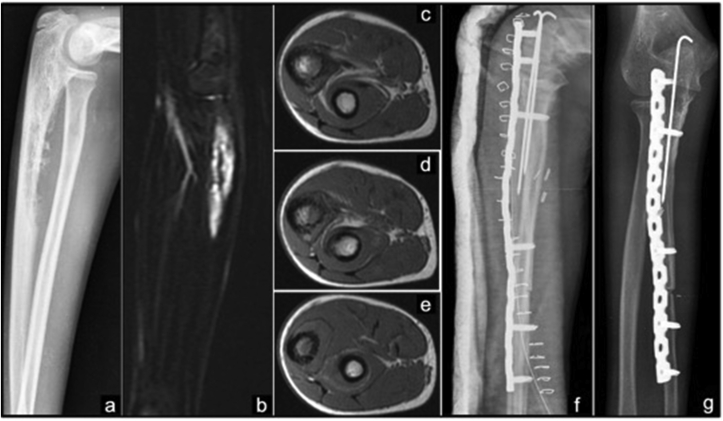
Fig. 5.
Giant cell Tumor of right proximal ulna GCT treated with proximal ulna resection and radio humeral-trochlea transfer.
a) Radiograph showing expansile lytic lesion with trabeculations. b) MRI showing a hypointense lesion in T1W sagittal, c&d) T2W axial images. e) Postoperative radiograph f) radiograph at 6 months follow-up.
Decision making is crucial for selecting the correct method of reconstruction. Factors related to the patient, disease, treatment, choice of surgical procedure and reconstruction option, surgical expertise, and availability of adequate infrastructure guides the surgical team in decision making. Careful case selection and meticulous surgical planning and execution are essential to achieve optimum results. Oncological clearance is of paramount importance to prevent an oncological failure (local or distant) and at the same time preservation of uninvolved host bone is essential for superior functional outcome. This has been documented in various studies where type II resection and reconstruction fares better than type III. For elderly patients, wherein rehabilitation and adaptability are challenging, non-biological options like megaprosthesis are better. Given the longevity of the construct, biological reconstruction options should be preferred in younger patients. Extracorporeal radiotherapy (recycled tumor bone and re-implantation) can be used for cases where post-operative radiotherapy is not needed. Surgical expertise and availability of adequate infrastructure like access to tissue bank and radiotherapy impact the choice of reconstruction and eventual functional outcome.
This review is one of the first attempts to address the complex issue of proximal ulna reconstruction post resection of tumors. Though beset with inherent issues of retrospective literature like lack of complete information on surgical planning and patient factors, this article aims to provide a roadmap for the selection of an apposite reconstruction option. A personalized reconstruction plan based on levels of resections, expertise, patient needs and the need for adjuvant treatment, is the cornerstone of success. We hope that the proposed classification will also ensure homogeneity in future research.
Contributor Information
Ashish Gulia, Email: aashishgulia@gmail.com.
Manish Pruthi, Email: manishpruthi@gmail.com.
Srinath Gupta, Email: srigups@gmail.com.
Shravan Nadkarni, Email: docshravan@gmail.com.
References
- 1.Sboui I., Jlalia Z., Riahi H. A new biological technique of elbow reconstruction following an extensive tumoral resection of the proximal ulna. Med Case Rep. 2017:1–5. 03(02) [Google Scholar]
- 2.Dean G.S., Holliger E.H., Urbaniak J.R. Elbow allograft for reconstruction of the elbow with massive bone loss. Long term results. Clin Orthop. 1997;341(8):12–22. [PubMed] [Google Scholar]
- 3.Rydholm A. Reconstruction after resection of the proximal ulna: report of a case of chondrosarcoma. Acta Orthop. 1987;58(6):671–672. doi: 10.3109/17453678709146513. [DOI] [PubMed] [Google Scholar]
- 4.Kharrazi F.D., Busfield B.T., Khorshad D.S., Hornicek F.J., Mankin H.J. Osteoarticular and total elbow allograft reconstruction with severe bone loss. Clin Orthop. 2008;466(1):205–209. doi: 10.1007/s11999-007-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houdek M.T., Gupta S., Griffin A.M., Wunder J.S., Ferguson P.C. Radial neck-to-humerus transposition for elbow reconstruction following oncologic resection of the proximal ulna: a report of two cases. JBJS Case Connect. 2019;9(4):1–6. doi: 10.2106/JBJS.CC.18.00451. [DOI] [PubMed] [Google Scholar]
- 6.Gundavda M.K., Agarwal M.G., Reddy R. Reconstructive challenges of proximal ulnar bone tumors: our experience with biological osteoarticular reconstruction using extracorporeal irradiation and reimplantation. Sarcoma. 2019 Apr 11;2019:7812018. doi: 10.1155/2019/7812018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan S.F.M., Athanasian E.A., Healey J.H. Radius neck-to-humerus trochlea transposition for elbow reconstruction after resection of the proximal ulna: report of 2 cases. J Hand Surg Am. 2008;33(8):1384–1387. doi: 10.1016/j.jhsa.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Kimura K., Tatezaki S., Ishii T., Yonemoto T. Hemiarthroplasty of the elbow with a vascularized fibular graft after excision of Ewing's sarcoma of the proximal ulna: a case report. Jpn J Clin Oncol. 2002;32(10):430–434. doi: 10.1093/jjco/hyf088. [DOI] [PubMed] [Google Scholar]
- 9.Chen F., Xia J., Wei Y. Radius neck-to-humerus trochlea transposition elbow reconstruction after proximal ulnar metastatic tumor resection: case and literature review. Eur J Med Res. 2012;17(1):1–8. doi: 10.1186/2047-783X-17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulko J. Elbow reconstruction following an extensive re- section of the proximal part of the ulna in a patient with Ewing sarcoma: a case report. JBJS Case Connect. 2013;3(4):111. doi: 10.2106/JBJS.CC.L.00003. [DOI] [PubMed] [Google Scholar]
- 11.Gianoutsos M.P., Marsden F.W., McCarthy S.W., Lee K.K. Ulnar Adamantinoma: en bloc excision and fibular osteoseptocutaneous free flap reconstruction. J Hand Surg. 1994;19(3):495–499. doi: 10.1016/0363-5023(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 12.Megas P., Kokkalis Z.T., Iliopoulos I. Ulnohumeral reconstruction with autogenous, nonvascularized, fibular graft for metastatic clear cell renal carcinoma of the proximal ulna: a case report. JSES Open Access. 2017;1:90–93. doi: 10.1016/j.jses.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalaiah K., Thejaswi S.G., Siddappa M. Reconstruction of elbow by free fibular graft in a case of osteoclastoma of proximal ulna: a rare case report. Case Rep Med. 2015:3. doi: 10.1155/2015/429309. Article ID 429309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C., Lin N. Ewing's sarcoma of the ulna treated with sub-total resection and reconstruction using a non-vascularized, autogenous fibular graft and hernia mesh: a case report. Oncol Lett. 2015;10:2067–2070. doi: 10.3892/ol.2015.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal T., Rastogi S., Tripathy S.K. Desmoplastic fibroma of ulna: excision and reconstruction of olecranon with a fibular graft. Indian J Orthop. 2013;47:207–210. doi: 10.4103/0019-5413.108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber K.L., Lin P.P., Yasko A.W. Complex segmental elbow reconstruction after tumor resection. Clin Orthop. 2003;415:31–44. doi: 10.1097/01.blo.0000093894.12372.53. [DOI] [PubMed] [Google Scholar]
- 17.Guo W., Tang S., Yang R.L., Ji T. Total elbow arthroplasty after resection of tumors at the elbow. Chin J Surg. 2008;46(22):1734–1737. [PubMed] [Google Scholar]
- 18.Sewell M.D., Hanna S.A., Pollock R.C. Proximal ulna endoprosthetic replacement for bone tumours in young patients. Int Orthop. 2012;36(5):1039–1044. doi: 10.1007/s00264-012-1483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri A., Gulia A., Byregowda S., Ramanujan V. Reconstruction of the elbow and forearm for Ewing sarcoma of ulna: a new biological technique. Int J Shoulder Surg. 2016;10(2):85–88. doi: 10.4103/0973-6042.180721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogose A., Hotta T., Shibata M., Kawashima H., Endo N. Combined use of free vascularised fibula graft and extra- corporeally irradiated osteochondral graft for osteosarcoma of the proximal ulna. Oncol Lett. 2010;1(1):133–135. doi: 10.3892/ol_00000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takenaka S., Araki N., Ueda T. Clinical Outcomes of Osteoarticular extracorporeal irradiated autograft for malignant bone tumor. Sarcoma. 2020 doi: 10.1155/2020/9672093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri A., Gulia A., Pruthi M., Koushik S. Primary cement spacers: a cost-effective, durable limb salvage option for knee tumors. Knee. 2012 Aug;19(4):320–323. doi: 10.1016/j.knee.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Minhas M.S., Mehmood G. Giant cell tumour of the proximal ulna. J Coll Phys Surg Pakistan. 2010;20(6):416–418. [PubMed] [Google Scholar]
- 24.Wang F., Zhu J., Peng X., Su J. The application of 3D printed surgical guides in resection and reconstruction of malignant bone tumor. Oncol Lett. 2017;14:4581–4584. doi: 10.3892/ol.2017.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal P., Gautam V., Saini N., Sharma Y. Rare giant cell tumor of olecranon bone!!!! J Orthop Case Rep. 2016 Sep-Oct;6(4):27–30. doi: 10.13107/jocr.2250-0685.556. [DOI] [PMC free article] [PubMed] [Google Scholar]