Abstract
Background:
Integrase strand transfer inhibitors(INSTIs) are first-line regimens for HIV treatment. We aimed to examine their impact on cognitive performance and depressive symptoms in women with HIV(WWH).
Setting:
Women’s Interagency HIV Study(WIHS) a multisite, prospective, cohort study
Methods:
WWH who started or switched to INSTI-based ART and completed neuropsychological(NP) testing and the Center for Epidemiological Studies-Depression(CES-D) scale before and after INSTI start/switch were included in the analyses. Primary outcomes were demographically corrected cognitive domain T-scores. Linear mixed effects models adjusted for relevant covariates were used to examine effects of start/switch of any INSTI and individual INSTI drugs on cognition and CES-D.
Results:
639 WWH, median age 49(interquartile range 12) years, 66% Black non-Hispanic, had NP and CES-D data before and after INSTI start/switch. While 14% started INSTI-based ART, the remainder switched to INSTI-based ART from another regimen. Overall, any INSTI use was associated with poorer learning post-INSTI. Specifically, use of elvitegravir, but not raltegravir, was associated with poorer learning. In analyses restricted to INSTI switch, any INSTI use, and specifically dolutegravir use, was associated with poorer learning. Among those switching from a PI-based regimen, INSTIs overall and dolutegravir remained associated with poorer learning; switching from an NNRTI to dolutegravir was also associated with poorer learning. INSTI start/switch was not related to depressive symptom changes.
Conclusions:
INSTI use was associated with poorer learning among WWH. These changes were mainly observed in elvitegravir and dolutegravir users, indicating that the impact of INSTI on cognition in WWH may not be a class effect.
Keywords: Women with HIV, cognition, learning, antiretroviral therapy, integrase strand transfer inhibitors
Introduction
Over the past decade, integrase strand transfer inhibitors (INSTIs) have emerged as an effective component of first line antiretroviral therapy (ART)1. INSTIs work by inhibiting the action of an enzyme, HIV integrase, which the virus needs in order to enter CD4 cells and replicate. To date, the Food and Drug Administration (FDA) has approved four INSTIs: raltegravir in 2007, elvitegravir in 2012, in 2013, and most recently bictegravir in 2018. Early clinical trials demonstrated that INSTIs are well tolerated with limited central nervous system (CNS) adverse events2,3, however following widespread use, reports of INSTI treatment discontinuation attributed to neuropsychiatric adverse events emerged, particularly with dolutegravir4,5. This remains controversial as not all published analyses demonstrate increased neuropsychiatric adverse events with INSTI use6. Furthermore, because studies reporting INSTI-based neuropsychiatric adverse events consist predominantly of men, these findings may not be generalizable to women with HIV (WWH). Women have been shown to have higher frequency of adverse events related to ART and other medications7–9.
One potential INSTI-related adverse neuropsychiatric side effect is cognitive impairment; however, little is known about the effect of INSTIs on cognition. Studies conducted predominantly in men with HIV on INSTI-based regimens demonstrated poorer verbal learning and memory compared to people with HIV (PWH) on non-INSTI-based regimens,10 and have indicated that long-term exposure to INSTIs, particularly, was associated with impaired cognitive profiles, such as globally low-performance or decreased performance in verbal learning and memory11. In contrast a recent study demonstrated no differences in NP performance between PWH initiating INSTI-based therapy and HIV-negative controls not on ART12; however, this is an all-male small sample study and the effect of INSTIs may have been offset by the effect of initiation of HIV treatment. Among WWH, we have previously demonstrated both positive and negative associations between specific INSTI drugs and cognition13. Raltegravir was associated with worse executive function among women with profound HIV legacy effects, but was associated with better memory among substance users with poorly controlled HIV. Additionally, was associated with better motor function among women primarily 36–55 years of age. Whether neuropsychiatric adverse events and poorer cognitive function are independent issues or represent a continuum of the same underlying etiology remains unknown.
A second INSTI-related adverse neuropsychiatric side effect that has garnered attention is depressive symptoms. Two retrospective cohort studies reported depression, amongst other neuropsychiatric side effects, as reasons for discontinuation of INSTI-based therapy, in particular, dolutegravir (DTG)4,5. However, the data surrounding increased neuropsychiatric side effects with INSTI therapy are inconclusive. Low rates of treatment discontinuation due to neuropsychiatric side effects are observed in larger analyses from the Swiss HIV Cohort Study and the Observational Pharmaco-Epidemiology Research & Analysis (OPERA) cohort, with the latter consisting of data from five randomized clinical trials6,14. Recently, data from a longitudinal cohort study, which included a planned switch to DTG, reported higher proportions of participants with moderate depressive symptoms following a switch to DTG, but no difference in the proportion with moderately severe symptoms using the Patient Health Questionnaire-9 (PHQ-9)15. While this analysis was limited to DTG, data on depressive symptoms with other INSTIs are not available. In addition, although the PHQ-9 is a brief, validated screening tool for depressive symptoms, it does not have the ability to differentiate symptoms on a sub-scale level.
In the present study, we aimed to examine the impact of starting or switching to, an INSTI on cognitive function and depressive symptoms in WWH, comparing neuropsychiatric assessments before and after INSTI initiation. We hypothesized that INSTI use would be associated with poorer neuropsychological outcomes and increases in depressive symptoms in WWH.
Methods
Study Population
Data from eligible participants in the Women’s Interagency HIV Study (WIHS) were used in this analysis. The WIHS study design and procedures have been described elsewhere16–18 but in brief, the WIHS is a multi-center, longitudinal cohort designed to expand our knowledge of HIV in women. Enrollment in the study occurred in four waves between October 1994 and September 2015. The first three enrollment waves occurred at six sites in Brooklyn, Bronx, Chicago, Washington DC, Los Angeles, and San Francisco) while the final enrollment wave included five additional Southern US sites in Chapel Hill, Atlanta, Miami, and Birmingham/Jackson.
Participants enrolled in WIHS engaged in semiannual study visits during which comprehensive medical histories including medication review and clinical examinations were performed. Participants also completed other study assessments including laboratory assessments, questionnaires and neuropsychological assessments. For the purpose of this study, only data from WIHS visits where neurocognitive assessments were performed was included totaling 18,363 observations. A further 7215 observations (from 1016 participants) were excluded as ART use “at study visit” and “since last study visit” (~ past 6 months) were different indicating an ART switch in that time period. This left 11,148 observations from 2418 participants. In addition, those not prescribed INSTIs were excluded leaving 4753 observations in 923 participants. Finally, we retained only those who has one neuropsychological assessment available before starting or switching to INSTIs and one after they started or switched to INSTI-based therapy leaving 1278 observations in 639 participants.
Outcome measures
Cognitive function
Participants completed a comprehensive neuropsychological (NP) battery which included the Hopkins Verbal Learning Test–Revised (HVLT-R)18, Trail Making Test (TMT), Stroop Test19, Symbol Digit Modalities Test (SDMT), Letter-Number Sequencing (LNS) Test, letter fluency, animal fluency, and Grooved Pegboard (GPEG). Each NP outcome of interest was transformed into demographically-corrected T-scores (M=50, SD=10) based on the WIHS HIV-uninfected women19,20 that were recruited into the study to be demographically similar to the WWH with respect to age, race/ethnicity, education, annual household income, and risk behaviors prior to study entry (i.e., substance use, risky sexual behavior)16–18. As in our previous studies, the NP outcomes were combined into domain-specific T-scores as follows; learning (HVLT-R total learning across trials 1–3), memory (HVLT-R delay free recall), verbal fluency (letter and animal fluency), fine motor skills (Grooved Pegboard dominant and non-dominant hands), processing speed (TMT-Part A, SDMT), attention/working memory (LNS attention and working memory conditions), and executive function (TMT-Part B, Stroop Trial 3). A global NP score was also computed for individuals with data for ≥4 domains. See details in our previous publication20. Global NP impairment was defined as a T-score ≤40.
Depressive Symptoms
The Centers for Epidemiologic Studies – Depression Scale (CES-D), a 20-item self-administered questionnaire, is administered to examine depressive symptoms in the WIHS. The CES-D queries symptoms experienced by respondents in the preceding week. This scale has been validated for use in people with HIV21–26. A CES-D score ⩾16 suggest a high level of depressive symptoms.
Covariates
We used covariates of interest that were available across the duration of WIHS in this analysis which included age, race/ethnicity, years of education, employment status, annual household income, and marital status, current smoking status, recent alcohol use, marijuana, crack, cocaine, and/or heroin use. We included clinical factors such as Center for Epidemiologic Studies Depression Scale (CES-D) scores (NP analyses only), Hepatitis C virus antibody status, body mass index (BMI), hypertension (systolic blood pressure ≥140, diastolic blood pressure ≥90, self-report or use of anti-hypertensive medications), and diabetes (self-reported anti-diabetic medication or any of fasting glucose ≥126 or HgbA1C >6.5% or self-reported diabetes) since past research has shown that these can affect cognitive and psychological status. HIV-related parameters such as HIV RNA (copies/ml), CD4+ T cell count (current and nadir; cells per mm3), and previous self-reported AIDS diagnosis were also included.
Statistical Analyses
A series of linear mixed effects models were used to examine cognitive and depressive symptom changes after starting or switching to INSTIs after adjusting for both time-invariant and time-varying covariates. We first examined the effects of all INSTIs followed by examining each of the three INSTI drugs separately (raltegravir, elvitegravir, or dolutegravir). Bictegravir was not included as there were insufficient observations. When effects were significant, we computed effect sizes using Cohen’s d methodology (effects, small=0.2; medium=0.5; large=0.8)27. Analyses were conducted in R version 3.5.2.
Results
Overall Study Population Characteristics pre-INSTI
Overall, 639 participants met the criteria for inclusion in the analysis, of whom 89 (14%) started INSTIs ‘starters’ and 550 (86%) switched to INSTIs ‘switchers.’ Demographically, starters were similar to switchers. Of those starting INSTIs, the median age was 46 (IQR 37, 54), 66% were Black-non Hispanic and 66% had high-school education or higher (Table 1).For those switching to INSTIs, the median age was 49 (IQR 42, 54), 65% were Black-non Hispanic and 68% had high-school education or higher (Table 2). The median current CD4+ T cell count for those starting INSTIs and those switching to INSTIs was 314 (IQR 174, 464) and 573 (IQR 369, 784) respectively. Thirty-six percent of those starting INSTIs and 40% of those switching to INSTIs had a self-reported diagnosis of AIDS. Of those who switched to INSTIs, 324 (59%) switched from PI-based regimens while 171 (31%) switched from NNRTI-based regimens. Switches from efavirenz accounted for 75% of those switched from NNRTIs. The median duration of ART use prior to switch was 12.4 (IQR 8.0 −16.2) years. Raltegravir, elvitegravir and dolutegravir were introduced in 38%, 24% and 38% of WWH, respectively.
Table 1.
Demographic, behavioral, and clinical characteristics at the WIHS visit before starting INSTI
| INSTI Overall (n=89) | Elvitegravir (n=22) | Raltegravir (n=38) | Dolutegravir (n=29) | |
|---|---|---|---|---|
| N (%) | n (%) | n (%) | n (%) | |
| Year of Age | ||||
| 26–35 | 19 (21) | 6 (27) | 7 (18) | 6 (21) |
| 36–45 | 23 (26) | 3 (14) | 11 (29) | 9 (31) |
| 45–55 | 32 (36) | 10 (45) | 14 (37) | 8 (28) |
| >55 | 15 (17) | 3 (14) | 6 (16) | 6 (21) |
| High school or less | 57 (64) | 13 (59) | 25 (66) | 19 (66) |
| White, non-Hispanic | 14 (16) | 3 (14) | 6 (16) | 5 (17) |
| Black, non-Hispanic | 59 (66) | 16 (73) | 21 (55) | 22 (76) |
| Hispanic | 15 (17) | 3 (14) | 10 (26) | 2 (7) |
| Other | 1 (1) | 0 (0) | 1 (3) | 0 (0) |
| Average Annual Household ncome ≤$12000 | 56 (63) | 11 (50) | 55 (39) | 24 (83) |
| Currently employed | 33 (37) | 11 (50) | 15 (39) | 7 (24) |
| Married | 26 (29) | 7 (32) | 13 (34) | 6 (21) |
| Currently smoking | 34 (38) | 5 (23) | 13 (34) | 16 (55) |
| Abstainer | 46 (52) | 14 (64) | 20 (53) | 12 (41) |
| 0–7 drinks/wk | 29 (32) | 8 (36) | 10 (26) | 11 (38) |
| >7 drinks/wk | 14 (16) | 0 (0) | 8 (21) | 6 (21) |
| Marijuana | 23 (26) | 2 (9) | 10 (26) | 11 (38) |
| Crack, cocaine, and/or heroin | 7 (8) | 0 (0) | 3 (8) | 4 (14) |
| Hepatitis C RNA positive | 20 (22) | 1 (5) | 10 (26) | 9 (31) |
| Body Mass Index ≥30 kg/m2 | 38 (43) | 14 (64) | 11 (29) | 13 (45) |
| Hypertension | 30 (34) | 8 (36) | 11 (29) | 11 (38) |
| Diabetes | 22 (25) | 3 (14) | 10 (26) | 9 (31) |
| CD4+ T cell count (cells/mm3), median (IQR) | ||||
| Current | 314 (145) | 364 (131) | 237 (109) | 372 (203) |
| Nadir | 219 (128) | 269 (117) | 140 (86) | 269 (149) |
| HIV RNA (copies/mL) | ||||
| Undetectable | 3 (3) | 2 (9) | 1 (3) | 0 (0) |
| ≤500 | 9 (10) | 2 (9) | 4 (11) | 3 (10) |
| >500 | 76 (85) | 18 (82) | 33 (87) | 25 (86) |
| Prior AIDS diagnosis | 32 (36) | 2 (9) | 20 (53) | 10 (34) |
| CESD score >16 | 29 (33) | 5 (23) | 12 (32) | 12 (41) |
Note. Current, refers to within the past week; recent, refers to within 6 months of the most recent WIHS visit. CESD, Centers for Epidemiological Studies Depression Scale
Table 2.
Demographic, behavioral, and clinical characteristics at the WIHS visit before switching to II from ART.
| Variable | INSTI Overall (n=550) | Elvitegravir (n=132) | Raltegravir (n=202) | Dolutegravir (n=216) |
|---|---|---|---|---|
| N (%) | n (%) | n (%) | n (%) | |
| Year of Age | ||||
| 26–35 | 40 (7) | 12 (9) | 20 (10) | 8 (4) |
| 36–45 | 170 (31) | 41 (31) | 75 (37) | 54 (25) |
| 45–55 | 225 (41) | 51 (39) | 76 (38) | 98 (45) |
| >55 | 115 (21) | 28 (21) | 31 (15) | 56 (26) |
| High school or less | 350 (64) | 84 (64) | 130 (64) | 136 (63) |
| White, non-Hispanic | 79 (14) | 11 (8) | 33 (16) | 35 (16) |
| Black, non-Hispanic | 359 (65) | 97 (73) | 124 (61) | 138 (64) |
| Hispanic | 89 (16) | 15 (11) | 40 (20) | 34 (16) |
| Other | 23 (4) | 9 (7) | 5 (3) | 9 (4) |
| Average Annual Household Income ≤$12000 | 256 (47) | 62 (47) | 98 (49) | 96 (44) |
| Currently employed | 189 (34) | 47 (36) | 66 (33) | 76 (35) |
| Married | 169 (31) | 33 (25) | 64 (32) | 72 (33) |
| Currently smoking | 196 (36) | 54 (41) | 76 (38) | 66 (31) |
| Abstainer | 330 (60) | 69 (52) | 132 (65) | 129 (60) |
| 0–7 drinks/wk | 184 (33) | 56 (42) | 60 (30) | 68 (31) |
| >7 | 36 (7) | 7 (5) | 10 (5) | 19 (9) |
| Marijuana | 93 (17) | 25 (19) | 28 (14) | 40 (19) |
| Crack, cocaine, and/or heroin | 29 (5) | 8 (6) | 9 (4) | 12 (6) |
| Hepatitis C RNA positive | 102 (19) | 14 (11) | 43 (21) | 45 (21) |
| Body Mass Index ≥ 30 (kg/m2) | 242 (44) | 68 (52) | 69 (33) | 105 (49) |
| Hypertension | 253 (46) | 68 (52) | 76 (38) | 109 (50) |
| Diabetes | 120 (22) | 27 (20) | 38 (19) | 55 (25) |
| Current | 573 (207) | 634 (185) | 458 (207) | 622 (192) |
| Nadir | 208 (120) | 298 (136) | 140 (88) | 239 (126) |
| HIV RNA (copies/mL) | ||||
| Undetectable | 252 (46) | 89 (67) | 18 (9) | 145 (67) |
| ≤500 | 208 (38) | 37 (28) | 118 (58) | 53 (25) |
| >500 | 90 (16) | 6 (5) | 66 (32) | 18 (8) |
| Prior AIDS diagnosis | 219 (40) | 27 (20) | 108 (53) | 84 (39) |
| CESD score >16 | 148 (27) | 34 (26) | 59 (29) | 55 (25) |
| Previous ART Therapy | ||||
| NNRTI based | 171 (31) | 69 (52) | 32 (16) | 70 (32) |
| PI based | 324 (59) | 54 (41) | 132 (65) | 138 (61) |
| Others | 55 (10) | 9 (7) | 38 (19) | 8 (7) |
Note. Current, refers to within the past week; recent, refers to within 6 months of the most recent WIHS visit. CESD, Centers for Epidemiological Studies Depression Scale
Due to the nature of the data collection in WIHS, the exact date of INSTI start/switch was not available but known to have occurred in the six month interval between study visits. The median time from baseline NP to start/switch was 690 (431–871) days. The median time from study visit at which start/switch was reported to NP test visit was 186 (IQR 0, 387) days. It is important to note that all switches occurred at some point in the six months prior to the study visit at which start/switch was reported. The median time between the two NP tests was 755 (721, 1294) days. Using the global NP score, 136 (21.3%) of participants were considered cognitively impaired at baseline. Eighty (58.8%) of those women impaired at baseline remained impaired post start/switch, while 52 (38.2%) were no longer impaired post start/switch. Fifty-six (14.5%) of those women who had normal scores at baseline were impaired on subsequent testing. The proportion of participants with CES-D scores ≥16 prior to start/switch was 29.7% compared to 31.1% post switch.
Associations of INSTI Use with Cognitive Domains
Overall, any INSTI use was associated with poorer learning after start/switch (before 49.5 (SD 9.8) versus after 48.1 (10.2); p<0.001; Cohen’s d= −0.16) (Figure 1). There were no differences in any other cognitive domain before and after INSTI use. When examined by individual INSTI drug, start/switch of elvitegravir (before 49.6 (SD 9.4) versus after 48.0 (10.2); p=0.02; Cohen’s d= −0.19) and dolutegravir (before 49.5 (SD 10.1) versus after 47.5 (10.5); p=0.002; Cohen’s d= −0.22) were associated with poorer learning, but raltegravir use was not (p=0.71) (Figure 1).
Figure 1.
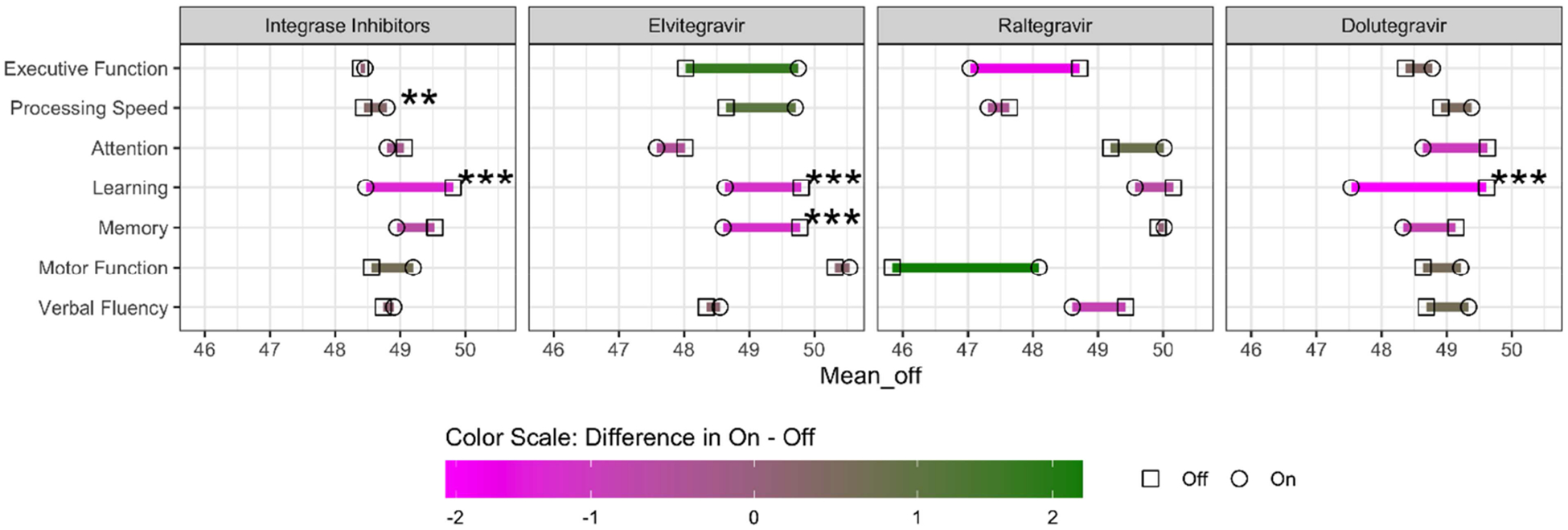
Mean T-score per cognitive domain before and after start/switch of integrase strand transfer inhibitor. Green corresponds to improvement and magenta to decline. Significance derived from linear effects model.
In analyses restricted to participants who switched to INSTI-based therapy (n=550), any INSTI use was associated with poorer learning (before 49.8 (SD 9.9) versus after 48.5 (10.3); p=0.009; Cohen’s d= −0.15), as was use of dolutegravir (p=0.004). When restricted to those started on INSTIs (n=89), overall INSTI use remained associated with poorer learning although this was not retained in the model after adjusting for covariates (before 47.7 (SD 9.1) versus after 45.9 (9.7); p=0.02; Cohen’s d= −0.20).
In adjusted analyses, overall INSTIs and dolutegravir remained associated with poorer learning among those switching from a PI-based regimen (p’s<0.05). Dolutegravir also remained associated with poorer learning among those switching from an NNRTI (before 51.7 (SD 10.3) versus after 48.7 (10.3); p=0.02; Cohen’s d= −0.33). In contrast, switching from an NNRTI to an INSTI was also associated with better processing speed, while switching from an NNRTI to EVG specifically was associated better executive function and processing speed (p’s<0.05).
Finally, when restricted to those who switched from efavirenz, overall INSTI use was associated with poorer memory (before 51.4 (SD 9.5) versus after 49.3 (10.7); p=0.02; Cohen’s d= −0.25) and learning (before 52.3 (SD 9.5) versus after 50.2 (10.0); p=0.02; Cohen’s d= −0.25). In contrast, better processing speed (before 49.0 (SD 8.1) versus after 50.1 (8.5); p=0.03; Cohen’s d= 0.15), and verbal fluency (before 48.3 (SD 9.1) versus after 49.9 (10.1); p=0.03; Cohen’s d= 0.20) were observed. Poorer learning was also observed in those switching from efavirenz to dolutegravir (before 51.4 (SD 10.1) versus after 47.5 (10.4); p=0.02; Cohen’s d= −0.43). All analyses were adjusted for covariates outlined above.
Associations of INSTI Use with Depressive Symptoms
Overall, INSTI use (start and switch combined) was not associated with changes in depressive symptoms as determined by CES-D scale scores. Similarly, start/switch to individual INSTIs (DTG, EVG or RAL) did not affect depressive symptom scores. When analysis was restricted to those who switched to INSTIs from any previous ART, again, there were no effects observed on depressive symptom scores. Similarly, switch from a PI-based regimen or an NNRTI regimen (overall or limited to EFV) to any INSTI or individual INSTIs did not affect depressive symptom scores (all p>0.05).
Discussion
As efforts continue to determine the effects of contemporary ART on cognitive function in PWH, we sought to examine the impact of starting on, or switching to INSTI-based therapy in WWH. Here, we found that overall INSTI use was associated with poorer performance, in verbal learning among WWH. This finding persisted in those who switched from other ART regimens to INSTI-based regimens. Interestingly, our data suggests that poorer learning observed in our cohort may not be related to an INSTI class effect but rather associated with specific drugs, namely elvitegravir and dolutegravir use. Both elvitegravir and dolutegravir were associated with poorer learning in the overall analysis that considered those starting and switching to INSTIs, while dolutegravir remained associated with poorer learning when the analysis was restricted to those switching ART irrespective of whether they switched from PI-based or NNRTI, including efavirenz-based regimens. Bictegravir, a recently approved INSTI, was not included in this analysis.
Our results in WWH align with data demonstrating worse learning and memory performance in a demographically similar group of men and WWH28. The potential mechanism for this is unclear; however, one possible contributor may relate to polymorphisms in UGT1A1, which influences INSTI metabolism. In support of this hypothesis, a recent study demonstrated that specific UGT1A1 gene polymorphisms, in addition to younger age, were associated with increased dolutegravir trough concentration and increased overall neuropsychiatric side effects29. However, high dolutegravir concentrations were not associated with worse cognition in a sample of older, predominantly White men30. Given the significant demographic difference between our study population and prior studies, investigating UGT1A1 gene polymorphisms in our cohort of predominantly Black, non-Hispanic women is warranted. Nevertheless, given the multifactorial nature of cognitive impairment in WWH, it also possible that indirect underlying mechanisms could be contributing to our findings.
To date, many of the larger studies examining the impact of INSTIs on neural function focus on more general neuropsychiatric side effects including depression, insomnia, and anxiety but not cognitive function4–6,14. Whether adverse mental health symptoms and poorer cognition are independent issues or a continuum of the same underlying etiology remains unknown with INSTI use. For this reason, our group recently examined and reported on the impact of INSTI on post-traumatic stress disorder (PTSD)31 and here we present data on depressive symptoms in WWH. Interestingly, we did not observe a worsening in depressive symptomatology when WWH were started on or switched to INSTIs as a class or individual INSTI drugs In addition, an elvitegravir-based ART regimen was associated with improved PTSD symptoms, in both those starting and switching INSTIs, while switching onto a raltegravir-based regimen was associated with worse PTSD symptoms but had no impact on cognition. Dolutegravir-based regimens either did not affect, or worsened PTSD symptoms, in those starting or switching to its use31. These data indicate that the impact of INSTIs on neural function in WWH are varied and suggest that assessment across a host of neuropsychiatric complications need to be considered to ensure that less clinically apparent impacts such as mild cognitive changes are noticed if patients do not present with other neuropsychiatric symptoms such as depression or anxiety.
This study has limitations. As is generally the case in studies that examine changes in the setting of ART switch, it is always worth considering that the observation being reported is a result of the removal of a previous drug rather than the addition of a new drug. In this case, we did see similar results in those starting INSTIs (who were currently not on ART) which would therefore indicate that our observations related to the addition of INSTIs rather than the withdrawal of non-INSTI drugs. Additionally, the exact date for starting or switching ART regimens were not collected in our cohort and cumulative drug effect was not examined. Furthermore, we measured cognition on one occasion before and one after INSTI initiation; additional time points would facilitate more fine-grained analyses to better understand acute and longer-term effects of starting or switching to an INSTI-based regimen and to understand their impact not only on neuropsychological testing parameters but also on functional status. Finally, although significant efforts were made to adjust for other factors that might impact cognition in this population it is possible that unmeasured factors could play a role.
In summary, switching to or starting an INSTI-based regimen was primarily associated with poorer learning among WWH. However, because this observation was not seen with raltegravir use, our data suggest that the impact of INSTIs on cognition in WWH may not be a class effect. Validation of our finding in other large cohorts in warranted, as is assessment of the neuropsychiatric effects of newer INSTIs including bictegravir and cabotegravir.
Acknowledgement
This work was supported by the Johns Hopkins University NIMH Center for novel therapeutics for HIV-associated cognitive disorders (P30MH075773) 2018 pilot award to Dr. Rubin, the Johns Hopkins University Center for AIDS Research NIH/NIAID fund (P30AI094189) 2019 faculty development award to Dr. Xu, and NSF DMS1918854 to Drs. Xu and Rubin. Dr. O’Halloran is a scholar on the Sustained Training in HIV and Aging (STAHR) program which is supported by the STAHR Training Grant (R25 MH108389). Dr. Williams effort was supported by K99DA044838. Dr. Lahiri is also supported by NIH/NIAID K23AI124913. AS has received funding from Gilead Sciences, Inc. Data in this manuscript were collected by the Women’s Interagency HIV Study, now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Footnotes
The authors report no conflicts of interest related to this work.
This work was presented at the Conference of Retroviruses and Opportunistic Infections, Boston, 2020
Financial Disclosures
AS has received funding from Gilead Sciences, Inc for unrelated work. The remaining authors have nothing to disclose.
References
- 1.Kamal S, Locatelli I, Wandeler G, et al. The Presence of Human Immunodeficiency Virus-Associated Neurocognitive Disorders Is Associated With a Lower Adherence to Combined Antiretroviral Treatment. Open Forum Infect Dis. 2017;4(2):ofx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. [DOI] [PubMed] [Google Scholar]
- 3.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. [DOI] [PubMed] [Google Scholar]
- 4.de Boer M, van den Berk G, van Holten N, et al. Intolerance of dolutegravir containing cART regimens in real life clinical practice. AIDS. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63. [DOI] [PubMed] [Google Scholar]
- 6.Fettiplace A, Stainsby C, Winston A, et al. Psychiatric Symptoms in Patients Receiving Dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamargo J, Rosano G, Walther T, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3(3):163–182. [DOI] [PubMed] [Google Scholar]
- 8.Karalis DG, Wild RA, Maki KC, et al. Gender differences in side effects and attitudes regarding statin use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) study. J Clin Lipidol. 2016;10(4):833–841. [DOI] [PubMed] [Google Scholar]
- 9.Kempf MC, Pisu M, Dumcheva A, Westfall AO, Kilby JM, Saag MS. Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr. 2009;52(3):336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Halloran JA, Cooley SA, Strain JF, et al. Altered neuropsychological performance and reduced brain volumetrics in people living with HIV on integrase strand transfer inhibitors. AIDS. 2019;33(9):1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amusan P, Power C, Gill JM, et al. Lifetime antiretroviral exposure and neurocognitive impairment in HIV. Journal of Neurovirology. 2020;ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Prats A, Martínez-Zalacaín I, Mothe B, et al. Central nervous system effects of therapy initiation with integrase inhibitors. Conference on Retroviruses and Opportunistic Infections, Seattle. 2019. [Google Scholar]
- 13.Rubin LH, Li Y, Fitzgerald KC, et al. Associations between Antiretrovirals and Cognitive Function in Women with HIV. J Neuroimmune Pharmacol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elzi L, Erb S, Furrer H, et al. Adverse events of raltegravir and dolutegravir. AIDS. 2017;31(13):1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan P, Goh O, Kroon E, et al. Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res Ther. 2020;17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 17.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki PM, Rubin LH, Valcour V, et al. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology. 2015;84(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin LH, Maki PM, Springer G, et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology. 2017;89(15):1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore J, Schuman P, Schoenbaum E, Boland B, Solomon L, Smith D. Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. Aids. 1999;13(17):2459–2468. [DOI] [PubMed] [Google Scholar]
- 22.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. [DOI] [PubMed] [Google Scholar]
- 23.Maki PM, Rubin LH, Cohen M, et al. Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Menopause. 2012;19(11):1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin LH, Cook JA, Grey DD, et al. Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. J Womens Health (Larchmt). 2011;20(9):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook JA, Cohen MH, Burke J, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30(4):401–409. [DOI] [PubMed] [Google Scholar]
- 26.Cook JA, Grey DD, Burke-Miller JK, et al. Illicit drug use, depression and their association with highly active antiretroviral therapy in HIV-positive women. Drug Alcohol Depend. 2007;89(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenhard W, Lenhard A. Calculation of effect sizes. Psychometrica Web site. https://www.psychometrica.de/effect_size.html. Published 2016. Accessed May 7, 2020.
- 28.O’Halloran JA, Cooley SA, Strain JF, et al. Altered neuropsychological performance and reduced brain volumetrics in people living with HIV on integrase strand transfer inhibitors. AIDS (in press). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagura H, Watanabe D, Kushida H, et al. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC infectious diseases. 2017;17(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliot ER, Wang X, Singh S, et al. Increased Dolutegravir Peak Concentrations in People Living With Human Immunodeficiency Virus Aged 60 and Over, and Analysis of Sleep Quality and Cognition. Clin Infect Dis. 2019;68(1):87–95. [DOI] [PubMed] [Google Scholar]
- 31.Kamkwalala AR, Wang K, O’Halloran J, et al. Starting or Switching to an Integrase Inhibitor-Based Regimen Affects PTSD Symptoms in Women with HIV. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


