Key Points
Question
How do the performance of leadless VVI pacemakers and transvenous VVI pacemakers compare in a contemporaneous Medicare population?
Findings
In this cohort study including 15 408 patients, despite significant differences in patient characteristics, patients in whom a leadless pacemaker was implanted were observed to have higher rates of pericardial effusion and/or perforation but lower rates of other device-related complications and requirements for device revision at 6 months.
Meaning
The results from this study further develop the evidence on leadless pacemakers in practice and can inform shared decision-making about device choice for patients and physicians.
This cohort study compares patient characteristics and complications among patients implanted with leadless VVI and transvenous VVI pacemakers.
Abstract
Importance
The safety and efficacy of leadless VVI pacemakers have been demonstrated in multiple clinical trials, but the comparative performance of the device in a large, real-world population has not been examined.
Objective
To compare patient characteristics and complications among patients implanted with leadless VVI and transvenous VVI pacemakers.
Design, Setting, Participants
The Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers (Micra CED) is a continuously enrolling observational cohort study evaluating complications, utilization, and outcomes of leadless VVI pacemakers in the US Medicare fee-for-service population. Patients implanted between March 9, 2017, and December 1, 2018, were identified and included. All Medicare patients implanted with leadless VVI and transvenous VVI pacemakers during the study period were enrolled. Patients with less than 12 months of continuous enrollment in Medicare prior to leadless VVI or transvenous VVI implant and with evidence of a prior cardiovascular implantable electronic device were excluded, leaving 5746 patients with leadless VVI pacemakers and 9662 patients with transvenous VVI pacemakers. Data were analyzed from May 2018 to April 2021.
Exposures
Medicare patients implanted with leadless VVI pacemakers or transvenous VVI pacemakers.
Main Outcomes and Measures
The main outcomes were acute (30-day) complications and 6-month complications.
Results
Of 15 408 patients, 6701 (43.5%) were female, and the mean (SD) age was 81.0 (8.7) years. Compared with patients with transvenous VVI pacemakers, patients with leadless VVI pacemakers were more likely to have end-stage kidney disease (690 [12.0%] vs 226 [2.3%]; P < .001) and a higher mean (SD) Charlson Comorbidity Index score (5.1 [3.4] vs 4.6 [3.0]; P < .001). The unadjusted acute complication rate was higher in patients with leadless VVI pacemakers relative to transvenous VVI pacemakers (484 of 5746 [8.4%] vs 707 of 9662 [7.3%]; P = .02). However, there was no significant difference in overall acute complication rates following adjustment for patient characteristics (7.7% vs 7.4%; risk difference, 0.3; 95% CI, −0.6 to 1.3; P = .49). Pericardial effusion and/or perforation within 30 days was significantly higher among patients with leadless VVI pacemakers compared with patients with transvenous VVI pacemakers in both unadjusted and adjusted models (unadjusted, 47 of 5746 [0.8%] vs 38 of 9662 [0.4%]; P < .001; adjusted, 0.8% vs 0.4%; risk difference, 0.4; 95% CI, 0.1 to 0.7; P = .004). Patients implanted with leadless VVI pacemakers had a lower rate of 6-month complications compared with patients implanted with transvenous VVI pacemakers (unadjusted hazard ratio, 0.84; 95% CI, 0.68-1.03; P = .10; adjusted hazard ratio, 0.77; 95% CI, 0.62-0.96; P = .02).
Conclusions and Relevance
In this study, despite significant differences in patient characteristics, patients in whom a leadless pacemaker was implanted were observed to have higher rates of pericardial effusion and/or perforation but lower rates of other device-related complications and requirements for device revision at 6 months. Understanding the benefits and risks associated with leadless VVI pacemakers compared with transvenous VVI pacemakers can help clinicians and patients make informed treatment decisions.
Introduction
Leadless pacemakers are an alternative pacing option for patients with bradyarrhythmias. Leadless technology was designed to reduce complications related to transvenous pacing leads and subcutaneous pockets—the most common sources of transvenous pacemaker-related complications.1,2 Following US Food and Drug Administration approval in 2016, the US Centers for Medicare & Medicaid Services (CMS) issued a national coverage determination for leadless pacing in January 2017. The national coverage determination requires coverage with evidence development (CED), which mandates all Medicare beneficiaries be enrolled in a CMS-approved study designed to assess the complications and outcomes of leadless pacing in practice. All CED clinical research study protocols must address prespecified research questions, adhere to standards of scientific integrity, and be revised and approved by CMS.3 CMS approved the Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers (Micra CED) study on March 9, 2017, initiating national coverage of leadless pacing for Medicare beneficiaries. This study relies on Medicare claims data to address the evidence gaps identified by CMS during its national coverage process, including a direct contemporaneous comparator cohort of patients implanted with transvenous VVI pacemakers. The study’s unique design and application of real-world data establishes a new paradigm for CMS to conduct CED by efficiently generating evidence of the impact of new technologies on Medicare beneficiaries.
The Micra CED study will enroll patients until CMS determines that sufficient evidence exists on leadless pacemakers to remove the CED requirement of the coverage decision. To that end, this report provides an initial assessment of outcomes in this ongoing study and a direct comparison between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers in the Medicare setting. The objective of this analysis is to compare the safety and complication rates of patients treated with leadless VVI pacemakers with a contemporary cohort of patients treated with transvenous VVI pacemakers.
Methods
Study Design and Population
The design of the Micra CED study4has been described previously.5 Briefly, the observational study is a continuously enrolling cohort study designed to evaluate complications, utilization, and outcomes of the leadless pacing system in the Medicare population. The study uses administrative claims data to enroll patients, ascertain patient characteristics, identify comorbidities, and measure outcomes. Additionally, patients receiving a transvenous VVI pacemaker, regardless of manufacturer, during the study period were included as a contemporaneous control group. The study was approved by the Western Institutional Review Board with a waiver of informed consent and is registered on ClinicalTrials.gov.4 The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Database
Medicare inpatient, outpatient, carrier claims, and enrollment data linked to manufacturer device registration data were used to identify Medicare fee-for-service beneficiaries implanted with a leadless pacemaker (model MC1VR01; Medtronic, Inc) from March 9, 2017, to December 1, 2018. The study period includes the first date of Medicare coverage for leadless VVI pacemakers and the most recent available administrative claims data. The methodology of linking the claims and manufacturer device registration data has been previously described.5 Patients with transvenous VVI pacemakers were identified with International Classification of Diseases, 10th Revision, Procedure Coding System codes for implants occurring in the inpatient hospital setting and Current Procedural Terminology codes for implants occurring in the outpatient hospital setting, as defined in eTable 1 in the Supplement. Patients with transvenous VVI pacemakers were included if the hospital at which they were treated was implanting leadless VVI pacemakers during the study period. These inclusion criteria minimize selection bias based on the assumption that any patient going to the hospital for a VVI pacemaker implant had the chance to receive either system.
The index or baseline date for outcomes ascertainment was the date of each patient’s first observed pacemaker implant procedure during the study period. Patients with less than 12 months of continuous enrollment in Medicare fee-for-service prior to implant and patients with evidence of a prior cardiovascular implantable electronic device were excluded to facilitate comparisons in patients who were receiving de novo pacemaker implants.
Baseline Comorbidities and Encounter Characteristics
Baseline patient comorbidities were ascertained using diagnosis and procedure codes present on any encounter during a 12-month lookback period, as defined in eTable 1 in the Supplement. Comorbidities included end-stage kidney disease (ESKD), kidney dysfunction, coronary artery disease, peripheral vascular disease, tricuspid valve disease, atrial fibrillation, left bundle branch block, supraventricular tachycardia, ventricular arrythmia, 6-month steroid use, diabetes, heart failure, chronic obstructive pulmonary disease, hyperlipidemia, and hypertension. Prior cardiovascular events and procedures (acute myocardial infarction, coronary artery bypass graft, transcatheter aortic valve, and percutaneous coronary intervention), concomitant transcatheter aortic valve replacement, and atrial ablation were also included as a measure of severity of comorbid illness. In addition, the Charlson Comorbidity Index score was calculated for each patient.6 Age, sex, and US region were identified in the CMS enrollment file. Hospital encounter characteristics were also identified, including inpatient or outpatient hospital setting, admission through an emergency department, admission during the weekend, and the number of days from hospital admission to implant procedure.
Study Objectives
The primary objectives of the Micra CED study were to estimate the acute (30-day) complication rate and the 2-year survival rate associated with the leadless pacing system. Secondary study objectives included a comparative analysis of acute and 6-month complication rates and device revisions between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers.
Outcomes
The present analysis focuses on a comparative analysis of acute (30-day) complications, a subset of these complications measured through 6 months, device revisions, and mortality between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers. Acute complications were prospectively defined as embolism and thrombosis, events at the puncture site, cardiac effusion and/or perforation, device-related complications, and other complications, including device-related acute myocardial infarction, postprocedural hematoma or hemorrhage, intraoperative cardiac arrest, pericarditis, vascular complications, hemothorax, and pneumothorax (eTables 1 and 2 in the Supplement). Device-related complications are defined as complications related to the mechanical integrity of the device or codes explicitly stating device relatedness (eg, device dislodgement, device infection, device pocket complication). The 6-month complications analysis was restricted to patients with implants occurring between the study start date and June 30, 2018, to allow for a complete 6-month follow-up. Six-month complications were measured from implant through 6 months but were limited to the subset of complications most likely attributable to the device implant or the device itself that may continue to occur outside the acute period (eTable 2 in the Supplement). Device-related complications, for example, can occur at any time after the index procedure, and thus are included in both the acute and 6-month outcome measures.
Statistical Analysis
Baseline characteristics were compared with t tests for continuous variables and χ2 tests for categorical variables. Balance was also assessed using standardized mean differences where values exceeding 0.1 suggested imbalance between groups.
To account for important patient and encounter characteristics, we used propensity score overlap weights to construct a weighted cohort of patients who differed with respect to pacemaker type (leadless VVI vs transvenous VVI) but were similar with respect to other observed characteristics.7 Specifically, propensity score overlap weights estimate the probability of treatment with the opposing therapy based on observed characteristics. To calculate the propensity score, the probability for each patient to be implanted with a leadless VVI pacemaker was estimated using a logistic regression model that included baseline patient and encounter characteristics. The resulting propensity scores were used to derive an overlap weight for each patient to adjust for differences between treatment groups. Propensity score overlap weights place the most emphasis on patients who are considered the most comparable and the least emphasis on patients who are least likely to be treated with the opposing therapy. The propensity score overlap weight is bound by 0 and 1 and thus eliminates the extreme weights and arbitrary trimming of the study population that may be introduced with alternative balancing techniques and has been shown to induce balance for the means of any selected covariates.8
Univariate logistic regression was used to compare the unadjusted and overlap weight–weighted adjusted acute complication rate between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers and compute risk differences. Unadjusted and overlap weight–weighted adjusted 6-month complication rates were estimated using the cumulative incidence function. Fine-Gray competing risk models were used to compare the unadjusted and adjusted risk of 6-month complications and device-related reinterventions between study groups, given that the competing risk of death may preclude these events. Unadjusted and overlap weight–adjusted Cox proportional hazards models were used to compare all-cause mortality through 6 months. Standard error was correlated at the hospital level in unadjusted and adjusted models to account for within-hospital correlation. Events occurring between 1 and 10 patients were suppressed to protect beneficiary privacy as required by CMS.9 Significance was set at P < .05, and all P values were 2-tailed. All statistical analyses were conducted in SAS version 9.4 (SAS Institute).
Sensitivity Analyses
Several sensitivity analyses were performed. First, analyses of the 6-month complications, reintervention, and mortality were compared between study groups using all patients in each cohort, regardless of implant date, with patients censored at their last follow-up. Second, cause-specific hazard ratios (HRs) were computed using Cox proportional hazards models. Additionally, the impact of the weighting scheme on the results was assessed by using inverse probability of treatment weighting rather than overlap weights. Finally, we did a falsification analysis using 6-month hip fractures to evaluate whether associations between pacemaker type and outcomes might be due to unmeasured patient characteristics.10
Results
Cohort Formation and Baseline Characteristics
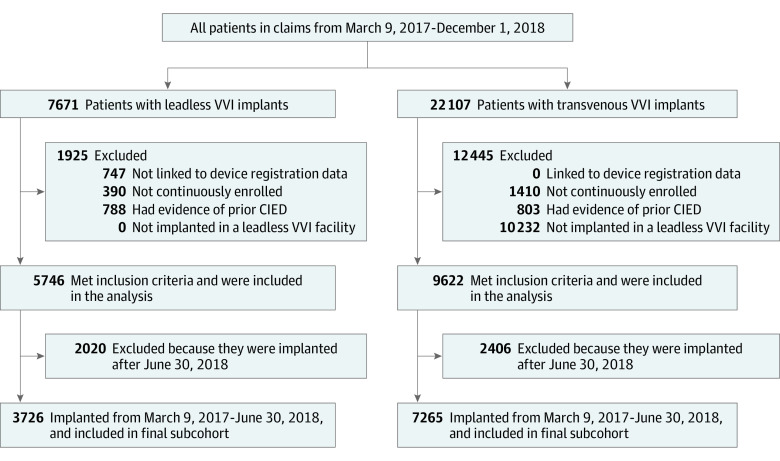
Of 15 408 patients, 6701 (43.5%) were female, and the mean (SD) age was 81.0 (8.7) years. Overall, there were 5746 leadless VVI pacemaker and 9662 transvenous VVI pacemaker de novo implant procedures identified during the study period. Among those patients, 3726 patients with leadless VVI pacemakers and 7256 patients with transvenous VVI pacemakers were implanted before June 30, 2018, and included in the subset with adequate follow-up time to observe 6-month complications (Figure 1). Patient baseline characteristics are detailed in Table 1. Compared with patients with transvenous VVI pacemakers, patients with leadless VVI pacemakers were more likely to have ESKD (690 [12.0%] vs 226 [2.3%]; P < .001) and kidney dysfunction (2792 [48.6%] vs 4057 [42.0%]; P < .001), as well as higher mean SD Charles Comorbidity Index score (5.1[ 3.4] vs 4.6 [3.0]; P < .001). Patients with leadless VVI pacemakers were less likely to be implanted in the inpatient setting, on weekends, or admitted through the emergency department (Table 1). Baseline characteristics were similar among the subset of patients included in the 6-month measures cohort (eTable 3 in the Supplement). After weighting, all measured baseline and encounter characteristics were well balanced with all standardized mean differences near 0 (eFigure in the Supplement).
Figure 1. Cohort Formation Flowchart.
Patient selection and exclusion criteria and the numbers of patients excluded/included at each step. CIED indicates cardiac implantable electronic device; TVPM, transvenous pacemaker.
Table 1. Baseline Characteristics of Patients Undergoing De Novo Implantation With a Leadless VVI Pacemaker vs a Transvenous VVI Pacemaker.
| Patient characteristics | No. (%) | SMD | P value | |
|---|---|---|---|---|
| Leadless VVI (n = 5746) | Transvenous VVI (n = 9662) | |||
| Demographic characteristics | ||||
| Age, mean (SD), y | 79.4 (9.5) | 82.0 (8.1) | 0.29 | <.001 |
| Female | 2509 (43.7) | 4192 (43.4) | 0.01 | .74 |
| Region | ||||
| Midwest | 1251 (21.8) | 2096 (21.7) | 0.002 | .91 |
| South | 2313 (40.3) | 3668 (38.0) | 0.05 | .005 |
| Northeast | 971 (16.9) | 2138 (22.1) | 0.13 | <.001 |
| Encounter characteristics | ||||
| Inpatient implant | 3064 (53.3) | 5470 (56.6) | 0.07 | <.001 |
| Time to implant, mean (SD), d | 2.5 (5.3) | 1.9 (3.6) | 0.12 | <.001 |
| Weekend implant | 147 (2.6) | 331 (3.4) | 0.05 | .003 |
| Admission through the ED | 219 (3.8) | 493 (5.1) | 0.06 | .<001 |
| Clinical characteristics | ||||
| ESKD | 690 (12.0) | 226 (2.3) | 0.38 | <.001 |
| Diabetes | 2597 (45.2) | 3994 (41.3) | 0.08 | <.001 |
| Atrial fibrillation | 4679 (81.4) | 8609 (89.1) | 0.22 | <.001 |
| Congestive heart failure | 3023 (52.6) | 5111 (52.9) | 0.01 | .73 |
| COPD | 1778 (30.9) | 2824 (29.2) | 0.04 | .02 |
| Chronic steroid use | 230 (4.0) | 311 (3.2) | 0.04 | .01 |
| Coronary artery disease | 3215 (56.0) | 5161 (53.4) | 0.05 | .002 |
| Supraventricular tachycardia | 436 (7.6) | 513 (5.3) | 0.09 | <.001 |
| Ventricular arrythmia | 895 (15.6) | 1333 (13.8) | 0.05 | .002 |
| Hyperlipidemia | 4410 (76.8) | 7163 (74.1) | 0.06 | <.001 |
| Left bundle branch block | 302 (5.3) | 520 (5.4) | 0.01 | .74 |
| Peripheral vascular disease | 1558 (27.1) | 2583 (26.7) | 0.01 | .61 |
| Prior CABG | 857 (14.9) | 1380 (14.3) | 0.02 | .28 |
| Prior acute myocardial infarction | 1141 (19.9) | 1589 (16.5) | 0.09 | <.001 |
| Prior percutaneous coronary intervention | 903 (15.7) | 1325 (13.7) | 0.06 | .<.001 |
| Kidney dysfunction | 2792 (48.6) | 4057 (42.0) | 0.13 | <.001 |
| Tricuspid valve disease | 1660 (28.9) | 2803 (29.0) | 0.003 | .87 |
| TAVR | 101 (1.8) | 147 (1.5) | 0.02 | .26 |
| Concomitant atrial ablationa | 793 (13.8) | 1069 (11.1) | 0.08 | <.001 |
| Concomitant TAVRa | 151 (2.6) | 449 (4.7) | 0.11 | <.001 |
| Charlson Comorbidity Index score, mean (SD) | 5.1 (3.4) | 4.6 (3.0) | 0.16 | <.001 |
Abbreviations: CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; ED, emergency department; ESKD, end-stage kidney disease; SMD, standardized mean difference; TAVR, transcatheter aortic valve replacement.
Concomitant procedures are defined as those occurring during the implant encounter. Atrial ablation includes Current Procedural Terminology codes 93650, 93653, 93656, 93657, and 02583ZZ with diagnosis of atrial fibrillation and may include atrial fibrillation as well as AV node ablation (eTable 1 in the Supplement).
Acute Complications
Table 2 shows the complete unadjusted and adjusted acute complication rates observed for patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers. The unadjusted overall acute complication rate was significantly greater in the patients with leadless VVI pacemakers compared with the patients with transvenous VVI pacemakers (484 of 5746 [8.4%] vs 707 of 9662 [7.3%]; P = .020); however, there was no significant difference in adjusted overall acute complications between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers (7.7% vs 7.4%; risk difference, 0.3; 95% CI, −0.6 to 1.3; P = .49). Cardiac effusion and/or perforation within 30 days was significantly higher among the patients with leadless VVI pacemakers compared with patients with transvenous VVI pacemakers in both the unadjusted and adjusted models (unadjusted, 47 of 5746 [0.8%] vs 38 of 9662 [0.4%]; P < .001; adjusted, 0.8% vs 0.4%; risk difference 0.4; 95% CI, 0.1 to 0.7; P = .004). Unadjusted and adjusted device-related complications, including dislodgement, infection, and pocket complications, were significantly lower in the patients with leadless VVI pacemakers compared with patients with transvenous VVI pacemakers (unadjusted, 81 [1.4%] vs 247 [2.6%]; P < .001; adjusted, 1.4% vs 2.5%; risk difference, −1.1; 95% CI, −1.5 to −0.6; P < .001). Pericarditis and vascular complications, while higher in the leadless group, were less than 1% in both groups.
Table 2. Unadjusted and Adjusted 30-Day Complication Rates in Patients With Leadless VVI Pacemakers vs Patients With Transvenous VVI Pacemakers .
| Measure | Unadjusted event, No. (%) | Adjusted rates | |||||
|---|---|---|---|---|---|---|---|
| Leadless VVI (n = 5746) | Transvenous VVI (n = 9662) | P value | % | Risk difference, % (95% CI) | P value | ||
| Leadless VVI (n = 5746) | Transvenous VVI (n = 9662) | ||||||
| Overall complications | 484 (8.4) | 707 (7.3) | .02 | 7.7 | 7.4 | 0.3 (−0.6 to 1.3) | .49 |
| Embolism and thrombosis | 202 (3.5) | 286 (3.0) | .07 | 3.2 | 3.1 | 0.1 (−0.5 to 0.7) | .81 |
| Deep vein thrombosis | 145 (2.5) | 176 (1.8) | .003 | 2.2 | 2.0 | 0.3 (−0.2 to 0.7) | .27 |
| Pulmonary embolism | 72 (1.3) | 128 (1.3) | .74 | 1.2 | 1.3 | −0.1 (−0.5 to 0.3) | .52 |
| Thrombosis due to cardiac device | ≤10a | ≤10a | .64 | ≤10a | ≤10a | 0 (0 to 0) | .52 |
| Embolism due to cardiac device | ≤10a | 0 | NA | ≤10a | NA | NA | NA |
| Events at puncture site | 78 (1.4) | 31 (0.3) | <.001 | 1.2 | 0.3 | 0.8 (0.5 to 1.2) | <.001 |
| Arteriovenous fistula | 40 (0.7) | ≤10a | <.001 | 0.5 | NA | NA | .003 |
| Vascular aneurysm | 49 (0.9) | 24 (0.3) | <.001 | 0.9 | 0.2 | 0.7 (0.4 to 0.9) | <.001 |
| Cardiac effusion and/or perforation | 47 (0.8) | 38 (0.4) | <.001 | 0.8 | 0.4 | 0.4 (0.1 to 0.7) | .004 |
| Device-related complicationb | 81 (1.4) | 247 (2.6) | <.001 | 1.4 | 2.5 | −1.1 (−1.5 to −0.6) | <.001 |
| Other complications | 136 (2.4) | 169 (1.8) | .01 | 2.1 | 1.7 | 0.4 (−0.1 to 0.9) | .10 |
| Device-related AMI | ≤10a | ≤10a | .71 | ≤10a | ≤10a | .0 (0 to 0.1) | .40 |
| Postprocedural hematoma | 30 (0.5) | 40 (0.4) | .33 | 0.5 | 0.4 | 0.1 (−0.1 to 0.3) | .45 |
| Postprocedural hemorrhage | 32 (0.6) | 11 (0.1) | <.001 | 0.5 | 0.1 | 0.4 (0.2 to 0.6) | <.001 |
| Intraoperative cardiac arrest | 20 (0.4) | 24 (0.3) | .30 | 0.3 | 0.3 | 0.1 (−0.2 to 0.3) | .51 |
| Pericarditis | 51 (0.9) | 23 (0.2) | <.001 | 0.8 | 0.3 | 0.6 (0.3 to 0.9) | <.001 |
| Vascular complication | 38 (0.7) | 16 (0.2) | <.001 | 0.6 | 0.2 | 0.4 (0.2 to 0.6) | <.001 |
| Hemothorax | 0 | 0 | NA | NA | NA | NA | NA |
| Pneumothorax | 0 | 77 (0.8) | NA | NA | 0.8 | NA | NA |
Abbreviations: AMI, acute myocardial infarction; NA, not applicable.
Cells with 10 or less patients were suppressed to protect beneficiary privacy as required by the US Centers of Medicare & Medicaid Services.
Includes complications related to the mechanical integrity of the device or codes explicitly stating device relatedness (eg, device dislodgement, device infection, device pocket complication). See eTable 1 in the Supplement for details.
6-Month Complications
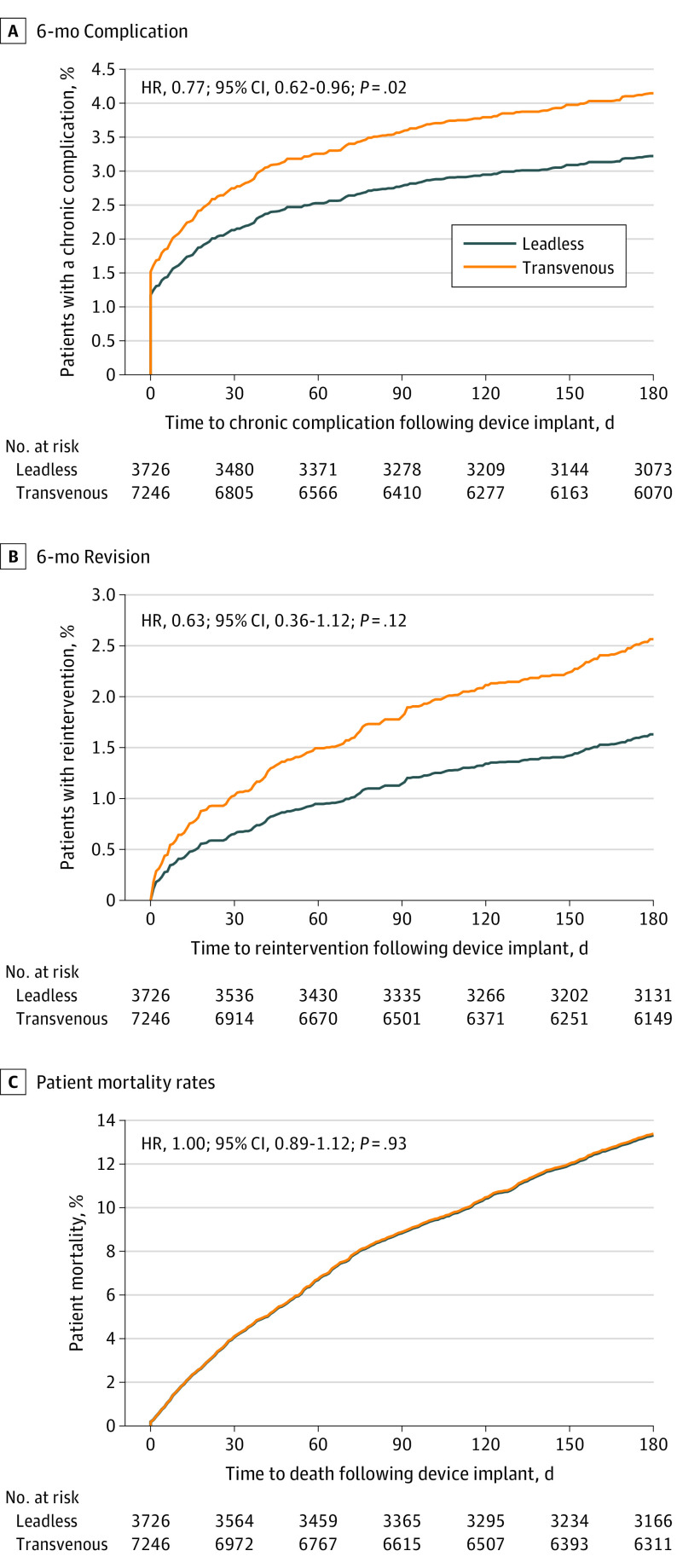
Table 3 details the adjusted rates for the subset of complications measured through 6-month follow-up. Patients implanted with a leadless VVI pacemaker had a reduction in 6-month complications compared with patients implanted with a transvenous VVI pacemaker, which was significant on adjustment (unadjusted HR, 0.84; 95% CI, 0.68-1.03; P = .10; adjusted HR, 0.77; 95% CI, 0.62-0.96; P = .02; Figure 2A). This reduction was also maintained when evaluating all patients who were implanted (adjusted HR, 0.77; 95% CI, 0.64-0.93). Unadjusted 6-month complications are shown in eTable 4 in the Supplement. Patients implanted with a leadless VVI pacemaker had a lower rate of device revision compared with patients who were implanted with a transvenous VVI pacemaker (unadjusted HR, 0.67; 95% CI, 0.38-1.20; adjusted HR, 0.63; 95% CI, 0.36-1.12; Figure 2B) and this lower risk was consistent when the entire cohort of patients who were implanted were included (adjusted HR, 0.59; 95% CI, 0.37-0.94).
Table 3. Adjusted Rates of Device-Related Complication at 6-Month Follow-up in Patients with Leadless VVI Pacemakers vs Patients with Transvenous VVI Pacemakers .
| Complication type | 6-mo Weighted CIF estimates, % (95% CI) | Relative risk reduction, % (95% CI) | |
|---|---|---|---|
| Leadless VVI (n = 3726) | Transvenous VVI (n = 7256) | ||
| Overall complications | 3.2 (2.9 to 3.6) | 4.1 (3.8 to 4.6) | 23 (4 to 38) |
| Embolism and thrombosis | ≤10a | ≤10a | 53 (−163 to 92) |
| Thrombosis due to cardiac device | ≤10a | ≤10a | 82 (−54 to 98) |
| Embolism due to cardiac device | ≤10a | ≤10a | −1 (−1430 to 93) |
| Device-related complicationb | 1.7 (1.5 to 1.9) | 3.3 (3.0 to 3.7) | 49 (33 to 61) |
| Other complications | 1.6 (1.3 to 1.8) | 0.8 (0.7 to 1.0) | −88 (−179 to 27) |
| Pericarditis | 1.2 (1.0 to 1.5) | 0.5 (0.4 to 0.6) | −161 (−316 to 63) |
| Hemothorax | 0.4 (0.3 to 0.5) | 0.4 (0.3 to 0.5) | 1 (−102 to 51) |
Abbreviation: CIF, cumulative incidence function.
Cells with 10 or less patients were suppressed to protect beneficiary privacy as required by the US Centers of Medicare & Medicaid Services.
Includes complications related to the mechanical integrity of the device or codes explicitly stating device relatedness (eg, device dislodgement, device infection, device pocket complication). See eTable 1 in the Supplement for details.
Figure 2. Time-to-Event Plots for Complications, Device Revision, and Mortality Out to 6-Month Follow-up in Patients Treated With Leadless VVI vs Transvenous VVI Pacing.
A, Hazard ratio (HR) and cumulative incidence function for 6-month complication based on the Fine-Gray competing risk model. B, HR and cumulative incidence function for 6-month device revision based on the Fine-Gray competing risk model. C, HR and patient mortality rates based on the Cox proportional hazards model. The comparisons of 6-month device revision and patient mortality were not significantly different between groups.
Survival
The 30-day all-cause mortality rate was not significantly different between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers (unadjusted, 245 of 5746 [4.3%] vs 366 of 9662 [3.8%]; P = .14; adjusted, 3.9% vs 4.1%; risk difference, −0.2%; 95% CI; −0.8 to 0.5 P = .61). The unadjusted 6-month all-cause mortality rate was significantly greater in the patients with leadless VVI pacemakers compared with the patients with transvenous VVI pacemakers (HR, 1.13; 95% CI, 1.01-1.27; P = .03); however, there was no difference in adjusted 6-month all-cause mortality rate between the patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers with 6 months of follow-up eligibility (HR, 1.00; 95% CI, 0.89-1.12; Figure 2C) or among the entire cohort of patients who were implanted (HR, 0.96; 95% CI, 0.87-1.06).
Sensitivity Analyses
Results from the sensitivity analyses show that the findings were robust to the specific analysis model (Fine-Gray vs Cox) or propensity score weighting scheme (overlap vs inverse probability of treatment weighting; refer to the eResults and eTable 5 in the Supplement). There was no significant difference in the adjusted 6-month rate of hip fracture between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers (eTable 6 in the Supplement).
Discussion
This nationwide study includes, to our knowledge, the largest population of patients who were implanted with leadless VVI pacemakers to date and is the first to compare the safety and complication rates with patients who were implanted with transvenous pacemakers in a contemporaneous Medicare population. Additionally, this study highlights the implications for the interpretation of nonrandomized data. Our findings include differences between adjusted and unadjusted results, underscoring the importance of careful interpretation of the data, and the relevance of addressing significantly different patient characteristics between the study cohorts via appropriate design and statistical techniques.
In this novel study, there are several findings relevant to leadless VVI pacing. First, despite significant differences in patient characteristics, the rate of the complications measured through 6 months was significantly lower in patients who were implanted with leadless VVI pacemakers compared with patients implanted with transvenous VVI pacemakers after adjustment. Second, patients with a leadless VVI pacemaker had a substantially lower rate of device revision in the 6 months after implantation. Finally, although the adjusted risk of cardiac effusion and/or perforation at 30 days was higher with leadless VVI pacing, device-related complications were significantly lower. While comparative safety analyses can be difficult to interpret when important safety events differ across devices, transparency around these event rates and better understanding of the risks and benefits can help clinicians and patients in making informed treatment decisions.
While pacemaker implantation is considered a routine and safe practice in contemporary clinical practice, observational studies have noted a striking rate of overall complications in patients who undergo transvenous pacemaker implantation. In a propensity-matched analysis from another US claims database, complication rates at 30 days after transvenous VVI pacemaker implantation were estimated at 9.4%.11 In comparison, we identified a lower rate of acute complications in the Medicare cohort (unadjusted rate, 8.4% for leadless VVI pacemakers and 7.3% for transvenous VVI pacemakers). However, when comparing the overall rate of acute complications within 30 days, we found no significant difference between leadless and transvenous pacemaker implantation (adjusted rate, 7.7% for leadless VVI pacemakers and 7.4% for transvenous VVI pacemakers). It is important to note that the adjusted rate of pericardial effusion and/or perforation was higher with leadless VVI pacemaker implantation compared with transvenous VVI pacemaker implantation (0.8% vs 0.4%); however the absolute rate was less than 1%, which is in line with prior reports12 and lower than that observed in the Micra investigational device exemption study.13 This finding is consistent with Vamos et al,14 who also identified a higher rate of pericardial effusion in patients treated with leadless pacemakers relative to transvenous pacemakers.
A common question in clinical practice is whether the findings in a pivotal clinical study will be generalizable to routine clinical practice. The Micra CED study provides valuable supplemental data beyond what is captured in clinical trials and postapproval registry studies. Notably, the cohort enrolled in this study was sicker than patients enrolled in both the investigational device exemption and postapproval registry trials.12,13 Despite the older patient age and higher comorbidity burden, outcomes of leadless pacing observed in the investigational device exemption and postapproval registry trials appear to be maintained in this study of general clinical practice. While the study design addresses CMS’s specific research questions about the impact of leadless pacemakers in the Medicare population, it also demonstrates a unique application of using administrative claims data to conduct CED and contributes to a larger body of research assessing the potential of administrative data for postmarket device evaluation.15,16
The pivotal investigational device exemption study identified a significant reduction (HR, 0.49; 95% CI, 0.33-0.75; P = .001) in overall major complications through 6 months in patients with leadless pacemakers relative to a transvenous pacemakers historical control group.13 Consistent with the findings in the pivotal study, in this US Medicare cohort, leadless VVI pacemaker implantation was associated with a 23% lower risk of complications measured through 6 months compared with transvenous pacing. This finding was mirrored by a 37% reduction in rates of device revision after adjustment for patient characteristics. Moreover, there was no difference in either 30-day or 6-month adjusted all-cause mortality. Finally, leadless pacing has demonstrated a lower risk of infection in previous studies; it is notable that in this study of 3726 patients with leadless VVI pacemakers with 6-month follow-up, there were no identified infections.13,17,18
Limitations
There are several limitations inherent to this nonrandomized observational study. First, it is possible that complications could be missed or inadequately documented in administrative claims. For example, certain device-related complications, such as elevated pacing thresholds, do not have specific diagnosis codes. For other complications, such as cardiac perforation, our prior analyses suggest that this probability is very low5; however, administrative claims data lack qualitative details related to complication complexity or severity. Second, like any nonrandomized study, the possibility of residual confounding cannot be completely eliminated. We used multivariable adjustment in our statistical analysis with propensity score overlap weighting. This technique has advantages relative to other propensity score weighting methods because it eliminates extreme values, creates covariate balance between treatment groups, emphasizes patients that conceivably could get either therapy, and maintains the entire patient population.7 Further, our falsification analysis suggests that residual confounding is unlikely, though it remains possible as in any observational study (eTable 6 in the Supplement). Additionally, this analysis was performed in a Medicare fee-for-service population, which primarily consists of patients 65 years and older with disabilities or ESKD. Medicare Advantage patients are not included in the Micra CED study analyses due to unavailability of Medicare Advantage claims data for research. Thus, the results may not be generalizable to populations outside the US Medicare fee-for-service population, particularly younger populations. Additionally, there is a 12-month to 18-month lag in the availability of fully adjudicated Medicare claims for research. Therefore, this analysis does not include leadless VVI pacemaker outcomes beyond 2018.
Conclusions
To our knowledge, this is the first Medicare CED study to report results based on administrative claims data. In this novel, postmarket comparative safety study, patients in whom a leadless pacemaker was implanted were observed to have higher rates of cardiac effusion or perforation within 30 days but lower device-related complication rates and requirements for device revision at 6 months. Additional studies are needed to ascertain the long-term impact of these differences in net clinical benefit in VVI pacemaker populations.
eResults Supplement to sensitivity analysis results.
eTable 1. Current definitions of procedural terminology for implants.
eTable 2. Differences between acute and 6-month complications.
eTable 3. Baseline characteristics of the subset of leadless VVI and transvenous VVI pacemaker patients included in the 6-month complication and device re-intervention measures.
eTable 4. Unadjusted 6-month complication rates.
eTable 5. Summary of sensitivity analyses.
eTable 6. Results from a falsification test of hip fracture through 6 months.
eFigure. Flow of information through the different phases of the review.
References
- 1.Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9(5):728-735. doi: 10.1016/j.hrthm.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 2.Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35(18):1186-1194. doi: 10.1093/eurheartj/eht511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health & Human Services, Centers for Medicare & Medicaid Services. CMS manual system . Accessed April 23, 2021. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017Downloads/R201NCD.pdf
- 4.Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers (Micra CED) . ClinicalTrials.gov identifier. NCT03039712. Updated February 21, 2021. Accessed February 21, 2021. https://clinicaltrials.gov/ct2/show/NCT03039712
- 5.Wherry K, Stromberg K, Hinnenthal JA, Wallenfelsz LA, El-Chami MF, Bockstedt L. Using Medicare claims to identify acute clinical events following implantation of leadless pacemakers. Pragmat Obs Res. 2020;11:19-26. doi: 10.2147/POR.S240913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 7.Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019;188(1):250-257. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat. 2018;113(521):390-400. doi: 10.1080/01621459.2016.126046629930437 [DOI] [Google Scholar]
- 9.Kucharska-Newton AM, Heiss G, Ni H, et al. Identification of heart failure events in Medicare claims: the Atherosclerosis Risk in Communities (ARIC) study. J Card Fail. 2016;22(1):48-55. doi: 10.1016/j.cardfail.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309(3):241-242. doi: 10.1001/jama.2012.96867 [DOI] [PubMed] [Google Scholar]
- 11.Cantillon DJ, Dukkipati SR, Ip JH, et al. Comparative study of acute and mid-term complications with leadless and transvenous cardiac pacemakers. Heart Rhythm. 2018;15(7):1023-1030. doi: 10.1016/j.hrthm.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 12.El-Chami MF, Al-Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15(12):1800-1807. doi: 10.1016/j.hrthm.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Reynolds D, Duray GZ, Omar R, et al. ; Micra Transcatheter Pacing Study Group . A Leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374(6):533-541. doi: 10.1056/NEJMoa1511643 [DOI] [PubMed] [Google Scholar]
- 14.Vamos M, Erath JW, Benz AP, Bari Z, Duray GZ, Hohnloser SH. Incidence of cardiac perforation with conventional and with leadless pacemaker systems: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017;28(3):336-346. doi: 10.1111/jce.13140 [DOI] [PubMed] [Google Scholar]
- 15.Center for Devices and Radiological Health. Examples of real-world evidence (RWE) used in medical device regulatory decisions . Accessed date April 23, 2021.https://www.fda.gov/media/146258/download
- 16.National Evaluation System for Health Technology Coordinating Center (NESTcc) Test-cases. Accessed April 23, 2021.https://nestcc.org/test-cases/
- 17.El-Chami MF, Bonner M, Holbrook R, et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm. 2020;17(8):1393-1397. doi: 10.1016/j.hrthm.2020.03.019 [DOI] [PubMed] [Google Scholar]
- 18.El-Chami MF, Soejima K, Piccini JP, et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. Pacing Clin Electrophysiol. 2019;42(8):1105-1110. doi: 10.1111/pace.13752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eResults Supplement to sensitivity analysis results.
eTable 1. Current definitions of procedural terminology for implants.
eTable 2. Differences between acute and 6-month complications.
eTable 3. Baseline characteristics of the subset of leadless VVI and transvenous VVI pacemaker patients included in the 6-month complication and device re-intervention measures.
eTable 4. Unadjusted 6-month complication rates.
eTable 5. Summary of sensitivity analyses.
eTable 6. Results from a falsification test of hip fracture through 6 months.
eFigure. Flow of information through the different phases of the review.