Introduction
Acute necrotizing encephalopathy (ANE) is typically a parainfectious phenomenon most commonly described in children and associated with viral infections such as influenza A. It is associated with a nonspecific clinical presentation, often with alteration in mental status occurring about 12 to 72 h after a viral illness, and imaging often displays symmetric thalamic lesions with edema, petechial hemorrhage, and necrosis [1]. Three cases of ANE have been reported in association with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and there are no reported cases of ANE occurring in pregnancy [2–4]. Treatment is with immunomodulating therapy and, if started early, can improve outcomes [5].
We report a rare case of ANE due to SARS-CoV-2 in a pregnant female who presented with acutely altered mentation and findings of bilateral thalamic lesions on cranial imaging. Our case highlights the need for consideration of this rare clinical entity in association with SARS-CoV-2.
Description of case
A 19-year-old female, G2P1001 at a 33-weeks gestation with a history of obesity, previous gestational hypertension, and limited prenatal care, presented to an outside institution with 1 day of confusion. She had a prodrome of chest pain, tachypnea, dyspnea, and reduced appetite with associated nausea and vomiting over the preceding week. Computed tomography (CT) brain revealed bilateral thalamic hypodensities, for which she was transferred to our tertiary institution with concern for a cerebrovascular event.
Upon arrival, she was tachycardic and tachypneic with increased work of breathing. She was awake but globally aphasic, had a right homonymous hemianopsia, left gaze preference, and reduced movement of the right upper extremity.
Laboratory findings are outlined in Table 1. Metabolic acidosis was due to severe starvation ketosis and normal saline infusions, resolving with aggressive isotonic fluid and bicarbonate infusions. SARS-CoV-2 PCR resulted positive. High-dose thiamine was started initially given the possibility of Wernicke’s encephalopathy.
Table 1.
Serum and urine studies upon admission. Laboratory studies for our patient, with abnormal values highlighted in italics. The laboratory studies in acute necrotizing encephalopathy often reveal transaminitis, elevated lactate dehydrogenase, creatinine kinase, glucose, and creatinine, metabolic acidosis, leukocytosis, thrombocytopenia, disseminated intravascular coagulation, proteinuria [5]
| Reference/units | Results | |
|---|---|---|
| Serum studies | ||
| White blood count | 4–10 K/uL | 6.68 |
| Hemoglobin | 12–16 g/dL | 11.2 |
| Hematocrit | 37–47% | 35.1 |
| Platelet count | 150–399 K/uL | 189 |
| Sodium | 137–147 mmol/L | 136 |
| Potassium | 3.4–5.3 mmol/L | 5 |
| Chloride | 99–108 mmol/L | 116 |
| CO2 total | 22–29 mmol/L | 6 |
| Anion gap (corrected) | 8–16 | 18 |
| Urea nitrogen | 8–21 mg/dL | 5 |
| Total protein | 6–8.2 g/dL | 7.3 |
| Albumin | 3.5–5.0 g/dL | 2.4 |
| Calcium | 8.7–10.7 mg/dL | 9.1 |
| Bilirubin total | 0.2–1.3 mg/dL | 0.3 |
| Alkaline phosphatase | 30–125 U/L | 119 |
| AST | 3–44 U/L | 28 |
| ALT | 0–40 U/L | 9 |
| Ketone | 0–0.6 mmol/L | 4.4 |
| LDH | 110–240 U/L | 406 |
| PT-INR | 0.83–1.23 | 0.91 |
| Activated PTT | 23–33 s | 36.9 |
| d-Dimer | 0–0.60 mg/L FEU | 21.14 |
| Fibrinogen | 190–395 mg/dL | 312 |
| Ethanol | Not detected | Not detected |
| Urine studies | ||
| Urine toxicology screen | None detected | None detected |
| Urine protein | < 20 mg/dL | 100 |
| Urine glucose | < 30 mg/dL | Normal |
| Urine ketones | Negative mg/dL | > 150 |
| Arterial blood gas | ||
| Arterial pH | 7.350–7.450 | 7.266 |
| Arterial PCO2 | 23–45 mm Hg | 17 |
| Arterial PO2 | 83–108 mm Hg | 117 |
| Arterial lactate | 0.4–1.3 mmol/L | 1.1 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; PT, prothrombin time; INR, international normalized ratio; PTT, prothrombin time
Obstetrics was consulted and non-reassuring fetal heart tones were detected. She subsequently required intubation for respiratory distress. Post-intubation, fetal heart tones became prolonged with recurrent late decelerations requiring emergent bedside cesarean section. The neonate was intubated and transferred to the neonatal intensive care unit (ICU).
Urgent head CT angiogram and venogram did not reveal large vessel occlusion or venous sinus thrombosis. Electroencephalogram (EEG) showed severe encephalopathy. Magnetic resonance imaging (MRI) findings were consistent with ANE (Fig. 1), presumably secondary to SARS-CoV-2. She was given 5 days of methylprednisolone (1 g/day) followed by a prednisone taper. Given significant thalamic edema, it was deemed unsafe to perform a lumbar puncture.
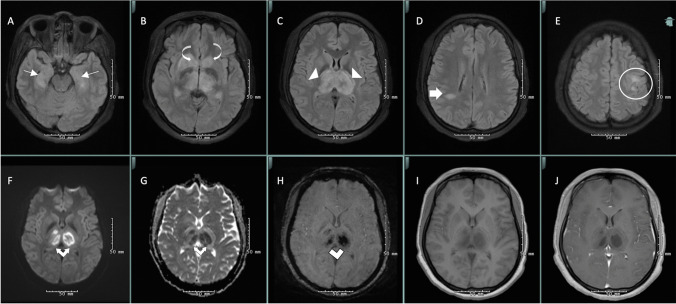
Fig. 1.
MRI brain was notable for T2-weighted-fluid-attenuated inversion recovery (T2-FLAIR) hyperintensities in bilateral thalami (white arrowheads) and caudate nuclei (curved white arrow) with hemorrhage (white double lines) and diffusion restriction (white double arrows), in addition to T2-FLAIR hyperintensities in bilateral hippocampi (thin white arrow), right parietal deep white matter (thick white arrow), and bilateral posterior frontal white matter (white circle), consistent with acute necrotizing encephalopathy due to SARS-CoV-2 (A–E axial T2-weighted-fluid-attenuated inversion recovery (FLAIR), F diffusion-weighted imaging, G apparent diffusion coefficient, H susceptibility-weighted imaging, I T1 pre-contrast, J T1 post-contrast)
The patient gradually improved allowing for extubation. By hospital day 22, she was awake and oriented with normal language and comprehension, intact cranial nerves, motor, and coordination examination but apathetic, requiring minor one-person assistance with ambulation. She was discharged to an acute rehabilitation facility. The baby was also ultimately discharged home in healthy condition.
Discussion
SARS-CoV-2 is a novel zoonotic pathogen which typically presents with respiratory or flu-like symptoms [6]. Over 35% of patients can present with neurologic symptoms, both central and peripheral [6]. Theories regarding nervous system manifestations of SARS-CoV-2 include disruption of the blood–brain barrier (BBB) causing direct invasion to cerebrospinal fluid (CSF) or direct nerve injury from immune system dysfunction, hypoxia, or disruption of ACE2 receptors [6].
Pregnant patients with SARS-CoV-2 present with similar manifestations to non-pregnant patients with comparable outcomes, though there is a high perinatal risk of prematurity (12%), and cesarean delivery (75%) [7].
Clinical presentation of ANE is nonspecific and may include seizures (40%), impaired consciousness (28%), and vomiting (20%) about 12 to 72 h after viral illness [1]. Several lab dyscrasias have been reported (Table 1). EEG often demonstrates encephalopathy [5]. Imaging should include cranial CT or MRI which display symmetric lesions in thalami, putamina, brainstem tegmentum, cerebral and cerebellar white matter, with edema, petechial hemorrhage, and necrosis [1, 5]. In the three previously reported cases of ANE due to SARS-CoV-2 and in our patient, there was an involvement of medial temporal lobes and subinsular regions (Fig. 1); this is not commonly described in ANE and could be specific to SARS-CoV-2 [2–4].
The mechanism of ANE is theorized to be immune-mediated or metabolic, related to cytokine storm damaging the BBB, resulting in edema and secondary necrosis [1, 4]. The differential diagnosis includes acute encephalitides (bacterial, viral, autoimmune inflammatory), posterior reversible encephalopathy syndrome (PRES), hypoxic-ischemic encephalopathy (HIE), Wernicke’s encephalopathy, and acute cerebrovascular disorders such as acute ischemic stroke involving the artery of Percheron or top of the basilar or cerebral venous sinus thrombosis involving the vein of Galen or straight sinus [8]. Based upon laboratory and imaging findings, we were able to exclude infectious and cerebrovascular etiologies. There were no clear antecedent events to cause PRES or HIE. It is useful to obtain CSF in these patients to aid in excluding certain infectious or inflammatory conditions. Two-thirds of ANE patients have elevated CSF protein with no pleocytosis [5]. The lack of inflammatory cells is a key differentiator from inflammatory immune-mediated processes such as acute demyelinating encephalomyelitis (ADEM) [5]. Unfortunately, lumbar puncture was unable to be performed in our case given significant thalamic edema. Based upon imaging, inflammatory conditions such as ADEM were deemed less likely in our patient, as this usually presents with asymmetric involvement of the central semiovale, basal ganglia, and thalamus [8].
Management involves supportive care with initial resuscitation and treatment of seizures [5]. Immunotherapy is recommended to suppress cytokine production, as these patients are thought to have an exaggerated immune response to viral infections resulting in excess production of pro-inflammatory cytokines [8]. Methylprednisolone 30 mg/kg/day for 3 days (1 g/day maximum) is recommended, improving the prognosis if started within 24 h [5]. Intravenous immunoglobulin and plasmapheresis are considerations though there is no current evidence for either [5]. Outcome reports vary widely, with some indicating 65% mortality or severe neurologic sequelae, and newer reports suggesting mild or no residual sequelae in 60% of cases [1]. Hemorrhage and tissue loss are associated with poor prognosis, while brainstem involvement may portend better recovery [1].
Conclusion
We describe the first reported case of ANE due to SARS-CoV2 in a pregnant female. Limitations of this report include the inability to obtain CSF studies, including CSF SARS-CoV-2. In SARS-CoV-2 infection, ANE should be considered in the differential diagnosis for bilateral thalamic lesions, as prompt detection and management can result in favorable outcomes. While bilateral thalamic lesions often portend a poor prognosis, our patient made an excellent recovery, suggesting outcomes depend on the etiology of the lesions.
Author contribution
All authors made substantial contributions to the conception and design of the manuscript, drafted the work or revised it critically, approved the version to be published, and agree to be accountable for all aspects of the work.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This manuscript adheres to ethical guidelines. This was not submitted to the Institutional Review Board at Rush University Medical Center as this is a case report.
Informed consent
Verbal informed consent was obtained from the individual participant included in this article. There is no identifying information included in the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hannah Breit, Email: Hannah_Breit@rush.edu.
Yazan Radaideh, Email: Yazan_Radaideh@rush.edu.
Sayona John, Email: sayona_john@rush.edu.
References
- 1.Wong AM, Simon EM, Zimmerman RA, et al. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. Am J Neuroradiol. 2006;27:1919–1923. [PMC free article] [PubMed] [Google Scholar]
- 2.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol-Neuroimmunol. 2020;7:e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virhammar J, Kumlien E, Fällmar D, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95:445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuguchi M, Ichiyama T, Imataka G, et al. Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev. 2021;43:2–31. doi: 10.1016/j.braindev.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. J Neurol Sci. 2020;41:1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matar R, Alrahmani L, Monzer N, et al. Clinical presentation and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Clin Infect Dis. 2020;72(3):521–533. doi: 10.1093/cid/ciaa828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevanović V, Barušić Z, Višković K, Rode O, Tešović G. Acute necrotizing encephalopathy of childhood associated with human herpes virus 6 in Croatia. J Neurol Sci. 2019;40(3):639–641. doi: 10.1007/s10072-018-3623-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.