Abstract
Background
The Medtronic Freestyle prosthesis has proven to be a promising recourse for aortic root replacement in various indications. The present study aims to evaluate clinical outcomes and geometric changes of the aorta after Freestyle implantation.
Methods
Between October 2005 and November 2020, the computed tomography angiography (CTA) data of 32 patients were analyzed in a cohort of 68 patients that underwent aortic root replacement using Freestyle prosthesis. The minimum and maximum diameters and areas of the aortic annulus, aortic root, ascending aorta, and the proximal aortic arch were measured at a plane perpendicular to the long axis of the aorta using 3D multiplanar reconstruction in both the preoperative (n = 32) and postoperative (n = 10) CTAs. Moreover, volumetric changes of the aortic root and ascending aorta were quantified.
Results
Mean age was 64.6 ± 10.6 years. Indications for surgery using Freestyle prosthesis were combined aortic valve pathologies, aortic aneurysm or dissection, and endocarditis, with concomitant surgery occurring in 28 out of 32 patients. In-hospital mortality was 18.6%.
Preoperative diameter and area measurements of the aortic annulus strongly correlated with the implanted valve size (p < 0.001). Bicuspid valve was present in 28.1% of the patients. Diameter and areas of the aortic root decreased after freestyle implantation, resulting in a reduction of the aortic root volume (45.6 ± 26.3 cm3 to 18.7 ± 4.5 cm3, p = 0.029). Volume of the aortic root and the ascending aorta decreased from 137.3 ± 65.2 cm3 to 54.5 ± 21.1 cm3 after Freestyle implantation (p = 0.023).
Conclusion
Implantation of the Freestyle prosthesis presents excellent results in restoring the aortic geometry. Preoperative CTA measurements are beneficial to the surgical procedure and valve selection and therefore, if available, should be considered in pre-operative planning.
Keywords: Aortic valve replacement, Aortic root, Bioroot, Computed tomography measurement
Background
The choice of valve prosthesis in aortic valve and root surgery must be tailored to individual patient needs and characteristics. Medtronic Freestyle prosthesis offers a promising recourse for patients when aortic root replacement becomes necessary. Indications include aortic stenosis or regurgitation in combination with an enlargement of the aortic root or ascending aorta, as well as aortic dissection. Furthermore, a stentless valve is often used in high-risk patients with infective endocarditis of the aortic valve with periannular abscess [1, 2]. Stentless aortic prosthesis provide good clinical outcomes and excellent hemodynamics, particularly in smaller aortic annuli, and further prevent patient-prosthesis mismatch [3, 4].
Echocardiography remains the gold standard for pre- and postoperative diagnosis of aortic valve disease [5]. Since surgical aortic valve sizing is intraoperatively performed using visual observation and measurement, computed tomography is largely rendered unnecessary for sizing measurements. In fact, computed tomography angiography (CTA) is only performed for special indications, typically when echocardiographic findings suggest an aortic pathology in the presence of a bicuspid aortic valve or in the case of minimally invasive surgery [6]. In accordance with guidelines, CT evaluation is helpful in gaining more insight into aortic geometry, improving preoperative planning, and contributes to the evaluation of the severity of the valvular disease [5]. Furthermore, due to aortic root asymmetry, it’s dimensions can be underestimated in echocardiography [7].
In this CTA based study, we assessed the geometry of the aortic valve, root, and ascending aorta before and after the implantation of the Freestyle prosthesis alongside the associated clinical outcomes.
Methods
Study design and population
This study is of a retrospective design with prospectively collected data and analysis of CTA images. The study was approved by the local ethics committee and patient consent was waived (21–9907-BO).
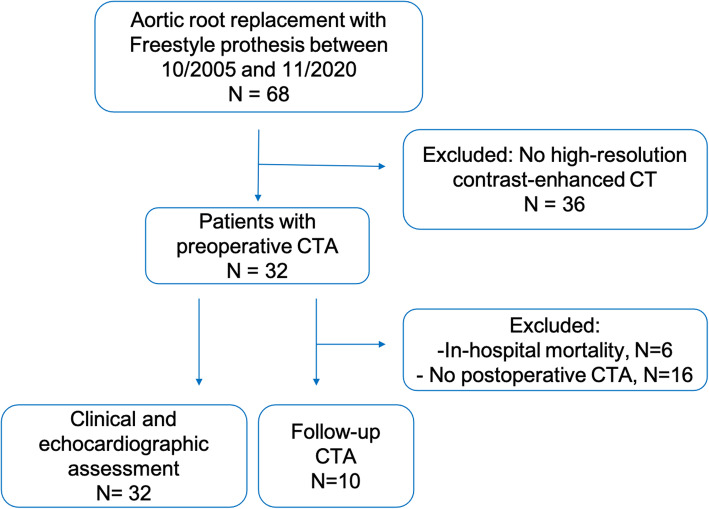
Between October 2005 and November 2020, 68 consecutive patients underwent aortic valve replacement (AVR) using the Medtronic Freestyle prosthesis (Medtronic Inc., Minneapolis, MN) at our institution. Of those, 32 patients received a preoperative CTA, with follow-up CTAs performed in 10 patients, after a median of 18 days [interquartile range (IQR): 12.5–457.0]. Indication for CTAs included ascending aortic aneurysm, aortic dissection, acute chest pain and unknown dyspnea, and previous cardiac surgery. All 32 aformentioned patients were included in this study (Fig. 1). Patient demographics, baseline clinical characteristics, echocardiographic findings, intraoperative parameters, and postoperative outcomes were evaluated. Aortic parameters were assessed in preoperative and postoperative CTAs using 3-dimentional (3D) multiplanar reconstructions (Fig. 2A-C). Preoperative mean transvalvular gradients were available in 12/32 patients.
Fig. 1.
Flowchart of patient inclusion
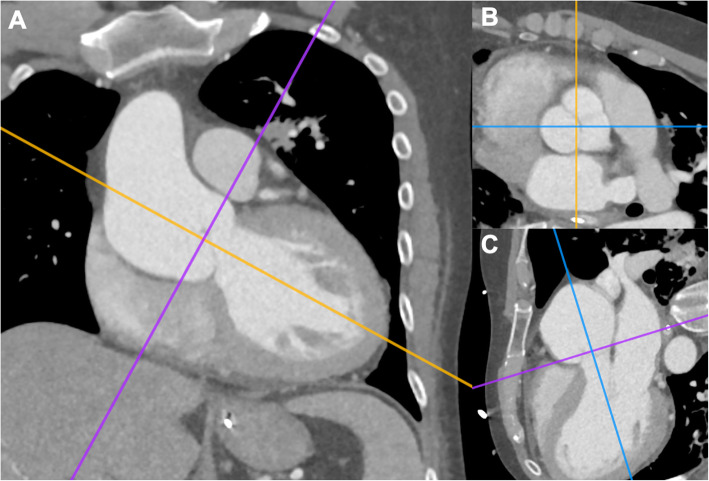
Fig. 2.
3-dimensional computed tomography reconstruction, with the visualization of the aortic root on a plane perpendicular to the long axis of the aorta (A-C)
Operative technique
All patients underwent surgery using the full root implantation technique, described by us thoroughly in previous literature [8]. In summary, aortic root replacement was performed via median sternotomy on cardiopulmonary bypass and in circulatory arrest. Cardiopulmonary bypass was achieved by cannulation of the ascending aorta and 2-stage venous cannulation. In case of an involvement of the aortic arch or an aortic dissection, arterial cannulation was achieved via the right subclavian artery with the use of an 8 mm vascular graft (n = 5). Cardioplegic arrest was enabled with cold crystalloid cardioplegia. After complete transection of the aorta, the aortic valve was resected. Implantation of the freestyle prosthesis was performed with pledgeted sutures. The coronary buttons were reimplanted into neo-ostia using continuous 5–0 polyprolene sutures. The bio-root was anastomosed either to the native aorta or, in the case of ascending aortic treatment, to the vascular graft. If further concomitant surgery was necessary, it was performed in a standard manner.
Follow up
Echocardiographic examinations were performed according to guidelines before discharge and within the first 6–12 months after surgery [5]. Thereafter, clinical assessment and echocardiography were recommended at annual intervals. CTA FU examinations were performed only for certain indications, such as aortic pathology in the downstream aorta, aortic dissection, or suspected valve dysfunction.
Imaging analysis
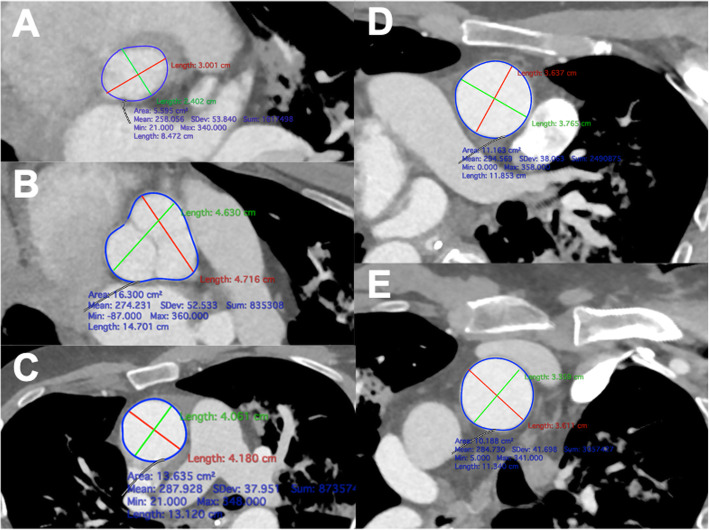
The CTA datasets were analyzed in Horos® (Nimble Co LLC d/b/a Purview in Annapolis, MD USA. 4 Version 3.3). Using 3D multiplanar reconstruction, the maximum and minimum diameter (mm) and aortic area (cm2) were measured in double-oblique planes at the level of the aortic annulus, sinus valsalva, sinotubular junction (STJ), proximal ascending aorta, and directly proximal to the origin of the brachiocephalic trunk (Fig. 3). A centerline was created from the aortic annulus to the most distal available part of the descending aorta to evaluate the length between the annulus and the aortic arch. Volumetric measurements of the aortic root and ascending aorta were obtained by manual segmentation and subsequent creation of a 3-dimentional model to enable automatic computation of the aortic volume.
Fig. 3.
The maximum and minimum diameter and areas of the aortic annulus (A), sinus valsalva (B), sinotubular junction (C), ascending aorta (D) and the proximal aortic arch (E) were measured using 3D multiplanar reconstruction
Statistical analysis
Statistical analysis was performed using SPSS (IBM SPSS, Version 27.0. Armonk, NY). Continuous and categorical variables were expressed as mean ± standard deviation or median and percentages, respectively. Normal assumption of continuous variables was validated using the Shapiro–Wilk test. Continuous variables were compared using the paired Student-t test. Correlations between continuous variables were calculated by Pearson’s test (Pearson’s coefficient r). All statistical tests were two sided with the alpha level set at 0.05 for statistical significance.
Results
Clinical characteristics
Preoperative patient characteristics of the entire cohort (n = 32) are displayed in Table 1. In the majority of patients, aortic root replacement was performed in either an elective (n = 19) or urgent (n = 8) setting. The indications were aortic regurgitation (n = 16), aortic stenosis (n = 12), or combined aortic valve pathology (n = 7), in combination with dilation of the aortic root (n = 14), ascending aorta (n = 18), or chronic aortic dissection (n = 4). Further indications were aortic valve endocarditis (n = 6) or double valve endocarditis (n = 3) with abscess formation (n = 5). All patients presented with multiple comorbidities, and the median EuroScore II was 7.4% [IQR: 4.46–22.75].
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| No. of patients | 32 |
| Demographics | |
| Age (years) | 64.6 ± 10.6 |
| Gender | |
| Male | 22 (68.7%) |
| Female | 10 (31.2%) |
| Body Mass Index (kg/m2) | 26.89 ± 5.89 |
| Body Surface Area (cm2) | 1.98 ± 0.26 |
| Clinical characteristics | |
| Arterial hypertension | 30 (93.7%) |
| Pulmonary hypertension | 7 (21.9%) |
| Chronic obstructive pulmonary disease | 8 (25.0%) |
| NYHA classification | |
| I | 12 (37.5%) |
| II | 7 (21.9%) |
| III | 10 (31.2%) |
| IV | 3 (9.4%) |
| Diabetes melltitus | 8 (25.0%) |
| Hyperlipoproteinemia | 19 (59.4%) |
| Chronic renal failure | 12 (37.5%) |
| Peripheral vascular disease | 5 (15.6%) |
| Cerebrovascular disease | 8 (25.0%) |
| Previous stroke | 4 (12.5%) |
| Previous cardiac surgery | 10 (31.2%) |
| AVR | 5 (15.6%) |
| AVR with ascending aorta replacement | 3 (9.4%) |
| CABG | 1 (3.1%) |
| CABG, MVR | 1 (3.1%) |
| Previous PCI | 4 (12.5%) |
| Previous endocarditis | 4 (12.5%) |
| EuroSCORE II (%) | 7.24 (4.46–22.75) |
| Echocardiographic findings | |
| Left ventricular ejection fraction (%) | 50 ± 13 |
| Aortic insufficiency | |
| Mild | 5 (15.6%) |
| Moderate | 7 (21.9%) |
| Severe | 8 (25.0%) |
| Aortic stenosis | |
| Mild | 1 (3.1%) |
| Moderate | 2 (6.25%) |
| Severe | 12 (37.5%) |
| Biscuspid valve | 9 (28.1%) |
| Mitral valve insufficiency | 21 (65.6%) |
| Tricuspid valve insufficiency | 14 (43.7%) |
AVR aortic valve replacement, CABG Coronary artery bypass grafting, PCI percutaneous coronary intervention
Aortic root replacement in emergency settings (5/32) were performed due to aortic regurgitation (n = 3), endocarditis (n = 2), aortic dissection (n = 2), or a combination of the above. Ten patients (31.2%) had a history of previous sternotomy, with eight (25.0%) being for AVR. Preoperative median aortic transvalvular gradient was 23.5 mmHg [IQR: 10.25–41].
Operative details and clinical outcomes
Concomitant procedures were necessary in 28 out of 32 patients (87.5%), including ascending aorta replacement, hemi arch replacement, additional valve surgery, and coronary artery bypass grafting (Table 2). Mean cardiopulmonary bypass time was 174 ± 87 min. Sizes of the implanted valve varied from 21 mm to 29 mm (mean 26.2 ± 2.4 mm), resulting in a mean effective orifice area of 1.19 ± 0.20 cm2.
Table 2.
Operative details and clinical outcome
| Operative details | Value |
|---|---|
| Cardiopulmonary bypass time (min) | 174 ± 87 |
| Crossclamp time (min) | 119 ± 53 |
| Valve Size (mm) | 26.2 ± 2.4 |
| Effective orifice area (cm2) | 1.19 ± 0.20 |
| Concomittant procedure | |
| Ascending aorta replacement | 21 (65.6%) |
| Aortic hemi-arch replacement | 6 (18.7%) |
| Mitral valve surgery | |
| replacement | 3 (9.4%) |
| repair | 1 (3.1%) |
| Tricuspid valve repair | 2 (6.25%) |
| Coronary artery bypass grafting | 12 (37.5%) |
| Mechanical circulatory support | |
| Extracorporeal life support | 4 (12.5%) |
| Intra-aortic balloon pump | 3 (9.4%) |
| Postoperative Outcomes | |
| Bleeding | 6 (18.7%) |
| Thromboembolic event | |
| Stroke | 3 (9.4%) |
| Myocardial infarction | 1 (3.1%) |
| Postoperative atrial fibrillation | 9 (28.1%) |
| Permanent pacemaker implantation | 4 (12.5%) |
| Dialysis | 11 (34.4%) |
| Sepsis | 5 (15.6%) |
| Prolonged ventilation | 12 (37.5%) |
| Re-intubation | 2 (6.25%) |
| Sternal wound infection | 1 (3.1%) |
| In-hospital mortality | 6 (18.7%) |
In-hospital mortality was 18.7%. Seven patients required temporary mechanical support in terms of an extracorporeal life support (12.5%) or an intra-aortic balloon pump (9.4%). Six patients (18.7%) underwent re-sternotomy due to bleeding complications. Neurological complications in terms of procedure-related stroke occurred in three patients (9.4%). Postoperative median aortic transvalvular gradient was 5.0 mmHg [IQR: 3.5–13.5].
Morphological findings
Preoperative minimum and maximum diameter at the level of the aortic annulus were 26.1 ± 3.2 mm and 28.1 ± 3.5 mm, respectively, with a measured aortic area of 6.2 ± 1.5 cm2 (Table 3). The implanted valve size (mean 26.2 ± 2.4 mm) and the calculated circular area (πr2, 5.4 ± 1.0 cm2) strongly correlated with the preoperative minimum and maximum diameter and aortic annulus area (r = 0.78, r = 0.85, r = 0.77, respectively, p < 0.001).
Table 3.
Pre- and postoperative measurements of the aortic root and ascending aorta
| Preoperative (n = 32) |
Postoperative (n = 10) |
P value* | |
|---|---|---|---|
| Aortic annulus | |||
| Diameter max. (mm) | 28.1 ± 3.5 | 26.8 ± 2.9 | 0.485 |
| Diameter min. (mm) | 26.2 ± 3.2 | 26.3 ± 3.0 | 0.049 |
| Area (cm2) | 6.2 ± 1.5 | 5.8 ± 1.5 | 0.025 |
| Sinus of Valsalva | |||
| Diameter max. (mm) | 40.6 ± 8.6 | 33.2 ± 6.3 | 0.092 |
| Diameter min. (mm) | 37.1 ± 7.5 | 30.6 ± 4.9 | 0.071 |
| Area (cm2) | 12.5 ± 4.7 | 5.9 ± 1.5 | 0.069 |
| Sinotubular junction | |||
| Diameter max. (mm) | 39.3 ± 9.8 | 29.8 ± 4.4 | 0.021 |
| Diameter min. (mm) | 36.0 ± 11.1 | 28.6 ± 4.1 | 0.518 |
| Area (cm2) | 12.1 ± 5.8 | 6.7 ± 1.8 | 0.035 |
| Mid-ascending aorta | |||
| Diameter max., (mm) | 44.1 ± 9.5 | 31.8 ± 3.5 | 0.069 |
| Diameter min. (mm) | 42.7 ± 9.5 | 30.1 ± 2.9 | 0.070 |
| Area (cm2) | 15.6 ± 6.3 | 7.9 ± 1.7 | 0.070 |
| Proximal aortic arch | |||
| Diameter max. (mm) | 37.5 ± 5.9 | 31.3 ± 4.7 | 0.087 |
| Diameter min. (mm) | 35.1 ± 5.9 | 29.6 ± 4.9 | 0.178 |
| Area (cm2) | 10.6 ± 3.4 | 8.0 ± 2.4 | 0.203 |
| Length root, ascending aorta (cm) | 10.8 ± 2.2 | 7.0 ± 1.3 | 0.005 |
| Volume aortic root, (cm3) | 45.6 ± 26.3 | 18.7 ± 4.5 | 0.029 |
| Volume root and ascending aorta, (cm3) | 137.3 ± 65.2 | 54.5 ± 21.1 | 0.023 |
*p-value: paired Student-t test (n = 10)
The minimum and maximum diameter of the sinus valsalva were 37.12 ± 7.5 mm and 40.59 ± 8.61 mm, respectively. Bicuspid valve was present in 28.1% of the patients. Preoperative measurements of the aortic root were also performed sinus-to-sinus (Fig. 4A) and sinus-to-commissures (Fig. 4B). Mean length of the sinuses of valsalva were 37.9 ± 5.5 mm, 36.7 ± 5.1 mm, 35.3 ± 4.9 mm sinus-to-sinus and 36.8 ± 5.0 mm, 35.3 ± 4.9 mm, 33.7 ± 5.1 mm sinus-to-commissure.
Fig. 4.
Preoperative measurements of the aortic root were performed sinus-to-sinus (A) and sinus-to-commissures (B)
The diagnosis of a root aneurysm varied depending on the use of the minimum or maximum diameter. Five patients had a maximum diameter > 5 cm, and in 12 patients > 4.5 cm, but only two had it > 5 cm and five had it > 4.5 cm when using the minimum diameter. In the mid-ascending aorta, the use of maximum and minimum diameter did not result in a change regarding the diagnosis.
Diameters at the STJ decreased significantly after implantation of the Freestyle prosthesis, but only in terms of the maximum diameter and area. Diameters and areas of the mid-ascending aorta and proximal arch changed depending on the implementation of ascending aortic surgery.
Volumetric changes
After Freestyle implantation, with or without ascending or hemi arch replacement, the length from the aortic root to the proximal arch decreased from 10.8 ± 2.2 cm to 7.0 ± 1.3 cm. Volume of the aortic root decreased from 45.6 ± 26.3 cm3 to 18.7 ± 4.5 cm3 (p = 0.029). The combined volume of the aortic root and ascending aorta decreased from 137.3 ± 65.2 cm3 to 54.5 ± 21.1 cm3 (p = 0.023) (Fig. 5).
Fig. 5.
Volumetric measurements were performed on pre- (A) and postoperative (C) CTAs, with the areas of interest drawn manually in each slice (B, D). These measurements demonstrate a decrease of the ascending aorta after Freestyle implantation
Discussion
The Freestyle stentless aortic bioprosthesis has demonstrated excellent long-term clinical and hemodynamic results [9]. The aim of this study was to evaluate the geometrical changes of the aorta following Freestyle prosthesis implantation in the context of clinical outcomes.
The use of a bioprosthesis is recommended in patients over the age of 65 years [5]. The choice between a biological and mechanical valve is also dependent on patient’s preference concerning the trade-off between the potential need for reintervention for valve destruction versus the risk associated with life-long anticoagulation. Further considerations are comorbidities, life expectancy, level of compliance and patient’s lifestyle [10]. If a reintervention becomes necessary, a transcatheter valve-in-valve procedure offers a good option, but entails the implantation of a smaller valve, which may result in patient-prothesis mismatch. Therefore, a mechanical valve is preferable in small annuli [11]. If patients’ characteristics nevertheless require the implantation of a biological valve, a Freestyle prothesis is preferred due to its larger effective orifice area. Furthermore, in case of destructive endocarditis or reoperation, especially if the aortic root tissue is fragile a Freestyle prothesis offers an excellent option.
In our cohort, Freestyle prosthesis was deployed in high-risk patients with infective endocarditis, aortic root enlargement, or aortic dissection. In the vast majority of these patients (87.5%), a concomitant procedure was necessary. Moreover, four patients (12.5%) underwent an isolated root replacement, two of which were redo procedures. One patient suffered from infective endocarditis and the other presented with isolated root dilatation. The population of this particular study group could be explained through the inclusion criteria for performing preoperative CTAs. Furthermore, this study population accounts for an in-hospital mortality of 18.6%, which is elevated when compared to isolated AVR with Freestyle prosthesis. Indeed, in their systematic review Sherrah et al., reported an in-hospital mortality of 5.2% after Freestyle implantation [12]. When our results are placed into perspective, and consideration is made of mortality rates in combined aortic root procedures, high-risk patients, reoperations, or freestyle prosthesis implantation in destructive endocarditis, the outcomes could be considered comparable [13–17].
Following AVR, a high residual transvalvular gradient constitutes a risk factor for worse outcomes, impaired left ventricular diastolic dysfunction, and incomplete regression of left ventricular hypertrophy [18, 19]. The Freestyle aortic bioprosthesis was designed to provide superior hemodynamic performance, more physiological flow patterns, and lower transvalvular gradients [20, 21]. Indeed, a reduction of the mean transvalvular gradient has been described by multiple studies [22]. Yun and colleagues, reported a 41% decrease after AVR with Freestyle prosthesis within the first 6 months, with a corresponding increase in EOA. After 6 months, the gradients remained relatively stable [23]. Accordingly, in our study median transvalvular gradients declined from 23.5 mmHg [IQR: 10.25–41] preoperatively to 5.0 mmHg [IQR: 3.5–13.5] during the postoperative course.
Echocardiographic diagnostics is the gold standard for AVR, evaluating not only the valve itself but also the dimensions of the aorta, which, currently remains the most influential parameter for assessing the risk for aortic dissection and deciding on surgery [24]. As a matter of fact, precise evaluation of the aortic diameter is essential for an accurate diagnosis and further planning of the surgical procedure [25, 26]. Due to the elliptical shape of the aortic annulus, with its maximum diameter lying in the coronary plane, its dimensions can be subject to significant underestimation when using echocardiography or only 2D CT measurements [27]. CT allows for the assessment of the valve anatomy, differentiation between bicuspid and tricuspid valves, and the shape and diameter of the aortic annulus and the left ventricular outflow tract. In our study, the measurements of the aortic annulus strongly correlated with the implanted size of the Freestyle prosthesis. Despite the advantage of sizing under direct vision, accurate pre-operative assessment is important for valve selection and the decision of whether additional surgery is necessary. Especially in patients with asymmetrical aneurysms and bicuspid aortic valve, a significant difference between the minimum and maximum diameter of the aortic root has been described [7]. In the present study, the comparison between the minimum and maximum measured diameter resulted in a more than 20% higher diagnosis rate of root aneurysms > 4.5 cm. On the other hand, 3D volume reconstructions allow the measurement of the entire volume of interest. Therefore, 3D volume measurements are more accurate in detecting small changes in the size of an aneurysm than diameter measurements [28]. Geisbüsch et al. assessed the volume of the ascending aorta in patients with aneurysms compared to a control group [29] and reported a volume of 132.9 ± 39.4 ml in patients with ascending aortic aneurysm and 78.0 ± 24.5 ml in the control group. In our study, the pre-operative volume of the aortic root and ascending aorta were similar (137.27 ± 65.24 cm3). Subsequent to Freestyle prosthesis implantation, the length from the aortic root to the proximal aortic arch decreased by approximately 3 cm. Moreover, the diameters and areas decreased to normal values, resulting in a mean volume of 54.5 ± 21.1 cm3 [30]. These volumetry results demonstrate an excellent restoration of the aortic root and ascending aortic geometry.
Study limitations
The presented study is an observational assessment of clinical and morphological outcomes. Patients were not randomly assigned to different therapies. The study is therefore limited by its retrospective, nonrandomized, single-center nature. We observed only patients with Freestyle prothesis, therefore we are not able to generalize the findings for different valve types.
The main limitation of this study is the relatively small cohort size, caused by the inclusion criterion for the availability of preoperative CTAs. Since we do not routinely perform a FU CTA, postoperative CT was not available in all patients.
Conclusions
Freestyle prosthesis is an excellent option for high-risk patients with concomitant aortic pathology or destruction of the aortic root due to endocarditis. CTA measurements provide substantial information for surgical planning and therefore, if available, should be considered in pre-operative planning.
Acknowledgements
None.
Abbreviations
- 3D
3-dimentional
- AVR
Aortic valve replacement
- CTA
Computed tomography angiography
- IQR
Interquartile range
- STJ
Sinotubular junction
Authors’ contributions
Concept/design: AO, AW, AZ; Data analysis/interpretation: AO, AW, AZ, KZ; Data collection and statistical analysis: AO, DW; Drafting article: AO, Critical revision of article: AZ, AW, AR, RV, Approval of article: BS, AR, AM.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The authors declare that all data supporting the findings of this study are available within the article.
Declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee and patient consent was waived (21–9907-BO).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gulbins H, Reichenspurner H. Which patients benefit from stentless aortic valve replacement? Ann Thorac Surg. 2009;88(6):2061–2068. doi: 10.1016/j.athoracsur.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 2.Easo J, Szczechowicz M, Hoelzl P, Horst M, Eichstaedt H, Zhigalov K, Mashhour A, Weymann A, Dapunt OE. Use of the Medtronic freestyle for aortic valve infection: a retrospective propensity score matched analysis. J Card Surg. 2019;34(10):957–964. doi: 10.1111/jocs.14176. [DOI] [PubMed] [Google Scholar]
- 3.Cohen G, Zagorski B, Christakis GT, Joyner CD, Vincent J, Sever J, Harbi S, Feder-Elituv R, Moussa F, Goldman BS, Fremes SE. Are stentless valves hemodynamically superior to stented valves? Long-term follow-up of a randomized trial comparing Carpentier-Edwards pericardial valve with the Toronto Stentless porcine valve. J Thorac Cardiovasc Surg. 2010;139(4):848–859. doi: 10.1016/j.jtcvs.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 4.Milano AD, Blanzola C, Mecozzi G, D'Alfonso A, De Carlo M, Nardi C, et al. Hemodynamic performance of stented and stentless aortic bioprostheses. Ann Thorac Surg. 2001;72(1):33–38. doi: 10.1016/S0003-4975(01)02672-8. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 6.Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, Khoynezhad A, Siu SC, Verma S, Hope MD, Cameron DE, Hammer DF, Coselli JS, Moon MR, Sundt TM, Barker AJ, Markl M, Della Corte A, Michelena HI, Elefteriades JA. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156(2):e41–e74. doi: 10.1016/j.jtcvs.2018.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plonek T, Berezowski M, Bochenek M, Filip G, Rylski B, Golesworthy T, Jasinski M. A comparison of aortic root measurements by echocardiography and computed tomography. J Thorac Cardiovasc Surg. 2019;157(2):479–486. doi: 10.1016/j.jtcvs.2018.07.053. [DOI] [PubMed] [Google Scholar]
- 8.Zubarevich A, Zhigalov K, Osswald A, Arjomandi Rad A, Vardanyan R, Wendt D, Sá MPBO, Schmack B, Ruhparwar A, Weymann A. Essen-commando: how we do it. J Card Surg. 2021;36(1):286–289. doi: 10.1111/jocs.15140. [DOI] [PubMed] [Google Scholar]
- 9.Bach DS, Kon ND. Long-term clinical outcomes 15 years after aortic valve replacement with the freestyle stentless aortic bioprosthesis. Ann Thorac Surg. 2014;97(2):544–551. doi: 10.1016/j.athoracsur.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 10.Varc-3 Writing C. Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42(19):1825–1857. doi: 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135(25):e1159–e1e95. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 12.Sherrah AG, Edelman JJ, Thomas SR, Brady PW, Wilson MK, Jeremy RW, et al. The freestyle aortic bioprosthesis: a systematic review. Heart Lung Circ. 2014;23(12):1110–1117. doi: 10.1016/j.hlc.2014.04.262. [DOI] [PubMed] [Google Scholar]
- 13.Szeto WY, Bavaria JE, Bowen FW, Geirsson A, Cornelius K, Hargrove WC, Pochettino A. Reoperative aortic root replacement in patients with previous aortic surgery. Ann Thorac Surg. 2007;84(5):1592–1598. doi: 10.1016/j.athoracsur.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 14.Heinz A, Dumfarth J, Ruttmann-Ulmer E, Grimm M, Muller LC. Freestyle root replacement for complex destructive aortic valve endocarditis. J Thorac Cardiovasc Surg. 2014;147(4):1265–1270. doi: 10.1016/j.jtcvs.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Keeling WB, Hunting J, Leshnower BG, Stouffer C, Binongo J, Chen EP. Salvage coronary artery bypass predicts increased mortality during aortic root operation. Ann Thorac Surg. 2018;106(6):1727–1734. doi: 10.1016/j.athoracsur.2018.06.079. [DOI] [PubMed] [Google Scholar]
- 16.Easo J, Weymann A, Holzl P, Horst M, Eichstaedt H, Mashhour A, et al. Hospital results of a single center database for Stentless xenograft use in a full root technique in over 970 patients. Sci Rep. 2019;9(1):4371. doi: 10.1038/s41598-019-40772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osswald A, Schmack B, Ruhparwar A. Impella 5.0 as short-term mechanical circulatory support following mitral valve surgery in high risk patients. Artif Organs. 2019;43(12):1182–1184. doi: 10.1111/aor.13515. [DOI] [PubMed] [Google Scholar]
- 18.Zenses AS, Dahou A, Salaun E, Clavel MA, Rodes-Cabau J, Ong G, et al. Haemodynamic outcomes following aortic valve-in-valve procedure. Open Heart. 2018;5(2):e000854. doi: 10.1136/openhrt-2018-000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinovic I, Farah I, Everlien M, Lindemann S, Knez I, Wittlinger T, Greve H, Vogt P. Eight-year results after aortic valve replacement with the CryoLife-O'Brien Stentless aortic porcine bioprosthesis. J Thorac Cardiovasc Surg. 2005;130(3):777–782. doi: 10.1016/j.jtcvs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Cartier PC, Dumesnil JG, Metras J, Desaulniers D, Doyle DP, Lemieux MD, et al. Clinical and hemodynamic performance of the freestyle aortic root bioprosthesis. Ann Thorac Surg. 1999;67(2):345–349. doi: 10.1016/S0003-4975(98)01350-2. [DOI] [PubMed] [Google Scholar]
- 21.Van den Eynde J, Sa M, Callahan CP, Dimagli A, Vervoort D, Kampaktsis PN, et al. Right ventricular outflow tract reconstruction with Medtronic freestyle valve in the Ross procedure: a systematic review with meta-analysis. Artif Organs. 2021;45(4):338–345. doi: 10.1111/aor.13837. [DOI] [PubMed] [Google Scholar]
- 22.Ennker J, Albert A, Ennker IC. Stentless aortic valves. Current aspects. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4(2):77–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Yun KL, Sintek CF, Fletcher AD, Pfeffer TA, Kochamba GS, Hyde MR, Torpoco JO, Khonsari S. Aortic valve replacement with the freestyle stentless bioprosthesis: five-year experience. Circulation. 1999;100(19 Suppl):II17–II23. doi: 10.1161/01.cir.100.suppl_2.ii-17. [DOI] [PubMed] [Google Scholar]
- 24.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 25.Elefteriades JA. Indications for aortic replacement. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S5–S9. doi: 10.1016/j.jtcvs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ, ESC Committee for Practice Guidelines 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 27.Hanneman K, Chan FP, Mitchell RS, Miller DC, Fleischmann D. Pre- and postoperative imaging of the aortic root. Radiographics. 2016;36(1):19–37. doi: 10.1148/rg.2016150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trinh B, Dubin I, Rahman O, Ferreira Botelho MP, Naro N, Carr JC, Collins JD, Barker AJ. Aortic Volumetry at contrast-enhanced magnetic resonance angiography: feasibility as a sensitive method for monitoring bicuspid aortic valve Aortopathy. Investig Radiol. 2017;52(4):216–222. doi: 10.1097/RLI.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisbusch S, Stefanovic A, Schray D, Oyfe I, Lin HM, Di Luozzo G, et al. A prospective study of growth and rupture risk of small-to-moderate size ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2014;147(1):68–74. doi: 10.1016/j.jtcvs.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Vriz O, Aboyans V, D'Andrea A, Ferrara F, Acri E, Limongelli G, Della Corte A, Driussi C, Bettio M, Pluchinotta FR, Citro R, Russo MG, Isselbacher E, Bossone E. Normal values of aortic root dimensions in healthy adults. Am J Cardiol. 2014;114(6):921–927. doi: 10.1016/j.amjcard.2014.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article.