Abstract
In diabetes mellitus, the wound healing process is impaired and delayed. Plants are actively investigated for safe, effective, and well-tolerated treatment options for wound. The plant Buddleja polystachya has a traditional claim for the treatment of wound. Fresh leaves are crushed and given for topical application. The objective of this study was to evaluate the wound healing activity of hydro-alcoholic extract and solvent fractions of leaves of B. polystachya fresen (Buddlejaceae) in normal and diabetic mice.
Method
B. polystachya leaves were extracted using 80% methanol and further fractionated using chloroform, ethyl acetate, and water. The wound healing activity was tested using excision, incision, and diabetic wound models. Area of wound contraction, time of epithelialization, and wound breaking strength were determined.
Result
Treatment with 5% w/w (P < 0 0.05) and 10% w/w (P < 0.001) crude extract of B. polystachya significantly lowered wound area as compared to simple ointment. Treatment of excision wound with 5% w/w and 10% w/w of chloroform, ethyl acetate, and aqueous fractions of B. polystachya significantly lowered wound area and epithelization period (P < 0.05). The 10% w/w extract of B. polystachya showed a significant area of diabetic wound contraction compared to 5% w/w extract and simple ointment. The 10% w/w and 5% w/w extracts of B. polystachya significantly increased wound breaking strength compared to untreated and simple ointment groups (P < 0.05).
Conclusion
The crude and the solvent fractions of B. Polystachya leaves possess wound healing activities as evidenced by an increase in tensile strength and wound contraction rate and decrease in the epithelialization period. This finding supports the traditional claims of B. polystachya for the healing of wounds.
Keywords: Buddleja polystachya, Excision, Incision, Diabetic wound, Mice
1. Introduction
Wound refers to a cut or break in the continuity of the skin caused by injury or operation [1,2]. A wound is classified as an acute and chronic wound [3]. In chronic wounds, the wound showed delayed healing [4]. Wounds associated with vascular insufficiency, diabetes mellitus (DM), and local-pressure effects are included in chronic wounds [5,6]. Based on different criteria; type of injury or presenting symptoms, clinical appearance, etiology, its depth, and location; wounds could be diabetic, cuts and bites, injuries, duodenal ulcers, and gastric [2].
Wounds need the expenditure of public resources and cause a physical and psychological deficiency or even death [7]. DM imposes high morbidity and health care cost due to impaired wound healing. A diabetic foot ulcer is one of the complications of DM [8].
There are several treatment options for acute and chronic wounds like nitrofurazone, mupirocin, silver sulfadiazine, and antibiotics [9]. Most of them are applied in the form of ointment. However, many of them have lowered efficacy, high costs and are associated with adverse effects on the patient, due to the long duration of treatment. Since there is no effective treatment for a diabetic foot ulcer, researchers are exploring new therapeutic strategies from medicinal plants [2,7,[10], [11], [12]]. Medicinal plants used for wound healing activity demonstrated that the various bioactive constituents contributing to mitogenic activities, anti-inflammatory, antioxidant, and antimicrobial are the main effects towards wound healing [13].
Plants of Buddlejaceae family such as Buddleja scordioides and Buddlejaglobosa leaf have wound healing activity [14,15]. Buddlejaglobosa has analgesic, anti-inflammatory, and antioxidant properties [16]. B. polystachya which is known as (“Anfar”) in Amharic, belongs to the family Buddlejaceae. This plant is endemic to Eritrea, Ethiopia, Saudi Arabia, Somalia, and Yemen. Sixteen chemical constituents including isobenzofuranone derivative, phenolic fatty acid ester, flavonoids, and triterpenic acids were isolated from the aerial parts of B. polystachya using various chromatographic techniques [17]. Sixteen bioactive compounds were isolated from the crude extract and solvent fractions of Buddleja polystachya through different chromatographic techniques and were identified using different 1D, 2D NMR, and mass spectrometry. Furthermore, the isolated constituents include 6-O-α-L-(4″-O-trans-cinnamoyl) rhamnopyranosylcatalpol, cirsimaritin, ursolic acid, luteolin, 1′ (4-hydroxyphenyl) ethanol ester of docosanoic, isobenzofuranone derivative (4-hydroxy-7-methylisobenzofuranone), sakuranetin, luteolin 7-(6″-caffeoyl)-O-β-D-glucopyranoside, kumatakenin, oleanolic acid, herbacetin 3,7,8-trimethyl ether, uvaol, 5- hydroxy-3,7,4′-trimethoxyflavone, verbascoside, linarin, luteolin 7-O-β-D-glucoside, and phenolic fatty acid ester [18].B. Polystachya used in traditional medicine as an anti-inflammatory, analgesic, antipyretic, hepatoprotective, hypotensive, hypoglycemic, neuroprotective, antimicrobial, molluscicidal, and amoebicidal remedies. B. polystachya is also used for the treatment of many skin disorders [19]. The plant B. polystachya has a traditional claim for the treatment of wound. Fresh leaves are crushed and given for topical application [20,21]. The plant extract has confirmed antidiarrheal and antispasmodic activities [22], antimicrobial, cytotoxic activity [23], antimalarial activity [24], antioxidant, hypoglycemic, and anti-inflammatory activities [25]. The practice of using plants for the treatment of wounds is also documented just like other ailments. The leaves of B. polystachya has been used in the treatment of wound without any scientific verification for safety and efficacy [20,21]. Thus, the present study aimed to investigate the in vivo wound healing activity of leaves of B. polystachya.
2. Methods and materials
2.1. Collection of plant material
The fresh leaves of B. polystachya were collected in December 2017 from Taragedam forest near Addis Zemen, North West Ethiopia. The plant was authenticated by a botanist Dr. Getnet Masresha and given a voucher number GA 007/2010 from the Department of Biology University of Gondar.
2.2. Experimental animals
Healthy Swiss albino mice of both sex weighing 25–35 g and 6–8 weeks of age and female Sprague-Dawley rats weighing 150–200 g of age 3–4 months were used. Animals were kept in a controlled environment provided with a 12:12 h light and dark cycle at a temperature of approximately 25 °C. Mice and rats were acclimatized for one week to the laboratory environment before the experiment is conducted. Apart from that, all experiments were performed following guidelines for use of animals issued by the institute of laboratory animal resources, national research council guideline, Washington, USA [26]. In all experiments using animals, each mouse was anesthetized with ketamine (70 mg/kg, I.P) and diazepam (5 ml/kg, I.P) injection to alleviate animal pain, and they were euthanized by cervical dislocation after completing experiments.
2.3. Plant extraction and solvent fractionation
The leaves of B. polystachya were washed under running tap water to remove adhering dirt and soil particles. The leaves then were shade dried at room temperature. The dried leaves of B. polystachya were coarsely powdered in a grinder and 1250 g of the powder was cold macerated with 80% methanol in the ratio of 1: 6 and then kept for three days at room temperature with occasional stirring and shaking [27]. After 72 h, the mixture was filtered using gauze followed by Whatman No. 1 filter paper. The residue was re-macerated twice for 72 h to exhaustively extract the active constituent of the plant. The extract was concentrated using an oven set at 40 °C. The hydro alcohol extract was further dried using a lyophilizer (Operan, Korea vacuum limited, Korea).
Solvent fractionation of the crude extract was carried out using chloroform, ethyl acetate, and water. 80 g of crude extract was dissolved completely in 480 ml of distilled water and an equal amount of chloroform was added within a separatory funnel. The chloroform layer (lower) was separated and this process was repeated. The combined chloroform filtrates were evaporated using an oven set at 40 °C. In the aqueous layer, an equal volume of ethyl acetate was added to the separatory funnel. The aqueous layer (lower) was separated and the procedure was repeated. The water in combined aqueous filtrates was evaporated using a lyophilizer (Operan, Korea vacuum limited, Korea). The remaining ethyl acetate fraction was evaporated using an oven set at 40 °C. Finally, the dried crude extract and solvent fractions were stored in a deep freezer set at 0 °C before use [28].
2.4. Preparation of ointment
The ointment fulfills the requirements stated under topical semi-solid preparations [29]. The formula used in British Pharmacopoeia was used for the preparation of a simple ointment base which contains wool fat (50 g), Hard paraffin (50 g), White soft paraffin (850 g), Cetostearyl alcoho1 (50 g), and the plant extract up to 1000 g [30]. Two types of ointment formulations were prepared from the extract: 5% W/W and 10% W/W. To prepare the ointments, 5 g and 10 g of the powdered extract were incorporated into a portion of a simple ointment base by levigating on the surface of the ointment slab to make an ointment of uniform consistency and smooth texture. The remainder of the simple ointment base was gradually added and mixed thoroughly. Finally, the extract ointment was transferred to a clean container for topical application during the experiment. The same procedure was followed for the preparation of each fraction ointment. Additionally, pre-formulated nitrofurazone ointment (0.2% w/w) was used as a standard drug for comparing the wound healing potential of the extract [31].
2.5. Acute dermal toxicity test
The evaluation of dermal acute toxicity was carried out as per the Organization for Economic Cooperation and Development (OECD 434) guideline [32]. Three female non-pregnant rats were randomly chosen. The animals were acclimatized to the laboratory condition for one week before the test. The hair was removed from the back trunks of the animals by shaving 24 h before ointment application. A limit dose of 2 g/kg hydro methanolic extract containing ointment was applied on the shaved back of rats within a range that is approximately 10% of the body surface area. The application site was covered with gauze attached with adhesive plaster. After 24 h, residual test substance was removed and the animals were observed for development of erythema, edema, inflammation, irritation, or redness, and the responses were scored at 24, 48, and 72 h after patch removal. Dermal reactions were graded, recorded and observation was continued daily for fourteen days [33]. The score of primary irritation (SPI) was calculated for each rat as follows:
The primary irritation index (PII) was calculated as the arithmetical mean of the SPI values of the rats. The irritation degree was categorized as negligible (PII = 0–0.4), or slight irritation (PII = 0.5–1.9), moderate irritation (PII = 2–4.9), or severe irritation (PII = 5–8).
2.6. Preliminary phytochemical screening
The qualitative phytochemical investigations of the chloroform, ethyl acetate, and aqueous fractions of B. polystachya were carried out using standard tests [24,34,35].
2.7. Wound healing activity test
2.7.1. Excision wound model
Ten groups, each containing six mice, were prepared for excision wound models. The first group was the negative control and was treated with simple ointment (SO). The second group was a positive control and treated with 0.2% w/w nitrofurazone (NF). The 3rd, 4th, 5th, and 6thgroups were treated with 5% w/w B. polystachya crude extract (BP5), 5% w/w chloroform fraction (CF5), 5% w/w ethyl acetate fraction (EF5), and 5% w/w aqueous fraction (AF5) respectively. The 7th, 8th, 9th, and 10thgroups were treated with 10% w/w B. polystachya crude extract (BP10), 10% w/w chloroform fraction (CF10), 10% w/w ethyl acetate fraction (EF10), and 10% w/w aqueous fraction (AF10), respectively.
Each mouse was anesthetized with ketamine (70 mg/kg, I.P) and diazepam (5 ml/kg, I.P) injection before the creation of wound. The back hair of each mouse was removed by shaver. The area covering 314 mm2 was marked with a marker and wound was created with 2 mm depth on the circular area by using sterilized scissors. After 24 h of wound creation, the ointments were applied gently to cover the wounded area once daily until complete healing was achieved. The area of wound contraction was determined. The area of wound was measured by applying a transparent plastic on the wound and transposing the area using pinpointed marker. Finally, the area on the transparent plastic was quantified using scale graph paper. By considering the first wounding day as day 0, the wound healing progress was evaluated by measuring wound areas on days 2, 4, 6, 8, 10, 12, 14, 16 until the wound completely heals [36,37].
Percentages of wound contraction and epithelialization period were calculated as follows:
Epithelialization time was the number of days required for falling eschar without any residual raw wound.
2.7.2. Diabetic wound model
In the diabetic wound model, five groups were used. Each group contains 6 mice. The first group was diabetic mice and treated with simple ointment (DSO) and the second group was nondiabetic mice and treated with simple ointment (NDSO); the third group was a positive control and treated with 2% mupirocin ointment (MP); The fourth and fifth group were treated with 5% w/w B. polystachya extract and 10% w/w B. polystachya extract, respectively.
DM in mice was induced by streptozotocin injection (150 mg/kg, i.p) in citrate buffer 0.1 M, PH 4.5 after the animals have fasted for 24 h and the baseline blood glucose level was measured. To prevent death from transient hypoglycemia, dextrose 10% was used as a water source for three days. After three days the blood glucose level was measured and mice having blood glucose greater than or equal to 250 mg/dl were included in the study. An excision wound was inflicted. The areas of wound contraction and epithelialization time were calculated like the excision wound model [37].
2.7.3. Incision wound model
Five groups, each containing six mice, were prepared for incision wound models. The first group was the negative control and was treated with simple ointment (SO). The second group was a positive control and treated with 0.2% w/w nitrofurazone (NF). The 3rd and 4th groups were treated with 5% w/w B. polystachya extract and 10% w/w B. polystachya extract, respectively. In the incision model, there was one extra group that was left untreated (UN).
First, the experimental animals were acclimatized to the laboratory environment for seven days. Each mouse was anesthetized with ketamine70 mg/kg and diazepam 5 ml/kg and the dorsal fur of the anesthetized animals were shaved with a shaver, and then paravertebral incisions (6 cm long) was done through the skin at a distance of 1.5 cm from the midline in a sterile condition. After incision, the parted skin was stitched together at intervals of 1 cm with surgical thread (No. 000) and curved needle (No. 11). The treatments for each group were applied. On the 8th post-wounding day sutures were removed and treatment was continued for 10 days. Finally, the skin breaking strength of the healed wound was measured on the 10th day by water flow technique.
On the 10th post-wounding day each mouse was anesthetized using ketamine (70 mg/kg, I.P) and diazepam (5 ml/kg, I.P) injection. The two forceps were firmly applied 1 cm away from healed tissue on the incised part of the skin onto the line facing each other. One of the forceps was fixed on stands, while the other was connected to a freely suspended. Water was allowed to flow into a plastic bag from tap water through the IV line. A slow increase in weight was passing on to the wound that pulls apart the wound edges. Water flow was stopped immediately after wound gaping appeared. The total volume of water collected in the container equal to breaking strength in grams [38].
TS – Tensile Strength, SO- simple ointment, UN- Left untreated
2.8. Statistical analysis
The experimental results were expressed as mean ± standard error of the mean (SEM), and analysis was done using SPSS version 23. Test of statistical significance was carried out by employing one-way analysis of variance (ANOVA) followed by Tukey's Post Hoc test. P < 0.05 is considered statistically significant.
3. Result
3.1. Percentage of extract yield
The percentage yield for the crude extract of B. polystachya was (283.8 g) 22.7% w/w. The percentage yield of the solvent fractions for chloroform, ethyl acetate, and aqueous fractions was (21.3 g) 7.5%, (32.6 g) 11.5%, and (221.4 g) 78% w/w, respectively.
3.2. Acute dermal toxicity
In acute dermal toxicity, the plant was safe with the dose of 10% w/w ointment using the limit dose of 2000 mg/kg. There was no sign of skin reaction, inflammation, erythema, irritation or redness, and any adverse reaction when the animals were followed for 14 days.
3.3. Excision wound model
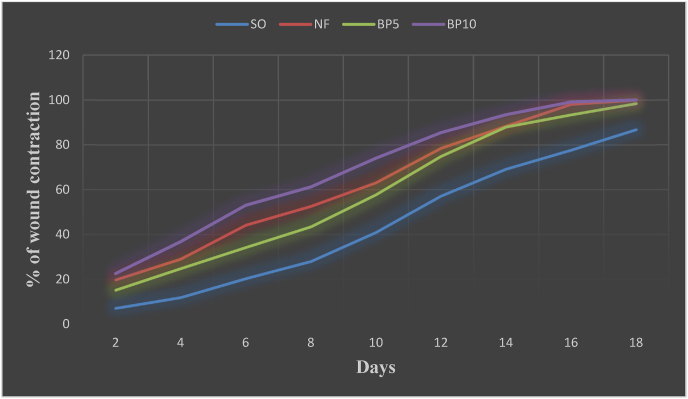
Topical application of hydro methanol extract of B. polystachya ointment showed wound healing activity. The BP10 ointment treated groups showed a significant effect starting from day two (P < 0.05) and BP5 ointment of treated group showed a significant effect starting from day four (P < 0.05). The BP10 ointment showed significant (P < 0.001) would healing activity on days 4, 6, 8, and 10 against the SO, also significant (P < 0.01) activity was observed on days 12, 14, 16, and 18 against the SO. The wound healing activity of the plant was greater than the NF on days 10, 12, and 14 but failed to reach statistical significance (Fig. 1, Table 1). As shown in Fig. 3, the percentage of wound contraction of BP10 ointment was greater than BP5 and NF ointments. On the 12th post wounding day, the percent of wound contraction of BP5, NF, and BP10 ointments were 74.9, 78.5, and 84.5 respectively. The period of epithelization of BP10 (16.67 ± 0.42), and NF (17.0 ± 0.45) were significantly lower than SO (20.0 ± 0.51) (P < 0.01). Animals treated with BP5 (17.83 ± 0.65) also showed a significant decrease in the epithelization period as compared to SO (P < 0.05) (Fig. 1).
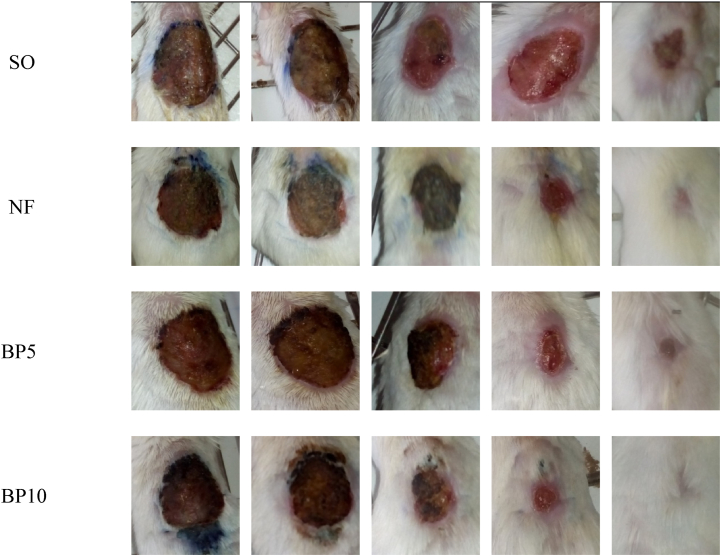
Fig. 1.
Appearance of a healing excision wound on 2nd, 6th, 10th, 14th and 18th post wounding days.
Abbreviations: SO = Simple Ointment, NF = Nitrofurazone ointment, BP5 = 5% BuddlejaPolystachya, BP10 = 10% BuddlejaPolystachya, Initial wound size was 314 mm2.
Table 1.
Effect of 80% methanol extract of B. polystachya on wound area and percent of contraction.
| Treatment | SO | NF | BP5 | BP10 |
|---|---|---|---|---|
| 2nd day | 291.79 ± 13.92 | 251.83 ± 4.43aA | 266.17 ± 7.81 | 243.17 ± 10.85Aa |
| (7.11%) | (19.79%) | (15.23%) | (22.56%) | |
| 4th day | 276.74 ± 15.14 | 222.67 ± 5.48aB | 236.00 ± 8.14aA | 203.67 ± 7.51aC |
| (11.89%) | (29.08%) | (24.84%) | (36.78%) | |
| 6th day | 250.27 ± 13.47 | 175.17 ± 5.60aC | 206.50 ± 4.04aA | 147.50 ± 9.01aC |
| (20.33%) | (44.21%) | (34.23%) | (53.03%) | |
| 8th day | 226.11 ± 15.66 | 148.83 ± 4.55aC | 177.50 ± 9.13aA | 121.83 ± 8.44aC |
| (28.02%) | (52.60%) | (43.47%) | (61.19%) | |
| 10th day | 185.35 ± 17.04 | 115.83 ± 7.75aB | 132.67 ± 12.33aA | 81.67 ± 10.6aC |
| (40.92%) | (63.11%) | (57.75%) | (73.99%) | |
| 12th day | 134.51 ± 21.13 | 63.83 ± 10.412aA | 78.83 ± 14.14aA | 45.83 ± 4.5aB |
| (57.16%) | (78.50%) | (74.89%) | (85.40%) | |
| 14th day | 96.81 ± 23.26 | 35.50 ± 8.049aA | 37.67 ± 13.68aA | 20.67 ± 1.23aB |
| (69.16%) | (88.37%) | (88%) | (93.41%) | |
| 16th day | 70.03 ± 20.11 | 6.00 ± 2.93aB | 20.67 ± 11.94aA | 2.83 ± 1.83aB |
| (77.65%) | (98.09%) | (93.42%) | (99.10%) | |
| 18th day | 41.50 ± 12.14 | 0.00 ± 00aB | 5.00 ± 3.41aB | 0.00 ± 0.00aB |
| (86.78%) | (100%) | (98.41%) | (100%) |
n = 6, Values are expressed as mean ± SEM, one way ANOVA, AP <0 0.05, BP < 0.01, Cp < 0.001, a as compared to the negative control, Abbreviations: SO = Simple Ointment, NF = Nitrofurazone ointment, BP5 = 5% BuddlejaPolystachya, BP10 = 10% BuddlejaPolystachya, Initial wound size was 314 mm2.
Fig. 3.
Effect of crude extracts of B. polystachyaleaves on percentage of wound contraction in excision wound model.
Abbreviations: SO = Simple Ointment, NF = Nitrofurazone ointment, BP5 = 5% BuddlejaPolystachya, BP10 = 10% BuddlejaPolystachya.
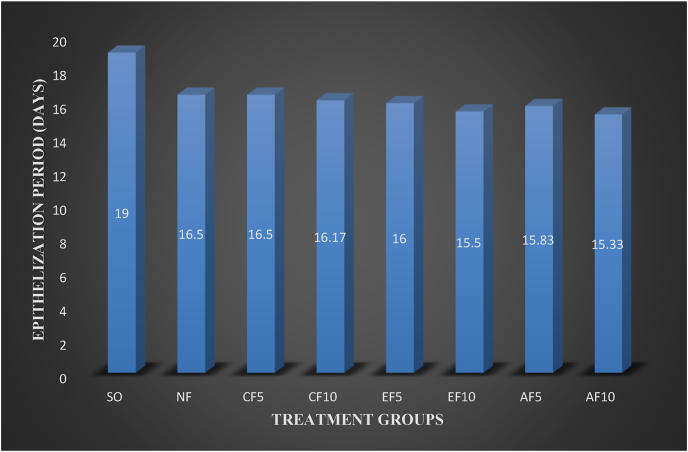
All the three fractions at 5% w/w and 10% w/w except EF5 and EF10 showed statistically significant wound healing effects starting from day 2. However, the EF5 and EF10 started to show significant wound healing effects starting from day 4 (P < 0.001). Wound contraction area at day 2 in CF5 (P < 0.05), CF10 (P < 0.01), AF5 (P < 0.05), and AF10 (P < 0.01) were significantly higher than SO. On day eight, the percent of wound contraction for AF10 and AF5 59.0 and 58.9% respectively. The percent of wound contraction was CF10 and CF5 were only 54.6% and 52% whereas in EF10 and EF5 were 58% and 57% respectively. Maximum percentage of wound contraction at day 16 was reported from AF10 (100%), AF5 (99.2%), CF10 (98.6%) and EF (98.6%). The chloroform, ethyl acetate, and aqueous fractions of B. polystachya showed activity on wound healing in terms of wound contraction and epithelialization. Among the three fractions, the aqueous fraction has the highest percentage of wound contraction and the chloroform fraction has a relatively lowest percentage of wound contraction (Table 2). The epithelization period of AF was shorter than EF and CF. All the 5% w/w and 10% w/w fractions showed a statistically significant effect on epithelization time (P < 0.001) as compared to SO (Fig. 4, Table 3).
Table 2.
Effect of solvent fractions of B. polystachya on wound area and percent of contraction.
| Group | Day2 | Day4 | Day6 | Day8 | Day10 | Day12 | Day14 | Day16 |
|---|---|---|---|---|---|---|---|---|
| SO | 294.33 ± 3.17 | 269.3 ± 3.21 | 237.0 ± 1.89 | 195.33 ± 2.23 | 146.33 ± 2.62 | 111.00 ± 3.32 | 74.50 ± 4.28 | 33.33 ± 6.09 |
| (6.26%) | (14.23%) | (24.52%) | (37.79%) | (53.40%) | (64.65%) | (76.27%) | (89.38%) | |
| NF | 274.0 ± 2.32aA | 245.3 ± 1.56aB | 192.2 ± 1.30aB | 145.50 ± 2.09aC | 103.3 ± 5.36aC | 68.83 ± 7.3aC | 37.83 ± 4.31aC | 5.50 ± 3.50aB |
| (12.74%) | (21.87%) | (38.80%) | (53.66%) | (67.09%) | (78.08%) | (87.95%) | (98.25%) | |
| CF5 | 275.2 ± 5.22aA | 240.8 ± 4.71aC | 198.3 ± 2.7aC | 150.83 ± 1.64aC | 113.7 ± 2.84aB | 70.33 ± 2.82aC | 41.67 ± 1.78aC | 9.33 ± 4.30aA |
| (12.37%) | (23.30%) | (36.84%) | (51.96%) | (63.80%) | (77.60%) | (86.73%) | (97.03%) | |
| CF10 | 273.2 ± 4.27aB | 239.8 ± 3.25aC | 187.3 ± 5.73aC | 142.50 ± 8.15aC | 99.33 ± 6.54aC | 65.00 ± 3.72aC | 38.00 ± 1.10aC | 4.33 ± 4.33aB |
| (13.00%) | (23.62%) | (40.34%) | (54.62%) | (68.36%) | (79.30%) | {87.90%) | (98.62%) | |
| EF5 | 283.33 ± 3.57 | 237.8 ± 5.71aC | 192.0 ± 7.01aC | 135.00 ± 8.08aC | 92.50 ± 9.68aC | 64.83 ± 5.91aC | 37.50 ± 4.74aC | 10.5 ± 6.67aA |
| (9.77%) | (24.26%) | (38.85%) | (57.01%) | (70.54%) | (79.35%) | (88.06%) | (96.65%) | |
| EF10 | 283.00 ± 4.20 | 238.0 ± 2.76aC | 182.3 ± 4.15aC | 131.83 ± 6.05aC | 91.00 ± 4.29aC | 59.50 ± 2.68aC | 34.83 ± 1.99aC | 4.33 ± 4.33aB |
| (9.87%) | (24.21%) | (41.93%) | (58.01%) | (71.02%) | (81.05%) | (88.91%) | (98.62%) | |
| AF5 | 277.0 ± 3.1 aA |
237.3 ± 5. 4aC |
186.7 ± 5.49aC | 129.00 ± 5.90aC | 90.67 ± 4.90aC | 59.00 ± 4.44aC | 38.00 ± 1.51aC | 2.67 ± 2.66aB |
| (11.78%) | (24.42%) | (40.55%) | (58.92%) | (71.13%) | (81.21%) | (87.90%) | (99.15%) | |
| AF10 | 270.3 ± 3.95aB | 241.0 ± 3.5aC | 188.0 ± 7.67aC | 128.67 ± 7.96aC | 89.33 ± 4.86aC | 57.17 ± 5.86aC | 35.17 ± 1.42aC | .00 ± .00aB |
| (13.91%) | (23.25%) | (40.13%) | (59.02%) | (71.55%) | (81.79%) | (88.80%) | (100%) |
n = 6, Values are expressed as mean ± SEM, one way ANOVA,AP <0 0.05, BP < 0.01, Cp < 0.001,aagainst the negative control, Abbreviations: SO = Simple Ointment, NF = Nitrofurazone ointment, CF5 = 5% chloroform fraction, CF10 = 10% chloroform fraction, EF5 = 5% ethyl acetate fraction, EF10 = 10% ethyl acetate fraction, AF5 = 5% aqueous fraction, AF10 = 10% aqueous fraction. Initial wound size was 314 mm2.
Fig. 4.
Effect of solvent fractions of B. polystachyaleaves on epithelialization days on excision wound model.
Abbreviations: SO = Simple Ointment, NF = Nitrofurazone ointment, CF5 = 5% chloroform fraction, CF10 = 10% chloroform fraction, EF5 = 5% ethyl acetate fraction, EF10 = 10% ethyl acetate fraction, AF5 = 5% aqueous fraction, AF10 = 10% aqueous fraction.
Table 3.
Effect of solvent fractions of B. polystachya on epithelization period.
| Group | Epithelization period (days) |
|---|---|
| SO | 19.00 ± 0.37 |
| NF | 16.50 ± 0.34aC |
| CF5 | 16.50 ± 0.22aC |
| CF10 | 16.17 ± 0.17Ac |
| EF5 | 16.00 ± 0.37aC |
| EF10 | 15.50 ± 0.34aC |
| AF5 | 15.83 ± 0.31aC |
| AF10 | 15.33 ± 0.21aC |
n = 6, Values are expressed as mean ± SEM, one way ANOVA,a against the control, Cp < 0.001, Abbreviations: SO = Simple Ointment, NF = Nitrofurazone ointment, CF5 = 5% chloroform fraction, CF10 = 10% chloroform fraction, EF5 = 5% ethyl acetate fraction, EF10 = 10% ethyl acetate fraction, AF5 = 5% aqueous fraction, AF10 = 10% aqueous fraction.
3.4. Diabetic wound model
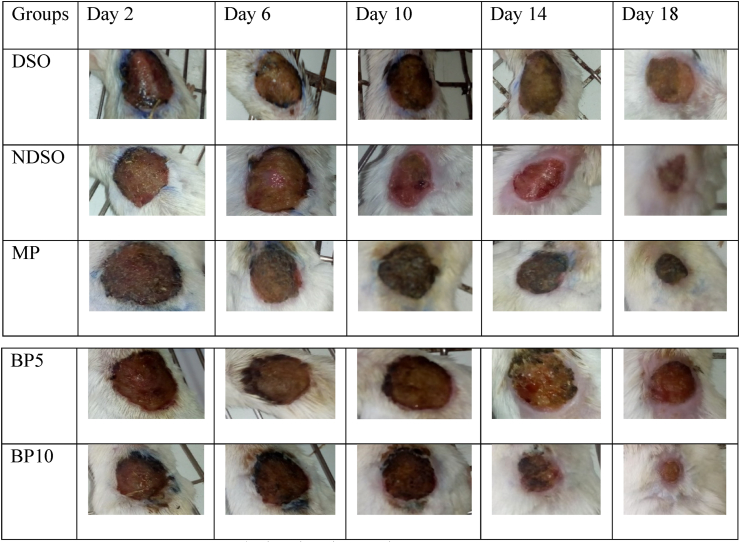
Wound contraction due to treatment with 5% w/w and 10% w/w crude extract of B. polystachya ointment, simple ointment base, and Mupirocin ointment is shown in Table 4. The 10% w/w ointment of the crude extract showed significant (P < 0.001) wound healing activity beginning from day 8th to day 18 when compared to NDSO and DSO. Likewise, B. polystachya5% w/w ointment has shown a significant wound contraction on 14th (P < 0.05), 16th (P < 0.05), and18th (P < 0.001) days of treatment when compared to DSO. The percentage contraction of Mupirocin ointment was significant on 6th (P < 0.05) day as compared to DSO and also significant wound contraction was observed on the 8th, 10th, and 12th day (P < 0.001 and P < 0.01 as compared to DSO and NDSO, respectively). Likewise, the percentage contraction of Mupirocin ointment was significant (P < 0.01) on 14th, 16th, and 18th day wound contraction was observed when compared to NDSO and DSO. Higher percentages of wound contraction (79.88% and 89.70%) were observed in animals treated with the 10% crude extract ointment from the 16th and 18th day, respectively. The non-diabetic group showed natural healing of wound and till day 16 didn't show significant effect against the diabetic control (Fig. 2, Table 4).
Table 4.
Effect of crude of B. polystachya in diabetic wound area and percent of contraction.
| Treatment | DSO | NDSO | MP | BP5 | BP10 |
|---|---|---|---|---|---|
| 2nd day | 298.50 ± 2.93 | 298.83 ± 1.72 | 297.17 ± 2.47 | 296.67 ± 2.81 | 295.67 ± 3.52 |
| 4.94% | 4.83% | 5.36% | 5.52% | 5.84% | |
| 4th day | 280.50 ± 3.92 | 278.83 ± 2.44 | 273.33 ± 2.23 | 273.67 ± 2.23 | 271.17 ± 4.89 |
| 10.67% | 11.20% | 12.95% | 12.84% | 13.64% | |
| 6th day | 260.17 ± 3.04 | 253.67 ± 2.62 | 242.5 ± 3.12aA | 250.5 ± 2.42 | 238.8 ± 5.98aB |
| 17.14% | 19.21% | 22.77% | 20.22% | 23.94% | |
| 8th day | 239.17 ± 3.17 | 231.50 ± 3.18 | 208.0 ± 5.13aCbB | 226.3 ± 2.09 | 210.7 ± 4.97aCbB |
| 23.83% | 26.27% | 33.76% | 27.92% | 32.91% | |
| 10th day | 218.17 ± 2.87 | 207.00 ± 5.99 | 179.0 ± 5.63aCbB | 203.67 ± 2.19 | 175.3 ± 5.64aCbB |
| 30.52% | 34.08% | 42.99% | 35.14% | 44.16% | |
| 12th day | 193.17 ± 3.97 | 181.17 ± 5.13 | 140.2 ± 5.37aCbB | 173.50 ± 2.26 | 139.5 ± 6.76aCbC |
| 38.48% | 42.30% | 55.36% | 44.75% | 55.57% | |
| 14th day | 169.00 ± 4.76 | 152.33 ± 3.59 | 101.0 ± 5.64aCbC | 144.50 ± 2.28aA | 109.2 ± 5.15aCbC |
| 46.18% | 51.49% | 67.83% | 53.98% | 65.23% | |
| 16th day | 141.50 ± 5.25 | 126.50 ± 4.24 | 58.3 ± 6.02aCbC | 118.8 ± 1.85aA | 63.2 ± 5.64aCbC |
| 54.94% | 59.71% | 81.42% | 62.16% | 79.88% | |
| 18th day | 117.33 ± 4.25 | 102.50 ± 5.44 | 27.2 ± 2.34aCbC | 88.17 ± 3.19aC | 32.3 ± 2.92aCbC |
| 62.63% | 67.35% | 91.35% | 71.92% | 89.70% |
n = 6, Values are expressed as mean ± SEM, one way ANOVA, AP <0 0.05, BP < 0.01, CP < 0.001, aagainst DSO, bagainst NDSO, Abbreviations: DSO = Diabetic mice treated with Simple Ointment, NDSO = Nondiabetic mice treated with Simple Ointment, MP = Mupirocin ointment, BP5 = 5% BuddlejaPolystachya, BP10 = 10% BuddlejaPolystachya. Initial wound size was 314 mm2.
Fig. 2.
Diabetic wound on day, 2nd, 6th, 10th, 14th and 18th postoperative days.
Abbreviations: DSO = Diabetic mice treated with Simple Ointment, NDSO = Nondiabetic mice treated with Simple Ointment, MP = Mupirocin ointment, BP5 = 5% BuddlejaPolystachya, BP10 = 10% BuddlejaPolystachya. Initial wound size was 314 mm2.
3.5. Incision wound model
The mean tensile strength in the group treated with a simple ointment base tended to increase by about 13.19% compared to untreated controls, which failed to reach statistical significance. The 10% w/w of the crude extract ointment treated groups showed a significant (P < 0.01) increase in tensile strength compared to simple ointment and the untreated control group. Similarly, the 5% w/w of the crude extract ointment treated groups showed a significant (P < 0.05 and P < 0.01) increase in tensile strength compared to simple ointment and the untreated control group, respectively. However, simple ointment treated groups did not show a statistically significant increase in breaking strength when compared to the untreated control group. The percentage of tensile strength of BP10, BP5, NF, and SO base were 57.4%, 51.2%, 52.2%, and 13.2% respectively (Table 5).
Table 5.
Effect of crude extract of B. polystachya on tensile strength.
| Group | Tensile strength in gm | % Tensile strength |
|---|---|---|
| UN | 166.83 ± 9.361 | |
| SO | 188.83 ± 9.645 | 13.18 |
| NF | 287.33 ± 15.64aBbA | 52.16 |
| BP5 | 285.5 ± 19.14aBbA | 51.19 |
| BP10 | 297.17 ± 32.17aBbB | 57.37 |
n = 6, Values are expressed as mean ± SEM, one way ANOVA, AP <0 0.05,BP < 0.01, aagainst UN,b against SO, Abbreviations: UN=Untreated group, SO = Simple Ointment, NF = Nitrofurazone ointment, BP5 = 5% BuddlejaPolystachya, BP10 = 10% BuddlejaPolystachya.
4. Discussion
In the present study, three different models were used to assess the effects of an ointment containing the hydro methanol extract of B. Polystachya. To assess the activity of drug on the various phases of wound which are independent and sometimes run concurrently, the use of two or more models is mandatory [36,39,40]. The models used in this study are excision, Incision, and diabetic wound models. The first two models are used to study the wound healing effect of B. polystachya on acute wounds. The diabetic wound model is used to study the effect of the plant extract on the chronic wound.
In our study, the crude extract and fractions of the plant B. polystachya treated groups had a greater area of wound contraction at 5% w/w and 10% w/w concentrations as compared with the simple ointment. Furthermore, wound contraction rate and tensile strength of 10% (w/w) of extract ointment were even better than the standard drug (Nitrofurazone). This is maybe due to enhanced activity of fibroblast, an increase in the synthesis of collagen, antioxidant, anti-inflammatory, and antimicrobial activity of the plant. Contraction of wounds decreases healing time because it decreases the size of the wound and reduces the amount of extracellular matrix needed to repair the defect. Contraction also facilitates re-epithelization by shortening the distance which migrating keratinocytes must travel [36].
Plants with antimicrobial and anti-inflammatory agents promote wound healing by forming a barrier against microbial infection in the wound especially against microbes like Staphylococcus aureus and Pseudomonas aeruginosa [41,42]. In previous studies, the antimicrobial activity of ethyl acetate extract of B. polystachya was confirmed against gram-positive bacteria like staphylococcus aureus, staphylococcus epidermidis, and streptococcus pyogenes [23]. The B. polystachya has also reported for anti-inflammatory activity using the carrageenan-induced paw edema model. Another possible mechanism for t
he wound healing effect could be its anti-oxidant effect. As per previously reported studies, the anti-oxidant activity has a significant effect on wound healing. Compounds that have antioxidant effects inhibit lipid peroxidation and prevent cell damage and increase collagen fibrillary endurance [43]. The anti-oxidant activity of B. polystachya might promote wound healing by inhibiting lipid peroxidation and increasing collagen fibrillary endurance [42]. In the previous study, constituents such as 6-O-α-L-(4″-O-trans-cinnamoyl) rhamnopyranosylcatalpol, cirsimaritin, ursolic acid, luteolin, 1′ (4-hydroxyphenyl) ethanol ester of docosanoic, isobenzofuranone derivative (4-hydroxy-7- methylisobenzofuranone), sakuranetin, luteolin 7-(6″-caffeoyl)-O-β-D-glucopyranoside, kumatakenin, oleanolic acid, herbacetin 3,7,8-trimethyl ether, uvaol, 5- hydroxy-3,7,4′-trimethoxyflavone, verbascoside, linarin, luteolin 7-O-β-D-glucoside, and phenolic fatty acid ester were isolated from the crude extract and solvent fractions of B. polystachya [18]. These bioactive compounds were some of the renowned compounds that were also isolated from the medicinal plant with a potential antimicrobial, anti-inflammatory, and antioxidant activity [38,[44], [45], [46]]. Thus, the significant wound healing activities of B. polystachya in both acute and chronic wounds could be due to the presence of the aforementioned constituents in the plant, which could act synergistically and/or independently.
Diabetic wounds showed a significantly delayed re-epithelialization period compared to non-diabetic wounds. The epithelialization period of healing of nondiabetic excision was found to be around 21 days which was shorter than the diabetic excision wound (followed for 30 days without epithelialization). This may be due to reduced levels of the growth factor-like including fibroblast growth factors, epidermal growth factors, platelet-derived growth factors, vascular endothelial growth factor, and tumor growth factor-β in diabetic wounds as compared to acute wounds [8]. Besides, endothelial cells damage may occur by occlusion of capillary vessels, leukocyte dysfunction, and reactive oxygen species generated due to increased blood glucose levels [47]. Moreover, increased oxidative stress leads to the activation of matrix metalloproteases (MMPs) which increases collagen degradation and decreases collagen synthesis.
The significant diabetic wound healing activity of the plant with 10%w/w strength may be due to dose-dependent activity of the extract, greater free radicals scavenging activity and increasing the formation of capillary vessels.
The BP10 ointment showed 57.7% tensile strength which was greater than SO, NF and BP5. The observed increase in tensile strength of incision wound may be due to significant activity in collagen synthesis and fibroblast formation. Further an increase in deposition of newly synthesized collagens at the wound site also responsible for the observed effect [48]. The anti-oxidant property of the plant may help for increasing the tensile strength of the wound as well. An increase in the skin breaking strength of the animals treated with the B. polystachya suggests the probable increase in the synthesis of aldehyde groups of collagen fibres. This aldehyde is responsible for cross-linkage resulting in greater tensile strength [49].
4.1. The strength and limitation of the study
Despite we have tried our best to collect accurate data, to include an adequate number of mice, to use appropriate animal models, to follow the mice relatively longer duration, and we have used our best efforts throughout the study, the study may not be out of limitation. As a limitation, histopathological samples from healed skin tissue of the mice were not taken to confirm the experimental results in terms of concentration of inflammatory cells, collagen fibers, fibroblasts, and angiogenesis in tissue at 18th post wounding days.
5. Conclusion
The crude extract and solvent fractions of B. Polystachya showed significant wound healing activity. Which was demonstrated by a significant rate of wound contraction, period of epithelization, and greater tensile strength. The wound healing activity may be due to the presence of biologically active secondary metabolites acting either individually or collectively to bring about the overall effect. The wound-healing effect of extract of B. Polystachya in our study confirmed the traditional claim related to its use for the treatment of a wound. In the future perspective, further investigation is required for the isolation and characterization of the active principle responsible for the wound healing activity of the extract.
Availability of data and materials
The data is available in the public library of University of Gondar in a form of a graduate student thesis.
Funding
None.
CRediT authorship contribution statement
Abiey Getahun: Writing – original draft, Data curation, Formal analysis, Supervision, contributed to designing the study, writing the final research, data interpretation, data analysis, final manuscript preparation, and supervision of the study. All authors read and approved the final manuscript. Zemene Demelash Kifle: Writing – original draft, Data curation, Formal analysis, Supervision, contributed to designing the study, writing the final research, data interpretation, data analysis, final manuscript preparation, and supervision of the study. All authors read and approved the final manuscript. Digambar Ambikar: Writing – original draft, Data curation, Formal analysis, Supervision, contributed to designing the study, writing the final research, data interpretation, data analysis, final manuscript preparation, and supervision of the study. All authors read and approved the final manuscript. Seyfe Asrade Atnafie: Writing – original draft, Data curation, Formal analysis, Supervision, contributed to designing the study, writing the final research, data interpretation, data analysis, final manuscript preparation, and supervision of the study. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgment
The authors would like to acknowledge University of Gondar for material support for this project.
Authors contribution statement
AG, SAA, DA, and ZDK contributed for designing the study, writing the final research, data interpretation, data analysis, final manuscript preparation, and supervision of the study. All authors have read and approved the final manuscript.
Ethical clearance
The ethical and legal approval was obtained from the school of pharmacy, University of Gondar with reference number SOP 005/2019. Further, all experiments were performed by following guideline for use of animals issued by the institute of laboratory animal resources, national research council guideline, Washington, USA.
References
- 1.Agrawal A.S. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccines Immunother. 2016;12(9):2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tessema Z., Yibeltal D., Molla Y. Evaluation of the wound healing activity of the crude extract of root bark of Brucea antidysentrica, the leaves of Dodonaea angustifolia and Rhamnus prinoides in mice. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2021.e05901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreifke M.B., Jayasuriya A.A., Jayasuriya A.C. Current wound healing procedures and potential care. Mater Sci Eng C. 2015;48:651–662. doi: 10.1016/j.msec.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S., Young A., McNaught C.-E. The physiology of wound healing. Surgery. 2017;35(9):473–477. [Google Scholar]
- 5.Mustoe T.A., O'Shaughnessy K., Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(7S):35S–41S. doi: 10.1097/01.prs.0000225431.63010.1b. [DOI] [PubMed] [Google Scholar]
- 6.Nunan R., Harding K.G., Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Disease models & mechanisms. 2014;7(11):1205–1213. doi: 10.1242/dmm.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gushiken L.F.S. Skin wound healing potential and mechanisms of the hydroalcoholic extract of leaves and oleoresin of Copaifera langsdorffii Desf. Kuntze in rats. Evid base Compl Alternative Med. 2017;14 doi: 10.1155/2017/6589270. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirapara H. Effects of ethanolic extract of Jasminum grandiflorum Linn. flowers on wound healing in diabetic Wistar albino rats. Avicenna journal of phytomedicine. 2017;7(5):401. [PMC free article] [PubMed] [Google Scholar]
- 9.Graça M.F. Electrospun asymmetric membranes as promising wound dressings: a review. Pharmaceutics. 2021;13(2):183. doi: 10.3390/pharmaceutics13020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson J.R. Current concepts in wound management and wound healing products. Vet Clin North Am Small Anim Pract. 2015;45(3):537–564. doi: 10.1016/j.cvsm.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Hilton J. Wound dressings in diabetic foot disease. Clin Infect Dis. 2004;39(Supplement_2):S100–S103. doi: 10.1086/383270. [DOI] [PubMed] [Google Scholar]
- 12.Elnahas R.A. Egyptian Olea europaea leaves bioactive extract: antibacterial and wound healing activity in normal and diabetic rats. Journal of Traditional and Complementary Medicine. 2021;11 doi: 10.1016/j.jtcme.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali A. A novel herbal hydrogel formulation of moringa oleifera for wound healing. Plants. 2021;10(1):25. doi: 10.3390/plants10010025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Mensah A. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J Ethnopharmacol. 2001;77(2):219–226. doi: 10.1016/s0378-8741(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Gutierrez R.M., Vargas-Solis R. Wound healing properties of triterpenes from Buddleia scordioides in diabetic rats. Pharmaceut Biol. 2008;46(9):647–653. [Google Scholar]
- 16.Backhouse N. Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J Ethnopharmacol. 2008;116(2):263–269. doi: 10.1016/j.jep.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Al Ati H.Y. Phytochemical and biological evaluation of Buddleja polystachya growing in Saudi Arabia. Pak J Pharm Sci. 2015;28(4):1533–1540. [PubMed] [Google Scholar]
- 18.Al Ati H.Y. Phytochemical and biological evaluation of Buddleja polystachya growing in Saudi Arabia. Pak J Pharm Sci. 2015;28(4):1533–1540. [PubMed] [Google Scholar]
- 19.El-Gamal A. Natural Product Research; 2017. Chemical composition of Buddleja polystachya aerial parts and its bioactivity against Aedes aegypti; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Enyew A. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. [Google Scholar]
- 21.Chekole G., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4. doi: 10.1186/1746-4269-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehman N.u. Antidiarrheal and antispasmodic activities of Buddleja polystachya are mediated through dual inhibition of Ca++ influx and phosphodiesterase enzyme. Phytother Res. 2015;29(8):1211–1218. doi: 10.1002/ptr.5367. [DOI] [PubMed] [Google Scholar]
- 23.Fawzy G.A., El Gamal A.A., Al Ati H.Y. Antimicrobial and cytotoxic potentials of Buddleja polystachya extracts. Bangladesh J Pharmacol. 2013;8(2):136–141. [Google Scholar]
- 24.Mohammed N., Abdulwuhab M., Mohammed F. Antimalarial activity of crude extract of Buddleja polystachya fresen (buddlejacea) against plasmodium berghei in mice. IOSR J Pharm Biol Sci. 2016;11(5):27–35. [Google Scholar]
- 25.Mothana R.A.A. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2009;64(4):260–268. [PubMed] [Google Scholar]
- 26.Council N.R. National Academies Press; 2010. Guide for the care and use of laboratory animals. [Google Scholar]
- 27.Tesfaye W.H., Alamneh E.A. In vivo antimalarial activity of the crude extract and solvent fractions of the leaves of Zehenria scabra (Cucurbitaceae) against Plasmodium berghei in mice. J Med Plants Res. 2014;8(42):1230–1236. [Google Scholar]
- 28.Fentahun S. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Compl Alternative Med. 2017;17(1):13. doi: 10.1186/s12906-016-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaur R. vol. 3. Crown, Inc. London; 2009. p. 9983. (Simple ointment: formulated preparations. British Pharmacopoeia). [Google Scholar]
- 30.Pharmacopoeia B. 2016. British pharmacopoeia. [Google Scholar]
- 31.Bhaskar A., Nithya V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L (Malvaceae) in Wistar albino rats. Indian J Pharmacol. 2012;44(6):694. doi: 10.4103/0253-7613.103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oecd O. 2015. Guideline for testing of chemicals: draft updated test guideline 402 on acute dermal toxicity. [Google Scholar]
- 33.Class A. 2001. Oecd guideline for testing OF chemicals. [Google Scholar]
- 34.Ayoola G. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharmaceut Res. 2008;7(3):1019–1024. [Google Scholar]
- 35.Wadood A. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2(4):1–4. [Google Scholar]
- 36.Mekonnen A. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638–646. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Morton J., Malone M. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196(1):117. [PubMed] [Google Scholar]
- 38.Mulisa E., Asres K., Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J.(Polygonaceae) in mice. BMC Compl Alternative Med. 2015;15(1):341. doi: 10.1186/s12906-015-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdulla M.A. Role of Ficus deltoidea extract in the enhancement of wound healing in experimental rats. Biomed Res. 2010;21(3) [Google Scholar]
- 40.Arun M., Satish S., Anima P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. leaves. Avicenna journal of phytomedicine. 2016;6(3):295. [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammed N., Abdulwuhab M., Mohammed F. Antimalarial activity of crude extract of Buddleja polystachya fresen (buddlejacea) against plasmodium berghei in mice. Journal of Pharmacy and Biological Sciences. 2016;11(5):27–35. [Google Scholar]
- 42.Atsbeha B., Mammo F., Kibret B. Phytochemical investigation on the leaves of Buddleja Polystachya (ethanol extract) International Journal of Integrative sciences, Innovation and Technology. 2014;3:7–10. [Google Scholar]
- 43.Tümen İ. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait) J Ethnopharmacol. 2018;211:235–246. doi: 10.1016/j.jep.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Sagbo I.J., Afolayan A.J., Bradley G. Antioxidant, antibacterial and phytochemical properties of two medicinal plants against the wound infecting bacteria. Asian Pacific Journal of Tropical Biomedicine. 2017;7(9):817–825. [Google Scholar]
- 45.Atsbeha B., Mammo F., Kibret B. Phytochemical investigation on the leaves of Buddleja Polystachya (ethanol extract) International Journal of Integrative sciences, Innovation and Technology. 2014;3:7–10. [Google Scholar]
- 46.Silambujanaki P. Wound healing activity of Glycosmis arborea leaf extract in rats. J Ethnopharmacol. 2011;134(1):198–201. doi: 10.1016/j.jep.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 47.Deshmukh P.T. Wound healing activity of Calotropis gigantea root bark in rats. J Ethnopharmacol. 2009;125(1):178–181. doi: 10.1016/j.jep.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Maurya H., Semwal M., Dubey S.K. Pharmacological evaluation of Chrozophora tinctoria as wound healing potential in diabetic rat's model. BioMed Res Int. 2016;2016 doi: 10.1155/2016/7475124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shivhare Y. Wound healing potential of methanolic extract of Trichosanthes dioica Roxb (fruits) in rats. J Ethnopharmacol. 2010;127(3):614–619. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available in the public library of University of Gondar in a form of a graduate student thesis.