Abstract
Background and Objective
Despite improvement in medical management, infective endocarditis (IE) remains a serious disease that may affect children with and without preexisting cardiac conditions with significant morbidity and mortality. Neurological complications of IE represent the worst with guarded prognosis. The aim of this study is to describe the incidence, etiology, characteristics, risk factors, and outcome of children with neurological complications associated with IE.
Material and methods
A retrospective cohort study was conducted from 2009 to 2019 where all pediatric patients who fulfilled the modified Duke criteria for IE were included. We divided the cases into 2 groups: IE with neurological complications and IE without neurological complications control group. We compared the two groups statistically and analyzed the results.
Results
We identified 31 (17 male, 14 female) patients with IE. Neurological complications occurred in 7/31 (23%) patients, mainly in the form of a stroke. Gram-positive microbes were the main causative agents for IE (52%) followed by gram-negative (14%), then fungal organisms (3%). Univariate analysis identified the following risk factors for neurological complications: lower body weight, higher C- reactive protein (CRP) level, and left-sided valvular lesions with P values of (0.0003, 0.0001, and 0.04), respectively.
Although mortality was higher in the neurological complications group, it was 43% in comparison to 21% in the control group and it did not reach statistical significance (P = .49). Large vegetation size (more than 10 mm) was seen in 57% of patients with neurological complications as compared to 16% in the control group (P = .052).
Conclusion
Neurological complications occurred in almost a quarter of children with IE. Possible risk factors include lower body weight, left-sided valvular lesion, and higher levels of inflammatory markers (CRP). Stroke was the most common neurological complication encountered with possible increased risk of mortality.
Keywords: Infective endocarditis, Neurological complications, Pediatrics
Abbreviations: IE, Infective Endocarditis; MRSA, Methicillin-resistant Staphylococcus aureus; CNS, Central Nervous System; PCICU, Pediatric cardiac intensive care unit; ECMO, Extracorporeal membrane oxygenation; WBC, White blood count; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; WBCs, White blood cells
1. Introduction
Infective Endocarditis (IE) is an unusual life-threatening disease that results from endocardial surface infection. In a systematic review that involves 10 countries, the incidence of IE ranges from 1.5 to 11.6 cases per 100,000 people per year [1]. Even though streptococci and staphylococci bacteria remain important causative organisms in around 80% of cases, methicillin-resistant Staphylococcus aureus (MRSA) has become more prevalent over the past few decades. A study conducted over 15 years reported that 86% of staphylococcus IE cases were MRSA [2]. In addition, many published reports described new hospital-acquired IE with enterococci and fungal infection [3]. Rheumatic heart disease remains one of the most common predisposing factors for IE. Its incidence has declined in the past 2 decades in the developed countries; yet, it is still a major contributing factor in the developing countries [4]. The incidence of IE in patients with congenital heart disease has significantly decreased over the past decades. This is probably due to antibiotic prophylaxis regimen, advances in cardiac surgeries, and improvement in intensive care management. In the absence of predisposing factors, IE may present in a severe form of acute congestive heart failure and embolic phenomena that may affect the central nervous system (CNS) [5].
Symptomatic neurological complications usually appear before the diagnosis of IE with reported incidence of 20%–40% [6]. Neurological complications occur because of embolization from endocardial vegetation that results in cerebral artery occlusion or CNS infection. There is paucity in the information related to neurological complications of IE in children. Hence, we conducted our study aiming to have a better understanding of neurological complications in children with IE.
The primary aim of this study is to describe the spectrum of neurological complications, incidence, and risk factors associated with pediatric IE. Furthermore, we aimed to compare the clinical, microbiological, and echocardiographic characteristics between patients with neurological complications and non-neurological complications.
2. Methodology
After obtaining approval from the Institutional Research Board, King Abdullah International Medical Research Center (IRBC/0596/19), we performed a retrospective chart review and follow up assessments of all pediatric patients diagnosed with IE from 2009 to 2019. The study was performed in a single tertiary center and included medical and surgical children under 14 years of age diagnosed with IE according to the modified Duke’s criteria [7]. We excluded patients who received extracorporeal membrane oxygenation support from this study.
After reviewing all patients who fulfilled the modified Duke’s criteria for IE, we identified among them cases with neurological complications. Patients were divided into two groups: control group, patients with IE without neurological complications, and neurological complications group, patients with IE associated with neurological complications. In both groups, we assessed and compared various characteristics, including patients’ demographics (age and gender), type of infective endocarditis (native valve IE or prosthetic valve), affected valve/s (aortic, mitral, pulmonary, or tricuspid valves), causal agents (bacterial and fungal), echocardiography findings, size of vegetation, cyanotic or noncyanotic lesions, type of cardiac surgery, early and late mortality, other complications during the course of the disease, and characters of neurological complications.
We compared both groups by univariate analysis to determine specific risk factors. We used the chi-square test to compare categorical variables and Student’s t-test for continuous variables. We considered P value less than 0.05 as significant.
3. Results
During the study period, we identified 31 children (17 male and 14 female) with IE. Their average age and weight were (73.5 [55.2] months and 25.3 [21.7] kg), respectively. Twenty-four subjects (77%) had no neurological complication in the control group, while neurological complications occurred in 7/31 (23%) subjects in the neurological complications group. Table 1 summarizes demographic and patient characteristics in both groups. The neurological complications group had lower body weight of 16.4 (10.9) kg in comparison to 28 (4.9) kg in the control group with P value = .0003. We isolated gram-positive organisms from 16/31 (51%) cases, gram-negative organisms from 4/31 (13%), fungal organism from 1/31 (3%), and no organism was isolated in 10/31 (35%) of IE cases. Both groups were statistically indifferent in terms of microbial causes of IE.
Table 1.
Summary of the difference between IE with the neurological complication (n = 7) and control groups (n = 24).
| Variable | Neurological complication group, n = 7 (%) | Control group n = 24 (%) | P value |
|---|---|---|---|
| Age in months | 62.4 | 79.1 | .19 |
| Weight (ave) | 16.4 | 28 | .0003 |
| Gender | 4 (57) | 13 (54) | .89 |
| Male % | |||
| Predisposing cardiac risk: | .6 | ||
| Normal structural heart | 2 (29) | 4 (17) | |
| Presence of cardiac condition | 5 (71) | 20 (83) | |
| Syndromic features | 1 (0) | 7 (29) | .16 |
| Cyanotic | 1 (14) | 5 (21) | 1 |
| Echocardiography | .29 | ||
| Positive Echo IE | 7 (100) | 18 (75) | |
| Negative Echo IE | 0 | 6 (25) | |
| Left-side heart lesion | 3 (43) | 1 (4) | .04 |
| Right-side heart lesion | 4 (57) | 17 (70) | .82 |
| Vegetation mass | |||
| > 10 mm | 4 (57) | 4 (17) | |
| < 10 mm | 2 (29) | 3 (13) | .05 |
| No mass detected | 1 (14) | 17 (70) | .66 |
| Microbial causes | |||
| Bacterial | 3 (43) | 13 (54) | |
| Staphylococcus aureus | 3 (43) | 7 (29) | |
| Streptococcus viridians | 0 (0) | 4 (16) | .69 |
| Gram-negative bacteria | 1 (14) | 3 (13) | 1 |
| Fungal | 1 (14) | 0 | .22 |
| Negative cultures | 2 (29) | 8 (33) | .81 |
| Inflammatory markers: (Ave) | |||
| WBC (109/L) | 12.9 ∓ 4.8 | 15 ∓ 11.4 | .64 |
| ESR (mm/hour) | 38.5 (50) | 38.1 | .97 |
| CRP (mg/L) | 100 | 36.4 | .0001 |
| Need for surgical management of IE (%) | 3 (43) | 11 (46) | .89 |
| PICU length of stay (ave days) | 19.6 (18.5) | 31 (41) | .66 |
| Hospital length of stay (ave days) | 57.8 (53.6) | 62 (54.6) | .62 |
| Mortality (%) | 3 (43) | 5 (21) | .49 |
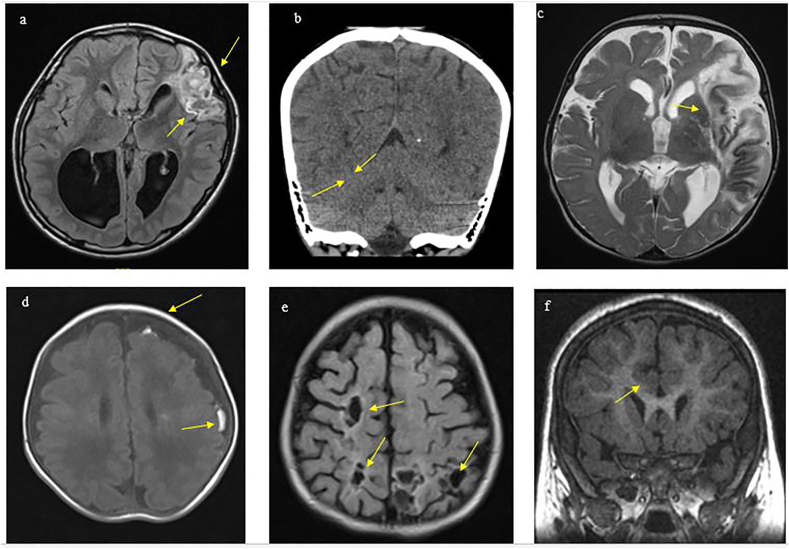
Neurological complications were in the form of a stroke in 4/7 (57%) patients. The first and second cases of ischemic stroke were in the region supplied by the middle cerebral artery and right thalamic region (Fig. 1A and B). The third case developed septic stroke associated with the stenosis of the internal carotid artery (Fig. 1C). The fourth case presented with hemorrhagic stroke in the periventricular area and subarachnoid region (Fig. 1D). In case 5 (Fig. 1E), the patient was infected with IE, which led to a left frontal brain abscess, while in case 6 (Fig. 1F) the patient developed neurological manifestations presented as delay myelination with hypoplasia of the posterior corpus callosum. For the last case 7, the patient was hemodynamically unstable with neurological functional deterioration and succumbed before an imaging study could be performed. Table 2 and Fig. 1 summarize imaging findings of computerized tomography or magnetic resonance imaging of pediatric IE cases with neurological complications.
Fig. 1.
Imaging of 5 patients with IE and neurological complications (a) Case 1: An 11-year-old boy, post-aortic valve replacement developed brucellosis IE that was complicated with multifocal infections in the left frontal lobe with abscess formation and adjacent gliosis.(b) Case 2: A 9-year-old boy, known to have congestive heart failure and multiple congenital heart diseases (Arcade mitral valve, severe mitral regurgitation, and atrial septal defect). He presented with symptoms of IE along with left-sided weakness and right facial weakness. Imaging was performed, which indicated a right thalamic infarct. (c) Case 3: A 4-year-old boy with coarctation of the aorta and atrial septal defect. He was diagnosed with fungal IE complicated with left-sided weakness and left middle cerebral artery stroke. (d) Case 4: Represents a 2-month-old boy known to have atrial septal defect diagnosed with MRSA sepsis IE, complicated with left frontal and partial subarachnoid hemorrhage. (e) Case 5: A 1-year-old girl, with Treacher Collins syndrome with bilateral anotia, Swiss cheese ventricular septal defects, and transposition of great arteries. The patient developed IE complicated with multiple septic infarctions and stenotic cavernous segment of the right carotid artery. (f) Case 6: A 5-year-old girl, not known to have any cardiac diseases, diagnosed with IE and complicated with right subclavian thrombosis. The patient developed seizure and hypotonia. Delayed myelination and hypoplasia of the corpus callosum were observed in her brain imaging.
Table 2.
Magnetic resonance imaging or computerized tomography of the brain findings in neurological cases.
| Patient number | Brain imaging findings |
|---|---|
| 1 (Fig. 1-a) | Left frontal brain abscess and vasogenic edema and left transverse sinus hypoplasia |
| 2 (Fig. 1-b) | Right thalamic lacunar infarct |
| 3 (Fig. 1-c) | Left middle cerebral artery infarction |
| 4 (Fig. 1-d) | Enlarged subarachnoid cerebrospinal fluid spaces with left frontal and parietal scattered subarachnoid hemorrhage. Tiny focus of left frontal periventricular hemorrhage. |
| 5 (Fig. 1-e) | Multiple focal hypodensities represent septic infarction, dense superior sagittal sinus with stenotic cavernous segment of the right internal carotid artery. |
| 6 (Fig. 1-f) | Delay myelination for the patient’s age with hypoplasia of the posterior corpus callosum. |
| 7 | Imaging studies could not be performed as the patient was hemodynamically unstable and succumbed later. |
Left-sided heart lesions were present in 43% of neurological complication cases versus 4% in the control group with P value = .04. Echocardiography identified vegetation in all cases of the neurological complication group with 57% of vegetation measuring >10 mm in size, while echocardiography recognized vegetation in 75% of the control group cases with only 17% of vegetation measuring >10 mm in size. Though there was a trend to have higher neurological complication when vegetation size is > 10 mm, this trend did not reach statistical significance with P value of .0528 (Table 1).
The inflammatory marker (CRP) was higher in the neurological complication group than in the control group with mean values of 100 ∓ 0 mg/L and 36.4 ∓ 12.8 mg/L, respectively, P value = .0001.
The univariate analysis confirmed that lower body weight, left-sided heart lesions, and higher CRP level were statistically significant as a predictor for neurological complications with IE. Because of the small sample size, a multivariate analysis was not performed between both groups. Moreover, when we compared the occurrence of neurological complications with left-sided cardiac lesions (3/4) with those of the right-sided cardiac lesions (4/21), we found a trend toward the left-sided lesion (P = .09); however, the difference did not reach statistical significance.
4. Discussion
Neurological complications are common and often considered as the silent complications of IE. Currently, the exact incidence of neurological complications in IE pediatric patients is still not established and most reported cases focus on the adult population. The incidence in previously reported studies was 20%–40% with the majority of cases linked to vascular events [8]. In a published local study, 28 out of 80 (35%) adult patients with IE developed neurological manifestations, with a high incidence of stroke that reached up to 49% [9]. A study conducted by Asaki and colleagues included 76 pediatric patients with radiology-confirmed ischemic stroke; they reported IE as the source of CNS embolization in 3% of cases [10]. In our study, the incidence of neurological complications in IE patients is 23% (7/31 patients) with 57% of them developed as a result of vascular events. The range of vascular events in our study was higher than that reported in literature, including adult populations [1,8].
The most commonly reported neurological complications were infarction with a median incidence of 13%–39% [11]. Emboli affecting the middle cerebral artery usually lead to hemiplegia, hemianopia, and other visual impairment. These findings are not only present in symptomatic patients, but it can also be present in asymptomatic individuals with IE. A study conducted among 109 patients with IE who did not develop neurological manifestations showed that 78 (71.5%) patients demonstrated a silent ischemic lesion in the brain magnetic resonance imaging [12]. Moreover, intracranial hemorrhage is one of the severe dramatic neurological complications described in anti-coagulated patients [13]. Other nonspecific manifestations, though rare, include brain abscess, meningitis, seizure, and headache [9,14]. Most of these neurological sequelae can lead to poor functional outcomes [15].
In our study, echocardiography identifies vegetation in 25/31 patients, including all seven patients with neurological complications. Our data indicate that left-sided IE patients are at higher risk of having ischemic stroke. Our findings were in concurrence with previous publications [15,16]. A study conducted by Walker et al. in which they reviewed 18 patients with stroke due to confirmed IE, found that 15 of them (83%) had left-sided heart lesions [17]. Another study conducted over 13 years, reported an incidence of 32% left-sided IE patients complicated with stroke [18]. Moreover, we reported four patients with neurological complications with right-sided lesions. Even though the prognosis of right-sided IE is often excellent, 3/4 patients died mostly due to other comorbidities. On the other hand, new cases of IE have been reported in the literature, in children with a normal heart. In our patients, 6/31 (19%) had no preexisting cardiac condition. A retrospective study assessed children with IE and reported that 35.4% of cases occurred in patients with a structurally normal heart [19]. Another study showed that 42% of children with IE had no preexisting cardiac disease [20].
Furthermore, the vegetation of >10 mm in size were likely to be associated with neurological complications in IE patients. According to Mugge et al., vegetation with large diameter (>10 mm) have significantly higher incidence of embolic events than smaller vegetation [21]. Several studies considered vegetation size and mitral valve involvements to be predictors of neurological complications [13,21]. However, other studies did not agree with this observation and emphasized on the causal organism. Blood cultures were positive in 68% of our patients, which is different in comparison to previously published papers. Studies from India reported a positivity rate between 21% and 47% (22), while literature from developed countries showed a higher rate of positive cultures in over 90% of cases [22,23]. Staphylococcus aureus was found to increase the risk of developing neurological complications 2–3 times more than other microorganisms [23]. Similarly, Staphylococcus aureus was the most identified organism in our patients with 32% among all cases, including 43% in the neurological complication group versus 29% in the control group.
The role of inflammatory biomarkers in the prediction of the prognosis of IE has been investigated thoroughly. A retrospective study conducted by Cornelissen et al. among patients diagnosed with IE found that high white blood cells and high CRP levels are prognosticators of complications in patients with heart diseases [24]. A large cohort study conducted in Asia emphasized the role of CRP levels in the prediction of adverse outcomes of IE, including neurological complications [25]. In our patients, we found significantly high CRP levels in patients with neurological complications. In addition, body weight is one of the factors that some authors hypothesized as an association with IE poor outcome. Even though adult population studies did not prove any association between body weight and neurological complications in IE patients, we could correlate lower body weight in children as a significant factor that contributes to neurological manifestations in our paper.
Timely administration of antibiotics and early cardiac surgery are usually advocated to reduce the incidence of neurological complications in IE patients. In addition, retrospective as well as prospective adult studies are still in debate with regard to the effect of antiplatelet therapy in IE. A cohort study conducted among adult IE populations revealed that the use of daily antiplatelets could decrease the risk of thrombotic events [26]. In contrast, Snygg-Martin et al. conducted a univariate analysis among 684 left-sided IE patients and found that a one-year mortality rate was higher among patients on antiplatelets therapy [27]. Moreover, there are no guidelines about the use of anticoagulation among pediatric IE and stroke. The United Kingdom guidelines recommended the use of aspirin therapy at a dose of 5 mg/kg/day in childhood ischemic stroke due to vasculopathy [1,28]. A retrospective review of pediatric IE cases reported a better outcome of aspirin in the prevention of CNS complications than in adults [1]. These findings suggest that aspirin could be beneficial in the reduction of the risk of neurological complications.
Our study had some limitations. First, the data presented in this study were derived from only one institution, which could affect the ability to generalize our findings to other facilities. However, our results are in line with the most recent findings reported in the literature. Second, the small number of sample size used in this study limited the scope of the statistical analysis to univariate analysis.
In conclusion, neurological complications occurred in almost a quarter of children with IE. Stroke is the most common neurological complication encountered among children with IE. Left-sided lesions are one of the risk factors for patients who develop neurological complications. Other possible risk factors include lower body weight and higher levels of inflammatory markers (CRP).
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Venkatesan C., Wainwright M.S. Pediatric endocarditis and stroke: a single-center retrospective review of seven cases. Pediatr Neurol. 2008;38(4):243–247. doi: 10.1016/j.pediatrneurol.2007.12.009.K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito S, Mayer A, Krzysztofiak A, Garazzino S, Lipreri R, Galli Let al. Infective endocarditis in children in Italy from 2000 to 2015. Expert Rev Anti-infect Ther. 2016 Mar 3;14(3):353-358. [DOI] [PubMed]
- 3.Alotaibi M.G., Rahman S., Al-Shalaan M.A., Omair A. Frequency of nosocomial infections in Pediatric Intensive Care Unit at King Abdulaziz Medical City, Riyadh, Saudi Arabia. J Infect Dis Ther. 2015;3:234. doi: 10.4172/2332-0877.1000234. [DOI] [Google Scholar]
- 4.Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, et al Infective endocarditis in childhood: 2015 update: a scientific statement from the American Heart Association. Circulation. 2015 Oct 13;132(15):1487-1515. [DOI] [PubMed]
- 5.Nasser BA, Al Qwaee A, Almesned AR, Akhfash A, Mohamad T, Chaikhouni F, et al Infective endocarditis in children with normal heart: indication for surgical intervention. J. Saudi Heart Assoc.. 2019 Apr 1;31(2):51-56. [DOI] [PMC free article] [PubMed]
- 6.Morris NA, Matiello M, Lyons JL, Samuels MA. Neurologic complications in infective endocarditis: identification, management, and impact on cardiac surgery. The Neurohospitalist. 2014 Oct;4(4):213-222. [DOI] [PMC free article] [PubMed]
- 7.Baddour LM, Wilson WR, Bayer AS, Fowler Jr VG, Tleyjeh IM, Rybak MJ, et al Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015 Oct 13 132(15):1435-1486. [DOI] [PubMed]
- 8.Dowling MM, Hynan LS, Lo W, Licht DJ, McClure C, Yager JY, et al International Paediatric Stroke Study: stroke associated with cardiac disorders. Int J Stroke. 2013 Oct;8(SA100):39-44. [DOI] [PMC free article] [PubMed]
- 9.Venkatesan C., Wainwright M.S. Pediatric endocarditis and stroke: a single-center retrospective review of seven cases. Pediatr Neurol. 2008;38(4):243–247. doi: 10.1016/j.pediatrneurol.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan S.A., Yaqub B.A., Al Deeb S.M. Neurological complications of infective endocarditis. Ann Saudi Med. 1996;16(3):254–256. doi: 10.5144/0256-4947.1996.254. [DOI] [PubMed] [Google Scholar]
- 11.Asakai H, Cardamone M, Hutchinson D, Stojanovski B, Galati JC, Cheung MM, et al Arterial ischemic stroke in children with cardiac disease. Neurology. 2015 Dec 8;85(23):2053-2059. [DOI] [PMC free article] [PubMed]
- 12.Hess A, Klein I, Iung B, Lavallee P, Ilic-Habensus E, Dornic Q, et al Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. Am J Neuroradiol. 2013 Aug 1;34(8):1579-1584. [DOI] [PMC free article] [PubMed]
- 13.Heiro M., Nikoskelainen J., Engblom E., Kotilainen E., Marttila R., Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160(18):2781–2787. doi: 10.1001/archinte.160.18.2781. [DOI] [PubMed] [Google Scholar]
- 14.Chen C.H., Lo M.C., Hwang K.L., Liu C.E., Young T.G. Infective endocarditis with neurologic complications: 10-year experience. J Microbiol Immunol Infect. 2001;34(2):119–124. [PubMed] [Google Scholar]
- 15.Sonneville R., Mourvillier B., Bouadma L., Wolff M. Management of neurological complications of infective endocarditis in ICU patients. Ann Intensive Care. 2011;1(1):10. doi: 10.1186/2110-5820-1-10. Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanter M.C., Hart R.G. Neurologic complications of infective endocarditis. Neurology. 1991;41(7):1015–1020. doi: 10.1212/wnl.41.7.1015. [DOI] [PubMed] [Google Scholar]
- 17.Walker K.A., Sampson J.B., Skalabrin E.J., Majersik J.J. Clinical characteristics and thrombolytic outcomes of infective endocarditis-associated stroke. Neurohospitalist. 2012;2(3):87–91. doi: 10.1177/1941874412446199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao G.F., Bi Q. Pediatric infective endocarditis and stroke: a 13-year single-center review. Pediatr Neurol. 2019;90:56–60. doi: 10.1016/j.pediatrneurol.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y.T., Hsieh K.S., Chen Y.S., Huang I.F., Cheng M.F. Infective endocarditis in children without underlying heart disease. J Microbiol Immunol Infect. 2013;46(2):121–128. doi: 10.1016/j.jmii.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Le Guillou S, Casalta JP, Fraisse A, Kreitmann B, Chabrol B, Dubus JC, et al Endocardite infectieuse sur cœur sain chez l’enfant: étude rétrospective de 11 cas. Arch Pediatr. 2010 Jul 1;17(7):1047-1055. [DOI] [PubMed]
- 21.Mügge A., Daniel W.G., Frank G., Lichtlen P.R. Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol. 1989;14(3):631–638. doi: 10.1016/0735-1097(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 22.Sadiq M., Nazir M., Sheikh S.A. Infective endocarditis in children incidence, pattern, diagnosis and management in a developing country. Int J Cardiol. 2001;78(2):175–182. doi: 10.1016/s0167-5273(01)00374-6. [DOI] [PubMed] [Google Scholar]
- 23.García-Cabrera E, Fernández-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A, et al Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation. 2013 Jun 11;127(23):2272-2284. [DOI] [PubMed]
- 24.Cornelissen C.G., Frechen D.A., Schreiner K., Marx N., Krüger S. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect Dis. 2013;13:272. doi: 10.1186/1471-2334-13-272. Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohanan S., Gopalan Nair R., Vellani H., C G.S., George B., M N.K. Baseline C-reactive protein levels and prognosis in patients with infective endocarditis: a prospective cohort study. Indian Heart J. 2018;70(Suppl 3):S43–S49. doi: 10.1016/j.ihj.2018.05.001. Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anavekar NS, Tleyjeh IM, Anavekar NS, Mirzoyev Z, Steckelberg JM, Haddad C, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis. 2007 May 1;44(9):1180-1186. [DOI] [PubMed]
- 27.Snygg-Martin U., Rasmussen R.V., Hassager C., Bruun N.E., Andersson R., Olaison L. The relationship between cerebrovascular complications and previously established use of antiplatelet therapy in left-sided infective endocarditis. Scand J Infect Dis. 2011;43(11–12):899–904. doi: 10.3109/00365548.2011.603742. [DOI] [PubMed] [Google Scholar]
- 28.DeVeber G. In pursuit of evidence-based treatments for paediatric stroke: the UK and Chest guidelines. Lancet Neurol. 2005;4(7):432–436. doi: 10.1016/S1474-4422(05)70120-4. [DOI] [PubMed] [Google Scholar]