Abstract
Iatrogenic left main coronary artery (LMCA) dissection is a complication inadvertently caused by the interventional cardiologist and can have significant consequences.
A 38-year-old man presented to hospital with non-ST-elevation myocardial infarction. Coronary angiography (CAG) revealed an obstructed proximal left circumflex artery (LCx) that was successfully treated with revascularization using a drug-eluting stent (DES). However, CAG after recanalization of the LCx demonstrated a spiral dissection of the left coronary artery from the mid-LMCA to the left anterior descending (LAD) artery and LCx. The dissection was classified as National Heart, Lung and Blood Institute type D in LAD and type F in LCx. Immediate exclusion stenting of the dissection flap by another DES and thrombolysis in myocardial infarction 3 flow were achieved in the LAD and LCx. The patient achieved hemodynamic stability with improvement in symptoms, despite residual dissection in the LAD. We, therefore, preferred careful observation over revascularization. The false lumen remained visible with a double-barrel appearance in the LAD on 6-month follow-up CAG, which disappeared at the 2-year follow-up. We report a rare case of a large double-barrel dissection that spontaneously occluded over time without any aggressive interventions.
<Learning objective: Iatrogenic left main coronary artery (LMCA) dissection is a rare but potentially life-threatening complication, with the associated risk of serious outcomes. Immediately after suffering a LMCA dissection, treatment strategies (conservative therapy, percutaneous coronary intervention, or coronary bypass grafting etc.) should be determined according to patient's symptoms and hemodynamic status. However, treatment strategies for chronic LMCA dissection are uncertain. Our case indicates that conservative therapy appears to be a potential option for the treatment of chronic asymptomatic and hemodynamically stable LMCA dissection.>
Keywords: Left main coronary artery, Dissection, Double-barrel, Acute coronary syndrome, Iatrogenic
Introduction
Iatrogenic left main coronary artery (LMCA) dissection is an extremely serious complication that can be induced by the interventional cardiologist. LMCA dissection is very rare, but it often results in acute pump failure and hemodynamic collapse if not promptly treated. We describe a patient with LMCA dissection that had a patent false lumen imaged with a double-barrel appearance at the 6-month follow-up, but had disappeared at the 2-year follow-up.
Case report
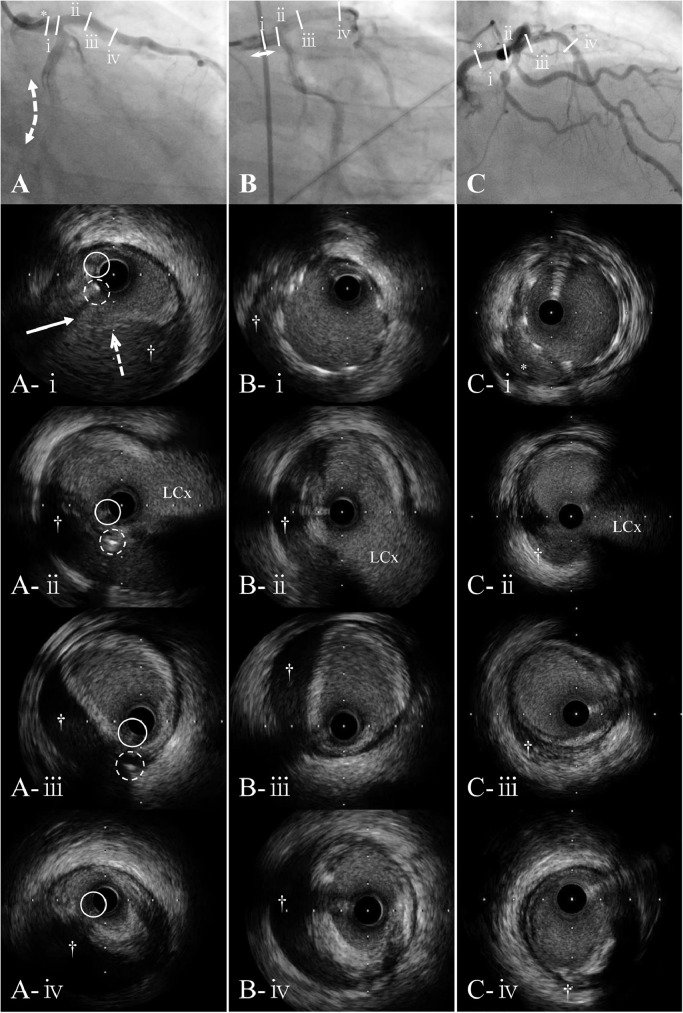
A 38-year-old man with a history of hypertension and dyslipidemia and an 18 pack-year current smoking underwent emergency coronary angiography (CAG) because of non-ST-elevation myocardial infarction. CAG showed a single-vessel total occlusion in the left circumflex artery (LCx) (Fig. 1A1). Percutaneous coronary intervention (PCI) was performed using a standard technique. A 6F Judkins-Left 4.0 guiding catheter (GC) (ASAHI INTECCⓇ, Seto, Japan) was used with deep intubation, because it was difficult to deliver the devices owing to coronary ectasia and the acute angle between LMCA and LCx artery (<90°). After pre-dilation, a drug-eluting stent (DES) (SYNERGYⓇ 3.0 × 38 mm, Boston ScientificⓇ, Natick, MA, USA) was implanted across the lesion (Fig. 1A2), followed by post-dilation with an optimized non-compliant balloon (DOBELⓇ 3.5 × 10 mm, FUKUDA DENSHI COⓇ., Tokyo, Japan). The procedure was ended because intravascular ultrasound (IVUS) (5F OpticrossⓇ, Boston ScientificⓇ) revealed a good expansion of DES. However, contrast infusion after removal of all devices from the coronary was complicated by a mid-LMCA dissection with an extension into the left anterior descending (LAD) artery and LCx, due to inappropriate positioning of the GC non-coaxially and vigorous contrast injection. While coronary blood flow (CBF) of the LCx was completely obstructed at the proximal edge of the implanted DES, that of the LAD was maintained (Fig. 1A3). After guidewires were carefully passed to the distal LAD and LCx again under the support of an intra-aortic balloon pump (IABP), IVUS was used to ensure that the guidewires were in the true lumen (Fig. 2A). IVUS also revealed that the entry of the coronary artery dissection existed in the mid-LMCA, from which the dissection extended to the LAD and LCx. Although the false lumen of the dissection could still be seen with IVUS image (Fig. 2B), PCI was ended because immediate exclusion stenting of the dissection flap by another DES (SYNERGYⓇ 4.0 × 12 mm, Boston ScientificⓇ) led the LCx flow to thrombolysis in myocardial infarction (TIMI) grade 3 (Fig. 1A4). He was transferred to the intensive care unit with the IABP for further monitoring. The maximum creatine kinase level during the entire course was 1541 IU/L.
Fig. 1.
Coronary angiography (CAG) images. (A1) Immediate CAG for non-ST-elevated myocardial infarction demonstrated a total occlusion in the proximal left circumflex coronary artery (LCx). (A2) LCx was successfully treated with a drug-eluting stent (DES) implantation (double dotted arrow). Guiding catheter was toward the ceiling of the left main coronary artery (LMCA) without coaxiality. (A3) Spiral dissection was visualized extending from LMCA to both the left anterior descending (LAD) artery and LCx. Coronary blood flow of the LCx was completely obstructed at the proximal edge of the DES. (A4) Antegrade flow in the LCx was improved after implantation of a DES at the LMCA body (double arrow). * indicates inappropriate positioning of the guiding catheter non-coaxially. Black arrow indicates first diagonal branch.
(B1 and B2) Pre-discharge CAG demonstrated obvious double-barrel dissection from mid-LMCA to mid-LAD. The true lumen (white arrow) was much poorer than the false lumen. (C1 and C2) The 6-month follow-up CAG showed that LMCA dissection remained. Vessel diameter of the true lumen (white arrow) became larger compared to pre-discharge. (D1 and D2) The 2-year follow-up CAG revealed LMCA dissection had disappeared except for the entry (*). Black arrow indicates first diagonal branch.
Fig. 2.
Coronary angiography (CAG) and intravascular ultrasound (IVUS) images. Slashes indicate the position of IVUS at 4 different levels on angiogram. From top to bottom panels, IVUS images are cross section of the mid-left main coronary artery (LMCA) (ⅰ), the distal LMCA (ⅱ), the left anterior descending (LAD) artery just proximal (ⅲ), and the proximal LAD (ⅳ). White arrow indicates the dissection entry, and white dotted arrow indicates intimal flap of the dissection. † indicates the false lumen.
(A) CAG (same as Fig. 1A3) and IVUS images immediately after LMCA dissection. IVUS images confirmed that the guidewire (white continuous circle) was in the true lumen. White dotted circle indicates another guidewire. It was located in the false lumen at the level of A-ⅱ & A-ⅲ, but that was transited to the true lumen through the dissection entry at the level of A-ⅰ. (B) CAG (same as Fig. 1A4) and IVUS images after bail-out stenting to LMCA. (C) CAG (same as Fig. 1D2) and IVUS images at the 2-year follow-up. The false lumen (†) was shrunk and occupied with organized thrombi, except for the mal-apposition at the mid-LMCA (*).
There were no complications during the acute phase, and the IABP was removed on day 2. There were neither symptoms nor significant electrocardiographic changes during cardiac rehabilitation. A second-look angiogram was performed 18 days later. CAG revealed a patent false lumen with distal LAD TIMI 3 flow (Fig. 1B). Coronary computed tomography angiography (CCTA) on day 19 showed a long, parallel, double-barrel dissection from mid-LMCA to mid-LAD (Fig. 3A). Both CAG and CCTA images indicated that the false lumen was dominant compared to the true one and that diagonal branch was arising from the false lumen. Owing to the absence of symptoms and the lesion complexity, conservative therapy was selected, and the patient was discharged.
Fig. 3.
Coronary computed tomography angiography and myocardial perfusion imaging (MPI) images. (A1 to A4) Multiple angles stretched curved planar reconstruction images at pre-discharge revealed double-barrel dissection from mid-left main coronary artery (LMCA) to mid-left anterior descending (LAD) artery (double arrow). 1st major septal branch was arising from true lumen (♯), but 1st diagonal branch was from false lumen (¶). Coronary artery dissection extended to the left circumflex coronary artery (LCx) (arrow). (B) The drug stress MPI demonstrated that the LAD main stem distribution was not ischemic, but the diagonal artery distribution was. This patient had 11.8% summed difference percent.
The patient did not complain of chest pain after hospital discharge, and at the 3-month follow-up, CCTA revealed the preserved double-barrel dissection. The patient remained asymptomatic at the 6-month follow-up, and although CAG showed that the double-barrel dissection had persisted (Fig. 1C), the true lumen was dilated compared to that observed at pre-discharge. Myocardial perfusion imaging (MPI) showed that the LAD main stem distribution was not ischemic; however, the diagonal artery distribution was ischemic (Fig. 3B). Continuation of optimal medical therapy (OMT) including dual antiplatelet therapy was decided for further monitoring. At the 2-year follow-up, he still did not have any complications, and CAG revealed that the false lumen had disappeared, except for the stump at the entry (Fig. 1D). Diagonal branch was collateralized from the LCx as Rentrop grade 1 flow. IVUS showed organized thrombi in the regressed false lumen and good apposition of almost all DES struts, except for the mal-apposition at the mid-LMCA (Fig. 2C). At this point, MPI demonstrated similar findings as before. The patient is being monitored in the outpatient department and is now asymptomatic.
Discussion
In the present case, a large double-barrel dissection including LMCA, which maintained patency up to the 6-month follow-up despite acute phase bail-out PCI, spontaneously occluded over time without any aggressive interventions.
The incidence and mortality rate associated with coronary artery dissection are 0.034% and 0.0028%, respectively [1]. Complicated lesions, such as “calcification, eccentricity, length, ulceration, and angulated coronary segments”, carry a higher risk for development of dissection. Risk factors for catheter-induced coronary artery dissection include atherosclerotic disease, catheterization for acute myocardial infarction, and vigorous contrast injection [2]. LMCA dissection is most frequently caused by the GC, with an incidence of 61.5% [3], and occurs due to non-coaxial positioning and deep intubation of the GC. Our case also occurred in such a situation, and we selected bail-out PCI. Backup support of GC was not enough due to the complex LCx lesion with coronary ectasia and angulated coronary in our case. As a result, the GC was repeatedly engaged and disengaged, and that led to physical trauma to the LMCA vessel wall. For reduction of vessel injury, it could be helpful to exchange the larger or more supportive GC, or to use guide extensions. And it was the most essential to keep the GC coaxially positioned constantly, and to take good care of appropriate contrast injection.
In case of an iatrogenic LMCA dissection, the treatment decision must be determined based on the extent and severity of the dissection, the clinical status of the patient, the operator's expertise, and availability of equipment to perform PCI to the LMCA successfully, as well as the time required to transfer the patient for coronary artery bypass grafting (CABG) [4]. In asymptomatic and hemodynamically stable patients with localized LMCA dissections without limitation of CBF, conservative management has been advocated under close monitoring with OMT. In contrast, in the presence of myocardial ischemia or acute vessel closure, we need to revascularize by PCI or CABG. The current management strategy of iatrogenic LMCA dissection with ischemia is PCI that can be performed immediately after the dissection, with a high success rate and acceptable short- and long-term outcomes [4].
When intervening for coronary dissection, insertion of the guidewire into the true lumen without extension of the dissection is the most important maneuver. IVUS may be the best imaging tool for readily achieving this purpose [5]. Using the useful IVUS information, such as media dissection, the existence and extent of intramural hematoma, calculation of adequate vessel sizing, and proper stent selection, we could negotiate the guidewires into the true lumen, and could implant the largest DES available in Japan.
Antegrade CBF in the LCx was improved after implanting another DES, but LMCA dissection persisted, and was the same at the 6-month follow-up. In hindsight, although we were successful during the acute phase, we probably should have added a post-balloon to the implanted DES of the LMCA. We discussed extensively whether to intervene in the chronic dissection or to continue OMT. Owing to sealing the entry site, implantation of a polytetrafluoroethylene (PTFE) covered stent might be useful. However, PTFE stents are associated with increased incidence of subacute stent thrombosis, restenosis, and target lesion intervention, which is higher than standard stents [6], and can occur later than with conventional stents. As for the MPI, patients with the summed difference percent (SD%) exceeding 10% are believed to benefit from revascularization [7]. This patient had 11.8% SD% with ischemia of the diagonal artery distribution. Considering this alone, it was better to intervene in the LMCA. However, if we intervened, it would be possible to provoke the patient to exhibit a new myocardial infarction because the diagonal branch was arising from the false lumen. It was considered that intervention for LMCA would not contribute to the improvement of ischemia. Because of the case complexity beyond this, we opted for conservative therapy. Two years later, the false lumen closed spontaneously without symptoms. Perfusion pressure in the true lumen is higher than that in the false lumen, which may lead to spontaneous regression of the false lumen with subsequent occlusion. Previous reports that recovery of the true lumen flow was observed in the chronic phase even if the true lumen was compressed by false lumen stenting, support our speculation [8], [9], [10].
Conclusion
We report a rare case of a large double-barrel coronary dissection, which occluded over a long period without any aggressive interventions. From this experience we can conclude that when confronted with a chronic double-barrel dissection, careful observation with OMT instead of immediate additional intervention appears to be one of the therapeutic options, if the patient is asymptomatic and hemodynamically stable, as spontaneous regression of the false lumen can be expected.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Funding sources
None declared
References
- 1.West R., Ellis G., Brooks N., Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London Complications of diagnostic cardiac catheterisation: results from a confidential inquiry into cardiac catheter complications. Heart. 2006;92:810–814. doi: 10.1136/hrt.2005.073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle A.J., Chan M., Dib J., Resar J. Catheter-induced coronary artery dissection: risk factors, prevention and management. J Invasive Cardiol. 2006;18:500–503. [PubMed] [Google Scholar]
- 3.Alfonso F., Almeria C., Fernandez-Ortiz A., Segovia J., Ferreirós J., Goicolea J., Hernández R., Bañuelos C., Gil-Aguado M., Macaya C. Aortic dissection occurring during coronary angioplasty: angiographic and transesophageal echocardiographic findings. Cathet Cardiovasc Diagn. 1997;42:412–415. doi: 10.1002/(sici)1097-0304(199712)42:4<412::aid-ccd16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Eshtehardi P., Adorjan P., Togni M., Tevaearai H., Vogel R., Seiler C., Meier B., Windecker S., Carrel T., Wenaweser P., Cook S. Iatrogenic left main coronary artery dissection: incidence, classification, management, and long-term follow-up. Am Heart J. 2010;159:1147–1153. doi: 10.1016/j.ahj.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Galassi A.R., Sumitsuji S., Boukhris M., Brilakis E.S., Di Mario C., Garbo R. Utility of intravascular ultrasound in percutaneous revascularization of chronic total occlusions: An Overview. JACC Cardiovasc Interv. 2016;9:1979–1991. doi: 10.1016/j.jcin.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 6.Campbell P.G., Hall J.A., Harcombe A.A., de Belder M.A. The Jomed Covered Stent Graft for coronary artery aneurysms and acute perforation: a successful device which needs careful deployment and may not reduce restenosis. J Invasive Cardiol. 2000;12:272–276. [PubMed] [Google Scholar]
- 7.Hachamovitch R., Hayes S.W., Friedman J.D., Cohen I., Berman D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2906. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 8.Omurlu K., Ozeke O. Side-by-side false and true lumen stenting for recanalization of the chronically occluded right coronary artery. Heart Vessels. 2008;23:282–285. doi: 10.1007/s00380-008-1052-y. [DOI] [PubMed] [Google Scholar]
- 9.Wassef A.W., Kirkpatrick I., Minhas K., Malik A., Kass M., Hussain F. The double helix angiography of right coronary arteries: false lumen stenting of a type F right coronary artery spiral dissection with late recanalization of the true lumen and occlusion of the stented false lumen. Heart Int. 2014;9:26–29. [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe Y., Fujino Y., Ishiguro H., Sunao N. Double-barrel coronary artery after subintimal stenting for chronic total occlusion. Cardiovasc Revasc Med. 2017;18:361–363. doi: 10.1016/j.carrev.2016.10.010. [DOI] [PubMed] [Google Scholar]