Highlights
-
•
Resistin levels have been associated with several pathological disorders such as metabolic disorders, cancers etc.
-
•
Resistin exists in three isoforms namely RELM-α, β and γ.
-
•
High resistin level activates inflammatory pathways, promotes metabolic disorders and is associated with carcinogenesis.
-
•
Increase in the resistin level impairs the therapeutic response by inducing stemness or resistance, in cancer cells.
-
•
Conventional drugs which alter resistin level could have therapeutic implications in several pathological disorders.
Keywords: Diabetes, Obesity, Cholesterol, Metabolism, Cancer, Resistin
Abbreviations: RELM, Resistin like molecule; ROS, Reactive oxygen species; CAP1, Adenylyl cyclase associated protein 1; ROR1, Receptor Tyrosine like Orphan Receptor 1; TLR4, Toll-like receptor 4; LPS, Lipopolysaccharide; CAD, Coronary Artery Disease; BMI, Body Mass Index; EPC, Endothelial Progenitor cells; EMT, Epithelial to Mesenchymal transition; LDL, Low density lipoprotein; LDLR, Low density lipoprotein receptor; PCSK9, Proprotein subtilisin kexin type 9; NAFLD, Non-alcoholic fatty liver disease; DTIC, Dacarbazine
Abstract
Resistin, a small secretory molecule, has been implicated to play an important role in the development of insulin resistance under obese condition. For the past few decades, it has been linked to various cellular and metabolic functions. It has been associated with diseases like metabolic disorders, cardiovascular diseases and cancers. Numerous clinical studies have indicated an increased serum resistin level in pathological disorders which have been reported to increase mortality rate in comparison to low resistin expressing subjects. Various molecular studies suggest resistin plays a pivotal role in proliferation, metastasis, angiogenesis, inflammation as well as in regulating metabolism in cancer cells. Therefore, understanding the role of resistin and elucidating its’ associated molecular mechanism will give a better insight into the management of these disorders. In this article, we summarize the diverse roles of resistin in pathological disorders based on the available literature, clinicopathological data, and a compiled study from various databases. The article mainly provides comprehensive information of its role as a target in different treatment modalities in pre as well as post-clinical studies.
Graphical abstract
Introduction
Resistin, a pro-inflammatory cytokine was initially discovered by Dr. Mitchell Lazar Group as a link between two diseases-diabetes and obesity in 2001. It was found to mediate insulin resistance – hence the name ‘Resistin’ was coined.1 Resistin is primarily secreted from macrophages in humans whereas in rodents’ its main source is adipocytes.1,2,3 It is a ~12.5 KDa hormone, rich in cysteine residues and is encoded by the RETN gene. It is commonly referred to as Adipocyte-specific secretory factor (ADSF), Fizz3, RSTN, or cysteine-rich protein 1(XCP1).[4], [5], [6], [7], [8], [9] The normal physiological range of resistin in human serum so observed is 7–22 ng/ml.3,10 Circulatory resistin is shown to oligomerize to higher-order structures and is up-regulated in some autoimmune disorders, metabolic diseases as well in cancerous conditions.[11], [12]
The immature resistin protein in humans consists of 108 amino acids whereas in rodents it was observed to be slightly longer with 114 amino acids. Human and mice resistin exhibit only 59% similarity in their sequence, and differ significantly in their secondary structures.7,13,14 It is quite intriguing that resistin exists in various isoforms, but their functions are yet to be understood in detail. The primary expression of resistin so observed in humans is mostly monocytes.15 These cells on being stimulated by pro-inflammatory mediators enable resistin to mediate the recruitment of other immune cells. This elevated level of resistin is often associated with chronic low-grade sub-clinical inflammation accompanied with obesity which involves macrophage infiltration in the adipose tissues. Moreover, human resistin also promotes the production of numerous inflammatory molecules like VCAM-1, ICAM-1 and MCP-1.[16], [17], [18] Additionally, resistin activates p38 MAPK signaling pathway which impairs insulin signaling and alters oxidative stress response as well as cell proliferation.[19], [20], [21]
Precursor human resistin forms a mature molecule of 12.5 kDa and it mainly exists in two conformations:
-
a)
Trimer with a molecular weight of 45 kDa
-
b)
Oligomer with a molecular weight of 660 kDa22
The assembly of these trimers and oligomers promotes secretion of TNF-α, IL-6, IL-8, IL-12 and IL-1β, generates reactive oxygen species (ROS) and inhibits eNOS.[22], [23], [24]
Mouse resistin is 11 kDa polypeptide. Several factors like glucocorticoids, growth hormones, testosterone and prolactin upregulate resistin in rat and mouse adipose cells, whereas insulin, epinephrine and somatotropin suppress its secretion.25,26 High serum resistin in rodents alter glucose homeostasis and insulin resistance in liver and skeletal muscle by impairing AMPK.[27], [28], [29] Reports suggest secretion of resistin in rodents is influenced by genetics as well as diet. Moreover, it has been reported that resistin increases in animals in response to hyperglycemia and promotes hepatic glucose production as well.1 As resistin exerts different regulatory mechanisms, assigning biological properties to rodent resistin remains obscure.
Resistin structure
Unraveling the structure of a molecule facilitates a better understanding of its structure-function relationship. The crystal structure of resistin and RELM-β was first determined by Patel et.al which revealed an unusual multimeric structure with each protomer consisting of carboxy-terminal disulfide-rich β-sheets as a ‘head’ domain and an amino-terminal alpha-helical region as ‘tail’ domain. Three stranded coiled coils have been reported to form by an association of the alpha-helical segment while the formation of a tail to tail hexamers are mediated by surface exposed interchain disulfide linkages.30 Structure of RELM-γ is very closely related to RELM-α but with varied tissue expression profiles.31 The secondary structure of human resistin was observed to be rich in α-helices whereas mice resistin mostly consists of β-sheets.4
There are three subtypes of RELMs identified till date:
-
a)
RELM-α or FIZZ1 is a secretory protein primarily present in the adipose tissue, which functions as a pro-inflammatory cytokine during murine allergic pulmonary inflammation.7,32 It is also detected in several pulmonary infections as well as in lung cells during fibrosis.33 Additionally, it has been observed to induce Th2 cytokine immune responses which negatively regulates Th2 responses in pulmonary granuloma formation by helminth parasites.34 FIZZ1 has been recognized as one of the main signatures of M2 or alternatively activated macrophages.[35], [36], [37], [38] Moreover, RELM-α is also involved in repair of tissues and promotes fibrosis by stimulating Th2 cytokines.39
-
b)
RELM-β, also known as FIZZ2, is mainly present in the goblet and epithelial cells of the gastrointestinal tract and promotes the proliferative capacity of the cells.4,40 It has also been reported to function as a chemoattractant for bone marrow cells, with a major focus on the bone-marrow derived CD11C+ dendritic cells.The human ortholog of FIZZ2 shows significant sequence homology to both rodent FIZZ2 and FIZZ1.41 Additionally, increased expression of RELM-β is observed in the airways of asthmatic patients wherein it regulates airway epithelial function. This establishes its role in pulmonary remodeling. It has also been reported to induce proliferation by enhancing expression of MUC5AC, ERK, MAPK, and PI3/Akt pathway, including TGFβ, EGF and VEGF.42 RELM-β plays contributory roles in metabolic dysfunction by suppressing insulin signaling in hepatocytes and activating MAPK pathway.43 Owing to its anti-microbial activity, both mouse and human RELM-β bind and permeabilize the membrane of gram-negative bacteria, thus acting on the pathogen and on the hosts.44
-
c)
RELM-γ is the least studied protein amongst the three and is expressed in white blood cells, spleen, thymus as well as the nasal respiratory epithelium of cigarette smoked rats.[45], [46], [47] It mainly exhibits cytokine-like functions in the hematopoietic tissues.46 It was also observed to interact with human neutrophil alpha-defensin and plays a pivotal role in the chemotaxis of bone-marrow-derived myeloid cells.47
Source
The sources of resistin are immune and epithelial cells, including peripheral blood mononuclear (PBMCs), macrophages and bone marrow cells, primarily in primates, pigs and dogs, while in rodents the main source is adipose tissue.1,22 Numerous literature indicate that resistin expression is not only restricted to adipose tissue but also detected in the stomach, small and large intestine, adrenal gland and skeletal muscle. Expression of resistin mRNA varies according to the deposition of white adipose tissue and gender specificity- the highest level being observed in female gonadal fat. 48,49
Receptors
CAP1, Decorin, ROR1 and TLR4-have been identified as receptors for resistin which causes activation of different signaling cascades.
-
a)
ROR1- ROR1 or Receptor Tyrosine like Orphan Receptor 1 is mostly expressed in 3T3-L1 preadipocytes which facilitate glucose uptake, adipogenesis, as well as development of the nervous system in embryonic stages. 50,51
-
b)
CAP1- One of the receptors of resistin present on monocytes is CAP1. It is an actin-binding protein found mainly in the cytosol. It regulates filamentous dynamics and activates the cyclic AMP pathway.52,53 Resistin is reported to bind directly to CAP1 in monocytes and upregulate cAMP, PKA and NFκβ dependent transcription of inflammatory cytokines.53
-
c)
Decorin- Resistin has been reported to bind to Decorin, a member of the leucine-rich proteoglycan family present on adipocytes. On binding with Decorin, it regulates WAT (White adipose tissue) expansion as well promotes proliferation and migration of 3T3-L1 cells.54
-
d)
TLR4- Additionally, TLR4 or Toll-like receptor 4 has been identified as one of the receptors from the TLRs. These receptors belong to type I transmembrane protein family but differ in their ligand specificity as well as their presence in different organisms.55,56 Resistin competes with LPS for binding to TLR457 which activates the Renin-angiotensin system via TLR4/p65/Agt pathway and induces hypertension.58 Direct binding of human resistin to TLR4 in the hypothalamus also causes activation of pro-inflammatory pathways and metastasis in cancerous conditions. 59
Functions
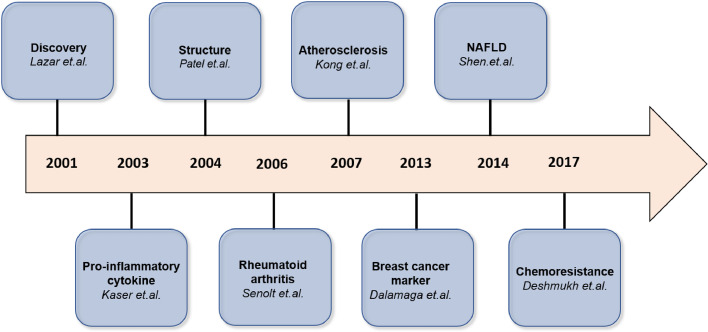
Several other functions such as blood glucose and lipid metabolism, modulation of satiety centers in hypothalamus and pituitary somatotrophic cells, central nervous system regulation, synthesis and secretion of different pro-inflammatory cytokines and differentiation of monocytes into macrophages, control of heart contractility, angiogenesis, smooth muscle cell activity, renal functioning and bone remodeling have been attributed to resistin.12,23,[60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74] For years, resistin has also been identified to play significant roles in various diseases too (Fig. 1).
Fig 1.
Resistin timeline.
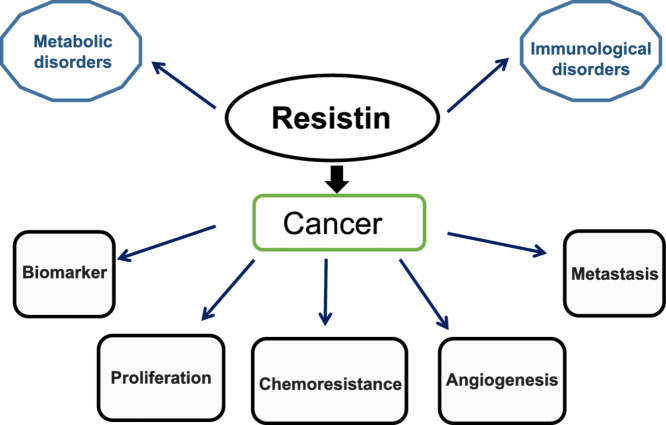
The review mainly highlights the roles of resistin in a) Cancer, b) Immunological disorders and c) Metabolic disorders. (Fig. 2).
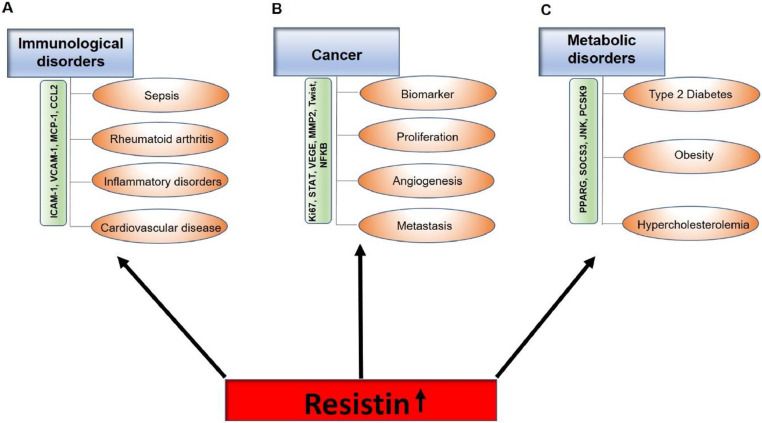
Fig 2.
Disorders associated with increased resistin condition A) Resistin is upregulated in various immunological disorders like sepsis, rheumatoid arthritis or cardiovascular diseases by regulating the inflammatory molecules B) Various hallmarks like proliferation, metastasis and angiogenesis are also regulated by elevated resistin level in the serum by modulation of molecules actively responsible for cancer C) Resistin plays a pivotal role in metabolic disorders like Type 2 diabetes, obesity and hypercholesterolemia by various pathways.
Resistin in cancer
Cancer is one of the most prevalent as well as life-threatening diseases worldwide. Pre-clinical and clinical studies indicated the presence of a high level of serum resistin in patients with various cancers. Interestingly, elevated resistin level is not only predominantly exhibited in obesity-influenced cancers i.e. breast, colon, etc. but also in cancers irrespective of obesity such as in lung, renal, etc.[75], [76], [77], [78], [79] Resistin has been linked to increased risk of progression, angiogenesis and metastasis in various cancer models80,81 (Fig. 3). Its role has been also associated with chemoresistance and stemness induction in cancer, yet related mechanistic details need further exploration.82
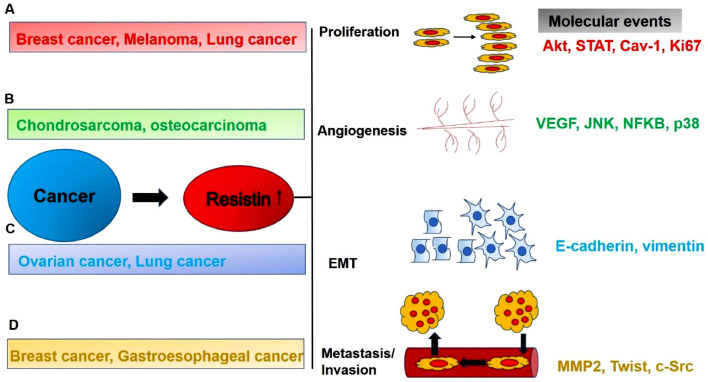
Fig 3.
Mechanistic details exploring resistin's role in different hallmarks of cancer. A) Resistin has been observed to promote proliferation in cancers like breast, melanoma and lung by inducing STAT, AKT or Ki67 pathways. B) It's also observed to promote angiogenesis in cancers like chondrosarcoma, osteocarcinoma etc. by upregulating molecules like VEGF, JNK, p38 etc. and dowregulate several miRNAs. C) Epithelial to mesenchymal transition is also reported to be regulated by increased resistin level in serum. Molecules responsible for EMT transition like vimentin, e-cadherin etc. are enhanced in these conditions. Several immune cells involved in cancer, like dendritic cells, also play an important role in secreting resistin and regulating EMT in cancer. D) In various cancers like breast and gastroesophageal, elevated resistin level induces invasion/metastasis via molecules like MMPs, c-Src etc.
Resistin as biomarker
Biomarker is a measurable characteristic of the pathophysiological condition of an individual, which can be useful in early diagnosis and understanding the therapeutic regime for any disease.83 Evidences suggest resistin as a potential prognostic and diagnostic biomarker in cancer.84
A diverse array of studies depicts high serum resistin levels in patients of breast cancer, lymphoma, esophageal squamous cell carcinoma, endometrial adenocarcinoma, gastric and colorectal cancer.85 Study done in 80 breast cancer patients and 50 healthy control indicated increased resistin levels in breast cancer patients compared to control. Moreover, patients with lymph node metastasis were also reported to have increased resistin level when compared with the non-lymph node metastatic patients.86 The relationship between a high level of resistin and increased risk of breast cancer has been observed to be independent of age, status of menopause, serum glucose, BMI and adiponectin. However, it is significantly associated with tumor and inflammatory markers, tumor size, cancer grade, stage and lymph node invasion.[83], [85]
Another case-control study involving 37 Caucasian endometrial female patients and 39 healthy controls who were BMI and age matched, revealed a significant difference in resistin level between the patients and the control group. The mean resistin level was observed to be 24.2 ng/ml in the affected individuals. Whereas, the level was found to be much less in healthy individuals (10.1 ng/ml).84 However the exact role of this elevated resistin in various cancers is yet to be explored.
Resistin in proliferation and arrest
Signaling pathways that link resistin with cancer include TLR4, PI-3 K, and NFκβ. In several cancers activation of diverse signaling pathways has been associated with proliferation. In prostate cancer, progression takes place via AKT pathway whereas in lung cancer it is mainly through PI-3 K, NFκβ, EGFR and TLR4 receptor.59,86,87 Few reports have indicated that proliferation in melanoma by resistin is mediated by pAKT and Cav-1, whereas in breast cancer progression has been attributed to IL-6 dependent STAT 3 signaling.88,89 Resistin-induced progression in ovarian cancer has been observed to be mostly via miR let-7a, miR-200c and miR-186.80 Yet, in gastric cancer cells resistin and visfatin synergistically increase cell proliferation by activating the expression of the telomerase gene.90 A similar report suggests upregulation of Human Telomerase Reverse Transcriptase (hTERT) by resistin treatment only.91 Recent studies suggest silencing of CAP1 along with TLR4 decreases proliferation of pancreatic cell lines mediated by STAT3, both in vitro and in vivo.92 Interestingly, another report implicates resistin's role in cell cycle arrest in colon cancer cells by upregulation of SOCS3.93 Numerous unambiguous explorations of resistin on the proliferation of cancer cells have opened up avenues for more detailed studies in the future.
Resistin in angiogenesis
Reports suggest resistin induces angiogenesis by regulating various pathways. Evidences indicate in chondrosarcoma cells, it induces VEGF-A expression through PI3K and Akt signaling pathway and downregulates microRNA expression (miR)−16–5p resulting in increased angiogenesis.69 Whereas, in ovarian cancer cell (HO-8910) resistin-induced expression of VEGF takes place through the PI3K/Akt-Sp1 pathway.94 According to available literature, resistin induces SDF-1 expression and promotes angiogenesis in human gastric cancer cells.95 However ERK, JNK and p38 pathways are mostly responsible for inducing VEGF mediated angiogenesis in osteocarcinoma cells.96 Inhibition of these signaling molecules reduce expression of VEGF-A and subsequently causes a decrease in EPC migration and tube formation.80
Resistin in metastasis
Metastasis is one of the main causes of cancer-related deaths worldwide. A comparative study including 42 endometrial cancer patients and 42 control individuals suggest high serum resistin level in endometrial patients.97 Similar studies with gastroesophageal cancer and postmenopausal breast cancer patients depict higher resistin levels in patients with distant metastasis.79,98 In breast cancer cells resistin induces phosphorylation of c-src, PP2A, PKCα, ezrin, radixin, moesin as well as increases expression of vimentin promoting cell invasion and metastasis of breast cancer cell.79 Resistin treatment also resulted in increased cell invasion and MMP-2 expression through AMPK and p38 pathway while suppressing miR 519d99 However, on being treated with resistin, ovarian cancer cells A2780 and SK-OV-3 secrete reduced levels of E-cadherin and enhanced levels of vimentin and ZEB1 resulting in EMT transition.80 Whereas Src/EGFR, NFκB, PI3K were also observed to be involved in signaling for invasion and migration in lung cancer cells upon resistin exposure.59 For the first time it has been demonstrated that tumor-associated dendritic cells (TADCs) of lung cancer, secrete resistin and treatment of condition media from TADCs upregulates Twist, an inducer of EMT.100 Strategies for targeting signaling molecules induced by resistin have been devised (Table 1).
Table 1.
Targeting molecules regulated by Resistin.
| Targets | Agents | Cancers | Phases | Reference NCT/PUBMED (PMID) |
|---|---|---|---|---|
| STAT3 | IONIS-STAT3Rx | Advanced cancers, DLBCL, Advanced lymphoma | Phase 1 Phase 2 | 01,563,302 |
| TTI-101 | Breast cancer, Head and neck cancer, Squamous cell carcinoma, Non-small cell lung cancer, Hepatocellular cancer, Colorectal cancer, Gastric cancer, Gastric adenocarcinoma, Melanoma, Advanced cancer | Phase 1 | 03,195,699 | |
| WP1066 | Brain tumor medulloblastoma, Brain metastases | Phase 1 | 04,334,863 | |
| WP1066 | Metastatic malignant neoplasm in brain, Metastatic melanoma, Recurrent brain neoplasm, Recurrent glioblastoma, Recurrent malignant, glioma | Phase 1 | 01,904,123 | |
| AZD9150 | Advanced adult hepatocellular carcinoma, Hepatocellular carcinoma metastatic | Phase 1 | 01,839,604 | |
| SAR302503 | Hematopoietic neoplasm | Phase 2 | 01,420,783 | |
| Pyrimethamine | Chronic lymphocytic leukemia, Small lymphocytic leukemia | Phase 1 Phase 2 | 01,066,663 | |
| Pyrimethamine | Myelodysplastic syndromes | Phase 1 | 03,057,990 | |
| TLR4 | CX-01, Azacitidine | Myelodysplastic syndromes (MDS), Acute Myeloid Leukemia (AML) | Phase 1 | 02,995,655 |
| NF-kB | Dimethylfumerate | Cutaneous T cell lymphoma | Phase 2 | 02,546,440 |
| Omaveloxolone Ipilimumab Nivolumab | Melanoma, Unresectable (stage3) melanoma, Metastatic (stage4) Melanoma | Phase 1 Phase 2 | 02,259,231 | |
| PI3K | Copanlisib Nivolumab | Unresectable or metastatic microsatellite stable solid tumor along with microsatellite stable colon cancer | Phase 1 Phase 2 | 03,711,058 |
| Pyruvate (13C) | Prostate cancer | Phase 1 Phase 2 | 02,913,131 | |
| BKM120 Trastuzumab Paclitaxel | HER-2 positive newly diagnosed, primary breast cancer | Phase 2 | 01,816,594 | |
| P38 | LY2228820 Midazolam Tamoxifan | Advanced cancer | Phase 1 | 01,393,990 |
| Rogarafenib | Metastatic colorectal cancer | Phase 2 | 01,949,194 | |
| VEGF | Pazopenib, 5-FU, Oxaliplatin, Leukovorin (FLO) | Advanced gastric cancer | Phase 2 | 01,503,372 |
| Bevacizumab Atezolizumab Entinostat | Metastatic cancer, Renal cancer | Phase 1 Phase 2 | 03,024,437 | |
| Bevacizumab | Adult primary hepatocellular carcinoma, Localized unresectable adult primary liver cancer, Recurrent adult primary liver cancer | Phase 2 | 00,055,692 | |
| Sunitinib | Urinary tract urothelial carcinoma | Phase 2 | 00,794,950 | |
| RAD001 Bevacizumab FOLFOX | Colorectal cancer | Phase 1 Phase 2 | 01,047,293 | |
| Docetaxel Vandetanib | Non-small cell lung cancer, Lung cancer | Phase 3 | 00,312,377 | |
| Aflibercept (Zivaflibercept, AVE0005-VEGF trap, ZALTRAP) | Neoplasms, Ovarian cancer | Phase 2 | 00,327,171 | |
| Itraconazole | Lung cancer | Phase 2 | 03,664,115 | |
| Cetuximab, BAY 43–9006 | Metastatic colorectal cancer | Phase 2 | 00,326,495 | |
| Lucitanib | Solid tumors | Phase 1 Phase 2 | 01,283,945 | |
| GSK1363089 (foretinib) | Neoplasm, Head and neck | Phase 2 | 00,725,764 | |
| Apatinib | Non-small cell lung cancer | Phase 2 | 02,515,435 | |
| Sunitinib | Ovarian cancer, Adverse effect | Phase 2 | 01,824,615 | |
| Dovitinib | Gastrointestinal stromal tumors | Phase 2 | 01,440,959 | |
| Pazopanib | Gastrointestinal stromal tumors | Phase 2 | 01,524,848 | |
| Ramucirumab Paclitaxel | Gastric adenocarcinoma, Gastroesophageal junction adenocarcinoma | Phase 2 | 02,628,951 | |
| Bevacizumab Sorafenib Tosylate | Recurrent melanoma, Stage 3 skin melanoma, Stage 4 skin melanoma | Phase 2 | 00,387,751 | |
| Ranibizumab | Neurofibromatosis type 1, Cutenous neurofibromas | Early phase 1 | 00,657,202 | |
| RAD001 | Renal cell carcinoma | Phase 4 | 01,206,764 | |
| Celecoxib | Lymphangioleiomyomatosis(LAM) | Phase 2 | 02,484,664 | |
| Bevacizumab and sorafenib | Metastatic colorectal cancer | Phase 2, in-vivo | PMID 32,201,506 |
Resistin and its effect on chemotherapy
Amongst several multifaceted roles of resistin, the least explored area has been its role in imparting therapeutic resistance. Few reports suggest resistin's involvement in promoting resistance towards different anticancer, antimetabolic or immunomodulatory drugs. Numerous factors contribute to this resistance such as impaired immune response, alterations in metabolic profile, inducing stemness, etc. Studies reported that increased resistin level induces chemoresistance to Gemcitabine in pancreatic cancer whereas resistin exposed breast cancer promotes resistance to Doxorubicin.92,101,102 It has also been shown that resistin abrogates doxorubicin-induced cell death through autophagy induction by activation of AMPK/mTOR/ULK1 and JNK signaling pathways.103 Studies in ovarian cancer have reported a significant correlation between high resistin level and poor prognosis. Additionally, it promotes chemoresistance to cisplatin by increasing stemness of ovarian cancer cells. Resistin is also known to induce chemoresistance in ovarian cancer by suppressing miRNAs let-7, miR-200c and miR-186. 104
Interestingly, a recent report suggests that treating colon cancer cells with exogenous resistin make them resistant to 5-fluorouracil because of the decreased drug uptake which is caused due to the arrest of cells in the G1 phase.90 Moreover, Pang et.al., reported that resistin inhibits chemotherapy-induced cleavage of caspases by the activation of NFκB and PI3K/Akt pathways in multiple myeloma. It decreases the expression of both DNA methyltransferases DNMT1 and DNMT3a as well as the methylation of ATP binding cassette (ABC) gene promoters which in turn increases the expression and the drug efflux function of ABC transporters in myeloma cells.105 Another recent study from our lab also demonstrates resistin impairs the efficacy of DTIC in melanoma by increasing and stabilizing the protein levels of Cav-1 and P-gp.88
From the available literature, it is evident that resistin is one of the key factors in imparting chemoresistance to various therapeutic interventions. However, detailed systemic and clinical studies are required to understand the mechanism underlying this phenomenon. The role of resistin in intervening chemotherapeutic responses should be investigated to elucidate the mode of action through which resistin impedes the efficacy of various drugs directly or indirectly.
Resistin in immunological disorders
Resistin being a pro-inflammatory cytokine, also plays a significant role in modulating immune functions in pathological conditions. It alters the secretory profile of factors like Intercellular adhesion molecule (ICAM-1), Vascular cell adhesion molecule (VCAM-1), Monocyte chemoattractant protein (MCP-1) and Chemokine (C—C motif) ligand 2 (CCL2) in human macrophages upon inflammation thereby promoting chemotaxis and recruitment of leukocytes to the inflamed sites. Resistin exhibits autocrine, paracrine as well as endocrine mechanisms on a wide range of cells and tissues by increasing the Th1 immune response system and directly activating the complement system as well.12,[106], [107], [108], [109] Bokarewa et.al reported resistin stimulates the synthesis and secretion of pro-inflammatory cytokines like TNF-α, IL-1 and IL-6 while Silswal et.al reports the stimulation takes place by inducing nuclear translocation of NFκB, activation of which in turn increases the production of IL-8 and MCP-1.24,110 Alternatively active murine macrophages express high levels of resistin like molecule (RELMα)- an effector protein with potent immunomodulatory roles.111 Circulating resistin levels are correlated with inflammatory and fibrinolytic markers such as CRP, TNF-α and IL-6 in the general population and in individuals with T2DM, coronary atherosclerosis, chronic kidney disease, rheumatoid arthritis and sepsis.112
-
a)
Sepsis- A study involving 95 patients of the Intensive Care Unit at Karolinska University Hospital and Center for Infectious Medicine, Karolinska Institute, Huddinge, Sweden, reported that resistin is elevated in patients suffering from sepsis or septic shock. It was the first report for establishing resistin as a biomarker for the severity of the disease as well as prolonged inflammatory state of critically ill patients.113 Sepsis-induced immunosuppression is a key factor contributing to morbidity and mortality of critically ill patients and polymorphonuclear neutrophil dysfunction is believed to be a hallmark of this immunosuppression.114
-
b)
Rheumatoid arthritis- Several studies have reported increased levels of resistin in the synovial fluid of rheumatoid arthritis patients. Evidences show that it increases osteoclastogenesis which induces a weak differentiation of pre-osteoblasts into osteoblasts.23 Resistin has been observed to increase the pathogenesis of rheumatoid arthritis by inducing the production of chemokines like CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 and CXCL8.115 Resistin has been reported to co-localize with macrophages, B lymphocytes and plasma cells suggesting a pathogenicity of resistin in arthritis condition.116
-
c)
Inflammatory disorder- Besides stimulating the production of numerous pro-inflammatory cytokines, resistin activates signal transduction pathways like p38, JNK and ERK.95 It activates ERK pathway inducing proliferation of smooth muscle which in turn affects vascular restenosis.68 Resistin inhibits eNOS gene expression and induces oxidative stress.117
Cardiovascular disorder
Despite reports demonstrating resistin being an important predictor of cardiovascular diseases, still, its relation remains controversial. In 2005, Reilly and his colleagues did the first landmark experiment to correlate elevated resistin level with a surrogate marker in atherosclerosis patients-coronary-calcium score.118 Reports suggest in patients with stable CAD, a stepwise increase in the resistin level can be observed with >50% stenosed coronary vessel.119 Moreover, resistin has been observed to play a major predictor in atherosclerosis (ATS) and related cardiovascular diseases such as myocardial infarction, heart failure and cardiac ischemic events 12,23,118,[120], [121], [122], [123], [124] Hyper-resistenimia state also increases the incidence of cardiovascular diseases even with a deregulated ischemia- perfusion injury, reduced contractility and hypertrophy.66,125 In women's health initiative observational study, elevated resistin level was strongly correlated with increased ischemic stroke in post-menopausal women.126 Frenkel et.al also performed a Framingham offspring study where a 26% increase in heart failure was observed with each 7.45 ng/ml increase in resistin level in serum.127 In vitro studies have shown high resistin level to induce various pro-atherogenic molecules like ET-1, VCAM-1 and MCP-1 and downregulate anti-atherogenic molecules like TRAF-3 which is an inhibitor of CD40 signaling in endothelial cells.128 Additionally, resistin contributes to the impairment of glucose uptake in cardiomyocytes which alters vesicle trafficking.129 Furthermore, resistin is observed in diabetic hearts, promoting cardiac hypertrophy, and decreasing myocyte contractility.130 Aortic stenosis is also a cardiac disorder associated with a high resistin condition which leads to an elevated vulvular calcium deposition and increased concentration of macrophages.2,3,118,123 However, the effect of resistin in human heart failure and aggravation should be explored in detail.
Resistin with metabolic disorders
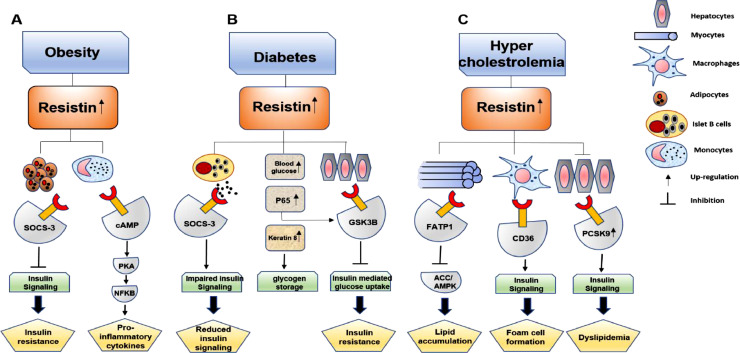
As stated earlier, resistin was first discovered by Steppan et.al. as a link between diabetes and obesity by imparting insulin resistance1. Reports suggest a correlation between increased resistin expression with various metabolic disorders since past few decades. Fig 4 summarizes mechanisms exhibited by resistin in these metabolic disorders.
Fig 4.
Different metabolic disorders regulated by resistin A) Resistin is observed to increase in obese conditions in which it regulates insulin signaling and imparts insulin resistance. This phenomenon is mediated by increase in SOCS-3. Resistin also induce inflammation in obesity by regulating cAMP and NFκB. B) In diabetes, resistin induces insulin resistance via several pathways. Apart from increase in SOCS-3 signaling, resistin induces p65 as well as GSK-3β signaling to regulate insulin signaling. C) Resistin maintains a hypercholesterolemic environment by various pathways. It increases FATP1 which inhibits ACC and AMPK to induce lipid accumulation. In macrophages, CD36 is induced which promotes increase in cholesterol level and foam cell formation. Resistin also induce PCSK9 and degrade LDLR in the hepatocytes to maintain a hypercholesterolemic environment.
Obesity
Few studies previously reported that resistin levels increase in diet-induced obese mouse but its role in obesity remains largely obscure.88 Also, there is an increase in adipose tissue and macrophages which secrete resistin in obese conditions.131 The obesity and low-grade chronic inflammation are synchronous in nature.132 In a study of 169 non-obese (mean body mass index [BMI] = 24.51–3.69 kg/m2) and 160 obese (mean BMI= 36–4.78 kg/m2) subjects, serum resistin levels were significantly associated with BMI (P=.047).133 Besides serum levels, the tissue-specific expression of resistin is dependent on various factors. It was observed that fasting downregulates resistin gene expression in adipose and pituitary tissue, but not in the hypothalamus. Resistin mRNA was decreased under starvation whereas it was up-regulated with an increase in visceral fat. Contrastingly, the pituitary levels of resistin were decreased in presence of both high (ob/ob) and low (fasting) adipose stores which are dependent on age and gender.134 In another study, resistin level was statistically correlated with insulin, BMI, body-fat content and homeostasis model assessment (HOMA).135 However it is an established fact that obesity has close relations with immune cells especially with macrophages- (a) the increased fat accumulation concomitant with infiltration,(b) polarization (c) and in-situ proliferation of macrophages.[136], [137], [138] It was characterized that CAP1 serves as the receptor for resistin in white adipose tissues of resistin humanized mice. However, the expression of CAP1 in adipose resident immune cells and the role of resistin is still an unexplored area. Surprisingly, resistin is also known to inhibit adipocyte differentiation and can function as a feedback regulator for adipogenesis. Moreover, the resistin mRNA and protein levels in ob/ob mice, (genetically obese mouse model), are suppressed by exogenous leptin treatment. These observations portray the highly contrasting role of resistin in disorders such as obesity and diabetes.139 The treatment of LPS and zymosan to human subcutaneous adipocytes increases secretion of resistin whereas treatment of human recombinant resistin in these cells significantly increases stimulation of TLR-2, IKKβ and JNK.139 The treatment of preadipocytes with PPARγ agonist GW501516 induces expression of PPARγ and resistin.140 Cyclolepis genistoides is a phytochemical known to modulate the expression of PPARγ and downregulate resistin.141 The treatment of chromium picolenate is also known to inhibit resistin secretion in insulin resistant and normal 3T3-L1 adipocytes by inhibiting AMPK.142 Strategies to target resistin in obesity are summarized in Table 2.
Table 2.
Anti-obesity drugs affecting resistin levels.
| Serial no. | Drug name | Phase | NCT no/PMID |
|---|---|---|---|
| 1. | Metformin | Clinical trials | NCT02438540 |
| 2. | Entacapone | Phase 1 | NCT02349243 |
| 3. | Ezetimibe add on to statin therapy | Clinical trials in obese patients with atherosclerosis | NCT00485121 |
| 4. | Dapagliflozin, Metformin | Phase 2 Phase 3 |
NCT03968224 |
| 5. | Losartan+Simvastatin Amlodipine+Simvastatin |
Phase 4 | NCT00669435 |
| 6. | Orlistat | Clinical Tests approved | 21,812,797 |
Diabetes
In the past few decades, resistin has been reported to play a significant role in Type 2 Diabetes Mellitus (T2DM). Insulin resistance is an important phenomenon exhibited in obese phenotype, which if manifested for extended time periods may lead to glucose intolerance and hyperglycemia. Several evidences suggest a strong correlation between resistin and obesity-associated diabetes. It is known to regulate glucose homeostasis and antagonizes hepatic insulin action. Exogenous resistin administration in mice causes an increase in glucose production and blood glucose levels.143 The renal alteration in diabetes is one of the complications which takes place and was observed to be negatively correlated to serum resistin level.144 Over-expression of resistin in L6 rat myotubes inhibits insulin-stimulated 2-Deoxy glucose uptake without affecting GLUT4 translocation, GLUT1 expression, and IRS signaling.[141], [145] The infusion of resistin or resistin-like molecule RELMβ induces hepatic insulin resistance which was denoted by increased hepatic glucose production in Adult male Sprague Dawley rats.[142], [146] The treatment of resistin in rat hepatocyte and mice with liver-specific resistin expression impair hepatic insulin action by decreasing phosphorylation of GSK3β at ser 9.147 A study was done in C57BL/6 J mice has reported that treatment of resistin decreases the storage of glycogen by increased expression of p65 which binds with the promoter of keratin 8 (K8) and increase its expression.138 In a case-control study it was observed that 68+ G to A phenotype of RETN may increase susceptibility to T2DM in the Thai population.148 Drugs to target resistin in Diabetes have been deduced. (Table 3)
Table 3.
Anti-diabetic drugs affecting resistin levels.
| Serial no. | Drug name | Phase | NCT no/PMID |
|---|---|---|---|
| 1. | Metformin Pioglitazone |
Phase 1 Phase 2 |
NCT01396564 |
| 2. | Pioglitazone | Phase 4 | NCT01223196 |
| 3. | Rosiglitazone Metformin/sulfonylurea |
Interventional clinical trial | NCT00486187 |
| 5. | Metformin glycinate Metformin hydroxychloride |
Phase 3 | NCT01386671 |
| 6. | Cenicriviroc 150 mg | Phase 2 | NCT02330549 |
| 7. | Alpha lipoic acid | Clinical trial | NCT02775266 |
| 8. | Liraglutide | Interventional Clinical trial | NCT02138045 |
Hypercholesterolemia
Several studies show a positive correlation of resistin with free cholesterol as well as LDL (Low-Density Lipoprotein) cholesterol. Increased ROS production and lipid accumulation in the intima of the vessels enhances hyper resistenimia. Monocytes which produce resistin and become macrophages are recuited- these subsequently take up cholesterol and become foam cells.25 Resistin is mainly observed to increase lipid accumulation as well as oxLDL in human macrophages accompanied by increased CD36 expression at both protein and mRNA level.149 Abrogating resistin in obese mice decreases hepatic steatosis, serum cholesterol as well as VLDL secretion which is a consequence of the reduced expression of genes involved in hepatic lipogenesis and VLDL export.150 NAFLD condition has also been associated with increase serum resistin level.151 Resistin has been observed to stimulate PCSK9, by enhanced gene expression and protein stability which leads to degradation of low-density lipoprotein receptor by 40%.[152], [153] Overexpression of resistin induces dyslipidemic condition by decreasing LDLR and Apolipoprotein A1 in the liver partially through enhanced secretion of lipoprotein.154 Whereas, in skeletal muscles, resistin induces FATP1 expression and decreases phosphorylation of AMPK and ACC promoting lipid accumulation.155 Interestingly, it has been reported that, in patients suffering from atherosclerosis, Pitavastatin significantly decreases hypercholesterolemia along with serum resistin and C-reactive protein.156 Strategies to target resistin in hypercholesterolemic condition has been enlisted (Table 4)
Table 4.
Anti-cholesterol drugs affecting resistin levels.
| Serial no. | Drug name | Phase | NCT no/PMID |
|---|---|---|---|
| 1. | Pitavastatin | Clinical study | 18,385,536 |
| 2. | PCSK9 monoclonal antibodies (AMG145, REGN727) | Phase 1 and Phase 2 | 24,217,159 |
Conclusion
With an increase in the sedentary lifestyle as well as epigenetic changes, incidences of pathological disorders are on the rise. Available evidences suggest a positive correlation between high resistin level and certain chronic diseases. Accumulative observations indicate that resistin might play a pivotal role in aggravating various disorders, which may contribute to increase in the mortality rate in subjects with pre-existing comorbidities. But the relation is still poorly understood. Additionally, from a molecular perspective, resistin has been reported to be involved in the regulation of several signaling pathways which include IGF-1, NFκβ, STAT, MAPK, PI3K etc. as well as some genetic recombination or polymorphisms. Further investigation will aid in understanding the complexity of resistin in these clinical disorders. This review along with recent literature has laid out the ground work for resistin's roles as a putative hallmark in numerous pathological disorders because of its varied roles in inflammation, regulating metabolism as well as in cancer. In cancers, resistin influences various hallmarks of cancers including chemotherapeutic responses. In conclusion, the comprehensive information of resistin in various pathological disorders represents an extensive area of research for targeting resistin as a diagnostic and prognostic tool which would help in better understanding its relevance in these comorbidities.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgements
A.D. thanks Department of Biotechnology (DBT), India for the research fellowship. B.D., P.R., and F.K.B., thank University Grants Commission (UGC), New Delhi, India for their research fellowship. The authors thank National center for Cell Science, Pune, India and Savitribai Phule Pune University, Pune, India for their support.
Author Contributions
Ankita Deb: Conceptualization, data research, literature survey, writing-original draft preparation, discussion of the content, writing- reviewing, editing, generation of figures and tables. Bhavana Deshmukh: Data research, literature survey, writing-reviewing, editing, generation of table. Pranay Ramteke: Data research, literature survey, writing-reviewing and editing. Firoz Khan Bhati: Data research, literature survey, writing-reviewing and editing. Manoj Kumar Bhat: Conceptualization, supervision, discussion of the content, writing- reviewing and editing. All the authors read and approved the manuscript before submission.
Funding
This work was supported by Intramural grant from National center for Cell Science (NCCS), an autonomous institution funded by Department of Biotechnology (DBT), Government of India. The funding agencies had no involvement data collection, interpretation and analysis, decision to publish, or writing of the manuscript.
References
- 1.Steppan C.M., Bailey S.T., Bhat S., Brown E.J., Banerjee R.R., Wright C.M., Patel H.R., Ahima R.S., Lazar M.A. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Jung H.S., Park K.H., Cho Y.M., Chung S.S., Cho H.J., Cho S.Y., Kim S.J., Kim S.Y., Lee H.K., Park K.S. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc. Res. 2006;69(1):76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Patel L., Buckels A.C., Kinghorn I.J., Murdock P.R., Holbrook J.D., Plumpton C., Macphee C.H., Smith S.A. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 2003;300(2):472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 4.Al Hannan F., Culligan K.G. Human resistin and the RELM of Inflammation in diabesity. Diabetol. Metab. Syndr. 2015;7(54) doi: 10.1186/s13098-015-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H., Chu W.S., Hemphill C., Elbein S.C. Human resistin gene: molecular scanning and evaluation of association with insulin sensitivity and type 2 diabetes in Caucasians. J. Clin. Endocrinol. Metab. 2002;87(6):2520–2524. doi: 10.1210/jc.87.6.2520. [DOI] [PubMed] [Google Scholar]
- 6.Kim K.H., Lee K., Moon Y.S., Sul H.S. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J. Biol. Chem. 2001;276(14):11252–11256. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb I.N., Kabakoff R.C., Chan B., Baker T.W., Gurney A., Henzel W., Nelson C., Lowman H.B., Wright B.D., Skelton N.J., Frantz G.D., Tumas D.B., Peale F.V., Jr, Shelton D.L., Hebert C.C. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19(15):4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chumakov A.M., Kubota T., Walter S., Koeffler H.P. Identification of murine and human XCP1 genes as C/EBP-epsilon-dependent members of FIZZ/Resistin gene family. Oncogene. 2004;23(19):3414–3425. doi: 10.1038/sj.onc.1207126. [DOI] [PubMed] [Google Scholar]
- 9.Cao H., Hegele R.A. Single nucleotide polymorphisms of the resistin (RSTN) gene. J. Hum. Genet. 2001;46(9):553–555. doi: 10.1007/s100380170040. [DOI] [PubMed] [Google Scholar]
- 10.Fain J.N., Cheema P.S., Bahouth S.W., Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochem. Biophys. Res. Commun. 2003;300(3):674–678. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 11.Raghu P., Ghosh S., Soundarya K., Haseeb A., Aruna B., Ehtesham N.Z. Dimerization of human recombinant resistin involves covalent and noncovalent interactions. Biochem. Biophys. Res. Commun. 2004;313(3):642–646. doi: 10.1016/j.bbrc.2003.11.156. [DOI] [PubMed] [Google Scholar]
- 12.Jamaluddin M.S., Weakley S.M., Yao Q., Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012;165(3):622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S., Singh A.K., Aruna B., Mukhopadhyay S., Ehtesham N.Z. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305(1):27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- 14.Patel S.D., Rajala M.W., Rossetti L., Scherer P.E., Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304(5674):1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 15.Savage D.B., Sewter C.P., Klenk E.S., Segal D.G., Vidal-Puig A., Considine R.V., O'Rahilly S. Resistin /Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001 Oct;50(10):2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 16.Al Hannan F., Culligan K.G. Human resistin and the RELM of Inflammation in diabesity. Diabetol. Metab. Syndr. 2015 Jun 18;7:54. doi: 10.1186/s13098-015-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrke M., Reilly M.P., Millington S.C., Iqbal N., Rader D.J., Lazar M.A. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004 Nov;1(2):e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett M.S., Lee C.W., Kinnaird T.D., Stabile E., Durrani S., Dullum M.K., Devaney J.M., Fishman C., Stamou S., Canos D., Zbinden S., Clavijo L.C., Jang G.J., Andrews J.A., Zhu J., Epstein S.E. The potential role of resistin in atherogenesis. Atherosclerosis. 2005 Oct;182(2):241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Chen C., Jiang J., Lü J.M., Chai H., Wang X., Lin P.H., Yao Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2010 Jul;299(1):H193–H201. doi: 10.1152/ajpheart.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y.H., Zhang L., Gan Y., Wang X., Wang J., LeMaire S.A., Coselli J.S., Wang X.L. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J. Biol. Chem. 2006 Mar 24;281(12):7727–7736. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- 21.Mu H., Ohashi R., Yan S., Chai H., Yang H., Lin P., Yao Q., Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc. Res. 2006 Apr 1;70(1):146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Codoñer-Franch P., Alonso-Iglesias E. Resistin: insulin resistance to malignancy. Clin. Chim. Acta. 2015;438:46–54. doi: 10.1016/j.cca.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Filkova M., Haluzik M., Gay S., Senolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin. Immunol. 2009;133(2):157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Silswal N., Singh A.K., Aruna B., Mukhopadhyay S., Ghosh S., Ehtesham N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem. Biophys Res. Commun. 2005;334(4):1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 25.Acquarone E., Monacelli F., Borghi R., Nencioni A., Odetti P. Resistin: a reappraisal. Mech. Ageing Dev. 2019;178:46–63. doi: 10.1016/j.mad.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz D.R., Lazar M.A. Human resistin: found in translation from mouse to man. Trends Endocrinol. Metab. 2011;22(7):259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muse E.D., Obici S., Bhanot S., Monia B.P., McKay R.A., Rajala M.W., Scherer P.E., Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J. Clin. Invest. 2004 Jul;114(2):232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee R.R., Rangwala S.M., Shapiro J.S., Rich A.S., Rhoades B., Qi Y., Wang J., Rajala M.W., Pocai A., Scherer P.E., Steppan C.M., Ahima R.S., Obici S., Rossetti L., Lazar M.A. Regulation of fasted blood glucose by resistin. Science. 2004 Feb 20;303(5661):1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 29.Satoh H., Nguyen M.T., Miles P.D., Imamura T., Usui I., Olefsky J.M. Adenovirus-mediated chronic "hyper-resistinemia" leads to in vivo insulin resistance in normal rats. J. Clin. Invest. 2004 Jul;114(2):224–231. doi: 10.1172/JCI20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel S.D., Rajala M.W., Rossetti L., Scherer P.E., Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304(5674):1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee R.R., Lazar M.A. Dimerization of resistin and resistin-like molecules is determined by a single cysteine. J. Biol. Chem. 2001;276(28):25970–25973. doi: 10.1074/jbc.M103109200. [DOI] [PubMed] [Google Scholar]
- 32.Raes G., Brys L., Dahal B.K., Brandt J., Grooten J., Brombacher F., Vanham G., Noël W., Bogaert P., Boonefaes T., Kindt A., Van den Bergh R., Leenen P.J., De Baetselier P., Ghassabeh G.H. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J. Leukoc. Biol. 2005;77(3):321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 33.Madala S.K., Edukulla R., Davis K.R. Resistin-like molecule alpha1 (Fizz1) recruits lung dendritic cells without causing pulmonary fibrosis. Respir. Res. 2012;13:51. doi: 10.1186/1465-9921-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesce J.T., Ramalingam T.R., Wilson M.S., Mentink-Kane M.M., Thompson R.W., Cheever A.W., Urban J.F., Jr, Wynn T.A. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009 Apr;5(4) doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loke P., Nair M.G., Parkinson J., Guiliano D., Blaxter M., Allen J.E. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002 Jul 4;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair M.G., Gallagher I.J., Taylor M.D., Loke P., Coulson P.S., Wilson R.A., Maizels R.M., Allen J.E. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 2005 Jan;73(1):385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raes G., De Baetselier P., Noël W., Beschin A., Brombacher F., Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 2002 Apr;71(4):597–602. [PubMed] [Google Scholar]
- 38.Jenkins S.J., Ruckerl D., Cook P.C., Jones L.H., Finkelman F.D., van Rooijen N., MacDonald A.S., Allen J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011 Jun 10;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gieseck R.L., 3rd, Wilson M.S., Wynn T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018 Jan;18(1):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 40.Lazar M.A. Resistin- and Obesity-associated metabolic diseases. Horm. Metab. Res. 2007;39(10):710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 41.Liu T., Baek H.A., Yu H., Lee H.J., Park B.H., Ullenbruch M., Liu J., Nakashima T., Choi Y.Y., Wu G.D., Chung M.J., Phan S.H. FIZZ2/RELM-β induction and role in pulmonary fibrosis. J. Immunol. 2011 Jul 1;187(1):450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang C., Meng Q., Wu H., Eid G., Zhang G., Zhang X., Yang S., Huang K., Lee T.H., Corrigan C.J., Ying S. Resistin-like molecule-β is a human airway remodelling mediator. Eur. Respir. J. 2012 Feb;39(2):458–466. doi: 10.1183/09031936.00107811. [DOI] [PubMed] [Google Scholar]
- 43.Pine G.M., Batugedara H.M., Nair M.G. Here, there and everywhere: resistin-like molecules in infection, inflammation, and metabolic disorders. Cytokine. 2018 Oct;110:442–451. doi: 10.1016/j.cyto.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Propheter D.C., Chara A.L., Harris T.A., Ruhn K.A., Hooper L.V. Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc. Natl. Acad. Sci. U S A. 2017 Oct 17;114(42):11027–11033. doi: 10.1073/pnas.1711395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerstmayer B., Küsters D., Gebel S., Müller T., Van Miert E., Hofmann K., Bosio A. Identification of RELMgamma, a novel resistin-like molecule with a distinct expression pattern. Genomics. 2003;81(6):588–595. doi: 10.1016/s0888-7543(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 46.Schinke T., Haberland M., Jamshidi A., Nollau P., Rueger J.M., Amling M. Cloning and functional characterization of resistin-like molecule gamma. Biochem. Biophys. Res. Commun. 2004 Feb 6;314(2):356–362. doi: 10.1016/j.bbrc.2003.12.100. [DOI] [PubMed] [Google Scholar]
- 47.Chumakov A.M., Kubota T., Walter S., Koeffler H.P. Identification of murine and human XCP1 genes as C/EBP-epsilon-dependent members of FIZZ/Resistin gene family. Oncogene. 2004 Apr 22;23(19):3414–3425. doi: 10.1038/sj.onc.1207126. [DOI] [PubMed] [Google Scholar]
- 48.Mishra A., Wang M., Schlotman J., Nikolaidis N.M., DeBrosse C.W., Karow M.L., Rothenberg M.E. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293(2):L305–L313. doi: 10.1152/ajplung.00147. [DOI] [PubMed] [Google Scholar]
- 49.Nogueiras R., Gallego R., Gualillo O., Caminos J.E., García-Caballero T., Casanueva F.F., Diéguez C. Resistin is expressed in different rat tissues and is regulated in a tissue- and gender-specific manner. FEBS Lett. 2003;548(1–3):21–27. doi: 10.1016/s0014-5793(03)00708-7. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez-Solana B., Laborda J., Baladrón V. Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol. Endocrinol. 2012;26(1):110–127. doi: 10.1210/me.2011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oishi I., Takeuchi S., Hashimoto R., Nagabukuro A., Ueda T., Liu Z.J., Hatta T., Akira S., Matsuda Y., Yamamura H., Otani H., Minami Y. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells. 1999;4(1):41–56. doi: 10.1046/j.1365-2443.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 52.Hasan R., Zhou G.L. The cytoskeletal protein cyclase-associated protein 1 (CAP1) in breast cancer: context-dependent roles in both the invasiveness and proliferation of cancer cells and underlying cell signals. Int. J. Mol. Sci. 2019;20(11):2653. doi: 10.3390/ijms20112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S., Lee H.C., Kwon Y.W., Lee S.E., Cho Y., Kim J., Lee S., Kim J.Y., Lee J., Yang H.M., Mook-Jung I., Nam K.Y., Chung J., Lazar M.A., Kim H.S. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014;19(3):484–497. doi: 10.1016/j.cmet.2014.01.01331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daquinag A.C., Zhang Y., Amaya-Manzanares F., Simmons P.J., Kolonin M.G. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9(1):74–86. doi: 10.1016/j.stem.2011.05.01732. [DOI] [PubMed] [Google Scholar]
- 55.Werling D., Jungi T.W. Toll-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 2003;91:1–12. doi: 10.1016/S0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 56.Vaure C., Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014;5:316. doi: 10.3389/fimmu.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarkowski A., Bjersing J., Shestakov A., Bokarewa M.I. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell. Mol. Med. 2010;14(6B):1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y., Lu L., Hu Y., Li Q., An C., Yu X., Shu L., Chen A., Niu C., Zhou L., Yang Z. Resistin induces hypertension and insulin resistance in mice via a TLR4-dependent pathway. Sci. Rep. 2016;6:22193. doi: 10.1038/srep22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong W.J., Liu J.Y., Yin J.Y., Cui J.J., Xiao D., Zhuo W., Luo C., Liu R.J., Li X., Zhang W., Zhou H.H., Liu Z.Q. Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-κB pathway. Cancer Sci. 2018;109(8):2391–2400. doi: 10.1111/cas.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McTernan P.G., Fisher F.M., Valsamakis G., Chetty R., Harte A., McTernan C.L., Clark P.M., Smith S.A., Barnett A.H., Kumar S. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J. Clin. Endocrinol. Metab. 2003;88(12):6098–6106. doi: 10.1210/jc.2003-030898. [DOI] [PubMed] [Google Scholar]
- 61.Yong Qi, Zhenying Nie, Yun-Sik Lee, Neel S. Singhal, Philipp E. Scherer, Mitchell A. Lazar, Rexford S. Ahima. Loss of resistin improves glucose homeostasis in leptin deficiency diabetes. 55 (11) (2006) 3083–3090, doi: 10.2337/db05-0615. [DOI] [PubMed]
- 62.Rodríguez-Pacheco F., Vázquez-Martínez R., Martínez-Fuentes A.J., Pulido M.R., Gahete M.D., Vaudry H., Gracia-Navarro F., Diéguez C., Castaño J.P., Malagón M.M. Resistin regulates pituitary somatotrope cell function through the activation of multiple signaling pathways. Endocrinology. 2009;150(10):4643–4652. doi: 10.1210/en.2009-0116. [DOI] [PubMed] [Google Scholar]
- 63.Broglio C., Gómez A., Durán E., Ocaña F.M., Jiménez-Moya F., Rodríguez F. Salas C Hallmarks of a common forebrain vertebrate plan: specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res. Bull. 2005;66(4–6):277–281. doi: 10.1016/j.brainresbull.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Pang S.S., Le Y.Y. Role of resistin in inflammation and inflammation-related diseases. Cell Mol. Immunol. 2006;3(1):29–34. [PubMed] [Google Scholar]
- 65.Suragani M., Aadinarayana V.D., Pinjari A.B., Tanneeru K., Guruprasad L., Banerjee S., Pandey S., Chaudhuri T.K., Ehtesham N.Z. Human resistin, a proinflammatory cytokine, shows chaperone-like activity. Proc. Natl. Acad. Sci. U. S. A. 2013;110(51):20467–20472. doi: 10.1073/pnas.1306145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M., Oh J.K., Sakata S., Liang I., Park W., Hajjar R.J., Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell Cardiol. 2008;45(2):270–280. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curat C.A., Wegner V., Sengenès C., Miranville A., Tonus C., Busse R., Bouloumié A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 68.Calabro P., Samudio I., Willerson J.T., Yeh E.T. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110(21):3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 69.Chen S.S., Tang C.H., Chie M.J., Tsai C.H., Fong Y.C., Lu Y.C., Chen W.C., Lai C.T., Wei C.Y., Tai H.C., Chou W.Y., Wang S.W. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death. Dis. 2019;10(1):31. doi: 10.1038/s41419-018-1241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Langheim S., Dreas L., Veschini L., Maisano F., Foglieni C., Ferrarello S., Sinagra G., Zingone B., Alfieri O., Ferrero E., Maseri A., Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am. J. Physiol. Heart Circ. Physiol. 2010;298(3):H746–H753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 71.Axelsson J., Bergsten A., Qureshi A.R., Heimbürger O., Bárány P., Lönnqvist F., Lindholm B., Nordfors L., Alvestrand A., Stenvinkel P. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69(3):596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 72.Thommesen L., Stunes A.K., Monjo M., Grøsvik K., Tamburstuen M.V., Kjøbli E., Lyngstadaas S.P., Reseland J.E., Syversen U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 2006;99(3):824–834. doi: 10.1002/jcb.20915. [DOI] [PubMed] [Google Scholar]
- 73.Ramteke P., Deb A., Shepal V., Bhat M.K. Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers (Basel) 2019;11(9):1402. doi: 10.3390/cancers11091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang C.H., Wang P.J., Hsieh Y.C., Lo S., Lee Y.C., Chen Y.C., Tsai C.H., Chiu W.C., Hu S.C.S., Lu C.W., Yang Y.F., Chiu C.C., Ou-Yang F., Wang Y.M., Hou M.F., Yuan S.S.F. Resistin facilitates breast cancer progression via TLR4- mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2018;37(5):589–600. doi: 10.1038/onc.2017.35738. [DOI] [PubMed] [Google Scholar]
- 75.Yang G., Fan W., Luo B., Xu Z., Wang P., Tang S., Xu P., Yu M. Circulating resistin levels and risk of colorectal cancer: a meta-analysis. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/7367485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demiray G., Değirmencioğlu S., Uğurlu E., Yaren A. Effects of serum leptin and resistin levels on cancer cachexia in patients with advanced-stage non–small cell lung cancer. Clin. Med. Insights Oncol. 2017;11 doi: 10.1177/1179554917690144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kallio J., Hämäläinen M., Luukkaala T., Moilanen E., Tammela T.L., Kellokumpu-Lehtinen P.L. Resistin and interleukin 6 as predictive factors for recurrence and long-term prognosis in renal cell cancer. Urol. Oncol. 2017;35(9) doi: 10.1016/j.urolonc.2017.05.005. 544e25-544.e31. [DOI] [PubMed] [Google Scholar]
- 78.Gong W.J., Zheng W., Xiao L., Tan L.M., Song J., Li X.P., Xiao D., Cui J.J., Li X., Zhou H.H., Yin J.Y., Liu Z.Q. Circulating resistin levels and obesity-related cancer risk: a meta-analysis. Oncotarget. 2016;7(36):57694–57704. doi: 10.18632/oncotarget.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J.O., Kim N., Lee H.J., Lee Y.W., Kim S.J., Park S.H., Kim H.S. Resistin, a fat derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci. Rep. 2016;6:18923. doi: 10.1038/srep18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiu L., Zhang G.F., Yu L., Wang H.Y., Jia X.J., Wang T.J. Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition. Sci. Rep. 2018;8(1):12522. doi: 10.1038/s41598-018-30978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devanoorkar A., Kathariya R., Guttiganur N., Gopalakrishnan D., Bagchi P. Resistin: a potential biomarker for periodontitis influenced diabetes mellitus and diabetes induced periodontitis. Dis. Markers. 2014;2014 doi: 10.1155/2014/930206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dalamaga M. Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives. Biomark. Med. 2014;8(1):107–118. doi: 10.2217/bmm.13.99. [DOI] [PubMed] [Google Scholar]
- 83.Dalamaga M., Sotiropoulos G., Karmaniolas K., Pelekanos N., Papadavid E., Lekka A. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin. Biochem. 2013;46(7–8):584–590. doi: 10.1016/j.clinbiochem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Hou W.K., Xu Y.X., Yu T., Zhang L., Zhang W.W., Fu C.L., Sun Y., Wu Q., Chen L. Adipocytokines and breast cancer risk. Chin. Med. J. (Engl.) 2007;120(18):1592–1596. [PubMed] [Google Scholar]
- 85.Assiri A.M., Kamel H.F., Hassanien M.F. Resistin, visfatin, adiponectin, and leptin: risk of breast cancer in pre- and postmenopausal saudi females and their possible diagnostic and predictive implications as novel biomarkers. Dis. Markers. 2015;2015 doi: 10.1155/2015/253519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hlavna M., Kohut L., Lipkova J., Bienertova-Vasku J., Dostalova Z., Chovanec J., Vasku A. Relationship of resistin levels with endometrial cancer risk. Neoplasma. 2011;58(2):124–128. doi: 10.4149/neo_2011_02_124. [DOI] [PubMed] [Google Scholar]
- 87.Kim H.J., Lee Y.S., Won E.H., Chang I.H., Kim T.H., Park E.S., Kim M.K., Kim W., Myung S.C. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108(2 Pt 2):E77–E83. doi: 10.1111/j.1464-410X.2010.09813.x. [DOI] [PubMed] [Google Scholar]
- 88.Malvi P., Chaube B., Singh S.V., Mohammad N., Vijayakumar M.V., Singh S., Chouhan S., Bhat M.K. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018;6:2. doi: 10.1186/s40170-018-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deshmukh S.K., Srivastava S.K., Bhardwaj A., Singh A.P., Tyagi N., Marimuthu S., Dyess D.L., Dal Zotto V., Carter J.E., Singh S. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6(13):11231–11241. doi: 10.18632/oncotarget.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammadi M., Zarghami N., Hedayati M., Ghaemmaghami S. Synergistic effects of resistin and visfatin as adipocyte derived hormones on telomerase gene expression in AGS gastric cancer cell line. Acta Med. Iran. 2017;55(10):621–627. [PubMed] [Google Scholar]
- 91.Mohammadi M., Hedayati M., Zarghami N., Ghaemmaghami S. Resistin effect on telomerase gene expression in gastric cancer cell line ags. Acta Endocrinol. (Buchar) 2016;12(2):145–149. doi: 10.4183/aeb.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang M., Yan L., Wang G.J., Jin R. Resistin effects on pancreatic cancer progression and chemoresistance are mediated through its receptors CAP1 and TLR4. J. Cell. Physiol. 2019;234(6):9457–9466. doi: 10.1002/jcp.27631. [DOI] [PubMed] [Google Scholar]
- 93.Singh S., Chouhan S., Mohammad N., Bhat M.K. Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS 3. FEBS Let. 2017;591(10):1371–1382. doi: 10.1002/1873-3468. [DOI] [PubMed] [Google Scholar]
- 94.Pang L., Zhang Y., Yu Y., Zhang S. Resistin promotes the expression of vascular endothelial growth factor in ovary carcinoma cells. Int. J. Mol. Sci. 2013;14(5):9751–9766. doi: 10.3390/ijms14059751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsieh Y.Y., Shen C.H., Huang W.S., Chin C.C., Kuo Y.H., Hsieh M.C., Yu H.R., Chang T.S., Lin T.H., Chiu Y.W., Chen C.N., Kuo H.C., Tung S.Y. Resistin-induced stromal cell-derived factor-1 expression through Toll-like receptor 4 and activation of p38 MAPK/NFκB signaling pathway in gastric cancer cells. J. Biomed. Sci. 2014;21(1):59. doi: 10.1186/1423-0127-21-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai H.C., Cheng S.P., Han C.K., Huang Y.L., Wang S.W., Lee J.J., Lai C.T., Fong Y.C., Tang C.H. Resistin enhances angiogenesis in osteosarcoma via the MAPK signaling pathway. Aging (Albany NY) 2019;11(21):9767–9777. doi: 10.18632/aging.102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ilhan T.T., Kebapcilar A., Yilmaz S.A., Ilhan T., Kerimoglu O.S., Pekin A.T., Akyurek F., Unlu A., Celik C. Relations of serum visfatin and resistin levels with endometrial cancer and factors associated with its prognosis. Asian Pac. J. Cancer Prev. 2015;16(11):4503–4508. doi: 10.7314/apjcp.2015.16.11.4503. [DOI] [PubMed] [Google Scholar]
- 98.Diakowska D., Markocka-Mączka K., Szelachowski P., Grabowski K. Serum levels of resistin, adiponectin, and apelin in gastroesophageal cancer patients. Dis. Markers. 2014;2014 doi: 10.1155/2014/619649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsai C.H., Tsai H.C., Huang H.N., Hung C.H., Hsu C.J., Fong Y.C., Hsu H.C., Huang Y.L., Tang C.H. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget. 2018;9(85):35598. doi: 10.18632/oncotarget.26303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuo C.H., Chen K.F., Chou S.H., Huang Y.F., Wu C.Y., Cheng D.E., Chen Y.W., Yang C.J., Hung J.Y., Huang M.S. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf–Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis. 2013;34(11):2600–2609. doi: 10.1093/carcin/bgt281. [DOI] [PubMed] [Google Scholar]
- 101.Du F., Yu L., Wu Y., Wang S., Yao J., Zheng X., Xie S., Zhang S., Lu X., Liu Y., Chen W. miR-137 alleviates doxorubicin resistance in breast cancer through inhibition of epithelial-mesenchymal transition by targeting DUSP4. Cell Death. Dis. 2019;10(12):922. doi: 10.1038/s41419-019-2164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deshmukh S.K., Srivastava S.K., Zubair H., Bhardwaj A., Tyagi N., Al-Ghadhban A., Singh A.P., Dyess D.L., Carter J.E., Singh S. Resistin potentiates chemoresistance and stemness of breast cancer cells: implications for racially disparate therapeutic outcomes. Cancer Lett. 2017;396:21–29. doi: 10.1016/j.canlet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Z., Shi A., Song D., Han B., Zhang Z., Ma L., Liu D., Fan Z. Resistin confers resistance to doxorubicin-induced apoptosis in human breast cancer cells through autophagy induction. Am. J. Cancer Res. 2017;7(3):574–583. [PMC free article] [PubMed] [Google Scholar]
- 104.Qiu L., Zhang G.F., Yu L., Wang H.Y., Jia X.J., Wang T.J. Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition. Sci. Rep. 2018;8(1):12522. doi: 10.1038/s41598-018-30978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pang J., Shi Q., Liu Z., He J., Liu H., Lin P., Cui J., Yang J. Resistin induces multidrug resistance in myeloma by inhibiting cell death and upregulating ABC transporter expression. Haematologica. 2017;102(7):1273–1280. doi: 10.3324/haematol.2016.154062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Won J.C., Park C.Y., Lee W.Y., Lee E.S., Oh S.W., Park S.W. Association of plasma levels of resistin with subcutaneous fat mass and markers of inflammation but not with metabolic determinants or insulin resistance. J. Korean Med. Sci. 2009;24(4):695–700. doi: 10.3346/jkms.2009.24.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shetty G.K., Economides P.A., Horton E.S., Mantzoros C.S., Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27(10):2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 108.Qi Q., Wang J., Li H., Yu Z., Ye X., Hu F.B., Franco O.H., Pan A., Liu Y., Lin X. Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur. J. Endocrinol. 2008;159(5):585–593. doi: 10.1530/EJE-08-0427. [DOI] [PubMed] [Google Scholar]
- 109.Qiu W., Chen N., Zhang Q., Zhuo L., Wang X., Wang D., Jin H. Resistin increases platelet P-selectin levels via p38 MAPK signal pathway. Diab. Vasc. Dis. Res. 2014;11(2):121–124. doi: 10.1177/1479164113513912. [DOI] [PubMed] [Google Scholar]
- 110.Bokarewa M., Nagaev I., Dahlberg L., Smith U., Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 2005;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 111.Krljanac B., Schubart C., Naumann R., Wirtz S., Culemann S., Krönke G., Voehringer D. RELM-expressing macrophages protect against fatal lung damage and reduce parasite burden during helminth infection. Sci. Immunol. 2019;4(35):eaau3814. doi: 10.1126/sciimmunol.aau3814. [DOI] [PubMed] [Google Scholar]
- 112.Park H.K., Ahima R.S. Resistin in Rodents and Humans. Metab. J. 2013;37(6):404–414. doi: 10.4093/dmj.2013.37.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sundén-Cullberg J., Nyström T., Lee M.L., Mullins G.E., Tokics L., Andersson J., Norrby-Teglund A., Treutiger C.J. Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit. Care Med. 2007;35(6):1536–1542. doi: 10.1097/01.CCM.0000266536.14736.03. [DOI] [PubMed] [Google Scholar]
- 114.Miller L., Singbartl K., Chroneos Z.C., Ruiz-Velasco V., Lang C.H., Bonavia A. Resistin directly inhibits bacterial killing in neutrophils. Intensive Care Med. Exp. 2019;7(1):30. doi: 10.1186/s40635-019-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sato H., Muraoka S., Kusunoki N., Masuoka S., Yamada S., Ogasawara H., Imai T., Akasaka Y., Tochigi N., Takahashi H., Tsuchiya K., Kawai S., Nanki T. Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res. Ther. 2017;19(1):263. doi: 10.1186/s13075-017-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Senolt L., Housa D., Vernerová Z., Jirásek T., Svobodová R., Veigl D., Anderlová K., Müller-Ladner U., Pavelka K., Haluzík M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann. Rheum. Dis. 2007;66(4):458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen C., Jiang J., Lü J.M., Chai H., Wang X., Lin P.H., Yao Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2010;299(1):H193–H201. doi: 10.1152/ajpheart.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reilly M.P., Lehrke M., Wolfe M.L., Rohatgi A., Lazar M.A., Rader D.J. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 119.Wang H., Chen D.Y., Cao J., He Z.Y., Zhu B.P., Long M. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin. Med. Sci. J. 2009 Sep;24(3):161–166. doi: 10.1016/s1001-9294(09)60082-1. [DOI] [PubMed] [Google Scholar]
- 120.Burnett M.S., Lee C.W., Kinnaird T.D., Stabile E., Durrani S., Dullum M.K., Devaney J.M., Fishman C., Stamou S., Canos D., Zbinden S., Clavijo L.C., Jang G.J., Andrews J.A., Zhu J., Epstein S.E. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182(2):241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 121.Langheim S., Dreas L., Veschini L., Maisano F., Foglieni C., Ferrarello S., Sinagra G., Zingone B., Alfieri O., Ferrero E., Maseri A., Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am. J. Physiol. Heart Circ. Physiol. 2010;298(3):H746–H753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 122.Pischon T., Bamberger C.M., Kratzsch J., Zyriax B.C., Algenstaedt P., Boeing H., Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes. Res. 2005;13(10):1764–1771. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 123.Ohmori R., Momiyama Y., Kato R., Taniguchi H., Ogura M., Ayaori M., Nakamura H., Ohsuzu F. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J. Am. Coll. Cardiol. 2005;46(2):379–380. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 124.Tsukahara T., Nakashima E., Watarai A., Hamada Y., Naruse K., Kamiya H., Nakamura N., Kato N., Hamajima N., Sekido Y., Niwa T., Tomita M., Oiso Y., Nakamura J. Polymorphism in resistin promoter region at -420 determines the serum resistin levels and may be a risk marker of stroke in Japanese type 2 diabetic patients. Diabetes Res. Clin. Pract. 2009;84(2):179–186. doi: 10.1016/j.diabres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 125.Rothwell S.E., Richards A.M., Pemberton C.J. Resistin worsens cardiac ischaemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2006;349(1):400–407. doi: 10.1016/j.bbrc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 126.Rajpathak S.N., Kaplan R.C., Wassertheil-Smoller S., Cushman M., Rohan T.E., McGinn A.P., Wang T., Strickler H.D., Scherer P.E., Mackey R., Curb D., Ho G.Y. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women's Health Initiative. Stroke. 2011 Jul;42(7):1813–1820. doi: 10.1161/STROKEAHA.110.607853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frankel D.S., Vasan R.S., D'Agostino R.B., Sr, Benjamin E.J., Levy D., Wang T.J., Meigs J.B. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J. Am. Coll. Cardiol. 2009 Mar 3;53(9):754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verma S., Li S.H., Wang C.H., Fedak P.W., Li R.K., Weisel R.D., Mickle D.A. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003 Aug 12;108(6):736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 129.Graveleau C., Zaha V.G., Mohajer A., Banerjee R.R., Dudley-Rucker N., Steppan C.M., Rajala M.W., Scherer P.E., Ahima R.S., Lazar M.A., Abel E.D. Mouse and human resistins impair glucose transport in primary mouse cardiomyocytes, and oligomerization is required for this biological action. J. Biol. Chem. 2005 Sep 9;280(36):31679–31685. doi: 10.1074/jbc.M504008200. [DOI] [PubMed] [Google Scholar]
- 130.Kim M., Oh J.K., Sakata S., Liang I., Park W., Hajjar R.J., Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell Cardiol. 2008 Aug;45(2):270–280. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013 doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ellulu M.S., Patimah I., Khaza'ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zayani N., Hamdouni H., Boumaiza I., Achour O., Neffati F., Omezzine A., Najjar M.F., Bouslama A. Resistin polymorphims, plasma resistin levels and obesity in Tunisian volunteers. J. Clin. Lab. Anal. 2018;32(2):e22227. doi: 10.1002/jcla.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morash B.A., Ur E., Wiesner G., Roy J., Wilkinson M. Pituitary resistin gene expression: effects of age, gender and obesity. Neuroendocrinology. 2004;79(3):149–156. doi: 10.1159/000077273. [DOI] [PubMed] [Google Scholar]
- 135.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bai Y., Sun Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015;16(2):127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Castoldi A., Naffah de Souza C., Câmara N.O., Moraes-Vieira P.M. The macrophage switch in obesity development. Front. Immunol. 2016;6:637. doi: 10.3389/fimmu.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wen F., Xia Q., Zhang H., Shia H., Rajesh A., Wu Y., Yang Y., Yang Z. Resistin activates p65 pathway and reduces glycogen content through keratin 8. Int. J. Endocrinol. 2020;2020 doi: 10.1155/2020/9767926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kusminski C.M., da Silva N.F., Creely S.J., Fisher F.M., Harte A.L., Baker A.R., Kumar S., McTernan P.G. The in vitro effects of resistin on the innate immune signaling pathway in isolated human subcutaneous adipocytes. J. Clin. Endocrinol. Metab. 2007;92(1):270–276. doi: 10.1210/jc.2006-1151. [DOI] [PubMed] [Google Scholar]
- 140.Huang X., Yang Z. Resistin's, obesity and insulin resistance: the continuing disconnect between rodents and humans. J. Endocrinol. Invest. 2016;39(6):607–615. doi: 10.1007/s40618-015-0408-2. [DOI] [PubMed] [Google Scholar]
- 141.Sato H., Ishikawa M., Funaki A., Kimura Y., Yoshida H., Fukata H., Hasegawa H., Ueno K. Cyclolepis genistoides D. Don (palo azul) promotes differentiation of adipocytes and regulates adipokine expression. Nutr. Res. 2013;33(11):922–931. doi: 10.1016/j.nutres.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 142.Wang Y.Q., Dong Y., Yao M.H. Chromium picolinate inhibits resistin secretion in insulin-resistant 3t3-l1 adipocytes via activation of amp-activated protein kinase. Clin. Exp. Pharmacol. Physiol. 2009;36(8):843–849. doi: 10.1111/j.1440-1681.2009.05164.x. [DOI] [PubMed] [Google Scholar]
- 143.Rajala M.W., Qi Y., Patel H.R., Takahashi N., Banerjee R., Pajvani U.B., Sinha M.K., Gingerich R.L., Scherer P.E., Ahima R.S. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53(7):1671–1679. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 144.Araújo L.S., da Silva M.V., da Silva C.A., Borges M.F., Palhares H., Rocha L.P., Corrêa R., Rodrigues V., Júnior, Dos Reis M.A., Machado J.R. Analysis of serum inflammatory mediators in type 2 diabetic patients and their influence on renal function. PLoS ONE. 2020;15(3) doi: 10.1371/journal.pone.0229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moon B., Kwan J.J., Duddy N., Sweeney G., Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. American journal of physiology. Am. J. Physiol. Endocrinol. Metab. 2003;285(1):E106–E115. doi: 10.1152/ajpendo.00457.2002. [DOI] [PubMed] [Google Scholar]
- 146.Rajala M.W., Obici S., Scherer P.E., Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J. Clin. Invest. 2003;111(2):225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Song R., Wang X., Mao Y., Li H., Li Z., Xu W., Wang R., Guo T., Jin L., Zhang X., Zhang Y., Zhou N., Hu R., Jia J., Lei Z., Irwin D.M., Niu G., Tan H. Resistin disrupts glycogen synthesis under high insulin and high glucose levels by down-regulating the hepatic levels of GSK3β. Gene. 2013;529(1):50–56. doi: 10.1016/j.gene.2013.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thammakun T., Laohasiriwong W., Kraiklang R., Saengprajak N. Association of +62 G>A polymorphism in the resistin gene with type 2 diabetes mellitus among thais: case-control study. J. Clin. Diagn. Res. 2017;11(2):BC15–BC20. doi: 10.7860/JCDR/2017/25072.9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kunjathoor V.V., Febbraio M., Podrez E.A., Moore K.J., Andersson L., Koehn S., Rhee J.S., Silverstein R., Hoff H.F., Freeman M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277(51):49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 150.Singhal N.S., Patel R.T., Qi Y., Lee Y.S., Ahima R.S. Loss of resistin ameliorates hyperlipidemia and hepatic steatosis in leptin-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2008;295(2):E331–E338. doi: 10.1152/ajpendo.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pagano C., Soardo G., Pilon C., Milocco C., Basan L., Milan G., Donnini D., Faggian D., Mussap M., Plebani M., Avellini C., Federspil G., Sechi L.A., Vettor R. Increased serum resistin in nonalcoholic fatty liver disease is related to liver disease severity and not to insulin resistance. J. Clin. Endocrinol. Metab. 2006;91(3):1081–1086. doi: 10.1210/jc.2005-1056. [DOI] [PubMed] [Google Scholar]
- 152.Melone M., Wilsie L., Palyha O., Strack A., Rashid S. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J. Am. Coll. Cardiol. 2012;59(19):1697–1705. doi: 10.1016/j.jacc.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 153.Cohen J., Pertsemlidis A., Kotowski I.K., Graham R., Garcia C.K., Hobbs H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005;37(2):161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 154.Sato N., Kobayashi K., Inoguchi T., Sonoda N., Imamura M., Sekiguchi N., Nakashima N., Nawata H. Adenovirus-mediated high expression of resistin causes dyslipidemia in mice. Endocrinology. 2005;146(1):273–279. doi: 10.1210/en.2004-0985. [DOI] [PubMed] [Google Scholar]
- 155.Palanivel R., Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 2005;579(22):5049–5054. doi: 10.1016/j.febslet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 156.Ohbayashi H. Pitavastatin improves serum resistin levels in patients with hypercholesterolemia. J. Atheroscler. Thromb. 2008;15(2):87–93. doi: 10.5551/jat.e536. [DOI] [PubMed] [Google Scholar]