Abstract

To improve the optical properties of polyimide (PI) films, we prepared two series of colorless transparent PIs from the dianhydride 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) and a diamine, either 2,2-bis(3-aminophenyl)hexafluoropropane (FDN) or 2,2-bis(3-amino-4-hydroxy-phenyl)hexafluoropropane (FDN-OH). Next, colorless PI (CPI) composite films were prepared by dispersing 0–1.00 wt % of organically modified clay (Cloisite 30B) in the intermediate poly(amic acid) (PAA) solution via solution intercalation, followed by imidization. The resultant CPI films had excellent optical transparency, which was achieved by reducing the charge-transfer effects by using a highly electronegative trifluoromethyl group and a kinked monomer structure. The thermal and mechanical properties, morphologies, and optical transparencies of the two as-synthesized CPI hybrid film series were investigated and compared. Electron microscopy observation of the two hybrid series revealed that the clay was well-dispersed with a nanoscale dispersion at all clay contents. However, agglomeration occurred at nanoclay loadings of 1.0 wt %. In addition, the effect of the presence of hydroxyl groups in the PI chain on various physical properties of the two CPI hybrids was also compared.
1. Introduction
Polyimide (PI) resins were developed by DuPont in the early 1960s and are highly heat-resistant polymer materials with a wide range of applications. To date, PI resins have been the subject of many studies.1,2 In general, PI is used in applications where high heat resistance and strength are required such as in electronics and vehicles.3,4 Although PI has many advantages, its use in electronics, for example, in display devices, is hindered because of its dark brown color.
In general, the coloration of PIs can be explained using charge transfer complex (CTC) theory. According to the CTC theory, the main chain of the polymer is responsible for the dark brown color, and PIs reflect yellow and red light rather than absorbing purple and cyan between 400 and 500 nm in the visible region.5,6 In particular, the electrons in the main imide chain are affected by intermolecular interactions, and the conjugated PI structure results in π-electron transitions. As the conjugation length increases, so does the number of π-electrons, which undergo chain-to-chain electron transfer through chain aggregation. The CTC reduction may be achieved if the resonance effect is minimized by limiting the electron movement. This is possible by introducing an electronegative element, such as fluorine in a trifluoromethyl (−CF3) group, into the imide chain. Alternatively, a colorless PI (CPI), which is transparent, can be synthesized by introducing a kinked monomer, resulting in an amorphous structure, thereby reducing chain stacking and the CTC effect between the chains.7,8 In addition, the use of a nucleating agent to increase transparency has also been reported. Zhou et al.9 used 1,3:2,4-di-p-methylbenzylidene sorbitol as a nucleating agent in poly(cyclohexylene dimethylene cyclohexane-dicarboxylate), a typical semicrystalline polymer, and the optical transparency and also thermomechanical properties were enhanced.
A suitable substrate for flexible displays should maintain device performance when its shape changes or it is bent. In addition, the substrate must be resistant to humidity and insoluble in general-purpose solvents. Traditionally, glass has been considered a good display material, and it is widely used in conventional displays. However, glass is heavy, brittle, and inflexible, and its roll-to-roll processing is difficult. Consequently, the development of polymeric substrates suitable for use in flexible displays has drawn attention. These “plastic” substrates have been popular since the first development of flexible displays because of their excellent insulating properties and flexibility, as well as ease of manufacture. For example, CPI is easy to synthesize, can be formed into thin films, and does not require cross-linking groups for curing.10,11 The processability of CPIs can be increased by the introduction of flexible bonds into the polymer backbone, the use of asymmetric diamine monomers and bulky side groups, or the incorporation of nonplanar and alicyclic monomers.12,13 Recently, CPIs have become widely integrated into electronics, such as transparent electrodes, liquid crystal displays, plasma display panels, and organic light-emitting devices, as well as lightweight and precision electronic products.14−16 Compared to heavy and brittle glass substrates, CPI is lightweight and flexible, making it an advantageous material for use as a flexible display substrate and the subject of many studies.15,16
Polymers are often composited with inorganic fillers to enhance their mechanical and thermal properties.17,18 In these organic–inorganic nanocomposites, a suitable (miscible and compatible) inorganic filler forms a nanoscale dispersion in the polymer matrix. There are many different inorganic fillers, for example, natural inorganic clays. These clays consist of several tens to hundreds of 1 nm thick lamellar sheets. Clays are hydrophilic because the sheets are covered with hydroxyl groups (−OH);19 therefore, polymers with hydrophilic −OH groups possess excellent miscibility and compatibility with clays through the formation of hydrogen bonds, facilitating the clay dispersion in the matrix polymer. Several studies have been conducted on nanocomposites of polymers and clays20−24 and hybrids with high thermal and mechanical properties have been synthesized by dispersing clay materials in polymers having −OH groups.
Herein, we introduce a new CPI structure that can form hydrogen bonds with clay, thus increasing the dispersibility and compatibility of the hydrophilic clay with the matrix CPI polymer. The physical properties of two synthesized CPI hybrid films having the same clay content were compared, and the effects of the presence or absence of hydrogen bonds were investigated.
The optical properties of the CPI can be improved by the CTC reduction. To achieve this, (1) a strong electron-withdrawing group is required, such as −CF3, and (2) a kinked monomer should be used in the PI main chain. Thus, we used 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) as a dianhydride and 2,2-bis(3-aminophenyl)hexafluoropropane (FDN), which does not contain a hydroxyl group, and 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (FDN-OH), which contains a hydroxyl group, as two diamines, yielding two CPI hybrid films FDN PI and FDN-OH PI, respectively. These two CPIs were also combined with varying amounts of organoclay (Cloisite 30B), from 0 to 1.00 wt %, and the thermomechanical, morphological, and optical transparency properties of the hybrid CPI films were investigated. In particular, we compared samples having the same organoclay contents. In addition, the effect of the presence or absence of the −OH group in the CPI hybrid was investigated.
2. Results and Discussion
2.1. Fourier-Transform Infrared (FT-IR) Spectra
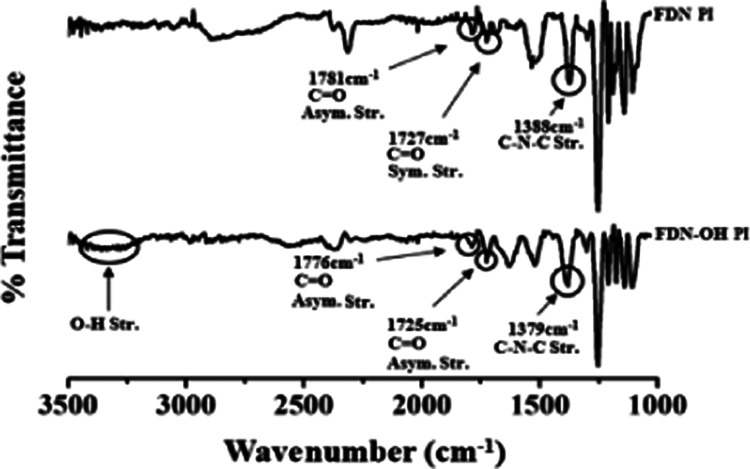
CPIs were synthesized using different diamine monomers, and the formation of the PIs was confirmed by FT-IR. In the spectrum of FDN PI shown in Figure 1, bands corresponding to C=O stretches were observed at 1781 and 1727 cm–1, and C–N–C stretching, which indicates PAA imidization, was observed at 1388 cm–1.25
Figure 1.
FT-IR spectra of FDN PI (top) and FDN-OH PI (bottom).
In general, the free O–H stretch shows a sharp peak at 3650–3500 cm–1, and the hydrogen bond of the O–H band shows a broad peak at 3400–3300 cm–1. Intramolecular hydrogen bonding usually shifts the broad O–H band to a lower frequency.25 Hydrogen bonds between molecular chains having a hydrogen donor and an acceptor in the main chain generally show an O–H stretching absorption band between 3500 and 3000 cm–1. Because FDN-OH PI is capable of hydrogen bonding with the nitrogen atoms in the adjacent imide bonds, as well as forming interchain hydrogen bonds between the −OH groups present in the main chain, −OH stretching peaks are commonly observed between 3500 and 3200 cm–1. In addition, hydrogen bonding is also possible between −OH contained in hydrophilic clay and CPI using the FDN-OH monomer.
In the spectrum of FDN-OH PI, C=O peaks were also observed at 1776 and 1725 cm–1, and the band corresponding to C–N–C indicating the imidization of FDN-OH PI was also observed at 1379 cm–1. Thus, both CPIs underwent complete imidization.
The chemical structures of the two PIs were also confirmed using solid-state 13C cross peak magic angle spinning (CP/MAS) nuclear magnetic resonance (NMR), and the NMR spectra are shown in Figure 2. Solid-state 13C CP/MAS NMR spectroscopy was used to measure the chemical shifts of key components, such as the phenyl rings and trifluoromethyl groups, in FDN PI and FDN-OH PI at room temperature. Each sample was spun at 10–12 kHz. In the NMR spectrum of FDN PI shown in Figure 2a, peaks corresponding to the phenyl ring were observed at 127.80, 132.06, and 137.41 ppm, and the peaks corresponding to C (a) adjacent to −CF3, C (b) of −CF3, and C (f) of C=O were observed at 64.67, 127.80, and 165.23 ppm.
Figure 2.

13C NMR chemical shifts of pure CPI films containing (a) 6FAm and (b) 6FAm-OH monomers. The spinning sidebands are marked with asterisks.
In the spectrum of FDN-OH PI, the peaks corresponding to the phenyl rings were observed at 118.18, 132.77, and 137.03 ppm. Moreover, peaks corresponding to C (a) adjacent to −CF3, C (b) of −CF3, and C (h) adjacent to −OH were observed at 63.96, 124.18, and 153.49 ppm, as shown in Figure 2b, respectively. In addition, a peak corresponding to C (f) of C=O was observed at 165.94 ppm. Thus, the NMR results are consistent with the structure of the synthesized PIs.25
2.2. Solubility
The solubility of the two CPIs in various solvents is summarized in Table 1. Generally, conventional PI films comprising molecules with a rigid, rodlike form are insoluble in common solvents, but our CPIs, which have a kinked structure, may be soluble in some solvents. In fact, the two CPIs were very soluble in polar solvents, including DMAc, dimethyl sulfoxide (DMSO), and N′-methyl-2-pyrrolidone (NMP), as well as common solvents, such as acetone, pyridine, and tetrahydrofuran (THF). The reason for the good solubility of the PIs in common solvents is the asymmetric meta-substituted monomer structure and isopropylidene group in the polymer main chain.13 Compared to traditional PIs, the CPIs synthesized in this study showed superior solubility. Notably, FDN-OH PI, which contains −OH groups, is more soluble in alcohol than in the FDN PI.
Table 1. Solubility of CPI Films with Different Diaminesa.
| PI | Act | CHCl3 | CH2Cl2 | DMAc | DMF | DMSO | EtOH | MeOH | NMP | Py | THF | Tol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FDN PI | ◎ | ◎ | ◎ | ◎ | ◎ | ◎ | × | △ | ◎ | ◎ | ◎ | ○ |
| FDN-OH PI | ◎ | × | △ | ◎ | ◎ | ◎ | ○ | ○ | ◎ | ◎ | ◎ | × |
◎: excellent, ○: good, △: poor, ×: very poor. Act: acetone, DMAc: N,N′-dimethylacetamide, DMF: N,N′-dimethylformamide, DMSO: dimethyl sulfoxide, EtOH: ethyl alcohol, MeOH: methyl alcohol, NMP: N′-methyl-2-pyrrolidone, Py: pyridine, THF: tetrahydrofuran, and Tol: toluene.
2.3. Electron Microscopy
In general, using electron microscopy (scanning electron microscopy (SEM) and transmission electron microscopy (TEM)), the interlayer distance of clay particles or intercalation, exfoliation, and the degree of agglomeration of clay layers can be visualized directly.26−28 In particular, TEM can be used to confirm the X-ray diffraction (XRD) results and quantify the amount of dispersed clay. Further, if the ultrafine part of the hybrid sample can be observed by TEM, then it is possible to confirm the formation of a nanocomposite in which clay is dispersed at the nanoscale.28
Figure 3 shows the TEM image of an FDN PI hybrid containing 0.5 and 1.00 wt % Cloisite 30B. The linear structures in the micrographs are clay particles. As shown in Figure 3a,b, for the 0.50 wt % hybrid, some clay particles were intercalated, although most of the clay particles were evenly exfoliated to a thickness of ∼20 nm over a large area. Conversely, in the 1.00 wt % CPI hybrid, the clay was more agglomerated, having particles with a thickness of ∼60–80 nm because the excess clay was not dispersed as in the 0.5 wt % hybrid, as shown in Figure 3c,d.
Figure 3.
TEM micrographs of FDN PI hybrid films containing (a, b) 0.50 and (c, d) 1.00 wt % Cloisite 30B.
The hydrophilic clay contains hydroxyl groups and has excellent compatibility and affinity with the CPI synthesized using FDN-OH; thus, we expected this sample to have an excellent clay dispersion. The TEM images of the FDN-OH PI with 0.50 and 1.00 wt % organoclay loadings are shown in Figure 4. Compared to the FDN PI hybrid described above (see Figure 3), the FDN-OH PI hybrid showed excellent dispersion for both the 0.50 and 1.00 wt % Cloisite 30B loadings. At a concentration of 0.50 wt % (see Figure 4a,b), the clay was uniformly dispersed in the PI matrix, having a size of less than 10 nm. However, when the concentration of the clay increased to 1.00 wt %, some of the clay agglomerated, forming particles of around 60 nm in size (see Figure 4c,d). Comparing the clay dispersions of the FDN PI and FDN-OH PI hybrids, dispersions of the latter are superior to that of the former. These results can be explained by the high affinity between the FDN-OH PI containing the −OH group and the hydrophilic clay. In addition to the hydrophilicity of clays, the good dispersibility and compatibility between the matrix polymer and filler have a great influence on the thermomechanical and optical transparency properties, which will be discussed in the next section.
Figure 4.
TEM micrographs of FDN-OH PI hybrid films containing (a, b) 0.50 and (c, d) 1.00 wt % Cloisite 30B.
2.4. Thermal Properties
Table 2 summarizes the thermal properties of the CPI hybrids. The glass transition temperature (Tg) values of the PI hybrid film containing the FDN monomer gradually increased from 230 to 242 °C as the clay content increased from 0 to 0.50 wt %. This increase in Tg occurs because the movement of the polymer chain in the clay layers is limited, even at high temperatures.29−31 However, the Tg value of the CPI hybrid decreased as the organoclay content increased above a critical concentration. When Cloisite 30B increased from 0.50 to 0.75 wt %, the Tg of the CPI hybrid decreased to 238 °C; moreover, when the organoclay content reached 1.00 wt %, the Tg further decreased to 234 °C. This decrease in Tg is a result of the agglomeration of excess clay above the critical concentration. When excess clay is agglomerated in the polymer structure, the crystalline packing of the polymer chain interferes with the normal segmental motion, which reduces the thermal properties.31 The DSC thermograms of the pure PI and PI hybrids are shown in Figure 5a, and the agglomeration of excess clay in PI has already been confirmed by TEM (see Figure 3).
Table 2. Thermal Properties of CPI Hybrid Films with Various Organoclay Contents.
| FDN
PI |
FDN-OH
PI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cloisite 30B in PI (wt %) | Tg (°C) | TDi a (°C) | wtR600 b (%) | CTEc (ppm/°C) | Tg (°C) | TDi (°C) | wtR600 (%) | CTE (ppm/°C) |
| 0 (pure PI) | 230 | 501 | 68 | 63 | 287 | 312 | 63 | 65 |
| 0.25 | 236 | 505 | 67 | 61 | 292 | 314 | 64 | 58 |
| 0.50 | 242 | 512 | 70 | 59 | 302 | 315 | 64 | 57 |
| 0.75 | 238 | 509 | 70 | 62 | 309 | 319 | 64 | 52 |
| 1.00 | 234 | 502 | 68 | 64 | 292 | 311 | 62 | 57 |
At a 2% initial weight-loss temperature.
Weight percent of residue at 600 °C.
Coefficient of thermal expansion obtained in the second heating cycle between 20 and 220 °C.
Figure 5.
DSC thermograms of PI hybrid films with various organoclay contents: (a) FDN PI and (b) FDN-OH PI.
FDN-OH PI and FDN PI showed the same trends in thermal properties; that is, the Tg value of the FDN-OH PI hybrid film with 0.75 wt % Cloisite 30B (309 °C) was 22 °C higher than that of pure PI (287 °C). However, when the organoclay content increased from 0.75 to 1.00 wt %, the Tg decreased to 292 °C (see Figure 5b). Thus, the critical concentration of the FDN-OH PI series was 0.75 wt %. Comparing the Tg values in the two series, overall, the Tg value of the hybrid of FDN-OH PI is higher than that of FDN PI, and the critical concentration of the organoclay was also higher. This result can be explained by the strong hydrophilicity and intermolecular hydrogen bonding caused by the −OH groups in FDN-OH PI and clay. These results are consistent with the TEM images of films having the same organoclay content (Figure 4).
Clay has excellent heat resistance, which contributes to the increased thermal stability of the CPI. Table 2 summarizes the initial decomposition temperature (TDi) of the two CPI hybrid series. As the Cloisite 30B content was increased from 0 to 0.50 wt %, the TDi value of the FDN PI hybrid gradually increased from 501 to 512 °C (see Table 2). As the temperature increases, the clay layer acts as an insulator and barrier to volatile products generated at high temperatures, thus increasing the TDi. The reason for this increase in thermal stability can be explained by the high thermal stability of the clay itself, as well as the interaction between the clay particles and the polymer matrix.21,32 However, when 1.00 wt % of Cloisite 30B was added to the CPI hybrid, the TDi value was 10 °C lower than that of the 0.50 wt % hybrid (502 °C). As for the Tg, the decrease in TDi is due to the agglomeration of excess clay. Figure 6a shows the TGA thermograms of the FDN PI hybrids with 0–1.00 wt % clay loadings. The same trend was also observed in CPI hybrids containing FDN-OH monomers. For example, when the organoclay content was increased from 0 to 0.75 wt %, the TDi value increased from 312 to 319 °C, but, when the clay content reached 1.00 wt %, the TDi value for the hybrid was reduced to 311 °C (see Table 2). This is because agglomerated clay does not effectively insulate the volatile components because of thermal decomposition under high-temperature conditions; so the thermal stability is lowered. As shown in Figure 6b, there are several pyrolysis steps in the TGA heating curve of FDN-OH PI. This is because PI having a −OH group proceeds to polybenzoxazole (PBO) through thermal rearrangement (TR) upon heating.33−35 PI containing a −OH group becomes PBO through heat treatment by the mechanism shown in Scheme 1.
Figure 6.
TGA thermograms of PI hybrid films with various organoclay contents: (a) FDN PI and (b) FDN-OH PI.
Scheme 1. Thermal Rearrangement of FDN-OH PI to Polybenzoxazole (PBO).
For the FDN PI and FDN-OH PI composites, the weight residues at 600 °C (wtR600)were approximately the same, regardless of the clay concentration. For example, for pure FDN PI, the weight residue was 68%, and, as the Cloisite 30B content was increased from 0.25 to 1.00 wt %, wtR600 remained constant at 67–70%. The results for FDN-OH PI are similar to those for FDN PI. That is, as the Cloisite 30B content in FDN-OH PI increased from 0 to 1.00 wt %, wtR600 remained approximately constant at 62–64% (see Table 2). The TDi and wtR600 values of both series reveal that the FDN PI hybrid is more thermally stable than the FDN-OH PI hybrid at all clay contents. This result is contrary to the trend in Tg and can be explained by the weak thermal stability of the -OH group present in the main chain of the FDN-OH PI.
The CTE value depends largely on the orientation of the platelike clay particles, the type of polymer inserted into the clay layer, the binding force between the polymer and clay, and the shape of the hybrid film. In particular, when heated, the planar molecules oriented in the film plane expand perpendicularly to the film plane (i.e., in the out-of-plane direction). However, the clay layer dispersed in the hybrid does not deform or expand as easily as the polymer molecules because of its simple, stronger structure. As a result, the clay layer suppresses the thermal expansion of the polymer matrix very effectively.36−38
Table 2 also summarizes the CTE values of the PI hybrids with organoclay contents. The CTE values of the FDN PI hybrid showed a minimum value at the critical content of 0.50 wt %, which increased as the clay content was increased to 1.00 wt %. For example, when the clay content was increased from 0 to 0.50 wt %, the CTE of the FDN PI hybrid decreased from 63 to 59 ppm/°C, but when the clay content was increased to 1.00 wt %, the CTE value also increased to 64 ppm/°C. Similar trends were observed in the FDN-OH PI hybrid. When the clay content increased from 0 to 0.75 wt %, the CTE value decreased from 65 to 52 ppm/°C, but, then, it increased again to 57 ppm/°C when the clay content was increased to 1.00 wt %. This is because the excess clay agglomerates, as explained above and observed in the TEM micrographs.
By comparing the CTE values of the two CPI hybrids, it can be seen that the critical concentration of clay dispersed in the matrix PI is higher in the FDN-OH hybrid than that in the FDN hybrid, and the overall CTE value of the FDN-OH PI hybrid is also superior to that FDN PI, as shown in Table 2. As previously explained, this results due to the presence of −OH groups in the polymer chain of FDN-OH PI, which has excellent dispersibility and compatibility with hydrophilic clay, and also forms a polymer with better thermal deformation through hydrogen bonding between polymer chains. The CTE results of the two CPI hybrid series with various Cloisite 30B contents are presented in Figure 7.
Figure 7.
TMA thermograms of PI hybrid films with various organoclay contents: (a) FDN PI and (b) FDN-OH PI.
2.5. Tensile Properties
Like the critical concentration observed for the thermal properties, the organoclay also had a significant effect on the mechanical properties. Table 3 summarizes the mechanical tensile properties of the CPI hybrid films having various organoclay contents. For example, in the case of the FDN PI hybrid, when the organoclay content was increased from 0 to 0.5 wt %, the tensile strength increased from 37 to 83 MPa. However, when the Cloisite 30B content was increased to 1.00 wt %, the ultimate tensile strength decreased to 45 MPa. This result can also be explained by the agglomeration of clay at the critical concentration. The FDN-OH PI hybrid showed a similar trend to that of FDN PI. The final tensile strength of pure CPI was 55 MPa, but when the amount of Cloisite 30B reached 0.75 wt %, the value increased by 240% (132 MPa). However, the tensile strength decreased to 55 MPa when the clay content increased to 1.00 wt %. Similar reports have been published for polymer hybrid systems from many other groups, including our research group. We have reported similar results in PI hybrids containing functionalized graphene and organoclays.39 Yano et al.40 also reported that the mechanical properties of cellulose composites decreased above the critical concentration of silica, explaining that the filler particles did not evenly disperse and agglomerate with one another above the critical concentration. In this study, we confirmed this phenomenon using TEM images.
Table 3. Mechanical Tensile Properties of CPI Hybrid Films with Various Organoclay Contents.
| FDN
PI |
FDN-OH
PI |
|||||
|---|---|---|---|---|---|---|
| Cloisite 30B in PI (wt %) | ult. str. (MPa) | ini. mod. (GPa) | E.B.a (%) | ult. str. (MPa) | ini. mod. (GPa) | E.B. (%) |
| 0 (pure PI) | 37 | 2.00 | 2 | 55 | 3.28 | 2 |
| 0.25 | 71 | 2.41 | 4 | 57 | 3.67 | 2 |
| 0.50 | 83 | 2.75 | 4 | 70 | 4.35 | 2 |
| 0.75 | 65 | 2.85 | 3 | 132 | 4.60 | 3 |
| 1.00 | 45 | 2.93 | 3 | 55 | 6.21 | 2 |
Percentage elongation at break.
However, unlike the ultimate tensile strength, the initial tensile modulus increased steadily in proportion to the amount of clay (see Table 3). In the FDN PI and FDN-OH series, when the amount of clay was increased from 0 to 1.00 wt %, the initial modulus increased gradually from 2.00 to 2.93 and from 3.28 to 6.21 GPa, respectively. The increase in the initial tensile modulus of the two hybrid series can be explained by the type of clay (rigid rod), the high aspect ratio and directionality of the clay layer, and the resistance of the clay itself to external forces.41,42
The ultimate tensile strength and initial tensile modulus of the FDN PI and FDN-OH PI hybrids were compared. Overall, the mechanical properties of the FDN-OH PI hybrid were superior to those of the FDN PI hybrid having the same organoclay content. These results contrast with those of the thermal stability described above. The formation of hydrogen bonds between FDN-OH PI with −OH groups and hydrophilic clays allows for a stronger form of hybrid film that can withstand external tensile forces. The mechanical properties of the two CPI hybrid film series with respect to organoclay contents are shown in Figure 8.
Figure 8.
Mechanical tensile properties of PI hybrid films with various organoclay contents: (a) FDN PI and (b) FDN-OH PI.
Over the range of Cloisite 30B contents, the elongation at break (EB) of the FDN PI and FDN-OH PI hybrids were 2–4 and 2–3%, respectively; thus, there is no significant difference between the two series (see Table 3). The EB value of our study was lower than that of other polymer hybrids. These results are typical of hybrid materials reinforced with inorganic materials, such as strong and brittle clays.42
2.6. Optical Transparency
The optical transparencies of the CPI hybrid film are quantified by the initial transmitted wavelength (λo), the transmittance at a wavelength of 500 nm (500 nmtrans), and the yellow index (YI). The UV–vis results of each film are shown in Figure 9, and the summarized results are presented in Table 4. For the FDN PI and FDN-OH PI hybrid series, as the clay content increased from 0 to 1.00 wt %, the λo values increased from 352 to 361 and 312 to 326 nm, respectively. Thus, the λo values of all CPI hybrids are below 400 nm, and all of the synthesized hybrid films transmit light before the visible region. As the clay content increased from 0 to 1.00 wt %, 500 nmtrans decreased from 89 to 83% in the FDN PI series and from 90 to 87% in the FDN-OH PI hybrid series, respectively. Nevertheless, the transmittance values of the two series were excellent.
Figure 9.
UV–vis transmittances of CPI hybrid films with various organoclay contents: (a) FDN PI and (b) FDN-OH PI.
Table 4. Optical Transparencies of CPI Hybrid Films with Various Organoclay Contents.
| FDN
PI |
FDN-OH
PI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cloisite 30B in PI (wt %) | thicknessa (μm) | λ0 (nm) | 500 nmtrans (%) | YIb | thickness (μm) | λ0 (nm) | 500 nmtrans (%) | YI |
| 0 (pure PI) | 54 | 352 | 89 | 3 | 54 | 312 | 90 | 4 |
| 0.25 | 58 | 354 | 89 | 3 | 57 | 317 | 90 | 4 |
| 0.50 | 53 | 359 | 88 | 5 | 58 | 323 | 89 | 5 |
| 0.75 | 55 | 361 | 85 | 4 | 53 | 323 | 88 | 5 |
| 1.00 | 58 | 361 | 83 | 5 | 56 | 326 | 87 | 6 |
Film thickness.
Yellow index.
The YI values range from 3 to 5 and 4 to 6 as the organoclay content increased from 0 to 1.00 wt % in the FDN PI and FDN-OH PI hybrid series, respectively (Table 4). The YI value gradually increases as the clay content increases because excess clay agglomerates and affects the optical properties. The optical clarity of the FDN-OH PI hybrid was lower than that of the FDN PI hybrid. The hydrogen bonds formed by the −OH groups present in FDN-OH PI form a conjugated structure between chains and increase the CTC.41,43 This conjugated structure facilitates π-electron transitions, and, thus, the YI value increases. However, the YI values of both series are the same as those for the actual film.
The solvent-cast films of the two CPI hybrids having Cloisite 30B contents of 0–1.00 wt % are almost colorless and transparent, as shown in Figures 10 and 11. This result reveals that the 1.00 wt % clay added to the PI matrix does not affect the transparency significantly. In addition, these results suggest that the phase domain of the hybrid film containing up to 1.00 wt % of organoclay is considerably smaller than the wavelength of visible light (400–800 nm). Therefore, the two hybrid series produced in this study exhibit excellent transparency because of the fine dispersion of the clay particles in the PI matrix.44
Figure 10.
Photographs of FDN PI hybrid films containing (a) 0 (pure PI), (b) 0.25, (c) 0.50, (d) 0.75, and (e) 1.00 wt % Cloisite 30B. Kapton 200KN (YI = 97.5) is shown in (f) for comparison (the photos were taken by L.K.K.). Figure courtesy of Jin-Hae Chang from ref (45).
Figure 11.
Photographs of FDN-OH PI hybrid films containing (a) 0 (pure PI), (b) 0.25, (c) 0.50, (d) 0.75, and (e) 1.00 wt % Cloisite 30B. Kapton 200KN (YI = 97.5) is shown in (f) for comparison (the photos were taken by L.K.K.). Figure courtesy of Jin-Hae Chang from ref (45).
As shown in Figures 10 and 11, the colorlessness and transparency are outstanding at all clay contents. For comparison with the film we made, we used DuPont Kapton 200KN polyimide film with a thickness of 50 μm.45 As shown in Table 4, the YI value was affected by the organoclay concentration, although these differences are not noticeable to the naked eye. As shown in the figures, the background logo is clearly visible through the hybrid film, and the text is legible.
To determine the optical transparency with respect to film thickness, three PI films were superimposed and placed on the logo. Figure 12 shows two photographs of the three stacked FDN PI and FDN-OH PI hybrid films containing 0.5 wt % Cloisite 30B. The three stacked films have thicknesses of 159 and 174 μm for FDN PI and FDN-OH PI, respectively. With increased thickness, the yellow color is more noticeable. However, even though the YI value increased, the logo is still clearly visible through the stacked layers.
Figure 12.
Photographs of PI hybrid films containing 0.50 wt % Cloisite 30B, The films are laminates of three CPI films. (a) Schematic showing orientation of films for (b) FDN PI and (c) FDN-OH PI films (the photos were taken by L.K.K.).
3. Conclusions
Colorless and transparent PI films were prepared. Our strategy involved preventing CTC from using a monomer with an electron-withdrawing −CF3 substituent and a kinked monomer structure. To achieve this, 6FDA was used as a dianhydride, and FDN and FDN-OH were used as diamines. Two CPI hybrid series were prepared via the solution intercalation method using different loadings of Cloisite 30B organoclay (0–1.00 wt % with respect to CPI).
The properties of the CPI hybrid films prepared using FDN and FDN-OH with various organoclay contents were compared. At the same clay contents, the FDN-OH PI hybrid was superior to the FDN PI hybrid with respect to thermal (Tg and CTE) and mechanical (ultimate tensile strength and initial tensile modulus) properties. These results can be explained by the strong hydrogen bonds formed between the main chain of the FDN-OH PI and the −OH groups of the clays. However, the thermal stability (TDi and wtR600) of the FDN PI hybrid film was superior to that of FDN-OH PI film because of the low thermal stability of the −OH groups present in the FDN-OH PI hybrid film. Concerning optical transparency, the values of λo and YI for FDN-OH PI were worse than those of FDN PI at the same clay content, and this is also due to the −OH groups present in the FDN-OH PI. Based on the thermomechanical properties of the FDN PI and FDN-OH PI hybrids, optimal organoclay concentrations were determined: 0.50 and 0.75 wt %, respectively. FDN-OH PI has a higher critical concentration than FDN PI because the former is capable of hydrogen bonding with the hydrophilic clay.
The preparation of polymer nanocomposites by dispersing clay at the nanoscale has long been challenging. However, it is thought that these difficulties can be easily overcome if a hydrophilic monomer structure is used in the polymer chain to increase the polymer compatibility and dispersibility with the hydrophilic clay. Although our current results are not excellent, we hope that our research will be a starting point for further studies in similar fields.
4. Experimental Method
4.1. Materials
FDN, FDN-OH, and 6FDA monomers were purchased from TCI (Tokyo, Japan). N,N′-dimethylacetamide (DMAc), which was used as a solvent, was purchased from Junsei (Tokyo, Japan), and molecular sieves (5 Å) used for water removal were purchased from TCI (Tokyo, Japan). Cloisite 30B (organically modified montmorillonite, MMT) was purchased from Southern Clay Product, Co. The source clay, Kunipia-F (Na+-MMT), was purchased from Kunimine Co.
4.2. Synthesis of PI Hybrid Films
The synthetic scheme for the formation of poly(amic acid) (PAA) and the PI nanocomposites from the monomers is shown in Figure 13. The synthetic method is the same for both monomers, so only that of FDN-OH is described. First, PAA was prepared. FDN-OH (8.84 g, 2.41 × 10–2 mol) was added to a three-necked flask containing 120 mL of DMAc, stirred, and completely dissolved. Next, 6FDA (10.7 g, 2.41 × 10 mol) in 120 mL of DMAc was added to the FDN-OH solution. The mixture was then stirred for 1 h at 0 °C under a nitrogen atmosphere until completely dissolved. Subsequently, the mixture was vigorously stirred under a nitrogen atmosphere at room temperature for 14 h, thus yielding the precursor PAA solution. The solid content of the obtained PAA solution was 8 wt %. The inherent viscosities of FDN PI and FDN-OH PI in DMAc at a concentration of 0.1 g/dL and 30 °C were 0.85 and 0.90, respectively.
Figure 13.
Synthesis of PI hybrid films with 6FDA, FDN, and FDN-OH.
The CPI nanocomposite films were prepared in the same way, and only the organoclay contents varied. For brevity, we describe the preparation of the PI nanocomposite film using FDN-OH with 0.50 wt % Cloisite 30B. Cloisite 30B (9.76 mg) was added to the PAA solution (1.955 g), and the clay was dispersed using a sonicator with vigorous stirring at 25 °C for 3 h. The obtained PAA solution was poured onto a glass plate and left to stabilize at 50 °C for 2 h in a vacuum oven. The solvent was removed from the PAA by heating for 1 h at 80 °C under vacuum. Subsequently, the film was obtained via a stepwise heat treatment because cracking would occur if the film is heated rapidly at high temperatures. The obtained film was then heated sequentially for 30 min each at 110, 140, and 170 °C, followed by 50 min each at 195 and 220 °C, and, finally, 2 h at 235 °C. Table 5 shows the heat treatment conditions used to obtain the hybrid CPI films. The resulting hybrid film was peeled from the glass plate, and the thickness of the obtained film was constant, ranging from 53 to 58 μm. The film size was 10 × 10 cm2.
Table 5. Heat Treatment Conditions of CPI Hybrid Films.
| samples | temp. (°C)/time (h)/pressure (Torr) |
|---|---|
| PAA | 0/1/760 → 25/14/760 |
| PAA hybrid | 25/3/760 |
| CPI hybrid | 50/2/1 → 80/1/1 → 110/0.5/1 → 140/0.5/1 → 170/0.5/1 → 195/0.8/1 → 220/0.8/1 → 235/2/1 |
4.3. Characterization
Fourier-transform infrared (FT-IR) spectra were obtained using a JASCO-460 spectrometer (JASCO, Tokyo, Japan). The spectra were obtained between 3500 and 1000 cm–1 using potassium bromide disks containing the samples. The 13C cross-polarization (CP)/magic-angle spinning (MAS) NMR (Bruker 400 DSX NMR, Berlin, Germany) experiment was conducted at a Larmor frequency of 100.61 MHz. Tetramethylsilane (TMS) was used as a standard to record the NMR spectra.
Transmission electron microscopy (TEM, JEOL, JEM 2100, Tokyo, Japan) was used to examine the clay dispersion in the hybrid films. Before observation, the specimens were cured in epoxy resin for 24 h at 70 °C. After applying a vacuum, a sample having a thickness of 90 nm was prepared using a microtome equipped with a glass knife. The TEM acceleration voltage was 120 kV.
Differential scanning calorimetry (DSC; NETZSCH F3, Berlin, Germany) and thermogravimetric analyses (TGA, TA Q500, New Castle, DE) were conducted at a heating rate of 20 °C/min from 30 to 350 and 30–700 °C, respectively. The coefficient of thermal expansion (CTE) was calculated from the second heating cycle between 20 and 220 °C. Thermomechanical analysis (TMA, TMA-SS6100, Tokyo, Japan) was carried out using samples measuring 5 × 30 mm2, and the heating rate was 5 °C/min at an expansion force of 0.1 N.
A universal testing machine (UTM, Model 5564, Instron, Seoul, Korea) was used to determine the mechanical properties. The measured sample size was 5 × 50 mm2, and the crosshead speed was 2 mm/min. To reduce errors, each sample was measured 15–20 times, and the average of the remaining values was excluded, except for samples with severe errors.
The yellow index (YI) was measured with a spectrophotometer (KONICA MINOLTA CM-3600D, Tokyo, Japan), and an ultraviolet–visible (UV–vis) spectrometer (SHIMADZU UV-3600, Tokyo, Japan) was used to measure the cut-off wavelength (λo) and light transmittance between 300 and 800 nm.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A03012069).
Author Contributions
J.-H.C. designed the project and wrote the manuscript. L.K.K. and H.G.K. prepared the samples and participated in the data analysis. All authors reviewed the manuscript.
The authors declare no competing financial interest.
References
- Yu X.; Zhao X.; Liu C.; Bai Z.; Wang D.; Dang G.; Zhou H.; Chen C. Synthesis and properties of thermoplastic polyimides with ether and ketone moieties. J. Polym. Sci., Part A: Polym. Chem. 2010, 48, 2878–2884. 10.1002/pola.24065. [DOI] [Google Scholar]
- Wang L.; Zhao Z.; Li J.; Chen C. Synthesis and characterization of fluorinated polyimides for pervaporation of n-heptane/thiophene mixtures. Eur. Polym. J. 2006, 42, 1266–1272. 10.1016/j.eurpolymj.2005.12.013. [DOI] [Google Scholar]
- Tian Y.; Liu S.; Ding H.; Wang L.; Liu B.; Shi Y. Formation of deformed honeycomb-patterned films from fluorinated polyimide. Polymer 2007, 48, 2338–2344. 10.1016/j.polymer.2007.02.028. [DOI] [Google Scholar]
- Wang P.-C.; MacDiarmid A. G. Integration of polymer-dispersed liquid crystal composites with conducting polymer thin films toward the fabrication of flexible display devices. Displays 2007, 28, 101–104. 10.1016/j.displa.2007.04.006. [DOI] [Google Scholar]
- Chen C.-J.; Yen H.-J.; Hu Y.-C.; Liou G.-S. Novel programmable functional polyimides: Preparation, mechanism of CT induced memory, and ambipolar electrochromic behavior. J. Mater. Chem. C 2013, 1, 7623–7634. 10.1039/c3tc31598c. [DOI] [Google Scholar]
- Nishihara M.; Christiani L.; Staykov A.; Sasaki K. Experimental and theoretical study of charge-transfer complex hybrid polyimide membranes. J. Polym. Sci., Part B: Polym. Phys. 2014, 52, 293–298. 10.1002/polb.23411. [DOI] [Google Scholar]
- Chang J.-H. Equibiaxially stretchable colorless and transparent polyimides for flexible display substrates. Rev. Adv. Mater. Sci. 2020, 59, 1–9. 10.1515/rams-2020-0003. [DOI] [Google Scholar]
- Shin H. I.; Kwark Y.-J.; Chang J.-H. Colorless and transparent copolyimides and their nanocomposites: Thermo-optical properties, morphologies, and gas permeabilities. Polymers 2019, 11, 585 10.3390/polym11040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B.; Zhou Y.-G. Improvement of transparencies and mechanical properties of poly(cyclohexylene dimethylene cyclohexanedicarboxylate) parts using a compounding nucleating agent to control crystallization. Materials 2019, 12, 563 10.3390/ma12040563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdipour-Ataei S.; Hatami M. Synthesis and characterization of novel heat resistant poly(amide imide)s. Eur. Polym. J. 2005, 41, 2010–2015. 10.1016/j.eurpolymj.2005.03.012. [DOI] [Google Scholar]
- Yang C. Y.; Hsu S. L. C.; Chen J. S. Synthesis and properties of 6FDA-BisAAF-PPD copolyimides for microelectronic applications. J. Appl. Polym. Sci. 2005, 98, 2064–2069. 10.1002/app.22410. [DOI] [Google Scholar]
- Kim S.-U.; Lee C.; Sundar S.; Jang W.; Yang S.-J.; Han H. Synthesis and characterization of soluble polyimides containing trifluoromethyl groups in their backbone. J. Polym. Sci., Part B: Polym. Phys. 2004, 42, 4303–4312. 10.1002/polb.20270. [DOI] [Google Scholar]
- Liaw D.-J.; Liaw B.-Y.; Yu C.-W. Synthesis and characterization of new organosoluble polyimides based on flexible diamine. Polymer 2001, 42, 5175–5179. 10.1016/S0032-3861(00)00822-3. [DOI] [Google Scholar]
- Choi M.-C.; Kim Y.; Ha C.-S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. 10.1016/j.progpolymsci.2007.11.004. [DOI] [Google Scholar]
- Burrows P. E.; Graft G. L.; Gross M. E.; Martin P. M.; Shi M. K.; Hall M.; Mast E.; Bonham C.; Bennett W.; Sullivan M. B. Ultra barrier flexible substrates for flat panel displays. Displays 2001, 22, 65–69. 10.1016/S0141-9382(00)00064-0. [DOI] [Google Scholar]
- Chiang C.-J.; Winscom C.; Bull S.; Monkman A. Mechanical modeling of flexible OLED devices. Org. Electron. 2009, 10, 1268–1274. 10.1016/j.orgel.2009.07.003. [DOI] [Google Scholar]
- Kim Y. M.; Chang J.-H.; Kim J. C. Optically transparent and colorless polyimide hybrid films with various clay contents. Macromol. Res. 2012, 20, 1257–1263. 10.1007/s13233-012-0182-3. [DOI] [Google Scholar]
- Min U. K.; Kim J. C.; Chang J.-H. Transparent polyimide nanocomposite films: Thermo-optical properties, morphology, and gas permeability. Polym. Eng. Sci. 2011, 51, 2143–2150. 10.1002/pen.22059. [DOI] [Google Scholar]
- Wang Z.; Lan T.; Pinnavaia T. J. Hybrid organic–inorganic nanocomposites formed from an epoxy polymer and a layered silicic acid (magadiite). Chem. Mater. 1996, 8, 2200–2204. 10.1021/cm960263l. [DOI] [Google Scholar]
- Sapalidis A. A.; Katsaros F. K.; Steriotis T. A.; Kanellopoulos N. K. Properties of poly(vinyl alcohol)– bentonite clay nanocomposite films in relation to polymer–clay interactions. J. Appl. Polym. Sci. 2012, 123, 1812–1821. 10.1002/app.34651. [DOI] [Google Scholar]
- Shin H. I.; Chang J.-H. Transparent polyimide/organoclay nanocomposite films containing different diamine monomers. Polymers 2020, 12, 135 10.3390/polym12010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.-E.; Ham M.-R.; Kim J.-C.; Chang J.-H. Characterizations of flexible clay-pVA hybrid films: Thermo-optical properties, morphology, and gas permeability. Polymer 2011, 35, 402–408. 10.7317/pk.2011.35.5.402. [DOI] [Google Scholar]
- Lazaratou C. V.; Vayenas D. V.; Papoulis D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. 2020, 185, 105377 10.1016/j.clay.2019.105377. [DOI] [Google Scholar]
- Glotov A.; Stavitskaya A.; Chudakov Y.; Ivanov E.; Huang W.; Vinokurov V.; Zolotukhina A.; Maximov A.; Karakhanov E.; Lvov Y. Mesoporous metal catalysts template on clay nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. 10.1246/bcsj.20180207. [DOI] [Google Scholar]
- Pavia D. L.; Lampman G. M.; Kriz G. S.; Vyvyan J. A.. Introduction to Spectroscopy; Cengage Learning: Boston, MA, 2008; pp 14–95. [Google Scholar]
- Ke K.; Lu J.; Zhao J.; Qi Z. The effects of promoter and curing process on exfoliation behavior of epoxy/clay nanocomposites. J. Appl. Polym. Sci. 2000, 78, 808–815. . [DOI] [Google Scholar]
- Vaia R. A.; Jandt K. D.; Kramer E. J.; Giannelis E. P. Microstructural evolution of melt intercalated polymer-organically modified layered silicates nanocomposites. Chem. Mater. 1996, 8, 2628–2635. 10.1021/cm960102h. [DOI] [Google Scholar]
- Morgan A. B.; Gilman J. W. Characterization of polymer-layered silicate(clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J. Appl. Polym. Sci. 2003, 87, 1329–1338. 10.1002/app.11884. [DOI] [Google Scholar]
- Agag T.; Takeichi T. Polybenzoxazine–montmorillonite hybrid nanocomposites: Synthesis and characterization. Polymer 2000, 41, 7083–7090. 10.1016/S0032-3861(00)00064-1. [DOI] [Google Scholar]
- Fornes T. D.; Yoon P. J.; Hunter D. L.; Keskkula H.; Paul D. R. Effect of organoclay structure on nylon 6 nanocomposite morphology and properties. Polymer 2002, 43, 5915–5933. 10.1016/S0032-3861(02)00400-7. [DOI] [Google Scholar]
- Chang J.-H.; Seo B.-S.; Hwang D.-H. An exfoliation of organoclay in thermotropic liquid crystalline polyester nanocomposites. Polymer 2002, 43, 2969–2974. 10.1016/S0032-3861(02)00125-8. [DOI] [Google Scholar]
- Ray S. S. Recent trends and future outlooks in the field of clay-containing polymer nanocomposites. Macromol. Chem. Phys. 2014, 215, 1162–1179. 10.1002/macp.201400069. [DOI] [Google Scholar]
- Chang J.-H.; Park K. M.; Lee S.-M.; Oh J. B. Two-step thermal conversion from poly(amic acid) to polybenzoxazole via polyimide: Their thermal and mechanical properties. J. Polym. Sci., Part B: Polym. Phys. 2000, 38, 2537–2545. . [DOI] [Google Scholar]
- Kostina J.; Rusakova O.; Bondarenko G.; Alentiev A.; Meleshko T.; Kukarkina N.; Yakimanskii A.; Yampolskii Y. Thermal rearrangement of functionalized polyimides: IR-spectral, quantum chemical studies, and gas permeability of TR polymers. Ind. Eng. Chem. Res. 2013, 52, 10476–10483. 10.1021/ie3034043. [DOI] [Google Scholar]
- Meis D.; Tena A.; Neumann S.; Georgopanos P.; Emmler T.; Shishatskiy S.; Rangou S.; Filiz V.; Abetz V. Thermal rearrangement of ortho-allyloxypolyimide membranes and the effect of the degree of functionalization. Polym. Chem. 2018, 9, 3987–3999. 10.1039/C8PY00530C. [DOI] [Google Scholar]
- Tyan H.-L.; Liu Y.-C.; Wei K.-H. Thermally and mechanically enhanced clay/polyimide nanocomposite via reactive organoclay. Chem. Mater. 1999, 11, 1942–1947. 10.1021/cm990187x. [DOI] [Google Scholar]
- Hsu S. L.-C.; Wang U.; King J.-S.; Jeng J.-L. Photosensitive poly(amic acid)/organoclay nanocomposites. Polymer 2003, 44, 5533–5540. 10.1016/S0032-3861(03)00626-8. [DOI] [Google Scholar]
- Min U.; Kim J.-C.; Chang J.-H. Transparent polyimide nanocomposite films: Thermo-optical properties, morphology, and gas permeability. Polym. Eng. Sci. 2011, 51, 2143–2150. 10.1002/pen.22059. [DOI] [Google Scholar]
- Kwon K.; Chang J.-H. Comparison of the properties of poly(lactic acid) nanocomposites with various fillers: Organoclay, functionalized graphene, or organoclay/functionalized graphene complex. Polymer 2014, 38, 232–239. 10.7317/pk.2014.38.2.232. [DOI] [Google Scholar]
- Yano K.; Usuki A.; Okada A. Synthesis and properties of polyimide-clay hybrid films. J. Polym. Sci., Part A: Polym. Chem. 1997, 35, 2289–2294. . [DOI] [Google Scholar]
- Do K.; Saleem Q.; Ravva M. K.; Cruciani F.; Kan Z.; Wolf J.; Hansen M. R.; Beaujuge P. M.; Brédas J.-L. Impact of fluorine substituents on π-conjugated polymer main-chain conformations, packing, and electronic couplings. Adv. Mater. 2016, 28, 8197–8205. 10.1002/adma.201601282. [DOI] [PubMed] [Google Scholar]
- Luo J.-J.; Daniel I. M. Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos. Sci. Technol. 2003, 63, 1607–1616. 10.1016/S0266-3538(03)00060-5. [DOI] [Google Scholar]
- Choi I. H.; Chang J.-H. Colorless polyimide nanocomposite films containing hexafluoroisopropylidene group. Polym. Adv. Technol. 2011, 22, 682–689. 10.1002/pat.1565. [DOI] [Google Scholar]
- Yano K.; Usuki A.; Okada A.; Krauchi T.; Kamigaito O. Synthesis and properties of polyimide-clay hybrid. J. Polym. Sci., Part A: Polym. Chem. 1993, 31, 2493–2498. 10.1002/pola.1993.080311009. [DOI] [Google Scholar]
- Choi I. H.; Chang J.-H. Characterization of colorless and transparent polyimide films synthesized with various amine monomers. Polymer(Korea) 2010, 34, 480–484. 10.7317/pk.2010.34.5.480. [DOI] [Google Scholar]