Abstract
Objective:
To examine the role of non-exenterative secondary cytoreductive surgery (SCS) compared with non-surgical treatments and identify predictors of improved survival for patients with recurrent endometrial cancer (EC).
Methods:
All patients undergoing primary surgical management for EC 1/1/2009–12/31/2017 who subsequently developed recurrence were retrospectively identified. Survival was determined from date of diagnosis of first recurrence to last follow-up and estimated using Kaplan-Meier method. Differences in survival were analyzed using Log-rank and Wald tests, based on Cox Proportional Hazards model.
Results:
Among 376 patients with recurrent EC, median time to recurrence was 14.3 months (range, 0.2–102.2), post-recurrence median survival 29 months, median follow-up 29.2 months (range, 0–116). Sixty-one patients (16.2%) received SCS, 257 (68.4%) medical management (MM) (chemotherapy and/or radiation therapy), 32 (8.5 %) hormonal therapy, 26 (6.9%) no further therapy. Patients selected for SCS were younger, had more endometrioid histology, more stage I disease at initial diagnosis, no residual disease after primary surgery, longer interval to first recurrence or progression, and the longest OS (57.6 months) (95% CI, 33.3–not reached). On multivariate analysis SCS was an independent predictor of improved survival. Among the 61 SCS patients, age <70 at time of initial diagnosis, and endometrioid histology, were associated with improved post-relapse survival univariately (p=0.008, 0.03, respectively).
Conclusions:
While MM was the most common treatment for first recurrence of EC, patients selected for surgery demonstrated the greatest survival benefit even after controlling for tumor size, site, histology, stage, time to recurrence. Careful patient selection and favorable tumor factors likely play a major role in improved outcomes. Surgical management should be considered whenever feasible in medically eligible patients, with additional consideration given to our suggested criteria.
Keywords: Recurrent endometrial cancer, Endometrial cancer, Surgical cytoreduction, Chemotherapy, Radiation therapy
INTRODUCTION
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States, with an estimated 65,620 new cases and 12,590 deaths in 2020 [1]. The incidence of EC is on the rise and is likely attributable, in part, to increasing life expectancy and higher prevalence of obesity and metabolic syndrome [2, 3]. The rise in incidence parallels an increase in mortality rate. The recurrence rate is stable, ranging between 11–14% [1, 4–6]. Patients with recurrent EC represent a heterogenous group that varies by histological subtype, previous adjuvant therapy, time interval to recurrence, and size and site(s) of disease. To date, the literature focusing on management of recurrent EC is primarily retrospective, with small case numbers [7]. Retrospective data suggest that previous therapy, site of recurrence, interval to recurrence, and patient performance status are significant factors, and these are primarily what clinicians rely upon for decision-making [8–11]. The management of recurrent EC remains a challenge. Therapeutic strategies vary significantly, and may include surgical resection, radiation therapy, cytotoxic chemotherapy, hormonal therapy, or a combination of these. More recently immunotherapy has been incorporated as a treatment option.
Historically, surgery was limited to curative-intent pelvic exenterations for patients with central pelvic recurrences [12, 13]. Subsequent studies have investigated the role of non-exenterative surgery for advanced or recurrent disease [6, 14–17]. In this study, we evaluated patients with EC who underwent primary surgical treatment at our institution and developed a recurrence. We aimed to identify key clinical and pathologic features which might influence treatment selection for recurrent disease and impact oncologic outcomes. In addition, we investigated key variables associated with survival among patients selected for non-exenterative surgery.
METHODS
Study design and data collection
After obtaining IRB approval, we retrospectively identified 2864 patients undergoing primary surgical management for EC at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1, 2009 and December 31, 2017. Histologically confirmed carcinosarcomas, sarcomas, or dedifferentiated carcinomas were excluded (n=296). The remaining 2568 patients had histologically confirmed endometrioid, serous, clear cell, or mixed histologies. Among these patients 396 were found to have recurred by December 31st, 2020. Demographic, clinical and pathologic data were abstracted from the medical records. Patients were grouped by type of subsequent therapy versus no further therapy at time of recurrence. Ultimately, 376 patients were analyzed in the study after excluding patients lost to follow-up, with no clinicopathologic information after diagnosis of recurrence (n=19), and patients who underwent IR-guided ablation, as there was an insufficient number of cases (n=1) to perform statistically meaningful comparisons. Patients undergoing pelvic exenteration at time of secondary cytoreduction were also excluded (Supplementary Figure 1).
Primary surgical staging, all of which was performed at MSKCC, included, at minimum, a hysterectomy. All additional procedures were performed within the guidelines of the National Comprehensive Cancer Network (NCCN) [18]. Adjuvant therapy after primary surgery was selected using NCCN guidelines and clinical best practice, including chemotherapy and/or radiation therapy, or no further therapy [18]. Clinical follow-up reflected best practice guidelines and included physical exam, symptom review, and imaging based on symptoms or exam findings [19]. Follow-up included clinical assessments every 3–6 months for the first 2 years, and every 6–12 months thereafter [18].
Recurrence data were abstracted from documented physical exam, imaging, and pathology in the medical record. Size and sites of recurrent disease were abstracted from operative and pathology reports. Sites of recurrence were categorized by anatomical location: (1) pelvic only; (2) nodal only; (3) pelvic and nodal; (4) distant (extra-pelvic); (5) peritoneal carcinomatosis only.
Management of recurrence was classified as follows: (1) secondary cytoreductive surgery (SCS); (2) medical management (MM); (3) hormonal therapy (4) no further therapy. Medical management comprised of radiation therapy (n=47), chemotherapy with or without radiation therapy (n=191), and a few cases of targeted or immune therapy on trial (n=19); it did not include hormonal therapy. Selection for SCS was at the discretion of the attending physician, as no validated guidelines exist. Each patient’s overall health, recurrence characteristics, feasibility and safety of resection, were considered in the decision-making process. Perioperative data were abstracted, and included total operative time, estimated blood loss (EBL), residual disease, length of stay (LOS), complications, and adjuvant therapy. Complications were graded based on published institutional parameters, in which grade 1 requires bedside care or oral medications, grade 2 requires intravenous medications or transfusions, grade 3 includes radiologic, endoscopic, or operative interventions, grade 4 results in chronic disability or organ resection; and grade 5 is death [20].
Statistical Analysis
Descriptive statistics were provided for the whole cohort, as well as across the four treatment modalities. The differences among all four treatment groups were tested using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. To examine the difference between SCS and MM, the Wilcoxon Rank-Sum test was used for continuous variables and Fisher’s exact test for categorical variables.
Progression-free survival (PFS) was calculated from date of surgery to date of first recurrence or progression (PFS1). Progression-free survival was also reported from first recurrence to date of second recurrence, progression, death, or last follow-up (PFS2). Overall survival (OS) was defined as time elapsed in months from date of first recurrence to date of death or last follow-up. Follow-up data were collected until January 1, 2020. The median survival time and 2-year survival rate were estimated using the Kaplan-Meier method. In the univariate setting, the Log-rank test and the Wald test, based on Cox Proportional Hazards model, were applied to obtain p-values for survival outcomes. Due to the time-dependent nature of selecting and administering a treatment modality for first recurrence, a landmark analysis was performed. Landmark time of 26 days from first recurrence was used, based on the data. Multivariate models to predict PFS2 and OS were built, based on the results of univariate analyses. Stepwise selection was also applied. The final multivariate models excluded patients who underwent no further therapy, due to the small sample size of this subgroup (N=26). Estimation of hazard ratios (HRs) was non-robust, as shown by wide confidence intervals (CIs). Statistical analysis was conducted using SAS 9.4.
RESULTS
Patient characteristics grouped by treatment modalities for recurrent disease
A total of 376 patients with recurrent EC, who met the inclusion criteria, were identified. The median time to first recurrence was 14.3 months (range, 0.2–102.2 months). Median age at time of primary surgical resection was 66 years (range, 28–93 years). Median BMI was 29.5 kg/m2 (range, 14.2–60.3 kg/m2). The histologic subtypes were as follows: 183 (48.7%) endometrioid; 146 (38.8%) serous; 47 (12.5%) clear cell or mixed. Initial surgical stage was equally distributed between early-stage (I/II) disease versus late-stage (III/IV) disease. After primary surgery, 314 (83.5%) patients received adjuvant therapy.
Of the 376 patients with recurrence, 61 (16.2%) were selected for SCS, 257 (68.4%) for MM, 32 (8.5%) for hormonal therapy, and 26 (6.9%) had no further therapy. Compared across treatment modalities, the median age at initial diagnosis for patients who had no further therapy and those who had hormonal therapy was 67.5 (range, 33–92) and 69.5 (range, 47–86) years, respectively, compared with 66 years (range, 28–92) for patients treated with MM; patients undergoing SCS had the youngest median age of 62 years (range, 39–83.3) (p=0.004). There was no significant difference in BMI across treatment modalities (p=0.198). Histology and stage did not differ significantly across treatment modalities; however, these variables did differ significantly between patients undergoing SCS versus MM. Thirty-seven (60.7%) of the 61 SCS patients had endometrioid histology, compared with 117 of the 257 (45.5%) MM patients (p=0.033). Among SCS patients, 31 (50.8%) had endometrioid FIGO grade 3 or high-grade histology, compared with 177 (68.9%) MM patients (p=0.022). Thirty-five (57.4%) SCS patients and 108 (42%) MM patients had stage I disease at initial diagnosis; there were only 6 (9.8%) SCS patients with stage IV disease at initial diagnosis, compared with 67 (26.1%) MM patients (p=0.014). Time to first recurrence or progression differed significantly across treatment modalities. Patients undergoing SCS had the longest median PFS1 after primary surgery: 19.4 months (range, 2.4–88.3; p<0.001). Table 1A.
Table 1.
Clinicopathologic characteristics among all recurrences compared across treatment modalities (N = 376).
| Variable | All recurrences | SCS | Medical management | Hormonal therapy | No therapy | p-Value† | p-Value |
|---|---|---|---|---|---|---|---|
| (N = 376) | (N = 61) | (N = 257) | (N = 32) | (N = 26) | (SCS vs MM)†† | ||
| 1A: Clinicopathologic characteristics at the time of initial diagnosis and primary surgery | |||||||
| Age at initial diagnosis, years | |||||||
| Median (range) | 66 (28–93) | 62 (39–83.3) | 66 (28–90) | 69.5 (47–86) | 67.5 (33–92) | 0.004 | 0.004 |
| Age ≤70 | 254 (67.6) | 50 (82) | 174 (67.7) | 16 (50) | 14 (53.8) | 0.005 | 0.029 |
| Age > 70 | 122 (32.4) | 11 (18) | 83 (32.3) | 16 (50) | 12 (46.2) | ||
| BMI at initial diagnosis, kg/m2 | |||||||
| Median (range) | 29.5 (14.2–60.3) | 25.3 (19.1–43) | 29.7 (14.2–60.3) | 28.5 (15.8–51) | 25.3 (19.1–43) | 0.349 | 0.739 |
| Normal (BMI < 25) | 93 (24.7) | 13 (21.3) | 62 (24.1) | 7 (21.9) | 11 (42.3) | 0.165* | 0.882 |
| Overweight (25 ≤BMI < 30) | 104 (27.7) | 16 (26.2) | 70 (27.2) | 14 (43.8) | 4 (15.4) | ||
| Obese (BMI ≥30) | 179 (47.6) | 32 (52.5) | 125 (48.6) | 11 (34.4) | 11 (42.3) | ||
| Progression-free survival (PFS1) (months) | |||||||
| Median (range) | 14.3 (0.2–102.2) | 19.4 (2.4–88.3) | 13.3 (0.2–102.2) | 15.5 (2.5–92.4) | 11.6 (1.9–62.2) | 0.002 | <0.001 |
| Histology | |||||||
| Endometrioid | 183 (48.7) | 37 (60.7) | 117 (45.5) | 18 (56.3) | 11 (42.3) | 0.214* | 0.033 |
| Serous | 146 (38.8) | 15 (24.6) | 109 (42.4) | 11 (34.4) | 11 (42.3) | ||
| Clear Cell/Mixed | 47 (12.5) | 9 (14.8) | 31 (12.1) | 3 (9.4) | 4 (15.4) | ||
| FIGO Grade | 0.016* | 0.022 | |||||
| G1 | 62 (16.5) | 12 (19.7) | 37 (14.4) | 11 (34.4) | 2 (7.7) | ||
| G2 | 71 (18.9) | 18 (29.5) | 43 (16.7) | 5 (15.6) | 5 (19.2) | ||
| G3/high-grade histology | 243 (64.6) | 31 (50.8) | 177 (68.9) | 16 (50) | 19 (73.1) | ||
| Stage | |||||||
| I | 162 (43.1) | 35 (57.4) | 108 (42) | 11 (34.4) | 8 (30.8) | 0.065* | 0.014 |
| II | 27 (7.2) | 2 (3.3) | 19 (7.4) | 3 (9.4) | 3 (11.5) | ||
| III | 96 (25.5) | 18 (29.5) | 63 (24.5) | 9 (28.1) | 6 (23.1) | ||
| IV | 91 (24.2) | 6 (9.8) | 67 (26.1) | 9 (28.1) | 9 (34.6) | ||
| ≥Grade 3 Complication | |||||||
| No | 363 (96.5) | 60 (98.4) | 250 (97.3) | 30 (93.8) | 23 (88.5) | 0.069 | 1 |
| Yes | 13 (3.5) | 1 (1.6) | 7 (2.7) | 2 (6.3) | 3 (11.5) | ||
| Length of hospital stay, days | |||||||
| Median (range) | 1 (0–49) | 1 (0–6) | 1 (0–49) | 2 (0–15) | 2.5 (0–18) | 0.002 | 0.003 |
| Residual disease after primary surgery | |||||||
| Absent | 348 (92.6) | 61 (100) | 234 (91.1) | 30 (93.8) | 23 (88.5) | 0.033 | 0.011 |
| Present | 28 (7.4) | 0 (0) | 23 (8.9) | 2 (6.3) | 3 (11.5) | ||
| Adjuvant Therapy after primary surgery | |||||||
| No | 62 (16.5) | 12 (19.7) | 41 (16) | 5 (15.6) | 4 (15.4) | 0.891 | 0.452 |
| Yes | 314 (83.5) | 49 (80.3) | 216 (84) | 27 (84.4) | 22 (84.6) | ||
| Adjuvant Chemotherapy after primary surgery | |||||||
| No | 133 (35.4) | 21 (34.4) | 91 (35.4) | 11 (34.4) | 10 (38.5) | 0.988 | 1 |
| Yes | 243 (64.6) | 40 (65.6) | 166 (64.6) | 21 (65.6) | 16 (61.5) | ||
| Adjuvant Radiation after primary surgery | |||||||
| No | 173 (46) | 19 (31.1) | 123 (47.9) | 15 (46.9) | 16 (61.5) | 0.039 | 0.022 |
| Yes | 203 (54) | 42 (68.9) | 134 (52.1) | 17 (53.1) | 10 (38.5) | ||
| Type of radiation therapy | |||||||
| IVRT | 144 (70.9) | 26 (61.9) | 100 (74.6) | 13 (76.5) | 5 (50) | 0.101 | 0.097 |
| EBRT | 53 (26.1) | 16 (38.1) | 30 (22.4) | 3 (17.6) | 4 (40) | ||
| IVRT/EBRT | 6 (3.0) | 0 (0) | 4 (3) | 1 (5.9) | 1 (10) | ||
| 1B: Clinicopathologic characteristics at the time of recurrence | |||||||
| Size of largest recurrent tumor (cm)^ | |||||||
| Median (range) | 2.2 (0.2–19.8) | 2.6 (0.5–12) | 2.2 (0.2–19.8) | 2.5 (0.5–12) | 2.4 (0.6–19.2) | 0.003 | 0.148 |
| Multiplicity of sites of recurrence | |||||||
| Single | 201 (53.5) | 46 (75.4) | 125 (48.6) | 16 (50) | 14 (53.8) | 0.002 | <0.001 |
| Multiple | 175 (46.5) | 15 (24.6) | 132 (51.4) | 16 (50) | 12 (46.2) | ||
| Sites of recurrence | |||||||
| Pelvic alone | 72 (19.1) | 11 (18) | 59 (23) | 0 (0) | 2 (7.7) | 0.004* | 0.109 |
| Nodal alone | 60 (16) | 10 (16.4) | 35 (13.6) | 11 (34.4) | 4 (15.4) | ||
| Pelvic and nodal | 16 (4.3) | 3 (4.9) | 11 (4.3) | 1 (3.1) | 1 (3.8) | ||
| Distant +/− pelvic | 195 (51.9) | 36 (59) | 124 (48.2) | 18 (56.3) | 17 (65.4) | ||
| Peritoneal carcinomatosis alone | 33 (8.8) | 1 (1.6) | 28 (10.9) | 2 (6.3) | 2 (7.7) | ||
| Subsequent lines of therapy | |||||||
| Median (range) | 1 (1–8) | 1 (1–6) | 2 (1–8) | 2 (1–7) | – | <0.001 | <0.001 |
Values are presented as numbers (%) unless otherwise specified.
SCS, secondary cytoreductive surgery; BMI, body mass index; IVRT, intravaginal radiation therapy; EBRT, external beam radiation therapy; FIGO, International Federation of Gynecology and Obstetrics.
Bold denotes significant p-values.
Twenty-four data points not available.
p-values with * are obtained using Monte Carlo estimation for exact test; other p-values are obtained using Kruskal Wallis test for continuous and Fisher’s exact test for categorical.
p-values are obtained using Fisher’s exact test for categorical and Willcoxon rank-sum test for continuous.
The presence of a grade 3 or greater complication at time of primary surgery did not differ across treatment modalities, ranging from 1 (1.6%) among SCS patients to 7 (2.7%) among MM patients (p=1). However, LOS after primary surgery differed. Patients receiving SCS or MM had a 1-day median LOS after primary surgery (range, 0–6; 0–49, respectively) compared with 2 (range, 0–15) and 2.5 (range, 0–18) days for patients receiving hormonal therapy or no further therapy, respectively (p=0.002). Absence of residual disease after primary surgery varied significantly in distribution between treatment modalities; all 61 (100%) SCS patients had no visible disease at completion of their primary surgery, regardless of disease stage, compared with 234 (91.1%) MM patients, 30 (93.8%) patients receiving hormonal therapy, and 23 (88.5%) receiving no further therapy (p=0.033). Adjuvant chemotherapy after primary surgery was equally distributed across treatment modalities, ranging from 80–85% (p=0.891). Distribution of adjuvant radiation therapy differed: 68.9% of SCS patients, 52.1% of MM patients, 53.1% of hormonally treated patients, 38.5% of patients who had no further therapy (p=0.039) (Table 1A).
The clinicopathological characteristics of recurrent disease are reported in Table 1B. Of the 376 recurrences, 175 (46.5%) presented with multiple sites of disease. Among those selected for SCS, 15 (24.6%) had multisite disease compared with 132 (51.4%), 16 (50%), and 12 (46.2%) selected for MM, hormonal therapy, and no further therapy, respectively (p=0.002). Anatomical site of recurrence differed across treatment modalities (p=0.004); however, there was no significant difference in site distribution between the MM and SCS cohorts (p=0.109). Patients treated surgically received a median of 1 (range 1–6) additional therapy, compared with 2 among patients receiving MM or hormonal therapy (range 1–8, 1–7; p <0.001) (Table 1B).
Secondary cytoreductive surgery characteristics
Table 2 shows the operative characteristics of the 61 patients undergoing SCS as initial treatment after first recurrence. Median operative time was 145 minutes (range, 11–405 minutes) and EBL was 75 mL (range, 5–1800 mL). Complete gross resection (CGR) was documented in 46 patients (75.4%), while the remaining 15 (24.6%) had visible tumor at completion of surgery. There were 9 reported complications within 30 days from surgery; two of these (4.9%) were grade 3 complications: 1 patient with an intra-abdominal fluid collection requiring an interventional procedure, and another patient with postoperative bleeding. There were no grade 4 or 5 complications. The remaining 7 complications were grade 1 or 2. The median LOS was 1 day (range, 0–13 days). Fifty-four (88.5%) patients received adjuvant therapy: 45 received postoperative chemotherapy and/or radiation therapy; the remaining 9 received adjuvant hormonal therapy (Table 2).
Table 2.
Intraoperative and postoperative characteristics of secondary cytoreductive surgery for recurrent disease (N = 61)
| Variable | |
|---|---|
| DOD of recurrence to SCS (days) | 26 (0–155) |
| Total Operative Time (min) | 145 (11–405) |
| Estimated Blood Loss (mL)* | 75 (5–1800) |
| Residual Disease | |
| CGR | 46 (75.4) |
| Non-CGR | 15 (24.6) |
| Complication | |
| No | 51 (85) |
| Yes | 9 (15) |
| Complication Grade | |
| 1 | 3 (4.9) |
| 2 | 4 (6.6) |
| 3 | 2 (3.3) |
| Length of stay (days)** | 1 (0–13) |
| Adjuvant therapy | |
| None | 7 (11.5) |
| Chemo | 26 (42.6) |
| RT | 7 (11.5) |
| Chemo-RT | 12 (19.7) |
| Hormone | 9 (14.8) |
Values are presented as numbers (%) or median (range)
DOD, Date of diagnosis of recurrence; SCS, secondary cytoreductive surgery; CGR, complete gross resection; Chemo, chemotherapy; RT, radiation therapy
One data point not available
Three data points not available
Survival across treatment modalities
Two hundred and eighty-eight patients progressed or died after their initial recurrence. Median follow-up was 29.2 months (range, 0–116 months). Median PFS after treatment for first recurrence (PFS2) for the entire cohort was 9.5 months (95% CI, 8.5–10.9); median OS after recurrence was 29 months (95% CI, 24.4–32.5). Patients selected for SCS had a median PFS2 of 14.9 months (95% CI, 10.1–41.8) and OS of 57.6 months (95% CI, 33.3-NE), which were statistically significantly longer than patients selected for other therapeutic modalities (p<0.001). Patients receiving MM had a median PFS2 of 8.6 months (95% CI, 7.5–10.6) and OS of 24.5 months (95% CI, 21–30.6), which differs significantly from the SCS group (p=0.002, p<0.001) (Table 3; Figure 1A).
Table 3.
Survival analysis based on treatment modality for initial recurrence (N = 376)
| 3A: Progression-free survival after recurrence (PFS2) | ||||||
|---|---|---|---|---|---|---|
| Variable | N | Progression/Death (n) | Median PFS2, months (95% CI) | 2-yr PFS2 rate (95% CI) | HR (95% CI) | p-Value |
| Whole cohort | 376 | 288 | 9.5 (8.5–10.9) | 25.5% (21.0–30.3) | ||
| All Modalities of Treatment for 1st Recurrence | <0.001 | |||||
| SCS | 61 | 37 | 14.9 (10.1–41.8) | 44.6% (31.2–57.1) | 0.13 (0.07–0.24) | |
| MM | 253 | 199 | 8.6 (7.5–10.6) | 22.6% (17.3–28.2) | 0.23 (0.13–0.38) | |
| Hormonal therapy | 31 | 28 | 5.9 (3.2–17.2) | 21.3% (8.9–37.2) | 0.29 (0.16–0.55) | |
| None | 16 | 15 | 1.1 (0.4–2.6) | Not Reached | 1 | |
| Surgical vs Medical Treatment for 1st Recurrence | ||||||
| SCS | 61 | 37 | 14.9 (10.1–41.8) | 44.6% (31.2–57.1) | 1 | 0.002 |
| MM | 253 | 199 | 8.6 (7.5–10.6) | 22.6% (17.3–28.2) | 1.74 (1.22–2.47) | |
| 3B: Overall survival (OS) after recurrence | ||||||
| Variable | N | Death (n) | Median OS, months (95% CI) | 2-yr OS rate (95% CI) | HR (95% CI) | p-Value |
| Whole cohort | 376 | 212 | 29 (24.4–32.5) | 56.4% (50.8–61.6) | ||
| All Modalities of Treatment for 1st Recurrence | <0.001 | |||||
| SCS | 61 | 21 | 57.6 (33.3-NE) | 80.9% (67.3–89.3) | 0.04 (0.02–0.07) | |
| MM | 255 | 149 | 24.5 (21–30.6) | 51.6% (44.7–58) | 0.08 (0.05–0.15) | |
| Hormone | 31 | 20 | 33.3 (22.7–46.7) | 66.5% (46.6–80.4) | 0.08 (0.04–0.16) | |
| None | 16 | 15 | 1.8 (0.5–2.6) | Not Reached | 1 | |
| Surgical vs Medical Treatment for 1st Recurrence | ||||||
| SCS | 61 | 21 | 57.6 (33.3-NE) | 80.9% (67.3–89.3) | 1 | <0.001 |
| MM | 255 | 149 | 24.5 (21–30.6) | 51.6% (44.7–58) | 2.32 (1.47–3.66) | |
PFS2, progression-free survival after recurrence; SCS, secondary cytoreductive surgery; OS, overall survival; MM, medical management; NE, not estimable.
Figure 1:
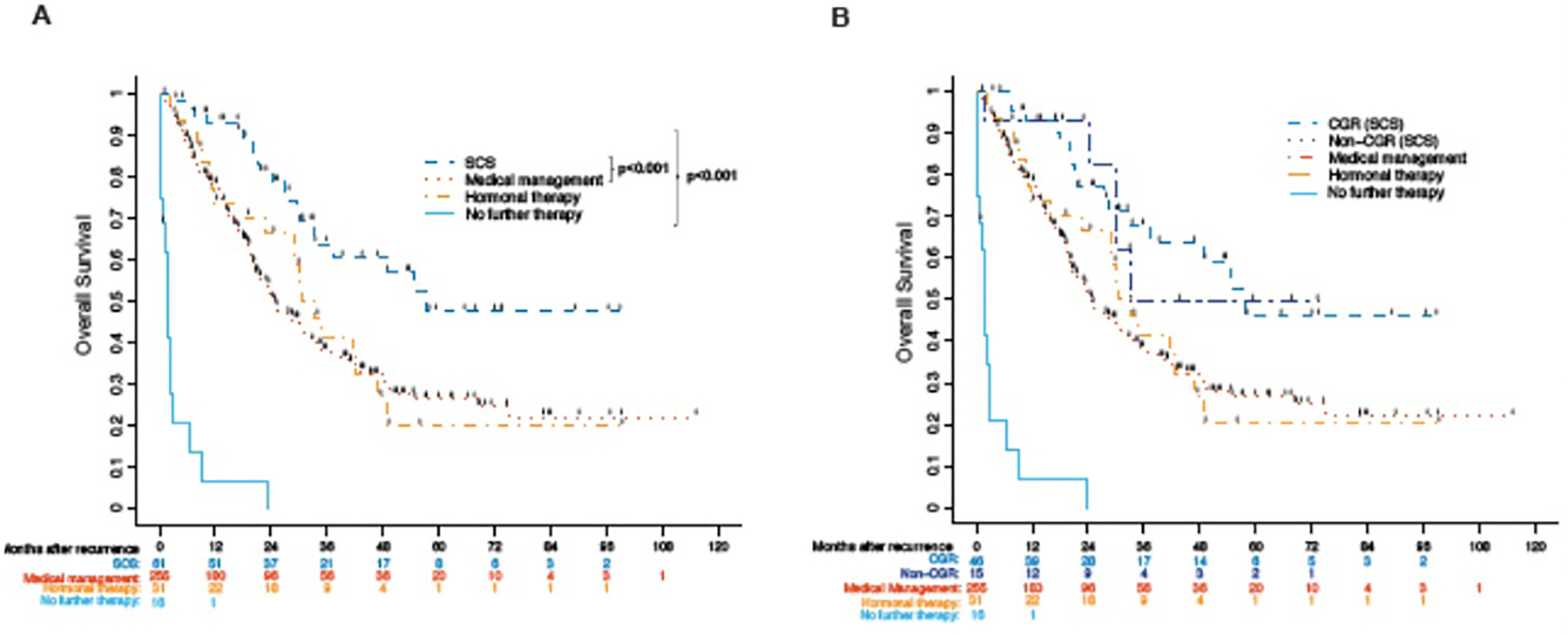
Overall survival for 376 patients with recurrent endometrial cancer from time of recurrence stratified by treatment modality (A) and further stratified by complete gross resection at time of SCS (B).
In addition to therapeutic modality, multiple other clinicopathologic variables were investigated, on univariate analysis, with respect to post-recurrence survival (Supplementary Table 1). Multivariate analysis was used to incorporate any variables identified as significant on univariate analysis; these are reported in Supplementary Table 2. The univariate variables significantly impacting PFS2 included: time to recurrence; factors identified at initial diagnosis and surgery—BMI, histology, FIGO grade, stage, presence of > grade 3 complication, LOS, residual disease, adjuvant therapy; and factors related to recurrence—size of largest tumor, multiplicity of sites, anatomical site distribution, treatment modality (Supplementary Table 1A). With the exception of BMI, these univariate variables also significantly impacted OS, as did age at initial diagnosis (Supplementary Table 1B). The final multivariate models, shown in Supplementary Table 2, exclude the no treatment subgroup. The multivariate model demonstrated that advanced stage, adjuvant therapy after initial surgery, size of tumor at recurrence, extrapelvic recurrence, and selection for non-surgical treatment at time of recurrence, were significantly associated with a decrease in PFS2. Specifically, PFS2 was significantly shorter among patients receiving MM (HR 2.0; 95% CI 1.4–3) and hormonal therapy (HR 2.2; 95% CI 1.4–4.2), compared with SCS (p<0.001) (Supplementary Table 2A). The multivariate model on OS demonstrated that age > 70 years at diagnosis, < 12 months’ time to initial recurrence, serous histology, advanced stage, adjuvant therapy after initial surgery, increased size of tumor at recurrence, extrapelvic recurrence, and non-surgical treatment at time of recurrence, were significantly associated with lower OS. SCS was an independent predictor of improved survival, compared with MM (HR of death 2.1; 95% CI 1.3–3.5) and hormonal therapy (HR 2.3; 95% CI 1.1–4.5) (p=0.012) (Supplementary Table 2B).
Further selection of the surgical patient
A univariate analysis was performed using clinicopathologic factors determined at time of primary surgery or at recurrence, to delineate potential prognostic factors among the 61 SCS patients. Table 4 summarizes the analysis of these factors: age, BMI, histology, grade, stage, complications, LOS, adjuvant therapy at time of primary surgery, time to first recurrence, size of largest recurrent tumor, multiplicity and sites of recurrence, and residual disease at SCS. Age (> 70 years vs. ≤ 70 years) at primary surgery was the only factor significantly associated with a lower PFS2 (HR 2.46; 95% CI 1.1–5.5; p=0.023). Age > 70 years at primary surgery portended worse OS (HR 3.52; 95% CI 1.3–9.5; p=0.008). Among SCS patients, histology also appeared to impact OS: endometrioid histology was associated with improved OS compared with serous (HR 3.4; 95% CI 1.3–8.9) and clear cell or mixed (HR 1.35; 95% CI 0.37–4.93) (p=0.030) histologies (Table 4A, 4B).
Table 4.
Survival after secondary cytoreductive surgery based on clinicopathologic and surgical characteristics (N = 61).*, **
| 4A: Progression-free survival after secondary cytoreduction surgery | ||||||
|---|---|---|---|---|---|---|
| Variable | Total N | Progression/Death# | Median PFS2, months (95% CI) | 2Yr PFS2 rate (95% CI) | HR (95% CI) | p-Value |
| Whole | 61 | 37 | 15.8 (10.9–42.7) | 44.6% (31.2–57.1%) | ||
| Age at initial diagnosis, years | ||||||
| Age ≤70 | 50 | 29 | 27.7 (12.2–56.8) | 50.6% (35.4–63.9%) | 1 | 0.023 |
| Age > 70 | 11 | 8 | 11.1 (2.8–17.7) | 13.3% (0.7–43.5%) | 2.46 (1.1–5.48) | |
| BMI at initial diagnosis, kg/m2 | ||||||
| Normal (BMI < 25) | 13 | 10 | 12.7 (7.4–66.9) | 38.5% (14.1–62.8%) | 1 | 0.707 |
| Overweight (25 ≤BMI < 30) | 16 | 11 | 21 (7.5–56.8) | 50% (24.5–71%) | 0.81 (0.34–1.92) | |
| Obese (BMI ≥30) | 32 | 16 | 17.7 (10.6–NE) | 43.2% (23.8–61.2%) | 0.71 (0.32–1.59) | |
| Progression-free survival (PFS1) | ||||||
| As continuous (6 months increase) | 0.89 (0.76–1.05) | 0.179 | ||||
| ≤12 months | 13 | 9 | 10.4 (5.7–NE) | 30.8% (9.5–55.4%) | 1 | 0.205 |
| >12 months | 48 | 28 | 18.7 (13.5–42.7) | 48.4% (32.7–62.5%) | 0.61 (0.29–1.32) | |
| Histology | ||||||
| Endometrioid | 37 | 19 | 29 (10.9–NE) | 54.2% (35.6–69.5%) | 1 | 0.310 |
| Serous | 15 | 11 | 11.1 (5.8–28.5) | 32% (10.9–55.7%) | 1.76 (0.82–3.77) | |
| Clear Cell/Mixed | 9 | 7 | 15.8 (7.4–66.9) | 33.3% (7.8–62.3%) | 0.99 (0.39–2.51) | |
| FIGO Grade | ||||||
| G1 | 12 | 4 | NR | 62.5% (26.8–84.6%) | 1 | 0.236 |
| G2 | 18 | 11 | 27.7 (10.9–66.9) | 56.5% (29.7–76.4%) | 1.51 (0.48–4.81) | |
| G3/high-grade histology | 31 | 22 | 15 (9.9–18.7) | 32.2% (16.4–49.2%) | 2.26 (0.77–6.58) | |
| Stage | ||||||
| I | 35 | 20 | 27.7 (12.2–66.9) | 53.6% (35–69%) | – | |
| II | 2 | 1 | 5.8, 2.0 (alive) | NR | ||
| III | 18 | 12 | 14.7 (5.7–42.7) | 32.6% (12.1–55.1%) | ||
| IV | 6 | 4 | 10.3 (3.4–NE) | 33.3% (4.6–67.6%) | ||
| ≥Grade 3 Complication | ||||||
| No | 60 | 36 | 15.8 (10.9–42.7) | 45.4% (31.9–58.1%) | – | |
| Yes | 1 | 1 | 12.7 | NR | ||
| Length of hospital stay (as continuous; 1-day increase) | 1.11 (0.87–1.41) | 0.409 | ||||
| Adjuvant therapy after primary surgery | ||||||
| No | 12 | 7 | 27.7 (6.9–56.8) | 56.3% (24.4–79.1%) | 1 | 0.777 |
| Yes | 49 | 30 | 15.7 (10.6–29) | 41.6% (27–55.7%) | 1.13 (0.49–2.58) | |
| Adjuvant Chemotherapy after primary surgery | ||||||
| No | 21 | 11 | 27.7 (8.9–56.8) | 52% (27.6–71.7%) | 1 | 0.616 |
| Yes | 40 | 26 | 15.7 (10.4–29) | 40.6% (24.8–55.9%) | 1.2 (0.59–2.44) | |
| Adjuvant Radiation after primary surgery | ||||||
| No | 19 | 13 | 13.5 (9.5–56.8) | 39.5% (17.9–60.5%) | 0.473 | |
| Yes | 42 | 24 | 17.7 (10.9–66.9) | 46.8% (30.2–61.8%) | 0.78 (0.39–1.54) | |
| Size of largest recurrent tumor (as continuous; 1 cm increase) | 1.09 (0.99–1.21) | 0.08 | ||||
| Multiplicity of Sites of Recurrence | ||||||
| Single | 46 | 26 | 27.7 (14.7–56.8) | 52.5% (36.3–66.4%) | 1 | 0.065 |
| Multiple | 15 | 11 | 11 (7.5–13.5) | 21.4% (5.2–44.8%) | 1.96 (0.95–4.04) | |
| Sites of recurrence | ||||||
| Pelvic alone | 11 | 5 | 29 (5.8–NE) | 62.3% (27.7–84%) | – | |
| Nodal alone | 10 | 5 | NR | 50% (18.4–75.3%) | ||
| Pelvic and nodal | 3 | 3 | 10.9 (7.5–12.7) | NR | ||
| Distant +/− pelvic | 36 | 23 | 17.7 (11.1–42.7) | 43.9% (26.4–60.1%) | ||
| Peritoneal carcinomatosis alone | 1 | 1 | 10.6 | NR | ||
| Sites of recurrence | ||||||
| Pelvic | 11 | 5 | 29 (5.8–NE) | 62.3% (27.7–84%) | 1 | 0.587 |
| All other sites of disease | 50 | 32 | 15.7 (10.9–28.5) | 41.5% (27.2–55.2%) | 1.3 (0.5–3.37) | |
| 4B: Overall surivival after secondary cytoreduction surgery | ||||||
| Variable | Total N | Progression/Death# | Median OS in months (95% CI) | 2Yr OS rate (95% CI) | HR (95% CI) | p-Value |
| Whole | 61 | 21 | 58.4 (34.1–NE) | 80.9% (67.3–89.3%) | ||
| Age at initial diagnosis, years | ||||||
| Age ≤70 | 50 | 15 | NR | 84.2% (69.6–92.2%) | 1 | 0.008 |
| Age > 70 | 11 | 6 | 29 (8.1–38.4) | 61.7% (20.7–86.3%) | 3.52 (1.31–9.47) | |
| BMI at initial diagnosis, kg/m2 | ||||||
| Normal (BMI < 25) | 13 | 5 | NR | 76.9% (44.2–91.9%) | 0.974 | |
| Overweight (25 ≤BMI < 30) | 16 | 8 | 49.7 (31.1–NE) | 93.8% (63.2–99.1%) | 1.14 (0.37–3.49) | |
| Obese (BMI ≥30) | 32 | 8 | NR | 74.4% (50.9–87.8%) | 1.08 (0.35–3.34) | |
| Progression-free survival (PFS1) | ||||||
| As continuous (6 months increase) | 0.91 (0.73–1.13) | 0.385 | ||||
| ≤12 months | 13 | 6 | NR | 61.5% (30.8–81.8%) | 1 | 0.222 |
| >12 months | 48 | 15 | 58.4 (34.1–NE) | 86.9% (71.2–94.3%) | 0.56 (0.21–1.44) | |
| Histology | ||||||
| Endometrioid | 37 | 10 | NR | 87.8% (70.5–95.2%) | 1 | 0.030 |
| Serous | 15 | 8 | 30.6 (21.1–38.4) | 67.9% (35–86.7%) | 3.4 (1.3–8.88) | |
| Clear Cell/Mixed | 9 | 3 | NR | 71.4% (25.8–92%) | 1.35 (0.37–4.93) | |
| FIGO Grade | ||||||
| G1 | 12 | 2 | 31.4 (8.5–90.0) | 100% | – | |
| G2 | 18 | 4 | NR | 87.5% (58.6–96.7%) | ||
| G3/high-grade histology | 31 | 15 | 31.1 (22.6–NE) | 69.8% (48.2–83.7%) | ||
| Stage | ||||||
| I | 35 | 8 | NR | 82.2% (62.3–92.2%) | – | |
| II | 2 | 1 | 2.0 (alive), 38.4 | 100% | ||
| III | 18 | 9 | 49.7 (21.1–NE) | 75.8% (47.3–90.2%) | ||
| IV | 6 | 3 | 31.1 (18.6–NE) | 83.3% (27.3–97.5%) | ||
| ≥Grade 3 Complication | ||||||
| No | 60 | 20 | NR | 80.5% (66.7–89.1%) | – | |
| Yes | 1 | 1 | 55.6 | 100% | ||
| Length of hospital stay (as continuous; 1-day increase) | 1.19 (0.88–1.61) | 0.253 | ||||
| Adjuvant therapy after primary surgery | ||||||
| No | 12 | 4 | 58.4 (11.3–NE) | 82.5% (46.1–95.3%) | 1 | 0.765 |
| Yes | 49 | 17 | 55.6 (31.1–NE) | 80.1% (63.9–89.6%) | 1.18 (0.4–3.52) | |
| Adjuvant Chemotherapy after primary surgery | ||||||
| No | 21 | 6 | NR | 84% (58.1–94.6%) | 1 | 0.600 |
| Yes | 40 | 15 | 55.6 (31.1–NE) | 79% (60.8–89.5%) | 1.29 (0.5–3.32) | |
| Adjuvant Radiation after primary surgery | ||||||
| No | 19 | 8 | 58.4 (30.6–NE) | 83.6% (57.3–94.4%) | 1 | 0.940 |
| Yes | 42 | 13 | NR | 78.9% (60.5–89.4%) | 0.97 (0.4–2.34) | |
| Size of largest recurrent tumor (as continuous; 1 cm increase) | 1.08 (0.94–1.23) | 0.273 | ||||
| Multiplicity of sites of recurrence | ||||||
| Single | 46 | 15 | NR | 79% (62.1–88.9%) | 1 | 0.693 |
| Multiple | 15 | 6 | 55.6 (29–NE) | 85.7% (53.9–96.2%) | 1.21 (0.47–3.13) | |
| Sites of recurrence | ||||||
| Pelvic alone | 11 | 4 | 38.4 (18.6–NE) | 77.8% (36.5–93.9%) | – | |
| Nodal alone | 10 | 2 | 33.6 (13.9–49.9) | 100% | ||
| Pelvic and nodal | 3 | 1 | 55.6 (28.7–90.0) | 100% | ||
| Distant +/− pelvic | 36 | 14 | 58.4 (29–NE) | 77.2% (57.9–88.5%) | ||
| Peritoneal carcinomatosis alone | 1 | 0 | 17.9 | – | ||
| Site of recurrence | ||||||
| Pelvic | 11 | 4 | 38.4 (18.6–NE) | 77.8% (36.5–93.9%) | 1 | 0.755 |
| All other sites of disease | 50 | 17 | 58.4 (33.9–NE) | 81.6% (66.4–90.4%) | 0.84 (0.28–2.52) | |
PFS2, progression-free survival after recurrence; NR, not reached; NE, not estimable.
Bold denotes significant p-values.
p-values are obtained using log-rank test for categorical variables and CoxPH model for continuous variables.
p-value and hazard ratio are not provided for a variable if its certain level of event count is <3.
Residual disease at time of SCS was investigated as a potential prognostic indicator of greater survival benefit from surgical management. Among the 61 SCS patients, CGR was achieved in 46 (75.4%) cases. There was no statistically significant difference in OS based on CGR (HR 0.96; 95% CI 0.35–2.63; p=0.938) versus non-CGR. Median survival was 57.6 months (95% CI 57.6-not estimable) in the CGR versus 33.1 months (95% CI 24.2--not estimable) in the non-CGR groups (Figure 1B).
DISCUSSION
In this study we examined the role of surgery compared with other treatment modalities for patients with recurrent EC. We investigated surgical selection criteria based on an updated patient group representing current surgical care and techniques. We found that surgical cytoreduction for recurrent disease has a significant impact on survival even when it is non-exenterative. In our study, median survival from time of diagnosis of recurrence was 29 months, with a 56.4% 2-year OS rate. SCS patients had the longest median OS of 57.6 months (95% CI, 33.3–not reached) resulting in an 80.9% 2-year OS rate. These patients required a median of 1 additional line of subsequent therapy. On univariate analysis, after accounting for all significant factors that differed across treatment modalities, the impact of surgery on survival remained significant. Compared with surgery, MM was less effective (HR 2.1; 95% CI 1.3–3.5; p<0.012); hormonal therapy was even less effective (HR 2.3; 95% CI 1.1–4.5; p=0.012). Based on our findings, surgery demonstrated improved oncologic outcomes compared with MM and should be considered, if deemed technically feasible, in medically eligible patients. Similar findings have been reported by others, suggesting that surgical cytoreduction for the treatment of recurrence, even when non-exenterative, confers a significant survival benefit [14–16]. Bristow et al. demonstrated a significant prolongation of post-recurrence survival in patients undergoing surgery, compared with patients receiving non-surgical intervention [16]. The survival benefit conferred by surgical cytoreduction has been more extensively investigated in the setting of ovarian cancer; some have suggested that it results in improved perfusion and subsequent drug delivery to residual disease, improving performance status; and that (by decreasing the amount of viable tumor cells), it decreases the potential for somatic mutations that can lead to drug resistance [7]. These effects may also explain the survival advantage seen in patients receiving SCS for recurrent EC. Although many variables are accounted for through multivariate analysis, there may be other prognostic factors preferentially selected in SCS patients, such as better performance status, fewer comorbidities, and favorable molecular characteristics. Additional prospective trials are necessary to validate the survival benefit observed in patients with recurrent EC receiving SCS.
In the current study, SCS conferred the greatest survival benefit compared with other treatment modalities. Based on this, it is crucial to determine which patients should be selected for SCS. To date, however, there is no established criteria, and selection remains arbitrary. Based on our analysis, we propose a list of criteria for selecting patients who might benefit from SCS (Table 5). We suggest higher consideration for SCS in patients with the following features from time of diagnosis: age ≤ 70, PFS1 ≥ 19 months, grade 1/2 endometrioid or clear cell histology, early stage (I/II) disease; the following features from time of primary surgery: no residual disease, short LOS (0–6 days), no more than two grade 3 complications, received adjuvant radiation therapy; and the following feature from time of recurrence: single site disease. These features were chosen based on variables that differed significantly between patients selected for SCS versus MM. Features that did not differ statistically in distribution between patients selected for SCS versus MM should merit less consideration. These include BMI at initial diagnosis, adjuvant chemotherapy after primary surgery, size of recurrent tumor, and distant site of recurrent disease. These criteria are based solely on descriptive statistics and warrant further investigation with a prospective trial to evaluate their utility in selecting patients for SCS versus MM. (Table 5).
Table 5.
Suggested criteria for the consideration of secondary cytoreductive surgery over medical management for recurrent endometrial cancer based on clinicopathologic and initial surgical features
| Higher Consideration | Lower Consideration | |
|---|---|---|
| Features from time of diagnosis | Age ≤ 70 at initial diagnosis PFS1 ≥ 19 months Endometrioid/Clear cell FIGO Grade 1/2 Early stage I/II disease at diagnosis |
BMI |
| Features from primary surgery | No residual disease Hospital stay ranging from 0–6 days Received adjuvant radiation therapy |
Received adjuvant chemotherapy |
| Features at time of recurrence | Single site of disease | Size of tumor Distant site of recurrence |
PFS1, time interval from initial surgery to first progression/recurrence; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics
In patients with recurrent EC who are selected for SCS, extensive surgical procedures may be required, including upper and extra-abdominal surgery. Perioperative morbidity and mortality are of concern. In this series we report acceptable adverse outcomes data, including a 15% complication rate among patients undergoing SCS, with few major complications (3.3%) and no perioperative deaths. There were no prolonged hospital stays (median 1 day; longest 6 days). These findings likely reflect appropriate patient selection based on performance status and medical co-morbidities, the increased use of minimally invasive surgery, and improved perioperative care utilizing the Enhanced Recovery after Surgery (ERAS) protocols. Continued improvements in surgical technique and perioperative care permit more flexibility in selecting patients for surgical resection.
We found that, among the 61 patients undergoing SCS, those with distant or nodal disease did not have a statistically different survival compared with patients with disease confined to the pelvis. Except in 1 case, however, all patients with peritoneal carcinomatosis received non-surgical treatment; therefore, our findings are not applicable in the setting of peritoneal carcinomatosis. Women with recurrent disease are often categorized as having a vaginal or pelvic recurrence versus nodal or distant disease. Those with a vaginal or pelvic recurrence in a previously irradiated field are considered candidates for pelvic exenteration with curative intent [13, 21]. In the current study, however, we found that surgery is a viable strategy in carefully selected patients who do not meet criteria for total pelvic exenteration. Our data indicate that surgical resection of nodal or distant disease is worth considering if it is deemed safe and feasible.
In our series, CGR did not demonstrate a statistically significant survival benefit. Given that a majority achieved CGR (75.4%; n=46), this limited statistical analysis to a small comparator group with those who did not have a documented CGR (n=15). With respect to survival outcomes, the not-estimable upper limit of the CI for both the CGR and non-CGR cases suggests that, with further follow-up, the estimates could change as more events occur. Among the patients in our study who had residual disease at SCS, all residual disease was ≤ 3 cm. The lack of any residual disease > 3 cm might also explain why survival among patients who did not achieve CGR was not significantly different from those who did, and why our median OS of 33.1 months in non-CGR patients is greater than that reported by others (14 to 20 months) [14, 16, 22]. Previously published studies have indicated a survival advantage to CGR or optimal cytoreduction in recurrent EC [15, 16, 23]. In a meta-analysis, Barlin et al. demonstrated that for each 10% increase in patients achieving CGR at debulking for advanced or recurrent EC, there was a significant improvement in survival of 9.3 months [7]. They also reported that for each 10% increase in patients with optimal resection (≤ 2 cm) there was a 16-month improvement in survival that approached statistical significance (p=0.05). Awtrey et al. reported a median survival of 43 months among patients with ≤ 2 cm of residual disease after SCS for recurrent EC, which differed significantly from those with residual disease > 2 cm [15]. While optimal debulking appears to confer survival benefit, further research is warranted to determine a minimal resection level associated with improved survival.
This study is limited by its retrospective design, which introduces bias, most notably regarding patient selection. As the study was not powered, comparisons between groups were numerically limited. The variability in follow-up intervals and screening methods is another limitation. No stringent follow-up protocol was implemented across providers; thus, recurrences could have been missed, or there could have been bias in detecting recurrences because of variations in time intervals and screening methods. Some patients were lost to follow-up. Additionally, not all surgeons have the same technical skills, not all institutions have perioperative care protocols in place, and in some institutions access to other surgical specialists may be limited. These factors limit comparison across multiple institutions.
Lastly, our study ended prior to the recent approval of pembrolizumab for MSI-high tumor and combined pembrolizumab and levatinib for MS-stable or MMR-proficient endometrial tumors. The impact of immunotherapeutic strategies compared with surgery remains to be seen, as does the potential for synergistic treatment. One of the strengths of this study is that it reports on one of the largest cohorts of recurrent EC to date. In addition, by limiting the cases to only those patients who underwent prior primary surgical staging at our institution, multiple possible confounders were excluded.
The landscape of treatment options for recurrent EC is changing. Until there is sufficient data to compare these newer modalities, however, our findings suggest that surgery should be strongly considered whenever it is deemed safe and surgically feasible. The additional criteria defined in this study may help guide treatment recommendations.
Supplementary Material
Supplementary Figure 1: Consort diagram outlining patient selection criteria. The 19 patients lost to follow up that were excluded lacked any clinicopathologic information regarding the recurrence.
Supplementary Table 1: Univariate analysis of survival after recurrence based on clinicopathologic and surgical characteristics (N=376)
Supplementary Table 2: Multivariate survival analysis (N=376)
Highlights.
In recurrent endometrial cancer, secondary cytoreductive surgery (SCS) confers better survival than other treatments.
Age <70 at initial diagnosis, and endometrioid histology, were associated with improved survival in SCS.
In well-selected, medically eligible patients, surgical management should be considered whenever technically feasible.
Funding:
This study was funded in part by the NIH/NCI Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: None declared.
DISCLOSURES: AI reports the following, outside the submitted work: Mylan (consultant; personal fees). NRA reports the following, outside the submitted work: grant from Stryker/Novadaq (paid to institution); grant from Olympus (paid to institution); grant from GRAIL (paid to institution). Memorial Sloan Kettering Cancer Center (MSK) has financial interests relative to GRAIL. As a result of these interests, MSK could ultimately potentially benefit financially from the outcomes of this research. MML is a consultant for Intuitive Surgical, outside the submitted work. DSC reports the following, outside the submitted work: Bovie Medical Co. (Medical Advisory Board; stock options); Verthermia Inc. (now Apyx Medical Corp.) (Medical Advisory Board; stock options); Biom’Up (Medical Advisory Board Meeting 4/19/2019; personal fees); Intuitive Surgical, Inc. (recent stock owner; sold Dec. 2018); TransEnterix, Inc. (recent stock owner; sold Dec. 2018).
REFERENCES
- 1.Society, A.C. Cancer Facts & Figures 2020. 2020. [cited 2020 August, 14th]; Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf.
- 2.Duska L, Shahrokni A, and Powell M., Treatment of Older Women With Endometrial Cancer: Improving Outcomes With Personalized Care. American Society of Clinical Oncology Educational Book, 2016(36): p. 164–174. [DOI] [PubMed]
- 3.Calle EE, et al. , Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med, 2003. 348(17): p. 1625–38. [DOI] [PubMed] [Google Scholar]
- 4.Aalders JG, Abeler V, and Kolstad P., Recurrent adenocarcinoma of the endometrium: a clinical and histopathological study of 379 patients. Gynecol Oncol, 1984. 17(1): p. 85–103. [DOI] [PubMed] [Google Scholar]
- 5.Huijgens AN and Mertens HJ, Factors predicting recurrent endometrial cancer. Facts Views Vis Obgyn, 2013. 5(3): p. 179–86. [PMC free article] [PubMed] [Google Scholar]
- 6.Legge F, et al. , Clinical outcome of recurrent endometrial cancer: analysis of post-relapse survival by pattern of recurrence and secondary treatment. Int J Gynecol Cancer, 2020. 30(2): p. 193–200. [DOI] [PubMed] [Google Scholar]
- 7.Barlin JN, Puri I, and Bristow RE, Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol Oncol, 2010. 118(1): p. 14–8. [DOI] [PubMed] [Google Scholar]
- 8.Bendifallah S, et al. , Patterns of recurrence and outcomes in surgically treated women with endometrial cancer according to ESMO-ESGO-ESTRO Consensus Conference risk groups: Results from the FRANCOGYN study Group. Gynecol Oncol, 2017. 144(1): p. 107–112. [DOI] [PubMed] [Google Scholar]
- 9.Creutzberg CL, et al. , Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecol Oncol, 2003. 89(2): p. 201–9. [DOI] [PubMed] [Google Scholar]
- 10.Robbins JR, et al. , Is time to recurrence after hysterectomy predictive of survival in patients with early stage endometrial carcinoma? Gynecol Oncol, 2012. 127(1): p. 38–42. [DOI] [PubMed] [Google Scholar]
- 11.van Wijk FH, et al. , Management of recurrent endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer, 2009. 19(3): p. 314–20. [DOI] [PubMed] [Google Scholar]
- 12.Morris M, et al. , Treatment of recurrent adenocarcinoma of the endometrium with pelvic exenteration. Gynecol Oncol, 1996. 60(2): p. 288–91. [DOI] [PubMed] [Google Scholar]
- 13.Barakat RR, et al. , Pelvic exenteration for recurrent endometrial cancer. Gynecol Oncol, 1999. 75(1): p. 99–102. [DOI] [PubMed] [Google Scholar]
- 14.Turan T, et al. , Salvage Cytoreductive Surgery for Recurrent Endometrial Cancer. Int J Gynecol Cancer, 2015. 25(9): p. 1623–32. [DOI] [PubMed] [Google Scholar]
- 15.Awtrey CS, et al. , Surgical resection of recurrent endometrial carcinoma. Gynecol Oncol, 2006. 102(3): p. 480–8. [DOI] [PubMed] [Google Scholar]
- 16.Bristow RE, et al. , Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol Oncol, 2006. 103(1): p. 281–7. [DOI] [PubMed] [Google Scholar]
- 17.McAlarnen LA, et al. , Salvage treatment in recurrent endometrial cancer of the pelvis and peritoneal cavity. Gynecol Oncol Rep, 2019. 29: p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Network, N.C.C. Uterine Neoplasms. July 24, 2020; Version 2.2020:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 19.Salani R, et al. , An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol, 2017. 146(1): p. 3–10. [DOI] [PubMed] [Google Scholar]
- 20.Strong VE, et al. , Development and assessment of Memorial Sloan Kettering Cancer Center’s Surgical Secondary Events grading system. Ann Surg Oncol, 2015. 22(4): p. 1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford LS, et al. , Advances in the management of recurrent endometrial cancer. Am J Clin Oncol, 2015. 38(2): p. 206–12. [DOI] [PubMed] [Google Scholar]
- 22.Shikama A, et al. , Predictors of favorable survival after secondary cytoreductive surgery for recurrent endometrial cancer. Int J Clin Oncol, 2019. 24(10): p. 1256–1263. [DOI] [PubMed] [Google Scholar]
- 23.Papadia A, et al. , Surgical Treatment of Recurrent Endometrial Cancer: Time for a Paradigm Shift. Ann Surg Oncol, 2015. 22(13): p. 4204–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Consort diagram outlining patient selection criteria. The 19 patients lost to follow up that were excluded lacked any clinicopathologic information regarding the recurrence.
Supplementary Table 1: Univariate analysis of survival after recurrence based on clinicopathologic and surgical characteristics (N=376)
Supplementary Table 2: Multivariate survival analysis (N=376)


