Abstract
Objectives
The study aimed to assess the knowledge, attitude and practices (KAP) of HCPs regarding the use of probiotics in different health conditions and to identify various barriers that are associated with their use.
Methods
A cross-sectional study was conducted on 405 HCPs by using a validated self- administered questionnaire for assessing their KAP towards probiotic use. The study data were analysed using descriptive statistics, χ2 test and binary logistic regression (BLR).
Results
Among the 405 participants, only 15.1 % of HCPs had good knowledge, while 15.6% had acceptable practices and 89.1% had a positive attitude towards probiotics. The professional position of HCPs was significantly associated with knowledge (p=0.001) and practice (p=0.001). Among all the HCPs, the pharmacists showed a significant association with good knowledge (p=0.016) and good practices (p=0.024) by using BLR. The lack of knowledge about probiotics was a major barrier to the utilisation of probiotics.
Conclusions
The poor knowledge and practices regarding the use of probiotics have been seen in the current study. While the participants showed a positive attitude towards the utilisation of probiotics. To transform HCPs’ positive attitude to their practices and to create awareness regarding probiotic use focused training programmes should be initiated by professional health organisations.
Keywords: public health, medical education & training, nutrition & dietetics
Strengths and limitations of this study.
We have reported for the very first time healthcare professionals (HCPs) knowledge, attitude, and practices (KAP) towards probiotic use in Southern Punjab, Pakistan.
The sample size was inflated by 10% to minimise the errors in completing the questionnaire and to identify significant influencing factors of KAP among the HCPs.
The response bias may be present in the current study due to the use of self-reporting data, which may affect the accuracy of the findings.
As the current study was cross-sectional, so the causal relationship can not be established.
The present study was conducted only in the HCPs of Southern Punjab (Multan), Pakistan and the results cannot be generalised to the HCPs working in the whole country.
Introduction
Trillions of bacteria reside in the human gastrointestinal tract (GIT) and collectively they are called gut microbiota.1 These microbes provide many benefits to the human body like regulation of the immune system, provision of energy, protection against pathogens and metabolism of lipids. Food products or supplements having such microorganisms can alter the composition of the microbial flora in GIT.2
The term ‘probiotic’ is a combination of ‘pro’ (Latin word) means ‘for’ and bios (Greek word) means ‘life’ and considered as opposite to ‘antibiotic’, which was the first time used in the 1960s.3 ‘Live microorganism that, when administered in adequate amounts, confers a health benefit on the host’ is the most accepted definition provided by WHO and the Food and Agriculture Organisation of the United Nations in regards to term probiotics.4 5 Bifidobacterium and Lactobacillus genera are the most common micro-organisms, which are mostly available in many probiotic products, while Lactococcus, Streptococcus, Enterococcus, Propionibacterium and Saccharomyces are less common.6
The change in human gut microbiota may cause many clinical complications like acute diarrhoea,7 antibiotic-associated diarrhoea,8 traveller’s diarrhoea,9 inflammatory bowel disease,10 irritable bowel syndrome,11 ulcerative colitis12 and Clostridium difficile infection.13 These complications can be treated and prevented by the use of probiotics. Moreover, probiotics are also beneficial in the management of health conditions other than GIT like urinary tract14 and respiratory tract infections,15 allergies,16 obesity,17 stress18 and cardiovascular disorders.19
Most of the previously published reports on probiotics were mainly focused on discovering their mechanism of action and possible health benefits. Unfortunately, there are only a few published studies on factors influencing the use of probiotics. It is known that the healthcare professionals’ (HCPs) knowledge and attitude towards probiotics could affect their prescribing behaviour and the knowledge provided by them directly affects the consumption of probiotics by the patients. The evidence supporting the safe and effective use of probiotics is increasing with every passing day and it has been proved by different clinical trials.9 20–23 However, less data are available about the usage and knowledge of healthcare workers (HCWs) regarding probiotics. The studies assessing knowledge, attitude and practices (KAP) of probiotics have been reported in different countries,24–28 but to date, there is no published report on this topic that has assessed HCPs KAP towards probiotics in Pakistan. The present study was designed to assess the KAP of HCPs regarding the use of probiotics and the various barriers that influence their prescribing in Pakistan.
Methods
Study design
A descriptive, cross-sectional study was conducted using a self-administered questionnaire (SAQ) as a tool to assess the KAP of HCPs regarding probiotics use in Multan, Pakistan. A convenient sampling technique was used for the data collection from January 2020 to March 2020. The registered doctors (ie, general physician, paediatrician, gastroenterologist) and retail pharmacists working in the private and public sectors who were involved in the prescribing and sale of probiotics were included in the study. Only those participants were given the SAQ, who filled and signed the informed consent form. The incomplete responses were excluded from the final study results.
Sample size
A sample size of 385 was calculated by using Raosoft, assuming a 95% CI, a response rate of 50%, Z of 1.96 and a margin of error of 5%. The calculated sample size was further 10% (N=38) increased to minimise any error that may occur while completing SAQ, making the final sample size of N=423 for this study.
Questionnaire development
A close-ended questionnaire was developed and modified according to the need after conducting an extensive literature review.27 28 To evaluate the clarity and utility of the questionnaire it was first circulated among the subject specialists (teaching pharmacists and physicians). The suggested changes from the subject specialists were then incorporated into the questionnaire. Afterward, to check the reliability and validity of the questionnaire, a pilot study was conducted on 30 participants. The Cronbach’s alpha value of 0.846 showed that the questionnaire was reliable.
The final questionnaire was divided into two sections, section 1 comprised questions/information related to demographics and the second section contained questions related to probiotics (online supplemental table 1). The demographic section included information on gender, age, marital status, profession, patient population and experience (year in practice) of the participants. The probiotic portion of the questionnaire was further subdivided into five sections. These sections included questions regarding commercially available probiotic products, knowledge about probiotics, attitude towards probiotic use, practices regarding probiotic use and barriers to prescribe probiotics. The probiotic drug (brand) section comprised seven items in which participant rated their knowledge regarding commonly available probiotic brands in the market on a Likert scale (0=not at all, 1=somewhat, 2=very much). Second subsection comprised knowledge about probiotics, where the first question was about the definition of probiotics. While second is composed of 13 different diseases or health conditions in which probiotics show promising benefits (assessed from literature review) and the participants rated their views on a Likert scale (0=not at all, 1=somewhat, 2=very much). The third subsection is comprised of three items to assess HCPs’ attitude about probiotics. The inverted score was used for a negative question. The total attitude score was 06, while participants having a score more than the mean of the total score (>03) were considered having a positive attitude. The fourth subsection was focused on assessing HCPs practices regarding prescribing probiotics in 13 different health conditions. The third and fourth subsections included a similar Likert scale as in previous portions. The Last segment of the questionnaire composed of the barriers related to the prescribing of probiotics. The scoring of the questionnaire has been described in online supplemental table 1.
bmjopen-2020-047494supp001.pdf (228.9KB, pdf)
Data collection
The printed copies of the developed questionnaire were distributed among eligible HCPs along with informed consent and instruction for filling the questionnaire. Total 423 responses were collected out of 500 distributed questionnaires. The detail of the data collection process is provided in online supplemental figure 1.
Statistical analysis
In the present study, the descriptive and inferential statistics were performed by using SPSS V.23.0 (IBM). In descriptive statistics, the frequencies and percentages were used to express the categorical variables. A χ2 test was used to analyse the KAP between the study subgroups. The binary logistic regression (BLR) models were used to find the possible determinants for participant’s positive attitude, good knowledge and practice regarding probiotics. The adjusted OR(AOR) and crude OR(COR) with a 95% CI were used to express the results of BLR. While the model was adjusted for gender, age, marital status, profession, patient population, experience (year in practice), and knowledge about probiotics brands of the participants In all tests, a p<0.05 was considered to be statistically significant.
Patient and public involvement
There were no patients involved in the current study as the study participants were HCPs only.
Results
From the total 423 study participants, only 405 were included in the final analysis, as 18 questionnaires were incomplete. Most of the participants were, female 55.6% (n=225), aged less than 25 years 53.1% (n=215) and were single 74.6% (n=302). Around 51.9% (n=210) of the participants were pharmacists while 37.0% (n=150) were physicians with highest patient population as adults 63.7% (n=258). The 80.5% (n=326) of the participants were having less than 4 years of professional experience. The 57.8% (n=234) of the participants had poor knowledge regarding the available probiotic products. The participants’ demographic characteristics are presented in table 1.
Table 1.
Demographic and knowledge about probiotic drugs brand of healthcare professionals (N=405)
| Variables | N (%) | |
| Gender | Male | 180 (44.4) |
| Female | 225 (55.6) | |
| Age | ≤25 years | 215 (53.1) |
| 26–30 years | 142 (35.1) | |
| ≥31 years | 48 (11.9) | |
| Marital status | Single | 302 (74.6) |
| Married | 103 (25.4) | |
| Professional position | Physician | 150 (37.0) |
| Pharmacist | 210 (51.9) | |
| Surgeon | 45 (11.1) | |
| Patient population | Paediatric | 27 (6.7) |
| Adult | 258 (63.7) | |
| Geriatric | 14 (3.5) | |
| Paediatric and adult | 8 (2.0) | |
| Adult and geriatric | 26 (6.4) | |
| Paediatric, adult and geriatric | 72 (17.8) | |
| Experience (year in practice) |
≤4 years | 326 (80.5) |
| 5–9 years | 52 (12.8) | |
| 10–14 years | 14 (3.5) | |
| ≥15 years | 13 (3.2) | |
| Knowledge about probiotic drugs brand* | Good knowledge | 171 (42.2) |
| Poor knowledge | 234 (57.8) | |
*Score range from 0 to 14. Poor knowledge (score of ≤7). Good knowledge (score of ≥8).
Among the participants, 15.1% (n=61) had good knowledge while 84.9% (n=344) had poor knowledge regarding the use of probiotics. The professional position of the respondent was significantly associated with knowledge (χ2=35.607, p<0.001). While the knowledge did not vary significantly between the gender, age, marital status, patient population and knowledge about different probiotics brands. The differences in KAP of HCPs regarding the use of probiotics can be seen in table 2.
Table 2.
Difference in knowledge, attitude and practices of healthcare professionals about probiotics by demographic
| Variables | Total | Knowledge* | Attitude† | Practice‡ | ||||||
| N (%) | Good | Poor | X2 (p value) | Positive | Negative | X2 (P) | Good | Poor | X2 (p value) | |
| Overall | 405 (100%) | 61 (15.1%) | 344 (84.9%) | 361 (89.1%) | 44 (10.9%) | 63 (15.6%) | 342 (84.4%) | |||
| Gender | 0.757 (0.384) | 1.225 (0.268) | 3.730 (0.053) | |||||||
| Male | 180 (44.4%) | 24 (13.3%) | 156 (86.7%) | 157 (87.2%) | 23 (12.8%) | 21 (11.7%) | 159 (88.3%) | |||
| Female | 225 (55.6%) | 37 (16.4%) | 188 (83.6%) | 204 (90.7%) | 21 (9.3%) | 42 (18.7%) | 183 (81.3%) | |||
| Age | 2.754 (0.252) | 1.255 (0.534) | 2.182 (0.336) | |||||||
| ≤25 years | 215 (53.1%) | 38 (17.7%) | 177 (82.3%) | 191 (93.8%) | 24 (11.2%) | 36 (16.7%) | 179 (83.3%) | |||
| 26–30 years | 142 (35.1%) | 16 (11.3%) | 126 (88.7%) | 125 (88.0%) | 17 (12.0%) | 23 (16.2%) | 119 (83.8%) | |||
| ≥31 years | 48 (11.9%) | 7 (14.6%) | 41 (85.4%) | 45 (93.8%) | 3 (6.3%) | 4 (8.3%) | 44 (91.7%) | |||
| Marital status | 0.027 (0.870) | 0.440 (0.507) | 0.104 (0.748) | |||||||
| Single | 302 (74.6%) | 46 (15.2%) | 256 (84.8%) | 271 (89.7%) | 31 (10.3%) | 48 (15.9%) | 254 (84.1%) | |||
| Married | 103 (25.4%) | 15 (%14.6) | 88 (85.4%) | 90 (87.4%) | 13 (12.6%) | 15 (14.6%) | 88 (85.4%) | |||
| Professional position | 35.607 (<0.001) | 0.256 (0.880) | 39.741 (<0.001) | |||||||
| Physician | 150 (37.0%) | 5 (3.3%) | 145 (96.7%) | 134 (89.3%) | 13 (10.7%) | 3 (2.0%) | 147 (98.0%) | |||
| Pharmacist | 210 (51.9%) | 53 (25.2%) | 157 (74.8%) | 134 (88.6%) | 24 (11.4%) | 55 (26.2%) | 155 (73.8%) | |||
| Surgeon | 45 (11.1%) | 3 (3.7%) | 42 (93.3%) | 41 (91.1%) | 4 (8.6%) | 5 (11.1%) | 40 (88.9%) | |||
| Patient population | 10.348 (0.066) | 13.197 (0.022) | 10.185 (0.070) | |||||||
| Paediatric | 27 (6.7%) | 5 (18.5%) | 22 (81.5%) | 23 (85.2%) | 4 (14.8%) | 5 (18.5%) | 22 (81.5%) | |||
| Adult | 258 (63.7%) | 42 (16.3%) | 216 (83.7%) | 228 (88.4%) | 30 (11.6%) | 42 (16.3%) | 216 (83.7%) | |||
| Geriatric | 14 (3.5%) | 5 (35.5%) | 9 (64.3%) | 11 (78.6%) | 3 (21.4%) | 4 (28.6%) | 10 (714%) | |||
| Paediatric and adult | 8 (2.0%) | 1 (12.5%) | 7 (87.5%) | 5 (62.5%) | 3 (37.5%) | 1 (12.5%) | 7 (87.5%) | |||
| Adult and geriatric | 26 (6.4%) | 4 (15.4%) | 22 (84.6%) | 24 (92.3%) | 2 (7.7%) | 7 (26.9%) | 19 (73.1%) | |||
| Paediatric, adult and geriatric | 72 (17.8%) | 4 (5.6%) | 68 (94.4%) | 70 (97.2%) | 2 (2.8%) | 4 (5.6%) | 68 (94.4%) | |||
| Experience (year in practice) |
6.755 (0.080) | 7.463 (0.059) | 5.850 (0.119) | |||||||
| ≤4 years | 326 (80.5%) | 49 (15.0%) | 277 (85.0%) | 290 (89.0%) | 36 (11.0%) | 54 (16.6%) | 272 (83.4%) | |||
| 5–9 years | 52 (12.8%) | 6 (11.5%) | 46 (88.5%) | 50 (96.2%) | 2 (3.8%) | 3 (5.8%) | 49 (94.2%) | |||
| 10–14 years | 14 (3.5%) | 1 (7.1%) | 13 (92.9%) | 10 (71.4%) | 4 (28.6%) | 4 (28.6%) | 10 (71.4%) | |||
| ≥15 years | 13 (3.2%) | 5 (38.5%) | 8 (61.5%) | 11 (84.6%) | 2 (15.4%) | 2 (15.4%) | 11 (84.6%) | |||
| Knowledge about probiotic drugs brand§ | 0.123 (0.726) | 25.363 (<0.001) | 5.699 (0.017) | |||||||
| Good knowledge | 171 (42.2%) | 27 (15.8%) | 144 (84.2%) | 168 (98.2%) | 3 (1.8%) | 18 (10.5%) | 153 (89.5%) | |||
| Poor knowledge | 234 (57.8%) | 34 (14.5%) | 200 (85.5%) | 193 (82.5%) | 41 (17.5%) | 45 (19.2%) | 189 (80.8%) | |||
P<0.05 was considered to indicate significance. Bold fonts show significant differences.
*Score range from 0 to 27. Poor knowledge (score of ≤15), good knowledge (score of ≥16).
†Score range from 0 to 27. Negative attitude (score of ≤3), positive attitude (score of ≥4).
‡Score range from 0 to 25. A score of ≤ 14 was set for poor practice, ≥15 for good practice.
§Score ranges from 0 to 14. Poor knowledge (score of ≤7). Good knowledge (score of ≥8).
89.1% (n=361) of the participants had a positive attitude regarding probiotics, while only 10.9% (n=44) had a negative attitude. The difference in the attitude of participants was significantly associated with the patient population (χ2=13.197, p=0.022) and knowledge about probiotic brands (χ2=25.363, p<0.001). The difference in attitude was not significantly associated with gender, age, marital status, professional position and experience.
The 15.6% (n=63) participants had good practices while 84.4% (n=342) had poor practices in prescribing probiotic products. The professional position (χ2=39.741, p<0.001) and the knowledge about the probiotic brands (χ2=5.699, p=0.00.17) were significantly associated with the HCPs practices. The age, gender, marital status, patient population and experience did not show any significant association with the HCPs practice.
The BLR showed that in comparison to other HCPs, the pharmacists had higher odds regarding good knowledge about probiotics in both adjusted and unadjusted models (AOR=6.162, 95% CI 1.401 to 27.111, p=0.016) and (COR=4.726, 95% CI 1.707 to 15.880, p=0.012) respectively. The groups with experience ≤4 and 5–9 years showed higher odds for good knowledge (COR=0.283, 95% CI 90.089 to 0.901, p=0.033), (AOR=0.127, 95% CI 0.025 to 0.653, p=0.013) and (COR=0.209, 95% CI 0.051 to 0.850, p=0.029), (AOR=0.107, 95% CI 0.017 to 0.666, p=0.017), respectively, in both adjusted and unadjusted BLR analysis (table 3).
Table 3.
Binary logistic regression for variables related to good knowledge of healthcare professionals
| Variables | Variables associated with good knowledge | ||||||
| COR | 95% CI | P value | AOR | 95% CI | P value | ||
| Gender | Male | 0.782 | 0.448 to 1.363 | 0.385 | 0.693 | 0.355 to 1.351 | 0.281 |
| Female* | |||||||
| Age | ≤25 years | 1.257 | 0.524 to 3.016 | 0.608 | 0.702 | 0.203 to 2.432 | 0.577 |
| 26–30 years | 0.744 | 0.286 to 1.934 | 0.544 | 0.465 | 0.127 to 1.709 | 0.249 | |
| ≥31 years* | |||||||
| Marital status | Single | 1.054 | 0.561 to 1.981 | 0.870 | 1.210 | 0.535 to 2.736 | 0.647 |
| Married* | |||||||
| Professional position | Physician | 0.483 | 0.111 to 2.104 | 0.332 | 0.390 | 0.076 to 2.011 | 0.261 |
| Pharmacist | 4.726 | 1.407 to 15.880 | 0.012 | 6.162 | 1.401 to 27.111 | 0.016 | |
| Surgeon* | |||||||
| Patient population | Paediatric | 3.864 | 0.953 to 15.666 | 0.058 | 2.287 | 0.482 to 10.851 | 0.298 |
| Adult | 3.306 | 1.144 to 9.552 | 0.027 | 2.090 | 0.642 to 6.806 | 0.221 | |
| Geriatric | 9.444 | 2.134 to 41.792 | 0.003 | 12.766 | 2.307 to 70.641 | 0.004 | |
| Paediatric and adult | 2.429 | 0.237 to 2.844 | 0.455 | 2.137 | 0.173 to 26.454 | 0.554 | |
| Adult and geriatric | 3.091 | 0.713 to 3.402 | 0.132 | 9.672 | 1.645 to 56.865 | 0.012 | |
| Paediatric, adult and geriatric* | |||||||
| Experience (year in practice) |
≤4 years | 0.283 | 0.089 to 0.901 | 0.033 | 0.127 | 0.025 to 0.653 | 0.013 |
| 5–9 years | 0.209 | 0.051 to 0.850 | 0.029 | 0.107 | 0.017 to 0.666 | 0.017 | |
| 10–14 years | 0.123 | 0.012 to 1.253 | 0.077 | 0.037 | 0.002 to 0.563 | 0.018 | |
| ≥15 years* | |||||||
| Knowledge about probiotic drugs brand † | Good knowledge | 1.103 | 0.637 to 1.909 | 0.726 | 0.953 | 0.500 to 1.819 | 0.885 |
| Poor knowledge* | |||||||
P<0.05 was considered to indicate significance. Bold fonts show significant differences.
*Indicates a reference group in the logistic regression.
†Score range from 0 to 14. Poor knowledge (score of ≤7). Good knowledge (score of ≥8).
AOR, adjusted OR; COR, crude OR.
The HCPs who were interacting with the population groups, ‘geriatric’ and ‘geriatric and adults’ showed significant association with positive attitude (COR 0.105, 95% CI 0.016 to 0.700, p=0.020), (AOR=0.042, 95% CI 0.004 to 0.465, p=0.010) and (COR=0.048, 95% CI 0.006 to 0.354), p=0.003), (AOR=0.018, 95% CI 0.001 to 0.232, p=0.002), respectively (table 4). The HCPs with good knowledge about probiotic brands showed higher odds for positive attitude in both unadjusted (COR 11.896, 95% CI 3.618 to 39.118, p<0.001) and adjusted (AOR=34.396, 95% CI 7.282 to 162.456), p<0.0001) models, respectively.
Table 4.
Binary logistic regression for variables linked with the positive attitude of healthcare professionals
| Variables | Variables associated with positive attitude | ||||||
| COR | 95% CI | P value | AOR | 95% CI | P value | ||
| Gender | Male | 0.703 | 0.375 to 1.316 | 0.270 | 0.463 | 0.213to 1.008 | 0.053 |
| Female* | |||||||
| Age | ≤25 years | 0.531 | 0.153 to 1.840 | 0.318 | 0.125 | 0.011 to 1.406 | 0.092 |
| 26–30 years | 0.490 | 0.137 to 1.752 | 0.273 | 0.146 | 0.013 to 1.655 | 0.120 | |
| ≥31 years* | |||||||
| Marital status | Single | 1.263 | 0.633 to 2.518 | 0.508 | 2.648 | 0.993 to 7.064 | 0.052 |
| Married* | |||||||
| Professional position | Physician | 0.817 | 2.259 to 2.581 | 0.731 | 1.876 | 0.440 to 8.002 | 0.395 |
| Pharmacist | 0.756 | 0.249 to 2.297 | 0.622 | 1.717 | 0.412 to 7.154 | 0.458 | |
| Surgeon* | |||||||
| Patient population | Paediatric | 0.164 | 0.028 to 0.956 | 0.044 | 0.224 | 0.031 to 1.606 | 0.136 |
| Adult | 0.217 | 0.051 to 0.931 | 0.040 | 0.334 | 0.071 to 1.575 | 0.166 | |
| Geriatric | 0.105 | 0.016 to 0.700 | 0.20 | 0.042 | 0.004 to 0.465 | 0.010 | |
| Paediatric and adult | 0.048 | 0.006 to 0.354 | 0.003 | 0.018 | 0.001 to 0.232 | 0.002 | |
| Adult and geriatric | 0.343 | 0.046 to 2.569 | 0.298 | 0.913 | 0.082 to 10.192 | 0.941 | |
| Paediatric, adult and geriatric* | |||||||
| Experience (year in practice) |
≤4 years | 1.465 | 0.312 to 6.873 | 0.629 | 2.867 | 0.387 to 21.214 | 0.302 |
| 5–9 years | 4.545 | 0.576 to 35.871 | 0.151 | 7.429 | 0.614 to 89.931 | 0.115 | |
| 10–14 years | 0.455 | 0.068 to 3.043 | 0.416 | 0.216 | 0.011 to 4.433 | 0.320 | |
| ≥15 years* | |||||||
| Knowledge about probiotic drugs brand† | Good knowledge | 11.896 | 3.618 to 39.118 | <0.001 | 34.396 | 7.282 to 162.456 | <0.01 |
| Poor knowledge* | |||||||
P<0.05 was considered to indicate significance. Bold values show significant differences.
*Indicates a reference group in the logistic regression.
†Score range from 0 to 14. Poor knowledge (score of ≤7). Good knowledge (score of ≥8).
AOR, adjusted OR; COR, crude OR.
The results of BLR for good practice showed that the male HCPs showed higher odds for good practice (OR=0.442, 95% CI 0.216 to 0.905, p=0.025). The unadjusted and adjusted BLR models showed that the physicians and pharmacists were significantly associated with the good practice (COR=0.163, 95% CI 0.037 to 0.713), p=0.016), (AOR=0.142, 95% CI 0.025 to 0.810, p=0.028) and (COR=2.839, 95% CI 1.066 to 7.558, p=0.037), (AOR=6.040, 95% CI 1.272 to 28.668, p=0.024), respectively (table 5).
Table 5.
Binary logistic regression for variables related to good practice of healthcare professionals
| Variables | Variables associated with good practice | ||||||
| COR | 95% CI | P | AOR | 95% CI | P | ||
| Gender | Male | 0.575 | 0.327 to 1.013 | 0.055 | 0.442 | 0.216 to 0.905 | 0.025 |
| Female* | |||||||
| Age | ≤25 years | 2.212 | 0.748 to 6.543 | 0.151 | 20.008 | 0.458 to 8.792 | 0.355 |
| 26–30 years | 2.126 | 0.696 to 6.494 | 0.186 | 0.449 | 0563 to 10.652 | 0.232 | |
| ≥31 years* | |||||||
| Marital status | Single | 1.109 | 0.591 to 2.078 | 0.748 | 0.998 | 0.431 to 2.314 | 0.997 |
| Married* | |||||||
| Professional position | Physician | 0.163 | 0.037 to 0.713 | 0.016 | 0.142 | 0.025 to 0.810 | 0.028 |
| Pharmacist | 2.839 | 1.066 to 7.558 | 0.037 | 6.040 | 1.272 to 28.668 | 0.024 | |
| Surgeon* | |||||||
| Patient population | Paediatric | 3.864 | 0.953 to 15.666 | 0.058 | 1.402 | 0.291 to 6.761 | 0.673 |
| Adult | 3.306 | 1.144 to 9.552 | 0.027 | 1.215 | 0.371 to 3.984 | 0.748 | |
| Geriatric | 6.800 | 1.463 to 31.614 | 0.014 | 7.957 | 1.166 to 54.302 | 0.034 | |
| Paediatric and adult | 2.429 | 0.237 to 24.844 | 0.455 | 3.049 | 0.253 to 36.792 | 0.380 | |
| Adult and geriatric | 6.263 | 1.657 to 23.672 | 0.007 | 13.912 | 2.197 to 88.104 | 0.005 | |
| Paediatric, adult and geriatric* | |||||||
| Experience (year in practice) |
≤4 years | 1.092 | 0.235 to 5.066 | 0.911 | 0.278 | 0.036 to 2.153 | 0.220 |
| 5–9 years | 0.337 | 0.050 to 2.263 | 0.263 | 0.070 | 0.006 to 0.765 | 0.029 | |
| 10–14 years | 2.200 | 0.329 to 14.726 | 0.416 | 0.770 | 0.069 to 8.553 | 0.831 | |
| ≥15 years* | |||||||
| Knowledge about probiotic drugs brand† | Good knowledge | 0.494 | 0.275 to 0.888 | 0.019 | 0.372 | 0.185 to 0.747 | 0.005 |
| Poor knowledge* | |||||||
P<0.05 was considered to indicate significance. Bold values show significant differences.
*Indicates a reference group in the logistic regression.
†Score range from 0 to 14. Poor knowledge (score of ≤7). Good knowledge (score of ≥8).
AOR, adjusted OR; COR, crude OR.
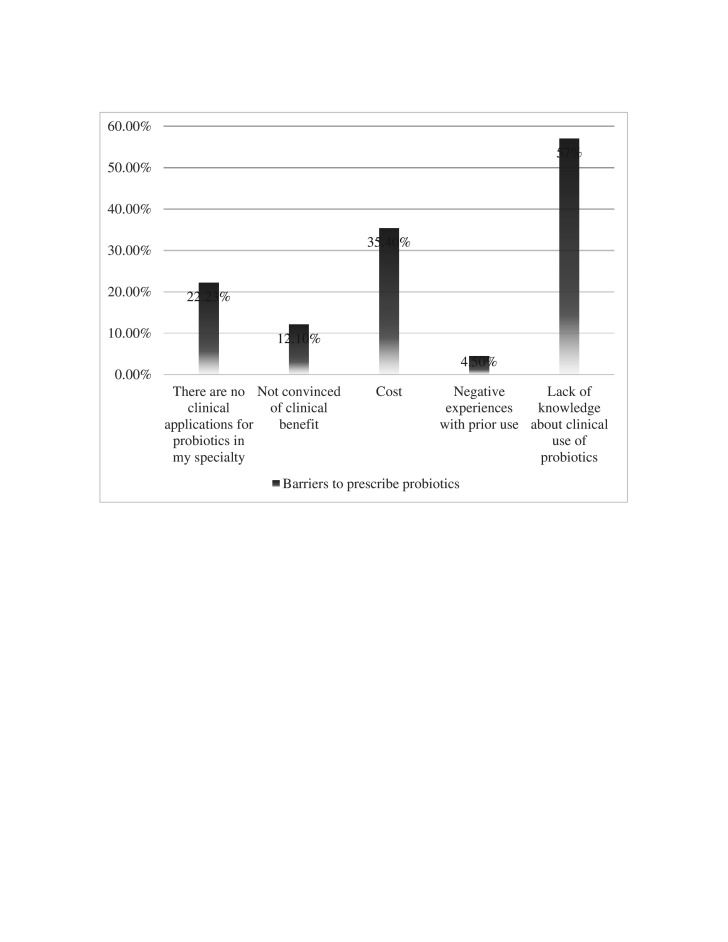
The lack of knowledge about the clinical use of probiotics (57%) was the most common reason among both doctors and pharmacists. The high cost of probiotics was the second common barrier (35.4 %) for HCPs for not recommending probiotics to their patients (figure 1).
Figure 1.
The barriers to recommend probiotics reported by healthcare professionals.
Discussion
The present study was aimed at assessing the KAP of HCPs regarding the use of probiotics and the barriers that are encountered in their prescribing. The results showed that only a small percentage of HCPs (15.1 %) had good knowledge regarding probiotic use, while the practising pharmacists were having better knowledge. These findings are not consistent with reports from Jordan (35.6%) and India (57.6%),25 29 as the HCPs in these countries were having better knowledge regarding probiotics. Similarly, a study from Nigeria and an international study showed that the practitioners in these regions had better probiotics knowledge.24 30 The differences seen in HCPs knowledge between our study and the previous reports may be because these countries have more developed probiotic industry and the HCPs are in more frequent contact with the probiotic product marketing professionals.31 32
Most HCPs (89.1%) had a positive attitude regarding the use of probiotics. This was consistent with studies conducted in Jordan, Nigeria, India and Europe.28 29 31 33 34 The positive attitude of the HCPs towards probiotic use can be related to the fact that most of the probiotic products have been introduced recently in Pakistan and there are no reports of any adverse drug reactions associated with their use. Moreover, it was seen that the female HCPs had a more positive attitude (56.5 %) towards probiotic use as compared with males (43.5 %). This finding can be related to the perception that the females are generally more concerned about dietary approaches for the management of various ailments.25
In the current study, only 15.6% of HCPs had good practices regarding the use of probiotics in different health conditions, but the pharmacists were having better probiotic use practices than other professionals. This percentage is lower than the previous studies from Jordan (41.0%), Nigeria (25.8%) and international study (79.0%).24–26 The less use of probiotic products in Pakistan can be attributed to the poor probiotics’ knowledge of HCPs. Moreover, the biggest barrier related to the non-prescribing of probiotics reported by the HCPs was also a lack of knowledge and this is consistent with previously published studies.25 27 30 35
The higher probiotics knowledge among the HCPs will enhance their confidence to offer safe and effective treatment to the patients.35 The results of this survey have highlighted the need for increasing HCPs' knowledge regarding the use of probiotics in Pakistan as most of them were having poor knowledge. To enhance the HCPs’ knowledge, educational activities like continuing medical education programmes or focused training should be conducted by professional medical associations. The increase in HCP’s knowledge regarding the use of probiotics will promote their safe and effective use in the population.
The present study was conducted among HCPs of Multan, Pakistan and the results cannot be generalised for the whole country. The influence of HCPs specialisation was not considered in this study, which can potentially influence their KAP regarding probiotics. Moreover, the knowledge section of the study questionnaire contained some indications that were not mentioned on the product label but were taken from the published literature. The response bias may be present in the current study due to the use of self-reporting data, which may affect the accuracy of the findings. As the current study was cross-sectional, so the causal relationship can not be established.
Conclusion
The poor knowledge and practices regarding the use of probiotics have been seen in the current study. While the participants showed a positive attitude towards the utilisation of probiotics. To transform HCPs’ positive attitude to their practices and to create awareness regarding probiotic use focused training programmes should be initiated by professional health organisations. Lastly, probiotics should be included in the undergraduate curriculum of medical and pharmacy professions, so that future practitioners may have better knowledge and practices regarding the use of probiotics.
Supplementary Material
Footnotes
Contributors: MSA, MFR, AM, II, BR, MS MI, OC and MAA made substantial contributions to conception and design. MSA, HS, AM, MA, MSA, OC, BR and MU performed data analysis and acquisition of data. MFR, MA, MAA, HS, MI, MU, II and FH took part in interpretation of data. All the authors were involved in the writing of initial draft and the approval of the final version of the manuscript. All authors gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. The ethical approval for the study was accorded by the ethical committee of the Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University Multan, Pakistan on 20 December 2019, against reference no. Acad/19/20/16. Each participant was asked to give written informed consent before the administration of the study questionnaire.
References
- 1.Rodríguez JM, Murphy K, Stanton C, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 2015;26:26050. 10.3402/mehd.v26.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J 2017;474:1823–36. 10.1042/BCJ20160510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morelli L, Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 2012;46 Suppl:S1–2. 10.1097/MCG.0b013e318269fdd5 [DOI] [PubMed] [Google Scholar]
- 4.FAO/WHO . Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, 2001. Available: http://www.fao.org/3/a-a0512e.pdf
- 5.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 6.Chugh B, Kamal-Eldin A. Bioactive compounds produced by probiotics in food products. Curr Opin Food Sci 2020;32:76–82. 10.1016/j.cofs.2020.02.003 [DOI] [Google Scholar]
- 7.Li Y-T, Xu H, Ye J-Z, et al. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: A systematic review with meta-analysis. World J Gastroenterol 2019;25:4999–5016. 10.3748/wjg.v25.i33.4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai J, Zhao C, Du Y, et al. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: systematic review with network meta-analysis. United European Gastroenterol J 2018;6:169–80. 10.1177/2050640617736987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers' diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis 2019;27:11–19. 10.1016/j.tmaid.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Derwa Y, Gracie DJ, Hamlin PJ, et al. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 2017;46:389–400. 10.1111/apt.14203 [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Guo L-M, Yang S-Y, et al. Effectiveness of probiotics in irritable bowel syndrome: methodological quality of meta-analyses and systematic reviews. Frontiers of Nursing 2019;6:115–21. 10.2478/FON-2019-0018 [DOI] [Google Scholar]
- 12.Astó E, Méndez I, Audivert S, et al. The efficacy of probiotics, prebiotic inulin-type fructans, and synbiotics in human ulcerative colitis: a systematic review and meta-analysis. Nutrients 2019;11:293. 10.3390/nu11020293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med 2016;9:27. 10.2147/IJGM.S98280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini M, Yousefifard M, Ataei N, et al. The efficacy of probiotics in prevention of urinary tract infection in children: a systematic review and meta-analysis. J Pediatr Urol 2017;13:581–91. 10.1016/j.jpurol.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 15.Amaral MA, Guedes GHBF, Epifanio M, et al. Network meta-analysis of probiotics to prevent respiratory infections in children and adolescents. Pediatr Pulmonol 2017;52:833–43. 10.1002/ppul.23643 [DOI] [PubMed] [Google Scholar]
- 16.Li L, Han Z, Niu X, et al. Probiotic supplementation for prevention of atopic dermatitis in infants and children: a systematic review and meta-analysis. Am J Clin Dermatol 2019;20:367–77. 10.1007/s40257-018-0404-3 [DOI] [PubMed] [Google Scholar]
- 17.Wang Z-B, Xin S-S, Ding L-N, et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2019;2019:1–14. 10.1155/2019/3862971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang N, Liao X, Zhang Y, et al. Probiotic supplements for relieving stress in healthy participants: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine 2019;98:e15416. 10.1097/MD.0000000000015416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondanelli M, Faliva MA, Perna S, et al. Using probiotics in clinical practice: where are we now? A review of existing meta-analyses. Gut Microbes 2017;8:521–43. 10.1080/19490976.2017.1345414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 2019;102:13–23. 10.1016/j.neubiorev.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther 2018;48:1044–60. 10.1111/apt.15001 [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Feng J, Chen P, et al. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin Res Hepatol Gastroenterol 2017;41:466–75. 10.1016/j.clinre.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Wardill HR, Van Sebille YZA, Ciorba MA, et al. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Curr Opin Support Palliat Care 2018;12:187–97. 10.1097/SPC.0000000000000338 [DOI] [PubMed] [Google Scholar]
- 24.Fijan S, Frauwallner A, Varga L, et al. Health Professionals’ Knowledge of Probiotics: An International Survey. Int J Environ Res Public Health 2019;16:3128. 10.3390/ijerph16173128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ababneh M, Elrashed N, Al-Azayzih A. Evaluation of Jordanian healthcare providers' knowledge, attitudes, and practice patterns towards probiotics. Expert Rev Pharmacoecon Outcomes Res 2020;20:93–7. 10.1080/14737167.2019.1609354 [DOI] [PubMed] [Google Scholar]
- 26.Chukwu OA. Assessing the awareness and knowledge on the use of probiotics by healthcare professionals in Nigeria. Journal of Young Pharmacists 2016;8:53. [Google Scholar]
- 27.Ensminger A, Haque RS. Clinical use of probiotics: a survey of physicians’ beliefs and practice patterns. J Am Diet Assoc 2011;111:A41. 10.1016/j.jada.2011.06.152 [DOI] [Google Scholar]
- 28.Oliver L, Rasmussen H, Gregoire MB, et al. Health Care Provider’s Knowledge, Perceptions, and Use of Probiotics and Prebiotics. Top Clin Nutr 2014;29:139–49. 10.1097/01.TIN.0000445898.98017.eb [DOI] [Google Scholar]
- 29.Soni R, Tank K, Jain N. Knowledge, attitude and practice of health professionals about probiotic use in Ahmedabad, India. NFS 2018;48:125–35. 10.1108/NFS-02-2017-0032 [DOI] [Google Scholar]
- 30.Chukwu E, Nwaokorie F, Yisau J, et al. Assessment of the knowledge and perception of probiotics among medical science students and practitioners in Lagos state. Br J Med Med Res 2015;5:1239–46. 10.9734/BJMMR/2015/13676 [DOI] [Google Scholar]
- 31.Anukam KC, Osazuwa E, Reid G. Knowledge of probiotics by Nigerian clinicians. International Journal of Probiotics and Prebiotics 2006;1:57. [Google Scholar]
- 32.Stanton C, Gardiner G, Meehan H, et al. Market potential for probiotics. Am J Clin Nutr 2001;73:476s–83. 10.1093/ajcn/73.2.476s [DOI] [PubMed] [Google Scholar]
- 33.Ali SI, Naqvi BS, Tasleem S. Physicians’knowledge and attitude concerning the clinical use of probiotics for the treatment of infantile diarrhea in sindh. Pakistan 2017. [Google Scholar]
- 34.Basrowi RW, Krisnamurti D, Wibowo Y. Factors influencing probiotics recommendation among pediatricians in Indonesia. Age;60:4. [Google Scholar]
- 35.Wilson Z, Whitehead K. A cross sectional survey to assess healthcare professionals' attitudes to and understanding of probiotics. Clin Nutr ESPEN 2019;34:104–9. 10.1016/j.clnesp.2019.08.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047494supp001.pdf (228.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.