Abstract
Hypertension adversely affects the quality of life in humans across modern society. Studies have attributed increased reactive oxygen species production to the pathophysiology of hypertension. So far, a specific drug to control the disease perfectly has not been developed. However, artichoke, an edible vegetable, plays an essential role in treating many diseases due to its potent antioxidant activities. The objective of this study is to evaluate the effect of artichoke bud extract (ABE) on heart tissue metabolomics of hypertensive rats. Spontaneously hypertensive rats and Wistar–Kyoto (WKY) rats were divided into six groups, then exposed to different doses comprising ABE, Enalapril Maleate, or 1% carboxylmethyl cellulose for 4 weeks. Their blood pressures were recorded at 0, 2, 3, and 4 weeks after the start of the test period. Thereafter, all rats were anesthetized, and blood was collected from their cardiac apexes. Then, we measured the levels for 15 kinds of serum biochemical parameters. An established orthogonal partial least square-discriminant analysis model completed the metabolomic analysis. Hypertensive rats in the ABE group exhibited well-controlled blood pressure, relative to those in the model group. Specifically, artichoke significantly lowered serum levels for total protein (TP), albumin (ALB), and uric acid (UA) in the hypertensive rats. This effect involved the action of eight metabolites, including guanine, 1-methylnicotinamide, p-aminobenzoic acid, NAD, NADH, uridine 5′-monophosphate, adenosine monophosphate, and methylmalonic acid. Collectively, these findings suggest that ABE may play a role in affecting oxidative stress and purine, nicotinate, and nicotinamide metabolism.
1. Introduction
While acknowledging significant advances in medical therapy, hypertension (mean systolic blood pressure, SP ≥ 140 mm Hg or mean diastolic blood pressure, DP ≥ 90 mm Hg) remains a global public health threat.1 For instance, the 2016 age-adjusted death rate of people primarily attributable to hypertension was 21.6 per 100,000. Based on the National Health And Nutrition Examination Survey (NHANES), the age-adjusted hypertension prevalence among US adults ≥20 years old was estimated at 46.0% between 2013 and 2016, comprising 49.0 and 42.8% males and females, respectively. Hypertension increases the mortality risks of cerebrovascular disease and other diseases.2 Additionally, hypertension is the leading risk factor for deaths and disabilities, including stroke, accelerated coronary and systemic atherosclerosis, heart failure, chronic kidney disease, diabetes, vascular remodeling, and endothelial-to-mesenchymal transition and cardiovascular-related mortalities.3−7
A large number of studies have shown that the degree of portal hypertension is an important prognostic factor for liver dysfunction.8,9 Additionally, necrotizing inflammation of the liver can promote the increase of hepatic vascular tension.10 At the same time, hypertension is also the main cause of chronic kidney disease.11 Retrospective analysis showed that the level of serum uric acid was negatively correlated with renal function. The level of serum uric acid is directly related to SP and DP.12 Notably, patients with hypertension are closely related to dyslipidemia.13
The occurrence and severity of this disease are attributed to lifestyle factors including high sodium intake, weight gain and obesity, excess alcohol intake, and use of certain medications, specifically nonsteroidal anti-inflammatory drugs, stimulants, and decongestants.14 A previous study reported that high blood pressure is a heritable trait with heritability estimates of 48 to 60% (SP) and 34 to 67% (DP) across families.15
Treatment of hypertension involves the use of nonpharmacologic (lifestyle change) and pharmacologic approaches.14,16 These approaches aim at lowering blood pressure to less than 140/90 mm Hg.3 A meta-analysis, comprising 24 studies (N = 961,035), estimated a 13.7% prevalence of apparent treatment-resistant hypertension at (95% CI, 11.2–16.2%).17
Animal and human studies indicated an increase in the production of reactive oxygen species (ROS), indicating a pathological physiological factor for high blood pressure.18,19 Also, these studies reveal numerous molecular pathways associated with the pathogenesis of hypertension, including nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, uncoupled endothelial nitric oxide synthase, mitochondrial electron transport chain, xanthine oxidase, vasoactive peptides, and Src Family of kinases and Rho-kinase.20 Functionally, NADPH oxidase primarily produces peroxides in the vascular system via redox reactions.21 Studies have also shown that excessive ROS production by mitochondria causes hypertension,22 with mitochondrial ROS inhibition, MitoQ effectively reducing blood pressure and myocardial hypertrophy in spontaneously hypertensive rats (SHRs).23 Xanthine oxidase catalyzes the conversion of ATP to hypoxanthine, eventually causing the production of O2– and H2O2. Jankov et al. reported that xanthine oxidase-mediated O2– production induced neonatal hypertension in rats.24
Antioxidant genes, such as superoxide dismutase 2 (SOD2), peroxidase 2 (PRDX2), glutathione reductase (GSR), and glutathione peroxidase 4 (GPX4), play an important role in oxidative stress-related diseases. SOD2 is a mitochondria-specific antioxidant enzyme. It dismutates the superoxide into hydrogen peroxide, and then the catalase and glutathione peroxidase convert the hydrogen peroxide into water.25 Down-regulation of SOD2 expression may aggravate pulmonary hypertension.26 PRDX2 is an endogenous peroxidase, which can reduce oxidative damage in cells, thus reducing cell injury and apoptosis.27 PRDX2 gene knockdown leads to an increase in intracellular ROS, oxidative damage, and double-stranded DNA breaks.28 GSR is a key member of the glutathione antioxidant defense system. It converts oxidized glutathione into reduced glutathione to maintain the redox state of glutathione in cells and protect cells from oxidative damage.29 The activity of selenium peroxidase GPX4 plays a key role in the process of antioxidant defense.30 GPX4 converts lipid hydroperoxides into lipid alcohols, which prevents the formation of iron (Fe)-dependent toxic lipid ROS. Inhibition of GPX4 leads to lipid peroxidation and may lead to ferroptosis, which is an iron-dependent, non-apoptotic form of cell death.31
SHR is an animal model used for the study of spontaneous hypertension. The control rat is a Wistar–Kyoto (WKY) rat. There are differences in the renin-angiotensin system function between WKY and SHR.32,33
Since ancient times, Egyptians, Greeks, and Romans have widely used Cynara scolymus L. (family Asteraceae), a perennial plant also known as “artichoke”, for medicinal purposes.34 Over the centuries, many populations have incorporated artichokes into their culture and food habits because of their efficacy and safety.35 Pre- and clinical studies show that artichoke bud extract (ABE) has potential as a lipid-lowering and hepatoprotective agent36,37 due to the presence of powerful antioxidants. For instance, animal experiments indicated that these extracts elevate superoxide dismutase, catalase, glutathione, and glutathione peroxidase activities in the liver and lower the contents of malondialdehyde in the liver and plasma.38 Additionally, artichoke can alleviate diabetes induced by a high-fat diet in mice.39
Analytically, the plant contains the following chemicals, caffeoylquinic acid derivatives, chlorogenic acid (3-caffeoylquinic acid), cynarin (1,3-O-dicaffeoylquinic acid), 3,5-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, luteolin-7-rutinoside, cynaroside, apigenin-7-rutinoside, and apigenin-7-O-β-d-glucopyranoside (detected by high-performance liquid chromatography, HPLC). Chlorogenic acid and cynarin are considered to be the main active substances.40−42
Roghani-Dehkordi et al. first reported the antihypertensive effect of artichokes in patients with mild hypertension. SP of patients who received 50 and 100 mg of artichoke juice concentrate for 12 weeks significantly reduced (−2.74 and −2.95 mm Hg for change of SP, respectively; P = 0.006 and P = 0.007). Similarly, changes for DP of patients who received 50 and 100 mg of artichoke juice concentrate were −2.5 and −3.02 mm Hg, respectively (P = 0.008 and P = 0.002), indicating a dose-dependent pattern of the artichokes.43 Additionally, the body mass index in hypertensive patients given artichoke powder (500 mg twice daily for 8 weeks) was remarkably improved.44 In addition to antioxidation, the effect of ABE on endothelial dysfunction may also contribute to its antihypertensive effect. Li et al. discovered the enhanced endothelium-dependent vasodilation of rat aorta ex vivo incubated with artichoke leaf extract.45 Nonetheless, the specific mechanism of the antihypertensive effect of ABE remains unknown. As such, we evaluated the effect of ABE on heart tissue metabolomics of SHR, relative to age-matched WKY rats and explored the antihypertensive mechanism of ABE.
2. Results
2.1. Effect of ABE on Liver Function, Kidney Function, and Serum Lipid Profile of SHRs
The mean SP and DP of rats in the hypertension model (HPM) group were 179.83 ± 6.05 and 146.86 ± 5.53, respectively. Figure S1A–D demonstrates that exposure of WKY rats to ABE did not cause any significant alteration to SP or DP. This indicates that this dose of ABE does not affect SP or DP of WKY rats.
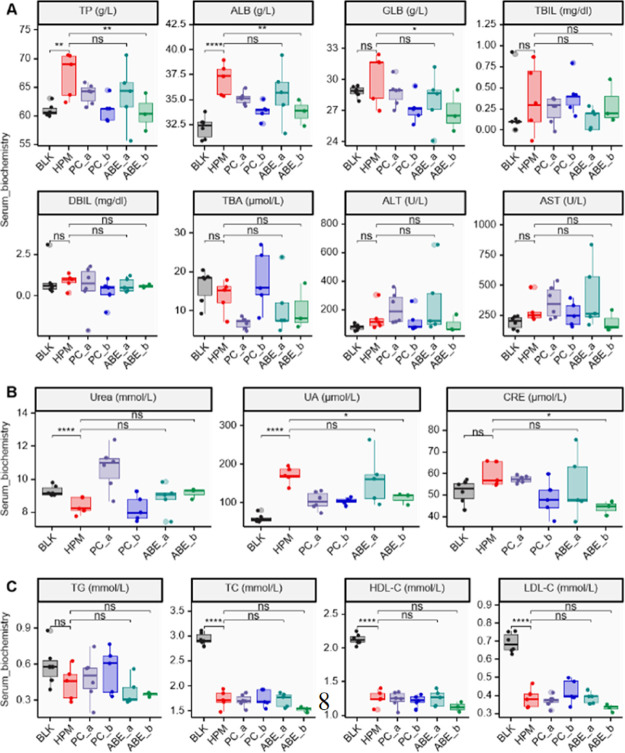
Rats in the group HPM exhibited higher levels of serum total protein (TP) and albumin (ALB) than those in the blank (BLK) group (P = 3.64 × 10–3 and P = 3.06 × 10–5 for TP and ALB, respectively) (Figure 1A). Interestingly, a comparison of these results revealed a significant reduction in serum TP and ALB levels after ABE treatment, relative to 1% carboxylmethyl cellulose for SHRs (P = 8.84 × 10–3 and P = 7.99 × 10–3 for TP and ALB, respectively).
Figure 1.
Effect of different dosage of ABE on the serum biochemical parameters of liver function (A), kidney function (B), and serum lipid profile (C). n(BLK) = 6; n(HPM) = 5; n(PC_a) = 6; n(PC_b) = 5; n(ABE_a) = 5; n(ABE_b) = 3. ns, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (ANOVA and post-hoc LSD test). TP, total protein; ALB, albumin; GLB, globulin; TBIL, total bilirubin; DBIL, direct bilirubin; TBA, total bile acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UA, uric acid; CRE, creatinine; TG, triglyceride; TC, cholesterol; HDL-C, high-density lipoprotein cholesterol c; LDL-C, low-density lipoprotein cholesterol c; BLK, blank; HPM, hypertension model; PC_a, positive drug control, 1 mg/kg/d Enalapril Maleate; PC_b, positive drug control, 20 mg/kg/d Enalapril Maleate; ABE_a, 25 mg/kg/d ABE; ABE_b, 50 mg/kg/d ABE.
Preliminary outcomes from serum biochemical indices targeting kidney function revealed significantly higher levels of UA in the group HPM than BLK (P = 4.08 × 10–6) (Figure 1B). Nevertheless, rats in the ABE_b group exhibited significantly lower UA (P = 1.44 × 10–2). Analysis of serum biochemical indices, targeting serum lipids, demonstrated that exposure of SHR rats to ABE did not cause any significant alteration to triglyceride (TG), cholesterol (TC), high-density lipoprotein cholesterol c (HDL-C), and low-density lipoprotein cholesterol c (LDL-C) levels (Figure 1C).
Figure S1E,F demonstrates that exposure of WKY rats to ABE did not cause any significant alteration on liver function or kidney function of control rats. WKY rats in the group TC exhibited lower levels of serum urea than those in the BLK group (P = 3.97 × 10–7) (Figure S1G).
2.2. Effect of ABE on Tissue Metabolites in SHRs
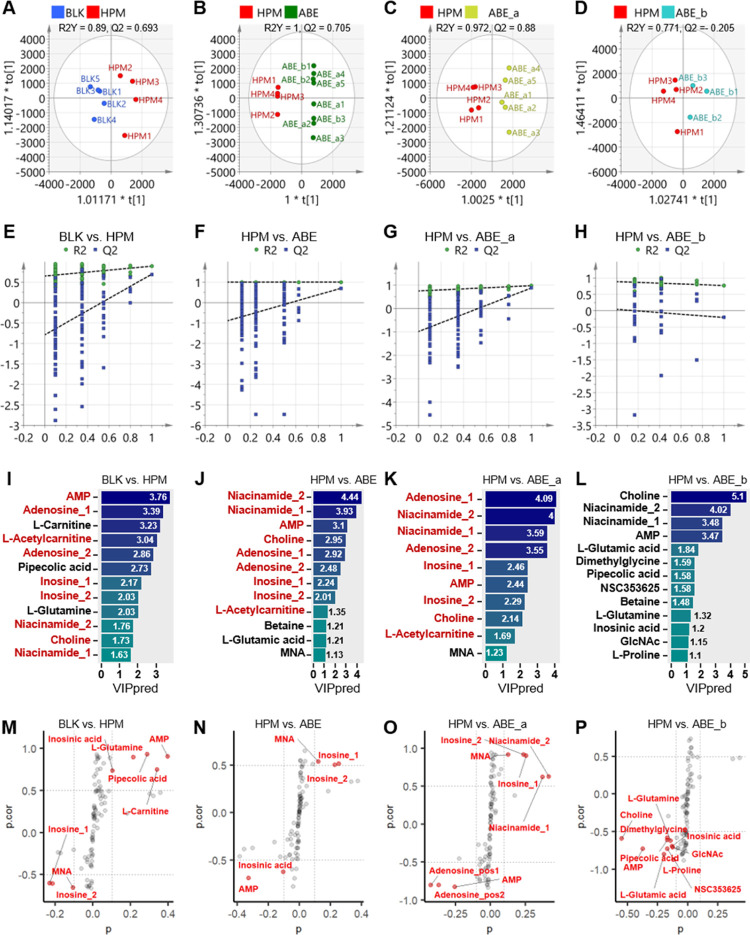
Orthogonal partial least square-discriminant analysis (OPLS-DA) was used to identify metabolites responsible for the observed differences across groups and distinguished metabolic profiles using score plots, 200-permutation tests, variable important projection (VIP) score bar plots, and S-plots (Figure 2).
Figure 2.
Effect of different dosages of ABE on the metabolomic profile of heart tissue from rats. (A–D) OPLS-DA model discriminating the metabolic profiles of rats. (E–H) 200-permutation test of different groups. (I–L) VIP scores of OPLS-DA analysis. (M–P) S-plot obtained from the OPLS-DA model, variables selected (|p| > 0.1 & |p.cor| > 0.5) are highlighted in red. BLK, blank; HPM, hypertension model; ABE_a, 25 mg/kg/d ABE; ABE_b, 50 mg/kg/d ABE; AMP, Adenosine monophosphate; MNA, 1-Methylnicotinamide; NSC353625, N-α-acetyllysine; GIcNAc, N-acetylglutamine.
Also, R2Y and Q2 were applied to validate the quality of OPLS-DA modeling using a model threshold of 0.5. Summarily, score plots revealed clear segregation between rats in the BLK and HPM groups (R2Y = 0.89, Q2 = 0.693, R2Y-Q2 < 0.2) (Figure 2A), indicating that the model had excellent quality. The predictive ability of the OPLS-DA model was further verified using a 200-permutation test. In the permutation test, the Q2Y-intercept was generally less than 0.05. Specifically, Q2Y-intercepts complied with the requirement (Q2Y-intercept = −0.787), indicating that our OPLS-DA model for BLK versus HPM was well-validated (Figure 2E). VIP values allowed the classification of metabolites with VIP >1 for BLK versus HPM groups (Figure 2I). S-plot of the variables for discriminating metabolites in the OPLS-DA model revealed a covariance against correlation p (cor) (Figure 2M). Significantly different metabolites, between BLK and HPM groups, were identified using absolute values of covariance p > 0.1 and absolute values of correlation p (cor) > 0.5 as selection criteria. Metabolites that showed significant influence on group separation were marked in red.
R2Y values in all OPLS-DA models for HPM versus those treated with ABE were more than 0.5, whereas Q2 ones for HPM versus ABE and HPM versus ABE_a were not less than 0.5 (Figure 2B–D). Notably, the difference between R2Y and Q2 for rats in HPM and ABE groups was less than 0.2, indicating that the model was excellent.
Results from the 200-permutation tests for rats in the HPM group versus The ABE group is shown in Figure 2F–H. Particularly, all Q2Y-intercepts for HPM versus ABE (−0.887), HPM versus ABE_a (−0.988), and HPM versus ABE_b (0.0443) complied with the requirement.
VIP bar plots for HPM versus groups treated with ABE are shown in Figure 2J–L. Score plot and 200-permutation test results indicate that OPLS-DA models for BLK versus HPM, HPM versus ABE, and HPM versus ABE_a were excellent and efficient (Figure 2). Interestingly, significant differences were found in six metabolites, including choline, niacinamide, adenosine, adenosine monophosphate (AMP), inosine, and l-acetylcarnitine, across all three excellent models. These are marked in red in Figure 2I–K.
S-plots of variables discriminating metabolites of OPLS-DA models for HPM and groups treated with ABE are presented in Figure 2N–P. Metabolites with a significant influence on group separation are marked in red (|p| > 0.1 & |p.cor| > 0.5). Notably, three shared differential metabolites, including inosine, MNA, and AMP, had remarkable discrimination in every OPLS-DA model.
2.3. Identification of Significantly Regulated Metabolites for ABE in SHRs
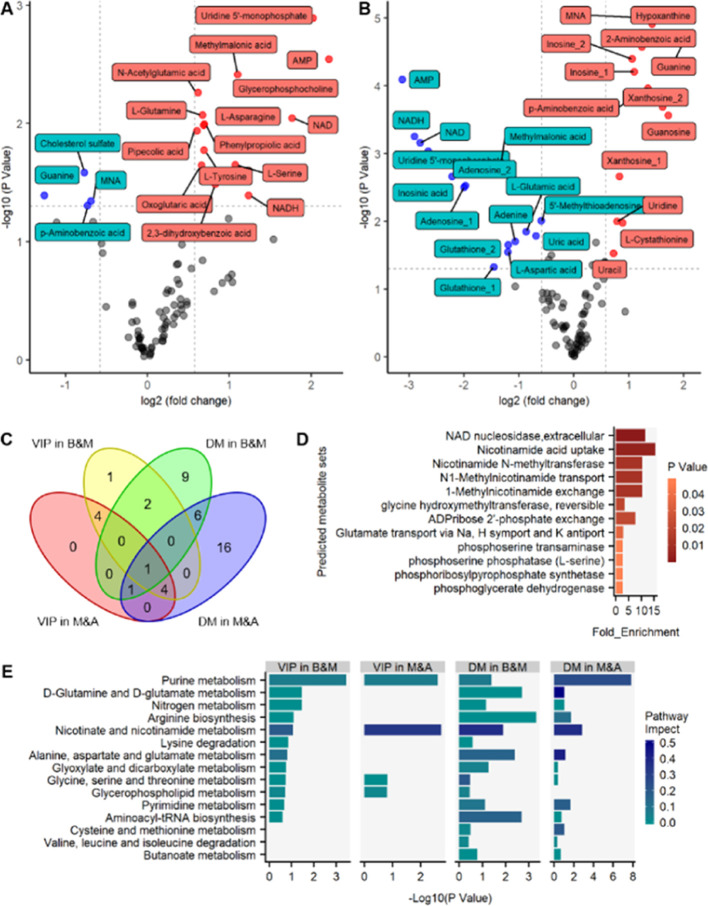
The metabolites were subjected to independent t-test and fold change (FC) analysis for comprehensive bioinformatics analysis. The resulting volcano plots of metabolite profiles revealed average changes in individual metabolite levels (Figure 3A,B), with red dots used to denote significantly upregulated metabolites in the HPM group versus BLK group in Figure 3A. In contrast, blue dots were used to represent those that were significantly downregulated in a similar group (FC > 1.5 or FC < 0.66, P < 0.05).
Figure 3.
Integrated analysis of uniquely distinguishing metabolites. (A) Volcano plot of metabolites in the heart tissue for (A) Group HPM vs Group BLK and (B) Group ABE_a vs Group HPM. Red dots are thus metabolites significantly up-regulated in Group HMP (A) or Group ABE_a (B), and blue dots are metabolites significantly down-regulated in Group HMP (A) or Group ABE_a (B). (C) Venn diagram depicting the overlaps of significantly changed metabolites found in VIP in B&M (yellow circle), VIP in M&A (red circle), DM in B&M (green circle), and DM in M&A (blue circle). (D) Enrichment analysis on predicted metabolite sets (P < 0.05) and (E) summary of pathway analysis on uniquely distinguishing metabolites with MetaboAnalyst 4.0. B&M, Group HPM vs Group BLK; M&A, Group ABE_a vs Group HPM; DM, significantly distinguishing metabolites.
Meanwhile, a volcano plot was used to show differential profiles of metabolites in ABE_a group versus HPM group, with red and blue dots denoting significantly up- and downregulated metabolites, respectively (FC > 1.5 or FC < 0.66, P < 0.05) (Figure 3B). Thereafter, we used a Venn diagram to depict overlapping metabolites of VIP in HPM versus BLK (yellow circle) groups, VIP in ABE_a versus HPM (red circle), significantly distinguishing metabolites for Group HPM versus Group BLK (green circle), and significantly distinguishing metabolites for Group ABE_a versus Group HPM (blue circle) (Figure 3C).
Since groups of metabolites are associated with either biological functions or pathways, an enrichment analysis was performed on predicted metabolite sets for functional annotation using MetaboAnalyst 4.0 (Figure 3D,E).
2.4. Metabolic Pathway Analysis of ABE in SHRs
Careful consideration of pathway impact and P values identified the pathways associated with “purine metabolism”, “nicotinate and nicotinamide metabolism”, and “glycerophospholipid metabolism” (Figure 3E). Consequently, they were visualized using the “Pathview” package implemented in R based on KEGG pathways (Figure S2A,B). Every circle in the pathway graph represented a metabolite, whereas the color of the circle indicated Log2 (fold change) (the left half of circle for Group HPM versus Group BLK; the right half of circle for Group ABE_a versus Group HPM).
2.5. Measurement of the Antioxidant Effect of ABE
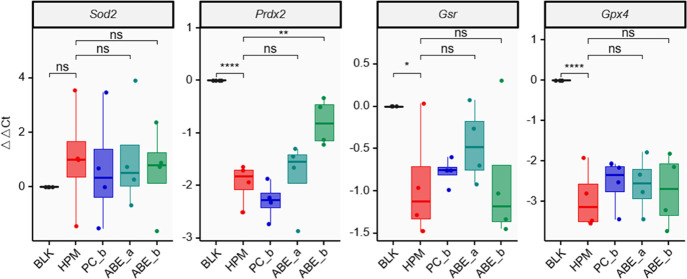
To measure the severity of oxidative stress, a quantitative PCR (qPCR) experiment was performed for the mRNA expression of oxidative stress-related genes. The results showed a reduction of Prdx2, Gsr, and Gpx4 in Group HPM versus Group BLK (P = 1.61 × 10–5 for Prdx2, P = 2.31 × 10–2 for Gsr P = 1.90 × 10–5 for Gpx4). Interestingly, 50 mg/kg/d ABE sharply increased the content of Prdx2 in Group ABE_b versus Group HPM (P = 2.18 × 10–3) (Figure 4).
Figure 4.
qPCR results were used to quantify the antioxidant effect of ABE. ns, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (ANOVA and post-hoc LSD test). BLK, blank; HPM, hypertension model; PC_b, positive drug control, 20 mg/kg/d Enalapril Maleate; ABE_a, 25 mg/kg/d ABE; ABE_b, 50 mg/kg/d ABE.
3. Discussion
3.1. Overview of ABE’ Antihypertensive Effect
Cynarin and chlorogenic acid are the main active substances of artichoke. Watanabe et al. reported the remarkable antihypertensive effect and suitable safety of chlorogenic acid in green coffee bean extract.46 Yao et al. discovered that chlorogenic acid has potential neuroprotective effects because it could directly neutralize free radicals and indirectly increase the content of Nrf2-related cytoprotective enzymes.47 Chlorogenic acid can significantly improve the aortic endothelium-dependent vasodilation induced by acetylcholine.48 Notably, chlorogenic acid could significantly reduce the activity of the angiotensin-1-converting enzyme [ACE, the key enzyme in the renin-angiotensin-aldosterone system (RAAS)], acetylcholinesterase (AChE), butrylcholinesterase (BChE), and arginase in hypertensive rats.49
3.2. Effect of ABE on Liver Function, Kidney Function, and Serum Lipid Profile of SHRs
Our findings revealed elevated levels of TP and ALB in Group HPM, although these were reversed following exposure to high ABE concentration. The elevated levels of TP and ALB mean damaged liver function. Interestingly, Senturk et al. reported a significantly higher level of plasma TP in pulmonary arterial hypertension patients.50 The decreasing effect on the content of TP is helpful to exert the antihypertensive effect.51,52 Hiraoka et al. discovered elevated levels of serum ALB in portal hypertension.53 Also, these findings were consistent with recent studies indicating that a steady rise of ALB was a significant predictor of hypertension (P < 0.001).54 Notably, the content of ALB is also related to the grade of hypertension.55 Interestingly, Feld et al. discovered that serum ALB levels were higher during early rather than late-life stages of SHR potentially because promotion of fluid retention during the early stages of SHR causes hypertension.56
Chronic kidney disease is a significant risk factor for hypertension,57−59 with results from animal models showing that kidneys from SHRs exhibit significant dysfunction.60 For SHR-associated renal transplanted rats, the genotype of the kidney of the donor rather than that of the recipient determined the blood pressure.61 Our findings revealed elevated levels of uric acid in Group HPM, although these were reversed following exposure to high ABE concentration. The lower level of uric acid in Group ABE_b possibly contributed to the antihypertensive effect of the ABE. Federica et al. first reported the relationship between serum UA and hypertension in 1870.62 Previous studies have shown that impaired excretion of renal uric acid and the final product of purine metabolism trigger hyperuricemia,63 whereas elevated levels enhance the development of hypertension in the general population.62,64 Nevertheless, Feig et al. discovered that reducing uric acid levels using allopurinol, an inhibitor of xanthine oxidase, lowered blood pressure.65 Antihypertensive drugs have been shown to affect uric acid levels, and, in turn, most hypouricemia drugs have been shown to have an effect on blood pressure.66,67 Animal and cell experiments revealed that the up-regulation of the RAAS contributed to the role of uric acid in promoting hypertension and inflammatory status.62 The up-regulation of thromboxane and endothelin-1 by uric acid may also contribute to UA-mediated hypertension.68,69
Interestingly, we found lower blood HDL-C levels in the SHR group than those in the WKY group. This was consistent with He et al. who showed that SHR had lower HDL-C concentrations than the control.70 Li et al. discovered that the HDL-C levels of hypertension patients were significantly lower than those of healthy people (P < 0.05). However, an increased level of HDL-C did not indicate improvement in the symptoms of hypertension.71 In our study, ABE failed to reverse the HDL-C change in SHR rats.
3.3. Significantly Regulated Metabolites for ABE in SHRs
Metabolomics provides a global snapshot of endogenous and exogenous small molecules in cells and biological fluids. So far, various studies have demonstrated the importance of metabolomics in unraveling the pathogenesis of hypertension.72 Herein, OPLS-DA models for HPM versus BLK and ABE_a versus HPM groups were excellent and efficient.
Among the characterized metabolites in HPM from BLK, 15 and 4 biomarkers were upregulated and down-regulated, respectively, in Group HPM, indicating changes of metabolites caused by hypertensive symptoms. On the other hand, 13 and 15 biomarkers were upregulated and down-regulated, respectively, in ABE_a. Interestingly, 8 metabolites, including guanine, 1-methylnicotinamide, p-aminobenzoic acid, NAD, NADH, uridine 5′-monophosphate, adenosine monophosphate, and methylmalonic acid, were represented in both volcano results. Among these metabolites, VIP (VIP > 1) selected adenosine monophosphate for both Group HPM versus Group BLK and Group ABE_a versus Group HPM, whereas 1-methylnicotinamide was selected for Group ABE_a versus Group HPM.
3.4. Purine Metabolism Pathway and Hypertension
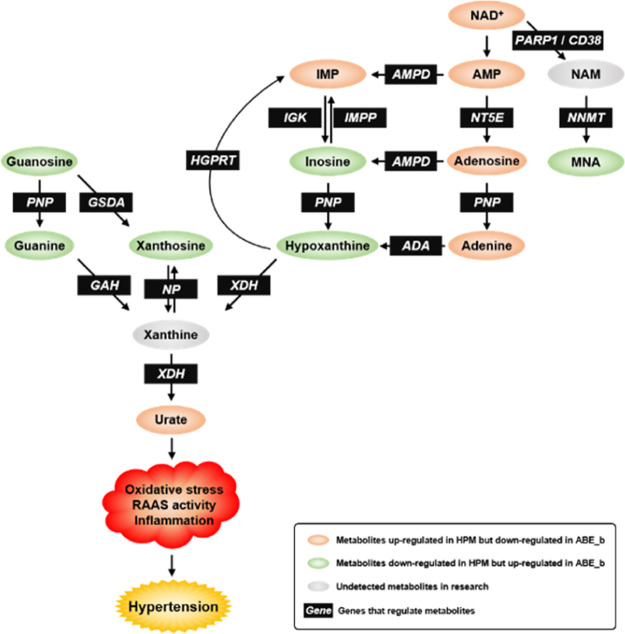
Enrichment and pathway analyses of the predicted metabolites revealed several underlying mechanisms of ABE action on hypertension, including “purine metabolism”, “nicotinate and nicotinamide metabolism”, and “glycerophospholipid metabolism” pathways. Pathview results further revealed distinct biomarkers between BLK and HPM groups, which was reversed after ABE treatment.
Figure S2A shows the purine metabolism pathway and 10 differentially expressed metabolites. Additionally, the hypertension model induced a significant elevation of guanosine, guanine, xanthosine, inosine, and hypoxanthine, while ABE treatment suppressed levels of these metabolites in hypertensive rats. An opposite effect was observed in IMP, AMP, adenosine, adenine, and urate. Numerous studies on hypertension have emphasized the correlation between urate with pulmonary arterial hypertension severity and mortality.73 A significant relationship exists between hyperuricemia and a high risk of hypertension. Consequently, allopurinol and febuxostat have been used for the management of hypertension and alleviation of chronic kidney disease by lowering uric acid.74 The release of adenine deaminase (ADA) and purine-nucleoside phosphorylase (PNP) from a hemolytic disease may increase the risk of hypertension, which may be achieved by eliminating the vascular protective effects of adenosine, inosine, and guanosine.75 The adenosinergic system regulates the tonicity of blood vessels through the complex system of adenosine, adenosine receptor (AR), and nucleoside transporters. Activation of adenosine A receptor (AR) contributed to lowering of intraocular pressure.76
3.5. Nicotinate and Nicotinamide Metabolism Pathway and Hypertension
Our findings indicated that the nicotinate and nicotinamide metabolism pathways might regulate the antihypertensive effect (Figure S2B). Specifically, we found elevated levels of NAD+ in the disease group, with exposure to ABE treatment lowering this index. Notably, an opposite trend was noted with regard to 1-methylnicotinamide. Previous studies reveal that patients with pulmonary arterial hypertension had lower α-tocopherol nicotinate levels and, similarly, lower antioxidants levels.77 Nicotinamide played an active role in the process of right ventricular function or pulmonary vascular remodeling, so it can improve the changes of pulmonary vascular and cardiac function in rats with pulmonary hypertension.78 Alleviation of renal impairment through the pathway linked to nicotinamide N-methyltransferase (NNMT) expression contributed to control of the progression of hypertensive nephropathy.79 Nicotinamide nucleotide transhydrogenase activity affected the redox balance of mitochondria and the development of hypertension in mice.80
3.6. Summary of This Study
We evaluated the effect of ABE on heart tissue metabolomics of SHR relative to age-matched WKY rats. The proposed molecular mechanism by which ABE regulates the metabolomics of hypertensive rats is shown in Figure 5, indicating that the extract plays the essential role of an antioxidant in antihypertensive therapies. We believe that metabolomics of SHR rats dosed with ABE provides very valuable information.
Figure 5.
Metabolic regulation of biomarker-associated pathways. Metabolites up-regulated in HPM but down-regulated in ABE_b are depicted in orange. Metabolites down-regulated in HPM but up-regulated in ABE_b are depicted in green. Undetected metabolites in this research are depicted in grey. Black boxes indicate genes that regulate metabolites. PARP, poly ADP-ribose polymerase; AMPD, adenosine monophosphate deaminase; NT5E, 5′-nucleotidase Ecto; NNMT, nicotinamide N-methyltransferase; ADA; PNP; IGK, inosine guanosine kinase; IMPP, IMP phosphatase; XDH, xanthine dehydrogenase; HGPRT, hypoxanthine phosphoribosyltransferase; GSDA, guanosine deaminase; GAH, guanine deaminase.
In conclusion, our findings demonstrate the efficacy of ABE in reducing serum TP, ALB, and UA in the SHR model. Functionally, artichokes may exert antioxidative effects regulated by purine metabolism and nicotinate and nicotinamide metabolism pathways in the SHR model. Further investigations are necessary to identify complex metabolic components regulating the therapeutic effect of artichokes in SHRs.
After all, more trials are needed to confirm or reject the antihypertensive impact of artichokes. It could be helpful to determine the effect of ABE on other oxidative stress markers, such as ROS production and lipid peroxidation with malondialdehyde formation in heart tissues.
4. Materials and Methods
4.1. Drugs and Regents
ABE was provided by Aikedao Biotechnology Co., Ltd. (Changde, China). 600 kg of immature artichoke buds were put into the extraction tank, then 3000 L of water was added into the tank and heated to boiling which was continued for 2 h. Each batch of the artichoke materials was extracted twice. After that, the solvent was collected, concentrated by a vacuum multistage evaporator, followed by spray-drying to produce the final crude extract. ABE was dissolved in 1% carboxylmethyl cellulose and orally administered by gastric intubation. In addition, 1% carboxylmethyl cellulose and Enalapril Maleate (Selleck, #S1941, USA), a known antihypertensive drug, were used as a negative and positive control, respectively.
HPLC analysis (symmetry 5 μm, 4.6 mm × 250 mm) on Waters 2695 RP-HPLC system equipped with a quaternary pump and PDA detector was performed on an ODS column. Detection parameters were set at a wavelength of 330 nm, a flow rate of 1 mL/min, a column temperature of 30 °C, and a sampling volume of 10 μL. The mobile phase comprised acetonitrile (A) and water with 0.2% phosphoric acid (B). Gradient elution was applied and the following procedures were used: 0–5 min, 5–20% A; 5–20 min, 20–30% A; 20–23 min, 30–50% A; 23.1–37 min, 5% A. Retention times for chlorogenic acid and cynarin were 11.4 and 14.5 min, respectively. HPLC results revealed that ABE contained 2.3 and 4.7% of chlorogenic acid and cynarin (dry weight), respectively. The resulting chromatograms and the standards (chlorogenic acid, cynarin) are shown in Figure S3, while chemical structures of active components in the ABE are illustrated in Figure S4.
4.2. Experimental Animals
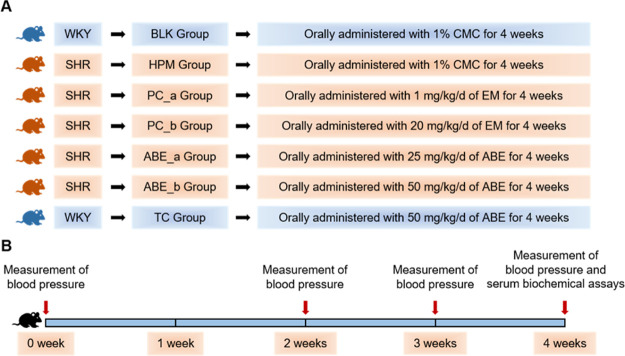
Twenty-week-old SHRs and WKY male rats were obtained from Vital River Laboratory (Beijing, China). All rats were individually housed under constant temperature (22 ± 2 °C) with a 12 h light/12 h dark cycle (lights off at 20:00) on a normal chow diet. The rats were randomly divided into seven groups (Figure 6, six rats per group) and administered with or without different doses of ABE (25 and 50 mg/kg/d) or Enalapril Maleate (1 and 20 mg/kg/d). At present, there are few clinical studies on the antihypertensive effect of ABE, and there is no recognized therapeutic dose. Therefore, according to a previous reference, we converted the drug dose for rats and set up the concentration gradient of 25 and 50 mg/kg/d in our study.43 The solution volume orally administered to the rats was 10 mL/kg rat. All ABE or Enalapril Maleate solutions were prepared freshly every day, before administration. Rats in the BLK group (Blank, WKY) were orally administered with 1% carboxylmethyl cellulose; HPM (Hypertension model group, SHR) were administered with 1% carboxylmethyl cellulose; PC_a (Positive drug control group, SHR) were administered with 1 mg/kg/d of Enalapril Maleate; PC_b (Positive drug control group, SHR) were administered with 20 mg/kg/d Enalapril Maleate; ABE_a (ABE group, SHR) were administered with 25 mg/kg/d of ABE, whereas ABE_b (ABE group, SHR) were administered with 50 mg/kg/d of ABE; and TC (toxicity control group, WKY) were administered with 50 mg/kg/d of ABE. Treatments spanned to 4 weeks, after which all rats were anesthetized with isoflurane, and blood samples were collected from the heart tip. The study was approved by the ethics committee of Xiangya Hospital of Central South University (ethics approval number: SCXK-2016-0004).
Figure 6.
Schematic overview for rats studies. All rats were randomly divided into six groups and fed with a standard chow diet for 4 weeks. Blood pressure was measured at 0 week, 2 weeks, 3 weeks, and 4 weeks after the start of the test period. After 4 weeks of treatment, all rats were anesthetized to take blood from the tip of the heart. n(BLK) = 6; n(HPM) = 6; n(PC_a) = 6; n(PC_b) = 6; n(ABE_a) = 6; n(ABE_b) = 6; n(TC) = 5. WKY, WKY rats; SHR, spontaneous hypertensive rats; BLK, blank; HPM, hypertension model; PC, positive drug control; ABE, ABE; CMC, carboxylmethyl cellulose; EM, Enalapril Maleate; TC, toxicity control.
4.3. Determination of Blood Pressure
Blood pressure was measured in the morning at 0, 2, 3, and 4 weeks after the start of the test period, using a noninvasive tail-cuff blood pressure series automatic noninvasive blood pressure system (BP-300A; Chengdu TME Technology, China). Rats were placed in a warming chamber, for a 10 min stabilization period, followed by recording of the three measurements as previously described.81,82
4.4. Serum Biochemical Assays
To separate and collect serum, blood samples were first centrifuged at 3000 rpm at 4 °C for 10 min (Centrifuge 5810 R, Eppendorf, Germany). Thereafter, levels of TP, ALB, globulin (GLB), total bilirubin (TBIL), direct bilirubin (DBIL), total bile acids (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, UA, creatinine (CRE), TG, TC, HDL-C, and LDL-C were measured at Xiangya Hospital, Changsha, China.
4.5. Quantitative PCR (qPCR)
Total RNA of heart tissue was extracted by RNAiso Plus (TaKaRa, #9109, Japan). Exactly 1 μg of RNA was reverse transcribed into cDNA using a PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, #RR047A, Japan) following the manufacturer’s instructions. The qRT-PCR was performed on a LightCycler@480II/96 (Roche Dignostics, Switzerland) using an SYBR Premix Dimer Eraser Kit (Takara, #RR091A, Japan). β-Actin was used to normalize the expression levels. A set of ΔCt replicates were used for statistical testing and estimation of the P values. The results shown are ΔΔCt versus BLK control. To measure the severity of oxidative stress, a qPCR experiment was performed for the mRNA expression of oxidative stress-related genes (Sod2, Prdx2, Gsr, and Gpx4). The primers were purchased from BioSune Biotechnology, and the sequences are detailed in Table S1.
4.6. Metabolomics Studies
Heart function is closely related to hypertension.83 Pathological features of left ventricular hypertrophy are always along with essential hypertension.84 Blood, urine, cerebrospinal fluid, and saliva may not accurately reflect the pathophysiological changes in specific tissues of hypertension patients.85 In view of this, we chose tissue samples for a metabolomic study. The heart tissues were collected under anesthesia, freeze-clamped in liquid nitrogen, and stored at −80 °C.86 Frozen tissue (50 mg) was ground into a fine powder, using a chilled mortar and pestle, then 80% (vol/vol) of HPLC-grade methanol (−80 °C, 1000 μL) was added. Exactly 600 μL of the mixture was transferred into a tube, vortexed for 45 s at 4 °C, and then incubated at −20 °C for 1 h. The suspension was centrifuged at 13,000 rpm at 4 °C for 15 min, and the supernatant was transferred into new 1.5 mL microcentrifuge tubes. The supernatant was lyophilized, redissolved in 100 μL of a solvent comprising HPLC grade 50% (vol/vol) chloroform and 50% (vol/vol) methanol, and centrifuged at 13,000 rpm at 4 °C for 15 min. Then the supernatant was injected into a sample bottle. The HPLC solvent system comprised buffer A (pH = 9.0; 95% (vol/vol) water, 5% (vol/vol) acetonitrile, 20 mM ammonium acetate) and buffer B (100% acetonitrile).87
Ion intensity was normalized from each detected peak, and then peak intensities were summed up. The data were imported into SIMCA-P V14.1 (Umetrics AB, Malmo, Sweden) for analysis using the OPLS-DA method. The model was validated using R2 and Q2 values, while biomarkers were selected using VIP values (VIP > 1.0). Significant differences among groups were determined using S-plot, with the OPLS-DA model validated with a 200 times permutation test. Uniquely distinguishing metabolites were identified using volcano plot filtering (FC > 1.5 or FC < 0.66, P < 0.05), whereas their closely associated potential pathways and predicted metabolite sets were analyzed using the MetaboAnalyst 4.0 (http://www.metaboanalyst.ca/).88 Differentially regulated pathways were visualized using the “Pathview” package implemented in R 3.6.0.89
4.7. Statistical Analysis
Data were presented as means ± standard deviations (SD). Comparisons between two groups were performed using a Student’s t-test, while comparisons between more than two groups were performed using ANOVA and post-hoc LSD test. For repeated measurements of blood pressure, ANOVA with repeated measurements was performed. Data followed by P < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81874327), the Natural Science Foundation of Hunan province, China (no. 2018JJ4020), and Fundamental Research Funds for the Central Universities of Central South University (2017zzts224). We thank Dr. Shipeng Guo (Chongqing Medical University) for the figure technology support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01135.
Toxicity assessment of ABE; Log2 fold changes of metabolites level mapped onto the KEGG pathway module; chromatograms of ABE used in this study and the standards (chlorogenic acid, cynarin); chemical structures of active components contained in ABE; and sequences of primers used for qRT-PCR (PDF)
Author Contributions
Z.-B.W. conducted the experiments and wrote the manuscript. Z.-B.W., Z.-Q.L., and X.-Y.M. designed the study. H.-H.Z. participated in the experimental design and provided financial and instrumental support. S.-L.J., J.-B.P., X.W., W.Z., T.L., and J.-W.G. gave help substantially in the experiment. X.-Y.M., Z.-Q.Y., S.H., and S.-B.L. revised the manuscript. Z.-Q.L. approved the final manuscript. Z.-Q.L., X.-Y.M., and Z.-Q.Y. contributed equally to this article.
The authors declare no competing financial interest.
Supplementary Material
References
- Whelton P. K.; Carey R. M.; Aronow W. S.; Casey D. E.; Collins K. J.; Dennison Himmelfarb C.; DePalma S. M.; Gidding S.; Jamerson K. A.; Jones D. W.; MacLaughlin E. J.; Muntner P.; Ovbiagele B.; Smith S. C.; Spencer C. C.; Stafford R. S.; Taler S. J.; Thomas R. J.; Williams K. A.; Williamson J. D.; Wright J. T. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Benjamin E. J.; Muntner P.; Alonso A.; Bittencourt M. S.; Callaway C. W.; Carson A. P.; Chamberlain A. M.; Chang A. R.; Cheng S.; Das S. R.; Delling F. N.; Djousse L.; Elkind M. S. V.; Ferguson J. F.; Fornage M.; Jordan L. C.; Khan S. S.; Kissela B. M.; Knutson K. L.; Kwan T. W.; Lackland D. T.; Lewis T. T.; Lichtman J. H.; Longenecker C. T.; Loop M. S.; Lutsey P. L.; Martin S. S.; Matsushita K.; Moran A. E.; Mussolino M. E.; O’Flaherty M.; Pandey A.; Perak A. M.; Rosamond W. D.; Roth G. A.; Sampson U. K. A.; Satou G. M.; Schroeder E. B.; Shah S. H.; Spartano N. L.; Stokes A.; Tirschwell D. L.; Tsao C. W.; Turakhia M. P.; VanWagner L. B.; Wilkins J. T.; Wong S. S.; Virani S. S.; Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Fisher N. D. L.; Curfman G. Hypertension—A public health challenge of global proportions. JAMA, J. Am. Med. Assoc. 2018, 320, 1757–1759. 10.1001/jama.2018.16760. [DOI] [PubMed] [Google Scholar]
- Qamar A.; Braunwald E. Treatment of Hypertension: Addressing a Global Health Problem. Jama 2018, 320, 1751–1752. 10.1001/jama.2018.16579. [DOI] [PubMed] [Google Scholar]
- He Y.; Zuo C.; Jia D.; Bai P.; Kong D.; Chen D.; Liu G.; Li J.; Wang Y.; Chen G.; Yan S.; Xiao B.; Zhang J.; Piao L.; Li Y.; Deng Y.; Li B.; Roux P. P.; Andreasson K. I.; Breyer R. M.; Su Y.; Wang J.; Lyu A.; Shen Y.; Yu Y. Loss of DP1 Aggravates Vascular Remodeling in Pulmonary Arterial Hypertension via mTORC1 Signaling. Am. J. Respir. Crit. Care Med. 2020, 201, 1263–1276. 10.1164/rccm.201911-2137oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Zha L.; Luo H.; Li S.; Zhao L.; He J.; Li X.; Qi Q.; Liu Y.; Yu Z. Galectin-3 Mediates Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Aging Dis. 2019, 10, 731–745. 10.14336/ad.2018.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Zhang J.; Liu W.; Su X. Determining the Prevalence of Primary Aldosteronism in Patients With New-Onset Type 2 Diabetes and Hypertension. J. Clin. Endocrinol. Metab. 2020, 105, 1079–1085. 10.1210/clinem/dgz293. [DOI] [PubMed] [Google Scholar]
- Pons M.; Augustin S.; Scheiner B.; Guillaume M.; Rosselli M.; Rodrigues S. G.; Stefanescu H.; Ma M. M.; Mandorfer M.; Mergeay-Fabre M.; Procopet B.; Schwabl P.; Ferlitsch A.; Semmler G.; Berzigotti A.; Tsochatzis E.; Bureau C.; Reiberger T.; Bosch J.; Abraldes J. G.; Genescà J. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am. J. Gastroenterol. 2020, 116, 723–732. 10.14309/ajg.0000000000000994. [DOI] [PubMed] [Google Scholar]
- Boyer-Diaz Z.; Morata P.; Aristu-Zabalza P.; Gibert-Ramos A.; Bosch J.; Gracia-Sancho J. Oxidative Stress in Chronic Liver Disease and Portal Hypertension: Potential of DHA as Nutraceutical. Nutrients 2020, 12, 2627. 10.3390/nu12092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer-Diaz Z.; Aristu-Zabalza P.; Andrés-Rozas M.; Robert C.; Ortega-Ribera M.; Fernández-Iglesias A.; Broqua P.; Junien J.-L.; Wettstein G.; Bosch J.; Gracia-Sancho J. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J. Hepatol. 2021, 74, 1188–1199. 10.1016/j.jhep.2020.11.045. [DOI] [PubMed] [Google Scholar]
- Teo B. W.; Chan G. C.; Leo C. C. H.; Tay J. C.; Chia Y. C.; Siddique S.; Turana Y.; Chen C. H.; Cheng H. M.; Hoshide S.; Minh H. V.; Sogunuru G. P.; Wang T. D.; Kario K. Hypertension and chronic kidney disease in Asian populations. J. Clin. Hypertens. 2021, 23, 475–480. 10.1111/jch.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubert M.; Bardin T.; Cohen-Solal A.; Diévart F.; Fauvel J.-P.; Guieu R.; Sadrin S.; Maixent J.; Galinier M.; Paganelli F. Hyperuricemia and Hypertension, Coronary Artery Disease, Kidney Disease: From Concept to Practice. Int. J. Mol. Sci. 2020, 21, 4066. 10.3390/ijms21114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury K. N.; Mainuddin A. K.; Wahiduzzaman M.; Islam S. M. Serum lipid profile and its association with hypertension in Bangladesh. Vasc. Health Risk Manage. 2014, 10, 327–332. 10.2147/VHRM.S61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler S. J. Initial treatment of hypertension. N. Engl. J. Med. 2018, 378, 636–644. 10.1056/nejmcp1613481. [DOI] [PubMed] [Google Scholar]
- Hottenga J.-J.; Boomsma D. I.; Kupper N.; Posthuma D.; Snieder H.; Willemsen G.; de Geus E. J. C. Heritability and stability of resting blood pressure. Twin Res. Hum. Genet. 2005, 8, 499–508. 10.1375/twin.8.5.499. [DOI] [PubMed] [Google Scholar]
- Oparil S.; Acelajado M. C.; Bakris G. L.; Berlowitz D. R.; Cífková R.; Dominiczak A. F.; Grassi G.; Jordan J.; Poulter N. R.; Rodgers A.; Whelton P. K. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. 10.1038/nrdp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achelrod D.; Wenzel U.; Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am. J. Hypertens. 2015, 28, 355–361. 10.1093/ajh/hpu151. [DOI] [PubMed] [Google Scholar]
- Higashi Y.; Sasaki S.; Nakagawa K.; Matsuura H.; Oshima T.; Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N. Engl. J. Med. 2002, 346, 1954–1962. 10.1056/nejmoa013591. [DOI] [PubMed] [Google Scholar]
- Laursen J. B.; Rajagopalan S.; Galis Z.; Tarpey M.; Freeman B. A.; Harrison D. G. Role of superoxide in angiotensin II–induced but not catecholamine-induced hypertension. Circulation 1997, 95, 588–593. 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- Hansen T.; Galougahi K.-K.; Celermajer D.; Rasko N.; Tang O.; Bubb K. J.; Figtree G. Oxidative and nitrosative signalling in pulmonary arterial hypertension - Implications for development of novel therapies. Pharmacol. Ther. 2016, 165, 50–62. 10.1016/j.pharmthera.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Sag C. M.; Schnelle M.; Zhang J.; Murdoch C. E.; Kossmann S.; Protti A.; Santos C. X. C.; Sawyer G.; Zhang X.; Mongue-Din H.; Richards D. A.; Brewer A. C.; Prysyazhna O.; Maier L. S.; Wenzel P.; Eaton P. J.; Shah A. M. Distinct regulatory effects of myeloid cell and endothelial cell NAPDH oxidase 2 on blood pressure. Lippincott Williams & Wilkins Open Access 2017, 135, 2163–2177. 10.1161/circulationaha.116.023877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Zhang X.; Wang F.; Zhou L.; Yin Z.; Fan J.; Nie X.; Wang P.; Fu X.-D.; Chen C.; Wang D. W. MicroRNA-21 Lowers Blood Pressure in Spontaneous Hypertensive Rats by Upregulating Mitochondrial Translation. Circulation 2016, 134, 734–751. 10.1161/circulationaha.116.023926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.; Huynh N. N.; Hamilton C. A.; Beattie E.; Smith R. A. J.; Cochemé H. M.; Murphy M. P.; Dominiczak A. F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. 10.1161/hypertensionaha.109.130351. [DOI] [PubMed] [Google Scholar]
- Jankov R. P.; Kantores C.; Pan J.; Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2008, 294, L233–L245. 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- Dosunmu-Ogunbi A. M.; Wood K. C.; Novelli E. M.; Straub A. C. Decoding the role of SOD2 in sickle cell disease. Blood Adv. 2019, 3, 2679–2687. 10.1182/bloodadvances.2019000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.; Hao S.; Liu Z.; Xie L.; Wu X.; Wu X.; Li S. SOD2 ameliorates pulmonary hypertension in a murine model of sleep apnea via suppressing expression of NLRP3 in CD11b(+) cells. Respir. Res. 2020, 21, 9. 10.1186/s12931-019-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Yang H.; Wang D.; Zhang L.; Ma T. Peroxiredoxin2 (Prdx2) Reduces Oxidative Stress and Apoptosis of Myocardial Cells Induced by Acute Myocardial Infarction by Inhibiting the TLR4/Nuclear Factor kappa B (NF-kappaB) Signaling Pathway. Med. Sci. Monit. 2020, 26, e926281 10.12659/MSM.926281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Chen Z.; Zhu S.; Lu H.; Peng D.; Soutto M.; Naz H.; Peek R. Jr.; Xu H.; Zaika A.; Xu Z.; El-Rifai W. PRDX2 protects against oxidative stress induced by H. pylori and promotes resistance to cisplatin in gastric cancer. Redox Biol. 2020, 28, 101319. 10.1016/j.redox.2019.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.; Kim M.-J.; Ding D.; Park H.-J.; White K.; Walker L.; Gu T.; Tanokura M.; Yamasoba T.; Linser P.; Salvi R.; Someya S. GSR is not essential for the maintenance of antioxidant defenses in mouse cochlea: Possible role of the thioredoxin system as a functional backup for GSR. PLoS One 2017, 12, e0180817 10.1371/journal.pone.0180817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F.; Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radical Biol. Med. 2020, 152, 175–185. 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- Forcina G. C.; Dixon S. J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- Prieto I.; Segarra A. B.; de Gasparo M.; Martínez-Cañamero M.; Ramírez-Sánchez M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. 10.1016/j.lfs.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Yuan P.; Zhang Q.; Fu Y.; Hou Y.; Gao L.; Zheng X.; Feng W. Acacetin improves endothelial dysfunction and aortic fibrosis in insulin-resistant SHR rats by estrogen receptors. Mol. Biol. Rep. 2020, 47, 6899–6918. 10.1007/s11033-020-05746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio V.; Kroon P. A.; Linsalata V.; Cardinali A. Globe artichoke: a functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- Santos H. O.; Bueno A. A.; Mota J. F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. 10.1016/j.phrs.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Kianpour P.; Mohtashami R.; Atkin S. L.; Butler A. E.; Jafari R.; Badeli R.; Sahebkar A. Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytother. Res. 2018, 32, 1382–1387. 10.1002/ptr.6073. [DOI] [PubMed] [Google Scholar]
- Sahebkar A.; Pirro M.; Banach M.; Mikhailidis D. P.; Atkin S. L.; Cicero A. F. G. Lipid-lowering activity of artichoke extracts: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2549–2556. 10.1080/10408398.2017.1332572. [DOI] [PubMed] [Google Scholar]
- Salekzamani S.; Ebrahimi-Mameghani M.; Rezazadeh K. The antioxidant activity of artichoke (Cynara scolymus): A systematic review and meta-analysis of animal studies. Phytother. Res. 2019, 33, 55–71. 10.1002/ptr.6213. [DOI] [PubMed] [Google Scholar]
- Shao T.; Yu Q.; Zhu T.; Liu A.; Gao X.; Long X.; Liu Z. Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br. J. Nutr. 2020, 123, 308–318. 10.1017/s0007114519002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Zhang H.; Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. 10.1021/jf0490192. [DOI] [PubMed] [Google Scholar]
- Farag M. A.; El-Ahmady S. H.; Elian F. S.; Wessjohann L. A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 2013, 95, 177–187. 10.1016/j.phytochem.2013.07.003. [DOI] [PubMed] [Google Scholar]
- D’Antuono I.; Garbetta A.; Linsalata V.; Minervini F.; Cardinali A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): in vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. 10.1039/c5fo00137d. [DOI] [PubMed] [Google Scholar]
- Roghani-Dehkordi F.; Kamkhah A.-F. Artichoke leaf juice contains antihypertensive effect in patients with mild hypertension. J. Diet. Suppl. 2009, 6, 328–341. 10.3109/19390210903280207. [DOI] [PubMed] [Google Scholar]
- Ardalani H.; Jandaghi P.; Meraji A.; Hassanpour Moghadam M. The Effect of Cynara scolymus on Blood Pressure and BMI in Hypertensive Patients: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. J. Complementary Med. Res. 2020, 27, 40–46. 10.1159/000502280. [DOI] [PubMed] [Google Scholar]
- Li H.; Xia N.; Brausch I.; Yao Y.; Förstermann U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. 10.1124/jpet.104.066639. [DOI] [PubMed] [Google Scholar]
- Watanabe T.; Arai Y.; Mitsui Y.; Kusaura T.; Okawa W.; Kajihara Y.; Saito I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. 10.1080/10641960600798655. [DOI] [PubMed] [Google Scholar]
- Yao J.; Peng S.; Xu J.; Fang J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. 10.1002/biof.1507. [DOI] [PubMed] [Google Scholar]
- Suzuki A.; Yamamoto N.; Jokura H.; Yamamoto M.; Fujii A.; Tokimitsu I.; Saito I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. 10.1097/01.hjh.0000226196.67052.c0. [DOI] [PubMed] [Google Scholar]
- Agunloye O. M.; Oboh G.; Ademiluyi A. O.; Ademosun A. O.; Akindahunsi A. A.; Oyagbemi A. A.; Omobowale T. O.; Ajibade T. O.; Adedapo A. A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. 10.1016/j.biopha.2018.10.044. [DOI] [PubMed] [Google Scholar]
- Senturk B.; Akdeniz B.; Yilmaz M. B.; Ozcan Kahraman B.; Acar B.; Uslu S.; Birlik M. Whole blood viscosity in systemic sclerosis: a potential biomarker of pulmonary hypertension?. Clin. Rheumatol. 2020, 39, 49–56. 10.1007/s10067-019-04603-4. [DOI] [PubMed] [Google Scholar]
- Wu L.; Gao Y.; Zhang S.; Fang Z. The Effects of Breviscapine Injection on Hypertension in Hypertension-Induced Renal Damage Patients: A Systematic Review and a Meta-Analysis. Front. Pharmacol. 2019, 10, 118. 10.3389/fphar.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong R.-C.; Qi M.; Yang Q.-M.; Li P.-F.; Wang D.-D.; Lan J.-P.; Wang Z.-T.; Yang L. Extract of Plantago asiatica L. Seeds Ameliorates Hypertension in Spontaneously Hypertensive Rats by Inhibition of Angiotensin Converting Enzyme. Front. Pharmacol. 2019, 10, 403. 10.3389/fphar.2019.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka A.; Kitahata S.; Izumoto H.; Ueki H.; Aibiki T.; Okudaira T.; Miyamoto Y.; Yamago H.; Iwasaki R.; Tomida H.; Mori K.; Kishida M.; Tsubouchi E.; Miyata H.; Ninomiya T.; Hirooka M.; Tokumoto Y.; Abe M.; Matsuura B.; Hiasa Y.; Michitaka K. Muscle volume loss a prognostic factor for death in liver cirrhosis patients and special relationship to portal hypertension. Hepatol. Res. 2018, 48, E354–E359. 10.1111/hepr.12984. [DOI] [PubMed] [Google Scholar]
- Deng Q.; Zhao D.; Wang W.; Qi Y.; Wang M.; Sun J.; Liu J.; Li Y.; Liu J. GW29-e1928 Serum albumin level predicts the risk of incident hypertension: A 10-year prospective longitudinal study. J. Am. Coll. Cardiol. 2018, 72, C232. 10.1016/j.jacc.2018.08.989. [DOI] [Google Scholar]
- Wang L.; Feng Y.; Ma X.; Wang G.; Wu H.; Xie X.; Zhang C.; Zhu Q. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS One 2017, 12, e0182969 10.1371/journal.pone.0182969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld L. G.; Springate J. E.; Van Liew J. B. Age-related changes in serum proteins of the spontaneously hypertensive rat. Proc. Soc. Exp. Biol. Med. 1988, 188, 480–484. 10.3181/00379727-188-42764. [DOI] [PubMed] [Google Scholar]
- Rossignol P.; Massy Z. A.; Azizi M.; Bakris G.; Ritz E.; Covic A.; Goldsmith D.; Heine G. H.; Jager K. J.; Kanbay M.; Mallamaci F.; Ortiz A.; Vanholder R.; Wiecek A.; Zoccali C.; London G. M.; Stengel B.; Fouque D. The double challenge of resistant hypertension and chronic kidney disease. Lancet 2015, 386, 1588–1598. 10.1016/s0140-6736(15)00418-3. [DOI] [PubMed] [Google Scholar]
- Ruilope L. M.; Bakris G. L. Renal function and target organ damage in hypertension. Eur. Heart J. 2011, 32, 1599–1604. 10.1093/eurheartj/ehr003. [DOI] [PubMed] [Google Scholar]
- You N.-N.; Jiang W.-H.; Lin M.-Y.; Li X.-G.; Wu Y.-Y.; Li J.-Y.; Zhou X.-Y.; Ding Z.-W.; Wang J.-W.; Zhao X.-X.; Chen H.-L.; Tang H.-T. The role of urinary renalase on early-stage renal damage in Chinese adults with primary hypertension. Exp. Biol. Med. 2020, 245, 576–582. 10.1177/1535370220909311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig R.; Folberth C.; Stauss H.; Kopf D.; Waldherr R.; Unger T. Role of the kidney in primary hypertension: a renal transplantation study in rats. Am. J. Physiol. 1990, 258, F606–F611. 10.1152/ajprenal.1990.258.3.f606. [DOI] [PubMed] [Google Scholar]
- Rettig R.; Grisk O. The kidney as a determinant of genetic hypertension: evidence from renal transplantation studies. Hypertension 2005, 46, 463–468. 10.1161/01.hyp.0000178189.68229.8a. [DOI] [PubMed] [Google Scholar]
- Piani F.; Cicero A. F. G.; Borghi C. Uric Acid and Hypertension: Prognostic Role and Guide for Treatment. J. Clin. Med. 2021, 10, 448. 10.3390/jcm10030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y.; Sim X.; Sim X.; Go M. J.; Wu J.-Y.; Gu D.; Takeuchi F.; Takahashi A.; Maeda S.; Tsunoda T.; Chen P.; Lim S.-C.; Wong T.-Y.; Liu J.; Young T. L.; Aung T.; Seielstad M.; Teo Y.-Y.; Kim Y. J.; Lee J.-Y.; Han B.-G.; Kang D.; Chen C.-H.; Tsai F.-J.; Chang L.-C.; Fann S.-J. C.; Mei H.; Rao D. C.; Hixson J. E.; Chen S.; Katsuya T.; Isono M.; Ogihara T.; Chambers J. C.; Zhang W.; Kooner J. S.; Albrecht E.; Yamamoto K.; Kubo M.; Nakamura Y.; Kamatani N.; Kato N.; He J.; Chen Y.-T.; Cho Y. S.; Tai E.-S.; Tanaka T. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 2012, 44, 904–909. 10.1038/ng.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr H. I.; Khowailed A. A.; Al-Fakharany R. S.; Abdel-Fattah D. S.; Taha A. A. Serum Uric Acid Level as a Predictive Biomarker of Gestational Hypertension Severity; A Prospective Observational Case-Control Study. Rev. Recent Clin. Trials 2020, 15, 227–239. 10.2174/1574887115666200709142119. [DOI] [PubMed] [Google Scholar]
- Feig D. I.; Soletsky B.; Johnson R. J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. Jama 2008, 300, 924–932. 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.-H.; Yen F.-S.; Li H.-L.; Wei J.-C.; Hsu C.-C.; Yang C.-C.; Hwu C.-M. Urate-lowering therapy exerts protective effects against hypertension development in patients with gout. J. Hum. Hypertens. 2021, 35, 351. 10.1038/s41371-020-0342-4. [DOI] [PubMed] [Google Scholar]
- Zhang J. X.; Lin X.; Xu J.; Tang F. Hyperuricemia Inhibition Protects SD Rats Against Fructose-Induced Obesity Hypertension Via Modulation of Inflammation and Renin-Angiotensin System in Adipose Tissue. Exp. Clin. Endocrinol. Diabetes 2021, 129, 314. 10.1055/a-1023-6710. [DOI] [PubMed] [Google Scholar]
- Zha D.; Wu S.; Gao P.; Wu X. Telmisartan Attenuates Uric Acid-Induced Epithelial-Mesenchymal Transition in Renal Tubular Cells. BioMed Res. Int. 2019, 2019, 3851718. 10.1155/2019/3851718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Zhuang J.; Tang P.; Li J.; Xiong X.; Deng H. The Role of the Gut Microbiota in Coronary Heart Disease. Curr. Atheroscler. Rep. 2020, 22, 77. 10.1007/s11883-020-00892-2. [DOI] [PubMed] [Google Scholar]
- He D.; Fan F.; Jia J.; Jiang Y.; Sun P.; Wu Z.; Li J.; Huo Y.; Zhang Y. Lipid profiles and the risk of new-onset hypertension in a Chinese community-based cohort. Nutr., Metab. Cardiovasc. 2021, 31, 911–920. 10.1016/j.numecd.2020.11.026. [DOI] [PubMed] [Google Scholar]
- Li X.; Su T.; Xiao H.; Gao P.; Xiong C.; Liu J.; Zou H. Association of the HDL-c Level with HsCRP, IL-6, U-NAG, RBP and Cys-C in Type 2 Diabetes Mellitus, Hypertension, and Chronic Kidney Disease: An Epidemiological Survey. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2020, 13, 3645–3654. 10.2147/dmso.s265735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett D. K.; Claas S. A. Omics of Blood Pressure and Hypertension. Circ. Res. 2018, 122, 1409–1419. 10.1161/circresaha.118.311342. [DOI] [PubMed] [Google Scholar]
- Kim S.; Rigatto K.; Gazzana M. B.; Knorst M. M.; Richards E. M.; Pepine C. J.; Raizada M. K. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertension 2020, 75, 1063–1071. 10.1161/hypertensionaha.119.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat S. G.; Al Kattar S.; Tanios B. Y.; Jurjus A. Hyperuricemia, Hypertension, and Chronic Kidney Disease: an Emerging Association. Curr. Hypertens. Rep. 2016, 18, 74. 10.1007/s11906-016-0684-z. [DOI] [PubMed] [Google Scholar]
- Bilan V. P.; Schneider F.; Novelli E. M.; Kelley E. E.; Shiva S.; Gladwin M. T.; Jackson E. K.; Tofovic S. P. Experimental intravascular hemolysis induces hemodynamic and pathological pulmonary hypertension: association with accelerated purine metabolism. Pulm. Circ. 2018, 8, 2045894018791557. 10.1177/2045894018791557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boia R.; Salinas-Navarro M.; Gallego-Ortega A.; Galindo-Romero C.; Aires I. D.; Agudo-Barriuso M.; Ambrósio A. F.; Vidal-Sanz M.; Santiago A. R. Activation of adenosine A3 receptor protects retinal ganglion cells from degeneration induced by ocular hypertension. Cell Death Dis. 2020, 11, 401. 10.1038/s41419-020-2593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Shults N. V.; Suzuki Y. J. Oxidative profiling of the failing right heart in rats with pulmonary hypertension. PLoS One 2017, 12, e0176887 10.1371/journal.pone.0176887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztormowska-Achranowicz K.; Jankowski Z.; Kocić I. Protective effect of nicotinamide and L-arginine against monocrotaline-induced pulmonary hypertension in rats: gender dependence. Pharmacol. Rep. 2020, 72, 1334–1346. 10.1007/s43440-020-00125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.-F.; Mao X.-J.; Jiang W.-M.; Fang Z.-Y. Qian Yang Yu Yin Granule protects against hypertension-induced renal injury by epigenetic mechanism linked to Nicotinamide N-Methyltransferase (NNMT) expression. J. Ethnopharmacol. 2020, 255, 112738. 10.1016/j.jep.2020.112738. [DOI] [PubMed] [Google Scholar]
- Leskov I.; Neville A.; Shen X.; Pardue S.; Kevil C. G.; Granger D. N.; Krzywanski D. M. Nicotinamide nucleotide transhydrogenase activity impacts mitochondrial redox balance and the development of hypertension in mice. J. Am. Soc. Hypertens. 2017, 11, 110–121. 10.1016/j.jash.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhao Z.; Cai J.; Gu B.; Lv Y.; Zhao L. The Frequency-Dependent Aerobic Exercise Effects of Hypothalamic GABAergic Expression and Cardiovascular Functions in Aged Rats. Front. Aging Neurosci. 2017, 9, 212. 10.3389/fnagi.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Bai W.; Gao L.; Jiang J.; Tang Y.; Niu Y.; Lin H.; Li L. Mangiferin alleviates hypertension induced by hyperuricemia via increasing nitric oxide releases. J. Pharmacol. Sci. 2018, 137, 154–161. 10.1016/j.jphs.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Berkin K. E.; Ball S. G. HYPERTENSION: Essential hypertension: the heart and hypertension. Heart 2001, 86, 467–475. 10.1136/heart.86.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R. E.; Messerli F. H. Hypertension and the heart. J. Hum. Hypertens. 2000, 14, 597–604. 10.1038/sj.jhh.1001044. [DOI] [PubMed] [Google Scholar]
- Nikolic S. B.; Sharman J. E.; Adams M. J.; Edwards L. M. Metabolomics in hypertension. J. Hypertens. 2014, 32, 1159–1169. 10.1097/hjh.0000000000000168. [DOI] [PubMed] [Google Scholar]
- McGarrah R. W.; Crown S. B.; Zhang G.-F.; Shah S. H.; Newgard C. B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. 10.1161/circresaha.117.311002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M.; Breitkopf S. B.; Yang X.; Asara J. M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J.; Soufan O.; Li C.; Caraus I.; Li S.; Bourque G.; Wishart D. S.; Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W.; Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. 10.1093/bioinformatics/btt285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.