Abstract
A 66-year-old Asian woman presented with severe kidney injury, microscopic haematuria and subnephrotic range proteinuria with elevated serum anti-glomerular basement membrane (anti-GBM) titre. She had a history of renal cell carcinoma. Renal biopsy revealed dual pathology with immunofluorescence showing 3+ linear glomerular IgG staining and 3+ IgA mesangial staining. Cellular crescents were present on light microscopy and electron microscopy revealed increased mesangial matrix. She was treated with plasma exchange and immunosuppression and remained in stage 4 chronic kidney disease. This case describes the coexistence of anti-GBM disease and IgA nephropathy, a phenomenon not well described in the literature. The report also explores the association of malignancy and glomerulonephritis as well as the role of genetics and the utility of human leukocyte antigen (HLA) typing in risk stratification.
Keywords: vasculitis, acute renal failure, proteinurea, genetics, pathology
Background
Concurrent presentation of two renal pathology is not an uncommon finding in kidney biopsy. Anti-glomerular basement membrane (anti-GBM) disease with antineutrophil cytoplasmic antibodies (ANCA) vasculitis and membranous nephropathy have been frequently described; however, only six cases on concurrent anti-GBM disease and IgA nephropathy have been reported. The expected clinical course, ideal treatment regimen and prognosis of these patients remain to be elucidated. Although distant, the history of renal cell carcinoma (RCC) in this patient highlights the association of malignancy and glomerulonephritis.
Case presentation
A 66-year-old retired nurse of Asian origin presented with asymptomatic severe acute renal injury. She presented with painless macroscopic haematuria with frothy urine and 4 kg of weight gain in 3 months. Serum creatinine at presentation was 320 µmol/L (estimated glomerular filtration rate (eGFR) of 11 mL/min/1.73 m2) with a baseline creatinine of 115 µmol/L 8 months ago. She had a left partial nephrectomy with complete loss of the left kidney due to vascular complications 8 years ago for chromophobe RCC, in complete remission since then. Her other medical issues include well-controlled bronchial asthma and impaired glucose tolerance. She presented clinically well and normotensive with bilateral pitting oedema. Her cardiac and respiratory examinations were unremarkable.
Investigations
A blood test showed urea of 12 mmol/L, creatinine of 320 µmol/L, potassium of 4.7 mmol/L, bicarbonate of 18 mmol/L and serum albumin of 31 g/L. Her Hba1C was 5.5%. She had normocytic, normochromic anaemia with a haemoglobin of 100 g/L with normal white cell and platelet counts. She had an elevated C reactive protein in the absence of infection. She had normal C3 of 1.32 g/L (0.83–1.93 g/L) and elevated C4 of 0.63 g/L (0.15–0.57 g/L). She was hepatitis B core antibody positive but hepatitis B surface antigen negative. The anti-GBM level was markedly elevated at 79 units/mL. A midstream urinalysis showed 3+ blood with no dysmorphic red cells or casts. A 24-h urine protein was 770 mg.
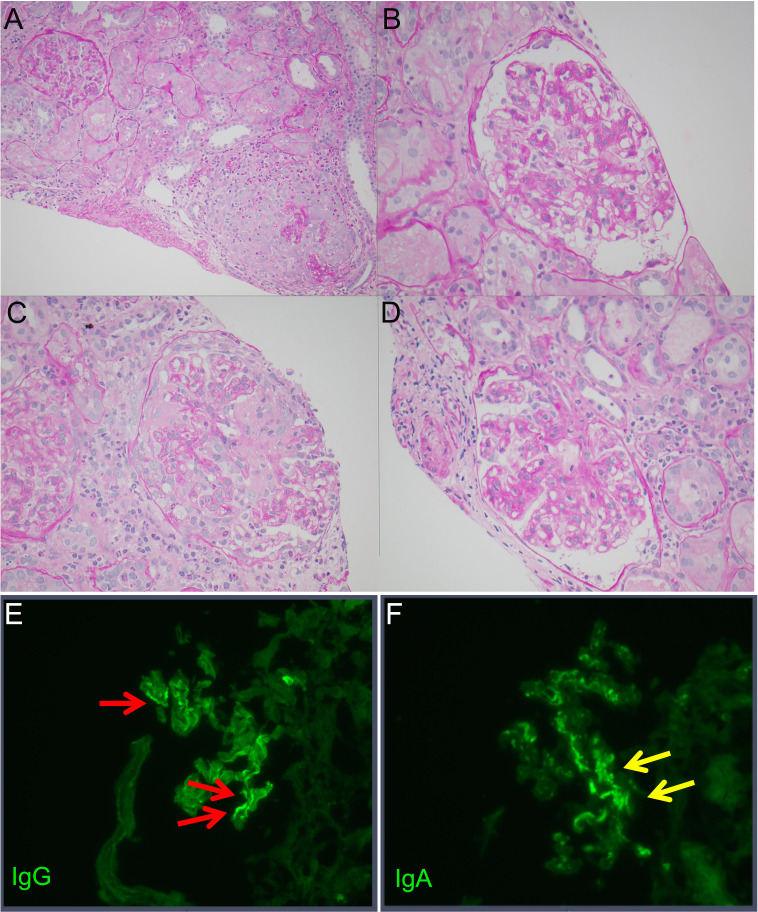
A chest CT revealed no evidence of pulmonary haemorrhage. A kidney biopsy showed multiple cellular crescents with fibrinoid necrosis involving 15% of glomeruli and mesangioproliferative hypercellularity and increased mesangial matrix (figure 1). Up to 40% of glomeruli are extensively sclerosed with associated fibrous crescents. There is moderate chronic renal damage with moderate tubulointerstitial fibrosis and diffuse interstitial inflammation. Immunofluorescence showed 3+ linear glomerular capillary IgG staining consistent with anti-GBM glomerulonephritis (figure 1E). In addition, immunofluorescence also showed 2–3+ granular IgA mesangial staining (figure 1F). In the context of dual pathology, the Oxford MEST-C (Mesangial hypercellularity, endocapillary hypercellularity, segmental sclerosis, tubular atrophy and interstitial fibrosis, crescents) score for IgA nephropathy is not applicable. The electron microscopy showed mild increase in mesangial matrix without immune deposits. Her human leukocyte antigen (HLA) typing was DRB1*07:01 and DRB1*10:01.
Figure 1.
Periodic acid-Schiff (PAS)-stained images of the kidney. (A) ×20 magnification of one glomerulus with fibrinoid necrosis, crescent and rupture of Bowman’s capsule and adjacent glomerulus with mildly hypercellular mesangium. (B) ×40 magnification of glomerulus with mild mesangial hypercellularity and mildly increased mesangial matrix. (C) ×40 magnification of glomerulus with fibrinoid necrosis. (D) ×40 magnification of glomerulus with mild mesangial hypercellularity and mildly increased mesangial matrix. Immunofluorescence shows (E) linear deposition of IgG along the glomerular capillaries typically observed in anti-glomerular basement membrane disease and (F) granular mesangial IgA deposits that are typical of IgA nephropathy.
Treatment
She was commenced on plasma exchange, three pulses of 1 g intravenous methylprednisolone and oral cyclophosphamide. This is followed by high dose (1 mg/kg/day) oral prednisolone in addition to opportunistic infection prophylaxis with bactrim and valganciclovir.
Outcome and follow-up
The anti-GBM level fell to 3.2 units/mL following six alternate days of plasma exchange. Her serum creatinine improved and plateaued at 180 µmol/L (eGFR 28 mL/min) 12 months after therapy. She remains well with stage 4 chronic kidney disease without evidence of disease recurrence.
Discussion
We report a case of concurrent anti-GBM disease and IgA nephropathy with a history of chromophobe renal cell carcinoma, who has responded well with standard therapy. Although it remains challenging to determine which renal pathology dominates her disease process, our report suggests several atypical presentations.
Anti-GBM disease is a rare aggressive form of glomerulonephritis characterised by autoantibodies against the non-collagenous domain of the alpha-3 subunit of type IV collagen. The restricted expression of this antigen in the glomerular and alveolar capillary basement membrane accounts for the predominantly renal and pulmonary manifestations.1 Anti-GBM disease has an estimated incidence of 0.6–1 per million population per year with a lower incidence in the Asian population compared with the Caucasian population.1 2 It is an autoimmune disease that occurs in genetically susceptible individuals exposed to certain environmental triggers. These triggers include pollutants such as hydrocarbon and cigarette smoke and viral infections accounting for the geographical and seasonal clustering of the disease.1 Patients typically present with rapidly progressive renal failure, microscopic haematuria and subnephrotic range proteinuria.3 4 The diagnosis requires immunofluorescence confirmation of strong linear IgG deposition along the glomerular basement membrane with less specific light microscopy findings of diffuse necrosis and cellular crescents.1 IgA and IgM staining are typically absent.1
In IgA nephropathy, there is aberrant galactosylation at the hinge region of IgA1 antibodies. Quite similar to anti-GBM disease, a multi-hit hypothesis is thought to trigger the formation of anti-Gal-antibody, anti-Gal-immunocomplex and other immune complex deposition in the glomeruli resulting in a chronic inflammatory response. The pathophysiology of concurrent anti-GBM disease with IgA nephropathy remains unclear but various theories have been postulated.5 Cytokines including interleukin-1, interleukin-6 and tumour necrosis factor as well as free oxygen radicals released by infiltrating inflammatory cells in IgA nephropathy are believed to be responsible for conformational changes in the glomerular basement membrane leading to exposure of the otherwise hidden glomerular basement membrane antigens.6 This triggers an autoimmune reaction to the modified antigens, resulting in the formation of anti-GBM antibodies.5 The other possible explanation is that deposition of aberrant galactose-deficient IgA1 antibodies resulting in an induction of novel glomerular basement membrane antigen formation triggering an autoimmune reaction.6 Seasonal viral infection and recurrent intestinal mucosal stimulation postulated as part of the pathophysiology of anti-GBM disease and IgA nephropathy, respectively, may be the common link in concurrent anti-GBM disease and IgA nephropathy.1 7 The fourth explanation is merely the coincidental occurrence of anti-GBM disease in one with IgA nephropathy, the most common glomerulonephritis in young adults worldwide.7
The clinical course, ideal treatment regimen and prognosis of patients with concurrent disease remains uncertain largely due to low incidence and partly due to under reporting. The treatment regimen of these two forms of glomerulonephritis is vastly different. Plasma exchange and high-dose cytotoxic agents to remove circulating antibody and suppress antibody production remains the therapeutic strategy for anti-GBM disease.1 Given the low risk of relapse of anti-GBM disease, cytotoxic therapy is often limited to a short duration of several months. On the other hand, IgA nephropathy has a broad spectrum of clinical manifestation varying from indolent course of microscopic haematuria to rapidly progressing crescentic glomerulonephritis. Immunosuppression in IgA nephropathy remains controversial limited to high-risk individuals.8 9 As in the case of coexistent anti-GBM disease and ANCA vasculitis, there may be a role for a more intense or prolonged immunosuppression in cases of concurrent anti-GBM disease and IgA nephropathy.1 The small series of concurrent anti-GBM disease and IgA nephropathy cases report variable responses towards standard immunosuppression with the majority reporting suboptimal response to immunosuppressant therapy (table 1). The small retrospective case series by Cui et al6 concluded that patients with concurrent anti-GBM disease and immune complex glomerulonephritis carries a worse prognosis and invariably requires plasma exchange and early intensive immunosuppression. This may be due to the aggressive clinical course with multi-organ involvement and late presentations of anti-GBM disease.
Table 1.
Case series of concurrent anti-GBM disease and IgA nephropathy
| Article | Histology crescents (%) | Anti-GBM level | Treatment | Renal outcome | Pulmonary involvement (Y/N) | Survival outcome (Y/N) |
| Wang et al5 | 14 glomeruli with cellular crescents, moderate interstitial inflammation and fibrosis | 93.5 units/mL | 3 pulses IV methyl prednisolone (0.5 g) followed by oral cyclophosphamide (1 mg/kg/day) and oral prednisone (1 mg/kg/day). Ten sessions of plasma exchange | Creatinine 375 µmol/L (at 30 days) | N | Y |
| Ge et al18 | 58% glomeruli with crescents, 6% globally sclerosed glomeruli. | N/A | 3 pulses IV methyl prednisolone (0.5 g), followed by oral prednisone (1 mg/kg/day) and IV cyclophosphamide (10 mg/kg) every 2 weeks. Six sessions of plasma exchange | ESRF (on dialysis) | N | Y |
| Annamalai et al19 | 70% glomeruli with fibrous and fibro-cellular crescents, 30% globally sclerosed glomeruli | 96 units/ mL | 3 pulses IV methyl prednisolone (0.5 g), followed by oral prednisone (0.5 mg/kg/day) | Non-dialysis-dependent CKD | N | Y |
| Cui et al6 | Cellular crescents in 100% glomeruli | N/A | Not documented | ESRF (on dialysis) | N | Y |
| Gao et al20 abstract) |
N/A | |||||
| Trpkov et al21 abstract) |
N/A | |||||
Anti-GBM, anti-glomerular basement membrane; CKD, chronic kidney disease; ESRF, end stage renal failure; N/A, not available; Y/N, yes/no.
Interestingly, none of the reported cases (including the current case) with concurrent anti-GBM disease and IgA nephropathy had pulmonary involvement. Up to 30%–60% of patients with anti-GBM disease have coexisting pulmonary involvement, and very rarely do patients present with pulmonary haemorrhage without renal involvement.1 Pulmonary haemorrhage is a severe complication of anti-GBM disease but is not a predictor of renal or overall survival prognosis.10
Genetic variability has been linked to the predisposition and clinical course of anti-GBM and IgA nephropathy. Anti-GBM disease is strongly associated with HLA-DR2 specificity.11 Individuals with HLA-DRB1*15 and HLA-DRB1*04 carry a worse prognosis in anti-GBM disease, whereas HLA-DRB1*07 confers a clinical protective course, while HLA-DRB1*15 is more commonly linked with anti-GBM disease in Asian ethnicity.1 12 However, HLA typing alone does not account for the entire pathogenesis and clinical manifestation of both anti-GBM disease and IgA nephropathy. Our patient who had the protective HLA-DRB1*07 typing developed anti-GBM disease. Although these alleles lack specificity as they are commonly associated with other autoimmune conditions, HLA-DRB1*07 may have conferred a less aggressive disease course in our patient.1 In IgA nephropathy, genome-wide association study (GWAS) have identified single nucleotide polymorphism that confer independent risk of developing IgA nephropathy found in the location of the HLA-DRB1, DQA1 and DQB1 genes.13 This may have contributed to our patient developing IgA nephropathy whose HLA typing was HLA-DRB1*07 and DRB1*10:01. Another GWAS study involving Europeans and Han Chinese found five susceptible loci; three on chromosome 6p21 and one on chromosome 1q32 and 22q12. The frequency of these risk alleles parallels the ethnic variations seen in IgA nephropathy where rates are highest in Asians followed by Europeans and lowest in Africans.7
Malignancy-associated glomerulonephritis is well described in some types of glomerulonephritis, in particular membranous nephropathy. In many cases, glomerulonephritis may precede the diagnosis of malignancy by many years and in membranous nephropathy, 10% of patients have an underlying malignancy.14 This relationship is less established in anti-GBM disease and IgA nephropathy.15 Mimura et al16 reported three cases of concurrent RCC and IgA nephropathy in patients more than 50 years old and reports an association of increased tumour size following steroid therapy for IgA nephropathy. Two of the three cases had significant reduction in proteinuria and haematuria post tumour resection. In another study where nephrectomised kidneys for RCC were examined histologically, 16 out of 60 patients had histological confirmation of immune-complex nephropathy, of which 11 had IgA nephropathy. Of the 16 patients, 11 had complete resolution of haematuria and proteinuria 3 months post nephrectomy,17 suggesting a possible paraneoplastic phenomenon. None had recurrence of RCC or other malignancies.17 In our case, chromophobe RCC preceded the diagnosis of anti-GBM and IgA nephropathy by 8 years making the association weak. No baseline urine analysis was available to confirm the presence of microscopic haematuria or proteinuria prior to or at the time of the RCC diagnosis. However, the association of glomerulonephritis and malignancy should be considered especially in the older patient and prior to immunosuppression commencement.
In conclusion, the coexistence of anti-GBM disease and IgA nephropathy is rare and not well described in literature, with few case reports describing poor renal prognosis despite aggressive therapy. Our report highlights a less aggressive concurrent disease course that may be due to favourable histology (15% glomeruli with crescents), timely intervention and favourable genetic factor (HLA-DRB1*07). Twelve months after initial presentation, the patient is doing well with a serum creatinine of 180 mmol/L and an eGFR of 28 mL/min. The association of glomerulonephritis with malignancy in the older patient should be excluded especially if immunosuppression is to be considered. Although the RCC preceded the onset of concurrent glomerulonephritis, our patient is currently under regular surveillance for contralateral RCC or other occult malignancy. Our case further highlights the possible utility of HLA typing for risk stratification and to guide therapy although more is to be discovered to better elucidate its association with these two forms of glomerulonephritis.
Learning points.
The occurrence of concurrent anti-glomerular basement membrane disease and IgA nephropathy is rare. Of the few cases reported, most described poor renal outcome despite aggressive interventions.
A more favourable outcome may be seen in patients with favourable histology and genetic profile who receive early aggressive interventions.
The exclusion of malignancy should be considered in elderly patients with glomerulonephritis.
Genetics including HLA typing may play a role in risk stratification to guide treatment decision however more research is required.
Footnotes
Contributors: CK conceived the idea of the case report, collected patient’s clinical information, analysed and interpreted investigation results, performed literature review, drafted and edited the case report and discussion, approved final version of the report, agreed to be accountable for all aspects of the work. MGW collected patient’s clinical information, analysed and interpreted investigation results, edited and critically revised case report and discussion and approved final version of the report, agreed to be accountable for all aspects of the work. JR provided the light microscopy and immunofluorescence renal biopsy images, provided the legend for the histopathology figure, edited and critically revised case report and discussion, approved final version of the report, agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 2017;12:1162–72. 10.2215/CJN.01380217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li FK, Tse KC, Lam MF, et al. Incidence and outcome of antiglomerular basement membrane disease in Chinese. Nephrology 2004;9:100–4. 10.1111/j.1440-1797.2003.00234.x [DOI] [PubMed] [Google Scholar]
- 3.Nasr SH, Collins AB, Alexander MP, et al. The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int 2016;89:897–908. 10.1016/j.kint.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Troxell ML, Houghton DC. Atypical anti-glomerular basement membrane disease. Clin Kidney J 2016;9:211–21. 10.1093/ckj/sfv140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang A, Wang Y, Wang G, et al. Mesangial IgA deposits indicate pathogenesis of anti-glomerular basement membrane disease. Mol Med Rep 2012;5:1212–4. 10.3892/mmr.2012.809 [DOI] [PubMed] [Google Scholar]
- 6.Cui Z, Zhao M-H, Wang S-X, et al. Concurrent antiglomerular basement membrane disease and immune complex glomerulonephritis. Ren Fail 2006;28:7–14. 10.1080/08860220500461195 [DOI] [PubMed] [Google Scholar]
- 7.Wyatt RJ, Julian BA. Iga nephropathy. N Engl J Med 2013;368:2402–14. 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 8.Lv J, Zhang H, Wong MG, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the testing randomized clinical trial. JAMA 2017;318:432–42. 10.1001/jama.2017.9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauen T, Eitner F, Fitzner C, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015;373:2225–36. 10.1056/NEJMoa1415463 [DOI] [PubMed] [Google Scholar]
- 10.Alchi B, Griffiths M, Sivalingam M, et al. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant 2015;30:814–21. 10.1093/ndt/gfu399 [DOI] [PubMed] [Google Scholar]
- 11.Fisher M, Pusey CD, Vaughan RW, et al. Susceptibility to anti-glomerular basement membrane disease is strongly associated with HLA-DRB1 genes. Kidney Int 1997;51:222–9. 10.1038/ki.1997.27 [DOI] [PubMed] [Google Scholar]
- 12.Luo H, Chen M, Cui Z, et al. The association of HLA-DQB1, -DQA1 and -DPB1 alleles with anti- glomerular basement membrane (GBM) disease in Chinese patients. BMC Nephrol 2011;12:21. 10.1186/1471-2369-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Yu X. Genetic study of immunoglobulin A nephropathy: from research to clinical application. Nephrology 2018;23 Suppl 4:26–31. 10.1111/nep.13470 [DOI] [PubMed] [Google Scholar]
- 14.Leeaphorn N, Kue-A-Pai P, Thamcharoen N, et al. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am J Nephrol 2014;40:29–35. 10.1159/000364782 [DOI] [PubMed] [Google Scholar]
- 15.McMahon RF, Lawler W, O'Donoghue DJ, et al. Goodpasture's syndrome in a patient with two endocrine tumours. Postgrad Med J 1989;65:582–6. 10.1136/pgmj.65.766.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimura I, Tojo A, Kinugasa S, et al. Renal cell carcinoma in association with IgA nephropathy in the elderly. Am J Med Sci 2009;338:431–2. 10.1097/MAJ.0b013e3181ae1b12 [DOI] [PubMed] [Google Scholar]
- 17.Magyarlaki T, Kiss B, Buzogány I, et al. Renal cell carcinoma and paraneoplastic IgA nephropathy. Nephron 1999;82:127–30. 10.1159/000045388 [DOI] [PubMed] [Google Scholar]
- 18.Ge Y-ting, Liao J-lan, Liang W, et al. Anti-glomerular basement membrane disease combined with IgA nephropathy complicated with reversible posterior leukoencephalopathy syndrome: an unusual case. Am J Case Rep 2015;16:849–53. 10.12659/ajcr.894619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annamalai I, Chandramohan G, Srinivasa Prasad ND, et al. Rapidly progressive glomerulonephritis due to anti-glomerular basement membrane disease accompanied by IgA nephropathy: an unusual association. Saudi J Kidney Dis Transpl 2017;28:1404–7. 10.4103/1319-2442.220866 [DOI] [PubMed] [Google Scholar]
- 20.Gao B, Li M, Xia W, et al. Rapidly progressive glomerulonephritis due to anti-glomerular basement membrane disease accompanied by IgA nephropathy: a case report. Clin Nephrol 2014;81:138–41. 10.5414/CN107213 [DOI] [PubMed] [Google Scholar]
- 21.Trpkov K, Abdulkareem F, Jim K, et al. Recurrence of anti-GBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol 1998;49:124–8. [PubMed] [Google Scholar]