Abstract
Background.
Research has demonstrated that chronic stress exposure early in development can lead to detrimental alterations in the orbitofrontal cortex (OFC)–amygdala circuit. However, the majority of this research uses functional neuroimaging methods, and thus the extent to which childhood trauma corresponds to morphometric alterations in this limbic-cortical network has not yet been investigated. This study had two primary objectives: (i) to test whether anatomical associations between OFC–amygdala differed between adults as a function of exposure to chronic childhood assaultive trauma and (ii) to test how these environment-by-neurobiological effects relate to pathological personality traits.
Methods.
Participants were 137 ethnically diverse adults (48.1% female) recruited from the community who completed a clinical diagnostic interview, a self-report measure of pathological personality traits, and anatomical MRI scans.
Results.
Findings revealed that childhood trauma moderated bilateral OFC–amygdala volumetric associations. Specifically, adults with childhood trauma exposure showed a positive association between medial OFC volume and amygdalar volume, whereas adults with no childhood exposure showed the negative OFC–amygdala structural association observed in prior research with healthy samples. Examination of the translational relevance of trauma-related alterations in OFC–amygdala volumetric associations for disordered personality traits revealed that trauma exposure moderated the association of OFC volume with antagonistic and disinhibited phenotypes, traits characteristic of Cluster B personality disorders.
Conclusions.
The OFC–amygdala circuit is a potential anatomical pathway through which early traumatic experiences perpetuate emotional dysregulation into adulthood and confer risk for personality pathology. Results provide novel evidence of divergent neuroanatomical pathways to similar personality phenotypes depending on early trauma exposure.
Keywords: Antagonism, childhood adversity, cortical volume, disinhibition, stress exposure, subcortical volume
Introduction
Trauma exposure in childhood is one of the most well-established and robust risk factors for the development of a range of psychiatric disorders, including increased risk for depression and suicidality (Carr, Martins, Stingel, Lemgruber, & Juruena, 2013; Pine & Cohen, 2002; Pompili et al., 2014). Given its transdiagnostic relevance, it is likely that early exposure to traumatic events disrupts the typical development of brain regions supporting affective systems, including the orbitofrontal cortex (OFC)–amygdala circuit. OFC is frequently implicated in the top-down processing of emotions (Garcia, Vouimba, Baudry, & Thompson, 2014; Ghashghaei, Hilgetag, & Barbas, 2007; Wright et al., 2008), such as recognizing and perceiving emotional responses (Adolphs, 2002), modulating fear responses (Phan et al., 2005), and reinforcement learning (Groman et al., 2019). Critical to these processes is OFC’s reciprocal communication with amygdala, a subcortical structure that supports the acquisition and expression of emotions, including fear expression (Öhman, 2005) and memory consolidation of emotional experiences (McGaugh, 2002). OFC is thought to play a critical regulatory role over amygdala, with the most replicated findings showing inverse correlations between prefrontal and OFC activity during tasks eliciting emotional responses (Ochsner et al., 2004; Stein et al., 2007) and at rest (Kim, Gee, Loucks, Davis, & Whalen, 2011; Roy et al., 2009) in healthy samples.
The OFC and amygdala are both highly susceptible to the effects of early life stress (Shonkoff et al., 2012) and essential to key regulatory processes (Bechara, Damasio, & Damasio, 2000; Phillips, Ladouceur, & Drevets, 2008), which is why we focused on these regions in this study. For example, extant research has identified alterations in the structural integrity of OFC and amygdala following exposure to early, repeated childhood stress, typically in the form of maltreatment exposure (Hanson et al., 2010; Hart & Rubia, 2012; Lim, Radua, & Rubia, 2014; Lupien, McEwen, Gunnar, & Heim, 1993; Moreno-López et al., 2020; Tottenham & Galván, 2016). Specifically, empirical studies have shown that childhood maltreatment is associated with reduced volume in cortical and subcortical regions, including in the OFC and amygdala (Cassiers et al., 2018; Edmiston & Blackford, 2013; Lim et al., 2019; Morey, Haswell, Hooper, & De Bellis, 2016) and alterations in amygdala–prefrontal circuitry (McLaughlin & Lambert, 2017; Paquola et al., 2019). While mounting evidence suggests that childhood trauma has detrimental effects on the functioning of OFC–amygdala interactions (Hart & Rubia, 2012), the extent to which alterations in OFC–amygdala circuitry related to early traumatic stress can be observed at a purely structural level has been relatively understudied. Based on recent arguments that examining structural covariation between brain regions is necessary to further understanding of brain networks initially established at a functional level (Gong, He, Chen, & Evans, 2012; He, Chen, & Evans, 2007; Zielinski, Gennatas, Zhou, & Seeley, 2010), the primary aim of this study was to address this gap in relation to OFC–amygdala connectivity.
To our knowledge, only two studies have investigated structural associations between amygdala and cortex. In a healthy youth sample, Albaugh et al. (2013) found that amygdala volume was inversely related to cortical thickness in several regions of PFC, including bilateral OFC. Blackmon et al. (2011) reported a similar inverse association between amygdala volume and thickness in the left lateral OFC (lOFC) in a sample of healthy adults. These studies provide a starting point for understanding morphometric associations between amygdala and OFC in healthy adult samples by providing preliminary evidence of inverse structural associations that mirror functional connectivity findings. Given research suggesting that early trauma exposure detrimentally impacts functional OFC–amygdala circuitry, it is likely that the structural associations between these regions may also be impacted by chronic childhood stress exposure. However, to our knowledge, this question has not yet been investigated.
Implications for the development of pathological personality
A large body of literature has implicated both trauma exposure early in life (e.g. Ball & Links, 2009; Goodman & Yehuda, 2002) and reductions in OFC cortical volume in the development of personality disorders (PDs), particularly those marked by emotional and behavioral dysregulation (e.g. borderline, antisocial PDs) (Chanen et al., 2018; Raine, 2018; Tebartz van Elst et al., 2003; Yang & Raine, 2009). Further, results from a prospective twin study showed that personality pathology is best understood in the context of both environmental (i.e. childhood maltreatment) and biological factors (Berenz et al., 2013). Research on the neurobiology of personality has consistently implicated alterations in OFC–amygdala circuitry that account for variation in trait-level personality dimensions central to PDs, including disinhibition, antagonism, and negative affectivity (Ameis et al., 2014; Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Blair, Tinkelman, Moita, & LeDoux, 2003; Coccaro, Lee, McCloskey, Csernansky, & Wang, 2015; Saxbe et al., 2018; Tebartz van Elst et al., 2003). For example, Coccaro et al. (2015) found that individuals with elevated impulsivity and aggression exhibited exaggerated amygdala reactivity and diminished OFC activation during a social threat task. Similarly, disruption in neural networks involving the amygdala and OFC has been implicated in emotion dysregulation among individuals with BPD (Soloff, Abraham, Ramaseshan, Burgess, & Diwadkar, 2017). Taken together, it appears that the OFC–amygdala relationship is critical to understanding links between trauma exposure and trait levels of emotional and behavioral dysregulation. However, the majority of this research has focused on either structural alterations in specific neural regions (e.g. OFC and/or amygdala individually) or in the functioning of OFC–amygdala circuitry. Research to date has yet to examine the clinical implications of early trauma exposure for OFC–amygdala morphometric associations and the manifestation of pathological personality traits in adulthood.
Current study
A gap in this line of work is knowledge of how structural covariation between the amygdala and OFC may be affected by developmental experiences that are known to impact emotion regulation, and in turn, confer risk for psychopathology. The primary objective of the current study was to investigate the extent to which morphometric associations between the amygdala and regions of the OFC vary as a function of childhood trauma exposure. We also explored the clinical relevance of these associations for pathological personality traits by examining the interactive effects of trauma exposure and OFC volume in regions found to covary with amygdala. We focused on assaultive trauma experiences, because these forms of childhood trauma have been robustly linked to chronic activation of the HPA-axis (Cross, Fani, Powers, & Bradley, 2017; Tarullo & Gunnar, 2006), which is posited to have long-term deleterious effects on the developing cortex (Gee & Casey, 2015; Tottenham & Galván, 2016). Consistent with past work in samples of healthy adults without trauma exposure (Albaugh et al., 2013; Blackmon et al., 2011), we hypothesized that there would be an inverse association between OFC and amygdala volume in those without a trauma history in our sample. Conversely, we hypothesized that this relationship would be absent or even positive in those with a trauma history.
Methods and materials
Participants
Participants were 137 ethnically diverse adults recruited from the community using flyers and online postings. Inclusion criteria: between ages 18 and 55, fluency in English. Exclusion criteria were current psychosis or a current diagnosis of a psychotic disorder, estimated IQ in the Intellectual Disability range, serious medical/neurological condition, history of head injuries with lasting effects, MRI contraindications, and imminent suicide risk. No other psychiatric conditions were considered exclusionary conditions. All participants with complete interview, self-report, and MRI data were included in the analysis. Two participants who reported more than one previous head injury were removed from analysis to reduce the potential impact of these events.
The final sample consisted of 135 participants [M(s.d.)age = 31.81(9.18); 48.1% men]. Sample characteristics are presented in Table 1. Approximately half of the sample self-identified as Caucasian/White (53.0%), followed by Black (35.1%), Asian (7.5%), or Biracial/Other (4.3%). Past year income for the sample ranged from $0 to $300 000 [M(s.d.) = $50 357.028 ($46 424.92)]. The majority of participants came from communities with high violent crime rates (www.neighborhoodscout.com/de/wilmington/crime on 06/07/2020), suggesting the sample is at relatively higher risk for exposure to assaultive trauma than other community samples.
Table 1.
Sample characteristics and zero-order correlations (N = 135) among study variables
| Mean | s.d. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Age (years) | 31.81 | 9.18 | ___ | |||||||
| (2) Sex | 0.48 | 0.50 | 0.04 | ___ | ||||||
| (3) Race | 0.46 | 0.50 | −0.13 | 0.05 | ___ | |||||
| (4) Income ($) | 50 357.28 | 46 424.92 | −0.09 | −0.05 | −0.02 | ___ | ||||
| (5) Childhood assaultive trauma exposure | 0.37 | 0.48 | 0.13 | −0.13 | 0.10 | −0.17 | ___ | |||
| (6) Antagonism | 2.81 | 2.59 | −0.02 | 0.06 | −0.01 | −0.15 | 0.12 | ___ | ||
| (7) Disinhibition | 3.73 | 3.68 | −0.01 | −0.06 | −0.10 | −0.24* | 0.21* | 0.57* | ___ | |
| (8) Negative affectivity | 5.31 | 3.92 | −0.02 | −0.23* | −0.10 | −0.02 | 0.12 | 0.40* | 0.47* | ___ |
Note. Sex was coded as 0-female; 1-male; Race was dummy-coded as 0-White, 1-Non-White; Trauma Exposure was coded as 0-absent, 1-present.
p < 0.05.
Procedures
Written and oral consent was obtained from all individuals before participation. Participants completed a battery of assessments, including a structured clinical interview and a neuroimaging protocol across two study visits. The University Institutional Review Board approved all protocols and procedures (Protocol #’s: 1073423–17, 1361164–1).
Measures
Childhood assaultive trauma exposure
The Detailed Trauma Screen from the SCID-5-RV Stressor Related Disorders module (First, 2014) was used to assess the history of exposure to traumatic events. Participants completed this measure via a clinical interview conducted by, or under the supervision of a licensed clinical psychologist (n = 72), or via a self-report survey (n = 63). Assessment method was not uniquely associated with any study variables of interest (p’s > 0.05); thus, the samples were combined in all analyses.
Consistent with previous research quantifying childhood trauma exposure (e.g. Bounoua, Miglin, Spielberg, & Sadeh, 2020), we compared adults with chronic exposure to childhood assaultive trauma, defined as experiencing multiple assaultive traumas prior to the age 13, to those individuals who either did not experience any assaultive trauma, only reported a single exposure in childhood, or reported assaultive trauma that only occurred later in life. Assaultive trauma was operationalized as direct exposure to threatened or actual physical violence (e.g. assault, domestic violence, or physical or sexual abuse). Non-assaultive events included exposure to life-threatening danger that did not involve threatened or actual physical violence (e.g. natural disasters) (Cisler et al., 2012; Sadeh, Miller, Wolf, & Harkness, 2015).
The sample reported a range of lifetime trauma exposure (Min/Max = 0/6 events), with a little over a third of the sample (37.0%, n = 50) meeting criteria for inclusion in the repeated childhood assaultive trauma group. Among individuals with repeated childhood assault exposure, the most common forms were physical abuse (82.0%), sexual abuse (20.0%), and witnessing violence perpetrated against others (14.0%).
Pathological personality traits
Three dimensions of pathological personality that have been associated with OFC–amygdala circuitry in prior work were assessed using the Personality Inventory for DSM-5-Brief Form (PID-5-BF; Krueger, Derringer, Markon, Watson, & Skodol, 2013). Participants rated the extent to which each item best described them from 0 (Very False/Often False) to 3 (Very True/Often True). An Antagonism score was created by summing five items measuring the degree to which individuals are self-focused and disregard the needs of others (e.g. ‘I use people to get what I want.’) [M/s.d. = 2.81/2.59]. A Disinhibition score was created by summing five items that measure the tendency to engage in risky and impulsive behaviors (e.g. ‘I feel like I act totally on impulse.’) [M/s.d. = 3.74/3.68]. A Negative Affectivity score was created by summing five items measuring the tendency to experience negative emotions (e.g. ‘I get emotional easily, often for very little reason.’) [M/s.d. = 5.31/3.92]. Average scores on these disordered personality traits in the current sample fell between means reported for clinical and non-clinical samples in previous work (Bach, Sellbom, & Simonsen, 2018). Previous work has demonstrated that the PID-5-BF shows excellent reliability and construct validity across a range of samples (Anderson, Sellbom, & Salekin, 2018).
MRI acquisition
Data were collected using a Siemens 3 T Magnetom Prisma scanner with a 64-channel head coil. A T1-weighted multi-echo MPRAGE anatomical scan (resolution = 1 mm3, TR = 2530 ms, TEs = 1.69, 3.55, 5.41,7.27 ms) was collected, which has the advantage of less distortion and higher contrast than standard MPRAGE sequences, resulting in more reliable cortical models (van der Kouwe, Benner, Salat, & Fischl, 2008). A T2-weighted variable flip-angle turbo spin-echo scan (resolution = 1 mm3, TR = 3200 ms, TE = 564 ms) was collected, which is used in FreeSurfer to better differentiate the gray-matter-dura boundary.
Amygdala volume
We used FreeSurfer’s (v6) probabilistic atlas tool to segment amygdala (Saygin et al., 2017). The tool is based on a generative model, where the registered atlas is combined with a subject-specific likelihood model to produce a segmentation specific to that individual’s brain.
OFC cortical volume
The cortical volume of each OFC vertex was estimated using FreeSurfer’s (v6) standard morphometric pipeline (Salat et al., 2004). Data were spatially smoothed using a Gaussian kernel of 15 mm full-width at half-maximum.
Data analytic plan
Age, biological sex, body mass index (BMI), and estimated intracranial volume (eTIV) were entered as covariates of no interest in all analyses, given associations between these variables, trauma exposure, and neural morphology in previous research (e.g. Chen, Sachdev, Wen, & Anstey, 2007; Taki & Kawashima, 2012). All analyses were conducted within each hemisphere separately, given evidence of lateralization of amygdala function (Baas, Aleman, & Kahn, 2004).
FreeSurfer’s QDEC application was used to examine associations between childhood trauma, amygdala volume, and OFC cortical volume using general linear models. We created an OFC mask by merging the lateral and medial orbitofrontal parcellations of the Desikan-Killiany Atlas. Main effects between amygdala and OFC cortical volume were examined. Next, OFC cortical volume was regressed on the interaction of childhood trauma and amygdala volume, with the main effects of childhood trauma and amygdala volume included in the model as well as the covariates (age, sex, BMI, and eTIV). To correct for multiple comparisons within OFC, we applied a standard correction procedure using the FreeSurfer analysis software. Volumetric results were corrected for the number tests conducted using pre-cached Gaussian Monte Carlo simulation (10 000 iterations, cluster-wise threshold: p < 0.05, sign: absolute) that was created based on the size of the ROI examined (Hagler, Saygin, & Sereno, 2006). Bonferroni correction was also applied to take into account the number of comparisons conducted across both hemispheres.
Follow-up linear regression analyses were conducted to test associations between OFC cortical volume in the identified clusters and disordered personality traits. Finally, to examine the clinical relevance of trauma-related differences in OFC–amygdala volumetric associations, we conducted moderation analyses to test whether cortical volume in OFC regions linked to amygdala volume was also related to pathological personality traits. Specifically, we modeled the interactive effect of childhood trauma and OFC cortical volume (in the regions associated with amygdala volume in the QDEC analysis) on disordered personality traits. Moderation analyses were conducted using PROCESS Macro v3.3 (Hayes, 2018) in SPSS v26 (IBM Corp, 2013). One participant had missing data on the PID-5-BF and was excluded from this analysis.
Results
Impact of childhood trauma on OFC–amygdala volumetric associations
We first examined whether the inverse association between amygdala volume and OFC structure reported in previous work (e.g. Albaugh et al., 2013) was present across our sample. Results revealed that no main effects of amygdala volume on OFC volume survived following correction for multiple comparisons.
Next, we examined whether associations between amygdala and OFC cortical volume differed between adults with and without assaultive trauma in childhood. Significant interaction effects emerged for both hemispheres (see Table 2 and Fig. 1), indicating that the relationship of amygdala with OFC cortical volume varied as a function of childhood trauma exposure.
Table 2.
Childhood trauma exposure moderates the volumetric associations between amygdala and clusters within the orbitofrontal cortex
| Cluster No. | Cluster | Peak F value | Peak MNI (x, y, z) | No. of vertices | Cluster size (mm2) |
|---|---|---|---|---|---|
| A | Left medial OFC/lateral OFC | 3.44 | −11.4, 15.7, −14.4 | 507 | 220.47 |
| B | Right medial OFC | 2.56 | 9.8, 39.6, −10.1 | 1053 | 532.18 |
OFC, orbitofrontal cortex.
Fig. 1.
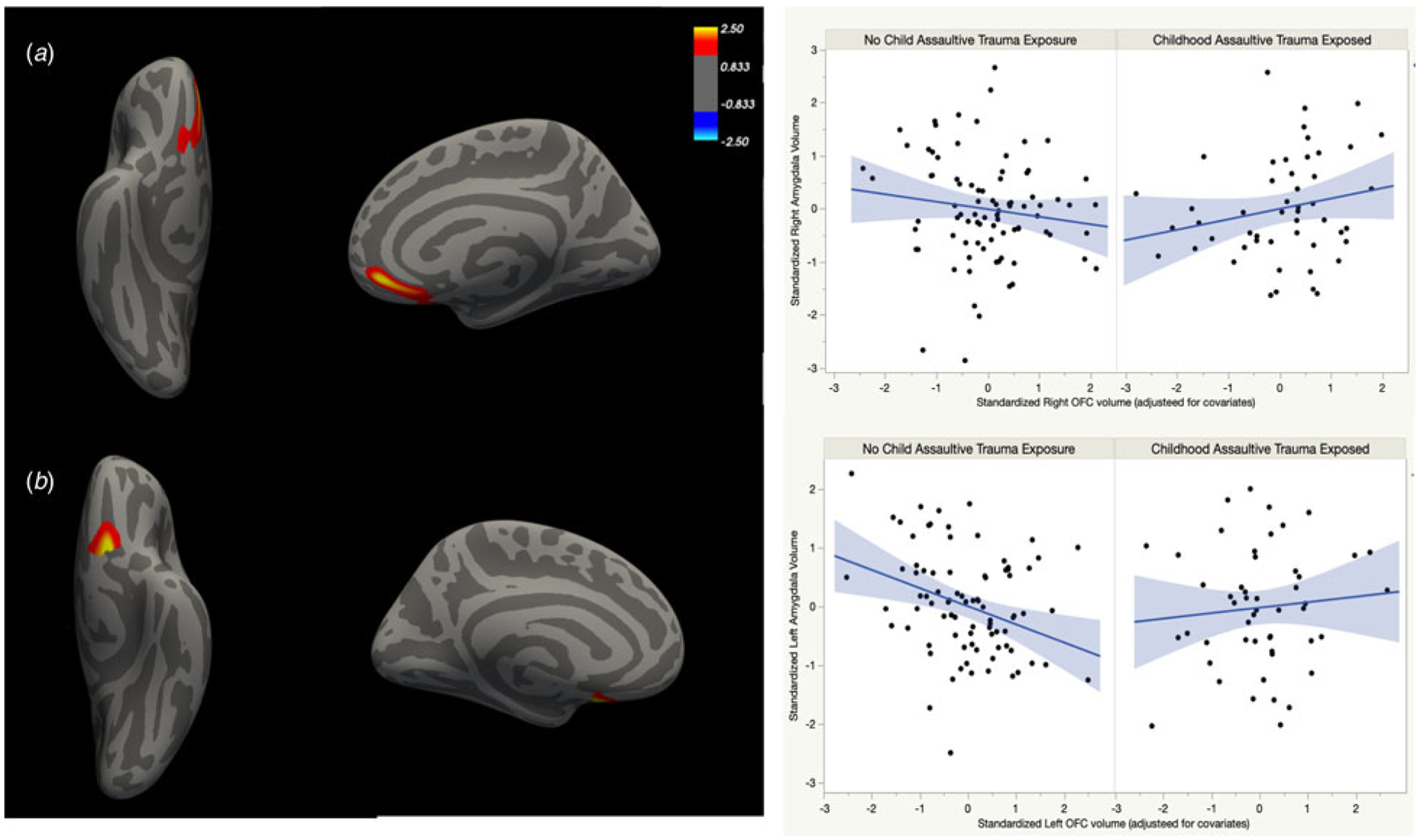
Chronic childhood trauma exposure moderates the volumetric association between amygdala and regions of the OFC, after controlling for age, sex, BMI, and eTIV. (a) Right medial OFC. (b) Left medial and lateral OFC.
In the right hemisphere, childhood trauma moderated the association between amygdala and cortical volume in a cluster that peaked in medial OFC (mOFC). Consistent with prior work, amygdala volume showed a trending inverse relationship with mOFC volume (t = −1.41; 95% CI −0.46 to 0.08) in individuals without a history of assaultive childhood trauma. Conversely, a significant positive association between amygdala and cortical volume in this cluster was observed in adults with chronic assaultive trauma exposure in childhood (t = 1.99; 95% CI 0.002–0.62).
A similar pattern of findings was evident in the left hemisphere. Childhood trauma moderated the association between amygdala and cortical volume in a cluster that peaked in mOFC and included lOFC. Follow-up analyses revealed a significant negative association between left amygdala and OFC cortical volume in the non-trauma exposed group (t = −2.93; 95% CI −0.31 to −0.06). Among adults with childhood assaultive trauma exposure, this relationship between amygdala and OFC cortical volume showed a trend in the positive direction (t = 0.65; 95% CI −0.10 to 0.19).
Exploratory examination of sex differences
Given extant research suggesting that biological sex moderates the effect of trauma exposure on neurobiological systems (e.g. van voorhees & Scarpa, 2004), we ran exploratory three-way interaction analyses to test whether sex moderated the observed associations. No significant results emerged [i.e. sex did not moderate the interactive effect of childhood trauma and amygdala volume on cortical volume in the left (t = 1.47, 95% CI −0.09 to 0.63) or right (t = −0.29, 95% CI −0.89 to 0.67) OFC clusters]. We also tested for, and did not find, any new cortical clusters where sex moderated the childhood trauma × amygdala association when we ran whole cortex analyses in QDEC.
Links with pathological personality
We investigated the translational relevance of trauma-related alterations in OFC–amygdala volumetric associations for understanding disordered personality traits. More specifically, we tested whether the OFC clusters identified above explained variance in disordered personality traits as a function of childhood trauma exposure. Interaction findings are depicted in Fig. 2.
Fig. 2.
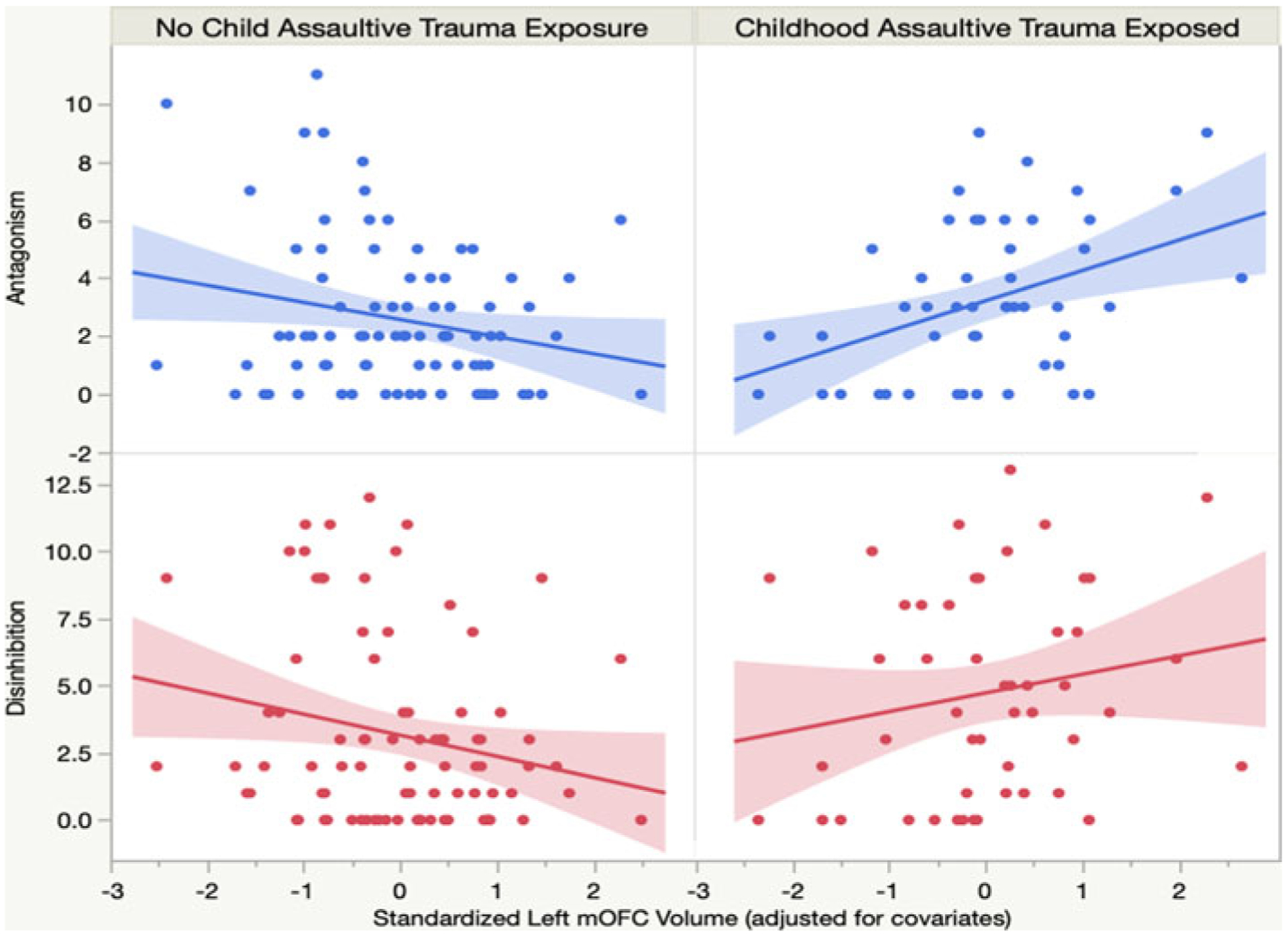
Childhood trauma exposure moderates associations between left OFC volume and disordered personality traits.
Antagonism
No main effects emerged for the left or right OFC clusters and antagonistic personality traits. However, childhood trauma exposure moderated the association between the left OFC cluster and antagonism (ΔR2 = 0.07, p = 0.003). In the trauma-exposed group, left OFC cortical volume was significantly and positively associated with trait antagonism (t = 2.03, 95% CI 0.0002 to 0.01), indicating that callous, manipulative, and grandiose traits increased as OFC cortical volume increased in this group. In contrast, in the non-trauma exposed group, left OFC cortical volume showed a negative trend with antagonistic personality traits (t = −1.25; 95% CI −0.01 to 0.001). No significant interaction emerged in the right hemisphere.
Disinhibition
No main effects emerged between cortical volume in the left or right amygdala-related OFC clusters and trait disinhibition. However, a significant interaction emerged between childhood trauma and OFC cortical volume for disinhibition (ΔR2 = 0.06, p = 0.004). Among individuals with childhood trauma, disinhibition showed a trending positive association with left OFC cortical volume (t = 1.21; 95% CI −0.003 to 0.01), indicating that more cortical volume in left OFC was associated with a greater tendency to act impulsively. In the non-trauma exposed group, left OFC cortical volume was significantly and inversely associated with disinhibition (t = −2.22; 95% CI −0.02 to −0.001), such that impulsivity increased as OFC cortical volume decreased in this group. No significant interaction emerged in the right hemisphere.
Negative affectivity
No significant main or moderation effects emerged for the left or right amygdala-related OFC clusters and negative affectivity.
Specificity of childhood assaultive trauma exposure
We conducted supplementary analyses to ensure the interactive effect of childhood trauma and amygdala volume on OFC volume could not be accounted for by assaultive trauma exposure in adulthood. These analyses revealed that childhood trauma remained a significant moderator of the association between amygdala and OFC volume when adult trauma exposure was entered into the model for both the left (p = 0.002) and right (p = 0.002) hemispheres. Similar analyses were conducted to test whether the moderating effect of childhood trauma on the association between left OFC volume and personality traits persisted after accounting for adult assaultive trauma exposure. Results indicated that childhood assaultive trauma remained a significant moderator of the effect of left OFC volume on antagonism (ΔR2 = 0.07, p = 0.003) and disinhibition (ΔR2 = 0.06, p = 0.004).
Discussion
Childhood trauma has been consistently implicated in a range of clinical disorders, particularly through its effect on underlying neurobiological processes (Heim & Nemeroff, 2001; Tottenham & Galván, 2016). The OFC–amygdala circuit is heavily involved in emotion regulatory processes (Bechara et al., 2000; Phillips et al., 2008) and is particularly vulnerable to early life stress (Tottenham & Galván, 2016). The present study revealed that trauma exposure moderates the anatomical associations between amygdala and OFC volume (see Fig. 1). Notably, volume in the identified clusters was also related to pathological personality traits in adulthood, such that disinhibition and antagonism exhibited different relationships with OFC volume in trauma-exposed v. non-exposed adults. Results provide new insights into the long-term neuroanatomical correlates of childhood trauma exposure and advance our understanding of the etiopathogenesis of pathological personality.
Importantly, supplementary analyses indicated that our findings appear to be relatively specific to childhood assaultive trauma. We found the effect of childhood trauma exposure on OFC–amygdala volumetric associations remained significant after accounting for assaultive trauma first experienced after the age of 18. This finding is consistent with existing work that underscores the vulnerability of amygdala–prefrontal circuitry to chronic stress early in development (Tottenham & Galván, 2016). Similarly, the moderating effect of childhood trauma on the association between left OFC volume and trait levels of antagonism and disinhibition remained significant after accounting for adult-only trauma exposure. Previous work has demonstrated that early trauma may lead to pathological personality traits via changes on neurobiological systems that support emotional and behavioral regulatory processes, including the amygdala and OFC (e.g. Raine, 2008). Taken together, these findings point to the impact of chronic childhood trauma exposure, in particular, on OFC–amygdala morphometric associations and associated links with inhibition and antagonism.
Impact of childhood trauma on OFC–amygdala morphology
We found that trauma exposure impacted the volumetric association between amygdala and bilateral mOFC, extending into lOFC/ACC in the right hemisphere. Specifically, amygdala and OFC volumes were positively correlated in adults with a history of childhood assaultive trauma, whereas this relationship was reversed in non-exposed individuals (Fig. 1). The inverse association in those without trauma is consistent with previous anatomical studies (Albaugh et al., 2013; Blackmon et al., 2011), and parallels the inverse frontolimbic connectivity patterns observed at rest among healthy adults (Roy et al., 2009). Thus, previous findings appear to reflect structural relationships only in those without childhood trauma.
To our knowledge, our findings provide the first evidence of the role of childhood trauma exposure on morphometric relations between amygdala and OFC. In particular, the positive volumetric association between OFC and amygdala among individuals with childhood trauma suggests a mechanism through which chronic stress may impact neurobiological development. Given that the OFC regions that we identified have previously been linked to impulsivity/decision making (Antonucci et al., 2006; Wang et al., 2016; Wilbertz et al., 2012) and reward anticipation (Hahn et al., 2009), a positive OFC–amygdala association may reflect the weakened or disturbed top-down regulation circuit that is well-documented in the trauma literature (van der Werff et al., 2013). This association may also reflect a mechanism by which repeated HPA-axis hyperactivation following chronic stress leads to amygdala hyperexcitability and concurrent structural atrophy in amygdala and OFC (Albaugh et al., 2013; Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009). Thus, the findings from this study underscore childhood trauma exposure as an important environmental factor that may impact critical frontolimbic circuitry.
Implications for etiological models of personality pathology
Present findings also have important implications for understanding heterogeneity in the etiology of PDs. In particular, we found that childhood trauma exposure modulated the relationship between volume in amygdala-linked OFC regions and pathological disinhibition and antagonism. As seen in Fig. 2, among non-exposed individuals, OFC volume was inversely associated with pathological personality, such that less OFC volume was associated with more trait impulsivity and antagonistic personality traits, a pattern that is consistent with much of the existing research (Antonucci et al., 2006; Blair, 2004; Coccaro et al., 2016; Johnson, Elliott, & Carver, 2020; Yang & Raine, 2007). In contrast, among trauma-exposed individuals, OFC volume was positively associated with impulsive and antagonistic personality traits. Interestingly, in a study of healthy adults, Cho et al. (2013) also found that mOFC volume was positively correlated with impulsivity, and authors speculated that this may be indicative of more engagement in OFC systems among impulsive individuals. Based on the findings of this study, it may be the case that trauma exposure is a critical moderating factor in the complex association between OFC morphometry and impulsivity in adulthood.
Furthermore, the divergent associations between OFC volume and impulsive/antagonistic personality traits in the exposed and non-exposed groups are intriguing in that they follow the pattern of findings for amygdalar volume. That is, among individuals with a history of assaultive childhood trauma, greater OFC volume was linked to both greater amygdalar volume and higher levels of pathological personality traits, whereas among individuals without this history, less OFC volume was associated with both greater amygdala volume and higher levels of pathological personality traits. Given the cross-sectional nature of the data, it is impossible to infer directionality in the relations among these variables. However, future research examining the joint influence of trauma exposure on the OFC–amygdala circuit and clinical phenotypes over time could clarify these interactive effects over the lifespan.
Although personality traits have a strong genetic basis and tend to remain stable over time (DeYoung, 2010; DeYoung et al., 2010), few studies have examined the neuroanatomical correlates of personality pathology. As research has already demonstrated personality pathology-related differences in functional coupling between amygdala and PFC (Kerr et al., 2015), examination of structural associations between amygdala and OFC provides sorely needed insights into the neural substrates of pathological personality. Indeed, prominent theories of personality development highlight the dynamic interplay between biology and environmental factors (Heim & Nemeroff, 2001; Linehan, 1993; Raine, 2008). Given the overwhelming evidence that many PDs are characterized by exposure to childhood adversity (DeLisi, Drury, & Elbert, 2019; Porter et al., 2020; Raine, 2008), the present findings shed light on potential divergent etiological pathways to PD development.
Strengths and limitations
The study had several strengths, including a vertex-wise analytic approach (v. treating OFC as a single entity), which is consistent with recommendations to examine amygdala connections with specific OFC locations (Ray & Zald, 2012), and recruitment of an ethnically diverse sample of adults with elevated rates of childhood trauma exposure. Nonetheless, results should be considered in light of some study limitations. First, in this study, we took a theory-driven approach and, consequently, focused specifically on the relations between OFC and amygdala. To comprehensively understand how childhood trauma impacts connections between subcortical regions and prefrontal regulatory processes, additional research is needed. For example, future examinations of whether childhood trauma exposure moderates structural and functional associations between amygdala (and other subcortical regions) with the whole cortex will be necessary to extend the current findings. Second, we used a conservative approach in our multiple comparison correction (i.e. we did not look at associations with the volume of the entire OFC), which may explain why we did not find significant associations between OFC cortical volume and trait levels of negative affectivity previously reported in the literature (Jackson, Balota, & Head, 2011; Kalin, Shelton, & Davidson, 2007; Mincic, 2015; Wright et al., 2006). Given research suggesting that biological sex moderates the effect of trauma exposure and neurobiological systems (e.g. van voorhees and Scarpa, 2004), future research should further explore potential sex differences in the impact of childhood trauma exposure on OFC–amygdala morphometry.
Conclusions
Taken together, these findings provide novel insights into the impacts of childhood trauma on OFC–amygdala morphometric relations, extending previous research primarily focused on functional alterations. Specifically, results indicate that the widely-cited impact of early life stress exposure on OFC–amygdala circuitry can be observed at a purely structural level. These findings may provide important insight in the examination of structurally-based networks of neural regions involved in key transdiagnostic regulatory processes by underscoring the importance of considering early environmental factors that may fundamentally alter morphometric associations apparent in adulthood. Further, present findings provide novel empirical evidence for an environment × neurobiology effect on pathological personality traits. While previous work has focused on direct associations between OFC volume and pathological personality presentations, these findings point to divergent neuroanatomical associations with disordered personality that depend on childhood trauma exposure. This knowledge has the potential to inform etiological models of personality development that may eventually have implications for the treatment of pathological personality traits.
Acknowledgements.
This research was supported in part by the National Institutes of General Medical Sciences [NS, 2P20GM103653-06-6527] and Mental Health [NB, 1F31MH120936-01A1], [NS, 1R01MH116228-01A1, L30MH117623], [JMS, L30MH117662].
Footnotes
Conflict of interest.
None.
References
- Adolphs R (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12(2), 169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, … Hudziak JJ, & Brain Development Cooperative Group (2013). Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: Implications for the neural substrates of emotion regulation. NeuroImage, 71, 42–49. doi: 10.1016/j.neuroimage.2012.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, … Karama S (2014). Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biological Psychiatry, 75(1), 65–72. doi: 10.1016/j.biopsych.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Sellbom M, & Salekin RT (2018). Utility of the personality inventory for DSM-5-brief form (PID-5-BF) in the measurement of mal-adaptive personality and psychopathology. Assessment, 25(5), 596–607. doi: 10.1177/1073191116676889. [DOI] [PubMed] [Google Scholar]
- Antonucci AS, Gansler DA, Tan S, Bhadelia R, Patz S, & Fulwiler C (2006). Orbitofrontal correlates of aggression and impulsivity in psychiatric patients. Psychiatry Research: Neuroimaging, 147(2–3), 213–220. doi: 10.1016/j.pscychresns.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, & Kahn RS (2004). Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Research. Brain Research Reviews, 45(2), 96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bach B, Sellbom M, & Simonsen E (2018). Personality inventory for DSM-5 (PID-5) in clinical versus nonclinical individuals: Generalizability of psychometric features. Assessment, 25(7), 815–825. doi: 10.1177/1073191117709070 [DOI] [PubMed] [Google Scholar]
- Ball JS, & Links PS (2009). Borderline personality disorder and childhood trauma: Evidence for a causal relationship. Current Psychiatry Reports, 11(1), 63–68. doi: 10.1007/s11920-009-0010-4. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, & Damasio AR (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex (New York, N.Y.: 1991: ), 10(3), 295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berenz EC, Amstadter AB, Aggen SH, Knudsen GP, Reichborn-Kjennerud T, Gardner CO, & Kendler KS (2013). Childhood trauma and personality disorder criterion counts: A co-twin control analysis. Journal of Abnormal Psychology, 122(4), 1070–1076. doi: 10.1037/a0034238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon K, Barr WB, Carlson C, Devinsky O, DuBois J, Pogash D, … Thesen T (2011). Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Research: Neuroimaging, 194(3), 296–303. doi: 10.1016/j.pscychresns.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2004). The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition, 55(1), 198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair HT, Tinkelman A, Moita MAP, & LeDoux JE (2003). Associative plasticity in neurons of the lateral amygdala during auditory fear conditioning. Annals of the New York Academy of Sciences, 985, 485–487. doi: 10.1111/j.1749-6632.2003.tb07106.x. [DOI] [PubMed] [Google Scholar]
- Bounoua N, Miglin R, Spielberg JM, & Sadeh N (2020). Childhood assaultive trauma and physical aggression: Links with cortical thickness in prefrontal and occipital cortices. NeuroImage: Clinical, 27, 102321. doi: 10.1016/j.nicl.2020.102321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CMS, Stingel AM, Lemgruber VB, & Juruena MF (2013). The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. The Journal of Nervous and Mental Disease, 201(12), 1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Cassiers LLM, Sabbe BGC, Schmaal L, Veltman DJ, Penninx BWJH, & Van Den Eede F (2018). Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: A systematic review of neuroimaging findings. Frontiers in Psychiatry, 9, 329. doi: 10.3389/fpsyt.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanen AM, Velakoulis D, Carison K, Gaunson K, Wood SJ, Yuen HP, … Pantelis C (2008). Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Research: Neuroimaging, 163(2), 116–125. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Chen X, Sachdev PS, Wen W, & Anstey KJ (2007). Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study. NeuroImage, 36(3), 691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, & Strafella AP (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26(3), 479–487. doi: 10.1007/s10548-012-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Begle AM, Amstadter AB, Resnick HS, Danielson CK, Saunders BE, & Kilpatrick DG (2012). Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: Data from the NSA-R. Journal of Traumatic Stress, 25(1), 33–40. doi: 10.1002/jts.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Keedy SK, Gorka SM, King AC, Fanning JR, Lee RJ, & Phan KL (2016). Differential fMRI BOLD responses in amygdala in intermittent explosive disorder as a function of past alcohol use disorder. Psychiatry Research. Neuroimaging, 257, 5–10. doi: 10.1016/j.pscychresns.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, McCloskey M, Csernansky JG, & Wang L (2015). Morphometric analysis of amygdala and hippocampus shape in impulsively aggressive and healthy control subjects. Journal of Psychiatric Research, 69, 80–86. doi: 10.1016/j.jpsychires.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D, Fani N, Powers A, & Bradley B (2017). Neurobiological development in the context of childhood trauma. Clinical Psychology: Science and Practice, 24(2), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, & Pruessner JC (2009). The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. NeuroImage, 47(3), 864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- DeLisi M, Drury AJ, & Elbert MJ (2019). The etiology of antisocial personality disorder: The differential roles of adverse childhood experiences and childhood psychopathology. Comprehensive Psychiatry, 92, 1–6. doi: 10.1016/j.comppsych.2019.04.001. [DOI] [PubMed] [Google Scholar]
- DeYoung CG (2010). Personality neuroscience and the biology of traits. Social and Personality Psychology Compass, 4(12), 1165–1180. [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, & Gray JR (2010). Testing predictions from personality neuroscience. Brain structure and the big five. Psychological Science, 21(6), 820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EK, & Blackford JU (2013). Childhood maltreatment and response to novel face stimuli presented during functional magnetic resonance imaging in adults. Psychiatry Research: Neuroimaging, 212(1), 36–42. doi: 10.1016/j.pscychresns.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB (2014). Structured clinical interview for the DSM (SCID). The Encyclopedia of Clinical Psychology, 1–6. [Google Scholar]
- Garcia R, Vouimba R-M, Baudry M, & Thompson RF (1999). The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature, 402(6759), 294–296. [DOI] [PubMed] [Google Scholar]
- Gee DG, & Casey BJ (2015). The impact of developmental timing for stress and recovery. Neurobiology of Stress, 1, 184–194. doi: 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage, 34(3), 905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Chen ZJ, & Evans AC (2012). Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. NeuroImage, 59(2), 1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Goodman M, & Yehuda R (2002). The relationship between psychological trauma and borderline personality disorder. Psychiatric Annals, 32(6), 337–345. [Google Scholar]
- Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, … Taylor JR (2019). Orbitofrontal circuits control multiple reinforcement-learning processes. Neuron, 103(4), 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr, Saygin AP, & Sereno MI, (2006). Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage, 33(4), 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis A-C, Plichta MM, Heinzel S, Polak T, … Fallgatter AJ (2009). Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage, 46(4), 1148–1153. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, & Pollak SD (2010). Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22), 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, & Rubia K (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis second edition: A regression-based approach. New York, NY: Ebook The Guilford Press . Google Scholar. [Google Scholar]
- He Y, Chen ZJ, & Evans AC (2007). Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cerebral Cortex, 17(10), 2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- Heim C, & Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry, 49(12), 1023–1039. doi: 10.1016/S0006-3223 [DOI] [PubMed] [Google Scholar]
- IBM SPSS (n.d.). Corp (2013). IBM SPSS statistics for windows, version 22.0 Armonk, NY: IBM Corp. [Google Scholar]
- Jackson J, Balota DA, & Head D (2011). Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of Aging, 32(12), 2162–2171. doi: 10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Elliott MV, & Carver CS (2020). Impulsive responses to positive and negative emotions: Parallel neurocognitive correlates and their implications. Biological Psychiatry, 87(4), 338–349. doi: 10.1016/j.biopsych.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2007). Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry, 62(10), 1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Avery JA, Barcalow JC, Moseman SE, Bodurka J, Bellgowan PSF, & Simmons WK (2015). Trait impulsivity is related to ventral ACC and amygdala activity during primary reward anticipation. Social Cognitive and Affective Neuroscience, 10(1), 36–42. doi: 10.1093/scan/nsu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, & Whalen PJ (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex, 21(7), 1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon KE, Watson D, & Skodol AE (2013). The personality inventory for DSM-5 – brief form (PID-5-BF) – adult. Washington, DC: American Psychiatric Association. [Google Scholar]
- Lim L, Hart H, Howells H, Mehta MA, Simmons A, Mirza K, & Rubia K (2019). Altered white matter connectivity in young people exposed to childhood abuse: A tract-based spatial statistics (TBSS) and tractography study. Journal of Psychiatry & Neuroscience: JPN, 44(4), E11–E20. doi: 10.1503/jpn.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Radua J, & Rubia K (2014). Gray matter abnormalities in childhood maltreatment: A voxel-wise meta-analysis. American Journal of Psychiatry, 171(8), 854–863. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- Linehan MM (1993). Skills training manual for treating borderline personality disorder (pp. xii). New York City, NY: Guilford Press. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2002). Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences, 25(9), 456–461. doi: 10.1016/S0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, & Lambert HK (2017). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. doi: 10.1016/j.copsyc.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincic AM (2015). Neuroanatomical correlates of negative emotionality-related traits: A systematic review and meta-analysis. Neuropsychologia, 77, 97–118. doi: 10.1016/j.neuropsychologia.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Moreno-López L, Ioannidis K, Askelund AD, Smith AJ, Schueler K, & van Harmelen A-L (2020). The resilient emotional brain: A scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 392–402. doi: 10.1016/j.bpsc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Hooper SR, & De Bellis MD (2016). Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in mal-treated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology, 41(3), 791–801. doi: 10.1038/npp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, & Gross JJ (2004). For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage, 23(2), 483–499. [DOI] [PubMed] [Google Scholar]
- Öhman A (2005). The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology, 30(10), 953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Paquola C, Wael RVD, Wagstyl K, Bethlehem RAI, Hong S-J, Seidlitz J, … Bernhardt BC (2019). Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biology, 17(5), e3000284. doi: 10.1371/journal.pbio.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, & Tancer ME (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, & Cohen JA (2002). Trauma in children and adolescents: Risk and treatment of psychiatric sequelae. Biological Psychiatry, 51(7), 519–531. doi: 10.1016/S0006-3223(01)01352-X. [DOI] [PubMed] [Google Scholar]
- Pompili M, Innamorati M, Lamis DA, Erbuto D, Venturini P, Ricci F, … Girardi P (2014). The associations among childhood maltreatment, ‘male depression’ and suicide risk in psychiatric patients. Psychiatry Research, 220(1), 571–578. doi: 10.1016/j.psychres.2014.07.056. [DOI] [PubMed] [Google Scholar]
- Porter C, Palmier-Claus J, Branitsky A, Mansell W, Warwick H, & Varese F (2020). Childhood adversity and borderline personality disorder: A meta-analysis. Acta Psychiatrica Scandinavica, 141(1), 6–20. doi: 10.1111/acps.13118. [DOI] [PubMed] [Google Scholar]
- Raine A (2008). From genes to brain to antisocial behavior. Current Directions in Psychological Science, 17(5), 323–328. doi: 10.1111/j.1467-8721.2008.00599.x. [DOI] [Google Scholar]
- Raine A (2018). Antisocial personality as a neurodevelopmental disorder. Annual Review of Clinical Psychology, 14(1), 259–289. doi: 10.1146/annurev-clinpsy-050817-084819. [DOI] [PubMed] [Google Scholar]
- Ray RD, & Zald DH (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews, 36(1), 479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, … Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage, 45(2), 614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Miller MW, Wolf EJ, & Harkness KL (2015). Negative emotionality and disconstraint influence PTSD symptom course via exposure to new major adverse life events. Journal of Anxiety Disorders, 31, 20–27. doi: 10.1016/j.janxdis.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, … Fischl B (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Saxbe D, Lyden H, Gimbel SI, Sachs M, Piero LBD, Margolin G, & Kaplan JT (2018). Longitudinal associations between family aggression, externalizing behavior, and the structure and function of the amygdala. Journal of Research on Adolescence, 28(1), 134–149. doi: 10.1111/jora.12349. [DOI] [PubMed] [Google Scholar]
- Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, … Augustinack JC (2017). High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: Manual segmentation to automatic atlas. NeuroImage, 155, 370–382. doi: 10.1016/j.neuroimage.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, The Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics, Siegel BS, Dobbins MI, Earls MF, Garner AS, … Wood DL (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Ramaseshan K, Burgess A, & Diwadkar VA (2017). Hyper-modulation of brain networks by the amygdala among women with borderline personality disorder: Network signatures of affective interference during cognitive processing. Journal of Psychiatric Research, 88, 56–63. doi: 10.1016/j.jpsychires.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, & Meyer-Lindenberg A (2007). A validated network of effective amygdala connectivity. NeuroImage, 36(3), 736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Taki Y, & Kawashima R (2012). Brain development in childhood. The Open Neuroimaging Journal, 6, 103–110. doi: 10.2174/1874440001206010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, & Gunnar MR (2006). Child maltreatment and the developing HPA axis. Hormones and Behavior, 50(4), 632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, … Ebert D (2003). Frontolimbic brain abnormalities in patients with borderline personality disorder: A volumetric magnetic resonance imaging study. Biological Psychiatry, 54(2), 163–171. doi: 10.1016/S0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Tottenham N, & Galván A (2016). Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience & Biobehavioral Reviews, 70, 217–227. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Salat DH, & Fischl B (2008). Brain morphometry with multiecho MPRAGE. NeuroImage, 40(2), 559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff SJA, Pannekoek JN, Veer IM, van Tol M-J, Aleman A, Veltman DJ, … van der Wee NJA (2013). Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychological Medicine, 43(9), 1825–1836. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- van voorhees E, & Scarpa A (2004). The effects of child maltreatment on the hypothalamic-pituitary-adrenal axis. Trauma, Violence, & Abuse, 5(4), 333–352. doi: 10.1177/1524838004269486. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L, … Xue G (2016). Dissociated neural substrates underlying impulsive choice and impulsive action. NeuroImage, 134, 540–549. doi: 10.1016/j.neuroimage.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Wilbertz G, Tebartz van Elst L, Delgado MR, Maier S, Feige B, Philipsen A, & Blechert J (2012). Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. NeuroImage, 60(1), 353–361. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Wright P, Albarracin D, Brown RD, Li H, He G, & Liu Y (2008). Dissociated responses in the amygdala and orbitofrontal cortex to bottom-up and top-down components of emotional evaluation. NeuroImage, 39(2), 894–902. doi: 10.1016/j.neuroimage.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, & Wedig MM (2006). Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex, 16(12), 1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Yang Y, & Raine A (2007). Functional and structural brain imaging research on psychopathy. International Handbook of Psychopathic Disorders and the Law, 1, 69–81. 10.1002/9780470516157.ch4. [DOI] [Google Scholar]
- Yang Y, & Raine A (2009). Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Research: Neuroimaging, 174(2), 81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, & Seeley WW (2010). Network-level structural covariance in the developing brain. Proceedings of the National Academy of Sciences of the USA, 107(42), 18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]


