Abstract
Maternal psychosocial stress during pregnancy can impact the developing fetal brain and influence offspring mental health. In this context, animal studies have identified the hippocampus and amygdala as key brain regions of interest, however, evidence in humans is sparse. We, therefore, examined the associations between maternal prenatal psychosocial stress, newborn hippocampal and amygdala volumes, and child social-emotional development.
In a sample of 86 mother-child dyads, maternal perceived stress was assessed serially in early, mid and late pregnancy. Following birth, newborn (aged 5–64 postnatal days, mean: 25.8 ± 12.9) hippocampal and amygdala volume was assessed using structural magnetic resonance imaging. Infant social-emotional developmental milestones were assessed at 6- and 12-months age using the Bayley-III.
After adjusting for covariates, maternal perceived stress during pregnancy was inversely associated with newborn left hippocampal volume (β = −0.26, p = .019), but not with right hippocampal (β = −0.170, p = .121) or bilateral amygdala volumes (ps > .5). Furthermore, newborn left hippocampal volume was positively associated with infant social-emotional development across the first year of postnatal life (B = 0.01, p = .011). Maternal perceived stress was indirectly associated with infant social-emotional development via newborn left hippocampal volume (B = −0.34, 95% CIBC [-0.97, −0.01]), suggesting mediation.
This study provides prospective evidence in humans linking maternal psychosocial stress in pregnancy with newborn hippocampal volume and subsequent infant social-emotional development across the first year of life. These findings highlight the importance of maternal psychosocial state during pregnancy as a target amenable to interventions to prevent or attenuate its potentially unfavorable neural and behavioral consequences in the offspring.
Keywords: Psychosocial stress, Pregnancy, Brain development, Newborn, Social-emotional development, Hippocampus
Highlights
-
•
Maternal perceived stress predicted smaller neonatal left hippocampal volume (HCV).
-
•
Neonatal left HCV was positively associated with infant social-emotional function.
-
•
Variation in HCV may mediate maternal stress-related effects on child mental health.
1. Introduction
Maternal psychosocial distress during pregnancy (perceived stress, anxiety and depressive symptomatology) is one of the most common perinatal health problems. Between 10 and 20% of women experience a mental health disorder (i.e., diagnosis of a mood or anxiety disorder) in the perinatal period (Kendig et al., 2017), with an even higher prevalence when subclinical forms of psychosocial distress are considered. Evidence from animal and human studies suggests that maternal psychosocial distress during pregnancy may influence the structure and function of the developing fetal brain (Buss et al., 2012; Entringer et al., 2015; Lautarescu et al., 2020). Stress-related biological signals in the intrauterine environment may elicit structural and functional changes in fetal cells, tissues, and organ systems during critical developmental periods of rapid cellular proliferation and differentiation that, in turn, may have long-term or permanent consequences. The fetal brain is particularly susceptible to exposure to environmental perturbations because major neurodevelopmental processes such as neuron proliferation, migration and synaptogenesis occur over a relatively short period of time during ontogeny (Buss et al., 2012).
Animal models have implicated the limbic system, particularly the hippocampus and amygdala as brain regions specifically affected by prenatal stress (Badihian et al., 2019; Charil et al., 2010; McEwen et al., 1992). The hippocampus is a key structure underlying cognitive function including memory, and it also plays a major role in regulating the stress response. The amygdala is a structure critical for affective evaluation and learning, including fear and reward learning, as well as the processing of salience and novelty (Fareri and Tottenham, 2016; Lindquist et al., 2012). Correspondingly, variation in hippocampal and amygdalar structure and function is implicated in many psychiatric disorders that are accompanied by social-emotional problems (e.g., major depression and anxiety) (Hamilton et al., 2008; Herman et al., 2005; Oler et al., 2016; Pruessner et al., 2010).
In humans, the unfolded hippocampus emerges by gestational week 13–14 and begins to resemble adult anatomy by the start of the third trimester (Kier et al., 1997). However, the subfields CA1 and dentate gyrus – a subfield relevant for later neurogenesis – do not reach anatomical maturity till late gestation, and neuronal proliferation in these areas likely continues until term and beyond (Arnold and Trojanowski, 1996). With regard to size, both postmortem and in utero studies reveal a linear increase in total hippocampal volume (HCV) throughout the fetal period (Ge et al., 2015; Jacob et al., 2011). In terms of the amygdala, the three major divisions (anterior, basolateral and centromedial) are discernible already at gestational week 7–8, and the first individual nuclei begin to be detectable at the same time. In the fetal period, growth of the amygdala continues by migration of cells from the ventricular eminences, followed by neuronal differentiation and the development of synapses and projections (Müller and O'Rahilly, 2006). Thus, due to their protracted development across gestation, the developmental trajectory of the embryonic and fetal hippocampus and amygdala may be susceptible to the influence of variation in environmental conditions.
In rodents and non-human primates, prenatal stress induces a global (i.e., not regionally-specific) decrease in HCV and inhibits local postnatal neurogenesis in regions including the dentate gyrus (Coe et al., 2003; Kawamura et al., 2006; Lemaire et al., 2000; Szuran et al., 1994). In adult rats, prenatal stress also is associated with a reduction of dendritic arborization and synaptic density in the CA1 and CA3 hippocampal subfields (Barros et al., 2006; Hayashi et al., 1998; Jia et al., 2010; Martínez-Téllez et al., 2009). Based on these findings and given its high levels of glucocorticoid receptors (Wang et al., 2013), the hippocampus has been considered a major target of alterations in gestational biology (particularly cortisol) associated with maternal psychosocial distress. Compared to the empirical evidence on the effects of prenatal stress on hippocampal morphology, animal models investigating the effects of prenatal stress on amygdala morphology are less abundant. Amygdalar nuclei volumes, neurons and glia have been demonstrated to be reduced in offspring of prenatally stress rats after birth, however, these effects appear to resolve after 45 days and at 80 days amygdala volume (AGV) was shown to be increased in PS-exposed offspring (Kraszpulski et al., 2006; Salm et al., 2004). These findings suggest that the developmental trajectory of the amygdala may be altered by prenatal stress but that the direction of the effect may be dependent on the developmental stage at assessment.
In human research, investigation of the impact of maternal psychosocial distress during pregnancy on offspring brain development has been rapidly developing over the last decade and increasing evidence supports an association between maternal stress during pregnancy and offspring brain anatomy and connectivity in offspring. Maternal prepartum stress-associated variation in offspring brain phenotypes has been shown in fetuses (De Asis-Cruz et al., 2020; van den Heuvel et al., 2021), neonates (Dean et al., 2018; Lautarescu et al., 2020b; Rifkin-Graboi et al., 2013; Scheinost et al., 2020), infants (Qiu et al., 2015), children (Davis et al., 2020; Donnici et al., 2021; Lebel et al., 2016), adolescents (McQuaid et al., 2019) and young adults (Favaro et al., 2015; Marečková et al., 2019) with phenotypes under investigation varying greatly including variation in brain anatomy as well as structural and functional brain connectivity. However, despite the conceptual underpinnings and empirical evidence in animals, there is little evidence in humans linking maternal psychosocial distress during pregnancy with offspring hippocampal and amygdalar volumetry to date.
In young adults, no association between exposure to stressful life events during gestation and HCV was observed (Marečková et al., 2018). However, a major challenge with studies relating conditions during gestation to child or adult brain structure or function is the high likelihood that postnatal conditions may alter the association between prenatal distress and HCV given the high degree of neuroplasticity during early postnatal life. For instance, it appears that the association between small-for-gestational age at birth (a proxy for a suboptimal gestational environment) and adult HCV was significant among only those subjects reporting low but not high postnatal maternal care (Buss et al., 2007). Despite the possibility that the association between exposure to maternal distress in utero and brain structure and function attenuates over time under certain postnatal environmental conditions, the relevance of variation in neonatal neurophenotypes for later cognitive function manifests itself in the fact that the developmental trajectory of a brain system (which is dependent on its initial set point) may in and of itself affect developmental outcomes such as cognitive function (Rasmussen et al., 2019). Thus, study designs that incorporate assessments of offspring brain development during gestation or soon after birth may prevent confounding by postnatal factors. Indeed, one recent study using fetal MRI reported an association between maternal psychosocial distress and fetal left HCV (Wu et al., 2020). In contrast, two other studies (Lehtola et al., 2020; Qiu et al., 2013) found no associations between maternal depressive and anxiety symptoms during pregnancy and newborn HCV (although maternal antenatal anxiety was associated with slower growth of the right hippocampus across the first 6 months of life (Qiu et al., 2013)).
Investigations of the association between maternal psychosocial stress and AGV have generated similar inconclusive results. One study in neonates observed no association between AGV and maternal depressive symptoms (Rifkin-Graboi et al., 2013), whereas another study reported smaller left and right AGV in association with maternal psychosocial stress, albeit only in male neonates (Lehtola et al., 2020). Sex-specific effects of maternal prenatal depression and anxiety have also been observed in two studies in preschool children. Both studies report a positive association between maternal depression or anxiety and AGV in girls, but not boys, which was restricted to the left amygdala in one study and to the right amygdala in the other (Acosta et al., 2019; Wen et al., 2017). One other study observed no associations between maternal anxiety during pregnancy and AGV in 3–7 year-old offspring, and did not report potential moderation by sex (Donnici et al., 2021).
A better understanding of the influence of psychosocial distress on the developing fetal hippocampus and amygdala may have clinical relevance. The hippocampus is involved in processing of emotions and social behavior (Immordino-Yang and Singh, 2013; Rubin et al., 2014), and hippocampal size has been related to behavioral and emotional problems in infants born very preterm (Rogers et al., 2012), with emotion dysregulation in adolescents (Barch et al., 2019), and with social phobia (Irle et al., 2010) and major depression (Videbech and Ravnkilde, 2004) in adults. AGV has been associated with variation in fear processing in infants (Tuulari et al., 2020), impaired impulse control and working memory in toddlers (Graham et al., 2018; Nolvi et al., 2021), with depression in children and adolescents (Merz et al., 2018; Rosso et al., 2005) and with pediatric anxiety (Milham et al., 2005). Common across these HCV- and AGV-related phenotypes are social-emotional problems, and social-emotional competence in infancy and early childhood constitutes an important predictor of subsequent mental health (Halligan et al., 2013; Skovgaard et al., 2008; Tang et al., 2020). Social-emotional problems are also robustly associated with maternal psychosocial distress during pregnancy. A recent meta-analysis including over 70 studies concluded that for mothers with more distress during pregnancy the odds of having children with behavioral and emotional difficulties was 1.5–2 times greater (Madigan et al., 2018). Thus, prenatal stress-associated structural alterations in hippocampus or amygdala may be underlying the persistent effects of maternal psychosocial distress on child social-emotional development.
Most human studies to date have examined associations between maternal psychosocial distress, child neurobiological substrates (such as brain structure) and child social-emotional outcomes in a piecemeal manner, but longitudinal research is still rare. We, therefore, in the present study, examined the prospective associations between maternal perceived stress across pregnancy, offspring HCV and AGV at birth, and infant social-emotional development over the first year of life.
2. Methods and materials
2.1. Study population
We conducted a prospective, longitudinal study at the University of California, Irvine, Development, Health and Disease Research Program, in a clinical convenience cohort of ethnically and socio-demographically diverse pregnant women receiving prenatal care at our university and other affiliated clinics. All participants had singleton, intrauterine pregnancies, with no known cord, placental, or uterine anomalies, fetal congenital malformations, or presence of any conditions known to be associated with dysregulated neuroendocrine function or systemic corticosteroid medication use. After birth, the participants’ children were assessed with structural magnetic resonance imaging (MRI) of the newborn brain and followed up at 6 and 12 months of age. The cohort comprised N = 131 mother-child dyads. The present study included a subsample of N = 86 mother-child dyads with good quality structural MRI data (see quality control measures below). All newborns included were born full-term (≥37 weeks gestational age) or late preterm (n = 7, range: 34.6–36.9 weeks), and had no known congenital, genetic or neurological disorders. Additional analyses were conducted excluding the late preterm infants to justify their inclusion in the data (see the Statistical Analysis section). Data on social-emotional development was available in a subset of n = 73 at 6 months and n = 63 at 12 months of age with n = 76 infants providing data for at least one time point.
Mother-child dyads with good quality MRI data (N = 86) did not differ from those that were not included in the analyses because no MRI scan was attempted or because of insufficient quality of the MRI scan (N = 45) with respect to maternal age, SES, race/ethnicity, smoking in pregnancy, parity, obstetric risk, mean PSS scores in pregnancy, gestational age at birth, birthweight, maternal sensitivity and infant social-emotional scores. Drop-out dyads had slightly lower PSS scores in late pregnancy (t = −2.05, p = .043) and were more often of female infant sex (Χ2 = 6.1, p = .017). All study procedures were approved by the university's IRB, and all participants (pregnant women, and parents on behalf of their infants) provided written informed consent. The demographic characteristics of the sample are provided in Table 1.
2.2. Procedure
Participants were recruited in early pregnancy (T1: mean (SD) = 12.8 (1.8) weeks of gestation) and followed with serial assessments in mid (T2: mean (SD) = 20.5 (1.4) weeks of gestation) and late pregnancy (T3: mean (SD) = 30.4 (1.3) weeks of gestation). Gestational age was confirmed by obstetric ultrasonographic biometry using standard clinical criteria (O'Brien et al., 1981). Study visit procedures included administration of structured socio-demographic and psychosocial interviews and questionnaires and abstraction of medical and previous obstetric history. Shortly after birth, MRI of the brain was performed in newborns during natural sleep. At 6 and 12 months of age, infant social-emotional development and environmental conditions were assessed with questionnaires and standardized/structured observational tests.
2.3. Maternal perceived stress in pregnancy
Maternal perceived stress was assessed at each pregnancy visit with the Perceived Stress Scale (PSS; Cohen et al., 1983). The 10-item PSS is a well-established and widely used self-report instrument that assesses the degree to which the respondent appraises situations in life as stressful, with a particular focus on the critical dimensions of unpredictability and uncontrollability. Data was available from at least two time points during pregnancy from all participants and 90.7% of participants had complete data from all three time points. Trimester-specific PSS scores did not change significantly across gestation (F2,145 = 1.68, p > .1) and showed moderate to high intercorrelations (all r > 0.6). Thus, a mean PSS score across pregnancy was computed for each subject and used in subsequent analyses (see descriptive statistics in Table 1 and the associations between the PSS at each time point during pregnancy and the outcomes in the Supplement).
2.4. Image acquisition and pre-processing
MRI was performed in the newborns during natural sleep using a Siemens 3T scanner (TIM Trio, Siemens Medical System Inc., Germany) at UCI Health Nikken Imaging Center. Before scanning, newborns were fed, swaddled, fitted with ear protection, and had their heads secured in a vacuum-fixation device (www.civco.com). A physician or nurse was present during each scan; a pulse oximeter was used to monitor heart rate and oxygen saturation.
T1-weighted images were obtained using a three-dimensional (3D) magnetization prepared rapid gradient echo (MP-RAGE) sequence (TR 2400 ms; TE 3.16 ms; TI 1200 ms; Flip Angle 8°; 6:18 min) and T2-weighted images were obtained with a turbo spin echo sequence (TR 3200 ms; TE1 13 ms; TE2 135 ms; Flip Angle 180°; 4:18 min). The spatial resolution was a 1 × 1 × 1 mm voxel for T1-weighted images and 1 × 1 × 1 mm voxel with 0.5 mm interslice gap for T2-weighted images. In N = 114 neonates an attempt was made to acquire an MRI scan; the complete MRI sequence was obtained in N = 94 neonates. Scan acquisition was not attempted in N = 17 infants because they did not fall asleep during the MRI study visit. Image quality control (QC) feedback was provided using a four point scale (1–4) developed for infant scanning, and based on a widely used visual QC protocol (Blumenthal et al., 2002). Criteria for exclusion was a QC score of 4 (N = 6 excluded), representing subjects with artifact contamination to a degree that renders image processing unreliable. A further N = 2 had to be excluded due to significant abnormalities as reviewed by a clinical neuroradiologist resulting in a final sample of N = 86 neonates for the current analyses.
Hippocampus and amygdala segmentation was performed using a multi-modality, multi-template based automatic method combining T1-and T2-weighted high-resolution images (Wang et al., 2014), followed by manual correction in ITK-Snap (Yushkevich et al., 2006). Scan/rescan stability tests for the automatic segmentation procedure conducted in a separate sample set indicated reliable and stable results at coefficients of variance <0.4% for all structures. Manual corrections were performed with data re-aligned such that the anterior-posterior direction was positioned along hippocampal long axis. Images were segmented in both original and left-right mirrored presentation to account for asymmetric presentation biases (Maltbie et al., 2012) and averaged for the final segmentation. Reliability for manual correction was established for raters on this dataset via a standard reliability study, in which 5 datasets were triplicated and randomized. These 15 datasets were then segmented automatically and manually corrected by two raters. Inter-rater correlation coefficients between the two raters and intra-rater correlation coefficients were established and were both high at r > 0.98.
Tissue segmentation was performed using a multi-atlas based iterative expectation maximization segmentation algorithm as previously described (Cherel et al., 2015; Gilmore et al., 2007). Brain tissue was classified as gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Taken together, these three tissue volumes comprise the intracranial volume (ICV). The descriptive statistics of hippocampi and ICV are shown in Table 1.
Bilateral HCV and AGV were corrected for ICV, length of gestation and postnatal age at MRI scan; the two latter variables were considered instead of a single variable representing postmenstrual age because of their different influence on the developing brain (Rasmussen et al., 2017). The correlation between length of gestation and postnatal age at MRI scan was r = −0.24, p = .024; the correlations for age at scan and HCV were r = 0.38 to 0.40, p < .001 and r = 0.22–0.25, p < .05 for length of gestation and HCV r = 0.32 to 0.37, p < .001, and for ICV and HCV r = 0.74 to 0.75, p < .001. The correlations for the age variables, ICV and AGV were r = 0.28 to 0.36, p < .01, r = 0.22 to 0.25, p < .05 and r = 0.65 to 0.74, p < .001, respectively. The residualized volumes were used in all analyses. Additionally, sensitivity analyses were performed excluding one neonate with bilateral HCV volumes that were relatively large compared to the HCV volumes of the other neonates included in the study, as well as the n = 7 neonates born before 37 weeks’ gestation (see more details in the section Statistical analysis).
2.5. Infant social-emotional development
At 6 and 12 months of age, infant social-emotional development was assessed using the Social-Emotional Scale of the Bayley Scales of Infant Development – Third Edition (Bayley, 2006). The Social-Emotional Scale is a standardized parent-report measure that was adapted from the Greenspan Social-Emotional Growth Chart (Greenspan, 2004). It assesses acquisition of social and emotional milestones in young children, including self-regulation and interest in the world, engagement in relationships and use of emotions in an interactive, purposeful manner. Additional information regarding the Bayley Social-Emotional Scale is provided in the Supplement. The age-adjusted scaled score (mean: 10 ± 3) was used in the analyses. Descriptive statistics of infant social-emotional development are provided in Table 1.
2.6. Covariates
A priori selection of the covariates was based on theoretical considerations and empirical evidence of possible associations with either both the outcome and primary predictor of interest (to account for potential confounding) or with only the outcome (to improve model precision). In addition to residualizing HCV for gestational length, postnatal age at scan and ICV, the following covariates were considered in statistical analyses: maternal socio-economic status (SES), obstetric complications in the index pregnancy, maternal smoking during pregnancy, infant sex assigned at birth, postnatal maternal behavior/care (i.e., maternal sensitivity).
Maternal socio-economic status (SES) was computed as the mean of maternal educational level (originally assessed in categories from less than high school to advanced degree and then recoded into values from 1 through 5) and household income (originally assessed in categories from ≤ 15,000$ to ≥ 100,000$ and then recoded into values from 1 through 5).
Presence of major obstetric risk complications in the index pregnancy (i.e., infection, hypertension, diabetes, anemia, vaginal bleeding) and pre-pregnancy body-mass-index (BMI) were abstracted from the antepartum and delivery medical records. A binary variable indicating presence of any obstetric risk factor vs. no obstetric risk factor was created and used in the analyses.
Maternal smoking during pregnancy was determined by maternal self-report and verified by measurement of urinary cotinine (COT) concentration. Urinary COT was assayed in maternal samples collected at each trimester using the Nicotine/COT(Cotinine)/Tobacco Drug Test Urine Cassette (http://www.meditests.com/nicuintescas.html). A binary variable was created indicating endorsement of smoking or detection of urinary COT in any trimester vs. absence of evidence for smoking in any trimester.
Gestational age at birth and infant sex were abstracted from the delivery medical records.
To control for postnatal maternal behavior/care, maternal sensitivity was assessed at 6 months of age with a semi-structured play situation in the infant's natural home environment that was recorded on videotape (duration 15 min). Two trained observers coded maternal sensitivity to non-distress, positive regard and intrusiveness using the coding manual of the NICHD Early Child Care Research Network (1999) (inter-rater reliability (ICC): 0.97–1). The scores were summed up (intrusiveness was inverted) to a total sensitivity score (M±SD = 10.17 ± 2.85, range: 3–15, n = 76), which was then used in the analysis.
2.7. Statistical analyses
First, the bivariate associations between maternal perceived stress, residualized neonatal HCV and AGV, social-emotional development and the covariates were quantified. Next, multiple linear regression analyses was performed to examine whether maternal perceived stress during pregnancy was associated with neonatal left and right HCV and AGV size after adjusting for pre-selected covariates: infant sex, maternal SES, maternal smoking during pregnancy and maternal obstetric complications during pregnancy. This analysis was conducted with and without the neonate who had a relatively large HCV, as well as the neonates born before 37 weeks’ gestation, to determine whether the observed associations between maternal PSS and neonatal HCV and AGV were affected by the inclusion of these infants. Furthermore, analyses were conducted including use of psychotropic medication and alcohol use as covariates. The results of these sensitivity analyses are presented in the Supplement.
Linear mixed effects models were performed to examine whether neonatal HCV and AGV were associated with infant social-emotional development at 6 and 12 months of age after controlling for pre-selected covariates: SES, maternal sensitivity and infant sex. Analyses were performed separately for left and right HCV as well as left and right AGV as the predictors of interest.
In the last step, a mediation analysis was performed using PROCESS for SPSS v 2.16 (www.afhayes.com). A mediation of the effect between maternal PSS and the mean social-emotional development at 6 and 12 months of age via HCV was tested, while adjusting for the afore-mentioned covariates. A bias-corrected bootstrap confidence interval (BC 95% CI) for the indirect effect was estimated based on 10,000 bootstrap samples.
3. Results
3.1. Maternal PSS during pregnancy and neonatal HCV and AGV
Bivariate associations between predictors/outcomes of interest and continuous covariates are summarized in Table 2. The bivariate associations between maternal PSS and neonatal HCV showed a significant negative association between maternal PSS and left but not right neonatal HCV. No association was detected between maternal PSS and AGV. Maternal SES was not significantly associated with HCV or AGV. Maternal sensitivity and maternal SES were significantly positively associated with M12 but not M6 social-emotional development score. Regarding the dichotomous covariates, no significant differences in corrected HCV or AGV were observed by infant sex, smoking or obstetric risk (p > .05). Infant M6 and M12 social-emotional scores did not differ by sex (p > .05). Furthermore, there was no association between PSS and age-corrected ICV (B = −0.03, p = .790).
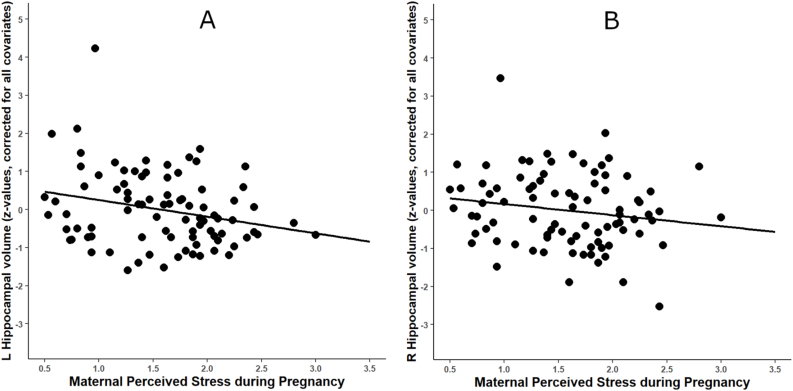
The results of the regression analyses are displayed in Table 3. When adjusting for the covariates, higher maternal PSS remained significantly associated with smaller neonatal left HCV, 1 SD change in PSS corresponding to 0.26 SD reduction in the age- and ICV-corrected left HCV (β = −0.260, p = .019, ΔR2 = 0.06). The association between PSS and neonatal right HCV was non-significant when adjusting for covariates (β = −0.170, p = .121; see Fig. 1), and no significant associations were found between PSS and bilateral AGV (ps > .542). No significant interaction between PSS and sex on left or right HCV or AGV were observed (Table 3).
Fig. 1.
Association between maternal perceived stress during pregnancy and newborn hippocampal volume. Note. Displayed are associations between PSS and (A) left (p = .019) and (B) right HCV (p = .12), N = 86; HCV values in the figures are corrected for postnatal age at scan, gestational age, ICV, infant sex, maternal smoking during pregnancy and maternal obstetric complications.
3.2. Neonatal HCV and AGV and infant social-emotional development
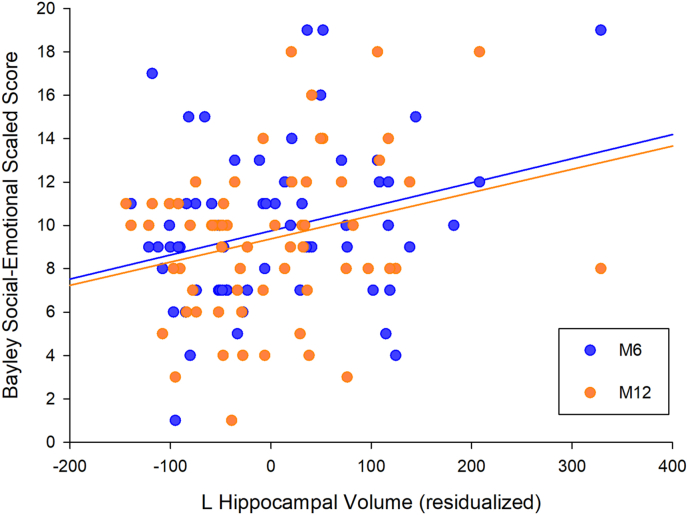
The zero-order associations between neonatal HCV and AGV and infant social-emotional development are presented in Table 2. Right HCV was not significantly associated with infant social-emotional development at any time point. Left HCV was significantly positively associated with both M6 and M12 infant social-emotional development. After adjusting for the covariates, infant left HCV was significantly positively associated with social-emotional development scores across the first year (between-subjects B = 0.011, 95% CI: 0.003 - 0.019, p = .010, N = 76; see Fig. 2 and Table 4). Infant right HCV was not significantly associated with infant social-emotional development across the first year in adjusted models (between-subjects B = 0.003, 95% CI: 0.004 - 0.011, p = .427, N = 76; see Table 4). Including an interaction term between time of social-emotional development assessment and HCV did not improve model fit and neither left nor right HCV predicted change in social-emotional development during the first year of life.
Fig. 2.
Association between newborn left hippocampal volume and social-emotional development. Note. Scatterplot depicting the association between left HCV corrected for postnatal age at scan, gestational age at birth and ICV and Bayley Social-Emotional scores at 6 months (blue) and 12 months of age (orange). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, neither left nor right AGV volumes were associated with social-emotional development in bivariate analyses (see Table 2) and in models adjusting for relevant covariates (left: between-subjects B = 0.015, 95% CI: 0.019 - 0.049, p = .379; right: between-subjects B = −0.003, 95% CI: 0.034 - 0.027, p = .843, N = 76).
3.3. Mediation analysis
Based on the above-described significant associations between maternal PSS and newborn left HCV and between newborn left HCV and infant social-emotional development as well as the negative association between PSS and social-emotional development observed in bivariate associations (see Table 2), a formal mediation analysis was conducted to examine the pathway between maternal PSS and infant social-emotional development via newborn left HCV. Controlling for all covariates included in the previous statistical models, higher maternal PSS during pregnancy was significantly indirectly associated with lower infant social-emotional development through its effect on newborn left HCV (B = −0.34, SE = 0.23, 95% CIBC [-0.97, −0.01], N = 76). Due to the small sample size, these results should, however, be interpreted as exploratory.
4. Discussion
In this study we report an inverse association between maternal perceived stress levels across pregnancy and offspring newborn HCV. While the direction of the association was similar for the right hippocampus, only the association with left HCV was statistically significant. Furthermore, left, but not right, HCV was positively associated with social-emotional development measured at two time points across the first year of life, and newborn left HCV mediated the association between maternal perceived stress in pregnancy and infant social-emotional development. No associations between maternal perceived stress and newborn AGV were detected, and AGV was also not associated with infant social-emotional outcomes.
A major limitation in previous studies investigating fetal programming of brain development by characterizing the developing brain in childhood or at later life stages is the difficulty of controlling the quality of the postnatal environment which may lead to confounding of the association between the prenatal environment and the developing brain. In the present study, hippocampal and amygdalar structure were measured in neonates, which limits the possible influence of the postnatal environment on offspring brain development. Thus, the reported results suggest a programming effect of maternal psychosocial distress on hippocampus that appears to have occurred during the intrauterine period. The findings regarding HCV are in line with several prior animal studies (Badihian et al., 2019; Charil et al., 2010) and a recent study employing fetal MRI in human pregnancy that reported an inverse association between maternal stress and anxiety and left fetal HCV (Wu et al., 2020). However, two other studies report no effect of maternal anxiety and depression symptoms on newborn HCV (Lehtola et al., 2020; Qiu et al., 2013). Instead, in the study by Qiu et al. (2013), antenatal anxiety was related to slower hippocampal growth over the first 6 months of life, and Ong et al. (2019) observed in the same cohort that the association between prenatal distress and HCV was moderated by genetic factors. Maternal perceived stress during pregnancy was not associated with offspring AGV in the present study, which is in concordance with observations by previous studies (Donnici et al., 2021; Rifkin-Graboi et al., 2013). Others have reported associations between maternal distress during pregnancy and offspring AGV that differed by sex (Acosta et al., 2019; Lehtola et al., 2020; Wen et al., 2017). Such moderation of the association between maternal distress during pregnancy and offspring AGV by offspring sex was not observed in the present study.
These discrepancies between the findings of the present study and those of previous studies may be due to the use of different measures of maternal distress during pregnancy. Although anxiety, depression and perceived stress share a large amount of variance, they do not represent identical constructs and thus may have differential effects on maternal-placental-fetal stress biology and consequently on the developing fetal brain. Furthermore, in contrast to some or all previous studies, the present study measured maternal psychosocial stress serially across pregnancy and employed multi-contrast (i.e., using both T1-and T2-weighted MR images) semi-automated segmentation to quantify newborn HCV and AGV, thereby improving the estimates of the predictor and outcome of interest.
In our study, maternal perceived stress explained 6% of the variation in the age- and ICV-adjusted left HCV. It is challenging to place the magnitude of this observed effect in the context of previous findings in the literature, given the small number of studies of this or similar research questions, differences in statistical approach, and/or absence of reported effect size estimates. However, it is apparent that the size of the effect of maternal perceived stress on newborn HCV in our study is consistent with that reported in studies of maternal psychosocial stress and other subcortical structures (Lehtola et al., 2020), and also with a study reporting a prospective association between serial measurements of perceived stress across a 20-year period and HCV in postmenopausal women (Gianaros et al., 2007). Moreover, the effect in our study of maternal stress on infant social-emotional development via offspring HCV may portend clinical relevance. Our association between HCV and social-emotional development is similar in size to that reported in previous studies linking hippocampal size with emotion regulation and empathic responding (Barch et al., 2019; Stern et al., 2019), and, as noted earlier, HCV-related social-emotional competence in childhood represents a key prognosticator of subsequent mental health and well-being (Bornstein et al., 2010; Burt et al., 2008). Thus, maternal psychosocial distress during pregnancy may, in part, program inter-individual differences in HCV that may in turn determine the vulnerability for psychopathology across the lifetime (Lupien et al, 2007, 2018).
There are several potential mechanisms underlying the observed association between maternal perceived stress during pregnancy and offspring HC volume. Due to its high number of glucocorticoid receptors, the hippocampus is a brain structure that is particularly sensitive to the influences of glucocorticoids (Aronsson et al., 1988; McEwen et al., 1992), and maternal cortisol concentrations have been associated with brain functional connectivity shortly after birth (Graham et al., 2019). Cortisol is a stress hormone secreted by the HPA axis specifically in response to situations with low controllability and predictability as well as high stressor chronicity (Dickerson and Kemeny, 2004), i.e. situations that are assessed with serial administrations of the PSS. However, previous studies in humans have not always detected an association between maternal cortisol concentrations during pregnancy and child hippocampal size (C. Buss et al., 2012). At the same time, there is conflicting evidence regarding the association between maternal psychosocial distress and cortisol concentrations during pregnancy (Harville et al., 2009; Himes and Simhan, 2011; Kalra et al., 2007; Seth et al., 2016), which may be due to large interindividual variability that masks intra-individual responses to stressful conditions in cross-sectional approaches (Lazarides et al., 2020). Other potential pathways underlying the association between maternal perceived stress during pregnancy and offspring HC volume include changes in the maternal-fetal-placental inflammatory milieu that is influenced by maternal distress (Coussons-Read et al., 2007) or reduced utero-placental perfusion in response to psychosocial distress, which may lead to fetal developmental alterations (Levine et al., 2016). For instance, prior studies have proposed that the levels of inflammatory cytokines may affect neonatal limbic structure volumes and functional connectivity, which, in turn, was associated with child cognitive development (Graham et al., 2018; Rudolph et al., 2018). These stress-induced changes in endocrine and immune maternal-placental-fetal biology may alter fetal hippocampal development for example by reducing the expression of mature brain-derived neurotrophic factor (mBDNF) and total BDNF micro-RNA (Badihian et al., 2019) as well as by increasing microglial activity in the hippocampus (Calcia et al., 2016).
The current study detected an association only between PSS and the left HCV as well as left HCV and infant social-emotional development; however, both, the left and right HCV showed links with the predictor and the outcomes in the same direction. Similar laterality effects on HCV have been previously observed. Wu et al. (2020) reported a significant inverse association of prenatal psychosocial distress with left but not right HCV. Structural asymmetries are likely linked with functional and biochemical asymmetries which may underlie the lateralized effects of exposure to maternal psychosocial distress. For instance, one of the main target receptors for glucocorticoids, the mineralocorticoid receptor (MR), exhibits asymmetrical distribution in the hippocampus, with a higher binding capacity in the right hippocampus (Neveu et al., 1998). The MR has been reported to mediate enhancement of neurogenesis and dendritic arborization (Fujioka et al., 2006) and to be downregulated in response to prenatal stress (Tamura et al., 2011). Thus, due to its overall higher MR levels, the right hippocampus may be somewhat protected from the inhibitory effects of stress on neuronal maturation via MR. Furthermore, the hippocampus also shows functional asymmetry in its associations with behavior and cognition (Hou et al., 2013; Robinson et al., 2016). Interestingly, some studies indicate that although both left and right hippocampi are involved in subcortical networks including the amygdala, the left hippocampus might be more engaged in these networks (Robinson et al., 2016), which would indicate a functional asymmetry of the hippocampus in neural circuits related to stress- and emotion regulation. However, empirical evidence on the functional asymmetry of the hippocampus in young children or infants is scarce. Further studies are needed to elucidate the mechanisms underlying the lateralized effects observed by the present study.
Finally, we observed a positive association between neonatal HCV and the attainment of social-emotional milestones across two time points within the first year of life, suggesting smaller neonatal hippocampal size, which may be programmed by higher levels of maternal perceived stress, may be related to delayed social-emotional development. Importantly, this association remained significant after controlling for maternal caregiving quality – a major contributor to offspring social-emotional development. We formally tested mediation by newborn HCV of the pathway between maternal perceived stress in pregnancy and infant social-emotional development and observed a significant indirect effect. Considering that the hippocampus plays an important role in stress regulation and is involved in processing social emotions and social behavior (Immordino-Yang and Singh, 2013; Rubin et al., 2014), this observation further strengthens the assumption of long-lasting prenatal programming of hippocampal function. Interestingly, the association between maternal perceived stress and infant social-emotional development was less strong at 12 months as compared to 6 months, which suggests that with increasing temporal distance to the exposure, other environmental determinants of social-emotional behavior may become increasingly relevant.
No association was observed between newborn AGV and infant social-emotional development across the first year of life. This may be due to different functions of the amygdala and hippocampus in stress regulation and social-emotional processing as well as the fact that the hippocampus matures faster than the amygdala. The specific role of the hippocampus and amygdala for different aspects of social-emotional functioning should be investigated longitudinally in infancy and childhood to gain a better understanding of their respective roles during different developmental phases.
The main limitation of the current study is the relatively small sample size, which limited the ability to reliably test mediation effects among the identified path and thus, findings from the mediation model need to be interpreted with caution. The observations should be replicated in larger samples with serial assessments of maternal distress during pregnancy, serial MRI scans of the developing brain and of social-emotional milestones before drawing final conclusions regarding mediating pathways. Another limitation is the relatively large age range of the neonates that underwent an MRI scan. Although we used HCV and AGV residualized for gestational age at birth and postnatal age at scan in all analyses and maternal perceived stress was not associated with either gestational length or age at scan, we cannot preclude that some unmeasured aspects of the postnatal environment may have affected neonatal brain volumes to a stronger degree with more time spent ex utero. Future studies should also include assessments of social-emotional development with observational measures. In the present study, social-emotional milestones were assessed via maternal report, which, while increasing ecological validity, may also be influenced by maternal perceptions and expectations of the infant. However, since we investigated the association between social-emotional development and an objective measure of HCV at birth, it appears unlikely that the observed association is the result of maternal biases. In addition, although a variety of covariates were included in the statistical models, it cannot be precluded that other, unmeasured variables (e.g., genetic factors) may have influenced the associations observed here.
5. Conclusion
To conclude, this is one of the first studies to demonstrate prospectively in humans that maternal perceived stress during pregnancy is associated with smaller newborn HCV, which in turn is related to infant social-emotional development across the first year of life. No associations between maternal perceived stress and AGV were detected in the current study. Thus, variation in newborn hippocampal morphology may be one mechanism through which maternal psychosocial distress during pregnancy may be related to her infant's social-emotional development in the first year of life. This finding has important implications for the development of timely and effective intervention strategies to prevent or attenuate the potential neurobiological and behavioral consequences of maternal psychosocial distress during pregnancy in the next generation.
CRediT authorship contribution statement
Nora K. Moog: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization. Saara Nolvi: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization. Theresa S. Kleih: Conceptualization, Formal analysis, Writing – review & editing. Martin Styner: Software, Writing – review & editing. John H. Gilmore: Software, Writing – review & editing. Jerod M. Rasmussen: Conceptualization, Investigation, Data curation, Writing – review & editing. Christine M. Heim: Writing – review & editing. Sonja Entringer: Conceptualization, Writing – review & editing, Project administration, Funding acquisition. Pathik D. Wadhwa: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition. Claudia Buss: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
None.
Acknowledgements
This work was supported, in part, by US PHS (NIH) grants R01 HD-060628, R01 MH-105538, UH3 OD-023349 and ERC grants ERC-Stg 639766, ERC-Stg 678073, Alexander von Humboldt Foundation and Emil Aaltonen Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100368.
Appendix
Table 1.
Sociodemographic characteristics of the sample
| Complete sample N = 86 | Mean ± SD or N (%) | Observed range |
|---|---|---|
| Maternal characteristics | ||
| Maternal age in 1st trimester, years | 28.0 ± 5.4 | 18–40 |
| Parity (primiparous) | 24 (39.5) | |
| SES index (education and income combined) | 3.1 ± 0.9 | 1.00–5.00 |
| Maternal race/ethnicity | ||
| Non-Hispanic White | 35 (40.7) | |
| Hispanic White | 27 (31.3) | |
| Other | 23 (26.7) | |
| Missing | 1 (1.2) | |
| Educational level | ||
| Less than high school | 3 (3.5) | |
| High school | 18 (20.9) | |
| Partial college or specialized training | 38 (44.2) | |
| Associate/Bachelor's degree | 18 (20.9) | |
| Advanced | 9 (10.5) | |
| Yearly income per household | ||
| Below $15,000 | 9 (10.5) | |
| $15,000–$29,999 | 19 (22.1) | |
| $30,000–$49,999 | 19 (22.1) | |
| $50,000–$100,000 | 30 (34.9) | |
| Over $100,000 | 5 (5.8) | |
| Missing | 4 (4.7) | |
| Maternal smoking during pregnancy | 9 (10.5) | |
| Obstetric complications, any | 20 (23.3) | |
| Severe infection | 7 (8.1) | |
| Hypertension | 1 (1.2) | |
| Diabetes | 4 (4.7) | |
| Anemia | 4 (4.7) | |
| Vaginal bleeding | 5 (5.8) | |
| PSS (mean across pregnancy) | 15.8 ± 5.6 | 5–30 |
| T1 PSS | 15.6 ± 6.0 | 1–33 |
| T2 PSS | 14.9 ± 6.7 | 4–35 |
| T3 PSS | 16.4 ± 6.3 | 3–29 |
| Child characteristics | ||
| Gestational age at birth, weeks | 39.2 ± 1.5 | 35–42 |
| Birthweight, grams | 3323 ± 515 | 1786–4906 |
| Age at MRI scan, days | 25.8 ± 12.9 | 5–64 |
| Child sex (male) | 51 (59.3) | |
| Intracranial volume (cm3) | 486.8 ± 58.8 | 355.9–639.8 |
| Hippocampal volume, right (cm3) | 1.20 ± 0.14 | 0.93–1.59 |
| Hippocampal volume, left (cm3) | 1.16 ± 0.14 | 0.92–1.49 |
| Amygdalar volume, right (cm3) | 0.28 ± 0.03 | 0.22–0.36 |
| Amygdalar volume, left (cm3) | 0.27 ± 0.03 | 0.20–0.36 |
| M6 Bayley social-emotional score, standardized | 9.77 ± 3.71 | 1–18 |
| M12 Bayley social-emotional score, standardized | 9.35 ± 3.63 | 1–19 |
Note. SES = socio-economic status; PSS = Perceived Stress Scale; MRI = magnetic resonance imaging.
Table 2.
Bivariate associations between hippocampal volume, amygdalar volume, maternal perceived stress, infant social-emotional development and pre-selected continuous covariates
| Right HCVres | Left HCVres | Right AGVres | Left AGVres | PSS | SES | M6 SE | M12 SE | |
|---|---|---|---|---|---|---|---|---|
| Right HCVres | ||||||||
| Left HCVres | .71** | |||||||
| Right AGVres | .30** | .19 | ||||||
| Left AGVres | .32** | .18 | .69** | |||||
| Maternal PSS | -.14 | -.24* | -.04 | .04 | ||||
| SES | -.07 | -.05 | -.03 | -.06 | -.18† | |||
| M6 Bayley SE score | .09 | .27* | -.07 | .02 | -.30** | .11 | ||
| M12 Bayley SE score | .13 | .26* | .01 | .13 | -.19 | .30* | .51** | |
| Maternal sensitivity | -.02 | .00 | -.16 | -.11 | .00 | .34** | .15 | .36** |
Note. **p < .01, *p < .05, †p < .10. HCV = hippocampal volume; AGV = amygdalar volume, res = residualized for age, length of gestation and intracranial volume; PSS = perceived stress scale, SES = socioeconomic status, SE = social-emotional. The associations with Bayley outcomes are examined within the mother-infant dyads with available data on social-emotional development (M6 N = 73 and M12 N = 63).
Table 3.
The linear regression models for left and right hippocampal and amygdalar volumes
| Left HCVres |
Right HCVres |
Left AGVres |
Right AGVres |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | p | B | SE | β | p | B | SE | β | p | B | SE | β | p | |
| Infant sex (girl) | −18.99 | 18.07 | −0.11 | .296 | −23.25 | 20.68 | −0.12 | .264 | −9.25 | 4.89 | −0.21 | 0.06 | −5.77 | 5.39 | −0.12 | .287 |
| Maternal smoking | 26.37 | 29.15 | 0.10 | .368 | 37.13 | 33.37 | 0.12 | .269 | 13.98 | 7.89 | 0.19 | 0.08 | 5.42 | 8.69 | 0.70 | .624 |
| SES | −8.18 | 9.88 | −0.09 | .410 | −8.54 | 11.31 | −0.08 | .452 | −0.79 | 2.67 | −0.03 | .768 | −0.82 | 2.94 | −0.03 | .782 |
| OB risk (ref: no risk) | 23.89 | 20.95 | 0.12 | .258 | 31.15 | 23.98 | 0.14 | .184 | −0.24 | 5.67 | −0.01 | .966 | 6.43 | 6.24 | 0.11 | .306 |
| PSS | −38.78 | 16.21 | −0.26 | .019 | −29.07 | 18.55 | −0.17 | .121 | 2.68 | 4.38 | 0.07 | .542 | −1.53 | 4.83 | −0.04 | .753 |
| PSS × infant sex | −43.03 | 32.93 | −0.63 | .195 | −46.51 | 37.73 | −0.60 | .221 | 4.89 | 8.98 | 0.27 | .588 | 5.33 | 9.90 | 0.273 | .592 |
Note. HCV = hippocampal volume, AGV = amygdalar volume, res = residualized for age, length of gestation and intracranial volume; SES = socioeconomic status, OB = obstetric, PSS = Perceived Stress Scale. The PSS by infant sex analyses were conducted in a separate step of the model.
Table 4.
Fixed effects estimates from the linear mixed effects models investigating the association between hippocampal volume and social-emotional development.
| Left HCVres |
Right HCVres |
|||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | t | p | B | SE | t | p | |
| Intercept | 5.38 | 1.77 | 3.05 | .003 | 5.55 | 1.84 | 3.02 | .003 |
| time | −0.16 | 0.48 | −0.34 | .737 | −0.19 | 0.48 | −0.39 | .696 |
| SES | 0.57 | 0.42 | 1.37 | .175 | 0.44 | 0.44 | 1.00 | .319 |
| Infant sex (ref: female) | 0.31 | 0.73 | 0.43 | .670 | 0.48 | 0.77 | 0.63 | .529 |
| Maternal sensitivity | 0.27 | 0.13 | 1.88 | .065 | 0.27 | 0.14 | 1.94 | .057 |
| HCVres | 0.01 | 0.00 | 2.61 | .011 | 0.00 | 0.00 | 0.80 | .427 |
Note. Analyses are performed separately for left and right HCV as predictor of interest. HCV = hippocampal volume, res = residualized for age, length of gestation and intracranial volume; SES = socioeconomic status.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Acosta H., Tuulari J.J., Scheinin N.M., Hashempour N., Rajasilta O., Lavonius T.I., Pelto J., Saunavaara V., Parkkola R., Lähdesmäki T., Karlsson L., Karlsson H. Maternal pregnancy-related anxiety is associated with sexually dimorphic alterations in amygdala volume in 4-year-old children. Front. Behav. Neurosci. 2019;13 doi: 10.3389/fnbeh.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.E., Trojanowski J.Q. Human fetal hippocampal development: I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J. Comp. Neurol. 1996;367:274–292. doi: 10.1002/(SICI)1096-9861(19960401)367:2%3c274::AID-CNE9%3e3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Aronsson M., Fuxe K., Dong Y., Agnati L.F., Okret S., Gustafsson J.A. Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc. Natl. Acad. Sci. Unit. States Am. 1988;85:9331–9335. doi: 10.1073/pnas.85.23.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badihian N., Daniali S.S., Kelishadi R. Transcriptional and epigenetic changes of brain derived neurotrophic factor following prenatal stress: a systematic review of animal studies. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.12.018. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Harms M.P., Tillman R., Hawkey E., Luby J.L. Early childhood depression, emotion regulation, episodic memory and hippocampal development. J. Abnorm. Psychol. 2019;128:81–95. doi: 10.1037/abn0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros V.G., Duhalde-Vega M., Caltana L., Brusco A., Antonelli M.C. Astrocyte–neuron vulnerability to prenatal stress in the adult rat brain. J. Neurosci. Res. 2006;83:787–800. doi: 10.1002/jnr.20758. [DOI] [PubMed] [Google Scholar]
- Bayley N. third ed. The Psychological Corporation; San Antonio, TX: 2006. Bayley Scales of Infant and Toddler Development. [Google Scholar]
- Blumenthal J.D., Zijdenbos A., Molloy E., Giedd J.N. Motion artifact in magnetic resonance imaging: implications for automated analysis. Neuroimage. 2002;16:89–92. doi: 10.1006/nimg.2002.1076. [DOI] [PubMed] [Google Scholar]
- Bornstein M.H., Hahn C.-S., Haynes O.M. Social competence, externalizing, and internalizing behavioral adjustment from early childhood through early adolescence: developmental cascades. Dev. Psychopathol. 2010;22:717–735. doi: 10.1017/S0954579410000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt K.B., Obradović J., Long J.D., Masten A.S. The interplay of social competence and psychopathology over 20 years: testing transactional and cascade models. Child Dev. 2008;79:359–374. doi: 10.1111/j.1467-8624.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss Claudia, Entringer S., Wadhwa P.D. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci. Signal. 2012;5 doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Lord C., Wadiwalla M., Hellhammer D.H., Lupien S.J., Meaney M.J., Pruessner J.C. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J. Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia M.A., Bonsall D.R., Bloomfield P.S., Selvaraj S., Barichello T., Howes O.D. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl.) 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charil A., Laplante D.P., Vaillancourt C., King S. Prenatal stress and brain development. Brain Res. Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Cherel M., Budin F., Prastawa M., Gerig G., Lee K., Buss C., Lyall A., Consing K.Z., Styner M. Automatic tissue segmentation of neonate brain MR images with subject-specific atlases. Proc. SPIE-Int. Soc. Opt. Eng. 2015;9413 doi: 10.1117/12.2082209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe C.L., Kramer M., Czéh B., Gould E., Reeves A.J., Kirschbaum C., Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile Rhesus monkeys. Biol. Psychiatr. 2003;54:1025–1034. doi: 10.1016/S0006-3223(03)00698-X. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Coussons-Read M.E., Okun M.L., Nettles C.D. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav. Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Davis E.P., Hankin B.L., Glynn L.M., Head K., Kim D.J., Sandman C.A. Prenatal maternal stress, child cortical thickness, and adolescent depressive symptoms. Child Dev. 2020;91:e432–e450. doi: 10.1111/cdev.13252. [DOI] [PubMed] [Google Scholar]
- De Asis-Cruz J., Krishnamurthy D., Zhao L., Kapse K., Vezina G., Andescavage N., Quistorff J., Lopez C., Limperopoulos C. Association of prenatal maternal anxiety with fetal regional brain connectivity. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22349. e2022349–e2022349. [DOI] [PubMed] [Google Scholar]
- Dean D.C., III, Planalp E.M., Wooten W., Kecskemeti S.R., Adluru N., Schmidt C.K., Frye C., Birn R.M., Burghy C.A., Schmidt N.L., Styner M.A., Short S.J., Kalin N.H., Goldsmith H.H., Alexander A.L., Davidson R.J. Association of prenatal maternal depression and anxiety symptoms with infant white matter microstructure. JAMA Pediatr. 2018;172:973–981. doi: 10.1001/jamapediatrics.2018.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Donnici C., Long X., Dewey D., Letourneau N., Landman B., Huo Y., Lebel C. Prenatal and postnatal maternal anxiety and amygdala structure and function in young children. Sci. Rep. 2021;11:4019. doi: 10.1038/s41598-021-83249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Buss C., Wadhwa P.D. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Tottenham N. Effects of early life stress on amygdala and striatal development. Dev. Cogn. Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro A., Tenconi E., Degortes D., Manara R., Santonastaso P. Neural correlates of prenatal stress in young women. Psychol. Med. 2015;45:2533–2543. doi: 10.1017/S003329171500046X. [DOI] [PubMed] [Google Scholar]
- Fujioka A., Fujioka T., Ishida Y., Maekawa T., Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141:907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Ge X., Shi Y., Li J., Zhang Z., Lin X., Zhan J., Ge H., Xu J., Yu Q., Leng Y., Teng G., Feng L., Meng H., Tang Y., Zang F., Toga A.W., Liu S. Development of the human fetal hippocampal formation during early second trimester. Neuroimage. 2015;119:33–43. doi: 10.1016/j.neuroimage.2015.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Jennings J.R., Sheu L.K., Greer P.J., Kuller L.H., Matthews K.A. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Prastawa M.W., Looney C.B., Vetsa Y.S.K., Knickmeyer R.C., Evans D.D., Smith J.K., Hamer R.M., Lieberman J.A., Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Entringer S., Ward E.B., Rudolph M.D., Gilmore J.H., Styner M., Wadhwa P.D., Fair D.A., Buss C. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatr. 2019;85:172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Rudolph M.D., Heim C.M., Gilmore J.H., Styner M., Potkin S.G., Entringer S., Wadhwa P.D., Fair D.A., Buss C. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 Years of age. Biol. Psychiatr. 2018;83:109–119. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan S.I. PsychCorp; San Antonio, TX: 2004. Greenspan Social-Emotional Growth Chart: a Screening Questionnaire for Infants and Young Children. [Google Scholar]
- Halligan S.L., Cooper P.J., Fearon P., Wheeler S.L., Crosby M., Murray L. The longitudinal development of emotion regulation capacities in children at risk for externalizing disorders. Dev. Psychopathol. 2013;25:391–406. doi: 10.1017/S0954579412001137. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Siemer M., Gotlib I.H. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol. Psychiatr. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville E.W., Savitz D.A., Dole N., Herring A.H., Thorp J.M. Stress questionnaires and stress biomarkers during pregnancy. J. Womens Health. 2009;18:1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Nagaoka M., Yamada K., Ichitani Y., Miake Y., Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int. J. Dev. Neurosci. 1998;16:209–216. doi: 10.1016/S0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Ostrander M.M., Mueller N.K., Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Himes K.P., Simhan H.N. Plasma corticotropin-releasing hormone and cortisol concentrations and perceived stress among pregnant women with preterm and term birth. Am. J. Perinatol. 2011;28:443–448. doi: 10.1055/s-0030-1270119. [DOI] [PubMed] [Google Scholar]
- Hou G., Yang X., Yuan T.-F. Hippocampal asymmetry: differences in structures and functions. Neurochem. Res. 2013;38:453–460. doi: 10.1007/s11064-012-0954-3. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang M.H., Singh V. Hippocampal contributions to the processing of social emotions. Hum. Brain Mapp. 2013;34:945–955. doi: 10.1002/hbm.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E., Ruhleder M., Lange C., Seidler-Brandler U., Salzer S., Dechent P., Weniger G., Leibing E., Leichsenring F. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J. Psychiatry Neurosci. JPN. 2010;35:126–131. doi: 10.1503/jpn.090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F.D., Habas P.A., Kim K., Corbett-Detig J., Xu D., Studholme C., Glenn O.A. Fetal hippocampal development: analysis by magnetic resonance imaging volumetry. Pediatr. Res. 2011;69:425–429. doi: 10.1203/PDR.0b013e318211dd7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N., Yang K., Sun Q., Cai Q., Li H., Cheng D., Fan X., Zhu Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev. Neurobiol. 2010;70:114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- Kalra S., Einarson A., Karaskov T., Uum S.V., Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin. Invest. Med. E103–E107. 2007 doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Chen J., Takahashi T., Ichitani Y., Nakahara D. Prenatal stress suppresses cell proliferation in the early developing brain. Neuroreport. 2006;17:1515–1518. doi: 10.1097/01.wnr.0000236849.53682.6d. [DOI] [PubMed] [Google Scholar]
- Kendig S., Keats J.P., Hoffman M.C., Kay L.B., Miller E.S., Moore Simas T.A., Frieder A., Hackley B., Indman P., Raines C., Semenuk K., Wisner K.L., Lemieux L.A. Consensus bundle on maternal mental health: perinatal depression and anxiety. Obstet. Gynecol. 2017;129:422–430. doi: 10.1097/AOG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier E.L., Kim J.H., Fulbright R.K., Bronen R.A. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am. J. Neuroradiol. 1997;18:525–532. [PMC free article] [PubMed] [Google Scholar]
- Kraszpulski M., Dickerson P.A., Salm A.K. Prenatal stress affects the developmental trajectory of the rat amygdala. Stress. 2006;9:85–95. doi: 10.1080/10253890600798109. [DOI] [PubMed] [Google Scholar]
- Lautarescu A., Craig M.C., Glover V. International Review of Neurobiology. Elsevier; 2020. Prenatal stress: effects on fetal and child brain development; pp. 17–40. [DOI] [PubMed] [Google Scholar]
- Lautarescu A., Pecheva D., Nosarti C., Nihouarn J., Zhang H., Victor S., Craig M., Edwards A.D., Counsell S.J. Maternal prenatal stress is associated with altered uncinate fasciculus microstructure in premature neonates. Biol. Psychiatry, Stress Mechanisms and Fear Memories. 2020;87:559–569. doi: 10.1016/j.biopsych.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides C., Ward E.B., Buss C., Chen W.-P., Voelkle M.C., Gillen D.L., Wadhwa P.D., Entringer S. Psychological stress and cortisol during pregnancy: an ecological momentary assessment (EMA)-Based within- and between-person analysis. Psychoneuroendocrinology. 2020;121:104848. doi: 10.1016/j.psyneuen.2020.104848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walton M., Letourneau N., Giesbrecht G.F., Kaplan B.J., Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children's brain structure in preschool. Biol. Psychiatry, Novel Signaling Mechanisms in Depression. 2016;80:859–868. doi: 10.1016/j.biopsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Lehtola S.J., Tuulari J.J., Scheinin N.M., Karlsson L., Parkkola R., Merisaari H., Lewis J.D., Fonov V.S., Louis Collins D., Evans A., Saunavaara J., Hashempour N., Lähdesmäki T., Acosta H., Karlsson H. Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. NeuroImage Clin. 2020;28:102380. doi: 10.1016/j.nicl.2020.102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V., Koehl M., Le Moal M., Abrous D.N. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T.A., Alderdice F.A., Grunau R.E., McAuliffe F.M. Prenatal stress and hemodynamics in pregnancy: a systematic review. Arch. Womens Ment. Health. 2016;19:721–739. doi: 10.1007/s00737-016-0645-1. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., Evans A., Lord C., Miles J., Pruessner M., Pike B., Pruessner J.C. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., Juster R.-P., Raymond C., Marin M.-F. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front. Neuroendocrinol., Stress and the Brain. 2018;49:91–105. doi: 10.1016/j.yfrne.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Madigan S., Oatley H., Racine N., Fearon R.M.P., Schumacher L., Akbari E., Cooke J.E., Tarabulsy G.M. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57:645–657. doi: 10.1016/j.jaac.2018.06.012. e8. [DOI] [PubMed] [Google Scholar]
- Maltbie E., Bhatt K., Paniagua B., Smith R.G., Graves M.M., Mosconi M.W., Peterson S., White S., Blocher J., El-Sayed M., Hazlett H.C., Styner M.A. Asymmetric bias in user guided segmentations of brain structures. Neuroimage. 2012;59:1315–1323. doi: 10.1016/j.neuroimage.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marečková K., Klasnja A., Bencurova P., Andrýsková L., Brázdil M., Paus T. Prenatal stress, mood, and gray matter volume in young adulthood. Cerebr. Cortex. 2019;29:1244–1250. doi: 10.1093/cercor/bhy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marečková K., Mareček R., Bencurova P., Klánová J., Dušek L., Brázdil M. Perinatal stress and human hippocampal volume: findings from typically developing young adults. Sci. Rep. 2018;8:4696. doi: 10.1038/s41598-018-23046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Téllez R.I., Hernández-Torres E., Gamboa C., Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gould E.A., Sakai R.R. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br. J. Psychiatr. Suppl. 1992:18–23. [PubMed] [Google Scholar]
- McQuaid G.A., Darcey V.L., Avalos M.F., Fishbein D.H., VanMeter J.W. Altered cortical structure and psychiatric symptom risk in adolescents exposed to maternal stress in utero: a retrospective investigation. Behav. Brain Res. 2019;375:112145. doi: 10.1016/j.bbr.2019.112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., Tottenham N., Noble K.G. Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. J. Clin. Child Adolesc. Psychol. 2018;47:312–323. doi: 10.1080/15374416.2017.1326122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Nugent A.C., Drevets W.C., Dickstein D.S., Leibenluft E., Ernst M., Charney D., Pine D.S. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol. Psychiatr. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Müller F., O'Rahilly R. The amygdaloid complex and the medial and lateral ventricular eminences in staged human embryos. J. Anat. 2006;208:547–564. doi: 10.1111/j.1469-7580.2006.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu P.J., Liège S., Sarrieau A. Asymmetrical distribution of hippocampal mineralocorticoid receptors depends on lateralization in mice. Neuroimmunomodulation. 1998;5:16–21. doi: 10.1159/000026322. [DOI] [PubMed] [Google Scholar]
- NICHD . NICHD Early Child Care Research Network; Developmental psychology: 1999. Child care and mother-child interaction in the first 3 years of life. [PubMed] [Google Scholar]
- Nolvi S., Tuulari J.J., Pelto J., Bridgett D.J., Eskola E., Lehtola S.J., Hashempour N., Korja R., Kataja E.-L., Saunavaara J., Parkkola R., Lähdesmäki T., Scheinin N.M., Fernandes M., Karlsson L., Lewis J.D., Fonov V.S., Collins D.L., Karlsson H. Neonatal amygdala volumes and the development of self-regulation from early infancy to toddlerhood. Neuropsychology. 2021;35:285–299. doi: 10.1037/neu0000724. [DOI] [PubMed] [Google Scholar]
- O'Brien G.D., Queenan J.T., Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am. J. Obstet. Gynecol. 1981;139:540–545. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- Oler J.A., Fox A.S., Shackman A.J., Kalin N.H. Living without an Amygdala. The Guilford Press; New York, NY, US: 2016. The central nucleus of the amygdala is a critical substrate for individual differences in anxiety; pp. 218–251. [Google Scholar]
- Ong M.-L., Tuan T.A., Poh J., Teh A.L., Chen L., Pan H., MacIsaac J.L., Kobor M.S., Chong Y.S., Kwek K., Saw S.M., Godfrey K.M., Gluckman P.D., Fortier M.V., Karnani N., Meaney M.J., Qiu A., Holbrook J.D. Neonatal amygdalae and hippocampi are influenced by genotype and prenatal environment, and reflected in the neonatal DNA methylome. Gene Brain Behav. 2019;18 doi: 10.1111/gbb.12576. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Pruessner M., Lord C., Buss C., Collins L., Dagher A., Lupien S.J. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Qiu A., Anh T.T., Li Y., Chen H., Rifkin-Graboi A., Broekman B.F.P., Kwek K., Saw S.-M., Chong Y.-S., Gluckman P.D., Fortier M.V., Meaney M.J. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry. 2015;5:e508. doi: 10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Rifkin-Graboi A., Chen H., Chong Y.-S., Kwek K., Gluckman P.D., Fortier M.V., Meaney M.J. Maternal anxiety and infants' hippocampal development: timing matters. Transl. Psychiatry. 2013;3:e306. doi: 10.1038/tp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J.M., Graham A.M., Entringer S., Gilmore J.H., Styner M., Fair D.A., Wadhwa P.D., Buss C. Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. 2019;185:825–835. doi: 10.1016/j.neuroimage.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J.M., Kruggel F., Gilmore J.H., Styner M., Entringer S., Consing K.N.Z., Potkin S.G., Wadhwa P.D., Buss C. A novel maturation index based on neonatal diffusion tensor imaging reflects typical perinatal white matter development in humans. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2017;56:42–51. doi: 10.1016/j.ijdevneu.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Bai J., Chen H., Hameed W.B., Sim L.W., Tint M.T., Leutscher-Broekman B., Chong Y.-S., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol. Psychiatr. 2013;74:837–844. doi: 10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Robinson J.L., Salibi N., Deshpande G. Functional connectivity of the left and right hippocampi: evidence for functional lateralization along the long-axis using meta-analytic approaches and ultra-high field functional neuroimaging. Neuroimage. 2016;135:64–78. doi: 10.1016/j.neuroimage.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Rogers C.E., Anderson P.J., Thompson D.K., Kidokoro H., Wallendorf M., Treyvaud K., Roberts G., Doyle L.W., Neil J.J., Inder T.E. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso I.M., Cintron C.M., Steingard R.J., Renshaw P.F., Young A.D., Yurgelun-Todd D.A. Amygdala and hippocampus volumes in pediatric major depression. Biol. Psychiatr. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Rubin R.D., Watson P.D., Duff M.C., Cohen N.J. The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M.D., Graham A.M., Feczko E., Miranda-Dominguez O., Rasmussen J.M., Nardos R., Entringer S., Wadhwa P.D., Buss C., Fair D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018;21:765–772. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm A.K., Pavelko M., Krouse E.M., Webster W., Kraszpulski M., Birkle D.L. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Dev. Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Scheinost D., Spann M.N., McDonough L., Peterson B.S., Monk C. Associations between different dimensions of prenatal distress, neonatal hippocampal connectivity, and infant memory. Neuropsychopharmacology. 2020;45:1272–1279. doi: 10.1038/s41386-020-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth S., Lewis A.J., Galbally M. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: a systematic literature review. BMC Pregnancy Childbirth. 2016;16:124. doi: 10.1186/s12884-016-0915-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgaard A.M., Olsen E.M., Christiansen E., Houmann T., Landorph S.L., Jørgensen T. Predictors (0–10 months) of psychopathology at age 1½ years – a general population study in the Copenhagen Child Cohort CCC 2000*. JCPP (J. Child Psychol. Psychiatry) 2008;49:553–562. doi: 10.1111/j.1469-7610.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- Stern J.A., Botdorf M., Cassidy J., Riggins T. Empathic responding and hippocampal volume in young children. Dev. Psychol. 2019;55:1908–1920. doi: 10.1037/dev0000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuran T., Zimmermann E., Welzl H. Water maze performance and hippocampal weight of prenatally stressed rats. Behav. Brain Res. 1994;65:153–155. doi: 10.1016/0166-4328(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Tamura M., Sajo M., Kakita A., Matsuki N., Koyama R. Prenatal stress inhibits neuronal maturation through downregulation of mineralocorticoid receptors. J. Neurosci. 2011;31:11505–11514. doi: 10.1523/JNEUROSCI.3447-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Crawford H., Morales S., Degnan K.A., Pine D.S., Fox N.A. Infant behavioral inhibition predicts personality and social outcomes three decades later. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:9800–9807. doi: 10.1073/pnas.1917376117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari J.J., Kataja E.-L., Leppänen J.M., Lewis J.D., Nolvi S., Häikiö T., Lehtola S.J., Hashempour N., Saunavaara J., Scheinin N.M., Korja R., Karlsson L., Karlsson H. Newborn left amygdala volume associates with attention disengagement from fearful faces at eight months. Dev. Cogn. Neurosci. 2020;45 doi: 10.1016/j.dcn.2020.100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.I., Hect J.L., Smarr B.L., Qawasmeh T., Kriegsfeld L.J., Barcelona J., Hijazi K.E., Thomason M.E. Maternal stress during pregnancy alters fetal cortico-cerebellar connectivity in utero and increases child sleep problems after birth. Sci. Rep. 2021;11:2228. doi: 10.1038/s41598-021-81681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P., Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatr. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wang J., Vachet C., Rumple A., Gouttard S., Ouziel C., Perrot E., Du G., Huang X., Gerig G., Styner M. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front. Neuroinf. 2014;8:7. doi: 10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Van Heerikhuize J., Aronica E., Kawata M., Seress L., Joels M., Swaab D.F., Lucassen P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging. 2013;34:1662–1673. doi: 10.1016/j.neurobiolaging.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Wen D.J., Poh J.S., Ni S.N., Chong Y.-S., Chen H., Kwek K., Shek L.P., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl. Psychiatry. 2017;7:e1103. doi: 10.1038/tp.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Lu Y.-C., Jacobs M., Pradhan S., Kapse K., Zhao L., Niforatos-Andescavage N., Vezina G., du Plessis A.J., Limperopoulos C. Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.19940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Piven J., Hazlett H.C., Smith R.G., Ho S., Gee J.C., Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.