Abstract
A 47-year-old man, positive for SARS-CoV-2, was diagnosed with acute coronary syndrome (ACS) complicated by myocarditis on a background of COVID-19 pneumonia. He was medically treated for ACS; however, 3 days into his admission, the patient developed neurological complications confirmed on MRI of the brain. MRI showed established infarcts involving a large part of the left temporal lobe and right occipital lobe, with minor foci of micro-haemorrhagic transformation in the left temporal lobe. A left ventricular mural thrombus was then confirmed on echocardiogram, and this was attributed as the cause of his neurological infarct. Further infarctions in the kidneys and spleen, and thrombi in the superior mesenteric and left femoral artery were also identified on imaging of the abdomen. The left ventricular mural thrombus was removed surgically via a midline sternotomy incision under general anaesthesia. Surgery was successful and the patient was discharged to a rehabilitation centre.
Keywords: COVID-19, cardiovascular medicine, neurology, cardiothoracic surgery
Background
Since the outbreak of the COVID-19 pandemic in December 2019, healthcare institutions worldwide have seen an overwhelming increase in hospitalisation of patients in view of the multiorgan involvement related to severe infection causing significant morbidity and mortality. The immune dysregulation characteristic of severe COVID-19 infection causes massive inflammation boosting cytokines, which increase the production of clotting factors leading to an increased risk of thromboembolic disease. The true prevalence of thrombosis associated with COVID-19 infection is unknown, as most literature to date does not include systematic investigation protocols; but one of the most consistent findings is that of a raised D-dimer level and the importance of thromboprophylactic agents in severely ill patients.1 We present a case of severe COVID-19 pneumonia complicated by multiorgan thrombosis including myocardial ischaemia, left ventricular thrombus, intracranial thrombosis and mesenteric involvement despite prophylactic anticoagulation to highlight the severe implications of this condition and to emphasise the high clinical suspicion of thromboembolic disease in such patients.
Case presentation
A 47-year-old man, positive for SARS-CoV-2, presented to the Accident and Emergency department 7 days after his positive swab test with sudden-onset central compressive chest pain radiating to his left shoulder. The pain was pleuritic in nature and it was associated with shortness of breath at rest. Over the past 7 days, the patient had lethargy, myalgias and a non-productive dry cough. The patient denied any other symptoms such as palpitations, loss of consciousness, diaphoresis, nausea or vomiting. He has a known case of dyslipidaemia and diabetes mellitus on oral hypoglycaemic treatment. His drug history included metformin 1 g three times per day, gliclazide 80 mg three times per day, vildagliptin 50 mg two times per day and fenofibrate 200 mg daily.
The patient was exposed to COVID-19 at work on a construction site. He was independent in his activities of daily living and lived with his partner. He was a non-smoker and had no family history of ischaemic heart disease or sudden cardiac death.
On initial examination, the patient’s observations showed a blood pressure of 120/89 mm Hg with a sinus tachycardia of 113 beats/min. He was afebrile, with oxygen saturation of 92% on room air, correcting at 96% on normal face mask at 5 L/min, and a respiratory rate of 16 breaths/min. On auscultation of his chest, there were sparse fine-end inspiratory crepitations at both lung bases, heart sounds were normal, abdomen was soft and non-tender, and his lower limbs had no pitting oedema or any signs of deep vein thrombosis.
Investigations
Initial blood investigations showed an elevated white cell count of 13.06×109/L (reference: 3.5–11×109/L) and neutrophil count of 10.31×109/L (reference: 2.5–7.5×109/L). Lymphocytes were slightly low at 1.22×109/L (reference: 1.6–3.5×109/L) and a normal eosinophil count. His haemoglobin was 149 g/L (reference: 115–165 g/L). The patient had a normal urea and electrolytes, and a slightly raised creatinine of 123 µmol/L (reference: 59–104 µmol/L). Procalcitonin was mildly raised at 0.192 ng/mL (reference: 0.02–0.046 ng/mL). Random blood glucose on admission was found to be high at 25 mmol/L (reference: 3.9–9 mmol/L) and C reactive protein (CRP) was raised at 115 mg/L (reference: 0–5 mg/L).
Admission chest X-ray showed diffuse patchy airspace shadowing throughout both lungs in keeping with COVID-19 pneumonia. ECG showed right heart strain with a new-onset right bundle branch block. D-dimer was grossly elevated at 5483 ng/mL (reference: 0–500 ng/mL). Initial troponins were also highly raised at 504 ng/L (reference: 3–14 ng/L), with repeat troponins uptrending to 1645 ng/L during the patient’s admission.
An urgent cardiology consultation was requested. CT pulmonary angiogram (CTPA) was suggested to exclude a pulmonary embolism and a bedside transthoracic echocardiogram (TTE) to be performed urgently thereafter. CTPA showed no evidence of pulmonary thromboembolic disease, however bilateral consolidations and widespread ground-glass changes in keeping with COVID-19 pneumonia were noted. There were no intrathoracic or axillary lymphadenopathy and the visualised upper abdominal organs were unremarkable. Bedside TTE revealed a globular dilated left ventricle with an ejection fraction of 35%, akinetic apical, anteroseptal and anterior walls, preserved contractility of inferolateral wall and no significant valvular abnormality. The right ventricle was borderline dilated with normal contractility. The inferior vena cava could not be visualised.
The initial impression and diagnosis was acute coronary syndrome (ACS) complicated by myocarditis on a background of COVID-19 pneumonia. The patient was started on remdesivir 200 mg stat dose, then 100 mg daily for a total of 5 days, dexamethasone 6 mg daily intravenously with omeprazole 20 mg daily. In view of his raised CRP and slightly elevated white cell count, co-amoxiclav 1.2 g intravenously three times per day was prescribed. Medical treatment for ACS was also initiated. Aspirin 300 mg and clopidogrel 600 mg stat were given, followed by aspirin and clopidogrel 75 mg daily, enoxaparin 1 mg/kg two times per day, enalapril 2.5 mg two times per day and atorvastatin 80 mg nocte. At the time of diagnosis, it was decided to treat the patient medically and not to proceed with a percutaneous coronary intervention in view of infection control limitations and no evidence of ST segment elevation myocardial infarction or new-onset left bundle branch block on ECG. The patient was admitted to the infectious diseases unit, where he remained haemodynamically stable during his admission and kept his oxygen saturation above 94% on room air. Oxygen was only needed on a PRN basis via normal face mask between 5 and 10 L/min.
Outcome and follow-up
Three days post-admission, the patient was noted to be drowsy and tachypnoeic with a respiratory rate of 22 breaths/min. On examination, the patient was afebrile, oxygen saturation was 97% on normal face mask 5 L /min, with a normal blood pressure and pulse. On examination, the patient appeared lethargic and slightly anxious, with a Glasgow Coma Score of 12; however, he was still able to transfer slowly from armchair to bed independently. It was noted that he was slurring his speech and had an episode of urinary incontinence. Pupils were equal and reactive, however neurological examination could not be performed adequately as the patient was not obeying and following all commands.
Arterial blood gases were taken on room air which showed type 1 respiratory failure with metabolic compensation—pH of 7.48 (reference: 7.35–7.45), partial pressure of oxygen of 67.6 mm Hg (reference: 80–100 mm Hg), partial pressure of carbon dioxide of 25.8 mm Hg (reference: 35–45 mm Hg), SO2 of 95.2% and a HCO3- of 19.7 (reference: 22–28). Repeat blood tests were taken which revealed a downtrending CRP of 75, downtrending troponins and normal white cell count. A provisional diagnosis for these neurological features included encephalopathy secondary to hypoxia or a cerebrovascular event.
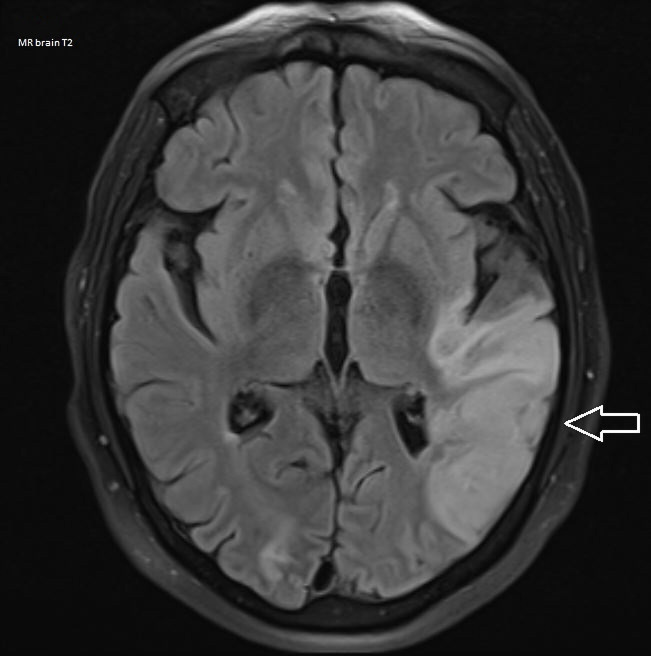
An urgent CT of the brain was carried out revealing a small area of low density in the right occipital lobe with no mass effect most likely secondary to an ischaemic infarct. These results were discussed with the neurologist on call and an urgent MRI of the brain and repeat TTE were advised. The MRI of the brain (figure 1) showed established infarcts involving a large part of the left temporal lobe and right occipital lobe. Minor foci of micro-haemorrhagic transformation were noted in the left temporal lobe. Slight sulcal oedema was seen within the affected areas with corresponding high signal on T2. In light of the neurological findings and the previously documented akinesia, a repeat TTE was indicated. Repeat TTE showed apical akinesia in keeping with a myocardial infarct, and a large apical mural thrombus. There was no pericardial effusion and no valvular abnormalities.
Figure 1.
T2 MRI of the brain: arrow showing established infarct involving a large part of the left temporal lobe.
A decision was taken to switch enoxaparin to unfractionated heparin (UFH) intravenous infusion 6000 units/6 hourly because of the risk of bleeding, in view of the micro-haemorrhagic infarcts noted on imaging, keeping the activated partial thromboplastin time (APTT) ratio between 2 and 3. Enalapril was increased to 5 mg two times per day, and carvedilol 3.125 mg two times per day was also added as heart failure treatment. Antiplatelets were stopped considering that the patient was on UFH intravenous pump. The patient improved from a neurological point of view by the following day. He became more coherent and able to follow commands. A thrombophilia screen, erythrocyte sedimentation rate (ESR) and auto-antibody screen were taken in view of these aforementioned findings. Total extractable nuclear antigen, anti-nuclear antibody, anti-double stranded DNA, complements and immunoglobulins were all normal. ESR was raised at 78 mm (reference: 8–12 mm 1st hour). Thrombophilia screen was negative for any mutations.
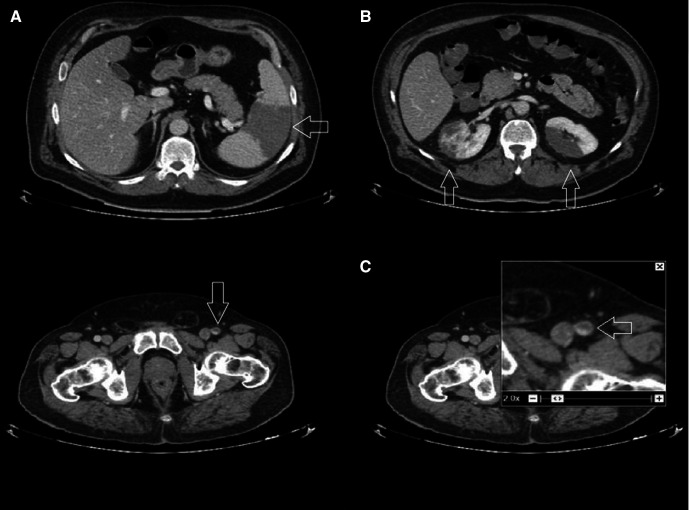
Two days after the initiation of UFH, warfarin was started at a loading dose of 5 mg on days 1 and 2—as per local hospital guideline. Subsequently, the patient developed acute abdominal pain. He was passing flatus and his abdomen was soft with no rigidity or guarding; however, he had generalised tenderness on palpation. A CT of the abdomen and pelvis was performed to exclude ischaemia of the bowel. CT showed infarctions in the spleen (figure 2A) and kidneys (figure 2B), with the renal lesions showing a preserved capsule. Thrombi were also seen in the superior mesenteric artery and left femoral artery (figure 2C). There were no obvious bowel ischaemic changes. After discussion with haematology and taking into consideration all the findings, the UFH infusion was switched to enoxaparin 1 mg/kg two times per day, with the aim to stop it and continue on warfarin once the international normalised ratio is more than 1.8.
Figure 2.
(A–C) CT of the abdomen and pelvis: arrows showing infarctions in the spleen (A), kidneys (B) and thrombus in the left femoral artery (C).
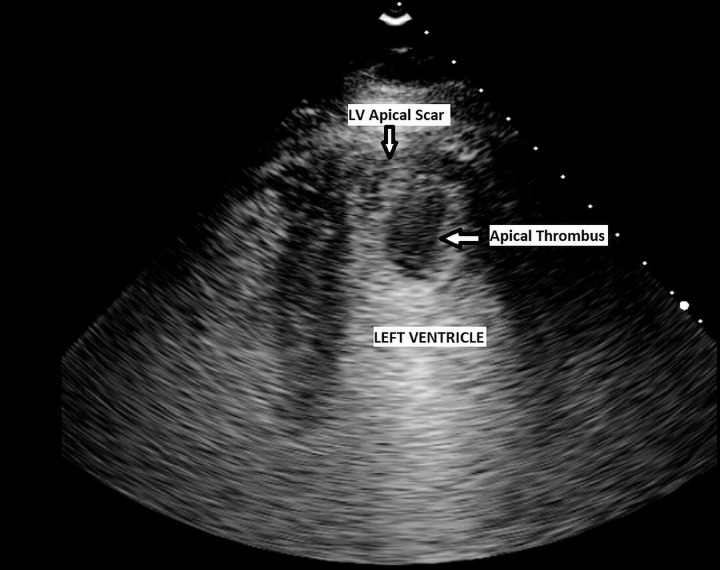
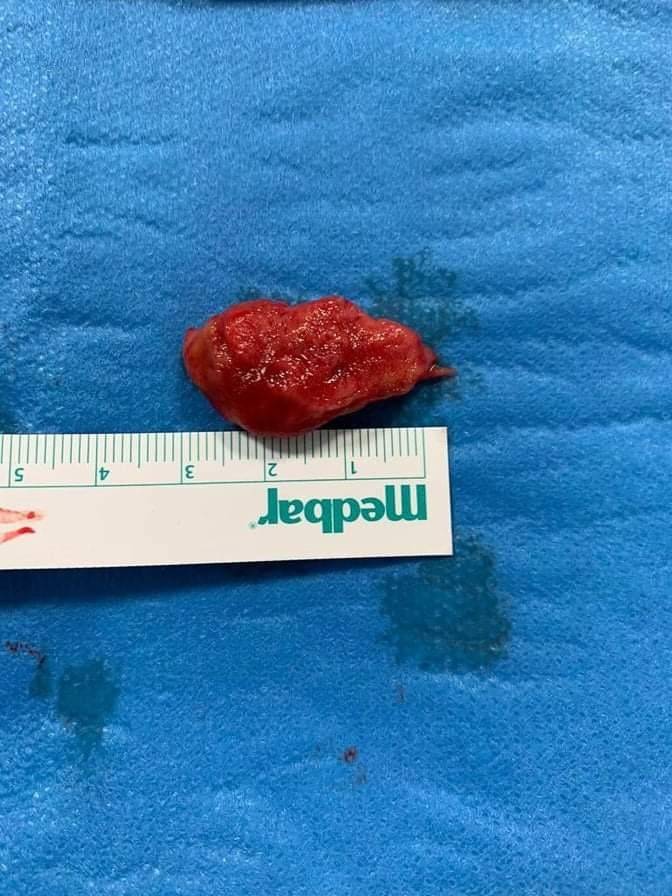
The patient tested negative on 2 separate consecutive days for SARS-CoV-2, 14 days from his initial positive swab. A contrast echocardiogram was performed which showed a large pedunculated left ventricular thrombus (figure 3). The case was discussed at a multidisciplinary team meeting which involved the cardiologists, cardiothoracic surgeons, neurologists and infectious diseases team, and it was decided to proceed with surgical removal of left ventricular thrombus via a midline sternotomy incision. Surgery was carried out successfully after reversing the warfarin and the thrombus was removed as a whole under direct vision (figure 4), leaving a drain in situ. Postoperatively, the patient was admitted to a cardiac intensive care unit (ICU). He was extubated 1 day postoperatively, kept on dopamine and furosemide infusion, and on UFH intravenous pump. He had no postoperative complications and was transferred to a rehabilitation centre 2 weeks post-surgery for further intense physiotherapy and occupational therapy.
Figure 3.
Contrast-enhanced apical four-chamber view of the left ventricle demonstrating a pedunculated apical thrombus.
Figure 4.
Resected left ventricular thrombus following surgical thrombectomy.
Discussion
SARS-CoV-2 infection is caused by an enveloped RNA beta-coronavirus. There are seven species of these beta-coronaviruses which are known to cause infections in humans, with four causing mild influenza-like symptoms and the remaining three resulting in potentially fatal illnesses: severe acute respiratory syndrome, Middle East respiratory syndrome and SARS-CoV-2. COVID-19 infection and pneumonitis have brought increasing awareness and attention by physicians to thromboembolic disease and associated complications. Useful biomarkers such as D-dimer levels are being taken on a routine basis, both to assess for thromboembolic events and also as a predictor of adverse outcomes. This pathophysiological process is not entirely understood, and most likely differs from the standard mechanisms of thrombosis in critically ill patients.1
Virchow’s triad is a well-known theory stating that an alteration in blood flow, vascular endothelial injury and a hypercoagulable state (inherited or acquired) result collectively in a higher risk of venous thromboembolism (VTE).2 3 In COVID-19 infection, a pro-inflammatory surge, also known as ‘cytokine storm’, is thought to be central to the pathogenesis of acute respiratory distress syndrome (ARDS) with hypercoagulability being an important hallmark of this inflammatory process. This cytokine storm involves activation of T cells, neutrophils, monocytes, macrophages and platelets, with resultant cytokine release including interleukin (IL) 1, IL-6, IL-10, tumour necrosis factor-alpha, monocyte-derived tissue factor and plasminogen activator inhibitor-1 expression, culminating in the development of microvascular and macrovascular thrombi. These pro-inflammatory cytokines are critically involved in abnormal clot formation and platelet hyperactivation, and also play an important role in the downregulation of important physiological anticoagulant pathways.4–6 Virchow’s triad applies greatly to our case as the patient was in a hypercoagulable state caused by the COVID-19 infection, vascular stasis caused by cardiac akinesia and endothelial injury caused by the widespread inflammation from the cytokine release. All these factors contributed significantly to extensive thromboembolic disease.
Although the respiratory tract is the primary target of SARS-CoV-2, the cardiovascular system also becomes involved in several different ways as represented in our report here. Direct myocardial injury occurs secondary to the SARS-CoV-2 entering the cells by binding to ACE-2 receptors, a membrane-bound aminopeptidase, which is highly expressed in the cardiorespiratory system. This binding leads to acute myocardial and/or lung injury leading to increased cardiometabolic demand. Moreover, the hypoxia caused by ARDS leads to impaired myocardial oxygen demand–supply relationship and leads to acute myocardial injury.7 8 In addition, it is reasonable to consider viral inclusion or associated myocardial inflammation as the pathogenesis of myocarditis. So far, there are limited reports showing pathological evidence that COVID-19 directly invades the heart. Sala et al showed viral particles with the morphology and size of coronaviruses in interstitial macrophages, however, there was no SARS-CoV-2 genomic material in the myocardium.9 10
Myocardial injury is a predictor of more severe illness and mortality in patients with COVID-19. Significant serum troponin elevation has been observed among several cases of severe infection and may be a reflection of supply–demand mismatch rather than myocardial injury.9 Our patient presented with ECG changes and grossly elevated troponin levels. CTPA was performed to exclude pulmonary embolism in view of the elevated D-dimer level. TTE confirmed akinesia, a complication of an ACS and also a complication of COVID-19 infection. Hence, our patient was at a higher risk of developing a left ventricular mural thrombosis, which ultimately resulted in widespread embolic events with significant sequelae.
TTE is an important first-line non-invasive test in the workup of such patients. In typical circumstances before this global pandemic, cardiac MRI would be a vital and integral test to confirm the diagnosis of myocarditis.9 However, in view of infection control practices surrounding this pandemic, physicians have to rely on a combination of clinical findings, biomarkers and bedside imaging to confirm diagnosis, such as in our case, with more elaborate investigations including interventional ones being postponed until the patient tests negative.
Although the incidence of VTE and arterial thromboembolism (ATE) seems to be higher in patients with COVID-19, further studies are surely needed as the actual prevalence is largely unknown. The International Society on Thrombosis and Hemostasis (ISTH) recommends measuring D-dimer, prothrombin time (PT), APTT and platelet counts in all hospitalised patients with COVID-19. Rapid increase in oxygen requirements might be a useful indicator of a VTE event, rather than relying solely on blood investigations. Given the logistical issues that hospitals and wards have encountered during this pandemic, it is likely that there is a higher threshold to perform diagnostic imaging in these patients. Appropriate VTE prophylaxis is an important aspect in the management of these patients.11
The Swiss Society of Hematology proposes the following guidelines which are based on the available literature and published recommendations from the ISTH, American Society of Hematology and from the Society for Thrombosis and Haemostasis Research. They recommend that all in-hospital patients with COVID-19 should receive pharmacological thromboprophylaxis according to a risk stratification score. They suggest regular monitoring of PT, D-dimer, fibrinogen, platelet count, lactate dehydrogenase, renal and liver function. Consideration to increase prophylactic anticoagulation to an intermediate or therapeutic dose needs to be given in critically ill patients admitted to a high-dependency unit or ICU who exhibit imminent respiratory failure or change in biochemistry and blood investigations. These guidelines also state that there are no data on the use of direct oral anticoagulants as thromboprophylaxis or as treatment of VTE (because of possible interactions with current proposed COVID-19 treatments and therapeutics).12
A meta-analysis examining the findings from 102 studies (64 503 patients) concluded that patients admitted to the ICU for severe COVID-19 had a high risk of VTE. This meta-analysis showed the frequency of COVID-19-related VTE was 14.7%. The overall prevalence rates of pulmonary embolism and leg deep vein thrombosis were 7.8% and 11.2%, respectively. The VTE prevalence was significantly higher in ICU. The frequency rates of overall ATE such as ACS, cerebrovascular accidents and other ATE were 3.9%, 1.6% and 0.9%, respectively.13
One can also find multiple case reports of VTEs and ATEs related to COVID-19 infection. Kumar et al14 report the first case in literature of a 71-year-old man with severe COVID-19 infection, diagnosed with pulmonary thromboembolism while having an ongoing gastrointestinal bleed. He was not on any prophylactic anticoagulation. Another case reports the admission of a 70-year-old woman with acute bilateral pulmonary emboli, thrombi within the aorta and complete occlusion of the right common iliac artery. This required emergency surgical thrombectomy followed by anticoagulation with low-molecular-weight heparin.15 This case shows the risk of widespread thromboembolic disease similar to our patient. Similarly, Hussain et al16 presented a case of a severe SARS-CoV-2 infection requiring around 25 days of ventilatory support who had presented with significantly elevated D‐dimer levels. He was maintained on VTE prophylaxis since initial presentation and was switched to empirical therapeutic anticoagulation on day 14 of admission on the development of worsening respiratory decline and new unstable arrhythmias. The patient remained on enoxaparin 90 mg two times per day for the majority of his ICU stay. CTPA performed after starting therapeutic anticoagulation still showed bilateral pulmonary emboli likely contributing to his prolonged ICU stay. This case questions the benefit of VTE prophylactic dosing in severe SARS-CoV-2 infection where therapeutic anticoagulation may also be unable to prevent thrombosis.16
In conclusion and with respect to our case presented here, the interplay of the cytokine storm induced by COVID-19 infection, leading to a thrombophilic state coupled with the myocardial injury secondary to the mechanisms mentioned above, could explain the massive left ventricular thrombus with subsequent widespread embolisation in an otherwise previously well patient. An earlier angiography might have prevented the formation of a left ventricular thrombus and the above-mentioned complications; however, in view of his COVID-19-positive state and infection control limitations, this was not a viable option at that particular time.
Learning points.
SARS-CoV-2 infection is a prothrombotic state secondary to systemic inflammation and cytokine storm.
Neurological symptoms and features in patients with SARS-CoV-2 require high clinical index of suspicion for thromboembolic disease.
Myocardial injury and myocarditis are not rare complications of COVID-19 infection.
Recurrent thromboembolic events should necessitate an echocardiography to exclude ventricular thrombi.
Limitations in the availability of procedures and investigations because of strict infection control measures during this pandemic might limit the care of secondary conditions which would otherwise be managed differently.
Footnotes
Contributors: ECB—acquisition of data, planning, manuscript editing and discussion. RC—imaging preparation, planning, manuscript editing and discussion. TX—manuscript editing and discussion. JB—imaging preparation and manuscript revision. CF—manuscript revision and guarantor of case integrity. CMA—manuscript revision and guarantor of case integrity.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Next of kin consent obtained.
References
- 1.Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care 2020;24:360. 10.1186/s13054-020-03077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teuwen L-A, Geldhof V, Pasut A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020;20:389–91. 10.1038/s41577-020-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–44. 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res 2020;69:1181–9. 10.1007/s00011-020-01401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miesbach W, Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost 2020;26:107602962093814. 10.1177/1076029620938149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colling ME, Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med 2020;25:471–8. 10.1177/1358863X20932640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID-19. J Thromb Haemost 2020;18:2103–9. 10.1111/jth.14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020;14:247–50. 10.1016/j.dsx.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and myocarditis: what do we know so far? CJC Open 2020;2:278–85. 10.1016/j.cjco.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861–2. 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aryal MR, Gosain R, Donato A, et al. Venous thromboembolism in COVID-19: towards an ideal approach to thromboprophylaxis, screening, and treatment. Curr Cardiol Rep 2020;22:52. 10.1007/s11886-020-01327-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casini A, Alberio L, Angelillo-Scherrer A, et al. Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 - a Swiss consensus statement by the Working Party Hemostasis.. Swiss Med Wkly 2020;150:w20247. 10.4414/smw.2020.20247 [DOI] [PubMed] [Google Scholar]
- 13.Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax 2021;2:thoraxjnl-2020-215383. 10.1136/thoraxjnl-2020-215383 [DOI] [PubMed] [Google Scholar]
- 14.Kumar MA, Krishnaswamy M, Arul JN. Post COVID-19 sequelae: venous thromboembolism complicated by lower Gi bleed. BMJ Case Rep 2021;14. 10.1136/bcr-2020-241059. [Epub ahead of print: 27 Jan 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Castillo-García S, Minguito-Carazo C, Echarte JC, et al. A case report of arterial and venous thromboembolism in a patient with severe COVID-19 pneumonia. Eur Heart J Case Rep 2020;4:1–6. 10.1093/ehjcr/ytaa350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain H, Sehring M, Aulakh BS. COVID-19-Associated coagulopathy: a case report of thrombosis despite therapeutic anticoagulation. Case Rep Crit Care 2020;2020:1–4. 10.1155/2020/8876932 [DOI] [PMC free article] [PubMed] [Google Scholar]