Abstract
The National Heart, Lung, and Blood Institute of the National Institutes of Health, together with the Longfonds BREATH consortium, convened a working group to review the field of lung regeneration and suggest avenues for future research. The meeting took place on May 22, 2019, at the American Thoracic Society 2019 conference in Dallas, Texas, United States, and brought together investigators studying lung development, adult stem-cell biology, induced pluripotent stem cells, biomaterials, and respiratory disease. The purpose of the working group was 1) to examine the present status of basic science approaches to tackling lung disease and promoting lung regeneration in patients and 2) to determine priorities for future research in the field.
Keywords: lung regeneration, stem cells, chronic lung disease, lung injury
During healthy life, the lung is a quiescent but highly complex organ with capacity for regeneration after injury. However, the mechanisms of lung regeneration and the factors that determine its outcome (i.e., productive restoration of homeostasis or destructive remodeling and scarring in disease) need to be better understood to realize the ambition of proregenerative therapies. Stem-cell populations have been described in the submucosal glands, the conducting airways, and the alveolar epithelium, with basal cells (proximal airways), club cells (distal airways), and alveolar type II (AT2) cells (alveolus) capable of self-renewal and differentiation toward region-specific cell types (1). These stem cells equip the lung to regenerate functional tissue from resident progenitors during homeostasis. However, with age and after severe or repetitive injury, these mechanisms can be overwhelmed and the response becomes characterized by remodeling, an adaptive but often irreversible process that prevents severe respiratory failure at the expense of long-term tissue function (2). As an example, mouse lungs exposed to severe infection with a mouse-adapted H1N1 strain of influenza A demonstrate abnormal appearance of KRT5+ cells in the distal airways and alveoli (3). Although these KRT5 “pods”—which bear striking similarity to peribronchiolar basaloid pods described in human cases (4)—might help to restore barrier function immediately after infection, they can persist and likely preclude optimal gas exchange (5).
Chronic lung diseases in patients are characterized by aberrant regeneration and failure to restore tissue homeostasis; thus, therapy development hinges on understanding how disease pathogenesis causes regenerative failure, at the same time as understanding how the lung is created and matures throughout pre- and postnatal lung development. To advance this cause, the National Heart, Lung, and Blood Institute and the Lung Foundation Netherlands (Longfonds) assembled a multidisciplinary working group for advancing lung regeneration work, involving basic, translational, and clinical investigators in May of 2019. The working group brainstormed opportunities for future lung regeneration research with a focus on identifying knowledge gaps whose resolution would help to bring research progress closer to the clinic for patients with chronic lung diseases. The meeting comprised four sessions, which addressed the subjects of technology development in lung research, cell therapy, bioengineering, and the challenges of understanding the human lung, followed by a group discussion on emerging themes and priorities. This report summarizes the topics highlighted, suggests areas of future investigation, and provides recommendations for moving the field forward.
Developing Tools to Study Lung Regeneration
To treat respiratory disease, it will be necessary to understand the mechanisms of development and adult homeostasis, as well as pathological deviations from these. Thanks to rapid advances in “omic” technologies and decreasing associated costs, it is becoming possible to study human patients in new detail. Practical and affordable whole-genome and whole-exome sequencing allow us to understand genotype and somatic alteration, whereas single-cell and single-nucleus RNA-sequencing technologies reveal the cellular actors of disease in new depths of resolution. In the lung, these technologies have already described epithelial-cell diversity and the processes relevant to regeneration by, for example, mapping the differentiation trajectories of epithelial progenitors during homeostasis (6, 7) and regeneration (8); defining rare cell populations such as the ionocyte population (9, 10), a population of CFTR-rich airway epithelial cells; and allowing deep phenotyping of lung tissues in smokers (11, 12), individuals with asthma (13), patients chronic obstructive pulmonary disease (COPD) (14–16), and patients with pulmonary fibrosis (16–21). Consortia efforts are now underway to characterize the processes of postnatal lung development (LungMAP [22]), homeostasis, and disease (Human Lung Cell Atlas [23]), and it will also be informative to analyze human embryonic and fetal lung development in utero (24). Because a majority of patients recover fully from serious insults during bacterial or viral pneumonia and acute respiratory distress syndrome, insights might also be gained by characterizing successful regeneration in the context of disease resolution.
Over the past decade, technology development has also substantially broadened the range of in vitro model systems available for the study of primary lung epithelial cells. New or improved human cell culture systems allow expansion of fetal lung-progenitor cells (25, 26), adult regional epithelial stem cells (27–29), and induced pluripotent stem cell (iPSC)-derived airway (30–32) and alveolar (33) epithelial cells. Notably, human alveolar epithelial cells change to resemble AT1-like cells in two-dimensional cultures and largely fail to establish the specialized cell types of the alveolus in three-dimensional organoids. However, methods for culturing murine and human AT2 cells as long-term proliferating organoid cultures have been a significant advance (34–37). The air–liquid interface culture system (38), in which freshly isolated or cultured basal cells are differentiated to recapitulate the pseudostratified epithelium, affords the airway field a robust primary cell culture system for studying differentiation and for subjecting the multiciliated epithelium to apical exposures. However, the throughput and cellular complexity of such cultures is low. Recent advances mean that it is now possible to culture the lung epithelium in a variety of other three-dimensional formats: organoids are predominantly reliant on culture in Matrigel basement-membrane extract but increasingly can be cultured in biological (39) or synthetic (40) alternatives, are feasible in high-throughput assays, and can be derived from both human (41, 42) and mouse (43) lungs. Lung-on-a-chip models have been developed, which, although low-throughput, allow recombination of the epithelium with mesenchymal and immune populations, the generation of directional flow, and the application of force to mimic the physical influence of breathing (44). At larger scale, bioreactor culture systems have the potential to allow tissue- and organ-scale investigations. An additional approach has been to maintain human tissue ex vivo for sufficient periods to perform experiments. At the whole-organ level, this is possible using ex vivo lung perfusion (45), a technique used to recondition and preserve lungs in the context of transplantation. Addition, after surgical resection of lung tissue, precision-cut lung slices can be maintained for periods of weeks to study complex cellular interactions within the native tissue organization (46). These approaches are potentially useful when tissue from diseased human lungs is available (47), but primary tissue is a scarce resource in many centers. Furthermore, there is a lack of standardization of many of these assays between laboratories at present, making it difficult to compare data.
iPSCs have added to the tool set available to model human lung disease, in theory having the capacity to provide an unlimited source of autologous cells for both in vitro modeling of disease and with the potential for cellular therapy. iPSCs can offer a number of advantages over primary cells or cell lines: they reflect the complete genotype of an individual, they can be propagated long-term in vitro, and they can be readily gene edited and clonally expanded. Purification of specific cell types throughout the differentiation process has been challenging because of a lack of cell-specific cell surface markers; protocols have been aided by the use of gene-editing approaches to knock-in reporters, specifically for the primordial lung-progenitor marker NKX2.1 and for SFTPC (33, 48). Although significant progress has been made in defining protocols to differentiate iPSCs into mature lung cells, more needs to be done to establish conditions representing all mature adult human lung cells (25, 30, 32, 48–51), and the field is only just starting to appreciate the extent of epithelial-cell heterogeneity in the human airway and alveolus. How close the iPSC-derived lung cells are to the cells of the adult human lung is currently under investigation. Although iPSC-derived alveolar cell culture protocols have advanced to the point at which a self-renewing population of AT2 cells can be generated through passage in spheroid format (33), it remains to be determined if they represent the full differentiation and functional potential of adult human AT2 cells.
In vivo animal models of lung injury have been a mainstay of regeneration research, and much has been gleaned from mouse models. However, given the limitations imposed on the preclinical development pathway by differences between murine and human lung biology (discussed below), the gap between basic lung biology and clinical translation is considerable, with just 3% of respiratory drug candidates reaching patients (52). Rabbits, ferrets, pigs, sheep, and rhesus monkeys all have respiratory systems that more closely resemble humans in some aspect, but large animal models are expensive and impractical for the majority of centers to develop and use (53). These less-frequently studied species also have downsides relating to genomic annotation, reagent availability, and protocol development. For example, iPSCs from pigs are still difficult to maintain in their undifferentiated state, and iPSCs from ferrets have yet to be established, limiting the tools available to study lung biology in these species. Regardless of species, histopathology has been the traditional means to determine the extent of regeneration in animals, but technology advances now allow more nuanced readouts by, for example, using genetic barcoding, advanced imaging (54), or single-cell RNA sequencing (55). These techniques will allow investigators to unravel the heterogeneity that is intrinsic to models and also present in patients. Regeneration occurring in severely damaged areas is likely to be governed by rules different from those occurring in less damaged regions, and there might be cross-talk between these that we do not yet fully appreciate—could a future therapy promote replenishment of damaged tissue by progenitors from less-affected regions? Such technologies will also be powerful tools in combination with assays that assess functional regeneration versus remodeling responses, such as pulmonary function tests including assessments of airflow and gas exchange.
Future Cell Therapies for Lung Disease
Multiple approaches have been taken to deliver cells to lungs, with the ultimate aim of functional restoration of lung tissue. Mesenchymal stromal cells (MSCs) have been widely used in regenerative medicine approaches as a result of the ease of their isolation and culture, their immunomodulatory effects, and the opportunity to use allogeneic cells as a result of immune privilege (56). MSCs have been widely trialed and have been shown to be safe in early-phase trials, including in chronic lung diseases, in which they might be of particular relevance given that they rapidly localize to the lungs after infusion. Although preclinical studies suggest safety in settings such as acute respiratory distress syndrome, allergic asthma, and emphysema, clinical trials have thus far failed to demonstrate therapeutic value. An overall lack of mechanistic insight into the therapeutic effects of MSCs in preclinical studies means that a large number of possible protocol modifications are now being explored to improve outcomes, including the MSC source (bone marrow, adipose, umbilical cord, etc.), isolation and culture methods, preconditioning (using hypoxia, serum starvation, oxidative stress, etc.), genetic modification approaches, and using MSC derivatives (exosomes, conditioned medium, etc.).
A major limitation of MSC therapy in the context of regeneration might be their short engraftment period. Live MSCs have been detected in the lungs 2 weeks after infusion (and 2 mo after infusion in skin), but the long-term engraftment of MSCs is limited (57). Although circulating bone marrow–derived MSCs were previously suggested to engraft and transdifferentiate within the lung epithelium, these cells were subsequently shown to definitively lack epithelial-lineage potential (58). Thus, although MSCs might be used as a tool to cyclically dampen inflammation and promote repair, they have not yet been validated as a truly corrective therapy. In pursuit of long-term engraftment in the lung and encouraged by improved cell culture methodologies, investigators are now considering alternative cell sources, including embryonic stem cell–derived AT2 cells, adult AT2 cells, lineage-negative epithelial progenitor cells, and fetal lung tissue. It is important to note that—despite the nascency of this field—epithelial-cell transplantation has been performed in patients, with culture-expanded SOX9+ human basal cells delivered to patients with bronchiectasis (59). Given these developments, it will be important for the field to acknowledge the potential to generate enthusiasm among patients for unlicensed stem-cell therapies (60) and attempt to direct resources and attention toward studied, validated therapies and further research and development of techniques (61).
With current techniques, it should be possible to assess the safety and potential of human progenitor cells in murine transplantation models and lung disease models, while also comparing alternative delivery methods and immunosuppressed mouse strains. Efforts to compare cell types and culture protocols directly would be of benefit, particularly using methods that unambiguously and comprehensively locate and quantify cells after xenotransplantation. In moving from small animal models into large animals and patients, cell delivery is a key challenge because delivery methods used in rodents are poorly applicable to large animal models and humans. To date, little cell engraftment is observed in the absence of recipient conditioning, which is generally achieved by causing extensive lung injury that would be unacceptable in clinical studies. Therefore, translational studies that investigate plausible cell delivery methods, perhaps drawing from the successful translation of bone marrow, epidermal, and corneal stem cell–based therapies or building on existing bronchoscopic or surgical techniques, will be required to advance the field. Gene-editing technology might also be used to determine donor-cell characteristics that promote engraftment and therapeutic effect.
Scale is also challenging for manufacture, and care must be taken that favorable characteristics of culture-expanded cells are not lost during scale-up. Using a rationale similar to that of gene-therapy approaches, total replacement of a cell population may not be necessary if introducing a subset can suffice. Indeed, restoring CFTR in 25% of epithelial cells can restore function in vitro (62), and recent evidence shows that correcting around 20% of lung epithelial cells (including AT2 stem cells) using in utero, intraamniotic delivery of adenoviral CRISPR-Cas9 gene-editing vectors can rescue the perinatal lethal congenital lung disease phenotype of Sftpc mutations in mice (63).
Although most efforts focus on the epithelium, it may become interesting to consider other cell types as more becomes understood about lung mesenchymal heterogeneity (64). Future therapies might enable the normalization of the diseased matrix, a particular problem in the context of cell therapy, as delivering cells to a disease niche may limit the efficiency of engraftment and the efficacy of therapies. There is some evidence for this concept from in vitro studies of recellularized lung scaffolds, where donor age and disease status affect the efficiency of reseeding (65), and from MSC infusions in patients, in whom lungs with milder disease retain MSCs for longer (66).
Although cell therapy clearly needs substantial preclinical work before it becomes feasible in routine clinical practice, an alternative avenue for more immediate exploitation is the mobilization of endogenous progenitor cells for repair. Deciphering pathways that enable progenitor-cell expansion in the context of diseased lungs will require not only an improved understanding of the pathways that control lung-progenitor self-renewal and differentiation but also work to determine how these cellular processes become dysfunctional during healthy aging and in disease microenvironments. Perhaps separable from future use in cell therapy, it is imperative for the field to have robust methods to test the potential of lung-cell populations in vivo as another strategy to delineate key pathways and molecules that may be altered in lung disease.
Bioengineering the Lung
The long-term objective of the field of lung bioengineering is to combine cell and materials approaches to produce functional, engineered lung tissue that could alleviate donor shortages and the requirement for immunosuppression in lung transplantation, which is indicated in end-stage COPD, pulmonary fibrosis, and cystic fibrosis, among other lung diseases. This clinical aspiration remains distant, in large part due to the cellular complexity of the lung (>50 cell types), but valuable insights into the lung extracellular matrix (ECM), the nature of cell–ECM interfaces, and the effects of mechanical force in lung regeneration are being generated. Technology development has led to novel approaches to expand cells at different scales in lung-on-a-chip devices or bioreactors, to maintain lung tissue in ex vivo lung slices or in ex vivo lung perfusion models, and to isolate high-quality lung ECMs for basic and translational research.
Lung decellularization techniques have improved markedly, with species-, tissue- and disease-specific protocols having been developed. Healthy and diseased ECMs can be compared using this approach, and because intraindividual differences are retained in patient decellularized lung scaffolds, these represent an opportunity to study the diseased ECM in detail. Efforts to recellularize scaffolds are currently relatively simplistic, with much focus being placed on the ability of scaffolds to support cells in a broad sense, often using immortalized cell lines. Increasingly, these studies have turned to primary cells to improve human relevance, seeding epithelial or endothelial cells. Although maintenance of both has been shown, the targeted delivery of cells to particular tissue locations is challenging, and the functionality of regeneration in these studies is difficult to ascertain. Restoration of functional interactions between the epithelium and the endothelial-, mesenchymal-, and immune-cell compartments is likely to be critically important given the roles of these cell types as niche cells in vivo (67–69) and the crucial role of ECM remodeling in lung disease pathogenesis (70), so efforts to generate increasingly complex recellularized structures will reveal new biology.
Additional insight might be gained by applying the bioengineering approach more broadly within regeneration research; investigating encapsulation, homing, and targeting of cells could overcome challenges in cell therapy; methods to focally decellularize and recellularized lung tissue would be beneficial; and engineered organoid techniques to assess hydrogel substrates, determine the influences of physical force, and acquire scalable platforms would expand our repertoire. Three-dimensional printing potentially also offers opportunities for progress, either by generating custom-made laboratory and/or surgical tools, or even by patterning cells and/or scaffolds.
Beyond Model Organisms
Much of our understanding of lung regeneration has been gleaned from studies in mice, whose genetic tractability has allowed precise delineation of stem-cell populations and interrogation of the roles of candidate genes, but there are major differences between mouse and human lung biology and anatomy (summarized in Table 1). Although there is broad conservation of stem and progenitor populations, with basal cells serving as upper airway progenitors and AT2 cells maintaining the alveolus, the relevance of club cells to human epithelial turnover remains unclear. Although club cells are generated from basal cells in human small airways (71), the composition and maintenance of the respiratory bronchiole, a simple epithelium within the smallest airways that is unique to humans, are very poorly understood and might be maintained by club-cell progenitors, as is seen in the intrapulmonary airways of mice.
Table 1.
Summary of Major Differences between Human and Mouse Lungs
| Human | Mouse | |
|---|---|---|
| Size | Trachea, 1.5–2 cm; >20 airway generations | Trachea, 1.5 mm; 13–17 airway generations |
| Mechanical forces | Mostly upright | Mostly prone |
| Cartilage | Trachea and intrapulmonary bronchi for several bronchial generations | Trachea and only extrapulmonary bronchi |
| Submucosal glands | Throughout cartilaginous airways | First three cartilage rings of trachea |
| Epithelial composition | Goblet cells, low number of club cells restricted to small airways | Few goblet cells (unless injured), club cells line all conducting airways |
| Basal cells | Trachea and extrapulmonary and intrapulmonary bronchi, extending to terminal bronchioles | Trachea and extrapulmonary bronchi only |
| Neuroendocrine cells | Cells found throughout airway epithelium; clusters found only within intrapulmonary airways | Mostly clustered |
| Respiratory bronchiole | Present | Absent |
| Proximal–distal patterning | SOX2 and SOX9 co-expression in tip progenitors in pseudoglandular stage | Sox2 expression absent in Sox9+ tip progenitors in pseudoglandular and canalicular stages |
| Developmental timing | Alveologenesis initiates before birth | Alveologenesis initiates after birth |
Definition of abbreviation: SOX = SRY-box transcription factors.
Perhaps owing to these differences in underlying biology, modeling some human lung disease in mice has proved challenging. In monogenic lung disease, there is variability in different genes for the similarity observed in mouse models and related human patients. When CFTR-knockout mice were created, some tissues recapitulated the phenotype well, but the critical airway ion transport defect that is the source of destructive lung disease in patients was not present because of differences in the acidity of human and mouse airway surface liquid (72). Conversely, recent data have indicated that a mouse model with a knock-in of the familial fibrosis-associated mutation SFTPCI73T can recapitulate several pulmonary and biomarker aspects of human fibrotic disease (73). In addition, injury models of clear utility in studying mice have variable relation to human disease. The bleomycin-induced lung injury model has been used as a surrogate for pulmonary fibrosis research, but the model has many drawbacks, including the fact that it is an acute injury that resolves in younger mice, making it poorly representative of the progressive disease course seen in patients. Conversely, the H1N1 influenza model has been widely adopted in recent years because of the similarity of injury seen in mice and humans. The regenerative capacity of mouse models has been seen as a drawback, but in fact, this resolution phase may provide an opportunity to understand new aspects that have heretofore been poorly characterized (74), potentially offering insight into how to achieve successful regeneration. Increasingly, chronic injury models have been sought, including using repeated, lower-dose exposure to bleomycin, but the acute nature of mouse injury models is a widespread limitation. Of note, the fibrotic phenotype of older mice is markedly different from the typically young mice studied in most applications, and few studies have focused on age-related decline in regeneration capacity because of the cost of working with aged animals. Such data would likely be relevant, as most lung disease occurs on an aged lung background.
Conclusions
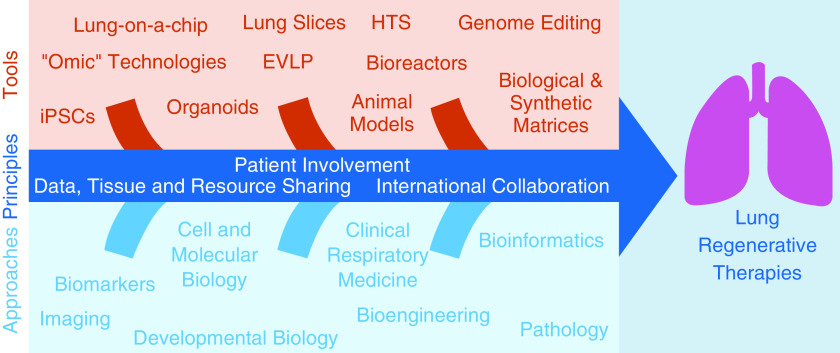
Despite significant recent advances, it is clear that extensive further work on lung regeneration is required to provide effective and realistic treatment options for the various pathologies causing lung tissue destruction, many of which are a direct consequence of failed regeneration. Considering the complexity of the lung, progress can only be made through a multidisciplinary approach, combining expertise in studying lung development, adult stem-cell biology, iPSCs, biomaterials, and respiratory disease (Figure 1). We have set the following specific scientific priorities and recommendations to bring us closer to effective lung regeneration.
Figure 1.
An integrated approach to the development of lung regenerative therapies. EVLP = ex vivo lung perfusion; HTS = high-throughput screening; iPSC = induced pluripotent stem cell.
Scientific Priorities to Advance Lung Regeneration
-
•
Deeply phenotype well-annotated patient samples using the suite of genomic, transcriptomic, epigenomic, and proteomic tools available, following up discoveries using in vitro and in vivo model systems.
-
•
Recognize and further investigate possible differences in lung biology among patients of different ages, sexes, and ethnic backgrounds.
-
•
Develop small and large animal models that recapitulate key aspects of human lung disease.
-
•
Take a multicenter approach to developing and implementing large animal models of lung disease.
-
•
Develop standards for in vitro model systems and in vivo transplantation methods, promoting working with well-characterized and transferable techniques.
-
•
Develop more complex model systems based on patients; for example, by considering aging, comorbidities, and exacerbations.
-
•
Refine human airway and alveolar in vitro model systems.
-
•
Develop new models and assays for understanding the cellular constituents and cell–cell interactions that regulate gas exchange in the human alveolar compartment and use these to compare both chronic, high-incidence lung diseases and rare lung diseases with related phenotypes, such as COPD and alpha-1 antitrypsin disorder.
-
•
Optimize emerging ex vivo assays for studying human lung-cell regeneration, including assays of organoids, tissue explants, and artificial matrices, to include additional cell types and architecture.
-
•
Develop new technologies for tracking cellular identity and responses in ex vivo human assay systems that include cell barcoding, emerging single-cell assays, and bioinformatic assessment of cell identity and relationships.
-
•
Leverage HTS technologies to identify transcriptional and signaling pathways that could be targeted by future therapies to awaken the facultative regenerative response in the lung.
-
•
Better understand the role of the lung scaffold in cellular regeneration and the requirements needed for lung repair.
-
•
Delineate the key factors that orchestrate functional repair and dysfunctional remodeling.
Recommendations to Advance These Aims
-
•
Actively promote the cause of evidence-based policy and education on the subject of stem cell–based therapies.
-
•
Develop and validate biobank platforms to enable more widespread access to viable human cells, viable and fixed tissue, and ECMs, including from patients with lung disease.
-
•
Encourage multidisciplinary team building that will be required to unravel new complexity; in particular, support integrated studies linking cell and developmental biologists with (bio)engineers and (bio)informaticians to tackle the complexities of lung disease and regeneration.
Acknowledgments
Acknowledgment
The authors thank the Dutch Lung Foundation Netherlands (Longfonds) for their support of this meeting and the wider BREATH consortium. Furthermore, they thank the investigators who also attended the meeting: Zea Borok (University of Southern California, Los Angeles, California), Jim S. Hagood (The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina), Connie C. W. Hsia (University of Texas Southwestern, Dallas, Texas), Finn Hawkins (Boston University, Boston, Massachusetts), Katie L. Leiby (on behalf of Laura Niklason; Yale University, New Haven, Connecticut), Anne K. T. Perl (Cincinnati Children’s Hospital, Cincinnati, Ohio), Hans-Willem Snoeck (Columbia University, New York, New York), Daniel J. Tschumperlin (Mayo Clinic, Rochester, Minnesota), Darcy E. Wagner (Lund University, Lund, Sweden), Jeffrey A. Whitsett (Cincinnati Children’s Hospital, Cincinnati, Ohio), and Yan Xu (Cincinnati Children’s Hospital, Cincinnati, Ohio).
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2020-0397WS on February 22, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor MS, Chivukula RR, Myers LC, Jeck WR, Waghray A, Tata PR, et al. A conserved distal lung regenerative pathway in acute lung injury. Am J Pathol. 2018;188:1149–1160. doi: 10.1016/j.ajpath.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanegai CM, Xi Y, Donne ML, Gotts JE, Driver IH, Amidzic G, et al. Persistent pathology in influenza-infected mouse lungs. Am J Respir Cell Mol Biol. 2016;55:613–615. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson JK, Rulands S, Wilkinson AC, Wuidart A, Ousset M, Van Keymeulen A, et al. Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep. 2015;12:90–101. doi: 10.1016/j.celrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deprez M, Zaragosi LE, Truchi M, Becavin C, Ruiz García S, Arguel MJ, et al. A single-cell atlas of the human healthy airways. Am J Respir Crit Care Med. 2020;202:1636–1645. doi: 10.1164/rccm.201911-2199OC. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz García S, Deprez M, Lebrigand K, Cavard A, Paquet A, Arguel M-J, et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development. 2019;146:dev177428. doi: 10.1242/dev.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida K, Gowers KHC, Lee-Six H, Chandrasekharan DP, Coorens T, Maughan EF, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578:266–272. doi: 10.1038/s41586-020-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldfarbmuren KC, Jackson ND, Sajuthi SP, Dyjack N, Li KS, Rios CL, et al. Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat Commun. 2020;11:2485. doi: 10.1038/s41467-020-16239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019;25:1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 14.Rao W, Wang S, Duleba M, Niroula S, Goller K, Xie J, et al. Regenerative metaplastic clones in COPD lung drive inflammation and fibrosis. Cell. 2020;181:848–864, e18. doi: 10.1016/j.cell.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauler M, McDonough JE, Adams TS, Kothapalli N, Schupp JS, Nouws J, et al. Single-cell RNA sequencing identifies aberrant transcriptional profiles of cellular populations and altered alveolar niche signalling networks in chronic obstructive pulmonary disease (COPD) [preprint] medRxiv 2020[accessed 2020 Sep 20]. Available from: https://www.medrxiv.org/content/10.1101/2020.09.13.20193417v1
- 16.Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6:eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6:eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J. 2019;54:1802441. doi: 10.1183/13993003.02441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carraro G, Mulay A, Yao C, Mizuno T, Konda B, Petrov M, et al. Single-cell reconstruction of human basal cell diversity in normal and idiopathic pulmonary fibrosis lungs. Am J Respir Crit Care Med. 2020;202:1540–1550. doi: 10.1164/rccm.201904-0792OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardini-Poleske ME, Clark RF, Ansong C, Carson JP, Corley RA, Deutsch GH, et al. LungMAP Consortium. LungMAP: the molecular atlas of lung development program. Am J Physiol Lung Cell Mol Physiol. 2017;313:L733–L740. doi: 10.1152/ajplung.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller HB, Montoro DT, Simon LM, Rawlins EL, Meyer KB, Strunz M, et al. The human lung cell atlas: a high-resolution reference map of the human lung in health and disease. Am J Respir Cell Mol Biol. 2019;61:31–41. doi: 10.1165/rcmb.2018-0416TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolić MZ, Sun D, Rawlins EL. Human lung development: recent progress and new challenges. Development. 2018;145:dev163485. doi: 10.1242/dev.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Reports. 2018;10:101–119. doi: 10.1016/j.stemcr.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids Elife 20176e26575</JRN> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19:217–231. doi: 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler CR, Hynds RE, Gowers KH, Lee DdoH, Brown JM, Crowley C, et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med. 2016;194:156–168. doi: 10.1164/rccm.201507-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters-Hall JR, Coquelin ML, Torres MJ, LaRanger R, Alabi BR, Sho S, et al. Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function. Am J Physiol Lung Cell Mol Physiol. 2018;315:L313–L327. doi: 10.1152/ajplung.00355.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell. 2017;20:844–857, e6. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang SX, Green MD, de Carvalho AT, Mumau M, Chen YW, D’Souza SL, et al. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat Protoc. 2015;10:413–425. doi: 10.1038/nprot.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, et al. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2014;111:E1723–E1730. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell. 2017;21:472–488, e10. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salahudeen AA, Choi SS, Rustagi A, Zhu J, van Unen V, de la O SM, et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature. 2020;588:670–675. doi: 10.1038/s41586-020-3014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youk J, Kim T, Evans KV, Jeong YI, Hur Y, Hong SP, et al. Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell. 2020;27:905–919, e10. doi: 10.1016/j.stem.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsura H, Sontake V, Tata A, Kobayashi Y, Edwards CE, Heaton BE, et al. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27:890–904, e8. doi: 10.1016/j.stem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- 39.Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019;10:5658. doi: 10.1038/s41467-019-13605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 41.Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Böttinger L, Klay D, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38:e100300. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL. Lung organoids: current uses and future promise. Development. 2017;144:986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. 2020;180:107–121, e17. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Proudfoot AG, McAuley DF, Griffiths MJ, Hind M. Human models of acute lung injury. Dis Model Mech. 2011;4:145–153. doi: 10.1242/dmm.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, Lindner M, Peschel B, Königshoff M, et al. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol. 2017;312:L896–L902. doi: 10.1152/ajplung.00084.2017. [DOI] [PubMed] [Google Scholar]
- 47.Woodcock HV, Eley JD, Guillotin D, Platé M, Nanthakumar CB, Martufi M, et al. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun. 2019;10:6. doi: 10.1038/s41467-018-07858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawkins F, Kramer P, Jacob A, Driver I, Thomas DC, McCauley KB, et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J Clin Invest. 2017;127:2277–2294. doi: 10.1172/JCI89950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCauley KB, Hawkins F, Kotton DN. Derivation of epithelial-only airway organoids from human pluripotent stem cells. Curr Protoc Stem Cell Biol. 2018;45:e51. doi: 10.1002/cpsc.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong AP, Rossant J. Generation of lung epithelium from pluripotent stem cells. Curr Pathobiol Rep. 2013;1:137–145. doi: 10.1007/s40139-013-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mestre-Ferrandiz J, Sussex J, Towse A.The R&D cost of a new medicineLondon, UK: Office of Health Economics; 2012 [Google Scholar]
- 53.Williams K, Roman J. Studying human respiratory disease in animals--role of induced and naturally occurring models. J Pathol. 2016;238:220–232. doi: 10.1002/path.4658. [DOI] [PubMed] [Google Scholar]
- 54.Pinar IP, Jones HD. Novel imaging approaches for small animal models of lung disease (2017 Grover Conference series) Pulm Circ. 2018;8:2045894018762242. doi: 10.1177/2045894018762242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie T, Wang Y, Deng N, Huang G, Taghavifar F, Geng Y, et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 2018;22:3625–3640. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 58.Ikonomou L, Wagner DE, Turner L, Weiss DJ. Translating basic research into safe and effective cell-based treatments for respiratory diseases. Ann Am Thorac Soc. 2019;16:657–668. doi: 10.1513/AnnalsATS.201812-890CME. [DOI] [PubMed] [Google Scholar]
- 59.Ma Q, Ma Y, Dai X, Ren T, Fu Y, Liu W, et al. Regeneration of functional alveoli by adult human SOX9+ airway basal cell transplantation. Protein Cell. 2018;9:267–282. doi: 10.1007/s13238-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner DE, Turner L, Panoskaltsis-Mortari A, Weiss DJ, Ikonomou L. Co-opting of ClinicalTrials.govby patient-funded studies. Lancet Respir Med. 2018;6:579–581. doi: 10.1016/S2213-2600(18)30242-X. [DOI] [PubMed] [Google Scholar]
- 61.De Luca M, Aiuti A, Cossu G, Parmar M, Pellegrini G, Robey PG. Advances in stem cell research and therapeutic development. Nat Cell Biol. 2019;21:801–811. doi: 10.1038/s41556-019-0344-z. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7:e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alapati D, Zacharias WJ, Hartman HA, Rossidis AC, Stratigis JD, Ahn NJ, et al. In utero gene editing for monogenic lung disease. Sci Transl Med. 2019;11:eaav8375. doi: 10.1126/scitranslmed.aav8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li R, Bernau K, Sandbo N, Gu J, Preissl S, Sun X. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. Elife. 2018;7:e36865. doi: 10.7554/eLife.36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–3297. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Armitage J, Tan DBA, Troedson R, Young P, Lam KV, Shaw K, et al. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur Respir J. 2018;51:1702369. doi: 10.1183/13993003.02369-2017. [DOI] [PubMed] [Google Scholar]
- 67.Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, et al. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell. 2017;21:120–134, e7. doi: 10.1016/j.stem.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170:1149–1163, e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018;73:77–104. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuo WL, Shenoy SA, Li S, O’Beirne SL, Strulovici-Barel Y, Leopold PL, et al. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med. 2018;198:1375–1388. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nureki SI, Tomer Y, Venosa A, Katzen J, Russo SJ, Jamil S, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128:4008–4024. doi: 10.1172/JCI99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glasser SW, Hagood JS, Wong S, Taype CA, Madala SK, Hardie WD. Mechanisms of lung fibrosis resolution. Am J Pathol. 2016;186:1066–1077. doi: 10.1016/j.ajpath.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]