Abstract
Aim
Obesity is a risk factor for COVID-19, but the underlying mechanisms are unclear. We investigated the role of adiponectin (an anti-inflammatory adipokine), leptin (a pro-inflammatory adipokine) and their ratio (Adpn/Lep) in this context.
Design
Single-centre, prospective observational study. Methods. Adiponectin and leptin were measured in 60 COVID-19 patients with mild (not hospitalised, n=11), moderate (hospitalised but not requiring intensive care, n=25) and severe (admission to the intensive care unit [ICU] or death, n=24) disease.
Results
Adiponectin and leptin levels were similar across severity groups, but patients with moderate severity had the highest Adpn/Lep ratio (1.2 [0.5; 2.0], 5.0 [1.6; 11.2], 2.1 [1.0; 3.6] in mild, moderate and severe disease; P = 0.019). Adpn/Lep, but not adiponectin or leptin alone, correlated with systemic inflammation (C reactive protein, CRP: Spearman's rho 0.293, P = 0.023). When dividing patients into Adpn/Lep tertiles, adiponectin was highest, whereas leptin was lowest in the third (highest) tertile. Patients in the highest Adpn/Lep tertile had numerically lower rates of obesity, diabetes and hypertension, and lower rates of death or admission to ICU versus other tertiles. At linear regression in the whole cohort, CRP significantly predicted Adpn/Lep (β 0.291, P = 0.022), while female gender (β -0.289, P = 0.016), diabetes (β -0.257, P = 0.028), and hypertension (β -239, P = 0.043) were negative predictors.
Conclusions
We speculate that the rise in Adpn/Lep, due to increased adiponectin and reduced leptin, is a compensatory response to systemic inflammation. In patients with worse cardiometabolic health (e.g. diabetes, hypertension) this mechanism might be blunted, possibly contributing to higher mortality.
Keywords: Adiponectin, Diabetes, Inflammation, Leptin, Obesity, SARS-CoV-2
Introduction
Obesity is an independent risk factor for severe coronavirus disease 2019 (COVID-19), increasing the risk of hospitalisation, severe pneumonia, admission to the intensive care unit (ICU), and death [1, 2]. Several mechanisms have been proposed to explain these associations. Mechanical factors such as reduced chest expansion, low respiratory muscle strength, and increased airway resistance all contribute to hypoventilation and worse respiratory outcomes [3]. Furthermore, emerging evidence links the amount of visceral adipose tissue (VAT) with COVID-19 severity [4, 5]. VAT inflammation is a key event triggering whole body metabolic abnormalities. The increase in inflammatory cytokines produced in adipose tissue (AT) such as leptin, tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) reflects excessive and dysfunctional AT [6], and could amplify systemic inflammation in COVID-19 patients with obesity [1, 3]. The role of adiponectin, an insulin-sensitizing and anti-inflammatory adipokine that is usually reduced in obesity [7], has been largely overlooked until recently, when a non-significant increase in adiponectin levels in patients with severe COVID-19 has been reported [8]. Of note, both leptin and adiponectin modulate lung inflammation [9]. Despite convincing theoretical bases, the evidence supporting the involvement of these two adipokines in the hyperinflammatory response in COVID-19 is scarce [8, 10, 11]. Furthermore, in almost all physiological conditions, leptin and adiponectin are regulated in an opposite manner, but no study has assessed both in the same cohort of COVID-19 patients. Based on this background, we sought to investigate the role of adiponectin, leptin and their ratio, which reflects AT dysfunction better than adiponectin or leptin alone [12], in patients with COVID-19 of different severity.
Material and methods
Study design
This was a sub-study of the COVID-BioB study, a large prospective observational investigation performed at San Raffaele University Hospital, Milan, Italy [13]. The study protocol complies with the Declaration of Helsinki, was approved by the Hospital Ethics Committee (protocol no. 34/int/2020) and was registered on ClinicalTrials.gov (NCT04318366). Full description of patient management and clinical protocols were previously published [13]. Signed informed consent was obtained from all patients.
Study subjects
Sixty adult (age ≥ 18 years) patients with a confirmed diagnosis of COVID-19 who had been admitted to the Emergency Department (ED) of San Raffaele University Hospital during the first wave of the pandemic (March 18th and May 5th, 2020) and whose anthropometric data were available were included in the present analysis. COVID-19 severity was classified as mild (patients not hospitalised, n=11), moderate (patients hospitalised but not requiring intensive care, n=25) and severe (admission to the intensive care unit [ICU] or death, n=24).
Study procedures
The following variables were collected for all patients: demographics, body mass index (BMI), PaO2/FiO2 (the ratio between the arterial partial pressure of oxygen measured on arterial blood gas analysis and the fraction of inspired oxygen, a unitless measure of pulmonary function; lower values indicate worse respiratory function, and values ≤ 300 – together with clinical and imaging features – are used in the definition of acute respiratory distress syndrome [ARDS] [14]), plasma glucose, estimated glomerular filtration rate (eGFR, as estimated by the CKD-EPI equation), lactate dehydrogenase (LDH) and high-sensitivity C-reactive protein (CRP) on admission to the ED, comorbidities (including history of hypertension, diabetes, coronary artery disease [CAD]). Adipokines were measured using ELISA kits (ab99968 and ab179884 for adiponectin and leptin, respectively; Abcam, Cambridge, UK). Median [25th – 75th percentile] time from hospital admission to venipuncture was 1 [0; 2] days.
Statistical analysis
Descriptive statistics were obtained for all study variables. Continuous variables were expressed as medians [25th – 75th percentile]. Categorical variables were summarised as counts and percentages. Fisher's exact test or χ2 test and Mann-Whitney U test or Kruskal–Wallis test were employed to assess differences in proportions and medians, respectively. Correlations were analysed using the Spearman rank correlation test. Simple and multiple linear regressions were carried out to identify predictors of the adiponectin/leptin (Adpn/Lep) ratio. Adpn/Lep, CRP and BMI were log-transformed due to non-normal distribution. All statistical tests were two-sided. A p-value of 0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
Patients were mostly males (68.3%), median age 59.3 [51.2; 66.6] years, BMI 27.0 [25.0; 30.5] kg/m2, leptin 5.7 [2.8; 10.3] ng/mL, adiponectin 14.8 [3.2; 29.5] µg/mL. Overall, 25.0% had a normal weight, 43.3% were overweight, 31.7% had obesity, and 20% had diabetes mellitus. Age, systemic inflammation as reflected by CRP and neutrophil to lymphocyte ratio (NLR), plasma glucose, LDH levels and hypoxia as reflected by PaO2/FiO2 increased with increasing COVID-19 severity (Table 1). Adiponectin and leptin levels did not differ across severity groups, but patients with moderate severity had the highest Adpn/Lep ratio (Table 1).
Table 1.
Comparison among patients with mild, moderate or severe COVID-19.
| Mild (not hospitalised)(n=11) | Moderate (no ICU, death)(n=25) | Severe (ICU, death)(n=24) | P | |
|---|---|---|---|---|
| Age (years) | 46.3 (34.2; 51.3) | 59.5 (52.1; 67.5)* | 62.7 (57.7; 70.3)* | 0.001 |
| Male gender | 4 (36.4) | 18 (72.0) | 19 (79.2)* | 0.043 |
| Obesity | 2 (18.2) | 8 (32.0) | 9 (37.5) | 0.499 |
| Hypertension | 2 (18.2) | 8 (32.0) | 11 (45.8) | 0.259 |
| CAD | 0 (0.0) | 1 (4.0) | 5 (20.8) | 0.049 |
| Diabetes | 1 (9.1) | 5 (20.0) | 6 (25.0) | 0.551 |
| CKD | 0 (0.0) | 0 (0.0) | 2 (8.3) | 0.152 |
| PaO2/FiO2 | 368.6 (333.8; 401.4) | 276.2 (221.4; 324.1)* | 151.6 (58.0; 271.8)*,# | 0.001 |
| NLR | 3.2 (2.2; 6.1) | 5.9 (3.9; 8.0) | 7.7 (5.4; 16.1)* | 0.002 |
| CRP (mg/dL) | 19.8 (8.2; 73.4) | 96.2 (38.0; 156.5)* | 126.2 (66.3; 267.6)* | 0.001 |
| Glucose (mg/dL) | 94.5 (86.8; 105.5) | 110.0 (101.5; 119.0) | 133.0 (107.8; 162.0)* | 0.001 |
| LDH (U/L) | 253.5 (197.2; 333.8) | 371.0 (307.8; 487.3)* | 539.0 (369.0; 725.0)* | 0.001 |
| Leptin (ng/mL) | 6.7 (3.8; 7.9) | 5.2 (1.3; 10.2) | 5.6 (2.9; 13.3) | 0.664 |
| Adiponectin (µg/mL) | 4.1 (2.7; 14.7) | 24.3 (3.4; 39.2) | 20.8 (3.5; 29.7) | 0.112 |
| Adpn/Lep | 1.2 (0.5; 2.0) | 5.0 (1.6; 11.2)* | 2.1 (1.0; 3.6) | 0.019 |
P 0.05 vs. mild;
P 0.05 vs. moderate. Categorical variables are expressed as counts (percentage). Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease; PaO2/FiO2, arterial partial pressure of oxygen measured on arterial blood gas analysis divided by the fraction of inspired oxygen (unitless); NLR, neutrophil to lymphocyte ratio (unitless), CRP, high-sensitivity C-reactive protein; LDH, lactate dehydrogenase; Adpn/Lep, adiponectin to leptin ratio (unitless).
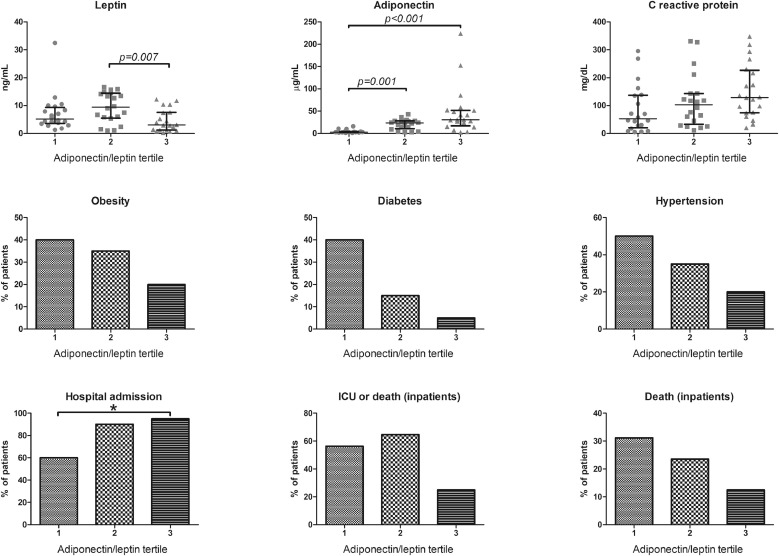
Leptin (Spearman's rho 0.480, P 0.001) significantly correlated with BMI. Neither leptin (P = 0.339) nor adiponectin (P = 0.113) alone correlated with the concentration of CRP. In contrast, Adpn/Lep ratio was significantly associated with CRP levels (Spearman's rho 0.293, P = 0.023) and CRP levels tended to increase with increasing Adpn/Lep tertile (P = 0.051, Fig. 1 ).
Fig. 1.
Leptin, adiponectin, C reactive protein and rates (%) of obesity, diabetes, hypertension, hospitalisation and (hospitalised patients only) admission to the intensive care unit (ICU) or death and death across tertiles of adiponectin/leptin ratio (33rd percentile: 1.46; 66th percentile: 3.59).
The proportion of patients with cardiometabolic disturbances (obesity, diabetes, hypertension) tended to decrease, while the proportion of patients admitted to hospital increased with increasing Adpn/Lep tertiles (P for trend = 0.010, Fig. 1). None of the patients managed at home died. Among hospitalized patients, the rate of death or admission to ICU was lowest in the highest Adn/Lep tertile (Fig. 1). Of note, mortality tended to decrease with increasing Adn/Lep tertiles.
The increase in Adpn/Lep reflects a compensatory response to inflammation [15]. Thus, we performed linear regression analyses to assess whether changes in CRP predicted changes in Adpn/Lep. At simple linear regression, CRP predicted Adpn/Lep, accounting for 9.7% of the explained variability in Adpn/Lep (P = 0.016). In a model including age, sex, BMI, diabetes and hypertension, CRP (β 0.291, P = 0.022) significantly and positively predicted Adpn/Lep, while female sex (β -0.289, P = 0.016) diabetes (β -0.257, P = 0.028) and hypertension (β -239, P = 0.043) emerged as significant negative predictors of Adpn/Lep (R2 = 0.391, P 0.001, adjusted R2 = 0.322).
Discussion
The correlation with the extent of systemic inflammation, along with the higher rate of hospital admissions with increasing Adpn/Lep tertiles, might suggest that the rise in Adpn/Lep ratio, driven by increased adiponectin and reduced leptin, reflects a compensatory response to inflammation and severe disease. Indeed, patients with the highest Adpn/Lep ratio (i.e. an adequate anti-inflammatory response) were those with the lowest rates of admission to ICU or death, which decreased with increasing Adpn/Lep tertile. Consistently, the prevalence of cardiometabolic risk factors (obesity, diabetes and hypertension), that are risk factors for mortality in COVID-19 and are associated with reduced Adpn/Lep [12], was lowest among patients with highest Adpn/Lep, and diabetes and hypertension were significant negative predictors of Adpn/Lep levels. We speculate that the role of increased Adpn/Lep ratio as a marker of a homeostatic protective mechanism limiting the damage associated with systemic inflammation is supported by the observation that patients with mild disease had the lowest Adpn/Lep ratio (less inflammation, minimal or no rise in Adpn/Lep), those with moderate COVID-19 had the highest Adpn/Lep (adequate response, better outcomes) and the group with severe COVID-19 had Adpn/Lep values similar to the mild disease group, possibly due to an inadequate anti-inflammatory response.
The role of adipokines in acute infection and critical illness is poorly understood. Previous studies have reported either low or high adiponectin levels in critically ill patients, and a biphasic pattern (low-high) has also been described [15]. Adiponectin being an anti-inflammatory adipokine, it has been hypothesised that an initial decrease in circulating adiponectin levels is permissive for an adequate inflammatory response, while a subsequent increase might reflect an anti-inflammatory response to suppress the over-activation of the immune system and to improve recovery [15]. Consistently, the only study that assessed adiponectin in COVID-19 patients found a positive correlation between adiponectin and IL-6, and a non-significant increase in adiponectin levels with increasing COVID-19 severity [8]. The latter is in agreement with our finding of numerically higher adiponectin levels in patients with moderate or severe disease. Both high and low leptin levels have been reported in critically ill patients [15]. Higher leptin levels have been found in COVID-19 patients admitted to the ICU as compared with non-COVID-19 ICU patients [10], and leptin was associated with greater monocyte activation, systemic inflammation, and disease progression in a small series of COVID-19 cases [11]. Different timing of assessment might explain the discrepancy with our finding. We measured adipokines shortly after hospital admission. It is possible that, in those with inadequate anti-inflammatory response at disease onset, leptin levels subsequently rose. Although neither adiponectin nor leptin correlated with systemic inflammation in our cohort, the Adpn/Lep ratio did, suggesting that the interplay between the two adipokines might predominate in the response to inflammatory triggers.
Our study has limitations: the sample size of each subgroup was relatively small, which might explain the lack of significant differences in some between-group comparisons. Further, adiponectin and leptin levels were measured only upon admission, and the descriptive nature of the study does not allow to draw conclusions on the causal relationship between Adpn/Lep ratio and clinical outcomes in COVID-19. In this light, our findings should be considered as hypothesis-generating, opening the route for further research on the role of adipokines in critical illness.
Conclusions
Reduced Adpn/Lep ratio is a marker of AT dysfunction, and strategies to increase this ratio have been proposed to reduce cardiometabolic risk. Our findings confirm that patients with cardiometabolic disturbances such as obesity, diabetes or hypertension have the lowest Adpn/Lep ratio, and suggest that this might contribute to worse outcomes in COVID-19. On the other hand, higher Adpn/Lep ratio might reflect a better cardiometabolic health, and possibly contribute to improved survival.
Author contributions
Conceptualization: CC, AG, AAM, PRQ; Data curation: LDF, RDL, CS, AC, NIL; Formal analysis: CC, LDF; Funding acquisition: CC, AAM, PRQ; Investigation: LDF, RDL,; Methodology: CC, AG, AAM, PRQ; Project administration: CC, AG, AAM, PRQ; Resources: AAM, PRQ; Supervision: CC, AAM, PRQ; Validation: CS, AC, NIL; Visualization: CC; Roles/Writing - original draft: LDF, CC; Writing - review editing: LDF, RDL, CS, AC, NIL, AG, AAM, PRQ, CC.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank the Institutional Biobank and the BioB Angels: Nicola Farina, Marco Battista, Domenico Grosso, Francesca Gorgoni, Carlo Di Biase, Alessio Grazioli Moretti, Lucio Granata, Filippo Bonaldi, Giulia Bettinelli, Elena Delmastro, Damiano Salvato, Chiara Maggioni, Giulia Magni, Monica Avino, Paolo Betti, Romina Bucci, Iulia Dumea, Simona Bossolasco, and Federica Morselli.
Funding sources
Part of this work was supported by a COVID-19 program project grant from the IRCCS San Raffaele Hospital and the grant COVID-2020-12371617 from the Italian Ministero della Salute and the EHA grant on COVID-19. CC is supported by the European Foundation for the Study of Diabetes Mentorship Programme 2019.
References
- 1.Aghili SMM, Ebrahimpur M, Arjmand B, Shadman Z, Pejman Sani M, Qorbani M, et al. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes (Lond) 2021;45:998–1016. doi: 10.1038/s41366-021-00776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battisti S, Pedone C, Napoli N, Russo E, Agnoletti V, Nigra SG, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43 doi: 10.2337/dc20-1333. e129-e30. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111 doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TMD. Adiponectin: role in physiology and pathophysiology. Int J Prev Med. 2020;11:136. doi: 10.4103/ijpvm.IJPVM_193_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterino M, Gelzo M, Sol S, Fedele R, Annunziata A, Calabrese C, et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci Rep. 2021;11:2941. doi: 10.1038/s41598-021-82426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Rourke RW, Lumeng CN. Pathways to severe COVID-19 for people with obesity. Obesity (Silver Spring) 2021;29:645–653. doi: 10.1002/oby.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Voort PHJ, Moser J, Zandstra DF, Muller Kobold AC, Knoester M, Calkhoven CF, et al. Leptin levels in SARS-CoV-2 infection related respiratory failure: a cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon. 2020;6:e04696. doi: 10.1016/j.heliyon.2020.e04696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Xu Y, Zhang X, Wang S, Peng Z, Guo J, et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J Leukoc Biol. 2021;110:9–20. doi: 10.1002/JLB.5HI1020-704R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruhbeck G, Catalan V, Rodriguez A, Gomez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovere-Querini P, Tresoldi C, Conte C, Ruggeri A, Ghezzi S, De Lorenzo R, et al. Biobanking for COVID-19 research. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.04168-3. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 15.Alipoor E, Mohammad Hosseinzadeh F, Hosseinzadeh-Attar MJ. Adipokines in critical illness: A review of the evidence and knowledge gaps. Biomed Pharmacother. 2018;108:1739–1750. doi: 10.1016/j.biopha.2018.09.165. [DOI] [PubMed] [Google Scholar]