Abstract
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease, with aging being considered the greatest risk factor for developing PD. Caveolin-1 (Cav-1) is known to participate in the aging process. Recent evidence indicates that prion-like propagation of misfolded α-synuclein (α-syn) released from neurons to neighboring neurons plays an important role in PD progression. In the present study, we demonstrated that cav-1 expression in the brain increased with age, and considerably increased in the brain of A53T α-syn transgenic mice. Cav-1 overexpression facilitated the uptake of α-syn into neurons and formation of additional Lewy body-like inclusion bodies, phosphorylation of cav-1 at tyrosine 14 was found to be crucial for this process. This study demonstrates the relationship between age and α-syn spread and will facilitate our understanding of the molecular mechanism of the cell-to-cell transmission of α-syn.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13041-021-00834-2.
Keywords: Parkinson’s disease, α-synuclein, Cell-to-cell transmission, Endocytosis, Caveolin-1, Aging
Introduction
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disease after Alzheimer's disease (AD), characterized by the loss of dopaminergic neurons located in the substantia nigra pars compacta and the presence of protein inclusions called Lewy bodies or Lewy neurites, which are composed of mainly α-synuclein (α-syn), a presynaptic protein [1]. Mutations and multiplication of α-syn have been identified in early-onset familial PD [2–4], suggesting that α-syn plays a major role in the pathogenesis of PD. Furthermore, recent studies have focused on the prion-like propagation of α-syn as a novel mechanism of PD progression [5, 6].
Despite extensive research, the pathogenesis of PD remains elusive. Both genetic and environmental factors are suspected to simultaneously contribute to the pathogenesis of PD. Mutations of genes such as SNCA, parkin, PINK1, DJ-1, and LRRK2 have been identified as genetic factors of PD [7]. Among environmental factors, age is regarded as the greatest risk factor for the development of PD [8, 9].
Caveolin-1 (cav-1) is a member of the caveolin family consisting of cav-1, 2, and 3, and is known to possess diverse functions, including regulation of membrane-initiated intracellular signaling via formation of caveolae [10, 11]. Although the role of cav-1 is poorly investigated in the brain, cav-1 is widely expressed in the central and peripheral nervous systems [12], regulating neurotrophin signaling pathways and synaptic remodeling [13, 14]. Additionally, it modulates neurotransmitter receptor signaling [15, 16].
Accumulating evidence indicates that cav-1 also participates in the aging process. Reportedly, cav-1 expression is upregulated in the brain of old rats and the aged human cortex [17, 18]. Old human diploid fibroblasts express higher levels of cav-1 when compared with younger cells [19]. In human and mice, cav-1 is also upregulated in the chronologically-aged skin [20]. Additionally, cav-1-overexpressing murine embryonic fibroblasts (MEFs) display a large, flat morphology and express high levels of senescence-associated β-galactosidase activity, revealing premature cellular senescence [21]. These studies have suggested that cav-1 overexpression may induce aging phenotypes.
A previous study has shown that cells derived from parkin knock-out (KO), or downregulating parkin using shRNA, demonstrate an increase in the cav-1 level, revealing that cav-1 is a substrate for parkin [22], for which loss-of-function mutations has been identified in familial PD [23]. Furthermore, parkin KO neurons have reported an increase in the cell-to-cell transmission of α-syn, proposing that each genetic risk factor of PD can share common pathological pathways with the other factors [22]. Given that aging is the greatest risk factor for PD and cav-1 can participate in the aging process, we hypothesized that age-related cav-1 expression may affect the cell-to-cell transmission of α-syn, contributing to the pathogenesis of PD. In the present study, we explored the association between cav-1 and cell-to-cell transmission of α-syn.
Material and methods
Antibodies and reagents
Antibodies against cav-1 (#610407) and α-syn (#610786) were purchased from BD Biosciences (Franklin Lakes, NJ). Antibodies against human α-syn (#ab138501), Tuj-1 (#ab18207) and mCherry (#ab167453) were purchased from Abcam (Cambridge, MA). Antibodies against pCav-1 (#3251S), pSrc (Y416) (#6943), and c-Src (#2123) were purchased from Cell signaling Technology (Danvers, MA). Antibody against GAPDH (#SC-32233) was procured from Santa Cruz Biotechnology (Santa Cruz, CA). Rhodamine-conjugated transferrin (#T23365) and boron-dipyrromethene (BODIPY) FL C5-LacCer (#B34402) were purchased from Molecular Probes (Leiden, The Netherlands). Saracatinib (#11497) was procured from Cayman Chemical (Ann Arbor, MI). SKI-1 (c-Src inhibitor 1, #S2075), retinoic acid (RA) (#r2625), and bafilomycin A1 (#B1793) were obtained from Sigma (St. Louis, MO). Antibodies against Iba-1 (#019-19741) and pSer 129 α-syn (#015-25191) was purchased from Wako (Richmond, VA). Antibody against glial fibrillary acidic protein (GFAP) (#RA22101) was purchased from Neuromics (Montreal, QC). Recombinant α-syn monomers and fibrils were prepared as described previously [24]. The status of fibrils was determined by thioflavin T binding assay.
Immunohistochemistry
C57BL/6 male mice (Orient Bio, Korea) and M83 male mice overexpressing A53T human α-syn under the control of mouse prion protein promoter (B6;C3-Tg(Prnp-SNCA*A53T)83Vle/J, The Jackson Laboratory) at indicated age were perfused, and their brains were fixed with 4% paraformaldehyde for 2 days. Frozen sections were cut at 35 μm in the coronal plane. The sections were stained using the free-floating method, adhering to the slide after staining. For 3,3’-diaminobenzidine (DAB) staining, the samples were twice rinsed with phosphate-buffered saline (PBS) containing 0.2% Triton X-100 (PBST), treated with 3% H2O2 for 5 min, and rinsed with PBST. After blocking non-specific binding by incubating with 1% bovine serum albumin (BSA) in PBST, the sections were incubated overnight at 4℃ with cav-1 antibody. Sections were rinsed three times with PBST, incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) and visualized using VECTASTATIN avidin-biotin complex (ABC kit) and DAB solution (0.05% DAB and 0.003% H2O2 in 0.1 M phosphate buffer). Next, the sections were mounted on slides and images were obtained and digitalized using a Axio scan.Z1 slide scanner (Carl Zeiss, Germany) at the Three-Dimensional Immune System Imaging Core Facility of Ajou University.
Plasmids
Plasmids for WT cav-1 and WT cav-1-EGFP were constructed using PCR. The plasmid for Y14A cav-1 was constructed using the Quick-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA). Each construct was subcloned into pCDH-EF1 for generating lentiviral vectors. All plasmids were verified via DNA sequencing and prepared using the Maxi prep Kit (Qiagen, Valencia, CA).
Cell culture
SH-SY5Y cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS). Primary cortical neurons were cultured from Sprague-Dawley rat embryos or human A53T α-syn overexpressing transgenic (TG) mice at embryonic day 18, maintained in neurobasal medium (Invitrogen, Carlsbad, CA) with L-glutamine and B-27 supplement (Invitrogen, Carlsbad, CA).
Generation of stable cell lines
WT cav-1, Y14A cav-1, WT cav-1-EGFP, and Y14A cav-1-EGFP overexpressing (OE) SH-SY5Y cells were prepared using lentiviral constructs and selected using puromycin, respectively. A53T α-syn-EGFP (A53T-E) and A53T α-syn-mCherry (A53T-M) were prepared as described previously [25]. WT cav-1/A53T-E and Y14A cav-1/A53T-E OE SH-SY5Y cells were prepared using lentiviral transfection of A53T-E in WT cav-1, Y14A cav-1 OE SH-SY5Y cells and selected using FACSAria III, respectively.
Western blot
Cells were washed with PBS and lysed in ice-cold RIPA buffer (50 mM Tris-HCl [pH7.4], 150 mM NaCl, 0.25% sodium deoxycholate and 1% Triton X-100) containing protease inhibitor cocktail (Calbiochem, Germany) and phosphatase inhibitor cocktail (GenDEPOT, Baker, TX). After brief sonication, the lysates were incubated on ice for 20 min and centrifugated at 15,700g for 30 min at 4 ℃. After centrifugation, the supernatant was collected. The protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (Bio-Rad, CA, USA). Proteins were separated by SDS-PAGE and transferred to PVDF membranes. The PVDF membrane was blocked with 1% BSA in Tris-buffered saline. After 1 h blocking, the membrane was immunoblotted with appropriate antibodies and visualized using an enhanced chemiluminescence (ECL) system (Thermo Fisher Scientific, Waltham, MA).
Dual chamber assay
The dual chamber assay was performed as described previously [24, 25]. WT α-syn or A53T-M OE SH-SY5Y cells as donor cells were differentiated by treatment with 50 μM RA for 5 days. Next, SH-SY5Y cells or primary cortical neurons cultured on coverslips in a 12-well plate as recipient cells were cocultured with differentiated WT α-syn or A53T-M OE SH-SY5Y cells cultured on the insert for 24 h. The recipient cells were prepared for staining with anti-α-syn or anti-mCherry antibody. To measure the amount of internalized α-syn, five random fields were selected and intensities of more than 100 cells were analyzed using the MetaMorph software (Molecular Devices).
Coculture assay
The coculture assay was performed as described previously [24, 25]. Briefly, A53T-E, WT cav-1/A53T-E or Y14A cav-1/A53T-E and A53T-M OE SH-SY5Y cells were cocultured in a 1:1 ratio for 5 days in the presence of 50 μM RA. After 5 days of coculture, the cells were then subcultured on coverslips in a 12-well plate. The cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min at room temperature, washed again with PBS, stained with DAPI for 10 min, and washed again with PBS before analysis. Then, the samples were observed under a confocal microscope. To quantify double fluorescence-labeled puncta, five random fields were selected and more than 100 cells containing double fluorescence-labeled puncta were counted manually. The number of A53T-E OE SH-SY5Y cells containing double fluorescence labeled puncta and the number of A53T-M OE SH-SY5Y cells containing double fluorescence-labeled puncta were counted separately and expressed as percentages of total cells analyzed.
Microfluidic chamber assay
Microfluidic chamber assay was performed as described previously with slight modification [26]. Briefly, triple compartment microfluidic devices (TCND1000) were obtained from Xona Microfluidic, LLC (Temecula, CA, USA). Glass coverslips were prepared and affixed to the microfluidic device according to the manufacturers’ instructions. Approximately 100,000 A53T TG cortical neurons (TP18) were plated per chamber. At 7 DIV, 0.5 μg of α-syn fibrils were added into chamber (C) 1, with lentiviruses for EGFP-only, WT cav-1-EGFP or Y14A cav-1-EGFP expression added into C2. To control the direction of flow, a 50 μl difference in media volume was maintained between C1 and C2, and C2 and C3, according to the manufacturers’ instructions. Neurons were fixed at 7 days after treatment with α-syn fibrils using 4% paraformaldehyde in PBS. Devices were then removed, and immunofluorescence assay was performed.
Confocal microscopy
Cells cultured on poly-D-lysine coated coverslips were washed twice with PBS and fixed in 4% paraformaldehyde for 10 min at room temperature. The fixed cells were then washed with PBS and incubated with PBS containing 0.1% Triton X-100 for 10 min at room temperature. After washing several times with PBS, cells were blocked with PBS containing 1% BSA for 1 h at room temperature, and then incubated overnight with indicated antibodies at 4 °C. The samples were then stained with fluorescence conjugated secondary antibodies for 1 h, mounted with VECTASHIELD (Vector Laboratories, Burlingame, CA), and observed under a confocal microscope (Zeiss, Germany).
Statistical analysis
All values are expressed as the mean ± SEM. Statistical significance was evaluated using Graphpad Prism (La Jolla, CA).
Results
The level of cav-1 expression increases in the brain with age
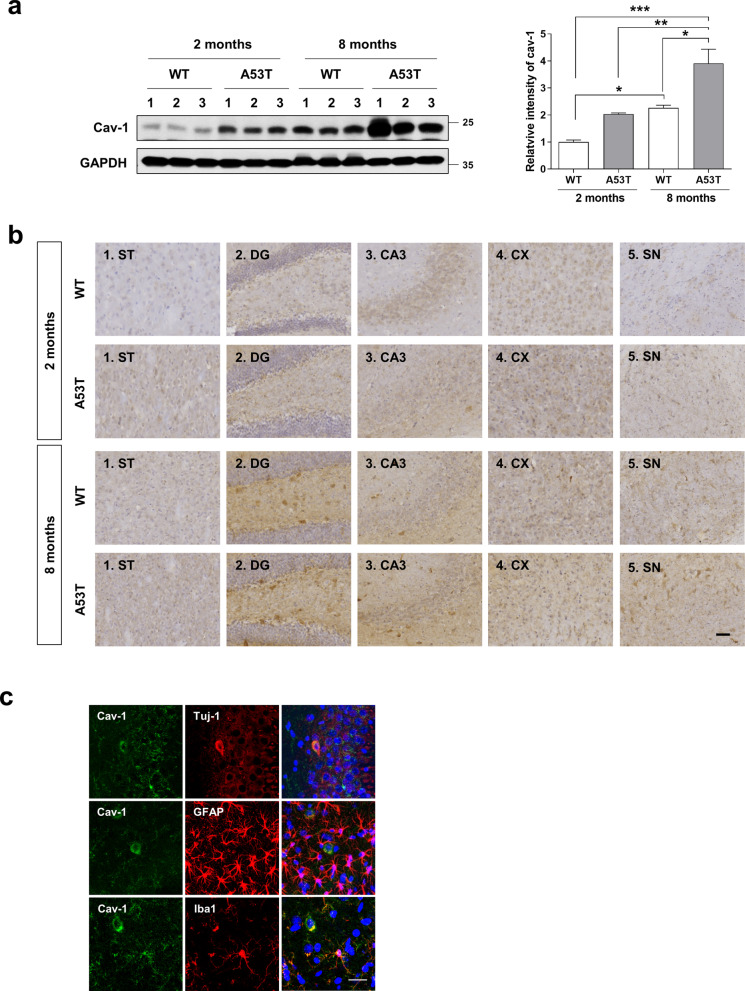
As cav-1 expression is reportedly related to aging [17, 19, 20], we first explored the age-related increase in cav-1 protein expression in mice. We observed that cav-1 protein expression was increased in whole brain lysates of mice in an age-dependent manner (Fig. 1a), consistent with a previous study [17]. Immunohistochemical analysis showed that cav-1 protein was widely expressed in the brain. Especially, cav-1 protein was predominantly expressed in the hippocampus when compared with the cortex, striatum, substantia nigra. Furthermore, the expression of cav-1 protein was increased with age (Fig. 1b). Additionally, we compared cav-1 protein expression between wild-type (WT) and A53T α-syn transgenic (TG) mice, a PD model demonstrating an improved simulation of PD progression as observed in patients [27]. Compared with WT mice at both ages, cav-1 protein was higher in A53T α-syn TG mice (Fig. 1a–b). In addition, cav-1 protein expression was increased mainly in neurons and microglia, but not astrocytes (Fig. 1c). These results suggest that cav-1 protein expression was increased in the brain with age, and A53T α-syn overexpression facilitated cav-1 protein expression in the brain.
Fig. 1.
Cav-1 expression increases in the brain with age. a Brain hemispheres obtained from WT and A53T transgenic (TG) mice at the age of 2 and 8 months were lysed and Western blot was performed with indicated antibodies. Values were derived from three individual mice (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA. b Immunohistochemistry for cav-1 at 2 months or 8 months was performed in WT or A53T TG mice. Images were derived from more than three individual mice. Scale bar indicates 50 μm. ST, triatum; DG, dentate gyrus; CX, cortex; SN, substantia nigra. c Brain tissues containing the hippocampus from A53T TG mice at the age of 8 months were immunostained with indicated antibodies and observed under a confocal microscope. Scale bar indicates 20 μm.
The uptake of ɑ-syn is increased in cav-1 overexpressing SH-SY5Y cells and rat primary cortical neurons
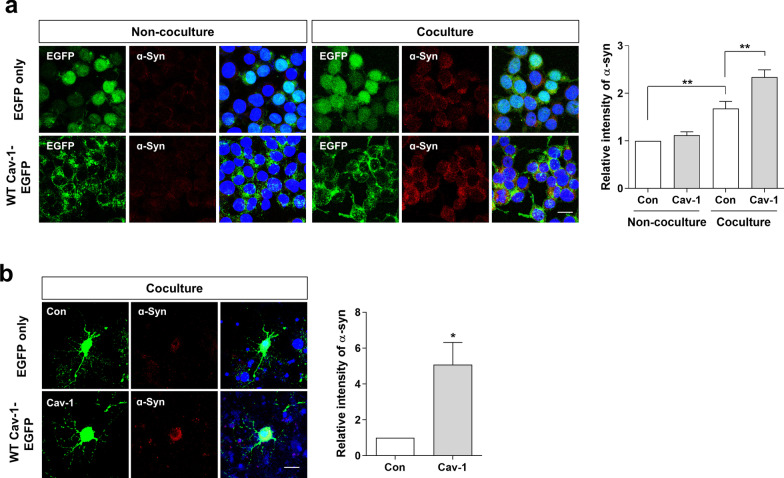
Next, we investigated the effect of cav-1 expression on the uptake of α-syn using a dual chamber system, which efficiently monitors cell-derived α-syn uptake into cells [24, 25]. We generated EGFP only or WT cav-1-EGFP overexpressing (OE) SH-SY5Y stable cell lines, EGFP only and WT cav-1-EGFP were confirmed to be well-expressed (Additional file 1: Fig. S1a–b). As shown in Fig. 2a, α-syn was rarely detected in SH-SY5Y cells overexpressing EGFP only or WT cav-1-EGFP without coculture, whereas α-syn was detected in both cells by coculturing with α-syn OE SH-SY5Y cells. Moreover, α-syn levels were higher in SH-SY5Y cells overexpressing WT cav-1-EGFP than in SH-SY5Y cells overexpressing EGFP only. Western blot further confirmed it (Additional file 1: Fig. S1c). Rat primary cortical neurons overexpressing cav-1 revealed similar results (Fig. 2b), suggesting that the uptake of cell-released α-syn was increased following cav-1 overexpression.
Fig. 2.
The uptake of α-syn increases in cav-1 overexpressing SH-SY5Y cells and rat primary cortical neurons. a EGFP only (Con) and WT cav-1-EGFP OE SH-SY5Y cells (Cav-1) were cocultured with differentiated α-syn OE SH-SY5Y cells cultured on the insert for 24 h. The samples were fixed and immunostained with α-syn antibody (BD) and observed under a confocal microscope. The intensity of five independent experiments was analyzed. b Rat primary cortical neurons were transfected with EGFP and WT cav-1-EGFP plasmids. After 1 day of transfection, the cells were cocultured with differentiated α-syn OE SH-SY5Y cells cultured on the insert for 24 h. The samples were fixed and immunostained with human α-syn antibody (Abcam) and observed under a confocal microscope. The intensity of four independent experiments was analyzed. Blue indicates DAPI staining. Scale bars indicate 20 μm. *p < 0.05, **p < 0.01, one-way ANOVA (a), unpaired t-test (b)
Phosphorylation of cav-1 at tyrosine 14 is important to regulate the uptake of ɑ-syn
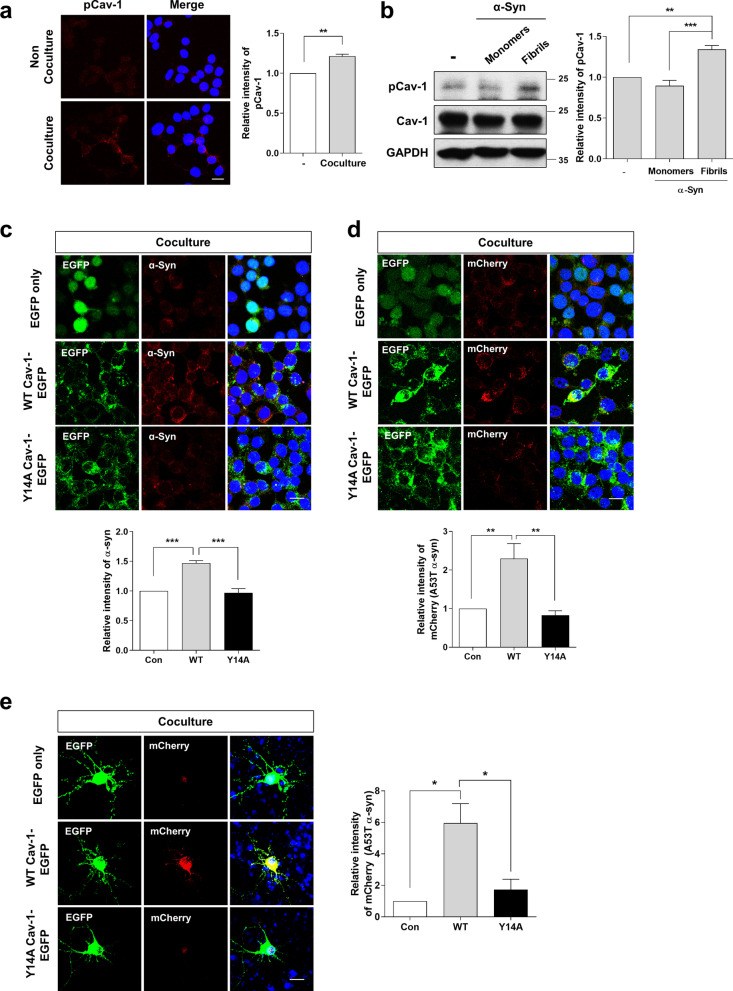
Cav-1 is a phosphoprotein, and the tyrosine 14 residue of cav-1 is considered the principal site for tyrosine phosphorylation [28–30]. We investigated whether the phosphorylation of cav-1 is involved in the uptake of α-syn. pCav-1 was increased in WT cav-1 OE SH-SY5Y cells by coculturing with α-syn OE SH-SY5Y cells (Fig. 3a). Variable forms of α-syn are released from cells [31–33]. Y14 of cav-1 was phosphorylated following treatment with α-syn fibrils, but not with monomeric α-syn (Fig. 3b), suggesting that α-syn fibrils induced the phosphorylation of cav-1. Next, we generated a cav-1 mutant stable cell line overexpressing Y14A cav-1-EGFP, which was confirmed by Western blot against the phosphorylation of cav-1 (Additional file 1: Fig. S2a). There was also no difference in intracellular localization between WT and Y14A cav-1 (Additional file 1: Fig. S1b). α-Syn uptake was increased in SH-SY5Y cells overexpressing WT cav-1-EGFP, but not in cells overexpressing Y14A cav-1-EGFP (Fig. 3c). On coculturing these cells with A53T α-syn-mCherry (A53T-M) OE SH-SY5Y cells using a dual chamber and staining with anti-mCherry antibody to rule out the possibility of detecting small amount of α-syn expressed endogenously, we observed similar results (Fig. 3d). Moreover, while the uptake of A53T-M was increased in primary neurons overexpressing WT cav-1-EGFP, this was not observed in cells overexpressing Y14A cav-1-EGFP (Fig. 3e). These results suggested that the phosphorylation of cav-1 at the tyrosine 14 residue is important for α-syn uptake.
Fig. 3.
Phosphorylation of cav-1 at tyrosine 14 is important to regulate the uptake of α-syn. a WT cav-1 OE SH-SY5Y cells were cocultured with differentiated α-syn OE SH-SY5Y cells cultured on the insert for 24 h. The samples were fixed and immunostained with pCav-1 antibody and observed under a confocal microscope. The intensity of three independent experiments was analyzed. b WT cav-1 OE SH-SY5Y cells were incubated in the presence of 1 μM α-syn monomers or α-syn fibrils (n = 5) for 10 min. The cells were then lysed, and Western blot was performed with the indicated antibodies. The band intensity of four independent experiments was analyzed. c Each stable cell line expressing EGFP only (Con), WT cav-1-EGFP (WT) and Y14A cav-1-EGFP (Y14A) was cocultured with differentiated α-syn OE SH-SY5Y cells for 5 days using a dual-chamber system (n = 5). After 24 h of coculture, the samples were stained with α-syn antibody (BD). d Each stable cell line was cocultured with differentiated A53T α-syn-mCherry OE SH-SY5Y cells (A53T-M) using the dual-chamber system (n = 4). After 24 h of coculture, the samples were stained with mCherry antibody. e Rat primary cortical neurons were transfected with EGFP, WT cav-1-EGFP and Y14A cav-1-EGFP plasmids. After one day of transfection, neurons were cocultured with A53T-M OE SH-SY5Y cells for 24 h (n = 3). The cells were stained with mCherry antibody. All images are observed under a confocal microscope. Blue indicates DAPI staining. Scale bars indicate 20 μm. The intensity of independent experiments was analyzed. *p < 0.05, **p < 0.01, ***p < 0.001, unpaired t-test (a), one-way ANOVA (b–e)
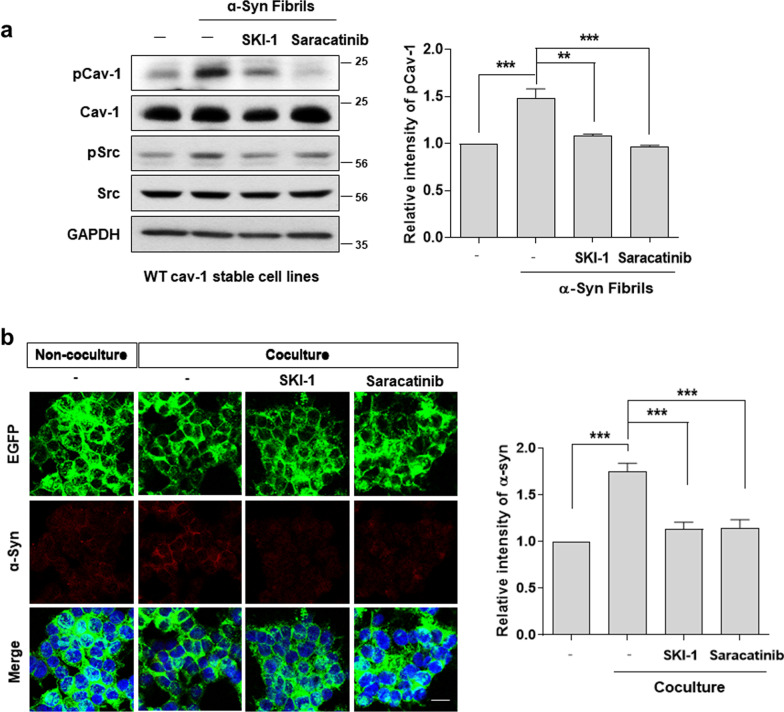
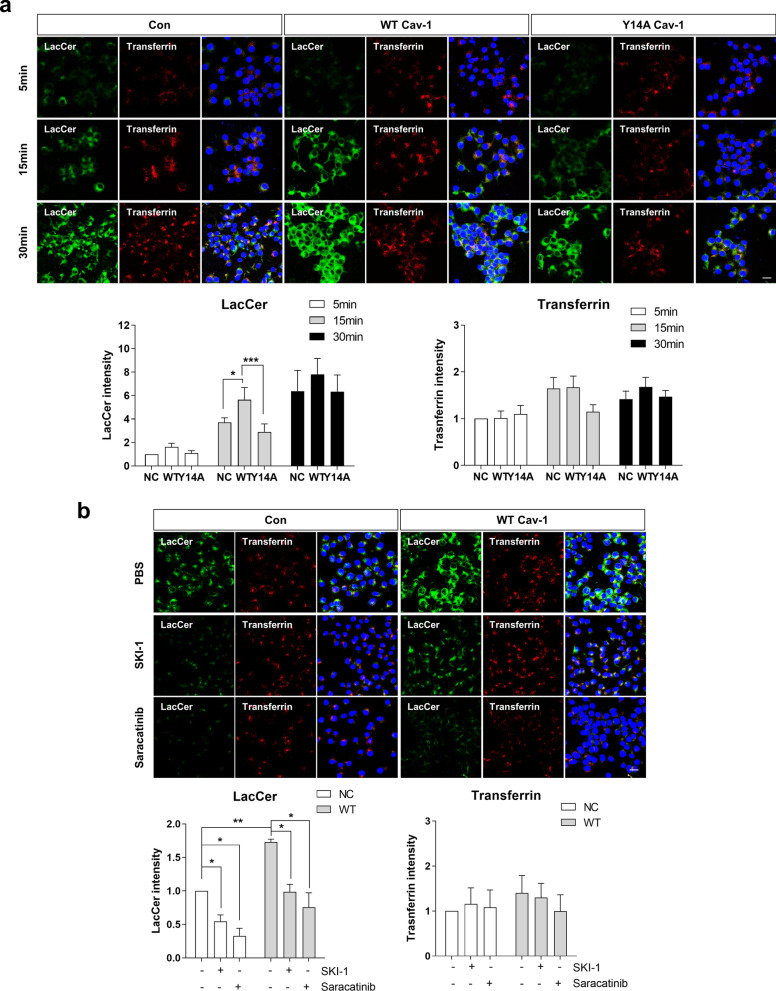
Reportedly, Y14 of cav-1 is mainly phosphorylated by Src tyrosine kinases [28–30]. To confirm the importance of cav-1 phosphorylation in α-syn uptake, we utilized the Src kinase inhibitor, SKI-1 (c-Src inhibitor 1), and saracatinib (c-Src/Abl dual kinase inhibitor). The increased Y14 phosphorylation of cav-1 by α-syn fibrils was significantly reduced by SKI-1 and saracatinib (Fig. 4a). Furthermore, the uptake of cell-derived α-syn into SH-SY5Y cells overexpressing WT cav-1 was attenuated following treatment with SKI-1 or saracatinib (Fig. 4b), suggesting that cav-1 was phosphorylated by Src kinases and the inhibition of c-Src activity attenuated α-syn uptake.
Fig. 4.
The phosphorylation of cav-1 is increased by α-syn fibrils and decreased by c-Src inhibitors. a WT cav-1 stable cell line was treated with 1μM α-syn fibrils for 10 min. Before treatment with α-syn fibrils, 10 μM SKI-1 or 0.1 μM saracatinib were pretreated for 30 min. Each lysate was then analyzed using Western blot. The band intensity of three independent experiments was quantified. b The WT cav-1-EGFP cell line was cocultured with α-syn OE SH-SY5Y cell using the dual-chamber system for 24 h in the presence of 10 μM SKI-1 or 0.1 μM saracatinib. The cells were stained with α-syn antibody (BD) and observed using confocal microscopy. Blue indicates DAPI staining. Scale bar indicates 20 μm. The intensity values are obtained from five independent experiments. **p < 0.01, ***p < 0.001, one-way ANOVA (a–b)
The phosphorylation of cav-1 at tyrosine 14 residue regulates lipid rafts dependent endocytosis.
It has been reported that cav-1 stimulates lipid rafts-dependent endocytosis [34]. Accordingly, to investigate whether the Y14 phosphorylation of cav-1 regulates endocytosis, we performed an in vitro endocytosis assay using LacCer as a marker of lipid rafts-dependent endocytosis [35] and transferrin as a marker of clathrin-dependent endocytosis [36]. As shown in Fig. 5a, LacCer uptake was transiently accelerated in WT cav-1 OE SH-SY5Y cells, compared with control cells, but not in Y14A cav-1 OE SH-SY5Y cells. In contrast, the level of transferrin uptake did not differ among cells. LacCer uptake was also decreased in the presence of c-src inhibitors (Fig. 5b), suggesting that cav-1 regulates lipid rafts-dependent endocytosis via cav-1 phosphorylation at tyrosine 14.
Fig. 5.
The phosphorylation of cav-1 regulates lipid rafts dependent endocytosis. a Each cell line was incubated with 50 nM BODIPY FL C5-Lactosylceramide (LacCer) (Green) and 2.5 μg/ml rhodamine-conjugated transferrin (Red) for 5, 15 and 30 min. b Each cell line was pretreated with 10 μM SKI-1 or 0.1 μM saracatinib for 30 min and further incubated with 50 nM BODIPY FL C5-Lactosylceramide (LacCer) (Green) and 2.5 μg/ml rhodamine-conjugated transferrin (Red) for 20 min. Next, the cells were fixed and observed via confocal microscopy. The intensity of five (a) or four (b) independent experiments were quantified. Blue indicates DAPI staining. Scale bars indicate 20 μm. *p < 0.05, ***p < 0.001, two-way ANOVA
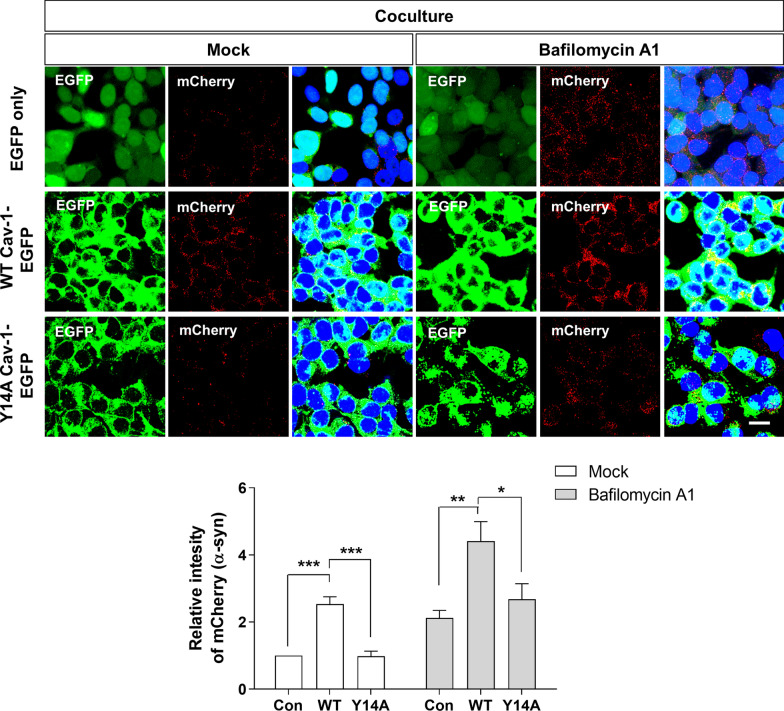
Cav-1 induces the uptake of ɑ-syn regardless of the degradation pathway
To confirm whether the increased levels of transferred α-syn in cav-1 OE cells could be attributed to the enhanced uptake of transferred α-syn into cells, or limited degradation of transferred α-syn in recipient cells via defects in the protein degradation system such as autophagy, a dual chamber assay was performed in the presence of bafilomycin A1, a well-known autophagy inhibitor. Despite the presence of bafilomycin A1, higher amounts of transferred α-syn were detected in WT cav-1-EGFP OE SH-SY5Y cells, when compared with control or Y14A cav-1-EGFP OE SH-SY5Y cells, although the relative amounts of detected transferred α-syn were increased (Fig. 6). This suggested that the higher amounts of transferred α-syn, detected in WT cav-1 OE SH-SY5Y cells, could be attributed to enhanced α-syn uptake, but not an impaired degradation system.
Fig. 6.
Cav-1 induces the uptake of α-syn regardless of the degradation pathway. Each cell line was cocultured with differentiated A53T-M OE SH-SY5Y cells in the absence or presence of 50 nM bafilomycin A1 using the dual-chamber system. After 24 h of coculture, the samples were stained with mCherry antibody. Scale bar indicates 20 μm. Blue indicates DAPI staining. The intensity was analyzed from five independent experiments. * p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA
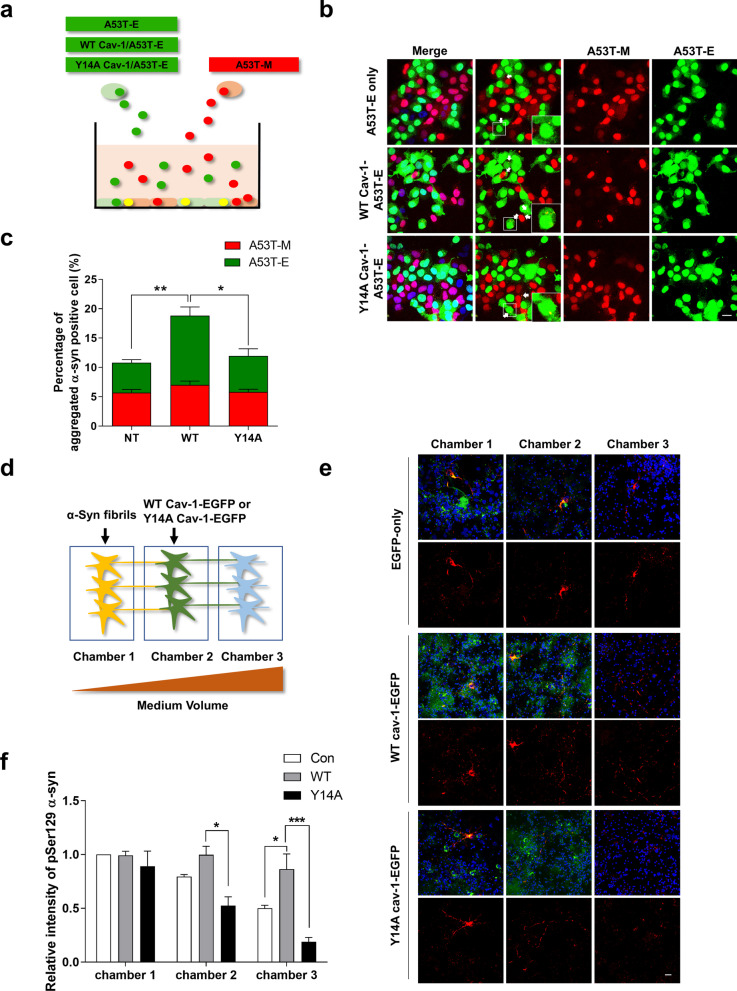
Formation of Lewy body-like inclusions is increased by WT cav-1 overexpression and attenuated by Y14A cav-1 overexpression
In a previous study, we developed an in vitro model system to explore the inclusion body formation by the cell-to-cell transmission of α-syn. In this model system, double fluorescence-labeled aggregation puncta are colocalized with pSer129 α-syn, which is abundantly found in Lewy body [37], suggesting that double fluorescence-labeled aggregation puncta are Lewy body-like inclusions [25]. To determine whether cav-1 expression regulates the inclusion body formation through the cell-to-cell transmission of α-syn, we generated stable cell lines overexpressing WT cav-1 or Y14A cav-1 with A53T α-syn-EGFP (A53T-E). These cells expressed A53T α-syn-EGFP similarly (Additional file 1: Fig. S2b) and were cocultured with A53T-M OE SH-SY5Y cells for 5 days in the presence of RA to induce differentiation (Fig. 7a). After 5 days of coculture, we counted cells containing double fluorescence-labeled aggregation puncta (yellow puncta) that were generated by the aggregation of both α-syn transferred from neighboring cells and expressed α-syn inside cells. The number of cells containing yellow puncta was increased in A53T-E OE SH-SY5Y cells overexpressing WT cav-1, but not in A53T-M OE SH-SY5Y cells. In contrast, the number of cells containing yellow puncta did not increase in A53T-E OE SH-SY5Y cells overexpressing Y14A cav-1 (Fig. 7b–c). Additionally, for further validation, we used microfluidic culture devices developed to monitor the cell-to-cell transmission of α-syn in primary neurons (Fig. 7d) [26]. Following addition of α-syn fibrils to Chamber 1 (C1) neurons with further incubation for 7 days, α-syn pathology were detected in C2 and C3 neurons. Next, we overexpressed WT cav-1 in C2 neurons. The addition of α-syn fibrils to C1 induced a higher α-syn pathology in C2 and C3 neurons, but not through Y14A cav-1 overexpression (Fig. 7e–f), suggesting that cav-1 is involved in the formation of Lewy body-like inclusions via the uptake of α-syn and those are regulated by Y14A phosphorylation of cav-1.
Fig. 7.
Cav-1 mediates Lewy body-like inclusion body formation. a Schematic diagram showing coculture assay. b–c A53T-E, WT cav-1-A53T-E and Y14A cav-1-A53T-E OE SH-SY5Y cells were cocultured with A53T-M OE SH-SY5Y cells for 5 days in the presence of 50 μM RA. Next, the samples were observed under a confocal microscope, and the number of cells containing double fluorescence-labeled puncta was analyzed. Arrows indicate double fluorescence-labeled puncta. In the graphs, green indicates the number of A53T-E cells, containing double fluorescence-labeled puncta, and red indicates the number of A53T-M cells, containing double fluorescence-labeled puncta. The values obtained are from five independent experiments. d Schematic diagram showing the microfluidic chamber assay. e Primary neurons from A53T TG mice were cultured in microfluidic chambers. At 7 DIV, 0.5 μg of α-syn fibrils were added into chamber 1, and lentiviruses of EGFP-only, WT cav-1-EGFP or Y14A cav-1-EGFP were added into chamber 2. Neurons were fixed at 7 days after treatment with α-syn fibrils. The devices were then removed. The cells were immunostained with pSer129 α-syn antibody. f The intensity was analyzed. Values were derived from four independent experiments. *P < 0.05, **p < 0.01, ***P < 0.001, one-way ANOVA. Blue indicates DAPI staining. Scale bars indicate 20 μm
Discussion
PD is a disabling neurodegenerative disorder that is strongly associated with aging [38, 39]. At the cellular level, postmortem tissues from sporadic patients with PD displayed an increased expression of senescent markers, including p16INK4a, and several senescence-associated secretory phenotype (SASP) factors, matrix metalloproteinase-3 (MMP-3), interleukin (IL)-6, IL-1a, and IL-8 [40]. Additionally, elevated p16INK4a and MMP-3 expression have been reported in cortical tissues of patients with AD [41], suggesting that the basic mechanisms of aging may be closely related to the pathogenesis of neurogenerative diseases such as AD and PD.
Reportedly, cav-1 expression has been associated with aging. Several previous studies using in vitro senescent cells [19] and in vivo aged tissues [17, 18] have suggested that cav-1 expression is increased with aging. Additionally, cav-1 overexpression displays senescence phenotypes [21, 42, 43], suggesting that increased cav-1 expression may play an important role in aging at the cellular level, although there have been conflicting observations that cav-1 knockout accelerates premature senescence in MEFs [44] and loss of cav-1 accelerates neurodegeneration and aging [45].
In the present study, we observed that cav-1 expression was increased in the brain in an aging-dependent manner, which is consistent with previous studies [17, 18]. Moreover, cav-1 expression was considerably elevated in the brain of A53T TG mice. In patients with PD, homozygous haplotypes have been observed upstream of human cav-1, which induce increased gene expression [46]. In vitro, overexpression of α-syn upregulates cav-1 expression [47, 48], supporting our data that demonstrates the association of elevated cav-1 expression with PD.
Accumulating evidence suggests that the mechanism of pathogenic α-syn spreading throughout the nervous system underlies the pathogenesis of PD [49], which remains poorly understood. We observed that cav-1 overexpression in neurons accelerated α-syn uptake into neurons and inclusion body formation via the cell-to-cell transmission of α-syn. It is well known that cav-1 regulates caveolae-dependent endocytosis [50]. Classical caveolae-mediated endocytosis may not occur in neurons as neurons lack caveolae [51, 52]. Nevertheless, a previous study has suggested that cav-1 also demonstrates caveolae-independent functions including trafficking of proteins to and from the plasma membrane [53]. Furthermore, α-syn is internalized into cells via various mechanisms in a species-dependent manner [54–56]. Accordingly, neuronal cav-1 may be involved in α-syn uptake via caveolin-dependent endocytosis. Our hypothesis was supported by the observation that cav-1 overexpression accelerates lipid rafts-dependent endocytosis, but not clathrin-dependent endocytosis. Interestingly, exogenously added α-syn fibrils induce lipid rafts-dependent endocytosis [25], with numerous PD-associated gene products, including parkin, DJ-1, and UCH-L1 also regulating lipid rafts-dependent endocytosis [22, 57, 58], indicating that the dysfunction of lipid rafts-dependent endocytosis may be associated with the pathogenesis of PD as a common pathological mechanism.
The activity of the degradation pathways, both autophagy and proteasome-mediated, are reduced during aging [59, 60]. Reportedly, cav-1 overexpression prevents autophagy in human osteosarcoma cells [61] and cav-1 deletion increases basal autophagy in the vascular endothelium [62]. Considering that α-syn is degraded by autophagy [63–65], we further investigated whether our finding that increased α-syn uptake into cav-1 OE cells resulted from enhanced cellular uptake of transferred α-syn or limited degradation of transferred α-syn following bafilomycin A1 treatment. Relative amounts of transferred α-syn detected in recipient cells were increased in the presence of bafilomycin A1. Inhibition of α-syn degradation induces the accumulation of α-syn, further releasing α-syn into the extracellular space via different secretory pathways [66–68]. Accordingly, α-syn accumulation could be attributed to increased α-syn release by inhibiting the autophagic degradation of α-syn in donor cells, and/or increased accumulation of α-syn in recipient cells. Nevertheless, α-syn was highly detected in cav-1 OE cells, suggesting that cav-1 overexpression enhanced the uptake of transferred α-syn into cells, although we were unable to comprehensively elucidate whether cav-1 overexpression affected the autophagy system to degrade transferred α-syn in our experimental condition.
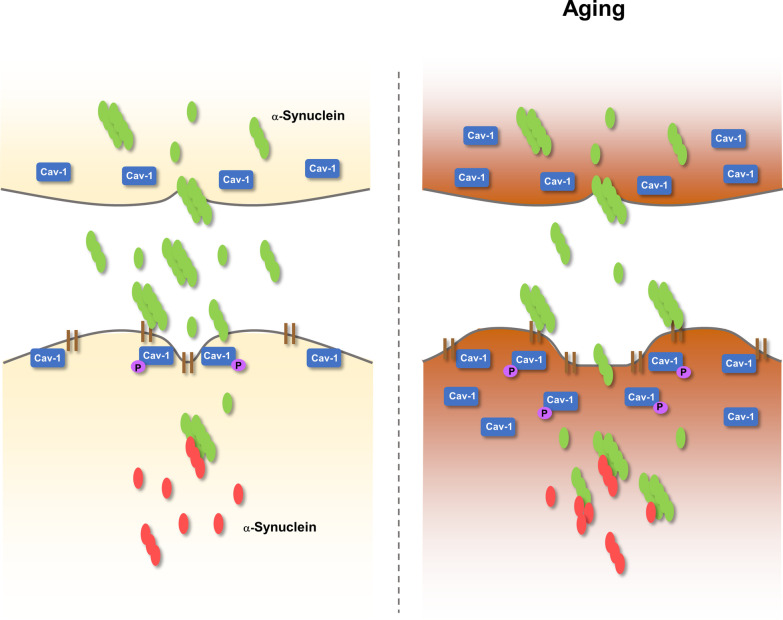
Given that cav-1 expression increases with age [17, 18] and cav-1 overexpression induces cellular senescence [21, 42, 43], and our observation that cav-1 overexpression accelerated α-syn uptake into neurons and inclusion body formation, α-syn propagation may be further accelerated in aging individuals (Fig. 8), which is consistent with a previous study revealing that aging-promoting genetic variations accelerate the rate of cell-to-cell transmission of α-syn aggregates in a C. elegans model [69].
Fig. 8.
Proposed model of the association of cav-1 in the uptake of α-syn. Cav-1 expression increases in the brain with age. Increased cav-1 expression facilitates the uptake of extracellular α-syn, as well as additional Lewy body-like inclusion formation with expressed α-syn inside cells. The phosphorylation of cav-1 is involved in the uptake of α-syn
The phosphorylation of cav-1 at the tyrosine 14 residue is related to macromolecular transcytosis as a specific form of the scaffold to recruit and organize multiple molecular components of the transcytotic machinery [70, 71]. Src-mediated phosphorylation of caveolin-1 Tyr-14 is necessary for caveolar endocytosis of EGFR under oxidative stress [72]. Accordingly, tyrosine 14 phosphorylation of cav-1 may regulate endocytosis in neurons. Furthermore, we observed that Y14A cav-1 overexpression failed to accelerate α-syn uptake into neurons, as well as inclusion body formation. Y14A cav-1 overexpression also failed to enhance LacCer uptake as a representative molecule for lipid rafts-dependent endocytosis, suggesting that the phosphorylation of cav-1 at the tyrosine 14 residue in neurons plays an important role in cell-to-cell transmission of α-syn by regulating lipid rafts-dependent endocytosis.
As cav-1 was first identified as a major phosphorylated protein in v-Src-expressing cells [73], it has been well documented that the tyrosine 14 residue of cav-1 is phosphorylated by Src kinases [29, 30, 74]. We observed that the phosphorylation of cav-1 at tyrosine 14 was induced by α-syn fibrils, but not by α-syn monomers. Moreover, the phosphorylation of cav-1 at tyrosine 14 induced by α-syn fibrils was dependent on c-Src kinase activity. Additionally, the inhibition of cav-1 phosphorylation by regulating c-Src activity attenuated the accelerated α-syn uptake by cav-1 overexpression. Previously, we have demonstrated that α-syn fibrils bind to FcγRIIB expressed in neurons and stimulate the cell-to-cell transmission of α-syn via SHP-1/-2 as a downstream mediator of FcγRIIB signaling [25]. Furthermore, the activation of SHP-1/-2 by α-syn fibrils stimulates c-Src phosphorylation, accelerating the cell-to-cell transmission of α-syn [24]. Accordingly, cav-1 may function as a downstream mediator of FcγRIIB-SHP-1/-2-c-Src for α-syn uptake.
Inhibiting cav-1 upregulation with selective cyclooxygenase (COX)-2 inhibitors attenuates the development of cellular senescence in human dermal fibroblasts [75]. Furthermore, an inhibitor of phosphatidylcholine-specific phospholipase C reduces the upregulation of cav-1 expression, as well as the number of replicative senescent bone marrow stromal cells [76]. Daidzein, known to inhibit cav-1 expression, restores memory deficits in an intracerebroventricular streptozotocin (ICV-STZ)-induced neurodegeneration rat model [77]. Additionally, our data support the postulation that inhibiting the upregulation of cav-1 expression or cav-1 phosphorylation can attenuate the progression of PD, as well as the aging process. In agreement with our hypothesis, reduced cav-1 expression reportedly extended lifespan and mitigated toxic protein aggregation by modulating the expression of age regulating and signaling-promoting genes [78].
Conclusions
We demonstrated that cav-1 expression was increased in the brain with age. Cav-1 overexpression in neurons increased cell-to-cell transmission of α-syn. Inhibiting cav-1 phosphorylation attenuated the cell-to-cell transmission of α-syn, further suppressing Lewy body-like inclusion body formation. This study will help elucidate the molecular mechanism of intercellular α-syn transmission, as well as to develop new therapeutics against PD.
Supplementary Information
Additional file 1: Figure S1. Protein expression of stable cell line and the uptake of α-syn in cav-1 overexpressing SH-SY5Y cells. a EGFP only, WT cav-1-EGFP and Y14A cav-1-EGFP OE SH-SY5Y cells were lysed, and Western blot was performed with the indicated antibodies. b EGFP only, WT cav-1-EGFP and Y14A cav-1-EGFP OE SH-SY5Y cells were observed under confocal microscopy. c EGFP only and WT cav-1-EGFP OE SH-SY5Y cells were cocultured with differentiated α-syn OE SH-SY5Y cells cultured on the insert for 24 h. The cells were then lysed, and Western blot was performed with the indicated antibodies. The intensity of four independent experiments was analyzed. ** p < 0.01, unpaired t-test. Figure S2. Protein expression of stable cell line. a EGFP only, WT cav-1-EGFP or Y14A cav-1-EGFP OE SH-SY5Y cells were treated with 1 μM α-Syn fibrils for 10 min. The cells were then lysed, and Western blot was performed with the indicated antibodies. b A53T-E only, WT cav-1/A53T-E and Y14A cav-1/A53T-E OE SH-SY5Y cells were lysed, and Western blot was performed with the indicated antibodies.
Acknowledgements
Not applicable.
Abbreviations
- PD
Parkinson's disease
- AD
Alzheimer's disease
- cav-1
Caveolin-1
- α-syn
α-synuclein
- MEFs
Murine embryonic fibroblasts
- KO
Knock-out
- DAB
3,3’-diaminobenzidine
- PBS
Phosphate-buffered saline
- PBST
PBS containing 0.2% Triton X-100
- BSA
Bovine serum albumin
- ABC
Avidin-biotin complex
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Fetal bovine serum
- A53T-E
A53T α-syn-EGFP
- A53T-M
A53T α-syn-mCherry
- OE
Overexpressing
- BCA
Bicinchoninic acid
- ECL
Chemiluminescence
- C
Chamber
- TG
Transgenic
- SASP
Senescence-associated secretory phenotype
- MMP-3
Matrix metalloproteinase-3
- IL
Interleukin
- COX
Cyclooxygenase
- ICV-STZ
Intracerebroventricular streptozotocin
- LacCer
BODIPY FL C5-Lactosylceramide
- DIV
Days in vitro
Authors' contributions
T-YH conducted overall experiments and participated in writing the manuscript. YRC carried out a microfluidic chamber assay. HRN assisted in western blot. S-HC conducted a preliminary study. J-BK carried out the making of lentiviral vectors. T-YH and SMP designed research studies and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (Ministry of Science and ICT) (Grant No. NRF-2017R1E1A1A01073713 to SMP, NRF-2019R1A5A2026045 to SMP, NRF-2019R1I1A1A01063711 to T-YH).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All experimental procedures used in this study followed national guidelines. All animal procedures were conducted according to the guidelines established by the Ajou University School of Medicine Ethics Review Committee (IACUC No. 2016-0047).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 3.Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 4.Chartier-Harlin MC, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–9. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 5.Duyckaerts C, Clavaguera F, Potier MC. The prion-like propagation hypothesis in Alzheimer's and Parkinson's disease. Curr Opin Neurol. 2019;32(2):266–271. doi: 10.1097/WCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 6.Vaquer-Alicea J, Diamond MI. Propagation of protein aggregation in neurodegenerative diseases. Annu Rev Biochem. 2019;88:785–810. doi: 10.1146/annurev-biochem-061516-045049. [DOI] [PubMed] [Google Scholar]
- 7.Karimi-Moghadam A, et al. Parkinson disease from mendelian forms to genetic susceptibility: new molecular insights into the neurodegeneration process. Cell Mol Neurobiol. 2018;38(6):1153–1178. doi: 10.1007/s10571-018-0587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie F, et al. Advances in the research of risk factors and prodromal biomarkers of Parkinson's Disease. ACS Chem Neurosci. 2019;10(2):973–990. doi: 10.1021/acschemneuro.8b00520. [DOI] [PubMed] [Google Scholar]
- 9.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell Mol Life Sci. 2010;67(22):3785–95. doi: 10.1007/s00018-010-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277(44):41295–8. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 12.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27(37):9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilderback TR, et al. Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways. J Biol Chem. 1999;274(1):257–63. doi: 10.1074/jbc.274.1.257. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, et al. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol. 2004;167(6):1205–15. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatnagar A, et al. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279(33):34614–23. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- 16.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29(11):3590–602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park WY, et al. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275(27):20847–52. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- 18.Kang MJ, et al. Caveolin-1 upregulation in senescent neurons alters amyloid precursor protein processing. Exp Mol Med. 2006;38(2):126–33. doi: 10.1038/emm.2006.16. [DOI] [PubMed] [Google Scholar]
- 19.Wheaton K, et al. Loss of functional caveolae during senescence of human fibroblasts. J Cell Physiol. 2001;187(2):226–35. doi: 10.1002/jcp.1071. [DOI] [PubMed] [Google Scholar]
- 20.Lee JA, et al. Methyl-beta-cyclodextrin up-regulates collagen I expression in chronologically-aged skin via its anti-caveolin-1 activity. Oncotarget. 2015;6(4):1942–53. doi: 10.18632/oncotarget.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volonte D, et al. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13(7):2502–17. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha SH, et al. Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: implications for Parkinson's disease. Mol Neurodegener. 2015;10:63. doi: 10.1186/s13024-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott WK, et al. Complete genomic screen in Parkinson disease: evidence for multiple genes. Jama. 2001;286(18):2239–44. doi: 10.1001/jama.286.18.2239. [DOI] [PubMed] [Google Scholar]
- 24.Choi YR, et al. The dual role of c-src in cell-to-cell transmission of alpha-synuclein. EMBO Rep. 2020;21(7):e48950. doi: 10.15252/embr.202050843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi YR, et al. Prion-like propagation of alpha-synuclein is regulated by the FcgammaRIIB-SHP-1/2 signaling pathway in neurons. Cell Rep. 2018;22(1):136–148. doi: 10.1016/j.celrep.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Tran HT, et al. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 2014;7(6):2054–65. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giasson BI, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34(4):521–33. doi: 10.1016/S0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271(7):3863–8. doi: 10.1074/jbc.271.7.3863. [DOI] [PubMed] [Google Scholar]
- 29.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277(11):8771–4. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinetti AR, Mastick CC. c-Abl is required for oxidative stress-induced phosphorylation of caveolin-1 on tyrosine 14. Cell Signal. 2003;15(3):289–98. doi: 10.1016/S0898-6568(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25(25):6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emmanouilidou E, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–51. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ejlerskov P, et al. Tubulin polymerization-promoting protein (TPPP/p25alpha) promotes unconventional secretion of alpha-synuclein through exophagy by impairing autophagosome-lysosome fusion. J Biol Chem. 2013;288(24):17313–35. doi: 10.1074/jbc.M112.401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh RD, et al. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14(8):3254–65. doi: 10.1091/mbc.e02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks DL, et al. Use of fluorescent sphingolipid analogs to study lipid transport along the endocytic pathway. Methods. 2005;36(2):186–95. doi: 10.1016/j.ymeth.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55(11):1439–66. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese V, et al. Aging and Parkinson's disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. 2018;115:80–91. doi: 10.1016/j.freeradbiomed.2017.10.379. [DOI] [PubMed] [Google Scholar]
- 39.Hou Y, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 40.Chinta SJ, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson's disease. Cell Rep. 2018;22(4):930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhat R, et al. Astrocyte senescence as a component of Alzheimer's disease. PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volonte D, et al. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6) J Biol Chem. 2015;290(7):4202–14. doi: 10.1074/jbc.M114.598268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69(7):2878–86. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu DM, et al. Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell. 2017;16(4):773–784. doi: 10.1111/acel.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Head BP, et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 2010;5(12):e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darvish H, et al. Biased homozygous haplotypes across the human caveolin 1 upstream purine complex in Parkinson's disease. J Mol Neurosci. 2013;51(2):389–93. doi: 10.1007/s12031-013-0021-9. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto M, et al. Alpha-synuclein up-regulates expression of caveolin-1 and down-regulates extracellular signal-regulated kinase activity in B103 neuroblastoma cells: role in the pathogenesis of Parkinson's disease. J Neurochem. 2003;85(6):1468–79. doi: 10.1046/j.1471-4159.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- 48.Madeira A, et al. Caveolin-1 interacts with alpha-synuclein and mediates toxic actions of cellular alpha-synuclein overexpression. Neurochem Int. 2011;59(2):280–9. doi: 10.1016/j.neuint.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Volpicelli-Daley L, Brundin P. Prion-like propagation of pathology in Parkinson disease. Handb Clin Neurol. 2018;153:321–335. doi: 10.1016/B978-0-444-63945-5.00017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161(4):673–7. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shyng SL, Heuser JE, Harris DA. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994;125(6):1239–50. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lang DM, et al. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol. 1998;37(4):502–23. doi: 10.1002/(SICI)1097-4695(199812)37:4<502::AID-NEU2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 53.Head BP, Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17(2):51–7. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Lee HJ, et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40(9):1835–49. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Park JY, et al. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem. 2009;110(1):400–11. doi: 10.1111/j.1471-4159.2009.06150.x. [DOI] [PubMed] [Google Scholar]
- 56.Hansen C, et al. Alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim KS, et al. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet. 2013;22(23):4805–17. doi: 10.1093/hmg/ddt332. [DOI] [PubMed] [Google Scholar]
- 58.Kang SJ, Kim JS, Park SM. Ubiquitin C-terminal hydrolase L1 regulates lipid raft-dependent endocytosis. Exp Neurobiol. 2018;27(5):377–386. doi: 10.5607/en.2018.27.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stroo E, et al. Cellular regulation of amyloid formation in aging and disease. Front Neurosci. 2017;11:64. doi: 10.3389/fnins.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21(12):1406–15. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 61.Guan J, et al. Overexpression of caveolin-1 reduces Taxol resistance in human osteosarcoma cells by attenuating PI3K-Akt-JNK dependent autophagy. Exp Ther Med. 2016;12(5):2815–2822. doi: 10.3892/etm.2016.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiroto T, et al. Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS One. 2014;9(2):e87871. doi: 10.1371/journal.pone.0087871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb JL, et al. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278(27):25009–13. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 64.Cuervo AM, et al. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 65.Vogiatzi T, et al. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283(35):23542–56. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jang A, et al. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113(5):1263–74. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- 67.Lee HJ, et al. Autophagic failure promotes the exocytosis and intercellular transfer of alpha-synuclein. Exp Mol Med. 2013;45:e22. doi: 10.1038/emm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poehler AM, et al. Autophagy modulates SNCA/alpha-synuclein release, thereby generating a hostile microenvironment. Autophagy. 2014;10(12):2171–92. doi: 10.4161/auto.36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim DK, et al. Anti-aging treatments slow propagation of synucleinopathy by restoring lysosomal function. Autophagy. 2016;12(10):1849–1863. doi: 10.1080/15548627.2016.1207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253(2):629–36. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 71.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995;270(24):14399–404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 72.Khan EM, et al. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006;281(20):14486–93. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 73.Turner CE, Glenney JR, Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111(3):1059–68. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abou-Sleiman PM, Healy DG, Wood NW. Causes of Parkinson's disease: genetics of DJ-1. Cell Tissue Res. 2004;318(1):185–8. doi: 10.1007/s00441-004-0922-6. [DOI] [PubMed] [Google Scholar]
- 75.Kim SR, et al. Selective COX-2 inhibitors modulate cellular senescence in human dermal fibroblasts in a catalytic activity-independent manner. Mech Ageing Dev. 2008;129(12):706–13. doi: 10.1016/j.mad.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Sun C, et al. Inhibition of phosphatidylcholine-specific phospholipase C prevents bone marrow stromal cell senescence in vitro. J Cell Biochem. 2009;108(2):519–28. doi: 10.1002/jcb.22282. [DOI] [PubMed] [Google Scholar]
- 77.Gupta A, et al. Alteration in memory cognition due to activation of caveolin-1 and oxidative damage in a model of dementia of Alzheimer's type. Indian J Pharmacol. 2019;51(3):173–180. doi: 10.4103/ijp.IJP_81_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roitenberg N, et al. Modulation of caveolae by insulin/IGF-1 signaling regulates aging of Caenorhabditis elegans. EMBO Rep. 2018 doi: 10.15252/embr.201745673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Protein expression of stable cell line and the uptake of α-syn in cav-1 overexpressing SH-SY5Y cells. a EGFP only, WT cav-1-EGFP and Y14A cav-1-EGFP OE SH-SY5Y cells were lysed, and Western blot was performed with the indicated antibodies. b EGFP only, WT cav-1-EGFP and Y14A cav-1-EGFP OE SH-SY5Y cells were observed under confocal microscopy. c EGFP only and WT cav-1-EGFP OE SH-SY5Y cells were cocultured with differentiated α-syn OE SH-SY5Y cells cultured on the insert for 24 h. The cells were then lysed, and Western blot was performed with the indicated antibodies. The intensity of four independent experiments was analyzed. ** p < 0.01, unpaired t-test. Figure S2. Protein expression of stable cell line. a EGFP only, WT cav-1-EGFP or Y14A cav-1-EGFP OE SH-SY5Y cells were treated with 1 μM α-Syn fibrils for 10 min. The cells were then lysed, and Western blot was performed with the indicated antibodies. b A53T-E only, WT cav-1/A53T-E and Y14A cav-1/A53T-E OE SH-SY5Y cells were lysed, and Western blot was performed with the indicated antibodies.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.