Abstract
Nineteen new thiazole-based derivatives were synthesized and their structures characterized with analytical and spectral data. The in vitro assessment of their acetylcholinesterase (AChE) inhibitory activity revealed that compounds 10 and 16 produced potent AChE inhibitory activities with IC50 values of 103.24 and 108.94 nM, respectively. Compounds 13, 17, 18, 21, 23, 31, and 33 displayed moderate activity with 25–50% relative potency compared to the known potent AChE inhibitor donepezil. Molecular docking studies of the active compounds docked within the active site cavity of AChE showed a binding orientation similar to that of donepezil, with good predicted binding affinities. These compounds could therefore be considered as potential lead compounds for the development of new and potentially improved AChE inhibitors.
1. Introduction
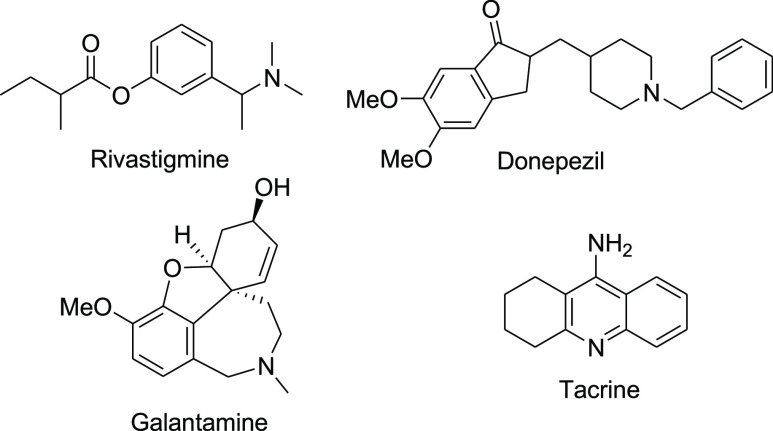
Alzheimer’s disease (AD) is the most predominant form of neurodegenerative disorder in the elderly worldwide.1−3 AD is a slowly progressive brain disease that begins many years before symptoms emerge.4 The early clinical symptoms of AD include difficulties in remembering recent conversations, names, or events in addition to apathy and depression. Later symptoms include confusion, disorientation, impaired communication, poor judgment, behavioral changes, and deterioration of speaking, swallowing, and walking capabilities.5 Although the reasons for the development and etiology of AD have not yet been fully explored, AD patients are characterized by histopathological brain changes including extracellular deposition of amyloid β-protein (Aβ) in amyloid plaques6 and by intraneuronal neurofibrillary tangles consisting of aggregated hyperphosphorylated τ-protein.7 To date, there is no cure for AD. Currently approved therapeutics for AD only offer symptomatic relief without the ability of slowing down disease progression.8 The formation of Aβ and τ-proteins is associated with the progressive loss of muscarinergic neurons and increased acetylcholinesterase (AChE) enzyme activity, consequently lowering the levels of brain acetylcholine (ACh).9 The AChE inhibitors rivastigmine, donepezil, galantamine, and tacrine (Figure 1) and the N-methyl-d-aspartate receptor antagonist memantine are the most commonly used symptomatic therapies for AD with limited success.10
Figure 1.
Currently used AChE inhibitors as anti-AD drugs.
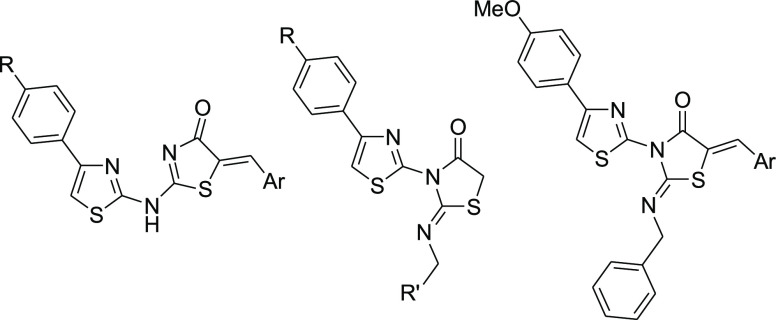
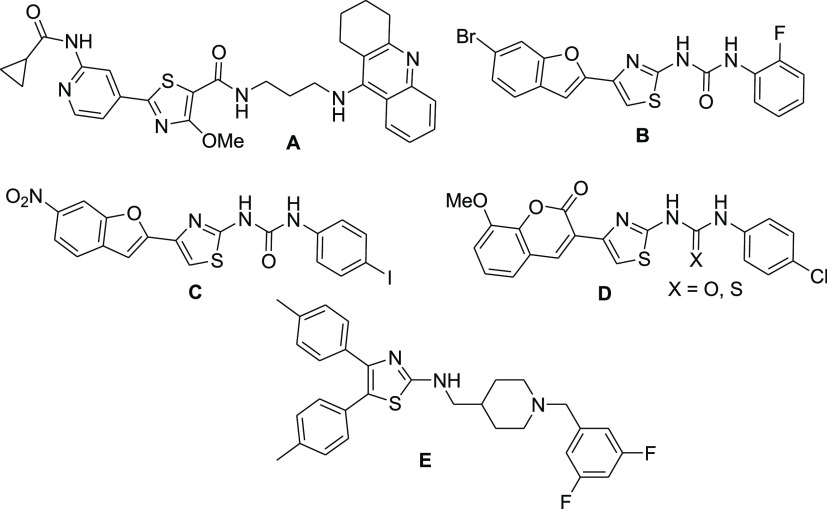
Structural modifications of the currently used clinical AChE inhibitors resulted in the exploration of the importance of the incorporation of thiazole moieties as essential building blocks for the optimization of AChE inhibitory activity (Figure 2).11 The acridine–thiazole hybrid derivative A showed potent AChE inhibitory activity with IC50 = 6.5 nM, exemplifying the importance of the acridine moiety of tacrine incorporated into this structure.12 However, the acridine moiety is known to cause hepatotoxicity, which has led to the removal of tacrine from the pharmaceutical market.10 In addition, the benzofuranylthiazole derivatives B and C displayed moderate dual AChE and butyrylcholinesterase (BuChE) inhibitory activities (IC50: 3.85 μM and 9.25 μM, respectively).13 Coumarylthiazole derivatives containing aryl urea/thiourea groups, represented by compound D (IC50 = 4.58 μM), were also reported as moderate AChE inhibitors.14 Furthermore, the benzylpiperidine-linked diarylthiazole derivative E exhibited promising AChE inhibitory activity with an IC50 value of 0.30 μM.15 In continuation for the search of improved thiazole-based AChE inhibitors without the acridine moiety, we synthesized a series of thiazole–thiazolidine-incorporated derivatives (Schemes 1 and 2, Table 1). The thiazolidine moiety acts as a bioisosteric replacement of the urea/thiourea moieties, as seen in compounds B–D, and the biological effect thereof was explored using an AChE inhibitory assay (Table 2).
Figure 2.
Thiazole-based AChE inhibitors (A–E) reported in the literature.
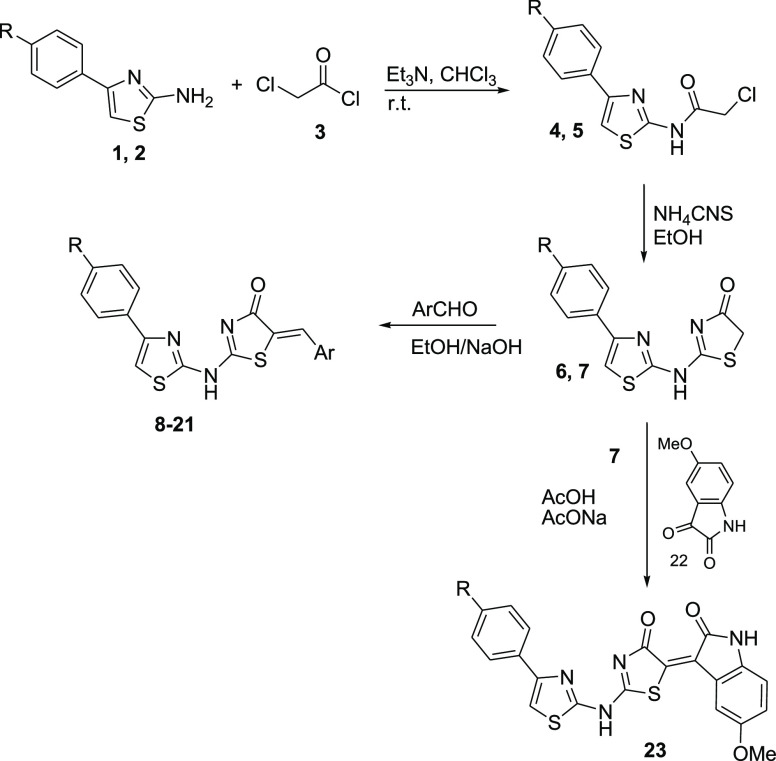
Scheme 1. Synthesis of Compounds 8–21 and 23.
Scheme 2. Synthesis of Compounds 28–33.
Table 1. Melting Points, Yield Percentages, Molecular Formulae, and Molecular Weights of Compounds 8, 10–21, 23, 24, and 26–33.
| comp. no. | R | R′/Ar | melting point (°C) | yield (%) | mol. formula (Mol. Wt.) |
|---|---|---|---|---|---|
| 8 | CH3 | 4-CH3C6H4 | 314–316 | 90 | C21H17N3OS2 (391.51) |
| 10 | CH3 | 3,4-(CH3O)2C6H3 | 290–292 | 85 | C22H19N3O3S2 (437.53) |
| 12 | CH3 | 2,4-Cl2C6H3 | 322–325 | 84 | C20H13Cl2N3OS2 (446.37) |
| 13 | CH3 | thiophen-2-yl | 328–330 | 90 | C18H13N3OS3 (383.51) |
| 14 | CH3 | 5-bromothiophen-2-yl | 320–322 | 86 | C18H12BrN3OS3 (462.41) |
| 15 | OCH3 | 4-CH3C6H4 | 308–310 | 90 | C21H17N3O2S2 (407.51) |
| 16 | OCH3 | 4-CH3OC6H4 | 322–325 | 90 | C21H17N3O3S2 (423.51) |
| 17 | OCH3 | 3,4-(CH3O)2C6H3 | 293–295 | 80 | C22H19N3O4S2 (453.53) |
| 18 | OCH3 | 4-ClC6H4 | 325–328 | 80 | C20H14ClN3O2S2 (427.93) |
| 19 | OCH3 | 2,4-Cl2C6H3 | 328–330 | 90 | C20H13Cl2N3O2S2 (462.37) |
| 20 | OCH3 | thiophen-2-yl | 325–327 | 80 | C18H13N3O2S3 (399.51) |
| 21 | OCH3 | 5-bromothiophen-2-yl | 288–290 | 80 | C18H12BrN3O2S3 (478.41) |
| 23 | 335–338 | 65 | C22H16N4O4S2 (464.52) | ||
| 24 | CH3 | CH3 | 220–222 | 79 | C13H15N3S2 (277.41) |
| 26 | OCH3 | CH3 | 210–212 | 69 | C13H15N3OS2 (293.41) |
| 27 | OCH3 | C6H5 | 215–217 | 79 | C18H17N3OS2 (355.48) |
| 28 | CH3 | CH3 | 208–210 | 64 | C15H15N3OS2 (317.43) |
| 29 | CH3 | C6H5 | 198–200 | 66 | C20H17N3OS2 (379.5) |
| 30 | OCH3 | CH3 | 215–215 | 66 | C15H15N3O2S2 (333.43) |
| 31 | OCH3 | C6H5 | 210–212 | 68 | C20H17N3O2S2 (395.5) |
| 32 | 3,4-(CH3O)2C6H3 | 217–218 | 79 | C29H25N3O4S2 (543.66) | |
| 33 | 2,4-Cl2C6H3 | 189–191 | 80 | C27H19Cl2N3O2S2 (552.49) |
Table 2. In Vitro AChE Inhibitory Potency of Compounds 8, 10, 12–21, 23, and 28–33 and Donepezil.
| compound no. | R | R′/Ar | IC50 (nM)a | relative potency (%) |
|---|---|---|---|---|
| 8 | CH3 | 4-CH3C6H4 | 500.56 ± 14 | 10.99 |
| 10 | CH3 | 3,4-(CH3O)2C6H3 | 103.24 ± 2.8 | 53.27 |
| 12 | CH3 | 2,4-Cl2C6H3 | 335.79 ± 9.1 | 16.38 |
| 13 | CH3 | thiophen-2-yl | 173.75 ± 4.7 | 31.66 |
| 14 | CH3 | 5-bromothiophen-2-yl | 379.81 ± 10 | 14.48 |
| 15 | OCH3 | 4-CH3C6H4 | 374.70 ± 10 | 14.68 |
| 16 | OCH3 | 4-CH3OC6H4 | 108.94 ± 3 | 50.49 |
| 17 | OCH3 | 3,4-(CH3O)2C6H3 | 175.93 ± 4.8 | 31.26 |
| 18 | OCH3 | 4-ClC6H4 | 193.48 ± 5.3 | 28.43 |
| 19 | OCH3 | 2,4-Cl2C6H3 | 731.33 ± 20 | 7.52 |
| 20 | OCH3 | thiophen-2-yl | 259.64 ± 7.1 | 21.18 |
| 21 | OCH3 | 5-bromothiophen-2-yl | 187.83 ± 5.1 | 29.28 |
| 23 | 151.0 ± 4.1 | 36.42 | ||
| 28 | CH3 | CH3 | 478.20 ± 13 | 11.50 |
| 29 | CH3 | C6H5 | 187.86 ± 5.1 | 29.28 |
| 30 | OCH3 | CH3 | 346.40 ± 9.4 | 15.88 |
| 31 | OCH3 | C6H5 | 152.45 ± 4.1 | 36.08 |
| 32 | 3,4-(CH3O)2C6H3 | 450.4 ± 12 | 12.21 | |
| 33 | 2,4-Cl2C6H3 | 187.9 ± 5.1 | 29.27 | |
| donepezil | 55.0 ± 0.004 | 100 |
Values are expressed as the mean ± standard error of the mean of three experiments.
2. Results and Discussion
2.1. Chemical Synthesis
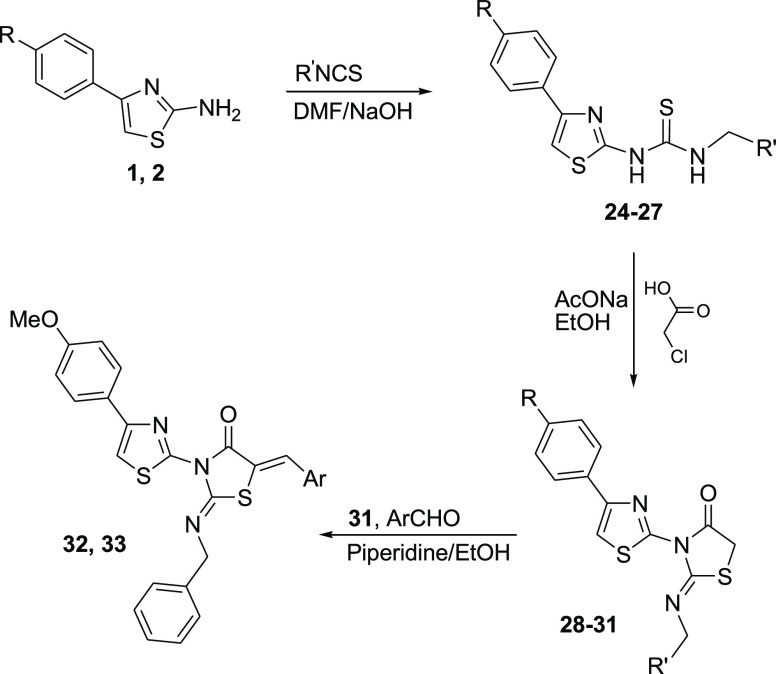
The required precursors 4-arylthiazol-2-amines 1 and 2 were prepared via condensation of thiourea and the appropriate aryl methyl ketone in the presence of iodine.16 The reaction of compounds 1 and 2 with chloroacetyl chloride 3 and triethylamine in chloroform at room temperature yielded the corresponding 2-chloro-N-thiazolyl acetamide derivatives 4 and 5,17,18 which were reacted with ammonium thiocyanate to yield the corresponding dihydrothiazol-4-one analogues 6(19) and 7.20 Compounds 6 and 7 were condensed with various aromatic aldehydes in the presence of sodium hydroxide to yield the corresponding 5-arylidene derivatives 8–21, and compound 7 was condensed with 5-methoxyindoline-2,3-dione in acetic acid in the presence of sodium acetate to yield compound 23 (Scheme 1, Table 1).
The aminothiazole derivatives 1 and 2 were reacted with ethyl or benzyl isothiocyanate in N,N-dimethylformamide (DMF) to yield the corresponding thiourea derivatives 24, 25,2126, and 27. The thiourea derivatives 24–27 were then reacted with chloroacetic acid in the presence of sodium acetate to yield the thiazolidin-4-one derivatives 28–31. The reaction of compound 31 with 3,4-dimethoxybenzaldehyde or 2,4-dichlorobenzaldehyde in ethanol in the presence of a catalytic amount of piperidine yielded the corresponding 5-arylidene analogues 32 and 33, respectively (Scheme 2, Table 1).
The structures of the newly synthesized compounds were confirmed by elemental analyses, 1H NMR, 13C NMR, and electron ionization mass spectrometry (EI-MS) spectral data.
2.2. In Vitro AChE Inhibitory Activity
The in vitro AChE inhibitory activity of the target compounds 8, 10, 12–21, 23, and 28–33 was investigated following the previously described modification of Ellman’s spectrophotometric method22,23 using donepezil hydrochloride as the reference drug (positive control). The IC50 values of AChE inhibitory activities and their relative potencies to donepezil hydrochloride are summarized in Table 2. The IC50 values ranged from 500.56 to 103.24 nM and their relative potencies from 10.99 to 53.27%. The optimum AChE inhibitory activity was attained by compounds 10 and 16 with IC50 values of 103.24 nM and 108.94 nM, respectively, and relative potencies of more than 50%. Compounds 13, 17, 18, 21, 23, 31, and 33 displayed moderate AChE inhibitory activities with 25–50% relative potencies. Meanwhile, the relative potencies of compounds 8, 12, 14, 15, 19, 20, 28, 29, 30, and 32 were less than 25%.
Within the 2-(4-arylthiazol-2-ylamino)-5-(4-methylbenzylidene)-4,5-dihydrothiazole-4-one derivatives 8–21, it could be concluded that the AChE inhibitory activity is mainly dependent on the nature of the arylidene substituents; the 4-methoxy- and 3,4-dimethoxybenzylidene substituents are optimal for activity, while the 4-methyl- and 2,4-dichlorobenzylidene substituents caused a sharp deterioration of the activity. The thiophen-2-yl, 5-bromothiophen-2-yl, and the 4-chlorobenzylidene substituents retained moderate activities. The activity of the 5-methoxyindolin-2-one analogue 23 is higher than that of the thiophen-2-yl, 5-bromothiophen-2-yl, and the 4-chlorobenzylidene analogues. In addition, it could also be concluded that the 4-aryl substituents (CH3 and OCH3) are almost equipotent.
In the 2-substituted imino-3-(4-arylthiazol-2-yl)thiazolidin-4-ones 28–31, the AChE inhibitory activities of the benzyl analogues 29 and 31 were superior to those of their ethyl analogues 28 and 30. Contrary to the activity of compounds 8–22, the activity of the 2,4-dichlorobenzylidene derivative 33 was found to be higher than that of its 3,4-dimethoxybenzylidene analogue 32.
2.3. Molecular Docking Studies
In order to obtain structural insights regarding the binding interactions and orientation of the most active AChE inhibitors within each series, molecular docking experiments were performed on representative active compounds 10, 13, 16, 23, and 31 (relative potencies = 31.66% to 53.27%, Table 2) using the X-ray crystal structure of AChE co-crystallized with the potent AChE inhibitor donepezil (PDB code: 4EY7, www.rcsb.org). Molecular Operating Environment (MOE) software24 was used to conduct the docking experiments, score the molecules according to their binding affinity, and determine potential binding interactions. To confirm if the docking parameters and computational procedure could reproduce the experimental results, the co-crystallized ligand, donepezil, was redocked (Figure 3). The docking pose obtained was comparable to the co-crystallized one, with a root-mean squared deviation (rmsd) of 0.23 Å and a binding affinity of −15.50 kcal/mol. In addition, compounds 8, 14, 19, 28, and 32 with some of the lowest relative potencies in the series (7.52 to 14.48%, Table 2) were also docked to observe any particular interactions or reduction in predicted binding affinity that could rationalize the reduced potency.
Figure 3.
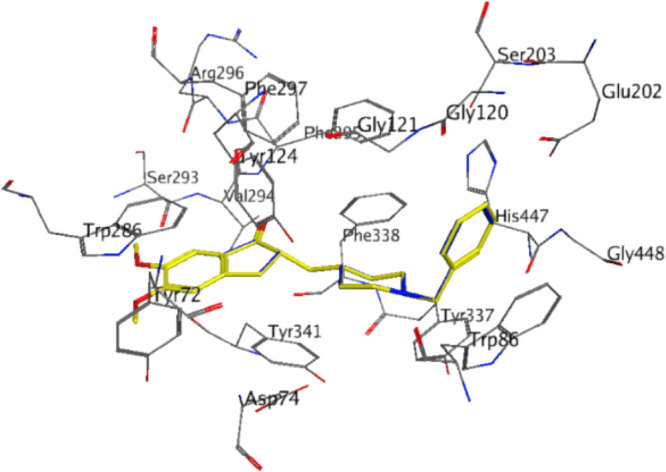
Co-crystallized pose of donepezil (yellow) vs the docked experimental pose of donepezil (blue) within the AChE active site. A very low rmsd value of 0.23 Å was obtained, indicating the accuracy and repeatability of the docking procedure.
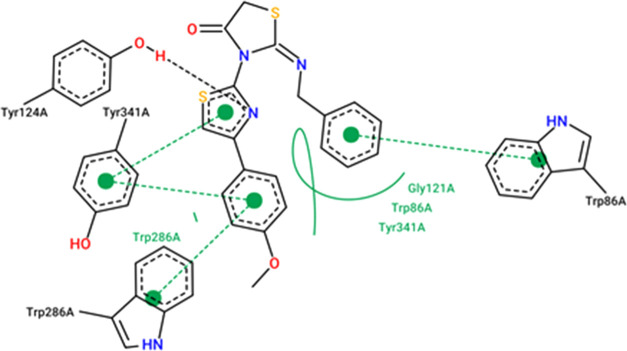
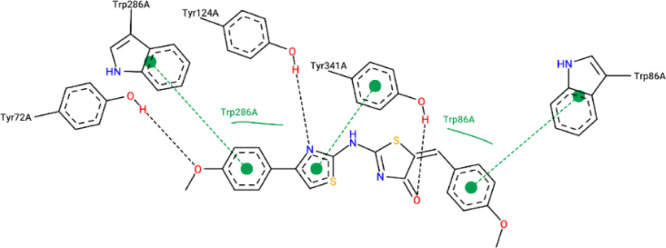
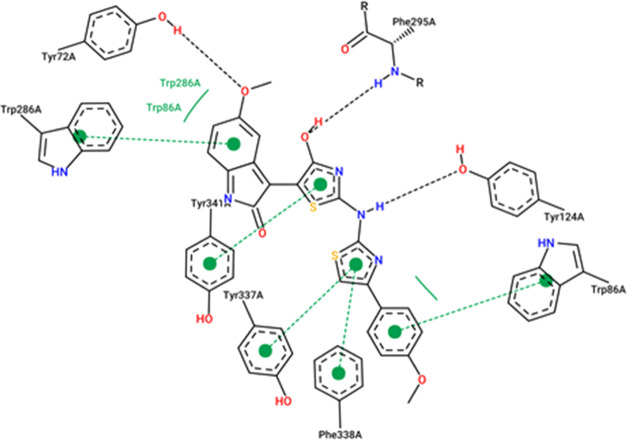
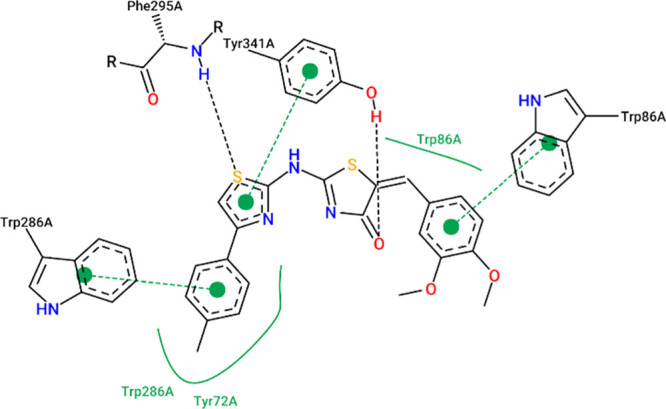
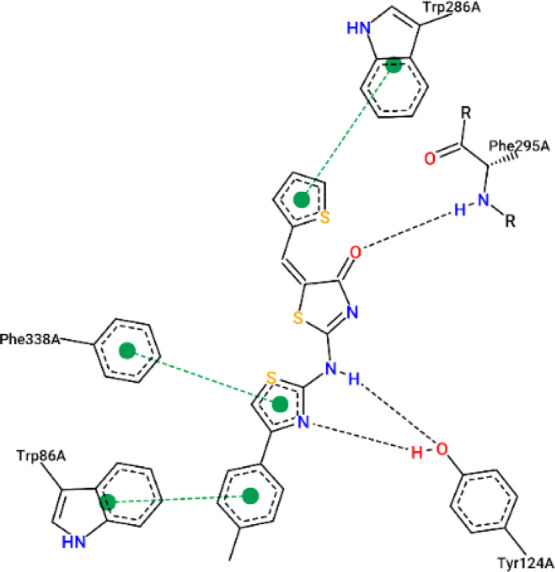
The results from the docking experiments revealed that compounds 10, 13, 16, 23, and 31 were able to bind in a comparable position and manner within the AChE active site to that of the co-crystallized ligand, donepezil. Figures 4–9 show the binding orientations, binding interactions, and binding affinities of donepezil and the active compounds (Table 3). The molecules were able to span from the catalytic anionic site (CAS) through the narrow active site gorge into the peripheral anionic site (PAS) of AChE. Important π–π interactions were observed for all the test compounds with Trp86 (within the CAS) and Trp286 (within the PAS), similar to donepezil. Interestingly, the thiazole–thiazolidine moiety within these structures was able to form a number of interactions with the residues lining the active site gorge of AChE (Figures 4–9, Table 3). In addition, the compounds presented with binding affinities (from −13.34 to −14.87 kcal/mol) that are within a close range to that of the potent AChE inhibitor donepezil (−15.50 kcal/mol). Table 3 illustrates the relationship between the predicted binding affinities and experimental IC50 values of the most potent AChE inhibitors within this series. A definite trend between the binding affinity and the experimental IC50 value of the test compounds is observed.
Figure 4.
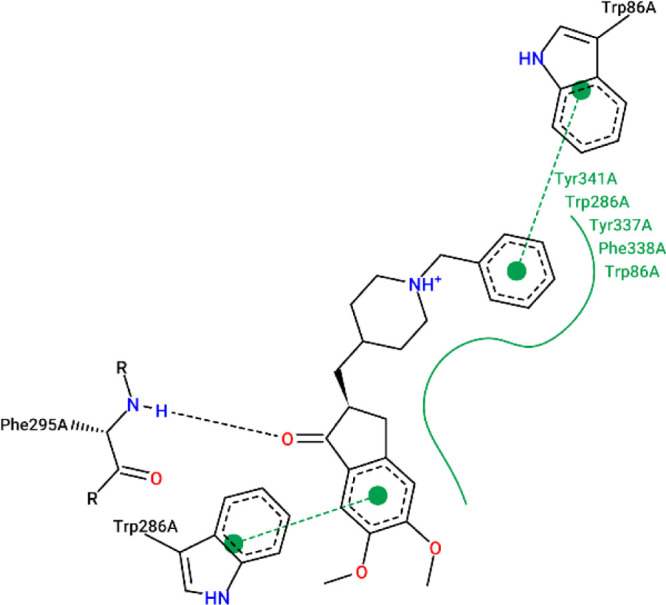
Interaction map of donepezil within the active site of AChE (BA = −15.50 kcal/mol).
Figure 9.
Interaction map of compound 31 within the active site of AChE (BA = −13.80 kcal/mol).
Table 3. Experimental IC50 Values, Binding Affinities, and Binding Interactions of the Representative Most Active Docked Compounds within This Series.
| compound | AChE IC50 (nM) | predicted binding affinity (kcal/mol) | binding interactions |
|---|---|---|---|
| donepezil | 55.0 ± 0.004 | –15.50 | Trp86, Phe295, Trp286 |
| 10 | 103.24 ± 2.8 | –14.87 | Trp86, Tyr341, Phe295, Trp286 |
| 16 | 108.94 ± 3.0 | –14.85 | Trp86, Tyr341, Tyr124, Trp286, Tyr72 |
| 23 | 151.00 ± 4.1 | –13.85 | Trp86, Tyr124, Phe295, Phe338, Tyr337, Tyr341, Trp286, Tyr72 |
| 31 | 152.45 ± 4.1 | –13.80 | Trp86, Tyr124, Tyr341, Trp286 |
| 13 | 173.75 ± 4.7 | –13.34 | Trp86, Phe338, Tyr124, Trp286 |
Figure 5.
Interaction map of compound 10 within the active site of AChE (BA = −14.87 kcal/mol).
Figure 6.
Interaction map of compound 13 within the active site of AChE (BA = −13.34 kcal/mol).
Figure 7.
Interaction map of compound 16 within the active site of AChE (BA = −14.58 kcal/mol).
Figure 8.
Interaction map of compound 23 within the active site of AChE (BA = −13.85 kcal/mol).
Additionally, the lower potency agents 8, 14, 19, 28, and 32 confirmed the structure–activity relationship observations made in the biological section. The predicted binding affinities of the compounds were slightly reduced (from 12.38 to 13.22 kcal/mol) when compared to that of the more potent compounds (Table 3), which is in correlation with the biological activities. In general, the interactions with Trp86 and Trp286 were retained, the thiazole–thiazolidine moiety also showed similar integrations with the residues within the active site gorge (including Tyr341, Tyr337, Tyr124, Phe295, and Phe338) as the more active compounds. This is to be expected as the least active compound 8 still showed an improved IC50 value of 500.56 ± 14 when compared to that of the known thiazole derivatives in the literature (see Scheme 1, compounds B–D).
3. Conclusions
Nineteen new thiazole-based derivatives were synthesized and evaluated as potential AChE inhibitors for the treatment of AD. The most active AChE inhibitory compounds were 10 and 16, which also represented the lowest IC50 values with relative potencies of more than 50% in comparison to the potent AChE inhibitor donepezil. Docking studies within the AChE active site revealed that compounds 10, 13, 16, 23, and 31 showed binding orientations similar to that of donepezil and presented with key interactions that explain their potent AChE activity observed. The thiazole moiety was also found to form important interactions with the amino acid residues lining the active site gorge of AChE. The results suggest that these new thiazole derivatives, especially 10 and 16, may serve as new lead compounds for further development of potent thiazole-based AChE inhibitors that do not incorporate the hepatotoxic acridine moiety.
4. Materials and Methods
4.1. General Information
Melting points (°C) were measured in open glass capillaries using a Stuart-SMP30 electro-thermal melting point apparatus and are uncorrected. Nuclear magnetic resonance (NMR) spectra were obtained on a JEOL ECA-500 II NMR spectrometer at 500.16 MHz for 1H and 125.77 MHz for 13C; the chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane as the internal standard; and the coupling constants (J) are expressed in Hz. Deuteriodimethyl sulfoxide (DMSO-d6) and deuterochloroform (CDCl3) were used as solvents. Elemental analyses (C, H, N, and S) were in good agreement with the proposed structures within ±0.4% of the theoretical values (Table S1, Supporting Information). Monitoring the reactions and checking the purity of the final products were carried out by thin layer chromatography using silica-gel-precoated aluminum sheets (60 F254, Merck) and visualization with ultraviolet light (UV) at 365 and 254 nm. The reference drug donepezil hydrochloride (CAS 120011-70-3) was purchased from Sigma-Aldrich Chemie GmbH, Germany. Compounds 9, 11,20 and 25(21) were prepared following the previously reported procedures.
4.2. General Procedure for the Synthesis of Compounds 8, 10, and 12–21
The appropriate aromatic aldehyde (10 mmol) was added to a solution of thiazolidinone 6 or 7 (10 mmol) and sodium hydroxide (1.0 g) in ethanol (30 mL), and the mixture was stirred at room temperature for 4 h. The precipitate formed was filtered, dried, and crystallized with ethanol.
4.2.1. 2-[4-(p-Tolylthiazol-2-yl)amino]-5-(4-methylbenzylidene)-4,5-dihydrothiazole-4-one (8)
1H NMR (DMSO-d6): δ 2.31 (s, 3H, CH3), 2.33 (s, 3H, CH3), 7.18 (d, 2H, Ar-H, J = 7.6 Hz), 7.28 (d, 2H, Ar-H, J = 8.2 Hz), 7.37 (s, 1H, thiazole-H), 7.42 (s, 1H, CH=C), 7.46 (d, 2H, Ar-H, J = 8.2 Hz), 7.78 (d, 2H, Ar-H, J = 7.6 Hz). 13C NMR: δ 20.83 (CH3), 21.0 (CH3), 108.76, 148.0, 159.50 (thiazole-C), 124.07, 125.44, 129.08, 129.57, 132.17, 132.77, 132.87, 136.13, 137.85, 172.0 (Ar-C, thiazoline-C, and arylidene-C), 182.0 (C=O). EI-MS, m/z (%): 393.56 (32.80), 100.95 (100).
4.2.2. 2-[4-(p-Tolylthiazol-2-yl)amino]-5-(3,4-dimethoxybenzylidene)-4,5-dihydrothiazole-4-one (10)
1H NMR (DMSO-d6): δ 2.31 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 7.38 (d, 2H, Ar-H, J = 8.2 Hz), 7.13–7.19 (m, 4H, Ar-H & NH), 7.36 (s, 1H, thiazole-H), 7.40 (s, 1H, CH=C), 7.78 (d, 2H, Ar-H, J = 7.6 Hz). 13C NMR: δ 20.90 (CH3), 55.46 (OCH3), 55.63 (OCH3), 108.73, 148.81, 167.80 (thiazole-C), 112.06, 122.04, 124.60, 125.51, 128.41, 129.15, 130.79, 132.90, 136.22, 149.01, 167.80, 171.98 (Ar-C, thiazoline-C, and arylidene-C), 180.41 (C=O). EI-MS, m/z (%): 438.83 (88), 117.37 (100).
4.2.3. 2-[4-(p-Tolylthiazol-2-yl)amino]-5-(2,4-dichlorobenzylidene)-4,5-dihydrothiazole-4-one (12)
1H NMR (DMSO-d6): δ 2.31 (s, 3H, CH3), 3.32 (s, 1H, NH), 7.18–7.20 (m, 2H, Ar-H & thiazole-H), 7.46 (s, 1H, CH=C), 7.59–7.61 (m, 2H, Ar-H), 7.61–7.69 (m, 1H, Ar-H), 7.73 (d, 1H, Ar-H, J = 2.0 Hz), 7.77 (d, 2H, Ar-H, J = 7.5 Hz). 13C NMR: δ 20.87 (CH3), 109.29, 149.10, 167.47 (thiazole-C), 117.83, 125.50, 128.09, 129.15, 129.50, 132.52, 132.76, 132.96, 134.54, 136.28, 137.55, 167.47, 170.96 (Ar-C, thiazoline-C, and arylidene-C), 179.43 (C=O). EI-MS, m/z (%): 444.96 (33.17), 445.99 (23), 446.98 (40.20), 377.20 (100).
4.2.4. 2-[4-(p-Tolylthiazol-2-yl)amino]-5-[(thiophen-2-yl)methylene]-4,5-dihydrothiazole-4-one (13)
1H NMR (DMSO-d6): δ 2.31 (s, 3H, CH3), 3.32 (s, 1H, NH), 7.18–7.20 (m, 3H, Ar-H & thiazole-H), 7.40 (d, 1H, thiophene-H, J = 3.5 Hz), 7.42 (s, 1H, CH=C), 7.61–7.82 (m, 4H, Ar-H & thiophene-H). 13C NMR: δ 20.89 (CH3), 108.95, 148.99, 167.67 (thiazole-C), 117.46, 125.53, 128.41, 128.88, 129.16, 130.19, 132.22, 132.85, 136.26, 140.80, 171.14 (Ar-C, thiazoline-C, thiophene-C, and arylidene-C), 179.79 (C=O). EI-MS, m/z (%): 385.47 (25), 381.76 (42), 147.66 (100).
4.2.5. 2-[4-(p-Tolylthiazol-2-yl)amino]-5-[(5-bromothiophen-2-yl)methylene]-4,5-dihydrothiazole-4-one (14)
1H NMR (DMSO-d6): δ 2.31 (s, 3H, CH3), 7.18–7.20 (m, 3H, Ar-H & thiazole-H), 7.25 (d, 1H, thiophene-H, J = 4.0 Hz), 7.31 (d, 1H, thiophene-H, J = 4.0 Hz), 7.44 (s, 1H, CH=C), 7.54 (s, 1H, NH), 7.78 (d, 2H, A-H, J = 7.5 Hz). 13C NMR: δ 20.87 (CH3), 109.08, 149.05, 167.55 (thiazole-C), 114.0, 116.58, 125.50, 129.11, 130.55, 131.72, 132.79, 133.22, 136.25, 142.77, 170.53 (Ar-C, thiazoline-C, thiophene-C, and arylidene-C), 179.42 (C=O). EI-MS, m/z (%): 460.93 (30), 462.90 (34%), 77.30 (100).
4.2.6. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-(4-methylbenzylidene)-4,5-dihydrothiazole-4-one (15)
1H NMR (DMSO-d6): δ 2.33 (s, 3H, CH3), 3.77 (s, 3H, OCH3), 6.94 (d, 2H, Ar-H, J = 8.5 Hz), 7.28 (d, 2H, Ar-H, J = 8.5 Hz), 7.33 (s, 1H, thiazole-H), 7.37 (s, 1H, CH=C), 7.45 (d, 2H, Ar-H, J = 7.5 Hz), 7.82 (d, 2H, Ar-H, J = 7.5 Hz). 13C NMR: δ 21.0 (CH3), 55.14 (OCH3), 107.68, 148.74, 167.74 (thiazole-C), 113.89, 124.14, 126.80, 128.41, 129.04, 129.59, 132.23, 132.78, 158.49, 159.89, 171.75 (Ar-C, thiazoline-C, and arylidene-C), 180.27 (C=O). EI-MS, m/z (%): 407.50 (7.21), 100.90 (100).
4.2.7. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-(4-methoxybenzylidene)-4,5-dihydrothiazole-4-one (16)
1H NMR (DMSO-d6): δ 3.77 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 6.94 (d, 2H, Ar-H, J = 8.0 Hz), 7.05 (d, 2H, Ar-H, J = 9.0 Hz), 7.32 (s, 1H, thiazole-H), 7.36 (s, 1H, CH=C), 7.51 (d, 2H, Ar-H, J = 8.5 Hz), 7.81 (d, 2H, Ar-H, J = 8.5 Hz). 13C NMR: δ 55.11 (OCH3), 55.31 (OCH3), 107.55, 148.72, 167.76 (thiazole-C), 113.90, 114.54, 124.10, 126.81, 128.08, 128.43, 129.0, 130.66, 158.49, 159.26, 171.83 (Ar-C, thiazoline-C, and arylidene-C), 180.42 (C=O). EI-MS, m/z (%): 423.50 (5), 139.04 (100).
4.2.8. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-(3,4-dimethoxybenzylidene)-4,5-dihydrothiazole-4-one (17)
1H NMR (DMSO-d6): δ 3.76 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 6.64 (d, 2H, Ar-H, J = 9.0 Hz), 7.09 (s, 1H, Ar-H), 7.13–7.15 (m, 2H, Ar-H), 7.32 (s, 1H, thiazole-H), 7.36 (s, 1H, CH=C), 7.81 (d, 2H, Ar-H, J = 8.5 Hz). 13C NMR: δ 55.10 (OCH3), 55.41 (OCH3), 55.58 (OCH3), 107.0, 148.76, 167.0 (thiazole-C), 112.02, 112.80, 113.86, 114.0, 122.50, 124.41, 126.77, 128.40, 131.50, 138.0, 148.93, 158.45, 172.0 (Ar-C, thiazoline-C, and arylidene-C), 180.50 (C=O). EI-MS, m/z (%): 453.40 (26), 117.45 (100).
4.2.9. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-(4-chlorobenzylidene)-4,5-dihydrothiazole-4-one (18)
1H NMR (DMSO-d6): δ 3.77 (s, 3H, OCH3), 6.94 (d, 2H, Ar-H, J = 8.0 Hz), 7.35 (s, 1H, thiazole-H), 7.39 (s, 1H, CH=C), 7.53 (d, 2H, Ar-H, J = 8.0 Hz), 7.58 (d, 2H, Ar-H, J = 8.0 Hz), 7.81 (d, 2H, Ar-H, J = 8.0 Hz). 13C NMR: δ 55.09 (OCH3), 107.86, 148.70, 167.50 (thiazole-C), 113.88, 122.56, 126.77, 128.98, 130.59, 132.41, 134.05, 134.53, 149.0, 157.40, 171.50 (Ar-C, thiazoline-C, and arylidene-C), 179.86 (C=O). EI-MS, m/z (%): 427.80 (13.03), 111.24 (100).
4.2.10. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-(2,4-dichlorobenzylidene)-4,5-dihydrothiazole-4-one (19)
1H NMR (DMSO-d6): δ 3.77 (s, 3H, OCH3), 6.94 (d, 2H, Ar-H, J = 8.0 Hz), 7.38 (s, 1H, thiazole-H), 7.59–7.73 (m, 4H, Ar-H & CH=C), 7.82 (d, 2H, Ar-H, J = 8.5 Hz). 13C NMR: δ 55.11 (OCH3), 108.14, 148.91, 167.46 (thiazole-C), 113.90, 117.78, 126.81, 128.05, 129.40, 129.47, 132.53, 132.93, 134.50, 137.57, 149.10, 159.80, 170.85 (Ar-C, thiazoline-C, and arylidene-C), 179.41 (C=O). EI-MS, m/z (%): 462.87 (100). (26), 117.45 (100).
4.2.11. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-[(thiophen-2-yl)methylene]-4,5-dihydrothiazole-4-one (20)
1H NMR (DMSO-d6): δ 3.78 (s, 3H, OCH3), 6.94 (d, 2H, Ar-H, J = 8.5 Hz), 7.18 (t, 1H, thiophene-H, J = 3.5 Hz), 7.37 (s, 1H, thiazole-H), 7.40 (d, 1H, thiophene-H, J = 3.5 Hz), 7.60 (s, 1H, CH=C), 7.73 (d, 1H, thiophene-H, J = 3.5 Hz), 7.81 (d, 2H, Ar-H, J = 8.5 Hz). 13C NMR: δ 55.09 (OCH3), 107.70, 148.50, 165.80 (thiazole-C), 113.87, 117.26, 126.78, 128.31, 128.39, 128.76, 130.04, 132.28, 140.79, 158.47, 171.95 (Ar-C, thiazoline-C, thiophene-C, and arylidene-C), 179.80 (C=O). EI-MS, m/z (%): 399.08 (42), 147.66 (100).
4.2.12. 2-[4-(4-Methoxyphenylthiazol-2-yl)amino]-5-[(5-bromothiophen-2-yl)methylene]-4,5-dihydrothiazole-4-one (21)
1H NMR (DMSO-d6): δ 3.77 (s, 3H, OCH3), 6.94 (d, 2H, Ar-H, J = 8.5 Hz), 7.25 (d, 1H, thiophene-H, J = 4.0 Hz), 7.31 (d, 1H, thiophene-H, J = 4.0 Hz), 7.36 (s, 1H, thiazole-H), 7.55 (s, 1H, CH=C), 7.81 (d, 2H, Ar-H, J = 8.5 Hz). 13C NMR: δ 55.10 (OCH3), 107.95, 149.96, 167.51 (thiazole-C), 112.05, 113.86, 116.47, 126.80, 127.44, 130.39, 131.68, 142.77, 158.50, 163.50, 170.40 (Ar-C, thiazoline-C, thiophene-C, and arylidene-C), 179.40 (C=O). EI-MS, m/z (%): 478.74 (16),104.22 (100).
4.3. Synthesis of 3-{2-([4-(4-Methoxyphenyl)thiazol-2-ylamino]-4-[4,5-dihydro-4-oxothiazol-5-ylidene])}5-methoxyindolin-2-one 23
A mixture of 2-[4-(4-methoxyphenyl)thiazol-2-ylamino]-3,4-dihydrothiazol-4-one 7 (1.53 g, 5.0 mmol), 5-methoxyindoline-2,3-dione 22 (886 mg, 5.0 mmol), and fused sodium acetate (820 mg, 10.0 mmol) in acetic acid (10 mL) was refluxed for 6 h. On cooling, the precipitated crude product was filtered, washed with water, dried, and crystallized from ethanol. 1H NMR (DMSO-d6): δ 3.56 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 4.89 (s, 1H, NH), 6.73 (d, 1H, indoline-H, J = 8.0 Hz), 6.82–6.84 (m, 1H, indoline-H), 6.94 (s, 1H, thiazole-H), 7.03 (d, 2H, Ar-H, J = 8.5 Hz), 7.70 (s, 1H, indoline-H), 7.90 (d, 2H, Ar-H, J = 8.0 Hz), 10.38 (s, 1H, indoline-NH). 13C NMR: δ 55.26 (OCH3), 55.29 (OCH3), 108.95, 149.96, 167.51 (thiazole-C), 110.42, 111.53, 114.20, 114.30, 114.40, 127.16, 128.83, 135.99, 151.06, 154.49, 159.51, 171.80 (Ar-C, indoline-C, and arylidene-C), 175.98 (C=O). EI-MS, m/z (%): 464.52 (30), 84.40 (100).
4.4. General Procedure for the Synthesis of the 1,3-Disubstituted Thiourea Derivatives 24, 26, and 27
Ethyl or benzyl isothiocyanate (0.025 mol) and sodium hydroxide (1.0 g, 0.025 mol) were added to a solution of the aminothiazoles 1 and 2 (0.025 mol) in DMF (15 mL), and the mixture was stirred for 1 h at room temperature. The reaction mixture was then poured onto ice-water (50 mL) and stirred for 30 min. The separated crude product was filtered, washed with water, dried, and crystallized from ethanol.
4.4.1. 1-Ethyl-3-[4-(p-tolylthiazol-2-yl)]thiourea (24)
1H NMR (DMSO-d6): δ 1.19 (t, 3H, CH2CH3, J = 7.0 Hz), 2.31 (s, 3H, CH3), 3.52 (q, 2H, CH2CH3, J = 7.0 Hz), 7.22 (d, 2H, Ar-H, J = 7.5 Hz), 7.44 (s, 1H, thiazole-H), 7.72 (d, 2H, Ar-H, J = 7.5 Hz), 9.55 (s, 1H, NH), 11.63 (s, 1H, NH). 13C NMR: δ 13.65 (CH2CH3), 20.81 (CH3), 39.50 (CH2CH3), 105.74, 148.54, 161.06 (thiazole-C), 125.51, 128.84, 129.3, 131.14, 137.25 (Ar-C), 177.49 (C=S).
4.4.2. 1-Ethyl-3-[4-(4-methoxyphenylthiazol-2-yl)]thiourea (26)
1H NMR (DMSO-d6): δ 1.19 (t, 3H, CH2CH3, J = 7.0 Hz), 3.55 (q, 2H, CH2CH3, J = 7.0 Hz), 3.77 (s, 3H, OCH3), 6.96 (d, 2H, Ar-H, J = 7.0), 7.35 (s, 1H, thiazole-H), 7.76 (d, 2H, Ar-H, J = 7.5 Hz), 9.60 (s, 1H, NH), 11.61 (s, 1H, NH). 13C NMR: δ 13.85 (CH2CH3), 20.81 (CH3), 39.50 (CH2CH3), 55.15 (OCH3), 104.54, 148.39, 161.04 (thiazole-C), 114.13, 126.59, 126.93, 159.04 (Ar-C), 177.45 (C=S).
4.4.3. 1-Benzyl-3-[4-(4-methoxyphenylthiazol-2-yl)]thiourea (27)
1H NMR (DMSO-d6): δ 3.76 (s, 3H, OCH3), 4.78 (s, 2H, benzylic CH2), 6.87 (d, 2H, Ar-H, J = 9.0), 7.31–7.44 (m, 8H, Ar-H and thiazole-H), 7.58 (d, 2H, Ar-H, J = 9.0 Hz), 10.16 (s, 1H, NH), 11.75 (s, 1H, NH). 13C NMR: δ 48.06 (benzylic CH2), 55.14 (OCH3), 104.61, 148.37, 161.10 (thiazole-C), 114.02, 126.87, 127.50, 127.87, 128.52, 128.68, 137.596, 159.02 (Ar-C), 177.64 (C=S).
4.5. General Procedure for the Synthesis of the 2-Substituted Imino-3-(4-arylthiazol-2-yl)thiazolidin-4-ones 28–31
A mixture of the appropriate thiourea derivative 24–27 (10 mmol), chloroacetic acid (0.95 g, 10 mmol), and fused sodium acetate (1.0 g) in ethanol (50 mL) was heated under reflux and refluxed for 8 h. On cooling, water (15 mL) was added with stirring for 4 h. The precipitated crude product was filtered, washed with water, dried, and crystallized from ethanol.
4.5.1. 2-Ethylimino-3-[4-(p-tolyl)thiazol-2-yl]thiazolidin-4-one (28)
1H NMR (DMSO-d6): δ 1.14 (t, 3H, CH2CH3, J = 7.0 Hz), 2.32 (s, 3H, Ar-CH3), 3.76 (q, 2H, CH2CH3, J = 7.0 Hz), 4.05 (s, 2H, thiazolidine-CH2), 7.24 (d, 2H, Ar-H, J = 7.5 Hz), 7.79 (s, 1H, thiazole-H), 7.84 (d, 2H, Ar-H, J = 7.5 Hz). 13C NMR: δ 12.44 (CH2CH3), 20.89 (Ar-CH3), 33.61 (thiazolidine-CH2), 37.76 (CH2CH3), 110.11, 151.03, 168.59 (thiazole-C), 125.69, 129.39, 131.30, 137.51 (Ar-C), 161.93 (thiazolidine-C2), 172.46 (C=O). EI-MS, m/z (%): 317.39 (43), 318.50 (62), 56.50 (100).
4.5.2. 2-Benzylimino-3-[4-(p-tolyl)thiazol-2-yl]thiazolidin-4-one (29)
1H NMR (DMSO-d6): δ 2.36 (s, 3H, CH3), 4.17 (s, 2H, thiazolidine-CH2), 4.97 (s, 2H, benzylic CH2), 7.25–7.36 (m, 7H, Ar-H), 7.75 (s, 1H, thiazole-H), 7.84 (d, 2H, Ar-H, J = 7.6 Hz). 13C NMR: δ 21.89 (Ar-CH3), 34.01 (thiazolidine-CH2), 46.12 (benzylic CH2), 110.68, 151.55, 168.76 (thiazole-C), 126.12, 128.0, 128.96, 129.84, 131.66, 135.0, 136.41, 138.04 (Ar-C), 162.27 (thiazolidine-C2), 173.17 (C=O). EI-MS, m/z (%): 379.75 (18), 331.22 (100).
4.5.3. 2-Ethylimino-3-[4-(4-methoxyphenyl)thiazol-2-yl]thiazolidin-4-one (30)
1H NMR (DMSO-d6): δ 1.14 (t, 3H, CH2CH3, J = 7.0 Hz), 3.78–3.79 (m, 5H, CH2CH3 & OCH3), 4.05 (s, 2H, thiazolidine-CH2), 6.99 (d, 2H, Ar-H, J = 8.0 Hz), 7.70 (s, 1H, thiazole-H), 7.87 (d, 2H, Ar-H, J = 8.0 Hz). 13C NMR: δ 12.45 (CH2CH3), 33.57 (thiazolidine-CH2), 37.76 (CH2CH3), 55.21 (OCH3), 108.89, 150.84, 168.53 (thiazole-C), 114.19, 126.80, 127.14, 161.79 (Ar-C), 161.79 (thiazolidine-C2), 172.45 (C=O). EI-MS, m/z (%): 334.92 (10), 333.35 (7), 65.54 (100).
4.5.4. 2-Benzylimino-3-[4-(4-methoxyphenyl)thiazol-2-yl]thiazolidin-4-one (31)
1H NMR (DMSO-d6): δ 3.78 (s, 3H, OCH3), 4.16 (s, 2H, thiazolidine-CH2), 4.95 (s, 2H, benzylic CH2), 6.99 (d, 2H, Ar-H, J = 9.0 Hz), 7.25–7.34 (m, 5H, Ar-H), 7.70 (s, 1H, thiazole-H), 7.87 (d, 2H, Ar-H, J = 9.0 Hz). 13C NMR: δ 33.57 (thiazolidine-CH2), 45.65 (benzylic CH2), 55.20 (OCH3), 109.05, 150.88, 168.23 (thiazole-C), 114.18, 127.13, 127.53, 127.62, 128.0, 128.41, 128.51, 136.02 (Ar-C), 161.76 (thiazolidine-C2), 172.17 (C=O). EI-MS, m/z (%): 396.38 (4), 395.01 (8), 196.81 (100).
4.6. General Procedure for the Synthesis of 5-Arylidene-2-(benzylimino)-3-[4-(4-methoxyphenyl)thiazol-2-yl]thiazolidin-4-ones 32 and 33
A mixture of compound 31 (791 mg, 2.0 mmol), the appropriate aromatic aldehyde (2.0 mmol), and piperidine (0.1 mL) in ethanol (20 mL) was heated under reflux and refluxed for 3 h, and the solvent was distilled off in vacuo. The residue was then washed with water, dried, and crystallized from ethanol.
4.6.1. 2-(Benzylimino)-5-(3,4-dimethoxybenzylidene)-3-[4-(4-methoxyphenyl)thiazol-2-yl]thiazolidin-4-one (32)
1H NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.22 (s, 2H, benzylic CH2), 6.93–6.97 (m, 3H, Ar-H), 7.17 (s, 1H, Ar-H), 7.21–7.23 (m, 2H, Ar-H), 7.26 (s, 1H, CH=C), 7.28–7.35 (m, 2H, Ar-H), 7.53 (d, 2H, Ar-H, J = 7.5 Hz), 7.82 (s, 1H, thiazole-H), 7.87 (d, 2H, Ar-H, J = 8.5 Hz). 13C NMR: δ 46.45 (benzylic CH2), 55.34 (OCH3), 55.98 (OCH3), 56.05 (OCH3), 108.59, 150.89, 168.35 (thiazole-C), 111.25, 111.59, 113.98, 120.5, 125.86, 126.75, 127.26, 127.91, 128.53, 128.93, 133.78, 135.87, 149.0, 149.25, 152.12, 154.60, 159.61 (Ar-C, thiazolidine-C, and CH=C), 166.66 (C=O). EI-MS, m/z (%): 543.37 (28), 198.04 (100).
4.6.2. 2-(Benzylimino)-5-(2,4-dichlorobenzylidene)-3-[4-(4-methoxyphenyl)thiazol-2-yl]thiazolidin-4-one (33)
1H NMR (CDCl3): δ 3.88 (s, 3H, OCH3), 5.21 (s, 2H, benzylic CH2), 6.95 (d, 2H, Ar-H, J = 9.0 Hz), 7.17 (s, 1H, Ar-H), 7.26–7.36 (m, 5H, Ar-H & CH=C), 7.52–7.55 (m, 3H, Ar-H), 7.68 (d, 1H, Ar-H, J = 9.0 Hz), 7.81–7.83 (s, 1H, Ar-H and thiazole-H), 8.10 (s, 1H, Ar-H). 13C NMR: δ 46.62 (benzylic CH2), 55.40 (OCH3), 108.89, 152.24, 168.30 (thiazole-C), 114.09, 127.32, 127.41, 128.59, 129.06, 153.87, 159.65 (Ar-C, thiazolidine-C, and CH=C), 165.84 (C=O). EI-MS, m/z (%): 552.75 (18), 196.32 (100).
4.7. Determination of In Vitro AChE Inhibitory Activity
The AChE inhibitory activity of the compounds 8, 10, 12–21, 23, and 28–33 was measured using Ellman’s colorimetric method.22,23 The concentration needed to inhibit half of the maximum biological response (IC50) was determined by constructing an absorbance and/or inhibition (%) curve and examining the effect of four different concentrations. Stock solutions of the compounds and donepezil hydrochloride in DMSO were prepared at concentrations of 0.01–10 μM. AChE was dissolved in 0.1 M phosphate-buffered saline (PBS) (pH 8.0) to obtain a solution of 0.35 U/mL. The compounds were dissolved in DMSO and diluted with the 0.1 M PBS to yield the corresponding test concentrations. AChE (20 μL) was incubated with 10 μL of tested compounds, 130 μL of 0.1 M PBS, and 20 μL of acetylthiocholine iodide (CAS 2260-50-6) for 10 min in 96-well microplates before the addition of 20 μL of 3.33 mM 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB, CAS # 69-78-3) solution. After the addition of DTNB, the 96-well microplates were read at 412 nm with a microplate reader for 15 min. One triplicate sample without inhibitors was always present to yield 100% AChE activity. All samples were assayed in triplicate.
4.8. Molecular Docking Method
Computer-assisted docking was carried out using the CHARMM force field and electric human AChE co-crystallized with donepezil (PDB ID: 4EY7), which was recovered from the Brookhaven Protein Database (www.rcsb.org/pdb). Docking simulations were performed on the test compounds using MOE24 with the following protocol: (1) the AChE enzyme structure was checked for missing atoms, bonds, and contacts. (2) Hydrogens and partial charges were added using the protonate 3D application in MOE. (3) The ligands were imported into MOE and were energy-minimized. (4) Ligands were docked within the AChE active site using the MOE Dock application, and the poses were generated by the Triangle Matcher placement method. (5) The retained best poses were visually inspected, and the interactions with the binding pocket residues were analyzed. To determine the accuracy of this docking protocol, the co-crystallized ligand, donepezil, was redocked into the AChE active site. This procedure was repeated three times, and the best ranked solutions of donepezil exhibited rmsd values of less than 0.5 Å from the position of the co-crystallized ligand. In general, rmsd values smaller than 2.0 Å indicate that the docking protocol is capable of accurately predicting the binding orientation of the co-crystallized ligand.25,26 This protocol was thus deemed to be suitable for the docking of inhibitors into the active site model of AChE.
Acknowledgments
The authors would like to thank the Holding Company for Biological Products & Vaccines (VACSERA), Cairo, Egypt, for performing the in vitro AChE inhibitory activity assay.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02549.
Elemental analyses’ data (C, H, N, and S) of the synthesized compounds 8, 10–21, 23, 24, and 26–33 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Matej R.; Tesar A.; Rusina R. Alzheimer’s disease and other neurodegenerative dementias in comorbidity: A clinical and neuropathological overview. Clin. Biochem. 2019, 73, 26–31. 10.1016/j.clinbiochem.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015, 11, 332–384. 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- King A.; Bodi I.; Troakes C. The neuropathological diagnosis of Alzheimer’s disease-the challenges of pathological mimics and concomitant pathology. Brain Sci. 2020, 10, 479. 10.3390/brainsci10080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B. A.; Blazey T. M.; Su Y.; Hari-Raj A.; Dincer A.; Flores S.; Christensen J.; McDade E.; Wang G.; Xiong C.; Cairns N. J.; Hassenstab J.; Marcus D. S.; Fagan A. M.; Jack C. R. Jr.; Hornbeck R. C.; Paumier K. L.; Ances B. M.; Berman S. B.; Brickman A. M.; Cash D. M.; Chhatwal J. P.; Correia S.; Förster S.; Fox N. C.; Graff-Radford N. R.; la Fougère C.; Levin J.; Masters C. L.; Rossor M. N.; Salloway S.; Saykin A. J.; Schofield P. R.; Thompson P. M.; Weiner M. M.; Holtzman D. M.; Raichle M. E.; Morris J. C.; Bateman R. J.; Benzinger T. L. S. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 2018, 17, 241–250. 10.1016/S1474-4422(18)30028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H.; Thal D. R.; Ghebremedhin E.; Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. 10.1097/nen.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Masters C. L.; Simms G.; Weinman N. A.; Multhaup G.; McDonald B. L.; Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 1985, 82, 4245–4249. 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I.; Iqbal K.; Tung Y. C.; Quinlan M.; Wisniewski H. M.; Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 1986, 83, 4913–4917. 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalley S. E. Expanding therapeutic opportunities for neurodegenerative diseases: A perspective on the important role of phenotypic screening. Bioorg. Med. Chem. 2020, 28, 115239. 10.1016/j.bmc.2019.115239. [DOI] [PubMed] [Google Scholar]
- Ballard C.; Gauthier S.; Corbett A.; Brayne C.; Aarsland D.; Jones E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Zemek F.; Drtinova L.; Nepovimova E.; Sepsova V.; Korabecny J.; Klimes J.; Kuca K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expet Opin. Drug Saf. 2014, 13, 759–774. 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- Gümüş M.; Yakan M.; Koca İ. Recent advances of thiazole hybrids in biological applications. Future Med. Chem. 2019, 11, 1979–1998. 10.4155/fmc-2018-0196. [DOI] [PubMed] [Google Scholar]
- Jiang X.-Y.; Chen T.-K.; Zhou J.-T.; He S.-Y.; Yang H.-Y.; Chen Y.; Qu W.; Feng F.; Sun H.-P. Dual GSK-3β/AChE Inhibitors as a New Strategy for Multitargeting Anti-Alzheimer’s Disease Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 171–176. 10.1021/acsmedchemlett.7b00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt B. Z.; Gazioglu I.; Basile L.; Sonmez F.; Ginex T.; Kucukislamoglu M.; Guccione S. Potential of aryl-urea-benzofuranylthiazoles hybrids as multitasking agents in Alzheimer’s disease. Eur. J. Med. Chem. 2015, 102, 80–92. 10.1016/j.ejmech.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Kurt B. Z.; Gazioglu I.; Sonmez F.; Kucukislamoglu M. Synthesis, antioxidant and anticholinesterase activities of novel coumarylthiazole derivatives. Bioorg. Chem. 2015, 59, 80–90. 10.1016/j.bioorg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Shidore M.; Machhi J.; Shingala K.; Murumkar P.; Sharma M. K.; Agrawal N.; Tripathi A.; Parikh Z.; Pillai P.; Yadav M. R. Benzylpiperidine-linked diarylthiazoles as potential anti-Alzheimer’s agents: Synthesis and biological evaluation. J. Med. Chem. 2016, 59, 5823–5846. 10.1021/acs.jmedchem.6b00426. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Zhu Y.; Ran K.; Liu Z.; Wang N.; Feng Q.; Zeng J.; Zhang L.; He B.; Ye T.; Zhu S.; Qiu X.; Yu L. Synthesis and biological evaluation of N-(4-phenylthiazol-2-yl)cinnamamide derivatives as novel potential anti-tumor agents. Med. Chem. Commun. 2015, 6, 1036–1042. 10.1039/c4md00573b. [DOI] [Google Scholar]
- Hassan G. S.; El-Messery S. M.; Al-Omary F. A. M.; El-Subbagh H. I. Substituted thiazoles VII. Synthesis and antitumor activity of certain 2-(substituted amino)-4-phenyl-1,3-thiazole analogs. Bioorg. Med. Chem. Lett. 2012, 22, 6318–6323. 10.1016/j.bmcl.2012.08.095. [DOI] [PubMed] [Google Scholar]
- Havrylyuk D.; Mosula L.; Zimenkovsky B.; Vasylenko O.; Gzella A.; Lesyk R. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 5012–5021. 10.1016/j.ejmech.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Dhakar D.; Ojha S.; Jat J. L.; Talesara G. L. Synthesis and characterization of some 3-N-alkoxyphthalimido-5-arylidene-2-{[4-(4-substituted phenyl)-1,3-thiazol-2-yl]imino}-1,3-thiazolidin-4-ones. J. Indian Chem. Soc. 2008, 85, 660–664. [Google Scholar]
- Hosseinzadeh N.; Hasani M.; Foroumadi A.; Nadri H.; Emami S.; Samadi N.; Faramarzi M. A.; Saniee P.; Siavoshi F.; Abadian N.; Mahmoudjanlou Y.; Sakhteman A.; Moradi A.; Shafiee A. 5-Nitro-heteroarylidene analogs of 2-thiazolylimino-4-thiazolidinones as a novel series of antibacterial agents. Med. Chem. Res. 2013, 22, 2293–2302. 10.1007/s00044-012-0224-6. [DOI] [Google Scholar]
- Lakhan R.; Sharma B. P.; Shukla B. N. Synthesis and antimicrobial activity of 1-aryl-2-amino-3-(4-arylthiazol-2-yl)/(benzothiazol-2-yl)guanidines. Farmaco 2000, 55, 331–337. 10.1016/s0014-827x(00)00032-x. [DOI] [PubMed] [Google Scholar]
- Magnotti R. A. Jr.; Eberly J. P.; Quarm D. E.; McConnell R. S. Measurement of acetylcholinesterase in erythrocytes in the field. Clin. Chem. 1987, 33, 1731–1735. 10.1093/clinchem/33.10.1731. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V. Jr.; Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Molecular Operating Environment MOE, Version 2019.01. Available from: https://www.chemcomp.com/Products.htm.
- Binda C.; Li M.; Hubálek F.; Restelli N.; Edmondson D. E.; Mattevi A. Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 9750–9755. 10.1073/pnas.1633804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström J.; Greenwood J. R.; Gottfries J. Assessing the performance of OMEGA with respect to retrieving bioactive conformations. J. Mol. Graphics Modell. 2003, 21, 449–462. 10.1016/S1093-3263(02)00204-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.