Abstract

This work using first-principles theory studies the sensing properties of Cu-decorated GaN (Cu–GaN) monolayers as a promising candidate for the detection of CO and HCHO in dry-type transformers. The Cu dopant prefers to be trapped on the TN site of the GaN surface with an Eb of −1.13 eV. Chemisorption is identified for the two gas adsorption systems, given the large adsorption energy (Ead) of −1.35 and −1.09 eV. Caused by the chemisorption, the electronic property of the Cu–GaN monolayer is significantly deformed, narrowing its band gap of 0.548 eV to 0.00 eV, exhibiting metallic property, in two gas systems. Combined with the desirable recovery property for CO and HCHO desorption from the Cu–GaN surface, it could be proposed that the Cu–GaN monolayer is a promising gas sensor for toxic gas detection in dry-type transformers, so as to evaluate the operation status of the power system and guarantee safe working conditions for the maintenances.
1. Introduction
Electrical transformers in the power system play a critical role in electricity transformation and distribution,1 which, generally speaking, in the modern urban power grid include two major categories, namely, oil-immersed transformers and dry-type ones.2 Different from the oil-immersed transformers whose insulation medium is dielectric oil preventing the possible insulating defects inside the devices,3 the insulation medium of the dry-type transformers is epoxy resin,4 a kind of solid insulation material, which with admirable insulation property can guarantee the safe operation of dry-type transformers in most cases.5 However, if there exist some cusps or rags inside the equipment, partial discharge would occur, and the energy engaged by the partial discharge can decompose the epoxy resin into several toxic gases, such as CO and HCHO, that are formed together and account for the dominant gas species inside the devices.6,7 Different from the oil-immersed transformers, in which the decomposed gases of the insulation oil would dissolve into the oil and deteriorate its insulation property,8,9 the formed toxic gases in the dry-type transformers would pervade the transformer substation,10 posing a great threat to the lives of the workers and maintenance persons. Therefore, the detection of CO and HCHO around the dry-type transformers is significant to, on one hand, evaluate its operation status in the power system and, on the other hand, guarantee safe working conditions for the maintenance persons.
In terms of gas detection, the nanosensing method can be regarded as a promising technique with rapid response, high sensitivity, and low cost.11−16 Recently, 2D nanomaterials with a large specific surface area, desirable electron mobility, and good chemical reactivity with the gas species are enormously explored for gas-sensing applications.17−20 Very recently, IV-group nitrides with a monolayer structure, such as InN and BN monolayers,21,22 have been reported with outstanding gas-sensing behavior and are full of potential for exploration of gas sensors.23 However, the GaN monolayer is not being focused, which in our opinion with desirable semiconducting property is a promising candidate for gas-sensing applications as well. Besides, 2D GaN has been successfully prepared using the migration-enhanced encapsulated growth technique.24 Such delightful progress stimulates us to perform a first-principles study exploring its sensing behavior for future research studies. In the meanwhile, the pure GaN monolayer, as reported, has limited chemical interactions with the gas molecules, such as NH3 and H2S.25 Therefore, we propose surface decoration with transition metal on the GaN monolayer to enhance the chemical reactivity of the whole system, aiming at promoting the adsorption and sensing performance of the GaN surface effectively.26,27
In this work, we propose Cu-decorated GaN (Cu–GaN) monolayers as a potential gas sensor for the detection of CO and HCHO, and the gas adsorption and sensing mechanism is expounded using first-principles theory. The Cu atom with a low cost and high catalytic property is widely applied for gas-sensing applications.28 Our calculations indicate the strong potential of the Cu–GaN monolayer as a resistance-type gas sensor in dry-type transformers. Also, it is our hope that our findings could be further verified by the following experimental research in the near future.
2. Results and Discussion
2.1. Cu Decoration Property on the GaN Monolayer
The Cu decoration process on the pristine GaN monolayer is presented in Figure 1, wherein Figure 1a is the pure GaN system, Figure 1b is the most stable configuration (MSC) of the Cu–GaN system, and Figure 1c is the charge density difference (CDD) of the Cu–GaN monolayer. To obtain the MSC of the Cu–GaN monolayer, several decoration sites are considered, including TN (on the top of the N atom), TGa (on the top of the Ga atom), BGa–N (on the bridge site of the Ga–N bond), and HGa–N (on the top of the hollow site of the Ga–N ring). Considering the lone-pair electron effect of the Cu dopant, we apply the auto, single, and double multiplicity to determine the ground state of the Cu–GaN system, and the results show that the double multiplicity has the lowest energy. Thus, the flowing calculations would all adopt the double multiplicity to obtain their energies. After geometric optimization, we only choose the one with the lowest Eb, which indicates the highest preference for Cu decoration on the GaN surface to further analyze the physicochemical properties of the Cu–GaN monolayer and to conduct the adsorption processes.
Figure 1.
Cu decoration process on the GaN monolayer. (a) Pristine GaN monolayer and (b, c) MSC of the Cu–GaN monolayer with related CDD. In CDD, the yellow areas are electron depletion, while the pink areas are electron accumulation with an isosurface of 0.01 eV/Å3.
In Figure 1a, it is seen that the GaN monolayer is a graphene-like structure with the Ga–N bond measured as 1.85 Å and the constant lattice obtained as 3.20 Å. These parameters agree with the previous report,29 indicating the desirable structure of the GaN monolayer in this work. In Figure 1b, we can find that the MSC for Cu decoration on the pure GaN surface is through the TN site, and the formed Cu–N bond is measured as 2.05 Å. The Eb for the TN site is obtained as −1.13 eV, much larger than 0.96 eV for the TGa site, suggesting the good stability and binding force in the Cu–N bond. Besides, the vibrational analysis shows that the frequencies of the Cu–GaN monolayer range at 113.67–1437.68 cm–1, which verifies its good chemical stability. Also, it is found that the BGa–N and HGa–N sites are not stable after Cu decoration, wherein the Cu dopant would undergo significant deformation and move to the TN site with a close Eb of −1.12 and −1.11 eV, respectively. This may be attributed to the large difference in the electronegativity between Ga and N atoms (1.81 for Ga and 3.04 for N), as proved by ref (30). Moreover, the Ga–N bond under the Cu dopant is slightly elongated to 1.89 Å, which suggests the geometric optimization for the GaN surface with Cu decoration.26
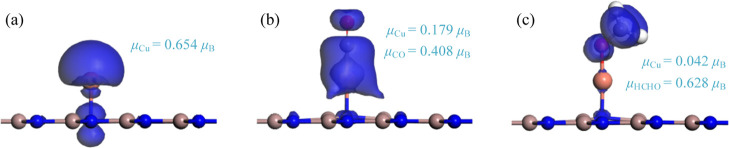
Based on the Hirshfeld analysis, the Cu dopant is positively charged by 0.028 e, as shown in Figure 1c, which may be due to the much smaller electronegativity of the Cu atom (1.90) compared with the N atom, thus giving rise to the electron-losing property for the Cu dopant. Apart from this, the magnetic moment of the Cu dopant is obtained as 0.654 μB in the total magnetic moment of 1 μB for the Cu–GaN system, as depicted in Figure 2a, which may result from the lone-pair electron effect of the Cu dopant. From the CDD, one can see the electron depletion on the Cu dopant and the below Ga–N bonds, which confirms the electron-losing behavior of the Cu dopant and the weakened binding force for the Ga–N bonds. Moreover, the electron accumulation on the Cu–N bond suggests the formation of new bonds where the electron hybridization occurs.31
Figure 2.
Magnetic property of (a) isolated Cu–GaN monolayer, (b) CO system, and (c) HCHO system. The blue areas are spin density with an isosurface of 0.01 eV/Å3.
To understand the Cu decoration behavior on the pure GaN monolayer, the band structure (BS) and density of state (DOS) of the bonding atoms are calculated, as displayed in Figure 3. One can see in Figure 3a that the band gap of the pristine GaN monolayer is calculated as 2.784 eV, and the top of the valence band and the bottom of the conduction band are not localized at the same point. These results manifest the wide band gap and indirect semiconducting property of the GaN system, which are in accordance with ref (30), wherein a band gap of 2.70 eV is obtained. In Figure 3b,c where the spin-up and spin-down of the Cu–GaN monolayer are shown, it is found that both spin-up and spin-down exhibit semiconducting property with different localizations of the top of the valence band and the bottom of the conduction band, and the band gap is obtained as 0.548 eV. These findings suggest that Cu decoration has little effect on the indirect semiconducting behavior of the GaN monolayer. However, the largely declined band gap in the Cu-decorated system can give rise to denser carrier concentration and stronger chemical reactivity compared with the pristine counterpart. From the atomic DOS, we can see that the spin-up and the spin-down of the Cu and N atoms are not symmetric, which manifest the magnetic property for the Cu–GaN system as analyzed above. Moreover, the Cu 3d orbital is hybridized with the N 2p orbital at −5.0, −2.0, 0, and 2.1 eV, which verifies the strong orbital interaction in the Cu–N bond, leading to the strong electron hybridization during its formation and verifying the strong binding force between the Cu dopant and N atom.
Figure 3.
BS of (a) pure GaN monolayer, (b, c) Cu–GaN monolayer, and (d) atomic DOS of bonding atoms. In the BS, the black values are band gaps of the related systems, and in the DOS, the dash line is the Fermi level.
2.2. Adsorption Behavior of the Cu–GaN Monolayer
Above the Cu dopant of the Cu–GaN monolayer, the CO and HCHO molecules approach with an atomic distance of appropriately 3.0 Å in various configurations to conduct the adsorption process and obtain the MSC for gas adsorption. With full geometric optimization, the MSC and related CDD and adsorption parameters for CO and HCHO adsorption on the Cu–GaN monolayer are presented in Figure 4, and the spin density is presented in Figure 2b,c.
Figure 4.

MSC and CDD for (a1, a2) CO system and (b1, b2) HCHO system. In the CDD, the yellow areas are electron depletion, while the pink areas are electron accumulation with an isosurface of 0.01 eV/Å3.
In the CO system, one can see that the CO prefers to be adsorbed on the Cu dopant through the C-end position, and the CO molecule stands vertically to the GaN surface, instead of the O-end counterpart, with the Ead calculated to be −1.35 eV. The new-formed Cu–C bond is measured to be 1.79 Å, and the Cu–N bond of the Cu–GaN monolayer is shortened to 1.91 Å. These findings suggest that the CO molecules are strongly trapped by the Cu dopant, impacting the geometry of the Cu–GaN monolayer accordingly.32 Based on the Hirshfeld analysis, the Cu dopant is positively charged by 0.182 e and the CO molecule is negatively charged by 0.150 e. These results show the electron-releasing property of the Cu dopant when interacting with the CO molecule, which donates 0.150 e to the CO molecule and 0.004 e to the GaN surface. From the CDD, the CO molecule is surrounded by the electron accumulation, while the Cu dopant is surrounded by the electron depletion, which confirms the charge-transfer behavior obtained by the Hirshfeld analysis well. Besides, strong electron accumulation and electron depletion on the Cu–C bond verify the strong electron hybridization during the formation of chemical bond and the chemisorption for CO adsorption, respectively. In the meanwhile, the magnetic moments of the Cu dopant and the CO molecule are obtained as 0.179 and 0.408 μB, respectively, and the total magnetic moment is obtained as 1.0 μB. This indicates over half the amount of the magnetic moment for the Cu dopant and the N atom in the Cu–GaN system (1.0 μB) and the weakened magnetic property of the Cu dopant after CO adsorption.
When it comes to the HCHO system, it is seen that the HCHO molecule tends to be trapped on the Cu dopant through the O-end position, and the O atom is captured by the Cu dopant through a new-formed Cu–O bond with a length of 1.83 Å. This is different from the CO system, which we assume may attribute to the strong chemical reactivity of the C=O bond.33 Besides, the HCHO molecule stands above the Cu dopant and, the molecular plane is vertical to the GaN surface. However, the Ead in the HCHO system is somewhat smaller than that in the CO system, which is calculated as −1.09 eV, suggesting the weaker adsorption performance of the Cu–GaN monolayer upon HCHO molecules. According to the Hirshfeld analysis, the HCHO molecule is negatively charged by 0.210 e, while the Cu dopant is positively charged by 0.242 e. These results reveal that the Cu dopant transfers 0.210 e to the HCHO molecule and very coincidently transfers 0.004 e to the GaN monolayer, the same amount of charge transfer to the GaN surface in the CO system. Moreover, the magnetic moments of the Cu dopant and HCHO molecule are obtained as 0.042 and 0.628 μB, respectively. These findings indicate that the majority of the magnetic moment of the Cu dopant has transferred to the HCHO molecule, and the Cu dopant almost exhibits no magnetic property after HCHO adsorption, while the HCHO molecule accounts for the largest magnetic moment in the system (1.0 μB).
In summary, the Cu dopant behaves as an electron donor, transferring charge to both the gas species and the GaN surface, while the CO and HCHO molecules exhibit strong electron-accepting behavior in the adsorptions. Given the large Ead of −1.35 and −1.09 eV in the CO and HCHO system, respectively, exceeding the critical value of 0.8 eV, as reported in ref (34), the chemisorption could be identified for the Cu–GaN monolayer in terms of interaction with the two gas species.35 Also, gas adsorption could largely impact the electronic property of the Cu–GaN monolayer, which may attribute to the charge-releasing property of the adsorbent system, distributing the charge density in the gas-adsorbed systems. For example, the magnetic property of the Cu dopant is largely affected after gas adsorption, and the magnetic moment of the Cu atom is reduced due to the weakened lone-pair electron effect in the gas-adsorbed systems. Some other deformations in the electronic property of the Cu–GaN monolayer would be expounded in the next section.
2.3. Electron Property in Gas Adsorption
Induced by gas adsorption, the electronic property of the Cu–GaN monolayer would be deformed. In this section, the BS and DOS of the gas-adsorbed systems are exhibited in Figure 5 to manifest this issue.
Figure 5.
BS and DOS of (a) CO system and (b) HCHO system. In the BS, the black values are the band gaps of related systems, and in the DOS, the dash line is the Fermi level.
From the BS configurations, one can see that the spin-up and spin-down of the CO and HCHO systems are asymmetric, indicating the retained magnetic property in the gas-adsorbed systems, which agrees with the Hirshfeld analysis. Besides, there appear several novel states in the spin-up and spin-down of the two systems crossing the Fermi level, giving rise to the 0.0 eV bandgap in the two gas systems. These findings suggest the metallic property of the Cu–GaN monolayer after adsorption of two gas species.36 In other words, the semiconducting property of the Cu–GaN monolayer would be modulated, thus exhibiting metallic property in the CO or HCHO environment.37 In this regard, the carrier density and carrier mobility of the Cu–GaN monolayer can be pronouncedly enhanced, leading to the obviously promoted electrical conductivity in the typical environment.38
From the atomic DOS of the bonding atoms in the CO system, it is found that the Cu 3d orbital is hybridized with the C 2p orbital at −5.9 and 2.0 eV, especially that around the Fermi level, which would largely impact the electronic property of the Cu–GaN monolayer. From the atomic DOS in the HCHO system, one can see that the Cu 3d orbital is hybridized with the O 2p orbital at −4.3, −3.0, and 1.7 eV. However, the orbital hybridization around the Fermi level in the Cu–O bond of the HCHO system is not as obvious as that in the Cu–C bond of the CO system. Therefore, from the electronic perspective, one can assume that the binding force of the Cu–O bond in the HCHO system is not as strong as that of the Cu–C bond in the CO system. This, to some extent, accounts for the smaller Ead in the HCHO system than that in the CO system.
These modifications in the BS of th etwo gas systems indicate the changed electrical conductivity of the Cu–GaN monolayer, which can provide the sensing mechanism to explore the Cu–GaN monolayer as a resistance-type gas sensor.39,40 In the meanwhile, the atomic DOS of bonding atoms reveals the binding force between them and shows the electronic nature of the formation of chemical bonds during gas adsorption.
3.4. Sensing Potential of the Cu–GaN Monolayer
The above-discussed section has mentioned the basic sensing mechanism of the Cu–GaN monolayer as a resistance-type gas sensor from the analysis of BS configuration before and after gas adsorption, that is, the detection of CO and HCHO can be realized using a Cu–GaN monolayer-based gas sensor if its electrical resistance is remarkably declined in a certain environment. On the other hand, to explore the potential of the Cu–GaN monolayer for possible application as a gas sensor, its recovery property should also be considered, which is another important parameter to evaluate the sensor’s performance for gas desorption from its surface. Based on ref (41), the recovery time could be obtained by the van’t Hoff–Arrhenius expression
| 1 |
in which A is the attempt frequency (1012 s–142), T is temperature, and KB is the Boltzmann constant [8.318 × 10–3 kJ/(mol·K)]. In this work, we assume the Ea (the potential barrier for gas desorption) to be equal as Ead. Therefore, we could infer from such an equation that the recovery time would be longer with larger Ead and lower temperature, and for a defined system, the increase in temperature can be beneficial for rapid gas desorption.
Figure 6 plots the recovery time of the Cu–GaN monolayer upon CO and HCHO desorption at various temperatures. One can see at the first glance that the recovery time could be significantly reduced as the temperature slightly increases. Specifically, at room temperature (298 K), the desorption for CO and HCHO requires 6.64 × 1010 and 2.67 × 106 s, respectively. Such time values would be quite long and unrealistic for applications. While the temperature is increased by 100 K, the recovery times in the CO and HCHO systems are declined remarkably to 1.22 × 105 and 62.66 s, respectively. On this occasion, it could be presumed that the desorption of HCHO would be feasible, and the Cu–GaN monolayer could be reused for circular gas sensing. Moreover, when the temperature increases to 498 K, the desorption of CO from the Cu–GaN surface, with a recovery time of 45.36 s, becomes realizable, thus giving rise to the possibility of the Cu–GaN monolayer for the recycled detection of CO.
Figure 6.

Recovery time of the Cu–GaN monolayer upon CO and HCHO desorption.
Combined with the desirable sensing performance of the Cu–GaN monolayer upon CO and HCHO, it could be concluded that the Cu–GaN monolayer has strong potential to be explored as a resistance-type gas sensor for the detection of two gas species with an admirable electrical response. However, the selective detection of such two species cannot be realized because of the similar sensing response and trend in the mixed gas atmosphere. On the other hand, selective detection under real working conditions does not result in a large significance because CO and HCHO are formed together. This gives the easier application of the gas sensor to diagnose the possible insulation defects of dry-type transformers. Moreover, the heating process should be conducted after the gas-sensing process before the recycled use of such a sensor. Considering the impossibility of selective detection of two gases in a real working environment, the heating temperature should be set as 498 K for about 1 min. Based on this, the possible existence of toxic gases inside the dry-type transformers could be identified using the Cu–GaN monolayer-prepared gas sensors with a desirable increase in its electrical conductivity. This would be meaningful to evaluate the working status and possible insulation defects of the dry-type transformers, thus guaranteeing the safe operation of the power system and the health of the maintenance persons.
3. Conclusions
In this work, we propose the Cu–GaN monolayer as a promising candidate for CO and HCHO detection around dry-type transformers to evaluate the operation status of the power system. The Cu dopant is decorated on the GaN surface through various configurations, and the gas adsorption and sensing properties are characterized and analyzed based on the MSC of the Cu–GaN monolayer. The main conclusions are as follows:
-
(i)
The Cu dopant prefers to be trapped on the pure GaN surface through the TN site, with an Eb of −1.13 eV, and the formed Cu–N bond is measured as 2.05 Å.
-
(ii)
Chemisorption could be identified for two gas adsorption systems, deforming the electronic property of the Cu–GaN monolayer as a result of the electron redistribution after adsorption.
-
(iii)
Cu–GaN monolayer has strong potential as a resistance-type gas sensor for the detection of two gas species with admirable electrical response and recovery property.
Our calculations mean to explore the strong potential of the Cu–GaN monolayer as a resistance-type gas sensor in dry-type transformers. Also, it is our hope that our findings could be further verified by the following experimental research in the near future.
4. Computational Details
The total geometric and electronic calculations based on first-principles theory were implemented in the DMol3 package,43 where the exchange correlation interaction was conducted using the Perdew–Burke–Ernzerhof function within the generalized gradient approximation.44,45 The DFT-D2 method proposed by Grimme was employed with corrected dispersion to consider the van der Waals force and long–range interactions.46 The Monkhorst–Pack k-point mesh of 10 × 10 × 1 was adopted for the Brillouin zone integration.47 We defined the energy tolerance accuracy, maximum force, and displacement to be 10–5 Ha, 2 × 10–3 Ha/Å, and 5 × 10–3 Å,48 respectively. A self-consistent loop energy of 10–6 Ha, global orbital cutoff radius of 5.0 Å, and smearing of 0.005 Ha were applied to ensure the accuracy of the total energy.49
A 4 × 4 × 1 GaN supercell was established with a vacuum region of 15 Å to perform the following simulations.50 The binding energy (Eb) was determined to evaluate the binding strength between the Cu dopant and the GaN surface, calculated by
| 2 |
where ECu–GaN, EGaN, and ECu represent the total energy of the Cu–GaN monolayer, pristine GaN monolayer, and isolated Cu atom, respectively. Besides, the adsorption energy (Ead) was defined to evaluate the adsorption performance of the Cu–GaN monolayer upon the gas molecules, obtained as51
| 3 |
whereECu–GaN/gas, ECu–GaN, and Egas represent the total energy of the gas adsorption system, isolated Cu–GaN monolayer, and isolated gas molecule, respectively. Moreover, we applied the Hirshfeld method to analyze the charge transfer (QT) of the gas species during adsorptions. For Cu–GaN and its gas adsorption systems, the one with the lowest energy would be analyzed in the following studies and would be called as the MSC accordingly.
Acknowledgments
This work is supported by the Key Research and Development Program of Hubei Province, China, (no. 2020BAA022), and Innovation Fund of China Electric Power Research Institute (no. SZ83-19-005).
The authors declare no competing financial interest.
References
- Cui H.; Chen D.; Zhang Y.; Zhang X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustainable Mater. Technol. 2019, 20, e00094 10.1016/j.susmat.2019.e00094. [DOI] [Google Scholar]
- Lu Y. C.; Wei L.; Chao W.; Peng W. The New Development Trend of Distribution Transformer. Appl. Mech. Mater. 2014, 672–674, 831–836. 10.4028/www.scientific.net/amm.672-674.831. [DOI] [Google Scholar]
- Ding J.; Li X.; Cao J.; Sheng L.; Yin L.; Xu X. New sensor for gases dissolved in transformer oil based on solid oxide fuel cell. Sens. Actuators, B 2014, 202, 232–239. 10.1016/j.snb.2014.05.061. [DOI] [Google Scholar]
- Rahimpour E.; Azizian D. Analysis of Temperature Distribution in Cast-resin Dry-type Transformers. Electr. Eng. 2007, 89, 301–309. 10.1007/s00202-006-0008-4. [DOI] [Google Scholar]
- Eslamian M.; Vahidi B.; Eslamian A. Thermal analysis of cast-resin dry-type transformers. Energy Convers. Manag. 2011, 52, 2479–2488. 10.1016/j.enconman.2011.02.006. [DOI] [Google Scholar]
- Xiong L.; Zhao Y.; Yang Z.; Song D.; He W. Temperature rise analysis and calculation of cast resin dry-type transformers. Gaodianya Jishu/High. Volt. Eng. 2013, 39, 265–271. 10.3969/j.issn.1003-6520.2013.02.002. [DOI] [Google Scholar]
- Zhang Y. L.; Wang L.; Xiong L.; Zhao Y. L.; Yang Z. K.; Wei H. E. On-line Temperature Monitoring and State Evaluation System for 10kV Dry-type Transformers. Electr. Meas. Instrum. 2012, 49, 33. [Google Scholar]
- Uddin A. S. M. I.; Yaqoob U.; Chung G.-S. Dissolved hydrogen gas analysis in transformer oil using Pd catalyst decorated on ZnO nanorod array. Sens. Actuators, B 2016, 226, 90–95. 10.1016/j.snb.2015.11.110. [DOI] [Google Scholar]
- Samsudin M. R.; Shee Y. G.; Mahamd Adikan F. R.; Abdul Razak B. B.; Dahari M. Fiber Bragg Gratings Hydrogen Sensor for Monitoring the Degradation of Transformer Oil. IEEE Sensor. J. 2016, 16, 2993–2999. 10.1109/jsen.2016.2517214. [DOI] [Google Scholar]
- Li Y.; Guan Y. J.; Li Y.; Li T. Y. Calculation of Thermal Performance in Amorphous Core Dry-Type Transformers. Adv. Mater. Res. 2014, 986–987, 1771–1774. 10.4028/www.scientific.net/amr.986-987.1771. [DOI] [Google Scholar]
- Gui Y.; Li W.; He X.; Ding Z.; Tang C.; Xu L. Adsorption properties of pristine and Co-doped TiO2 (1 0 1) toward dissolved gas analysis in transformer oil. Appl. Surf. Sci. 2020, 507, 145163. 10.1016/j.apsusc.2019.145163. [DOI] [Google Scholar]
- He X.; Gui Y.; Xie J.; Liu X.; Wang Q.; Tang C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. 10.1016/j.apsusc.2019.144030. [DOI] [Google Scholar]
- Gui Y.; Shi J.; Li T.; Tang C.; Xu L. Platinum modified MoS2 monolayer for adsorption and gas sensing of SF6 decomposition products: A DFT study. High. Volt. 2020, 5, 454. 10.1049/hve.2019.0170. [DOI] [Google Scholar]
- Xu L.; Gui Y.; Li W.; Li Q.; Chen X. Gas-sensing properties of Ptn-doped WSe2 to SF6 decomposition products. J. Ind. Eng. Chem. 2021, 97, 452–459. 10.1016/j.jiec.2021.02.030. [DOI] [Google Scholar]
- Pan Q.; Li T.; Zhang D. Ammonia gas sensing properties and density functional theory investigation of coral-like Au-SnSe2 Schottky junction. Sens. Actuators, B 2021, 332, 129440. 10.1016/j.snb.2021.129440. [DOI] [Google Scholar]
- Zhang D.; Liu J.; Jiang C.; Liu A.; Xia B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Actuators, B 2017, 240, 55–65. 10.1016/j.snb.2016.08.085. [DOI] [Google Scholar]
- Bradley D. Graphene gas sensor. Mater. Today 2012, 15, 233. 10.1016/s1369-7021(12)70105-0. [DOI] [Google Scholar]
- Yue Q.; Shao Z.; Chang S.; Li J. Adsorption of gas molecules on monolayer MoS2 and effect of applied;electric field. Nanoscale Res. Lett. 2013, 8, 425. 10.1186/1556-276x-8-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y.; Shi J.; Xu L.; Ran L.; Chen X. Aun (n = 1–4) cluster doped MoSe2 nanosheet as a promising gas-sensing material for C2H4 gas in oil-immersed transformer. Appl. Surf. Sci. 2021, 541, 148356. 10.1016/j.apsusc.2020.148356. [DOI] [Google Scholar]
- Zhang D.; Wu J.; Li P.; Cao Y. Room-temperature SO2 gas-sensing properties based on a metal-doped MoS2 nanoflower: an experimental and density functional theory investigation. J. Mater. Chem. A 2017, 5, 20666–20677. 10.1039/c7ta07001b. [DOI] [Google Scholar]
- Guo Y.; Zhang Y.; Wu W.; Liu Y.; Zhou Z. Transition metal (Pd, Pt, Ag, Au) decorated InN monolayer and their adsorption properties towards NO2: Density functional theory study. Appl. Surf. Sci. 2018, 455, 106–114. 10.1016/j.apsusc.2018.05.116. [DOI] [Google Scholar]
- Zhou X.; Chu W.; Zhou Y.; Sun W.; Xue Y. DFT simulation on H2 adsorption over Ni-decorated defective h-BN nanosheets. Appl. Surf. Sci. 2018, 439, 246–253. 10.1016/j.apsusc.2017.12.238. [DOI] [Google Scholar]
- Lin J.-H.; Zhang H.; Cheng X.-L.; Miyamoto Y. Single-layer group IV-V and group V-IV-III-VI semiconductors: Structural stability, electronic structures, optical properties, and photocatalysis. Phys. Rev. B 2017, 96, 035438. 10.1103/physrevb.96.035438. [DOI] [Google Scholar]
- Al Balushi Z. Y.; Wang K.; Ghosh R. K.; Vilá R. A.; Eichfeld S. M.; Caldwell J. D.; Qin X.; Lin Y. C.; Desario P. A.; Stone G. Two-dimensional gallium nitride realized via graphene encapsulation. Nat. Mater. 2016, 15, 1166. 10.1038/nmat4742. [DOI] [PubMed] [Google Scholar]
- Chen G.-X.; Li H.-F.; Wang D.-D.; Li S.-Q.; Fan X.-B.; Zhang J.-M. Adsorption of toxic gas molecules on pristine and transition metal doped hexagonal GaN monolayer: A first-principles study. Vacuum 2019, 165, 35–45. 10.1016/j.vacuum.2019.04.001. [DOI] [Google Scholar]
- Fan Y.; Zhang J.; Qiu Y.; Zhu J.; Zhang Y.; Hu G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. 10.1016/j.commatsci.2017.06.029. [DOI] [Google Scholar]
- Ma D.; Ju W.; Li T.; Zhang X.; He C.; Ma B.; Lu Z.; Yang Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. 10.1016/j.apsusc.2016.04.171. [DOI] [Google Scholar]
- Sharma A.; Anu; Khan M. S.; Husain M.; Khan M. S.; Srivastava A. Sensing of CO and NO on Cu-doped MoS2 Monolayer Based Single Electron Transistor: A First Principles Study. IEEE Sensor. J. 2018, 18, 2853. 10.1109/jsen.2018.2801865. [DOI] [Google Scholar]
- Xingxing Q.; Zhenzhen N.; Chen L.; Xiong Z.; Zhao Q. Tuning magnetism of monolayer GaN by vacancy and nonmagnetic chemical doping. J. Phys. Chem. Solids 2016, 91, 1–6. 10.1016/j.jpcs.2015.12.002. [DOI] [Google Scholar]
- Mu Y. Chemical Functionalization of GaN Monolayer by Adatom Adsorption. J. Phys. Chem. C 2015, 119, 20911–20916. 10.1021/acs.jpcc.5b04695. [DOI] [Google Scholar]
- Cui H.; Jia P.; Peng X. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863. 10.1016/j.apsusc.2020.145863. [DOI] [Google Scholar]
- Cui H.; Zhang G.; Zhang X.; Tang J. Rh-doped MoSe2 as toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 1, 772–780. 10.1039/c8na00233a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Wang Z.; Ye H.; Zhang K.; Chen X.; Zhang G. Promoting sensitivity and selectivity of HCHO sensor based on strained InP3 monolayer: A DFT study. Appl. Surf. Sci. 2018, 459, 554–561. 10.1016/j.apsusc.2018.08.014. [DOI] [Google Scholar]
- Cui H.; Liu T.; Zhang Y.; Zhang X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sensor. J. 2019, 19, 5249–5255. 10.1109/jsen.2019.2899966. [DOI] [Google Scholar]
- Huang J.; Chu J.; Wang Z.; Zhang J.; Yang A.; Li X.; Gao C.; Huang H.; Wang X.; Cheng Y.; Rong M. Chemisorption of NO2 to MoS2 Nanostructures and its Effects for MoS2 Sensors. ChemNanoMat 2019, 5, 1123–1130. 10.1002/cnma.201900350. [DOI] [Google Scholar]
- Ma D.; Ju W.; Li T.; Zhang X.; He C.; Ma B.; Tang Y.; Lu Z.; Yang Z. Modulating electronic, magnetic and chemical properties of MoS2 monolayer sheets by substitutional doping with transition metals. Appl. Surf. Sci. 2016, 364, 181–189. 10.1016/j.apsusc.2015.12.142. [DOI] [Google Scholar]
- Zhou Q.; Ju W.; Liu Y.; Li J.; Zhang Q. Influence of defects and dopants on the sensitivity of arsenene towards HCN. Appl. Surf. Sci. 2020, 506, 144936. 10.1016/j.apsusc.2019.144936. [DOI] [Google Scholar]
- Zhang Y.-H.; Yue L.-J.; Gong F.-L.; Li F.; Zhang H.-L.; Chen J.-L. Highly enhanced H2S gas sensing and magnetic performances of metal doped hexagonal ZnO monolayer. Vacuum 2017, 141, 109–115. 10.1016/j.vacuum.2017.03.033. [DOI] [Google Scholar]
- Cui H.; Zhang X.; Zhang G.; Tang J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. 10.1016/j.apsusc.2018.11.230. [DOI] [Google Scholar]
- Zhang D.; Mi Q.; Wang D.; Li T. MXene/Co3O4 composite based formaldehyde sensor driven by ZnO/MXene nanowire arrays piezoelectric nanogenerator. Sens. Actuators, B 2021, 339, 129923. 10.1016/j.snb.2021.129923. [DOI] [Google Scholar]
- Zhang Y.-H.; Chen Y.; Zhou K.-G.; Liu C. Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 2009, 20, 185504. 10.1088/0957-4484/20/18/185504. [DOI] [PubMed] [Google Scholar]
- Peng S.; Cho K.; Qi P.; Dai H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. 10.1016/j.cplett.2004.02.026. [DOI] [Google Scholar]
- Li P.; Hong Q.; Wu T.; Cui H. SOF2 sensing by Rh-doped PtS2 monolayer for early diagnosis of partial discharge in the SF6 insulation device. Mol. Phys. 2021, 119, e1919774 10.1080/00268976.2021.1919774. [DOI] [Google Scholar]
- Cui H.; Jia P.; Peng X.; Li P. Adsorption and sensing of CO and C2H2 by S-defected SnS2 monolayer for DGA in transformer oil: A DFT study. Mater. Chem. Phys. 2020, 249, 123006. 10.1016/j.matchemphys.2020.123006. [DOI] [Google Scholar]
- Zhou Q.; Ju W.; Yong Y.; Liu Y.; Li J. Quantum capacitance of supercapacitor electrodes based on germanene influenced by vacancy and co-doping: A first-principles study. Comput. Mater. Sci. 2021, 188, 110131. 10.1016/j.commatsci.2020.110131. [DOI] [Google Scholar]
- Tkatchenko A.; DiStasio R. A.; Head-Gordon M.; Scheffler M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 094106. 10.1063/1.3213194. [DOI] [PubMed] [Google Scholar]
- Cui H.; Zhang X.; Li Y.; Chen D.; Zhang Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. 10.1016/j.apsusc.2019.07.218. [DOI] [Google Scholar]
- Cui H.; Yan C.; Jia P.; Cao W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. 10.1016/j.apsusc.2020.145759. [DOI] [Google Scholar]
- Ju W.; Li T.; Su X.; Li H.; Li X.; Ma D. Au cluster adsorption on perfect and defective MoS2 monolayers: structural and electronic properties. Phys. Chem. Chem. Phys. 2017, 19, 2073. 10.1039/c7cp03062b. [DOI] [PubMed] [Google Scholar]
- Sanders N.; Bayerl D.; Shi G.; Mengle K. A.; Kioupakis E. Electronic and Optical Properties of Two-Dimensional GaN from First-Principles. Nano Lett. 2017, 17, 7345–7349. 10.1021/acs.nanolett.7b03003. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Ju W.; Yong Y.; Zhang Q.; Liu Y.; Li J. Effect of the N/P/S and transition-metal co-doping on the quantum capacitance of supercapacitor electrodes based on mono-and multilayer graphene. Carbon 2020, 170, 368–379. 10.1016/j.carbon.2020.08.045. [DOI] [Google Scholar]