Abstract
The nucleus accumbens (NAc), consisting of core (NAcC) and shell (NAcS) sub-regions, has primarily been studied as a locus mediating the effects of drug reward and addiction. However, there is ample evidence that this region is also involved in regulating aversive responses, but the exact role of the NAc and its subregions in regulating associative fear processing remains unclear. Here, we investigated the specific contribution of the NAcC and NAcS in regulating both fear expression and fear extinction in C57BL/6J mice. Using Arc expression as an indicator of neuronal activity, we first show that the NAcC is specifically active only in response to an associative fear cue during an expression test. In contrast, the NAcS is specifically active during fear extinction. We next inactivated each subregion using lidocaine and demonstrated that the NAcC is necessary for fear expression, but not for extinction learning or consolidation of extinction. In contrast, we demonstrate that the NAcS is necessary for the consolidation of extinction, but not fear expression or extinction learning. Further, inactivation of mGluR1 or ERK signaling specifically in the NAcS disrupted the consolidation of extinction but had no effect on fear expression or extinction learning itself. Our data provide the first evidence for the importance of the ERK/MAPK pathway as the underlying neural mechanism facilitating extinction consolidation within the NAcS. These findings suggest that the NAc subregions play dissociable roles in regulating fear recall and the consolidation of fear extinction, and potentially implicate them as critical regions within the canonical fear circuit.
Keywords: Pavlovian fear conditioning, mGluR1, ERK, Extinction
1. Introduction
Excessive associative fear and the inability to extinguish learned fear are some of the major hallmarks of several disabling anxiety disorders and trauma disorders such as post-traumatic stress disorder (PTSD) (Rothbaum and Davis, 2003; Cain et al., 2012; Mahan and Ressler, 2012). In a clinical setting, anxiety disorders, apart from a broad spectrum of co-existing symptoms, are also highly comorbid with substance abuse (Merikangas et al., 1998; Lai et al., 2015; Stewart et al., 2016). While failure to extinguish fear is an underlying mechanism contributing to anxiety disorders and PTSD, failure to extinguish appetitive behavior can lead to drug relapse, and PTSD patients have some increased likelihood of relapse to drugs of abuse (Brown et al., 1996; Breslau et al., 1997; Najavits et al., 2007). Thus, identifying common neural circuits associated with conditioned aversive responses and drug reinforcement will be fundamental to elucidating the pathophysiology of anxiety disorders and for developing broad pharmacologic interventions. One putative component of such a circuit is the nucleus accumbens (NAc), which acts as a hub guiding both aversive and appetitive behaviors (For review see Berridge, 2019). While the contribution of the NAc in appetitive behaviors, such as drug seeking, is well-established, its role in aversive conditioning remains less clear.
The NAc, a critical node for limbic-motor integration, is a heterogenous structure divided into core and shell subregions that differ in their connectional and cellular framework (Voorn et al., 1994; Meredith et al., 1996; Baliki et al., 2013). In addition, the two NAc subregions show distinctive innervation patterns by brain regions heavily implicated in emotional learning (Brog et al., 1993; Brauer et al., 2000). Data from a number of labs using a wide variety of behavioral methods suggest that the NAc is critically involved in aversive behavior and fear learning (McCullough et al., 1993; Salamone, 1994; Beck and Fibiger, 1995; Levita et al., 2002a; Reynolds and Berridge, 2002, 2003; Thomas et al., 2002; Schwienbacher et al., 2004; Muschamp et al., 2011; Richard and Berridge, 2011; Wendler et al., 2014a; Ramirez et al., 2015), but the underlying neurobiology is poorly understood and only a few studies have made functional distinctions in NAc anatomy in the same study (Reynolds and Berridge, 2002; Richard and Berridge, 2011, 2013). In fact, the specific dissociable role of the two sub-regions in Pavlovian fear learning remains unclear.
In addition to identifying brain regions involved in fear expression and extinction, it is also imperative to study the underlying mechanisms within the NAc regulating fear processing. In addition to AMPA and NMDA receptors, the NAc expresses large numbers of postsynaptic group I metabotropic glutamate receptors (mGluRs; mGluR1 and mGluR5) that are substantially contacted by terminals from the basolateral amygdala and infralimbic cortex (Mitrano and Smith, 2007; Mitrano et al., 2010). mGluRs, widely distributed across brain regions critical for aversive emotional regulation, have been implicated in memory consolidation, fear learning and extinction, and synaptic plasticity (Riedel et al., 2003; Kim et al., 2007; Xu et al., 2009; Menard and Quirion, 2012). At the molecular level, signaling via mGluR1 is coupled to the extracellular signal-regulated kinases/mitogen-activated protein kinases (ERK/MAPK) pathway which has also been strongly implicated in the acquisition, consolidation and retention of extinction memory (Schafe et al., 2000; Santini et al., 2004; Herry et al., 2006; Fischer et al., 2007). However, no study has investigated how mGluR1s and the ERK/MAPK signaling pathway within the NAc regulate fear learning processes.
In the current study we found that the NAc core is important for fear expression, but not extinction learning or the consolidation of extinction. We found that the NAc shell regulates the consolidation of extinction through mGluR1 activation and ERK/MAPK signaling. Our findings strongly implicate the NAc as an important node in the canonical fear circuit but also demonstrate that the NAc core and shell subregions have dissociable roles in fear processing.
2. Methods
2.1. Animals
All experiments used 7–9 weeks old adult male C57BL/6J mice which were generated from a breeding colony in the Department of Psychological Sciences at Kent State University, from breeders originally purchased from Jackson Laboratories (Stock# 000664). Mice were of F4 generation or less to avoid genetic drift, group housed with 2–5 mice/cage, and on a 12:12 light/dark cycle with free access to food and water. Mice used for experiments were seven weeks or older. All experiments were conducted with approval from the Kent State University Institutional Animal Care and Use Committee (IACUC) and in a facility accredited by the Association for Assessment and Accreditation and Laboratory Animal Care (AALAC), in accordance with the NIH guidelines for the Care and Use of Laboratory Animals, 8th edition.
2.2. Stereotaxic surgery
Mice at 7–9 weeks of age underwent stereotaxic surgery for cannula implantation. Mice were anesthetized with a subcutaneous injection of Ketamine (75 mg/kg) + Xylazine (10 mg/kg) and Acepromazine (2 mg/kg) cocktail prior to the surgery. Following anesthesia, the mice received a single subcutaneous administration of ketoprofen (5 mg/kg) for analgesia. The mice were then mounted on a stereotaxic apparatus (David Kopf instruments, Tujunga, CA) and the scalp of each mouse was retracted. The skull was adjusted so that lambda and bregma were within 0.5 mm of each other. Two guide cannulae (26-gauge, 4 mm pedestal height; Plastics One, Ranaoke, VA) were bilaterally implanted aimed at the nucleus accumbens core (+1.18 mm AP; +2.20 mm ML; −4.52 mm DV; angled at 14°) or nucleus accumbens shell (+1.30 mm AP; +1.60 mm ML; −4.40 mm DV; angled at 10°), secured with screws and cranial cement. Dummy cannulae were inserted in the guide cannulae after the completion of the surgeries. Upon completion of surgeries, the anesthesia was reversed with a subcutaneous injection of atipamezole (0.5 mg/kg). All mice were given one week to recover prior to the start of behavioral procedures.
3. Behavioral procedures
3.1. Apparatus
Fear conditioning was performed in four identical conditioning chambers containing two Plexiglas walls, two aluminum sidewalls, and a stainless-steel grid-shock floor, and positioned inside an isolation chamber (Coulbourn Instruments, Allentown, PA). A speaker was positioned along the sidewall to generate a tone to provide the conditional stimulus (CS). The cued fear training context, Context A, consisted of the conditioning chamber with a polka-dot insert attached to the rear Plexiglas wall, dim illumination, and the stainless-steel grid floors were cleaned with 70% ethanol. Extinction training and retention tests occurred in a distinct context (Context B) which contained no visible illumination (illuminated only with an infrared light) and flat brown Plexiglas floors which were cleaned with 2% Quatricide (Pharmacal Research Laboratories).
3.2. Cued fear conditioning and extinction
All animals were handled for 5 min a day, for two consecutive days prior to context exposure. Mice were pre-exposed to Context A for 5 min. Twenty-four hours later, mice were returned to Context A and after a 180s acclimation period, received three pairings of a 30 s tone conditional stimulus (CS; 75 dB, 6 kHz) and unconditional stimulus (US), foot-shock (2s, 0.8 mA). For each of the pairings the US was delivered as soon as the CS ended, and each pairing was separated by an inter-tone interval of 182 s. Mice were removed from the apparatus 30s after the last shock and returned to their home cage. The following day, animals were tested for fear expression in Context B which also served as extinction training. For expression testing and extinction training, mice received 21 unreinforced CS presentations of 30 s with an ITI of 60 s. Twenty-four hours after extinction training, mice were tested for extinction memory in Context B and were presented with 15 unreinforced 30 s CS presentations. Following the extinction memory test, infusion sites were verified, and brains collected for immunofluorescence. For experiment 1 examining immediate early gene activation in response to fear expression or fear extinction, there were three additional control groups: home cage, tone only, and explicitly unpaired. For the tone only and explicitly unpaired groups, mice were pre-exposed to Context A for 5 min on Day 1. On Day 2, mice in the tone only group were exposed to 3 tones (CS) with an ITI of 182 s and then tested in context B, 24 h later for freezing to the same CS as the day before. For animals in the explicitly unpaired group, training involved exposure to 3 tones (CS) and 3 shock (US) which were unassociated and had an ITI of 60 s in between. Expression testing and extinction training paradigm remained the same as above.
3.3. Pharmacology and procedure
All localized infusions were performed through an acute infusion cannula (Plastics One; 33 gauge) inserted into the guide cannula. Infusion cannulae were connected to PE 50 polyethylene tubing that were connected to Hamilton syringes, and mounted on an infusion pump. Pharmacological inhibitors were bilaterally infused at a rate of 0.1 μl/min for a total infusion volume of 0.2 μl. The infusion needle was left in place for an additional minute. To temporarily inactivate the NAc core or shell, mice received localized infusions of vehicle (Phosphate buffered saline; PBS) or 4% (w/v) lidocaine HCL (pH of ~7.0) dissolved in PBS (Frankland et al., 2004; Cullen et al., 2015). Lidocaine is a voltage-dependent sodium channel blocker and was used to temporarily inactivate the sub-region of interest (Butterworth and Strichartz, 1990) as we have done previously for fear expression (Cullen et al., 2015). Mice were trained on the cued fear conditioning task as described above. Twenty-four hours later, mice received an infusion of Lidocaine or vehicle into the NAc core or shell. Infusions lasted 2 min and expression testing/extinction training began 5 min after the end of infusions. The mice were then tested for extinction retention 24 h after extinction training. To investigate the role of mGluR1s in the core and shell, the mice received bilateral infusions of either 0.55nmol/side of the mGluR1 antagonist, 1-aminoindan-1,5, dicarboxylic acid (AIDA), or the vehicle (0.9% NaCl) 5 min prior to extinction training. To test the involvement of ERK/MAPK pathway in facilitating extinction consolidation within the shell, the mice received bilateral infusions of vehicle (50% DMSO) or 1,4-diamino-2,3-dicyano-1,4-bis (o-aminophenylmercapto) butadiene (U0126; 0.5 μg/side or 1 μg/side), a MEK inhibitor, 5 min prior to, or immediately after extinction training based on prior literature (Duvarci et al., 2005; Miller and Marshall, 2005; Fischer et al., 2007; Lynch et al., 2016). The mice were then tested for extinction memory 24 h after extinction training in the absence of drug.
3.4. Locomotor behavior
Locomotor behavior was assessed using an open field apparatus to confirm that freezing behavior was not altered by lidocaine infusions into the NAc core. We did not verify if NAc shell inactivation influenced locomotion because no immediate difference in freezing behavior was observed after lidocaine infusions. Square opaque acrylic open field boxes measuring 42 cm W × 42 cm D × 39 cm H (Coulbourn Instruments, Allentown PA) served as testing arenas. The boxes were cleaned with 70% ethanol after the testing for each animal was completed. Mice underwent stereotaxic surgeries for guide cannula implantation targeted at the core subregion. Mice received local infusions of vehicle or lidocaine (4%) as previously described and 5 min later were gently placed in the center of the open field boxes. Locomotor behavior was measured for 5 min using AnyMaze software and total distance traveled in meters was analyzed.
3.5. Histological verification of cannula placements and immunofluorescence
Calbindin immunohistochemistry is commonly used to delineate the two NAc sub-regions because the core is more densely populated by calbindin positive neurons compared to the shell (Brog et al., 1993; Meredith et al., 1996; Tan et al., 1999). Thus, we performed calbindin immunofluorescence to delineate the shell and core subregions. In addition, to precisely verify canula placements in the respective subregions and estimate spread of infused pharmacological substances, mice received localized infusions of quisqualic acid (0.012M), an excitotoxic amino acid which has been used to destroy cholinergic neurons when used in higher concentrations (0.12M). However, when infused at a lower concentration of 0.012M as above, it results in the induction of immediate early genes (IEG) such as cFos and Arc (Page et al., 1993). As with all other pharmacological substances, quisqualic acid was bilaterally infused at a rate of 0.1 μl/min for a total infusion volume of 0.2 μl. Thus, we combined local infusions of Quisqualic acid (0.012 M) with co-immunofluorescence for calbindin and Arc to verify that our infusions were restricted to the shell or core only.
One hour following completion of infusions, mice were deeply anesthetized with sodium pentobarbitol (Fatal plus; Vortech Pharmaceutics Ltd.). The mice were then transcardially perfused with ice-cold 0.9% saline for 3 min followed by 4% paraformaldehyde (PFA) in 0.1M sodium phosphate buffer, Ph 7.4 for 7 min and the brains were immediately extracted. Following extraction, the brains were post-fixed in PFA overnight, followed by 24–72 h cryoprotection in 30% sucrose in 0.1M phosphate buffer solution with 0.02–0.05% sodium azide to avoid bacterial growth. Coronal sections of 40 μm thickness were cut on a cryostat and immunofluorescence was performed for Arc and calbindin expression. Coronal brain sections were co-stained with antibodies against arc and calbindin. Free floating sections were rinsed in 0.1M phosphate buffer (PB), followed by blocking in 0.1M PBS containing 0.9% NaCl, 0.5% Triton X-100 and 5% normal alpaca serum for 2 h. After blocking, the sections were incubated at 4 °C for 24 h with primary antibodies- 1:2000 rabbit anti-arc (Synaptic systems; 156 003) and 1:500 mouse anti-calbindin (Abcam; ab108404), diluted in 0.1M PB containing 0.5% Triton X-100. The following day, sections were thoroughly rinsed in 0.1M PB and incubated in secondary antibodies (1:1000 anti-rabbit alexafluor 488 and 1:500 anti-mouse Cy3; Jackson ImmunoResearch) for 2 h at room temperature followed by 1:2000 DAPI for 1 h and room temperature. Sections were washed in 0.1M PB, mounted onto slides, and coverslipped with Prolong Gold Antifade Mountant (Invitrogen).
3.6. Immunofluorescence for arc analysis
For arc analysis, mice were deeply anesthetized and transcardially perfused as described above either 60 min after the end of a fear expression test consisting of 5 tones or 75 min after extinction training as described above. Because Arc expression peaks at 60 min and then declines thereafter after fear conditioning (Lonergan et al., 2010; Young and Williams, 2013), we chose a slightly longer extinction-to-perfusion interval for the extinction experiment. This was done to ensure that any Arc expression observed was not the result of fear expression to the first several presentations of the CS during the extinction session, which might have obscured Arc expression due solely to extinction (Fig. 1). Three additional control groups were included in this experiment: home cage controls, tone only controls, which were only exposed to the CS in the absence of shock, and explicit unpaired training in which the tone and shock presentations were separated by 60 s. Coronal sections of 40 μm thickness were cut on a cryostat and immunofluorescence was performed for Arc and calbindin protein. Arc is an IEG which has been implicated in LTP and memory consolidation. It has been found to be localized in dendrites with neuronal stimulation and commonly used as a cell activity marker. Immunofluorescence was conducted as described above. Imaging was conducted on an Olympus FluoView 3000 confocal microscope. The software program FIJI (NIH) was used to quantify the signal intensities of the nucleus accumbens subregions of interest. Each image was despeckled to filter image noise and the background was subtracted using a rolling bar radius of 5.0. Each image had a designated upper and lower signal threshold using the auto threshold feature and were then converted to a binary image for each of the different channels. Binary images were then further adjusted using watershed segmentation to automatically separate particles that were touching (Cullen et al., 2015). Analysis involved counting of the Arc positive cells and DAPI+ cells within each region and expressing the Arc+ cells as a percentage of DAPI positive cells (Arc+/DAPI+ x 100) within the NAC core and shell for all the different groups keeping the parameters for circularity and cell size constant across all analysis (Lacagnina et al., 2019). Three bilateral measurements were taken for each region of each mouse from every other consecutive section. The Arc+ cell counts as a percentage of DAPI+ cells for the 3 sections were then averaged together. There was no difference observed in the Arc+ count between animals sacrificed post expression and post extinction in the “tone only” and “explicitly unpaired” group and therefore, their measurements were collapsed into one.
Fig. 1.
The nucleus accumbens core is selectively active during fear expression whereas the shell is selectively active following fear extinction. (A) Schematic of experimental procedure for analysis of Arc+ neurons in the NAc during fear expression. (B) Schematic of experimental procedure for analysis of Arc+ neurons in the NAc following fear extinction. (C) Behavior during training, expression, and extinction. During training (Left), mice receiving traditional tone-shock pairings increased freezing with each successive presentation of the tone. Tone only mice displayed minimal freezing in response to the tone presentations. Explicitly unpaired mice froze in response to shocks but did not exhibit any association between the shock and tones the next day during the expression or extinction test. Twenty-four hours after training, mice underwent an expression test in context B and were presented with 5 tones (Middle). Tone-shock paired mice (Exp Testing) displayed significantly more freezing to the 5 tone presentations compared to the Explicitly Unpaired and Tone Only groups. During extinction (Right), mice that were trained with tone-shock pairings (Extinction Training) were exposed to 15 tones in the absence of shock and displayed high freezing during the initial tones but then exhibited extinction in response to repeated tone presentations. Mice that were in the Tone Only or Explicitly Unpaired groups displayed minimal freezing to tones throughout the procedure. Values are displayed as average percent freezing (±SEM). (D) Calbindin staining can be used to differentiate between the NAc core and shell regions. Representative image of calbindin expression showing intense staining in the NAc core, but little staining in the shell (Left). Representative image showing absence of colocalization between calbindin and Arc in a the NAc shell subregion (Center), and colocalization between Arc and calbindin expression in the core (Right). (E) Arc+/DAPI+ cell analysis for mice exposed to 5 tone presentations during a fear expression test. The NAc core subregion is selectively active during fear expression. There were significantly more Arc+ cells in the NAc core as compared to the shell after a fear expression test. Arc expression in the NAc shell was near baseline and no different than tone only and explicitly unpaired groups. Values are displayed as mean (Arc+/DAPI+) x 100 (±SEM). Significance values were set at p < 0.05 (*** = p < 0.01). F) Arc+/DAPI+ cell analysis for mice exposed to 15 tone presentations during fear extinction. The NAc shell subregion is selectively active during fear extinction. There were significantly more Arc+ cells in the NAc shell as compared to the core after a fear extinction. Arc expression in the NAc core was near baseline and no different than tone only and explicitly unpaired groups. Values are displayed as mean (Arc+/DAPI+) x 100 (±SEM). Significance values were set at p < 0.05 (*** = p < 0.01). (G) Representative images of arc expression in the NAc core and shell in mice that underwent normal cued fear conditioning and then tested for fear expression (Top; from mice in Fig. 1E) and after fear extinction (Bottom; from mice in Fig. 1F). Arc expression was significantly greater in the NAc core during a fear expression test (Fig. 1E and G top left) compared to the NAc shell (Fig. 1E and G top right). In contrast, Arc expression was significantly greater in the NAc shell after fear extinction (Fig. 1F and G bottom right) compared to the NAc core (Fig. 1F; Bottom left).
3.7. Statistical analysis
Freezing during extinction training and extinction testing were analyzed using a two-way repeated measures factorial analysis of variance (ANOVA) (treatment X tones) on Graphpad Prism statistical software. Arc+/DAPI+ cell counts were analyzed using a two-way ANOVA (subregion X behavioral test). Statistically significant ANOVAs were followed up with Sidak's multiple comparison. Effect sizes were calculated for completed experiments along with post-hoc power analyses using G*Power 3.
4. Results
4.1. The NAc core is preferentially active during fear expression but not during extinction learning
To identify if the NAc shell and core are preferentially involved in fear expression versus extinction, we first ran mice on cued fear conditioning, which were sacrificed after fear expression testing (Fig. 1A) or after extinction training (Fig. 1B) and brains processed for Arc expression. First, calbindin was used as a marker to delineate the two NAc subregions. Calbindin is highly expressed in the NAc core compared to the shell. To confirm our analyses were restricted to the core or the shell, we first verified colocalization of calbindin positive and Arc positive cells in each subregion for each slice. On average across all sections, 27% of the Arc positive cells were colocalized with calbindin in the shell, whereas 64% of the arc positive cells were colocalized with calbindin within the core verifying our neuroanatomical specificity of our analyses (Fig. 1D). In addition to a home cage (HC) control group, additional control groups were run using tone only training or explicitly unpaired training protocols. Freezing behavior was as expected in each of the control and experimental groups, with explicitly unpaired and tone only groups displaying minimal freezing during the fear expression test and extinction training, whereas the normally trained mice displayed elevated freezing (Fig. 1C). A total of 48 mice were used in the fear expression experiment and a two-way ANOVA revealed a significant main effect of behavioral test (F(3,40) = 111.1 p < 0.001), a main effect of subregion (F(1,40) = 57.94, p < 0.001) and a significant behavioral test × subregion interaction (F(3,40) = 65.22, p < 0.001). There were significantly more Arc+/DAPI+ cells in the NAc core as compared to the shell during fear expression testing (Fig. 1E, G) (p < 0.05). Further, there were significantly more Arc+/DAPI+ cells in the NAc core after mice underwent a cued fear expression test as compared to mice that underwent the explicitly unpaired training (p < 0.05). There was no significant difference in Arc expression in the NAc core or shell between tone only and the explicitly unpaired groups (p > 0.999). Finally, there was no significant difference in Arc+/DAPI+ cells in the NAc shell as compared to the core or shell of mice in the explicitly untrained group suggesting little activation of the NAc shell subregion during the fear expression test for the two control groups. These data suggest that the increase in the arc activity in the NAc core was due to fear recall and not due to exposure to shock or tones alone and was specific to the NAc core. In contrast, there was significantly more Arc+/DAPI+ cells in the NAc shell as compared to the core in mice that underwent fear extinction (Fig. 1F and G) (P < 0.05). A total of 48 animals were used in the fear extinction experiment and a two-way ANOVA revealed a significant main effect of behavioral test (F(3,40) = 267.4, p < 0.001), a main effect of subregion (F(1,40) = 176.1, p < 0.001) and a significant behavioral test × subregion interaction (F(3,40) = 163.9, p < 0.001). Again, Arc expression in the shell was significantly more in the fear extinction group as compared to the core and shell in the explicitly unpaired group (Fig. 1F) (p < 0.05). Finally, the number of Arc+/DAPI+ cells in the NAc core subregion of mice that underwent fear extinction was no different than the core or shell subregions in mice of the explicitly unpaired control group suggesting that the NAc core subregion is not active during fear extinction (Fig. 1F). Taken together, these data suggest that a dissociation between activity of the NAc core and shell subregions during fear expression versus fear extinction. Whereas the NAc core subregion is recruited during fear expression, activity of the NAc shell is recruited during fear extinction and the activity in both regions was dependent upon associative learning.
4.2. Inactivation of the NAc core disrupts fear expression but has no effect on extinction learning or extinction retention
To evaluate the influence of the core subregion in regulating fear expression and extinction, all mice were trained and tested for cued fear conditioning and received local infusions of either lidocaine or vehicle into the core subregion 5 min prior to expressing testing/extinction training (Fig. 2A). Twenty-four hours later the animals were tested for extinction retention. A total of 25 mice were used for these experiments, 6 animals were removed from the analysis either due to unilateral infusions or complete missed targets. Mice that had their core subregion inactivated showed attenuated freezing to the tones during the expression testing/extinction training session (Fig. 2B). A two-way ANOVA revealed a significant main effect of treatment (F(1, 23) = 11.340, p = 0.003) and a significant tone × treatment interaction (F(14, 322) = 2.02, p = 0.016). When tested for extinction retention 24 h later, there was no main effect of treatment (F(1, 23) = 0.004, p = 0.948), a main effect of tone (F(14, 322) = 2.956, p < 0.001), and no significant interaction (F(14, 23) = 0.56, p = 0.894). Because the NAc core strongly influences motor behavior we wanted to verify that the effects of lidocaine on freezing were not due to its effects on locomotor activity. Thus, mice were infused with lidocaine into the core subregion and tested in an open field. Distance traveled during a 10 min test revealed no significant differences in the total distance traveled between lidocaine-treated and vehicle-treated animals (t(18) = 0.263, p = 0.7953), suggesting that the reduced freezing as a result of NAc core inactivation was not due to alterations in NAc control of motor behavior (Fig. 2C).
Fig. 2.
Inactivation of the nucleus accumbens core disrupts fear expression but not extinction learning or the consolidation of extinction. (A) Schematic representation of the experimental paradigm. All mice underwent cued fear conditioning (FC) in Context A. Twenty-four hours later, mice underwent a fear expression test that also served as extinction training in Context B (Exp Test/Ext). Mice received infusions of lidocaine (Lid) or vehicle (Veh) 5 min before the expression test/extinction training. Twenty-four hours after extinction training, mice were tested for extinction memory in Context B (Ext Ret). (B) Inactivation of the nucleus accumbens core attenuated fear expression during the expression test but did not disrupt extinction learning or the consolidation of extinction. Inactivation of the NAc core prior to the fear expression test had no effect on consolidation of extinction. The percent freezing of mice that were infused with lidocaine (filled circles) were compared with those that were infused with vehicle (open circles). There were no significant differences between lidocaine-treated and vehicle-treated mice during the extinction retention test. Values are represented as % freezing (±SEM). Statistical significance was set at p < 0.05) (C) To verify that temporary inactivation of the nucleus accumbens core did not alter freezing by modulating motor activity, mice were tested in an open field apparatus after an infusion of lidocaine or vehicle in the nucleus accumbens core. Distance traveled during a 10 min test revealed no significant differences in the total distance traveled between lidocaine-treated (filled bars) and vehicle-treated animals (white bars) (t(16) = 0.264, p = 0.7953). (D) Site verification of lidocaine infusions. (Left) Region of interest in the representative image of Arc spread used to verify canula placements in the nucleus accumbens core. Verification of infusions were accomplished through colocalization of calbindin (Red) and Arc (Green) expression. The core subregion displays elevated calbindin compared to the shell subregion. (Right) Detailed mapping representing the maximum spread (light red), and the average spread (dark red) of Arc expression in mice used in this experiment. Arc expression was largely restricted to the nucleus accumbens core, with minimal spread into the shell subregion. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
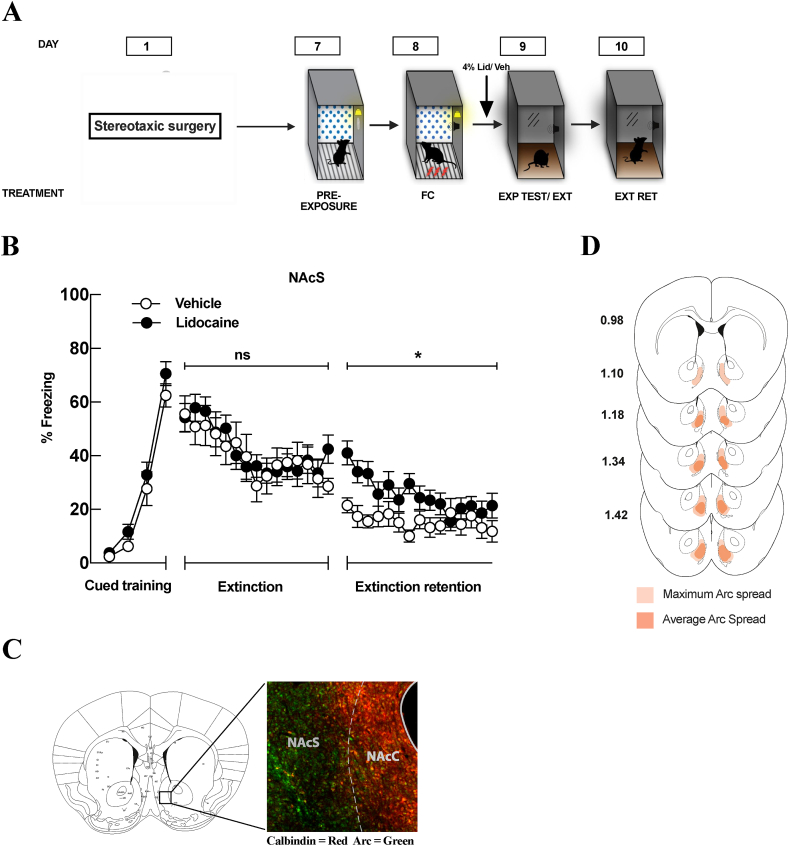
4.3. Inactivation of the NAc shell disrupts the consolidation of fear extinction but has no effect on fear expression or extinction learning
Given that Arc expression in the NAc shell was associated with fear extinction, we next wanted to evaluate how inactivation of the NAc shell would affect fear expression and extinction. A total of 32 mice were used for these experiments, 9 mice were removed from the analysis either due to unilateral infusions or due to complete missed targets. Mice underwent cued fear conditioning, testing/extinction, and an extinction retention test as above (Fig. 3A). Five minutes before the expression test/extinction session, mice received bilateral infusions of lidocaine or vehicle into the NAc shell (Fig. 3A). During the expression test and extinction training session, there was no main effect of treatment (F(1, 30) = 0.09, p = 0.77), a main effect of tone (F(14, 420) = 8.650, p < 0.001) suggesting successful within session extinction, and no significant treatment × tone interaction (F(14, 420) = 0.87, p = 0.593) (Fig. 3B). However, when the animals were tested for extinction retention 24 h later, a two-way ANOVA revealed that there was a main effect of treatment (F (1, 30) = 6.86, p = 0.01) and a significant treatment × tone interaction (F (14, 420) = 1.854, p = 0.03). Lidocaine-treated mice froze significantly more to the tone compared to the vehicle-treated animals (Fig. 3B). These data suggest that the shell subregion is necessary for the consolidation and retention of extinction. Taken together, these data suggest that the NAc shell is necessary for the consolidation of extinction, but not for fear expression or extinction learning itself.
Fig. 3.
Inactivation of the NAc shell disrupts the consolidation of fear extinction but has no effect on fear expression or extinction learning. (A) Schematic representation of the experimental paradigm. All mice underwent cued fear conditioning in Context A (FC). Twenty-four hours later, mice underwent a fear expression test that also served as extinction training in Context B (Exp Test/Ext). Mice received infusions of lidocaine (Lid) or vehicle (Veh) 5 min before the expression test/extinction training. Twenty-four hours after extinction training, mice were tested for extinction memory in Context B (Ext Ret). (B) Inactivation of the nucleus accumbens shell had no effect on fear expression or extinction learning. The percent freezing of mice that were infused with lidocaine (filled circles) were compared with those that were infused with vehicle (open circles). Values are represented as % freezing (±SEM). Significance was set at p < 0.05. However, when mice were tested for extinction retention 24 h later, lidocaine-treated mice froze significantly more to the tone compared to the vehicle-treated mice (p < 0.05) suggesting a disrupted consolidation of extinction. (C) (Left) Region of interest in the representative image of Arc spread used to verify canula placements in the nucleus accumbens shell. (Right) Representative image of Arc spread in the NAc shell. The nucleus accumbens core shows extensive calbindin staining (Red) which is absent in the shell subregion, where Arc expression is restricted (Green). (D) Site verification of lidocaine infusions. Detailed mapping representing the maximum spread (light red), and the average spread (dark red) of Arc expression in mice used in this experiment. Arc expression was largely restricted to the nucleus accumbens shell, with minimal spread into the core subregion. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.4. Group I metabotropic glutamate receptors within the NAc shell mediate the consolidation of extinction
Given the effects of inactivating the NAc shell on extinction, we next investigated if glutamatergic modulation through mGluR1 in the NAc shell is necessary for regulating extinction learning or consolidation. A total of 15 mice were used in these experiments. As in the experiments above, all mice underwent cued fear conditioning and 24 h later were infused with either AIDA or vehicle 5 min prior to fear expression testing/extinction training (Fig. 4A). There was no main effect of treatment (F(1, 13) = 0.750, p = 0.40), a main effect of tone (F (14, 182) = 4.437, p < 0.001) and no significant treatment × tone interaction (F(14, 182) = 1.554, p = 0.10) on fear expression and extinction training. Thus, there was no effect on within session extinction (Fig. 4B). However, when the mice were tested 24 h later for extinction retention there was a main effect of treatment (F(1, 13) = 20.01, p < 0.001), a main effect of tone (F(14,182) = 3.0, p < 0.001) but no tone × treatment interaction (F(14,182) = 1.7, p = 0.057). Specifically, AIDA-treated mice displayed more freezing compared to the vehicle-treated animals (Fig. 4B). These data suggest that signaling through mGluR1 within the shell is not necessary for fear expression or within-session extinction but is necessary for the consolidation of extinction.
Fig. 4.
Group I metabotropic glutamate receptors within the nucleus accumbens shell mediate the consolidation of fear extinction. (A) Schematic representation of the experimental paradigm. All mice underwent cued fear conditioning in Context A (FC). Twenty-four hours later, mice underwent a fear expression test that also served as extinction training in Context B (Exp Test/Ext). Twenty-four hours after extinction training, mice were tested for extinction memory in Context B (Ext Ret). Five minutes prior to the expression testing/extinction training, mice received local infusions of AIDA or vehicle as indicated by the arrow. Twenty-four hours later, mice were tested for extinction retention (Ext Ret). (B) mGluR1 within the shell is necessary for the consolidation of extinction. Analysis of freezing between mice receiving AIDA (filled circle) and vehicle (open circle), showed no effect of treatment on fear expression and extinction training. However, when the mice were tested 24 h later for extinction retention there was a main effect of treatment with AIDA-treated mice displaying higher levels of freezing compared to the vehicle-treated animals, suggesting disruption of fear extinction consolidation (p < 0.05). (C) Site verification of AIDA infusions within the shell subregion. Detailed mapping representing the maximum spread (light red), and the average spread (dark red) of Arc expression in mice used in these experiments. (D) mGluR1 within the core is not involved in fear expression, extinction learning or the consolidation of extinction. There was no effect of treatment on fear expression and extinction training between mice that received AIDA (filled circle) and those that received vehicle (open circle). Additionally, when the mice were tested for extinction retention 24 h later, there was no significant effect of treatment, suggesting no effect on the consolidation of extinction. Values are represented as % freezing (±SEM). Significance was set at p < 0.05. (E) Site verification of lidocaine infusions within the core subregion. Detailed mapping representing the maximum spread (light red), and the average spread (dark red) of Arc expression in mice used in these experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.5. Group I metabotropic glutamate receptors within the NAc core do not mediate fear expression or extinction learning
In the above experiments, we demonstrated that the NAc core is necessary for the expression of fear as measured by freezing, whereas the shell regulates the consolidation of extinction through mGluR1. We next investigated if glutamatergic modulation through mGluR1 is necessary for regulating these fear processes within the core. We hypothesized that inactivation of mGluR1 in the core would have no effect on fear expression or extinction. A total of 17 animals were used in this experiment., All mice underwent cued fear conditioning as above and 24 h later were infused with the mGluR1 antagonist, AIDA, or vehicle 5 min prior to expression testing/extinction training (Fig. 4A). A two-way repeated measures ANOVA revealed no main effect of treatment (F(1, 15) = 2.349, p = 0.15) and no significant interaction (F(14, 210) = 1.131, p = 0.33) on fear expression and extinction training. Analysis revealed a main effect of tone (F(14, 210) = 7.65, p < 0.001) showing that both groups extinguished their fear to the tones by the end of the extinction session (Fig. 4D). Additionally, when the mice were tested for extinction retention 24 h later, there was no main effect of treatment (F(1, 15) = 0.010, p = 0.92), a main effect of tone (F(14,210) = 8.4, p < 0.001) and no treatment × tone interaction (F(14, 210) = 0.632, p = 0.84). These data suggest that mGluR1 activation in the NAc core is not involved in the expression of fear or its extinction and extinction retention (Fig. 4D).
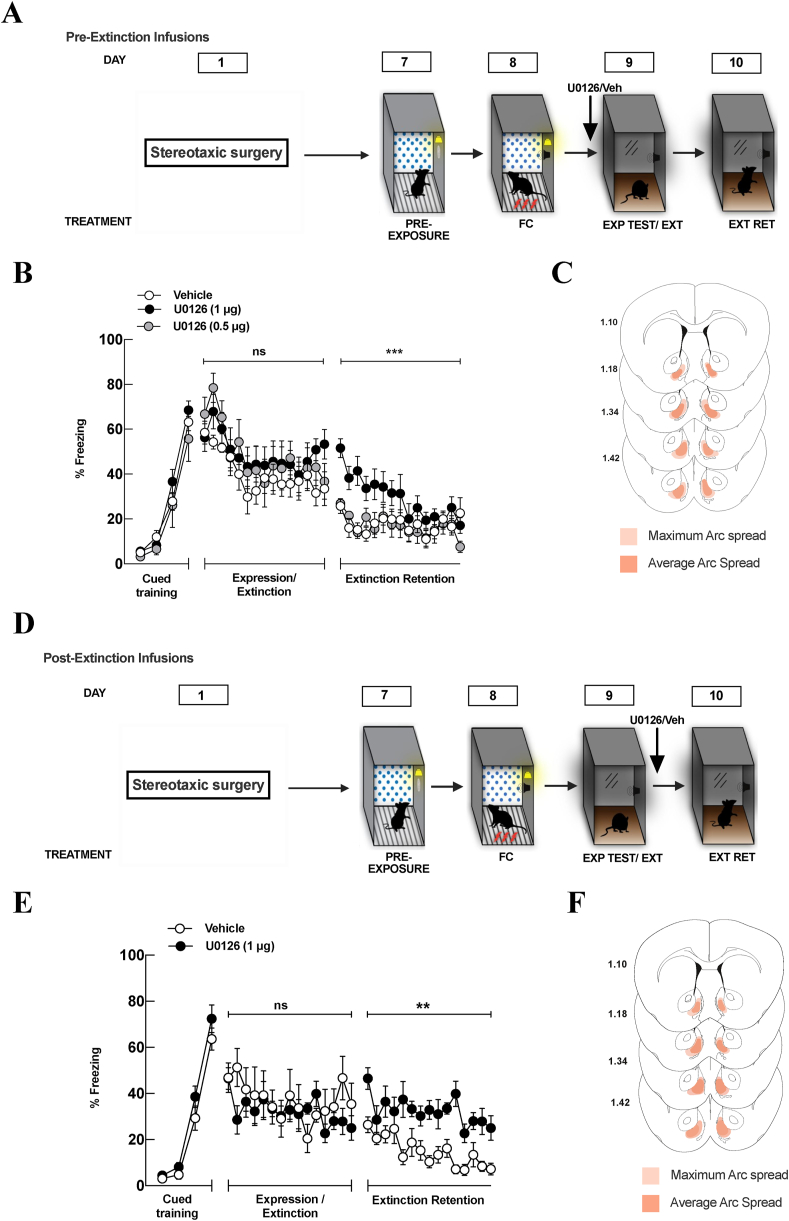
4.6. The consolidation of fear extinction requires ERK/MAPK in the NAc shell
Given our previous data suggesting that mGluR1 are important for the consolidation of fear extinction we hypothesized that their downstream signaling through the ERK/MAPK pathway within the NAc shell would be necessary for fear extinction retention. A total of 66 mice were used for these experiments. Nine animals were removed because they received unilateral infusions. Mice underwent cued fear conditioning as before and initially received bilateral, local infusions of either 0.5 μg/side or 1 μg/side of the MEK inhibitor, U0126, into the NAc shell 5 min prior to fear expression testing/extinction training session (Fig. 5A). These doses have been used previously in the amygdala to investigate the involvement of the ERK/MAPK pathway in auditory fear conditioning (Schafe et al., 2000; Duvarci et al., 2005; Herry et al., 2006). During the extinction training session, a two-way ANOVA revealed no main effect of treatment (F(2, 24) = 0.901, p = 0.42) and no significant treatment × tone interaction (F(28, 336) = 0.828, p = 0.72). However, when mice were tested 24 h later for extinction memory there was a main effect of treatment (F(2, 24) = 19.290, p < 0.001), a main effect of tone (F(14,366) = 2.398, p = 0.003) and no treatment × tone interaction (F(28,336) = 1.447, p = 0.70). Specifically, mice that received 1 μg/side of U0126 displayed enhanced fear as compared to mice that received 0.5 μg/side U0126 and vehicle-treated mice (Fig. 5B). These data indicate that ERK/MAPK signaling within the NAc shell is necessary for the consolidation of extinction, but not for fear expression or extinction learning. However, we did observe a small non-significant within-session extinction training effect of the 1 μg/side dose with pre-extinction infusions only during the final 2 tones of extinction training. Thus, we ran an additional group of mice that underwent the same training procedure and received local infusions of 1 μg/side of U0126 immediately after the extinction training session (Fig. 5D). During the extinction training there was no main effect of treatment (F(1,15) = 0.424, p = 0.525), there was a main effect of tone, (F(14,210) = 2.278, p = 0.006), and no treatment × tone interaction (F(14,210) = 1.734, p = 0.051). During the extinction retention test, there was a significant main effect of treatment (F (1,15) = 10.60, p = 0.005), a main effect of tone (F(14,210) = 5.394, p < 0.001) but no tone X interaction (F(14,210) = 1.333, p = 0.190). Specifically. mice that received local infusions of U0126 into the NAc shell immediately after extinction exhibited more freezing to the tones compared to the vehicle-treated group during the extinction retention test suggesting a disruption of extinction consolidation (Fig. 5E). These data are the first to show that the ERK/MAPK signaling within the NAc shell is critical for the appropriate consolidation of extinction.
Fig. 5.
Extinction consolidation requires ERK/MAPK activation in the nucleus accumbens shell. (A) Schematic representation of the experimental paradigm. All mice underwent cued fear conditioning in Context A (FC). Twenty-four hours later, mice underwent a fear expression test that also served as extinction training in Context B (Exp Test/Ext). Five minutes prior to the expression testing/extinction training, animals received local infusions of either U0126, or vehicle as indicated by the arrow. Twenty-four hours after extinction training, mice were tested for extinction memory in Context B (Ext Ret). (B) Pre-extinction infusions of 1ug/side of U0126 (black filled circles) into the NAc shell disrupted the consolidation of extinction but no effect was observed with a 0.5ug/side (grey circles) infusion as compared to vehicle (white circles). Thus, there was no effect of blocking ERK/MAPK on expression of fear or extinction training with either of the doses of U0126. However, animals that received 1ug/side of U0126 displayed enhanced fear as compared to the 0.5ug/side U0126-treated and vehicle-treated group (white circles) when tested for extinction retention 24 h later. Values are represented as % freezing (±SEM). Significance was set at p < 0.05. These data suggest that the ERK/MAPK signaling pathway in the nucleus accumbens shell is necessary for appropriate consolidation of fear extinction. (C) Verification of cannula placements. Detailed mapping representing the maximum spread (light red), and the average spread (dark red) of arc expression of animals within the nucleus accumbens shell with pre-extinction infusions. (D) Schematic representation of the experimental paradigm. All mice underwent cued fear conditioning in Context A (FC). Twenty-four hours later, mice underwent a fear expression test that also served as extinction training in Context B (Exp Test/Ext). Mice received infusions of U0126 or vehicle immediately after extinction training as indicated by the arrow. Twenty-four hours later, animals were tested for extinction retention in context B (Ext Ret). (E) Mice infused with U0126 post-extinction displayed significantly more freezing to tone when they were tested for extinction memory 24 h later, again suggesting that the ERK/MAPK pathway in the NAc shell is necessary for the appropriate consolidation of extinction. Values are represented as % freezing (±SEM). Significance was set at p < 0.05. (F) Verification of cannula placements. Detailed mapping representing the maximum spread (light red), and the average spread (dark red) of arc expression of animals within the nucleus accumbens shell with post-extinction infusions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
5. Discussion
Several studies using lesions, as well as investigations into dopamine signaling within the NAc subregions provide evidence that the core and shell have distinct roles regulating memory, approach, fear conditioning and instrumental conditioning (Parkinson et al., 1999; Corbit et al., 2001; Bassareo et al., 2002; Jongen-Relo et al., 2003; Blaiss and Janak, 2009). Whereas it is clear that this region plays an important role in regulating aversive motivation (Levita et al., 2002b, 2009; Reynolds and Berridge, 2003; Chen et al., 2012; Wenzel et al., 2015; Soares-Cunha et al., 2019), the precise functions of the core and shell in specific fear processes have not been fully delineated. Here, we show that the NAc core subregion is distinctly activated during fear retrieval but not following extinction. In contrast, the NAc shell is active only after extinction, but not during fear retrieval. Then, pharmacological inactivation of each subregion separately demonstrated that the NAc core promotes fear expression but is not necessary for fear extinction learning. In contrast, we found that the NAc shell is necessary for the consolidation of extinction, but not for fear expression or extinction learning itself. Additionally, we demonstrate that mGluR1 and ERK/MAPK signaling specifically within the nucleus accumbens shell promote the consolidation of extinction but are not necessary for fear expression or extinction learning. Combined, the same differential results observed in the NAc core and the NAc shell in fear expression versus extinction in Arc expression and three separate pharmacological manipulations suggest dissociable roles of the NAc core and shell subregions in specific control of fear expression and extinction.
We show that the NAc core is specifically active during fear retrieval versus fear extinction, whereas the NAc shell is specifically active following fear extinction (Fig. 1). We note three prior studies in which results suggest that the NAc shell is involved in the expression of fear memory based on IEG analyses (Campeau et al., 1997; Thomas et al., 2002; Knapska and Maren, 2009). One of the above studies also found the NAc core to be active during fear expression (Thomas et al., 2002), which is in agreement with our findings, and a final study found that the NAc was not responsive at all for any fear behavior (Huang et al., 2013). Several specific methodological differences can explain the discrepancy in the findings, including differences in behavioral tasks (fear-potentiated startle, fear renewal), IEG analysis (zif 268, c-fos), and that we rigorously defined the NAc core and shell based on known differences in colocalization of calbindin (Meredith et al., 1996; Tan et al., 1999). Overall, our Arc data suggest that the NAc shell is specifically involved in the extinction of fear to an explicit CS, which is supported by our additional pharmacological and behavioral experiments (Fig. 2, Fig. 3, Fig. 4, Fig. 5).
We next demonstrated that the core promotes freezing, but is not necessary for extinction learning, whereas the NAc shell promotes the consolidation of extinction (Fig. 2, Fig. 3). We note a recent study, however, that found an opposite role for the NAc shell in fear expression (Piantadosi et al., 2020). In that study, investigators used appetitive lever suppression ratio as an indirect measure of fear behavior and showed that infusions of a mixture of GABAA and GABAB receptor antagonists into the NAc shell significantly disrupted CS-induced lever suppression. That is, when the NAc shell was inactivated rats expressed less fear behavior. Inactivation of the NAc core, on the other hand, had no effect on fear expression (Piantadosi et al., 2020). Several methodological and pharmacological differences between the current study and the one by Piantadosi and colleagues could explain the opposite effect in the NAc shell observed in the two studies. The current study used lidocaine to inactivate the NAc shell, whereas Piantadosi and colleagues used a mixture of Baclofen and Muscimol, GABAB and GABAA receptor antagonists, respectively. The other major difference is in the behavioral measure of fear – CS-induced freezing (current study) versus CS-induced suppression of lever pressing (Piantadosi). Freezing is a simple and direct measure of threat responding in rodents that does not need to account for the effects of motivational states or operant appetitive learning. Thus, the NAc shell might be more important for suppressing rewarding behaviors (e.g., feeding, drinking, social interaction) when threatening stimuli are present than for generating freezing in response to threat. This could explain why we did not observe immediate effects of lidocaine on fear expression when infused into the NAc shell – we only observed effects during an extinction retention test. One issue of note is the use of lidocaine in the current study, which has its limitations. Because it is a sodium channel blocker it blocks activity in the region of interest as well as in fibers of passage. It is important to note that mPFC fibers pass through the NAc core before they terminate in the NAc shell (Gorelova and Yang, 1997). A recent report using an inactivation disconnect experiment to disrupt communication between the prelimbic cortex and the NAc shell showed that PL projections to the NAc shell are important for fear expression when using conditioned lever suppression as a measure of fear (Piantadosi et al., 2020). Thus, it is possible that our results of NAc core inactivation reducing freezing could be due to inactivation of PL fibers of passage that terminate in the NAc shell. We think this possibility does not explain our results for the following reasons: 1) we did not also observe immediate effects of NAc shell inactivation on freezing, 2) our additional experiments using more specific pharmacology replicated the regional specificity of the lidocaine experiments and lacked immediate effects on freezing. When AIDA was infused into the NAc shell only we again observed effects on fear during the extinction retention test, but not during the extinction training session. Moreover, infusions of U0126 into the NAc shell also did not disrupt freezing during extinction training. We again only observed effects during the extinction retention test. Thus, we think the differences between the current study and that of Piantadosi et al. (2020), are likely due to differences in NAc shell control of appetitive behavior versus freezing. Further validation of this hypothesis is necessary, however.
Several studies have implicated dopamine actions in the nucleus accumbens in conditioned fear. For example, dopamine activity in the nucleus accumbens is implicated in the blocking of learned fear responses (Iordanova et al., 2006). Furthermore, infusions of a dopamine receptor-2 antagonist increases fear expression and delays within session extinction as well as disrupting extinction retention (Holtzman-Assif et al., 2010). However, a limited number of studies have examined the different contributions of the core and shell and their signaling mechanisms in fear learning and its extinction. One study examined dopamine release in the nucleus accumbens core and shell subregions during context fear expression in the training context or a novel context. Elevated dopamine was observed in both the shell and core subregions only when rats were tested in the training context, suggesting that dopamine release in both regions is associated with contextual fear expression (Martinez et al., 2008). In addition, two separate studies found that lesioning of the core subregion disrupted fear expression to a CS (Parkinson et al., 1999; Wendler et al., 2014b). However, in one of the studies the animals were tested in the training context, making it difficult to distinguish between context and pure CS elicited freezing (Wendler et al., 2014b). Here we provide direct evidence that the core subregion is necessary for the expression of cued fear but not for its extinction. Temporary inactivation of the NAc core attenuated freezing to the CS when mice were tested in a different context but had no effect on the consolidation of extinction (Fig. 2B). Additionally, the significant difference in arc expression between the fear expression group when compared to the tone only or the explicitly unpaired group suggests that the NAc core is required for recall of the association between the CS and the US (Fig. 1E). These data support earlier findings showing increased expression of Zif268 within the core during retrieval of cued fear memories (Thomas et al., 2002). Taken together, our data suggest that the neuronal activity within the NAc core is necessary for fear expression but is not involved in extinction learning. We confirmed that the reduction of freezing observed during fear testing could not be interpreted as an effect on locomotor activity using an open field (Fig. 2C). Thus, our histological and behavioral pharmacology data suggest that the nucleus accumbens core contributes to the expression of fear during memory recall but is not necessary for extinction learning or its consolidation. Pharmacological inactivation of the NAc shell specifically disrupted fear extinction consolidation as evidenced by elevated freezing during the extinction retention test but did not disrupt expression of fear or extinction learning itself (Fig. 3B). It is possible, although we think unlikely given our additional more specific pharmacological experiments, that the lack of an effect on freezing during extinction training was due to an increase in locomotion. Thus, our findings suggest a dissociation between the NAc core and shell in regulating fear expression/recall and extinction consolidation. Our evidence for behavioral dissociation here is supported by additional anatomical evidence in that the core and shell display differential innervation patterns from the prefrontal cortices. The core receives projections from the prelimbic cortex, which is involved in fear expression, whereas the infralimbic cortex, necessary for fear extinction, projects preferentially to the shell subregion (Brog et al., 1993; Sierra-Mercado et al., 2010). Combined, these data suggest that separate inputs from medial prefrontal cortex to the nucleus accumbens may support different forms of learned fear responses.
We next demonstrated that blocking mGluR1 activity within the NAc shell subregion prior to extinction training disrupts consolidation of extinction but has no effect on fear expression or extinction learning (Fig. 4B). In contrast, inactivation of mGluR1 within the NAc core had no effect on fear expression, extinction learning, or on the consolidation of extinction (Fig. 4D). The current findings, to the best of our knowledge, are the first to show that mGluR1 activity within the NAc shell subregion is required for consolidation of extinction memories.
A major signaling pathway downstream of mGluR1 is ERK/MAPK (Ferraguti et al., 1999; Thandi et al., 2002; Wang et al., 2007; Mao et al., 2008; Willard and Koochekpour, 2013; Mao and Wang, 2016; Yang et al., 2017). We therefore determined if ERK/MAPK activity is necessary for extinction consolidation within the NAc shell. This pathway within the amygdala and hippocampus is implicated in extinction consolidation of auditory and contextual fear conditioning (Herry et al., 2006; Fischer et al., 2007; Peng et al., 2010), but has not been examined in the NAc. Pre-extinction infusions of U0126 disrupted extinction retention, but also had a small non-significant effect on freezing during the last few tone presentations during extinction training. To eliminate any possible effect of extinction learning on differences observed during the extinction retention test we used a post extinction infusion procedure in the next experiment. Here, we observed that U0126 infusion into the NAc shell disrupted the consolidation of extinction (Fig. 5). These data, to the best of our knowledge, are the first to indicate that the ERK/MAPK signaling within the NAc shell is necessary for the consolidation of fear extinction.
Excessive associative fear or the inability to suppress fear responses is associated with anxiety disorders and stress/trauma-related disorders such as PTSD. Brain imaging studies also implicate the nucleus accumbens in anxiety disorders by showing NAc activation in response to aversive stimuli and anxiety-dependent active avoidance behavior (Jensen et al., 2003; Levita et al., 2012). There may be some overlap in appetitive and aversive circuits within the NAc. For instance, the NAc core is involved in the self-administration of rewarding stimuli (LaLumiere and Kalivas, 2008; Bull et al., 2014), but the NAc shell is reported to be involved in the context-dependent control of both reinstatement and extinction of drug seeking (Peters et al., 2009; Millan et al., 2010; Gibson et al., 2019). Thus, the regional associations have some overlap for expression and extinction of appetitive and aversive responses but are not strictly segregated. Given the high comorbidity between anxiety disorders and substance abuse disorder, understanding the common domains that regulate both aversive and appetitive memories is crucial. Taken together, our data, in addition to previous findings, suggest that the NAc plays a prominent role in regulating fear memory and the consolidation of fear extinction and could perhaps be considered part of the canonical fear memory circuitry and has potential to be a target for the development of combined therapeutic intervention strategies.
Funding and disclosure
These experiments were funded by NIH grant R15MH118705 to AMJ. The NIH did not have any role in the design, collection, analysis, interpretation, writing or manuscript submission processes for the experiments and data described herein. All authors declare no conflicts of interest.
Author contributions
S.D. and A.M.J designed research; S.D., J.B., and C.J.H performed research; S.D. and A.M.J. analyzed data; S.D. and A.M.J. wrote and edited the paper.
CRediT authorship contribution statement
Sohini Dutta: Writing – review & editing, designed research, performed research, analyzed data, wrote the paper, edited and revised the manuscript. Jasmin Beaver: performed research. Carly J. Halcomb: Writing – review & editing, performed research, edited and revised the manuscript. Aaron M. Jasnow: Writing – review & editing, designed research, wrote the paper, edited and revised the manuscript.
Acknowledgments
We acknowledge and thank Dr. David Riccio and Dr. T. Lee Gilman for their intellectual contributions. We want to thank Samantha Ortiz for suggestions and help with statistical analysis. We also acknowledge the research assistance of Danielle Rogers and Jacob Corey with behavior and data collection. We also acknowledge the contributions of the animal care staff in the Department of Psychological Sciences. These experiments were funded by NIH grant R15MH118705 to AMJ.
References
- Baliki M.N., Mansour A., Baria A.T., Huang L., Berger S.E., Fields H.L., Apkarian A.V. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J. Neurosci. 2013;33:16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V., De Luca M.A., Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J. Neurosci. 2002;22:4709. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C.H., Fibiger H.C. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. Affective valence in the brain: modules or modes? Nat. Rev. Neurosci. 2019;20:225–234. doi: 10.1038/s41583-019-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss C.A., Janak P.H. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav. Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer K., Hausser M., Hartig W., Arendt T. The core-shell dichotomy of nucleus accumbens in the rhesus monkey as revealed by double-immunofluorescence and morphology of cholinergic interneurons. Brain Res. 2000;858:151–162. doi: 10.1016/s0006-8993(00)01938-7. [DOI] [PubMed] [Google Scholar]
- Breslau N., Davis G.C., Peterson E.L., Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch. Gen. Psychiatr. 1997;54:81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- Brog J.S., Salyapongse A., Deutch A.Y., Zahm D.S. The patterns of afferent innervation of the core and shell in the "accumbens" part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brown P.J., Stout R.L., Mueller T. Posttraumatic stress disorder and substance abuse relapse among women: a pilot study. Psychol. Addict. Behav. 1996;10:124–128. [Google Scholar]
- Bull C., Freitas K.C.C., Zou S., Poland R.S., Syed W.A., Urban D.J., Minter S.C., Shelton K.L., Hauser K.F., Negus S.S., Knapp P.E., Bowers M.S. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39:2835–2845. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C.K., Maynard G.D., Kehne J.H. Targeting memory processes with drugs to prevent or cure PTSD. Expet Opin. Invest. Drugs. 2012;21:1323–1350. doi: 10.1517/13543784.2012.704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S., Falls W.A., Cullinan W.E., Helmreich D.L., Davis M., Watson S.J. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Chen Y.W., Rada P.V., Bützler B.P., Leibowitz S.F., Hoebel B.G. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Corbit L.H., Muir J.L., Balleine B.W. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J. Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P.K., Gilman T.L., Winiecki P., Riccio D.C., Jasnow A.M. Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiol. Learn. Mem. 2015;124:19–27. doi: 10.1016/j.nlm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Duvarci S., Nader K., LeDoux J.E. Activation of extracellular signal-regulated kinase– mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Ferraguti F., Baldani-Guerra B., Corsi M., Nakanishi S., Corti C. Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur. J. Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- Fischer A., Radulovic M., Schrick C., Sananbenesi F., Godovac-Zimmermann J., Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol. Learn. Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland P.W., Bontempi B., Talton L.E., Kaczmarek L., Silva A.J. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Gibson G.D., Millan E.Z., McNally G.P. The nucleus accumbens shell in reinstatement and extinction of drug seeking. Eur. J. Neurosci. 2019;50:2014–2022. doi: 10.1111/ejn.14084. [DOI] [PubMed] [Google Scholar]
- Gorelova N., Yang C.R. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706. doi: 10.1016/s0306-4522(96)00380-6. [DOI] [PubMed] [Google Scholar]
- Herry C., Trifilieff P., Micheau J., Luthi A., Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur. J. Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Holtzman-Assif O., Laurent V., Westbrook R.F. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn. Mem. 2010;17:71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- Huang A.C.W., Shyu B.-C., Hsiao S., Chen T.-C., He A.B.-H. Neural substrates of fear conditioning, extinction, and spontaneous recovery in passive avoidance learning: a c-fos study in rats. Behav. Brain Res. 2013;237:23–31. doi: 10.1016/j.bbr.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Iordanova M.D., Westbrook R.F., Killcross A.S. Dopamine activity in the nucleus accumbens modulates blocking in fear conditioning. Eur. J. Neurosci. 2006;24:3265–3270. doi: 10.1111/j.1460-9568.2006.05195.x. [DOI] [PubMed] [Google Scholar]
- Jensen J., McIntosh A.R., Crawley A.P., Mikulis D.J., Remington G., Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Butterworth JFt, Strichartz G.R. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo A.L., Kaufmann S., Feldon J. A differential involvement of the shell and core subterritories of the nucleus accumbens of rats in memory processes. Behav. Neurosci. 2003;117:150–168. doi: 10.1037//0735-7044.117.1.150. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee S., Park H., Song B., Hong I., Geum D., Shin K., Choi S. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem. Biophys. Res. Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Knapska E., Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn. Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina A.F., Brockway E.T., Crovetti C.R., Shue F., McCarty M.J., Sattler K.P., Lim S.C., Santos S.L., Denny C.A., Drew M.R. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci. 2019;22:753–761. doi: 10.1038/s41593-019-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H.M.X., Cleary M., Sitharthan T., Hunt G.E. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031. [DOI] [PubMed] [Google Scholar]
- LaLumiere R.T., Kalivas P.W. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J. Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L., Dalley J.W., Robbins T.W. Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behav. Neurosci. 2002;116:539–552. doi: 10.1037//0735-7044.116.4.539. [DOI] [PubMed] [Google Scholar]
- Levita L., Dalley J.W., Robbins T.W. Nucleus accumbens dopamine and learned fear revisited: a review and some new findings. Behav. Brain Res. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Levita L., Hare T.A., Voss H.U., Glover G., Ballon D.J., Casey B.J. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L., Hoskin R., Champi S. Avoidance of harm and anxiety: a role for the nucleus accumbens. Neuroimage. 2012;62:189–198. doi: 10.1016/j.neuroimage.2012.04.059. [DOI] [PubMed] [Google Scholar]
- Lonergan M.E., Gafford G.M., Jarome T.J., Helmstetter F.J. Time-dependent expression of Arc and zif268 after acquisition of fear conditioning. Neural Plast. 2010;2010:139891. doi: 10.1155/2010/139891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.F., Winiecki P., Vanderhoof T., Riccio D.C., Jasnow A.M. Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol. Learn. Mem. 2016;130:83–92. doi: 10.1016/j.nlm.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Mahan A.L., Ressler K.J. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L.-M., Wang J.Q. Regulation of group I metabotropic glutamate receptors by MAPK/ERK in neurons. J. Nat. Sci. 2016;2:e268. [PMC free article] [PubMed] [Google Scholar]
- Mao L.-M., Zhang G.-C., Liu X.-Y., Fibuch E.E., Wang J.Q. Group I metabotropic glutamate receptor-mediated gene expression in striatal neurons. Neurochem. Res. 2008;33:1920–1924. doi: 10.1007/s11064-008-9654-4. [DOI] [PubMed] [Google Scholar]
- Martinez R.C., Oliveira A.R., Macedo C.E., Molina V.A., Brandao M.L. Involvement of dopaminergic mechanisms in the nucleus accumbens core and shell subregions in the expression of fear conditioning. Neurosci. Lett. 2008;446:112–116. doi: 10.1016/j.neulet.2008.09.057. [DOI] [PubMed] [Google Scholar]
- McCullough L.D., Sokolowski J.D., Salamone J.D. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience. 1993;52:919–925. doi: 10.1016/0306-4522(93)90538-q. [DOI] [PubMed] [Google Scholar]
- Menard C., Quirion R. Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front. Pharmacol. 2012;3:182. doi: 10.3389/fphar.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith G.E., Pattiselanno A., Groenewegen H.J., Haber S.N. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J. Comp. Neurol. 1996;365:628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Merikangas K.R., Mehta R.L., Molnar B.E., Walters E.E., Swendsen J.D., Aguilar-Gaziola S., Bijl R., Borges G., Caraveo-Anduaga J.J., Dewit D.J., Kolody B., Vega W.A., Wittchen H.-U., Kessler R.C. Comorbidity of substance use disorders with mood and anxiety disorders: results of the international consortium in psychiatric epidemiology. Addict. Behav. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Millan E.Z., Furlong T.M., McNally G.P. Accumbens shell–hypothalamus interactions mediate extinction of alcohol seeking. J. Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.A., Marshall J.F. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mitrano D.A., Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J. Comp. Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Mitrano D.A., Pare J.F., Smith Y. Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J. Comp. Neurol. 2010;518:1315–1329. doi: 10.1002/cne.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp J.W., Van't Veer A., Parsegian A., Gallo M.S., Chen M., Neve R.L., Meloni E.G., Carlezon W.A. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J. Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits L.M., Harned M.S., Gallop R.J., Butler S.F., Barber J.P., Thase M.E., Crits-Christoph P. Six-month treatment outcomes of cocaine-dependent patients with and without PTSD in a multisite national trial. J. Stud. Alcohol Drugs. 2007;68:353–361. doi: 10.15288/jsad.2007.68.353. [DOI] [PubMed] [Google Scholar]
- Page K.J., Saha A., Everitt B.J. Differential activation and survival of basal forebrain neurons following infusions of excitatory amino acids: studies with the immediate early gene c-fos. Exp. Brain Res. 1993;93:412–422. doi: 10.1007/BF00229357. [DOI] [PubMed] [Google Scholar]
- Parkinson J.A., Robbins T.W., Everitt B.J. Selective excitotoxic lesions of the nucleus accumbens core and shell differentially affect aversive Pavlovian conditioning to discrete and contextual cues. Psychobiology. 1999;27:256–266. [Google Scholar]
- Peng S., Zhang Y., Zhang J., Wang H., Ren B. ERK in learning and memory: a review of recent research. Int. J. Mol. Sci. 2010;11:222–232. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Kalivas P.W., Quirk G.J. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi P.T., Yeates D.C.M., Floresco S.B. Prefrontal cortical and nucleus accumbens contributions to discriminative conditioned suppression of reward-seeking. Learn. Mem. 2020;27:429–440. doi: 10.1101/lm.051912.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F., Moscarello J.M., LeDoux J.E., Sears R.M. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 2015;35:3470–3477. doi: 10.1523/JNEUROSCI.1331-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S.M., Berridge K.C. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. J. Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S.M., Berridge K.C. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur. J. Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Richard J.M., Berridge K.C. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D1 alone for appetitive eating but D1 and D2 together for fear. J. Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J.M., Berridge K.C. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol. Psychiatr. 2013;73:360–370. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G., Platt B., Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rothbaum B.O., Davis M. Roots of Mental Illness in Children. New York Academy of Sciences; New York, NY, US: 2003. Applying learning principles to the treatment of post-trauma reactions; pp. 112–121. [DOI] [PubMed] [Google Scholar]
- Salamone J.D. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Santini E., Ge H., Ren K., Pena de Ortiz S., Quirk G.J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., Atkins C.M., Swank M.W., Bauer E.P., Sweatt J.D., LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 2000;20:8177. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienbacher I., Fendt M., Richardson R., Schnitzler H.U. Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats. Brain Res. 2004;1027:87–93. doi: 10.1016/j.brainres.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D., Padilla-Coreano N., Quirk G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral Hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2010;36:529. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C., de Vasconcelos N.A.P., Coimbra B., Domingues A.V., Silva J.M., Loureiro-Campos E., Gaspar R., Sotiropoulos I., Sousa N., Rodrigues A.J. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol. Psychiatr. 2019;25(12):3241–3255. doi: 10.1038/s41380-019-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.H., Grant V.V., Mackie C.J., Conrod P.J. vol. 2. Oxford University Press; New York, NY, US: 2016. Comorbidity of anxiety and depression with substance use disorders; pp. 149–186. (The Oxford Handbook of Substance Use and Substance Use Disorders). [Google Scholar]
- Tan Y., Williams E.S., Zahm D.S. Calbindin-D 28kD immunofluorescence in ventral mesencephalic neurons labeled following injections of Fluoro-Gold in nucleus accumbens subterritories: inverse relationship relative to known neurotoxin vulnerabilities. Brain Res. 1999;844:67–77. doi: 10.1016/s0006-8993(99)01890-9. [DOI] [PubMed] [Google Scholar]
- Thandi S., Blank J.L., Challiss R.A.J. Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J. Neurochem. 2002;83:1139–1153. doi: 10.1046/j.1471-4159.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- Thomas K.L., Hall J., Everitt B.J. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur. J. Neurosci. 2002;16:1789–1796. doi: 10.1046/j.1460-9568.2002.02247.x. [DOI] [PubMed] [Google Scholar]
- Voorn P., Brady L.S., Schotte A., Berendse H.W., Richfield E.K. Evidence for two neurochemical divisions in the human nucleus accumbens. Eur. J. Neurosci. 1994;6:1913–1916. doi: 10.1111/j.1460-9568.1994.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Wang J.Q., Fibuch E.E., Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J. Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Wendler E., Gaspar J.C., Ferreira T.L., Barbiero J.K., Andreatini R., Vital M.A., Blaha C.D., Winn P., Da Cunha C. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol. Learn. Mem. 2014;109:27–36. doi: 10.1016/j.nlm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Wendler E., Gaspar J.C.C., Ferreira T.L., Barbiero J.K., Andreatini R., Vital M.A.B.F., Blaha C.D., Winn P., Da Cunha C. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol. Learn. Mem. 2014;109:27–36. doi: 10.1016/j.nlm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Wenzel J.M., Rauscher N.A., Cheer J.F., Oleson E.B. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem. Neurosci. 2015;6:16–26. doi: 10.1021/cn500255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard S.S., Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013;9:948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhu Y., Contractor A., Heinemann S.F. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.H., Mao L.-M., Choe E.S., Wang J.Q. Synaptic ERK2 phosphorylates and regulates metabotropic glutamate receptor 1 in vitro and in neurons. Mol. Neurobiol. 2017;54:7156–7170. doi: 10.1007/s12035-016-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Williams C. Differential activation of amygdala Arc expression by positive and negatively valenced emotional learning conditions. Front. Behav. Neurosci. 2013;7:191. doi: 10.3389/fnbeh.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]