Key Points
Question
Are experiences of racial discrimination associated with neural response patterns throughout the brain during attention to threat cues?
Findings
This cross-sectional study of 55 Black women in the US found that those with more racial discrimination experiences showed proportionally greater response in brain regions associated with threat vigilance and regulation of threat response.
Meaning
This study suggests that heightened activation in brain regions associated with threat vigilance and regulation of threat response may disproportionately encumber and drain these resources over time, serving to enhance vulnerability for race-related health disparities.
Abstract
Importance
Racial discrimination has a clear impact on health-related outcomes, but little is known about how discriminatory experiences are associated with neural response patterns to emotionally salient cues, which likely mediates these outcomes.
Objective
To examine associations of discriminatory experiences with brainwide response to threat-relevant cues in trauma-exposed US Black women as they engage in an attentionally demanding task.
Design, Setting, and Participants
A cross-sectional study was conducted from May 1, 2014, to July 1, 2019, among 55 trauma-exposed US Black women to examine associations of racial discrimination experiences with patterns of neural response and behavior to trauma-relevant images in an affective attentional control task. Posttraumatic stress disorder (PTSD) symptoms and trauma exposure were entered as covariates to isolate variance associated with experiences of racial discrimination.
Exposures
Varying levels of trauma, PTSD symptoms, and experiences of racial discrimination.
Main Outcomes and Measures
Experiences of Discrimination Questionnaire (EOD) (range, 0-9) for count of the number of situations for which each participant reported having unfair treatment for a racial reason. Experiences of trauma and PTSD symptoms were assessed with the Traumatic Events Inventory (TEI) (number of times the person was exposed to trauma; score range, 0-112) and PTSD Symptom Scale (PSS) (score range, 0-51). Response to trauma-relevant vs neutral distractor cues were assessed via functional magnetic resonance imaging during performance of an affective Stroop (attentional control) task. Statistical analyses were conducted at a whole-brain, voxelwise level with familywise error correction.
Results
In this study of 55 Black women in the US (mean [SD] age, 37.7 [10.7] years; range, 21-61 years), participants reported a mean (SD) TEI frequency of 33.0 (18.8) and showed moderate levels of current PTSD symptoms (mean [SD] PSS score, 15.4 [12.9]). Mean (SD) EOD scores were 2.35 (2.44) and were moderately correlated with current PTSD symptoms (PSS total: r = 0.36; P=.009) but not with age (r = 0.20; P = .15) or TEI frequency (r = –0.02; P = .89). During attention to trauma-relevant vs neutral images, more experiences of racial discrimination were associated with significantly greater response in nodes of emotion regulation and fear inhibition (ventromedial prefrontal cortex) and visual attention (middle occipital cortex) networks, even after accounting for trauma and severity of PTSD symptoms (brainwide familywise error corrected; r = 0.33 for ventromedial prefrontal cortex; P = .02). Racial discrimination was also associated with affective Stroop task performance; errors on trials with threat-relevant stimuli were negatively correlated with experiences of racial discrimination (r = −0.41; P = .003).
Conclusions and Relevance
These findings suggest that experiences of racial discrimination associate with disproportionately greater response in brain regions associated with emotion regulation and fear inhibition and visual attention. Frequent racism experienced by Black individuals may potentiate attentional and regulatory responses to trauma-relevant stressors and lead to heightened modulation of regulatory resources. This may represent an important neurobiological pathway for race-related health disparities.
This cross-sectional study examines associations of discriminatory experiences with brainwide response to threat-relevant cues in Black women in the US exposed to trauma as they engage in an attentionally demanding task.
Introduction
Racial discrimination, which refers to the differential and unfair treatment because of a person’s racial or ethnic background, is increasingly recognized as a substantial influence on physical and mental health outcomes in communities of color.1 Discriminatory and racist experiences have been defined as both overt and implicit ways in which a person experiences unfair or disadvantaged treatment (within social, occupational, or other situations) as a result of their race/ethnicity.2 Experiences of racial discrimination are common for racial and ethnic minority populations in the US, with an overwhelming majority (ie, 90%-98%) of Black individuals reporting experiences of racial discrimination during their lives.3,4,5 Prior social neuroscience research has identified neural correlates of prejudicial responses, which primarily encompass stress response networks.6 However, this research has generally focused on understanding the neural basis of racial prejudice, rather than the effects of such prejudice among racial minority groups.
As such, there is a paucity of data on the effects of racism on the brain, particularly among Black communities that have faced frequent trauma exposure and lifetime adversity; the need for this type of neurobiological research has been recently highlighted.7 Some evidence suggests that racial discrimination has an adverse, additive effect with respect to existing mental health difficulties. Lower total brain matter volume has been found in adults with depression who reported experiences of racial discrimination.8 In 1 study using a social interaction paradigm with Black participants, the authors found increased response in networks associated with pain and emotion regulation (insula, ventrolateral prefrontal cortex, and rostral anterior cingulate cortex) in participants during social exclusion trials and reported that attributions of social exclusion to discrimination were associated with increased activity in regulatory prefrontal brain regions, including the rostral anterior cingulate cortex.9 Furthermore, a recent study of mostly Black participants observed that greater spontaneous amygdala activation at rest and greater connectivity between the amygdala and regions involved with expression of threat response, such as the dorsal anterior cingulate cortex, were associated with more experiences of racial discrimination.10 These studies highlight the involvement of threat detection and regulation systems as well as social pain networks in association with experiences of racism.
Prior studies also provide support for the hypothesis that racism, similar to other types of social stress,11 may have a kindling effect on the brain, specifically with respect to threat-processing networks.12 A heightened attentional detection of racism-related threat, as well as increased efforts to regulate response to these threats over time, may emerge in people who have been exposed to chronic racism. The kindling hypothesis is also a proposed mechanism for the development of stress-related disorders such as posttraumatic stress disorder (PTSD).13 Over time, experiences of racism, such as discrimination, may sensitize threat response systems, whereby more frequent exposure to racism produces chronically heightened vigilance for potential threat. Racial discrimination is a form of racism that chronically activates the stress-response system via anticipation of further discrimination, as previously described.14 This heightened threat vigilance can encumber the body’s stress response system, thereby affecting available physical and mental resources.15 There is some evidence to suggest that exposure to racism depletes cognitive reserve and impairs performance on attentional control and executive functioning tasks, such as the Stroop task.16 Overall, experiences of racism may adversely impact cognitive and emotional processes (evident in cross-sectional17,18 and longitudinal studies19) through increased vigilance for racism-related threats and regulation of response to these threats. This experience may enhance the risk for health problems ranging from cardiac and metabolic disorders to infectious disease.20
The neuroscience of racism has been extensively investigated from the perspective of the perpetrator (eg, implicit bias experiments with White participants21). However, there are few data on how racism affects neural circuitry in the individuals who are targets of racism. These individuals already bear a disproportionate burden of risk for adverse psychological and health outcomes. Racist experiences, via their association with threat vigilance and threat regulation systems, may place a burden on attentional processes. This burden can affect the efficiency of these systems as well as the availability of attentional resources for goal-directed activities.
Although 1 study has examined associations between racial discrimination and amygdala response at rest, to our knowledge, no studies have examined the unique links between racial discrimination and neural response patterns throughout the brain during attention to threat cues in a trauma-exposed, community sample of Black individuals. Given that threat-processing networks appear to be associated with experiences of discrimination,10,14 our goal in this cross-sectional study was to examine the unique associations of racial discrimination with brainwide patterns of response during performance of an attentional control task that includes threat-relevant stimuli: the affective Stroop task. We were interested in exploring brain and behavior responses that were relevant to experiences of discrimination. To isolate the associations of racial discrimination with neural resonse to threat in our sample, we accounted for variance associated with trauma exposure and PTSD symptoms. We also explored the association between racial discrimination and disruptions in attentional control from a behavioral perspective, investigating how racial discrimination was uniquely associated with affective Stroop task performance for threat-relevant trials (with respect to error rates and response times). These data may reveal a way through which cumulative experiences of racial discrimination may be associated with response in regions within networks involved with detection and regulation of threat response, highlighting a potential mechanism for race-related health disparities.
Methods
Participants
This study was conducted from May 1, 2014, to July 1, 2019. Fifty-five Black women in the US aged 22 to 61 years (mean [SD] age, 37.7 [10.7] years) were recruited from a National Institutes of Health–funded study of attentional control in women with PTSD (study MH101380); this study was part of the Grady Trauma Project, which represents a collaboration of studies investigating risk and resilience for trauma-related disorders (including studies HD071982, MH071537, and MH094757). As part of the Grady Trauma Project, participants are approached in the general medical clinics (obstetrics-gynecology, diabetes, and internal medicine) of a publicly funded hospital serving primarily low-income individuals in inner-city Atlanta, Georgia. After participants provided informed consent, clinical assessments, including assessments of trauma and PTSD symptoms, were administered. Participants who reported trauma and either no PTSD symptoms or varying degrees of PTSD symptoms were asked to return for a magnetic resonance imaging (MRI) scan; procedures are further described in the eMethods in the Supplement. Clinical and demographic characteristics are detailed in Table 1. Race was self-identified by participants in this study. The Emory University institutional review board approved this study, and written informed consent was received from all participants.
Table 1. Demographic and Clinical Characteristics.
| Characteristic | Value (N = 55) |
|---|---|
| Age, mean (SD) [range], y | 37.7 (10.7) [21-61] |
| TEI frequency total, mean (SD) [range] | 33.0 (18.8) [13-104] |
| PSS total score, mean (SD) [range] | 15.4 (2.9) [0-45] |
| Reexperiencing | 4.2 (3.9) |
| Avoidance | 6.1 (5.5) |
| Hyperarousal | 5.1 (4.5) |
| EOD total score, mean (SD) [range] | 2.4 (2.4) [0-9] |
| Educational level, No. (%) | |
| <12th Grade | 6 (10.9) |
| High school graduate or GED | 18 (32.7) |
| Some college or technical school | 24 (43.6) |
| College graduate | 3 (5.5) |
| Graduate school | 4 (7.3) |
| Monthly income, $, No. (%)a | |
| ≤249 | 5 (9.1) |
| 250-499 | 4 (7.3) |
| 500-999 | 19 (34.5) |
| 1000-1999 | 15 (27.3) |
| ≥2000 | 11 (20.0) |
Abbreviations: EOD, Experiences of Discrimination Questionnaire; GED, General Educational Development certification; PSS, PTSD Symptom Scale; PTSD, posttraumatic stress disorder; TEI, Traumatic Events Inventory.
Data missing for 1 participant.
Clinical Assessments
We administered the following assessments, detailed further in the eMethods in the Supplement. The Traumatic Events Inventory (TEI) was administered to measure lifetime trauma exposure, inclusive of childhood and adult trauma; trauma frequency (number of times the person was exposed to trauma; score range, 0-112) was the TEI index included as a covariate in statistical analyses. The PTSD Symptom Scale (PSS; score range, 0-51)22 was administered on the day of the MRI scan, and the result was used as a covariate for all analyses, given the presence of PTSD symptoms in some participants. Participants also completed the Experiences of Discrimination Questionnaire (EOD).2 The EOD total score (range, 0-9) reflects a count of the number of situations for which each participant reported having unfair treatment for a racial reason; this score was used as an index of racial discrimination.
MRI Acquisition and Image Processing
Magnetic resonance imaging scanning was conducted on a research-dedicated Siemens 3-T TIM-Trio scanner (12-channel head coil) at Emory University Hospital. Acquisition parameters and image-preprocessing details are provided in the eMethods in the Supplement.
Functional MRI Task: Affective Stroop Task
The affective Stroop task is a measure of attentional control that has been detailed in earlier studies23,24,25,26 (further described in the eMethods in the Supplement). Participants were asked to rapidly identify, via button press, the amount of numbers displayed (3, 4, 5, or 6) while ignoring distractor images (trauma-relevant, positive, and neutral scenes) that appeared prior to and after each number stimulus (eFigure 1 in the Supplement). In some trials, the amount of numbers was incongruent with the actual number displayed. In addition, the task included trials with no cognitive demands (“view-only” trials). Error rates and response times were recorded and analyzed. Behavioral data were excluded from behavioral data analyses for 2 participants because of excessive errors on trials (>50%); these participants reported that their performance was affected by high levels of anxiety.
Functional MRI Data Analysis
We describe patterns of brainwide response in the entire sample to different affective Stroop task trials, including trauma-relevant (eTable 2 and eFigure 2 in the Supplement) and positive distractor trials (eTable 2 and eFigure 3 in the Supplement), in the eResults in the Supplement. Our primary objective was to examine the association between neural responses to trauma-relevant (both number-incongruent and number-congruent trials) vs neutral distractor trials (both number-incongruent and number-congruent trials) and racial discrimination (EOD total).
To examine blood oxygen–level dependent signal change to task stimuli, a first-level, fixed-effects analysis was conducted. Onset times for each task condition were entered into a general linear model, convolved with a hemodynamic response function, and linear contrasts between conditions were estimated. Participant-specific motion parameters were also included in the model as effects of noninterest. Given our interest in examining neural response to threat-related stimuli, and in accordance with our earlier studies using this task, our primary contrast of interest was trauma-relevant vs neutral distractor trials (including number-incongruent and number-congruent trials, as well as view-only trials).27 Events included the distractor and number stimuli for task trials and distractors for view-only trials.
Given that our primary objective was to examine associations between racial discrimination and response to threat-relevant vs neutral images, the EOD total score was entered as a participant-level regressor in a multiple regression model in SPM8 software (The Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology). Given that some participants reported current PTSD symptoms, we accounted for the effects of PTSD symptoms in analyses; PSS total scores were entered as covariates for this contrast of interest. Statistical analyses were conducted at a whole-brain, voxelwise level for this contrast; given that few studies examined neural responses associated with racism, we did not restrict analyses to any particular regions of interest. To reduce type I error inflation due to multiple comparisons testing, we used a whole-brain statistical threshold of P < .05 with voxelwise, familywise error (FWE) correction (provided in SPM8). To account for the possible effects of trauma, we repeated this analysis using trauma exposure (TEI frequency) as a covariate; given that trauma exposure and PTSD symptoms are collinear in this population,28 including this sample (r = 0.32; P = .02), these covariates were entered in 2 separate models to avoid model overfitting.
Statistical Analysis
Partial correlations were completed to explore associations between racial discrimination and affective Stroop task performance (error rate and response time) during trauma-relevant trials (number-congruent and number-incongruent trials combined). Similar to functional MRI analyses, we accounted for variance associated with PTSD symptoms and trauma exposure in 2 separate models. Given that the covariates were examined in separate models, we used a Bonferroni-corrected threshold of P < .025, 2-tailed, to define statistical significance. Analyses were conducted with SPSS, version 25 (IBM Corp).
Results
PTSD Symptoms, Trauma, and Racial Discrimination
Descriptive statistics on trauma exposure, PTSD symptoms, and racial discrimination for this sample of 55 Black women living in the US (mean [SD] age, 37.7 [10.7] years; range, 21-61 years) are provided in Table 1. Participants reported a mean (SD) TEI frequency of 33.0 (18.8) (similar to previous Grady Trauma Project studies28,29,30) and showed moderate levels of current PTSD symptoms (mean [SD] PSS, 15.4 [12.9]). Racial discrimination (EOD) scores ranged from 0 to 9 and were moderately correlated with current PTSD symptoms (PSS total: r = 0.36; P = .009), but not with age (r = 0.20; P = .15) or TEI frequency (r = −0.02; P = .89).
Functional MRI Data
After accounting for current PTSD symptoms, greater racial discrimination experiences were significantly associated with increased blood oxygen level–dependent (BOLD) responses within visual attention regions (middle occipital cortex) and emotion regulation and fear inhibition regions (ventromedial prefrontal cortex [vmPFC]) during trauma-relevant distractor trials vs neutral distractor trials (Table 2). Figure 1A illustrates the regions that showed significant positive correlations with EOD score during these trauma-relevant vs neutral distractor trial conditions. These findings were sustained when analyses were repeated with trauma exposure as a covariate (Table 3). Figure 1B illustrates the correlation between vmPFC activation and EOD score (r = 0.33). Activation in this region was not associated with age (r = 0.07; P = .62). In terms of negative correlations, no results survived the voxelwise FWE threshold.
Table 2. Anatomical Locations of Voxelwise BOLD Response to Affective Stroop Trauma-Relevant vs Neutral Distractor Trials (PTSD Symptom Scale Covaried)a.
| Characteristic | Brodmann area | MNI coordinate | Cluster size, mm3 | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive correlation | |||||
| Middle occipital gyrus | 37 | −42 | −52 | −17 | 430 |
| Middle occipital gyrus | 19 | −48 | −79 | 4 | NA |
| Middle temporal gyrus (angular gyrus) | 39 | −51 | −70 | 13 | NA |
| Cerebellum | 42 | −46 | −20 | 487 | |
| Middle temporal gyrus | 19 | 54 | −64 | 13 | NA |
| Superior temporal gyrus | 39 | 45 | −55 | 19 | NA |
| Medial frontal gyrus (vmPFC) | 11 | 3 | 47 | −14 | 27 |
| Negative correlation | |||||
| NS | NA | NA | NA | NA | NA |
Abbreviations: BOLD, blood oxygen level–dependent; MNI, Montreal Neurologic Institute; NA, not applicable; NS, not significant; PTSD, posttraumatic stress disorder; vmPFC, ventromedial prefrontal cortex.
P < .05; familywise error corrected, MNI coordinates, and PTSD Symptom Scale covaried.
Figure 1. Associations of Racial Discrimination With Neural Response During Attention to Threat-Relevant Affective Stroop Trials (Posttraumatic Stress Disorder Symptoms Covaried).
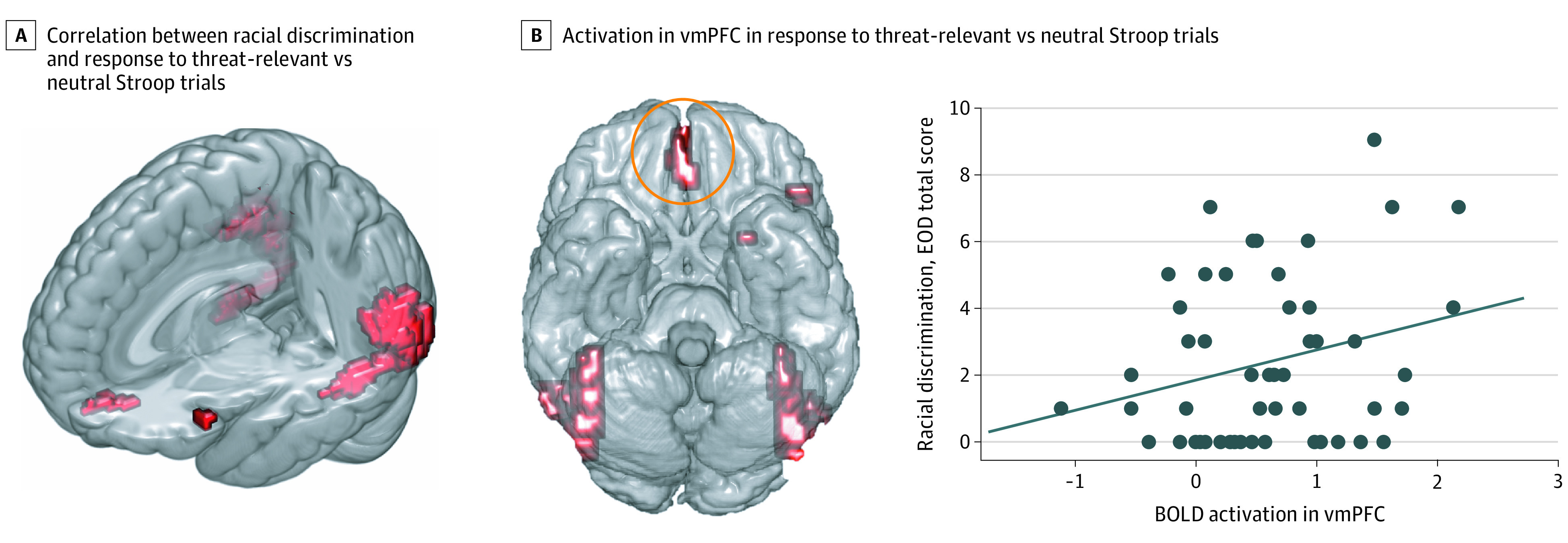
A, Brainwide correlation between racial discrimination (Experiences of Discrimination Questionnaire [EOD] total) and response to threat-relevant vs neutral affective Stroop trials. B, Increased activation in the ventromedial prefrontal cortex (vmPFC, circled in orange) to threat-relevant vs neutral trials corresponds with more experiences of racial discrimination, seen in the correlation plot (r = 0.33; P = .02). The diagonal line in the plot indicates the line of best fit. BOLD indicates blood oxygen level–dependent.
Table 3. Anatomical Locations of Voxelwise BOLD Response to Affective Stroop Trauma-Relevant vs Neutral Distractor Trials (Traumatic Events Inventory Covaried)a.
| Characteristic | Brodmann area | MNI coordinate | Cluster size, mm3 | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive correlation | |||||
| Middle occipital gyrus | 37 | −42 | −52 | −17 | 441 |
| Middle occipital gyrus | 19 | −48 | −79 | 1 | NA |
| Middle temporal gyrus (angular gyrus) | 39 | −51 | −70 | 13 | NA |
| Cerebellum | NA | 42 | −46 | −20 | 484 |
| Middle temporal gyrus | 19 | 54 | −64 | 13 | NA |
| Superior temporal gyrus | 19 | 45 | −67 | −14 | NA |
| Medial frontal gyrus (vmPFC) | 11 | 3 | 44 | −17 | 25 |
| Negative correlation | |||||
| NS | NA | NA | NA | NA | NA |
Abbreviations: BOLD, blood oxygen level–dependent; MNI, Montreal Neurologic Institute; NA, not applicable; NS, not significant; vmPFC, ventromedial prefrontal cortex.
P < .05; familywise error corrected, MNI coordinates, and Traumatic Events Inventory covaried.
Behavioral Data
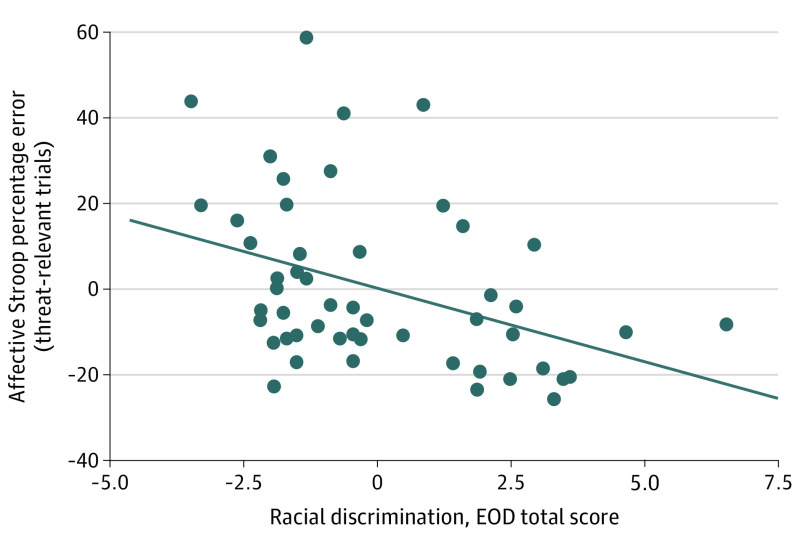
Descriptive statistics on affective Stroop task performance, including mean error rates and response times to affective Stroop task trials, are provided in eTable 1 in the Supplement. We examined associations between experiences of discrimination and affective Stroop task performance. After accounting for current PTSD symptoms (PSS total), racial discrimination experiences (EOD total) were associated with significantly fewer overall errors on trauma-relevant distractor trials (r = −0.41; P = .003; partial correlation plot with residuals provided in Figure 2). Similarly, after accounting for trauma exposure (TEI frequency), racial discrimination experiences were associated with significantly fewer overall errors in trauma-relevant distractor trials (r = −0.39; P = .004). No significant correlations were observed between discriminatory experiences and response time for trauma-relevant distractor trials after accounting for PTSD symptoms or trauma experiences.
Figure 2. Association of Experiences of Racial Discrimination With Affective Stroop Performance on Threat-Relevant Trials (Posttraumatic Stress Disorder Symptoms Covaried).
Percentage errors on affective Stroop threat-relevant trials negatively correlated with racial discrimination experiences, seen in the partial correlation plot of residuals (r = −0.41; P = .003). The diagonal line in the plot indicates the line of best fit. EOD indicates Experiences of Discrimination Questionnaire.
Discussion
The objective of the present study was to identify unique associations of racial discrimination with neural responses during performance of an affective attentional control task in a community sample of Black women living in the US exposed to trauma. To our knowledge, this is the first study to examine associations of racial discrimination experience with brainwide patterns of neural response to emotionally salient images among a Black population with high trauma exposure. We observed that, compared with participants who reported fewer racially discriminatory experiences, participants who reported more racial discrimination experiences showed heightened reactivity to trauma-relevant images in components of visual attention and emotion regulation and fear inhibition networks, including the vmPFC, even after accounting for variance associated with PTSD symptoms or trauma. These findings were robust, surviving a conservative FWE correction at the whole-brain level, indicating moderate correlations between experiences of racial discrimination and heightened response in nodes of these 2 networks, irrespective of PTSD symptoms or trauma exposure. Furthermore, experiences of racial discrimination were associated with affective Stroop task performance in trauma-relevant distractor trials. Our data suggest a possible neurobiological pathway of relevance to racism-related stress.
These findings indicate that experiences of racism were associated with increased response in critical nodes of networks involved with threat detection and regulation of threat response. These results align with a study of Black adults without a reported history of trauma exposure that showed increased vmPFC activity associated with feelings of exclusion based on race during performance of a dynamic social interaction task.9 Another study observed that increased spontaneous activation in the amygdala, as well as increased amygdala connectivity to occipitotemporal regions, was positively associated with racial discrimination.10 Earlier research in mostly White individuals has also revealed activation in threat-processing networks, including the dorsal anterior cingulate cortex, during experiences of social exclusion.31 The amygdala and dorsal anterior cingulate cortex are critical to the detection of threat and expression of threat response, respectively.32 Occipitotemporal regions comprise the ventral visual attention network, which engages to deploy attentional resources to salient stimuli,33 and the vmPFC is involved with regulation of threat response.34 In the present analyses, we observed an association between racially discriminatory experiences and neural reactivity to trauma-relevant (ie, threat) cues within regions of the visual attention network, including middle occipital gyrus regions, as well as the vmPFC. Taken together, our findings suggest that experiences of racism are associated with greater vigilance for potential threat and heightened response within threat-inhibition networks in response to threat cues. As such, these discriminatory experiences may be associated with greater preparedness for threat and suppression of the threat response.
We observed a negative association between discrimination and affective Stroop task performance on more difficult threat-relevant distractor trials. Thus, experiences of racism were not associated with deficiencies in attentional control, but rather with heightened activation in emotion regulation and threat inhibition regions, which can disproportionately encumber and drain these resources over time. One proposed pathway linking racism to adverse health outcomes is via increased self-regulation (ie, heightened attempts to manage responses to racism).35 Experiences of racism have a resource cost, both with respect to threat detection and regulation of threat response,35 and some studies have shown that racist experiences adversely affect performance on attention and executive functioning tasks, such as the Stroop task.16 Although discriminatory experiences were not associated with poorer Stroop performance in our study, it is possible that, in participants with more discriminatory experiences, task performance was preserved at the cost of efficiency in regulatory networks. The use of compensatory strategies at the cost of efficiency is also observed in the context of general anxiety. Attentional control theory describes ways in which high anxiety may not impair performance effectiveness on attentional control tasks, but the use of compensatory strategies encumbers attention network efficiency.36
There are different potential pathways through which racism can exert adverse effects on biological systems, including chronic overactivation of the hypothalamic-pituitary-adrenal axis and subsequently high allostatic load.37 Theoretical models applied to anticipatory race-related stress, such as prolonged activation theory, elaborate on ways in which racism can be associated with overburdened systems via increased vigilance for racism-related threat38,39,40,41; this stress may, in turn, increase vulnerability for physical and mental health problems.37
Our findings complement a growing literature on the associations between disparate stressor exposure and neurobiological outcomes in marginalized racial/ethnic groups. Although the prevalence of PTSD symptoms clearly differs between Black and White individuals in the US,42 limited studies have investigated how differences in response to stressors play a role in these disparities. Disproportionate stressor exposure has been reported to be linked to racial differences in neural reactivity to threat within key nodes such as the vmPFC.43 Our findings demonstrate that racial discrimination—a stressor experienced by an overwhelming majority of Black individuals—was associated with increased vmPFC activity to trauma-relevant cues. Racial discrimination is known to be associated with biological stress systems, and can exacerbate the development and expression of PTSD symptoms. Given the critical role of the vmPFC in PTSD, our findings suggest that racism has unique associations with the function of this regulatory region, which has implications for the neurobiology of PTSD and threat response in general in minoritized populations. These data highlight the need for further PTSD research that addresses the association of the intersection of trauma and racial inequities with neurobiological response.
Alternatively, the proportionally greater vmPFC response observed in association with discrimination may represent a resilient adaptation to stress. Prior literature on trauma exposure and PTSD symptoms suggest that increased vmPFC response (and volume) reflects resilience to trauma44,45,46 and adaptive threat response, including efficient extinction of conditioned fear responses.47 As such, it is possible that the findings observed in this study indicate a relatively enhanced ability to manage stress related to discrimination experiences. Coupled with the behavioral data that demonstrated better attention performance on threat-relevant affective Stroop task trials in association with discrimination experiences, the functional MRI findings may illustrate a biological marker of resilience and/or protective adaptation in the face of racism-related stressors in this sample of Black women in the US exposed to trauma.
Limitations
We acknowledge some limitations to the present study. For one, only Black women were included in the sample, thus precluding our ability to assess potential sex differences in behavioral or neural responses associated with discrimination. Future studies should seek to replicate these findings in larger, racially, ethnically, and sexually diverse cohorts. Furthermore, we did not examine mechanistic pathways for the outcomes observed; promising avenues of future investigation include investigations of the association of discrimination with stress regulation and the immune system. The current analysis also used a broad assessment of racial discrimination. A more detailed measurement of racist acts and behaviors, as well as identification of events linked to rumination, is warranted to characterize specificity of neurobiological response to these stressors. Furthermore, experimental manipulations, including social interaction tasks, may reveal ways in which experiences of discrimination (occurring in real time) may be associated with goal-directed attention.
Conclusions
This cross-sectional study of 55 Black women in the US exposed to trauma found that in the context of attention to threat-relevant stimuli, those who experienced more racism showed increased response within key nodes of visual attention and emotion and fear regulation network regions, even after accounting for variance associated with trauma and PTSD symptoms. These findings highlight the value of assessing racial discrimination in trauma research, and demonstrate associations between racism and responses to threat stimuli. Taken together, the results of this study reveal how racial discrimination is associated with threat vigilance and modulation of attentional response. The findings suggest a potential pathway through which health disparities may emerge for Black individuals; conversely, these data could also illustrate an adaptive calibration to racism-related stress.
eMethods.
eResults.
eTable 1. Affective Stroop Performance
eTable 2. Anatomical Locations of Voxel-Wide BOLD Response to Affective Stroop Distractor Trials
eFigure 1. Schematic Representation of Affective Number Stroop
eFigure 2. Brain-Wide Pattern of Response to AS Trauma-Relevant Distractor Trials
eFigure 3. Brain-Wide Pattern of Response to AS Positive Distractor Trials
eReferences.
References
- 1.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135(4):531-554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61(7):1576-1596. doi: 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Helms JE, Nicolas G, Green CE. Racism and ethnoviolence as trauma: enhancing professional and research training. Traumatology. 2012;18(1):65-74. doi: 10.1177/1534765610396728 [DOI] [Google Scholar]
- 4.Pachter LM, Bernstein BA, Szalacha LA, García Coll C. Perceived racism and discrimination in children and youths: an exploratory study. Health Soc Work. 2010;35(1):61-69. doi: 10.1093/hsw/35.1.61 [DOI] [PubMed] [Google Scholar]
- 5.Landrine H, Klonoff EA. The schedule of racist events: a measure of racial discrimination and a study of its negative physical and mental health consequences. J Black Psychology. 1996;22(2):144-68. doi: 10.1177/00957984960222002 [DOI] [Google Scholar]
- 6.Chekroud AM, Everett JA, Bridge H, Hewstone M. A review of neuroimaging studies of race-related prejudice: does amygdala response reflect threat? Front Hum Neurosci. 2014;8:179. doi: 10.3389/fnhum.2014.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harnett NG. Neurobiological consequences of racial disparities and environmental risks: a critical gap in understanding psychiatric disorders. Neuropsychopharmacology. 2020;45(8):1247-1250. doi: 10.1038/s41386-020-0681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer CS, Schreiner PJ, Lim K, Battapady H, Launer LJ. Depressive symptomatology, racial discrimination experience, and brain tissue volumes observed on magnetic resonance imaging. Am J Epidemiol. 2019;188(4):656-663. doi: 10.1093/aje/kwy282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masten CL, Telzer EH, Eisenberger NI. An FMRI investigation of attributing negative social treatment to racial discrimination. J Cogn Neurosci. 2011;23(5):1042-1051. doi: 10.1162/jocn.2010.21520 [DOI] [PubMed] [Google Scholar]
- 10.Clark US, Miller ER, Hegde RR. Experiences of discrimination are associated with greater resting amygdala activity and functional connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(4):367-378. doi: 10.1016/j.bpsc.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarlane AC. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry. 2010;9(1):3-10. doi: 10.1002/j.2051-5545.2010.tb00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waller RJ. Application of the kindling hypothesis to the long-term effects of racism. Soc Work Ment Health. 2003;1(3):81-9. doi: 10.1300/J200v01n03_06 [DOI] [Google Scholar]
- 13.Stam R. PTSD and stress sensitisation: a tale of brain and body, part 1: human studies. Neurosci Biobehav Rev. 2007;31(4):530-557. doi: 10.1016/j.neubiorev.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 14.Hicken MT, Lee H, Hing AK. The weight of racism: vigilance and racial inequalities in weight-related measures. Soc Sci Med. 2018;199:157-166. doi: 10.1016/j.socscimed.2017.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Dev Psychopathol. 2011;23(3):921-938. doi: 10.1017/S095457941100040X [DOI] [PubMed] [Google Scholar]
- 16.Bair AN, Steele JR. Examining the consequences of exposure to racism for the executive functioning of Black students. J Exp Soc Psychol. 2010;46:127-132. doi: 10.1016/j.jesp.2009.08.016 [DOI] [Google Scholar]
- 17.Salvatore J, Shelton JN. Cognitive costs of exposure to racial prejudice. Psychol Sci. 2007;18(9):810-815. doi: 10.1111/j.1467-9280.2007.01984.x [DOI] [PubMed] [Google Scholar]
- 18.Thames AD, Hinkin CH, Byrd DA, et al. Effects of stereotype threat, perceived discrimination, and examiner race on neuropsychological performance: simple as black and white? J Int Neuropsychol Soc. 2013;19(5):583-593. doi: 10.1017/S1355617713000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahodne LB, Kraal AZ, Sharifian N, Zaheed AB, Sol K. Inflammatory mechanisms underlying the effects of everyday discrimination on age-related memory decline. Brain Behav Immun. 2019;75:149-154. doi: 10.1016/j.bbi.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattan BD, Wei KY, Cloutier J, Kubota JT. The social neuroscience of race-based and status-based prejudice. Curr Opin Psychol. 2018;24:27-34. doi: 10.1016/j.copsyc.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 22.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459-473. doi: 10.1002/jts.2490060405 [DOI] [Google Scholar]
- 23.Blair KS, Smith BW, Mitchell DG, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430-440. doi: 10.1016/j.neuroimage.2006.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair KS, Vythilingam M, Crowe SL, et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2012;43(1):85-95. doi: 10.1017/S0033291712000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White SF, Costanzo ME, Blair JR, Roy MJ. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. Neuroimage Clin. 2014;7:19-27. doi: 10.1016/j.nicl.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fani N, King TZ, Clendinen C, et al. Attentional control abnormalities in posttraumatic stress disorder: functional, behavioral, and structural correlates. J Affect Disord. 2019;253:343-351. doi: 10.1016/j.jad.2019.04.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fani N, King TZ, Powers A, et al. Cognitive and neural facets of dissociation in a traumatized population. Emotion. 2019;19(5):863-875. doi: 10.1037/emo0000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillespie CF, Bradley B, Mercer K, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505-514. doi: 10.1016/j.genhosppsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon HD, Michopoulos V, Gluck RL, et al. Trauma exposure and stress-related disorders in African-American women with diabetes mellitus. Endocrinol Diabetes Metab. 2020;3(2):e00111. doi: 10.1002/edm2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers A, Fani N, Murphy L, et al. Attention bias toward threatening faces in women with PTSD: eye tracking correlates by symptom cluster. Eur J Psychotraumatol. 2019;10(1):1568133. doi: 10.1080/20008198.2019.1568133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? an FMRI study of social exclusion. Science. 2003;302(5643):290-292. doi: 10.1126/science.1089134 [DOI] [PubMed] [Google Scholar]
- 32.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62(10):1191-1194. doi: 10.1016/j.biopsych.2007.04.032 [DOI] [PubMed] [Google Scholar]
- 33.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150-159. doi: 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiser J, Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83(8):638-647. doi: 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol. 2007;58:201-225. doi: 10.1146/annurev.psych.57.102904.190212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336-353. doi: 10.1037/1528-3542.7.2.336 [DOI] [PubMed] [Google Scholar]
- 37.Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18(1):1-10. doi: 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- 38.Brosschot JF. Markers of chronic stress: prolonged physiological activation and (un)conscious perseverative cognition. Neurosci Biobehav Rev. 2010;35(1):46-50. doi: 10.1016/j.neubiorev.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 39.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60(2):113-124. doi: 10.1016/j.jpsychores.2005.06.074 [DOI] [PubMed] [Google Scholar]
- 40.Hoggard LS, Hill LK, Gray DL, Sellers RM. Capturing the cardiac effects of racial discrimination: do the effects “keep going”? Int J Psychophysiol. 2015;97(2):163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utsey SO, Belvet B, Hubbard RR, Fischer NL, Opare-Henaku A, Gladney LL. Development and validation of the prolonged activation and anticipatory race-related stress scale. J Black Psychol. 2013;39(6):532-59. doi: 10.1177/0095798412461808 [DOI] [Google Scholar]
- 42.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harnett NG, Wheelock MD, Wood KH, et al. Negative life experiences contribute to racial differences in the neural response to threat. Neuroimage. 2019;202:116086. doi: 10.1016/j.neuroimage.2019.116086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holz NE, Tost H, Meyer-Lindenberg A. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Mol Psychiatry. 2020;25(2):379-396. doi: 10.1038/s41380-019-0551-9 [DOI] [PubMed] [Google Scholar]
- 45.Bolsinger J, Seifritz E, Kleim B, Manoliu A. Neuroimaging correlates of resilience to traumatic events—a comprehensive review. Front Psychiatry. 2018;9:693. doi: 10.3389/fpsyt.2018.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno-López L, Ioannidis K, Askelund AD, Smith AJ, Schueler K, van Harmelen AL. The resilient emotional brain: a scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(4):392-402. [DOI] [PubMed] [Google Scholar]
- 47.Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci. 2011;5:44. doi: 10.3389/fnbeh.2011.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eTable 1. Affective Stroop Performance
eTable 2. Anatomical Locations of Voxel-Wide BOLD Response to Affective Stroop Distractor Trials
eFigure 1. Schematic Representation of Affective Number Stroop
eFigure 2. Brain-Wide Pattern of Response to AS Trauma-Relevant Distractor Trials
eFigure 3. Brain-Wide Pattern of Response to AS Positive Distractor Trials
eReferences.