Abstract
Peripheral neuropathy is one of the most common complications of both type 1 and type 2 diabetes. Up to half of patients with diabetes develop neuropathy during the course of their disease, which is accompanied by neuropathic pain in 30–40% of cases. Peripheral nerve injury in diabetes can manifest as progressive distal symmetric polyneuropathy, autonomic neuropathy, radiculo-plexopathies, and mononeuropathies. The most common diabetic neuropathy is distal symmetric polyneuropathy, which we will refer to as DN, with its characteristic glove and stocking like presentation of distal sensory or motor function loss. DN or its painful counterpart, painful DN, are associated with increased mortality and morbidity; thus, early recognition and preventive measures are essential. Nevertheless, it is not easy to diagnose DN or painful DN, particularly in patients with early and mild neuropathy, and there is currently no single established diagnostic gold standard. The most common diagnostic approach in research is a hierarchical system, which combines symptoms, signs, and a series of confirmatory tests. The general lack of long-term prospective studies has limited the evaluation of the sensitivity and specificity of new morphometric and neurophysiological techniques. Thus, the best paradigm for screening DN and painful DN both in research and in clinical practice remains uncertain. Herein, we review the diagnostic challenges from both clinical and research perspectives and their implications for managing patients with DN. There is no established DN treatment, apart from improved glycaemic control, which is more effective in type 1 than in type 2 diabetes, and only symptomatic management is available for painful DN. Currently, less than one-third of patients with painful DN derive sufficient pain relief with existing pharmacotherapies. A more precise and distinct sensory profile from patients with DN and painful DN may help identify responsive patients to one treatment versus another. Detailed sensory profiles will lead to tailored treatment for patient subgroups with painful DN by matching to novel or established DN pathomechanisms and also for improved clinical trials stratification. Large randomized clinical trials are needed to identify the interventions, i.e. pharmacological, physical, cognitive, educational, etc., which lead to the best therapeutic outcomes.
Keywords: diabetic neuropathy, painful diabetic neuropathy, diagnostic challenges, implication for management
Jensen et al. review the main diagnostic challenges associated with the most common neuropathy: the distal symmetric polyneuropathy that occurs in type 2 diabetes. They argue that the best approach is a hierarchical one based on symptoms, signs and additional tests.
Introduction
Peripheral neuropathies represent a heterogeneous group of neurological disorders that affect the peripheral nerves, causing sensory, motor, or autonomic symptoms or signs, or most frequently, a combination thereof. Neuropathies are present in 1–8% of the general population1–4 and vary in aetiologies, including metabolic, toxic, nutritional, inflammatory, and hereditary.5–9 The cause is unknown in up to 40% of patients with neuropathy. Unless treated, neuropathies are associated with an increase in morbidity, with pain, frequent falls, and, in more severe cases, a high risk for foot ulcers, Charcot arthropathy, and amputations, which increase mortality.10 It is therefore important to carefully screen for an underlying aetiology in all patients suspected of having neuropathy.
The most common causes of peripheral neuropathy are type 2 diabetes and prediabetes. Neuropathy occurs in approximately half of all patients with diabetes, of which 30–40% develop neuropathic pain, such that approximately one in five diabetic patients develop painful neuropathy.11 Diabetes gives rise to different types of nerve damage and clinical presentations, which includes distal symmetric polyneuropathy, autonomic neuropathy, radiculo-plexopathies, and mononeuropathies.12,13 By far the most common form of diabetic neuropathy is distal symmetric polyneuropathy, which we will refer to as DN, and encompasses small and large fibre neuropathy. DN is usually characterized by a sensory disturbance involving the feet, which ascends to the calves over time, and, in more advanced cases, also eventually involves the upper limbs. This review will focus on DN, while the other neuropathy subtypes, including autonomic neuropathy, will not be discussed in any depth.
DN accounts for 80–90% of diabetic neuropathies and is thus termed typical diabetic neuropathy, while other less common diabetic neuropathies are called atypical diabetic neuropathies.14,15 In contrast to other major diabetes complications, such as retinopathy and nephropathy, no single gold standard diagnostic test exists for DN. In some instances, damage occurs solely as a pure small fibre neuropathy (SFN), which also lacks a gold diagnostic standard. Further, improved glycaemic control is the only DN therapy, which is more effective in type 1 than type 2 diabetes. Therefore, definitive diagnosis can be challenging and therapy is often limited to symptomatic treatment of pain in patients with painful DN. With diabetes and prediabetes burden continuing to rise worldwide, it is anticipated that DN incidence and prevalence will also increase dramatically within the next decades. As a result, there is a critical need to address the major diagnostic challenges for this diabetic complication, by identifying the best strategies for diagnosing DN early in the disease course. Following DN progression will also generate useful prospective data for evaluating preventive and therapeutic DN interventions in future clinical studies.
Search strategy and selection criteria
References for this review were identified by searching PubMed for articles in English with no language restrictions for articles published mainly from 2010 to 2020. We used the search terms ‘Diabetes Mellitus’ [All Fields] OR ‘type 1 diabetes’ [All Fields] OR ‘type 2 diabetes’ [All Fields] OR ‘neuropathy’ [All Fields] OR ‘Diabetic peripheral neuropathy’ [All Fields]. Additional keywords were ‘diagnosis’ [All Fields] OR ‘diagnostic criteria’ [All Fields] OR ‘nerve conduction’ [All Fields] OR ‘small fibre neuropathy’ [All Fields] OR ‘skin biopsy’ [All Fields] or ‘quantitative sensory testing’ [All Fields] OR ‘cornea confocal microscopy’ [All Fields] OR ‘treatment’. Keywords were initially used as single search items and then combined using the Boolean operator ‘AND’. The final reference list is based on this search, supplemented with references from the authors’ own dataset.
Diagnostic challenges
The diagnostic criteria of DN and painful DN vary considerably in questionnaires,16 electrophysiological techniques,17 hierarchical classification schemes,18–20 and pathological and imaging diagnostic tools.21,22 Each of these criteria have their pros and cons. There is a need for identifying criteria and classifications that consider both general practice and research requirements. To date, no single gold standard diagnostic test exists for DN or painful DN. It is still unknown which clinical questionnaires and neurological assessment parameters best distinguish individuals with DN and painful DN from those without these conditions. DN diagnosis is complex and no specific biomarker is available for the condition. To paraphrase England and Asbury23 in a review over 15 years ago, diagnosing neuropathy depends on the examiner’s skill for tying together symptoms, signs, and diagnostic test results. Unfortunately, this is still the case today.
Definitions and hierarchical classifications of DN and painful DN
Numerous DN definitions have been suggested.8,15,17,24–26 The Toronto Consensus Panel on Diabetic Neuropathy15,17 distinguishes between typical and atypical DN, and defines typical DN as ‘a symmetrical length-dependent sensorimotor polyneuropathy attributable to chronic hyperglycaemia, associated metabolic derangements, cardiovascular risk covariates, and microvessel alteration’. The Toronto definition also requires excluding other neuropathy causes, but does not specify which conditions require evaluation. The American Diabetes Association (ADA)8 suggests that a simple definition for clinical practice is ‘the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes’. Although electrodiagnostic tests are conventional for assessing large nerve fibre dysfunction, the ADA definition does not require an abnormal electrodiagnostic test for clinical neuropathy diagnosis. This position is supported by studies indicating that electrodiagnostic tests rarely change the aetiology and/or management of patients meeting a clinical DN definition.27,28 For research purposes, however, it may be necessary to include additional tests to provide quantitative information on nerve injury and greater certainty that participants have DN.
Because of the lack of a gold standard test for diagnosing DN, hierarchical systems have been proposed over the years. In 2005, a report from three different American associations concluded that ‘the combination of symptoms, signs and electrodiagnostic findings provides the most accurate diagnosis of distal symmetric polyneuropathy’.29 The report further added that multiple symptoms and signs combined with electrodiagnostic examinations provide the highest degree of certainty for polyneuropathy. A limitation of this approach is that it does not specify which symptoms and signs should be used, nor does it specify clear DN categories.
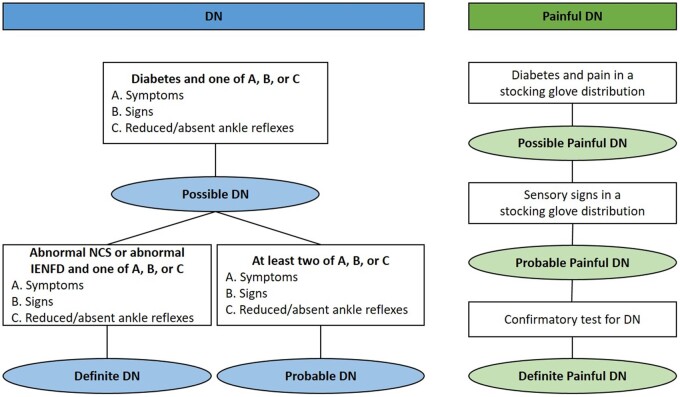
Similarly, the Toronto Consensus Panel proposed a hierarchical DN classification, but provided distinct DN categories graded as possible, probable, or definite neuropathy (Fig. 1). Fulfilling the Toronto Consensus Panel definition for a definite DN diagnosis requires symptoms or clinical signs of nerve dysfunction together with a positive confirmative test of either small or large nerve fibre dysfunction.15,17 Although DN usually causes injury to both small and large nerve fibres, a pure or isolated SFN also exists. SFN represents a separate entity characterized by specific damage to unmyelinated C and thinly myelinated Aδ type fibres.9,31–35 Like DN, no gold standard exists for diagnosing pure or isolated SFN. Therefore, a grading system has been suggested for SFN in diabetes, similar to the one proposed by the Toronto Consensus panel for DN.9
Figure 1.
Research definition of DN by the Toronto classification 15 and painful DN, modified from the NeuPSIG grading system of neuropathic pain. 30
Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory nervous system.36 Accordingly, painful DN can be defined as pain caused by damage to the peripheral somatosensory system attributable to diabetes and manifests as sensory abnormalities in the innervation territory of the damaged nerves. Like DN, no gold standard definition of painful DN exists; however, a hierarchical grading scheme has been proposed for neuropathic pain, which can be applied to painful DN.30,37 Specifically, painful DN can be rated as either possible (diabetes and pain distribution in stocking glove distribution), probable (sensory signs in a stocking glove distribution), or definite (confirmatory DN test) (Fig. 1). The grading system represents the level of certainty that the pain is neuropathic in nature, which is naturally based on a clinical judgement in which the skills and experience of the examiners are important elements.
Currently, hierarchical systems that combine symptoms, signs, and confirmatory tests are recommended for clinical research. However, issues remain, including clarification of the specific symptoms, signs, and confirmatory tests to use, as well as the incorporation bias that typically exists when comparing other neuropathy outcomes to these hierarchical systems. In clinical practice, confirmatory tests are often not needed, but challenges remain, for weighing specific symptoms and signs to draw a clinical diagnosis.
Sensory manifestations of DN
DN is characterized by multiple manifestations of which sensory symptoms and examination findings are the most common and earliest features. Diagnosing the sensory manifestations of DN relies on the history and presence of clinical signs (Fig. 2). DN manifests as bilateral, symmetric, and length-dependent nerve fibre damage, which affects the longest and most susceptible nerve fibres first and progresses distally-to-proximally in a stocking glove pattern.23 Thus, the history is generally straightforward, with symptoms of negative or positive sensations, starting in the toes and soles of the feet, and gradually ascending to involve the lower leg. When symptoms have reached the upper shins or knees, they often begin to appear in the fingertips and may subsequently spread further up the arms.10,11,38,39 Negative sensations include numbness or a lack of sensation to feeling; for SFN, it is specifically loss of pain and thermal sensation. Positive sensations are multiple in character, reflecting activity in nerve fibres of varying calibres and serving various sensory modalities.
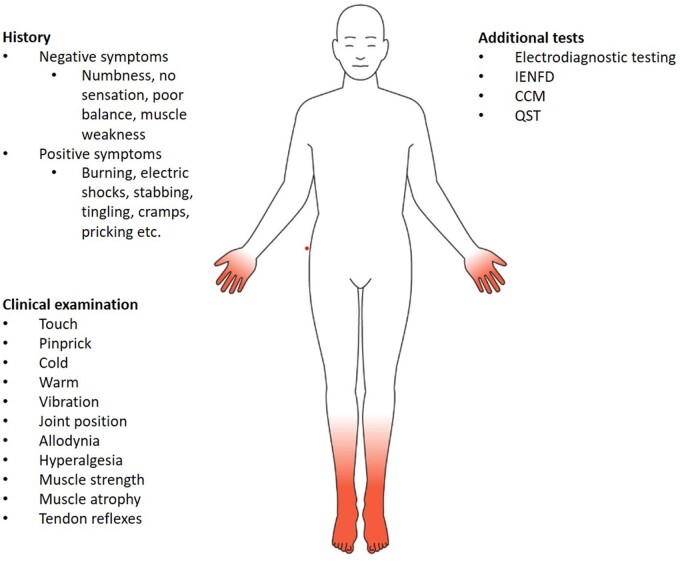
Figure 2.
Summary of symptoms, examination for signs, and additional tests to diagnose DN or painful DN. Clinical testing should fulfil typical DN pattern, i.e. symmetrical presentation with a distal-to-proximal gradient. Touch examination is performed with a 10 g monofilament, vibration with a 128 Hz tuning fork, pinprick with a sharp pin, and cold and warm with standardized cold and warm thermorollers.
Many patients with sensory DN are asymptomatic and abnormalities may only be revealed during the clinical examination. The sensory examination in DN usually reveals impaired nerve function with reduced or abolished sensation to touch, pinprick, temperature, vibration, and, more rarely, proprioception. Clinically evaluating small fibre function can involve determining the distal sensation to pinprick and a cold or warm thermal stimulus. The proximal limit of sensory abnormality is determined by moving the individual test paradigms proximally. Generally, sensory losses have the same characteristic stocking glove-like distribution as the sensory symptoms. Positive signs in DN, such as allodynia and hypersensitivity to different sensory stimuli, can be quantitated by measuring the intensity or area of these phenomena.40 However, in DN, these positive signs are rare compared to other types of peripheral nerve disorders with pain. Clinically assessing large fibre function can include examining vibration sensation to a 128 Hz tuning fork, light touch perception with a 10 g monofilament on the dorsal aspect of the great toe,41 and position sense of the same toe. Reduction or loss of ankle reflexes and other deep tendon reflexes is also a common DN feature. However, reduced ankle reflexes are common in older individuals without other neuropathy symptoms or signs.
DN sensory examinations are challenging, in part due to the large degree of variation in study populations and how, when, and where clinicians should test for different clinical signs.42 For example, the modified version of the Toronto Clinical Neuropathy Score20 excluded the tendon reflex score from the earlier version43 because of its low sensitivity for detecting early DN. In a study by Dyck and colleagues44 of 12 experienced physicians who assessed in a blinded manner 24 diabetes individuals with and without neuropathy for the presence of clinical neuropathy signs, there was ‘considerable and excessive variability among physician judgement of signs, symptoms, and diagnosis’. This study indicates that clinical findings have limitations, which may be surmounted in a setting where specific tests or scales are performed (see ‘Clinical scaling of DN’ section).
It is generally held that DN begins with small unmyelinated nerve fibre injury, followed by damage to small myelinated nerve fibres, and, as the disease progresses, injury to large myelinated nerve fibres.12,45 However, no prospective longitudinal studies are available to confirm or refute this idea. The recent Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen Detected Diabetes in Primary Care (ADDITION-Denmark) study showed that progression did not necessarily occur from small to large fibre symptoms based on the Michigan Neuropathy Screening Instrument questionnaire (MNSIq).46 Future studies are needed to longitudinally examine the symptoms and pathological changes in DN. Because of the uncertain course in the relative temporal dysfunction in and large fibre neuropathy, a single diagnostic DN criterion may not be expected to be valid throughout DN course. Currently, the ADA recommends that the annual clinical assessment should include a careful history, examination of vibration sensation using a 128-Hz tuning fork (large fibre function), and temperature or pinprick sensation (small fibre function) assessed at the base of the first toe to follow the progression of neuropathy. On the other hand, a 10-g monofilament sensory test in specified areas on the plantar surface of the feet has been proposed as a test to determine the risk for development of foot ulcers. However, since most diabetes patients are reviewed by general practitioners or at busy diabetic clinics, where neuropathy is only one among several possible complications, there is limited time for examining the patient. As a result, rapid sensitive and specific tests that do not require specialty training will ultimately be needed.
Motor manifestations of DN
Motor dysfunction in DN can be due to different causes, including neuropathy or myopathy. Motor neuropathy only appears clinically in a small proportion of patients, primarily in more advanced DN stages. Motor neuropathy in DN may lead to motor dysfunction, resulting in postural instability, impaired gait, frequent falls, severe injuries, and, ultimately, higher morbidity and mortality.47 Motor dysfunction negatively affects independence and capability to perform daily living activities, lowering quality of life. Motor neuropathy can be diagnosed by a clinical examination, including muscle strength and muscle atrophy evaluation combined with nerve conduction studies (NCS) to assess motor nerve function. Axonal degeneration in motor neuropathy also leads to in muscle fibre denervation. Collateral reinnervation compensates for this denervation48; however, as this compensatory mechanism fails, muscle atrophy and concomitant neurogenic muscle weakness develops.49–51 In a previous DN scale proposed by Dyck and colleagues,52 the inability to stand on heels was used to define more severe DN (stage 2B), as it was thought to reflect motor neuropathy. However, this test has not been validated in larger cohorts.
In diabetes, motor neuropathy is commonly based on NCS abnormalities, which even occur in the earlier disease stages.53 Milder forms of motor neuropathy are often undiagnosed. This may partly be explained by a lack of screening tests for motor neuropathy in standardized DN examinations. If a diabetes patient presents with substantial motor dysfunction early in the DN course, aetiologies other than classical DN should be considered, including focal diabetic neuropathies (radiculo-plexopathies) and immune-mediated neuropathies, such as chronic inflammatory demyelinating polyneuropathy,54 as well as a differential diagnosis for Charcot-Marie-Tooth disease (CMT) or other hereditary neuropathies.
In addition to a motor neuropathy, more recent studies indicate that diabetes may also give rise to a mild myopathy resulting in lower muscle strength due to lower muscle quality.55 Thus, in diabetes, muscle weakness may develop secondary to DN, but myopathic changes may also contribute to impaired muscle function. Alterations in muscle morphology lead to impaired muscle function, insulin sensitivity, glucose utilization, and decreased energy reserves. However, due to fat infiltration and fibrosis, the muscle may have a normal cross-sectional size. The contribution of diabetic motor neuropathy and diabetic myopathy on muscle dysfunction cannot be differentiated clinically. NCS combined with muscle biopsies and imaging (MRI and ultrasonography) may lead to a more detailed understanding of the relative importance of motor neuropathy versus myopathy in DN patients. Prospective studies of longitudinal MRI changes are needed to determine if such MRI findings are early markers of motor impairment.
In summary, DN motor dysfunction occurs much later than sensory dysfunction and is multifactorial through motor neuropathy and myopathy. The complexity of motor dysfunction in diabetes hinders identifying the underlying causes of motor weakness, poor ambulation, and falls, and hence management. Given its high morbidity, future work should focus on understanding motor disturbances as an important diabetes complication.
Additional diagnostic tests in DN
In addition to simple bedside tests, quantitative diagnostic tests have been developed to improve the likelihood of a clinical DN diagnosis and as potential outcomes in research studies. Examples include electrophysiological examinations, quantitative sensory testing (QST), skin biopsies, cornea confocal microscopy (CCM), and point-of-care devices (POCD).56 We briefly review the most frequently used confirmatory tests below.
Electrophysiology
Although NCS are not typically required for clinical DN evaluation, they are an important tool for quantitatively assessing large fibre involvement in DN for research studies and clinical care of patients with atypical neuropathy and/or unclear symptom localization. The sural nerve is most often examined in DN electrodiagnostic studies, usually through antidromic surface recordings, although its sensitivity is lower than needle electrode recordings.57 However, needle recordings are only used at a few centres, mainly because it is time consuming and unpleasant. In a recent study, examination of the distal segment of the sural and medial plantar nerves with surface electrodes was as sensitive as needle recordings in polyneuropathy of different aetiologies.53,58
Several studies have documented a higher sensitivity from NCS at sensory nerves more distal than sural nerve examination for DN diagnosis, but at the expense of specificity.59,60
Taking into account the length-dependent feature of DN, electrodiagnostic studies should include lower extremity nerves, i.e. sural, peroneal, and tibial nerves. Since DN is usually symmetric, bilateral NCS are usually not necessary for evaluating DN.53,61 The best NCS criteria for diagnosing DN are difficult to establish given the lack of a gold standard test with which to compare different NCS definitions. Experts have proposed using an abnormality in the sural nerve with at least one other NCS abnormality.17,53,62 Abnormality in either velocity or amplitude is often enough based on DN recommendations17 and in polyneuropathy of different aetiologies.53 Another approach recommended by Dyck and co-workers17 is to use composite scores from multiple nerves calculated by taking the sum of their Z-scores compared to the same set of nerve NCS from a control population. Further studies are necessary to determine the best NCS definition for DN. While NCS is unlikely to change the management of patients with DN, these tests are essential for patients with atypical neuropathy and/or difficulty localizing symptoms within the nervous system. NCS are also key research tests to quantitatively assess large nerve fibre function.
Quantitative sensory testing
QST is a psychophysical method for assessing somatosensory functions, which was originally presented as a simple perceptive response measure to thermal and vibratory stimuli.63–65 It has now developed into a detailed standardized tool where the response to multiple, well-defined painful and non-painful stimuli are measured. The QST protocol developed by the German Research Network on Neuropathic Pain66,67 assesses 13 parameters, including thermal and mechanical detection, pain thresholds, vibration thresholds, dynamic mechanical allodynia, wind-up ratio, and pressure pain threshold. These different tests measure the function of all afferent fibre classes, Aβ, Aδ, and C fibres. Raw QST data can be transformed into Z-scores normalized to age, sex, and body location, to generate a sensory profile, which includes sensory loss and hypersensitivity to thermal and mechanical stimuli.66,67 As such, QST can assess the functional status of sensory receptor systems, their afferent projections via large and small afferents, and how this somatosensory input is processed in the CNS.68–70
In patients with various types of neuropathic pain, regardless of underlying aetiology, it is possible to delineate distinct patient clusters based on their sensory profile. For example, Baron and colleagues71 found that three different patient clusters could be delineated by 13 different types of sensory stimulation paradigms. One cluster was dominated by sensory loss, one by thermal hyperalgesia, and one by mechanical hyperalgesia. These different clusters may represent distinct pathophysiological mechanisms that drive pain. For example, patients with diabetic and other neuropathies have more signs of sensory loss than hypersensitivity, suggesting that deafferentation rather than peripheral sensitization is the most likely mechanism in this profile. However, future studies are needed to confirm this hypothesis.
Most of the functions examined in the current QST protocols reflect sensory activity from skin, but not sensory functions from deep tissue innervation. It is possible that the pain generator in certain patients with painful DN may be related to abnormalities in deep tissue somatosensory innervation, but this requires further investigation.
In conclusion, QST represents a tool to identify separate specific sensory DN profiles if used in a standardized way, possibly reflecting separate underlying pathophysiological mechanisms. As such, QST may be used in clinical trials as an instrument to link mechanisms of pain with specific drug actions, as reviewed below. However, much work is needed to clarify the role of QST in research and clinical care.
Skin biopsy
Skin biopsy with intraepidermal nerve fibre density (IENFD) quantification is considered the pathological gold standard for diagnosing small fibre pathology in diabetes9 (Fig. 3). IENFD is reproducible with good diagnostic specificity and sensitivity, as well as favourable positive and negative predictive values.72 This test is not usually needed for clinically diagnosed DN, because it has not been shown to affect clinical management. However, skin biopsies are the best quantitative small fibre measure for research studies, and are more likely to improve upon reinnervation than NCS. A joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society published guidelines regarding the use of skin biopsy for demonstrating small fibre pathology.21 The recommendation is to take a 3-mm punch biopsy from the distal leg, 7–10 cm above the lateral malleolus, and compare to normative reference values based on distal leg in age- and sex-matched populations, both for bright field microscopy and for indirect immunofluorescence.73,74 An additional biopsy can be taken from the lateral distal thigh (7–10 cm above the knee) or at the lateral proximal thigh (7–10 cm below the greater trochanter) for evaluating small fibre pathology severity and distribution (length-dependent versus non-length-dependent).75,76 Since DN is typically length-dependent, the distal biopsy will have fewer fibres per millimetre than at the proximal biopsy.
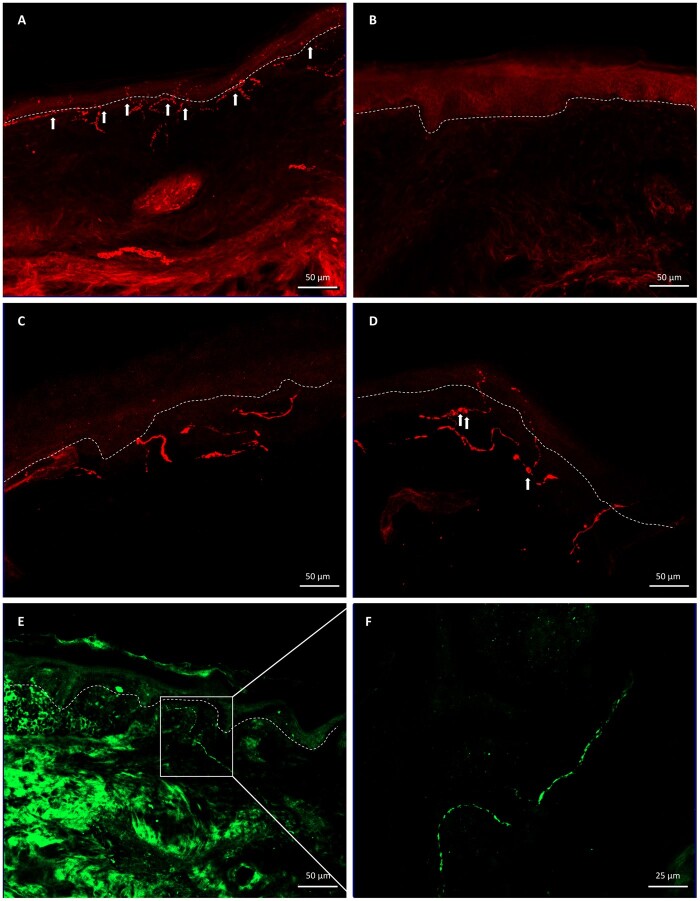
Figure 3 Different skin nerve fibre changes in diabetic neuropathy.
(A) PGP9.5+ intraepidermal nerve fibres (arrows) in a healthy individual. (B) DN patient with severe fibre loss. (C) High magnification of intraepidermal nerve fibres in a healthy individual (note absence of axonal swellings). (D) High magnification of intraepidermal nerve fibres in a DN patient (note presence of axonal swellings, arrows). (E) CGRP+ nerve fibres in a patient with painful DN. (F) High magnification of a CGRP+ nerve. CGRP = calcitonin gene related peptide; PGP9.5 = neuronal marker.
An important pathophysiological issue in diabetic neuropathies concerns the relationship between structural findings and function. It is still unclear whether reduced IENFD correlates with DN symptomatology, such as development or maintenance of neuropathic pain. A recent systematic review found that only 44% of studies reported associations between IENFD and symptoms, such as patient-reported pain.77 The same review also concluded that IENFD correlated better with objective tests, such as contact heat- or laser-evoked potentials, than with neuropathy instruments that quantify symptoms and signs (e.g. MNSI, Toronto Neuropathy Score). Importantly, the review also revealed that there may be better association between IENFD and QST measurements in DN than in non-diabetic neuropathies. Indeed, straightforward nerve fibre quantification only informs their density, not their physiological condition (e.g. whether they are hypersensitive, hyposensitive, or normal).
While most DN studies solely quantify IENFD from skin biopsies, it is also possible to measure other morphological changes in the skin, such as dermal nerve fibre length, nerve branching, and axonal swellings (Fig. 4).77,79–82 Axonal swellings, which possibly represent degenerating small nerve fibres,75 are elevated in DN,83,84 and may precede nerve fibre loss, as suggested by a recent study.85 Molecular changes can also be determined from diabetic skin biopsies. For instance, recent data indicate that patients with painful DN have higher densities of nerve fibres that are positive for substance P, calcitonin gene-related peptide,86 and growth associated protein 43,80 compared to patients without painful neuropathy (Fig. 4). Macrophages and ‘nociceptive’ Schwann cells are other biomarker examples that can be examined in skin biopsies. Specifically, recent studies suggests that DN patients have increased macrophage infiltration into the skin, which may be leveraged as novel treatment targets for neuropathic pain in diabetes.87,88
Figure 4.
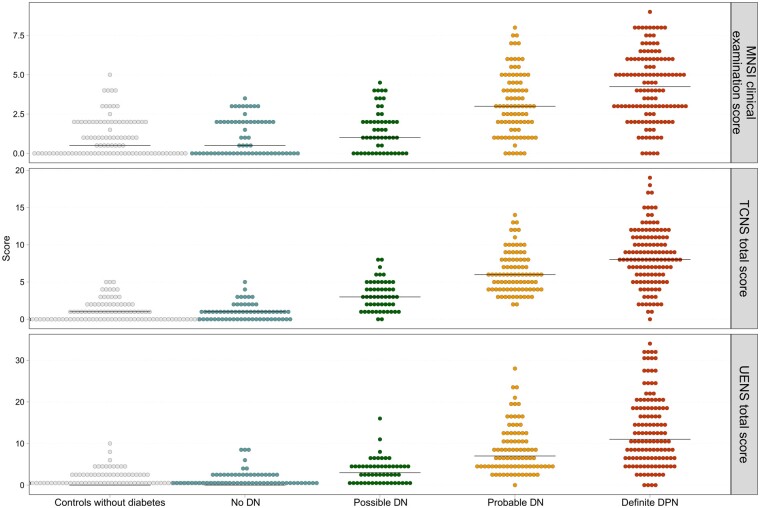
Correlation of median scores of the MNSI examination part, TCNS and UENS across DN groups, including controls without diabetes, and receiver operating characteristic (ROC) areas. MNSI: rs 0.61, P < 0.001, ROC area: 0.71; Toronto Clinical Neuropathy Score (TCNS): rs 0.79, P < 0.001, ROC area: 0.69; Utah Early Neuropathy Scale (UENS): rs 0.73, P < 0.001, ROC area: 0.66. Modified from Gylfadottir et al.78
In summary, skin biopsies represent an important tool for detecting small fibre abnormalities in diabetes through straightforward structural changes in reduced epidermal nerve fibre density and in quantifiable molecular changes. IENFD continues to be an important method for understanding the structure-function relationship and a useful instrument to prospectively track small fibre structural changes induced by different interventions. The main disadvantages of skin biopsy are invasiveness, relatively high cost, and requirement for highly specialized and trained staff. Moreover, the results are highly dependent on tissue handling and staining quality.
Cornea confocal microscopy
CCM is a non-invasive measure of corneal small fibre damage, which has emerged as an alternative to skin biopsies. CCM quantifies corneal nerve fibre density (CNFD), corneal nerve fibre length (CNFL), and corneal nerve branch density (CNBD), which some studies have shown are reduced in DN, especially CNFD and CNFL.22,89,90 However, a recent study reported that CCM could not distinguish type 2 diabetes patients with and without neuropathy.91 The largest study of diabetes patients to date (n = 998), using optimal CNFL thresholds and automated analysis, found CNFL had 73% sensitivity and 69% specificity for detecting type 1 diabetes DN, and 69% sensitivity and 63% specificity for detecting type 2 diabetes DN.92 Other studies also found good, but not great, CCM sensitivity and specificity for diagnosing DN.90,93
There are methodological challenges for objectively sampling and recording CCM images because the examiner is typically not blinded during image acquisition and the selection method for ‘good’ images for analysis varies between studies. Additionally, due to corneal curvature, its borders are out of focus when its centre is in focus, and the size of the focused area varies. A recent methodological study addressed these issues using stereology randomized sampling and adjusted area calculation, which was compared head-to-head to the usual method.94,95 The updated method, which avoids subjective CCM image selection bias, increased the absolute CCM values by 8–40% versus the usual method, and showed comparable differences between healthy individuals and DN patients. Furthermore, although CCM can be assessed manually and automatically, normative reference values are only available for the manual method, whose values are higher than by the automatic method.96 Thus, the best measurement method remains to be determined.
Another important challenge is the unclear relationship between CCM measures and IENFD from skin biopsy. The diagnostic characteristics of these two techniques seem comparable, but variation exists amongst studies and head-to-head comparisons are not available.90,93,95 CCM is promising as a non-invasive measure of small nerve fibre loss in diabetes, but more work remains to establish its role in research studies, including patient preference between CCM and skin biopsy. Importantly, the clinical roles of CCM and skin biopsies are also unclear, as neither are established tests for routinely assessing DN.97
Point-of-care devices
Most of the above additional tests are time consuming, some are invasive, and they all require specific expertise; therefore, they are not suitable for routine screening in general practice. More recently, a series of non-invasive, easy and rapid POCDs have been introduced to facilitate nerve function examination in patients suspected of having a neuropathy. These POCDs include examination of sural nerve conduction velocity,98–100 sweat production on the foot plantar,101 and other devices to measure sudomotor function in both hands and feet.102 A recent review demonstrated these POCDs have acceptable sensitivity10 and may be useful as future screening tools in patients to evaluate risk of deleterious outcomes, such as foot ulceration and amputation.10 However, there is a need for large, prospective studies to rigorously evaluate these POCDs versus conventional test procedures and/or clinical examination tools, to determine their value for detecting DN early.
Clinical scaling of DN
An important issue for clinically evaluating patients with suspected or established DN is early detection and subsequent follow-up of progression. A wide range of clinical scales exist to detect and track DN progression. These scales often combine symptom assessments with bedside evaluations of clinical DN signs, and they are mostly validated against NCS or IENFD. Recently, Gewandter and colleagues42 reviewed a series of clinical scales for distal symmetric axonal polyneuropathies, which revealed a large variation in motor and sensory examination items used in the different test measures, as well as great variability in the proportion of tests devoted to assess reflexes and motor, sensory, and autonomic functions. A particular problem in assessing the usefulness of a specific test concerns its validity (Box 1). Table 1 presents an overview of the most frequently used DN scales, along with a description of the potential overlap in measures between the diagnostic and reference test. Most of the tests include clinical signs and fewer symptoms or confirmatory tests, possibly because clinical signs are easier to quantify and follow than symptoms.20 Some of the clinical scales contain disproportionate tests of motor function, despite the late clinical appearance in the course of DN.
Box 1.
Validity
The validity of any test involves three aspects: construct, content, and criterion validity.
Construct validity is a test’s ability to measure the concept it is intended to measure and is key for determining general method validity. The construct for neuropathy cannot be directly observed, but it can be assessed by combining other indicators associated with it, such as a series of symptoms and signs. Construct validity for a neuropathy questionnaire requires it to only include questions that measure neuropathy indicators, but exclude questions not relevant to neuropathy. Construct validity can then be measured by determining the score difference between DN patients and individuals with diabetes alone.
Content validity is similar to face validity and examines whether a test can measure all aspects of the concept, in this case neuropathy. For example, for a DN questionnaire, it may be important for the instrument to include symptoms of autonomic or motor functions rather than only focusing on sensory symptoms.
Criterion validity determines how well the test results correspond to results from a different and independent test. For DN, the test score should correlate to the results of quantitative sensory nerve fibre functions, such as NCS, vibration threshold, or thermal sensitivity measures. Without any objective gold standard for either neuropathy or pain, there is always a bias risk and a possibility for violating the criterion validity. Ideally, the new diagnostic test or questionnaire should be completely independent from the reference standard. As discussed in detail in the main text, for both neuropathy and painful neuropathy, the reference test is usually based on the combination of history, neurological examination, and other specific measures.
In validity testing of various neuropathy tests, such as the Neuropathy Symptom Score,122 Michigan Neuropathy Screening Instrument (MNSI),17,123 Neuropathic Disability Score,25 Toronto Clinical Neuropathy Score,20 Diabetic Neuropathy Score,124 and Utah Early Neuropathy Scale,105 questions or items in these tests are also among the criteria in the reference test, which is called incorporation bias. A similar incorporation bias problem exists when measuring neuropathic pain, for example, in the Leeds Assessment of Neuropathic Symptoms and Signs,125 the German Pain Detect,126 and the French Douleur Neuropathique 4 (DN4).127 In all these cases, the reference standard is partly used to determine the outcome of the new diagnostic test. Incorporation bias128
is likely to lead to an overestimation of the diagnostic accuracy of the test.
Table 1.
Symptoms and signs from different diagnostic tests, which are also inherent in the Toronto classification15,17
| Toronto | Reflexes | Other clinical signs |
Symptoms |
Confirmatory tests |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tools | Reflexes | Muscle Strength | Vibration | Touch pressure | Joint position | Pin- Prick | Allodynia |
Cold/
warm |
Pain | Paraesthesias | Numbness |
Cold
Warm |
Weakness | NCS | IENFD |
| DNE | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| DNS | ✓ | ✓ | ✓ | ||||||||||||
| MDNSa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| MNSIqa | ✓ | ✓ | ✓ | ✓ | |||||||||||
| MNSIex | ✓ | ✓ | |||||||||||||
| TCNS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| UENS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| NIS-LL | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| NIS/NDS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| NIS-LL +7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
A tick indicates that the item is included in the test.
DNE = Diabetic Neuropathy Examination; DNS = Diabetic Neuropathy Symptom score (plus unsteadiness walking); TCNS = Toronto Clinical Neuropathy Score; UENS = Utah Early Neuropathy Score; NIS-LL = Neuropathy Impairment score for Lower Limbs; NIS/NDS = Neuropathy Impairment Score/Neuropathy disability Score; NIS-LL +7 = Neuropathy Impairment score for Lower Limbs + 7 tests; MNSI examination (MNSIex) = foot inspection; MNSIq = MNSI questionnaire(): more questions. aMNSI (questionnaire and clinical examination) and Michigan Diabetic Neuropathy Score (MDNS) are designed as a two-step screening and staging process, MDNS confirms the MNSI diagnosis (screening instrument) with a positive assessment of a quantitative neurological examination and nerve conduction studies.
The clinical scales also highlight the large variation in symptoms and clinical signs that qualify for the different classification grades using the Toronto classification. This is demonstrated in Fig. 4, where three different clinical scales, MNSI, Toronto Clinical Neuropathy Score, Utah Early Neuropathy Scale, are plotted against the Toronto Consensus definition of DN in a population of recently diagnosed type 2 diabetes patients.78 There is a positive correlation between increasing certainty of neuropathy from possible to definite with increasing scores on the neuropathy scales, but also a large variation. This Toronto hierarchical system has good criterion validity, but there is considerable variation and overlap between the DN groups on all three of these clinical scales. Currently, challenges exist in the diagnosis of DN, whether painful or non-painful, with a need to develop an easy and more uniform method to diagnose DN both for clinical and research purposes.
DN severity
Assessing DN severity presents its own challenges. Dyck and colleagues17 proposed a method to determine DN severity by successively adding signs and then symptoms to different electrophysiological abnormalities, generating five degrees of severity. An alternative approach is to determine severity based on different sum scores or composite scores, as presented in questionnaires or different assessment scales.16,18–20,103–105 Since few long-term prospective studies have been performed, the usefulness of these composite scores to grade severity is limited. However, using this principle of composite scoring, Dyck and colleagues104 showed worsening of DN status using the Neuropathy Impairment Score of the Lower Limbs [NIS (LL)] +7 tests in a longitudinally monitored patient cohort. Whether these changes associated with clinically relevant outcomes, such as falls, ulcerations, and quality of life, remained unclear. More studies are needed to investigate the best way to evaluate DN severity, including direct comparisons between approaches and associations of changes in severity with clinically relevant outcomes. Such studies could potentially lead to consensus definitions of DN severity, which could be used consistently across studies, facilitating better comparisons.
Phenotyping DN and painful DN and implication for treatment
Phenotyping patients with DN versus painful DN may be important for identifying new or better treatments for painful DN. Three studies used the German research network protocol to investigate differences in sensory profiles between painful and non-painful DN.106,107 In the UK Pain in Neuropathy Study (PiNS),106 patients with painful DN had more sensory loss, particularly small fibre function, and greater spread of proximal clinical signs compared to those with painless DN. Dynamic brush evoked allodynia was only observed in patients with painful DN. Similar findings were seen in two other studies,107 one of which also demonstrated a predominant sensory loss to thermal stimuli that correlated to the severity of neuropathic pain and neuropathy. Consistently, painful DN exhibits more extensive sensory function loss, supporting the notion that loss of afferent input, mainly related to small fibre function, is important for the development of neuropathic pain.70
Phenotypic profiling may be important for identifying the optimal treatment for patients with painful DN. Current pharmacological therapies for painful DN are insufficient, mainly due to a lack of approved therapies targeting the underlying pain mechanisms.8,39,108–110 As shown in Fig. 5, most of the current symptomatic pharmacological treatments are of poor efficacy with numbers need to treat (NNT) of ∼7 for the most frequently used therapies. This means that less than one in seven patients with painful DN obtain a pain sufficient relief. It is worth noting that the studies from which the different symptomatic guidelines were generated110,112–115 were almost entirely based on patients with painful DN, but without specific sensory profiling. The lack of highly efficacious therapies for painful DN raises the question of whether additional tests such as QST, nerve fibre assessment from skin biopsies, genetic analysis, or other biomarkers116 may aid in further phenotyping patients to improve clinical trial design and outcome measures.
Figure 5.
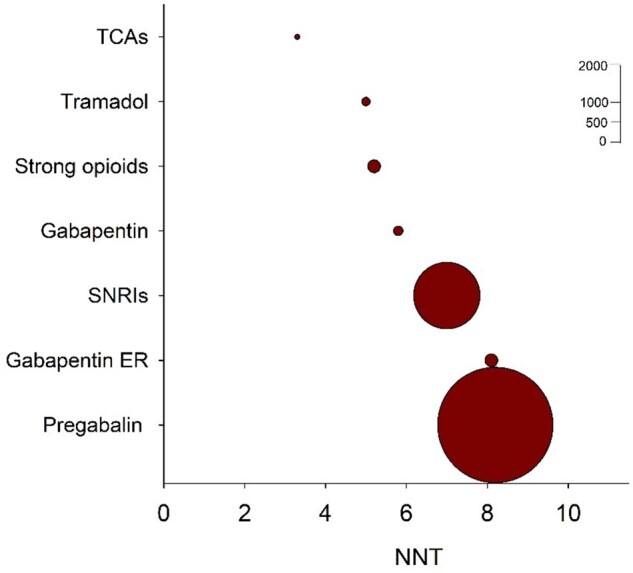
Combined numbers needed to treat values for drug classes recommended for painful DN. The circle sizes indicate the relative number of patients, who received active treatment drugs in studies for which dichotomous data were available. Gabapentin ER = gabapentin extended release or enacarbil; NNT = needed to treat; SNRIs = serotonin noradrenaline reuptake inhibitors; TCAs = tricyclic antidepressants. Updated from Finnerup et al.110,111
Recent studies suggest that more precise phenotyping of painful neuropathies, including DN, may identify patient subgroups likely to respond to an existing compound, which otherwise may be ineffective in unselected patients. One randomized controlled trial in peripheral neuropathic pain, including painful DN, stratified patients a priori to test the concept of a mechanism-based treatment.117 In that study, patients with the so-called ‘irritable nociceptor phenotype’ based on QST responded better to the sodium-channel blocker oxcarbazepine than those without this phenotype.117 A recent study Han and colleagues118 described how carbamazepine, a structural oxcarbazepine analogue, may correct hyperexcitability caused by a novel Nav1.8 mutation in a patient with diabetes and neuropathic pain. This novel mode of action of carbamazepine is similar to that seen in another sodium channel subtype, Nav1.7.119 These findings may be of value in identifying patients that are more likely to respond to carbamazepine or related compounds, but future studies are needed to clarify this possibility.
The anticonvulsant lacosamide, a Nav1.7 and Nav1.8 sodium channel blocker, has been tested in small trials of patients with painful DN, but did not significantly reduce pain compared to placebo.120 However, in a recent double-blind placebo controlled cross-over study in patients with Nav1.7-related SFN, lacosamide significantly reduced pain versus placebo.121 These findings indicate that pharmacological efficacy may be boosted in specific patient subgroups.
Taken together, these studies suggest it may be possible to apply precision medicine to treating neuropathic pain by utilizing detailed phenotypic profiling of DN patients. However, additional studies are needed to clarify the role of detailed phenotyping of diabetic patients, including consideration of specific mechanisms of pharmacological agents.
Conclusion and suggestions for the future
The diagnosis of DN and painful DN has changed over the last few decades, shifting from a descriptive delineation to a more detailed distinction, based on specific pathophysiological mechanisms. However, despite the introduction of new diagnostic tests, novel potential biomarkers, and a series of rather small intervention studies utilizing detailed phenotypic profiling, the management of DN and painful DN has remained largely unchanged. For DN, glycaemic control remains the sole intervention. For painful DN, no specific disease-modifying intervention exists and symptomatic management is still the treatment mainstay. To advance the current state of the field, large, prospective, cohort studies are needed to determine the value of the different diagnostic tests, e.g. NCS, IENFD, for diagnosing DN and painful DN and tracking progression. Furthermore, pharmacological intervention studies in a research setting using the principles of precision medicine may facilitate discovery of new disease-modifying therapies for DN and painful DN for ultimate clinical application. Given the high prevalence and morbidity associated with DN and painful DN, advances in the diagnosis and treatment of these patients will have a great impact on the health of a large number of patients worldwide.
Funding
All authors have been funded by a grant from the Novo Nordisk Foundation (grant no: 14OC0011633). T.S.J. and P.K. are supported by a grant from the Novo Nordisk Foundation (grant no: 18OC0052301). T.S.J., N.B.F., A.C.T., and D.L.B. are members of the DOLORisk Consortium funded by the European Commission Horizon 2020 (ID633491). A.J.T. is supported by the Independent Research Fund Denmark (grant no: 0134-00223B). E.L.F. is supported by National Institutes of Health (NIH) grants R24 DK082841 and R01 DK107956. B.C.C. is supported by NIH R01 DK115687.
Competing interests
D.L.B. has acted as a consultant for Amgen, Bristows, CODA therapeutic, LatigoBio, Lilly, Mundipharma, Orion, Regeneron, and Theranexus on behalf of Oxford University Innovation. D.L.B. has an MRC Industrial Partnership grant with Astra Zeneca funded by the BBSRC. N.B.F. has received consultation fees from Almirall, Merck, Vertex Pharmaceuticals, Mitshubishi Tanabe Pharma, Novartis Pharma, and NeuroPN, and research funding from IMI2 PainCare project of EU a public-private consortium and the companies involved are: Grunenthal, Bayer, Eli Lilly, Esteve, and Teva. B.C.C. consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology.
Glossary
- CCM
cornea confocal microscopy
- DN
distal symmetric polyneuropathy
- IENFD
intraepidermal nerve fibre density; MNSI = Michigan Neuropathy Screening Instrument
- NCS
nerve conduction studies
- QST
quantitative sensory testing; SFN = small fibre neuropathy
References
- 1. Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D.; ILSA Working Group. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68(18):1460–1467. [DOI] [PubMed] [Google Scholar]
- 2. Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA.. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology. 2016;87(18):1892–1898. [DOI] [PubMed] [Google Scholar]
- 3. Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA.. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31(1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martyn CN, Hughes RA.. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62(4):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callaghan BC, Price RS, Feldman EL.. Distal symmetric polyneuropathy: A review. JAMA. 2015;314(20):2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ.. Chronic painful peripheral neuropathy in an urban community: A controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–982. [DOI] [PubMed] [Google Scholar]
- 7. Davies M, Brophy S, Williams R, Taylor A.. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–1522. [DOI] [PubMed] [Google Scholar]
- 8. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS.. The diagnostic challenge of small fibre neuropathy: Clinical presentations, evaluations, and causes. Lancet Neurol. 2017;16(11):934–944. [DOI] [PubMed] [Google Scholar]
- 10. Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–948. [DOI] [PubMed] [Google Scholar]
- 11. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL.. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feldman EL, Nave KA, Jensen TS, Bennett DLH.. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain. Neuron. 2017;93(6):1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonçalves NP, Vægter CB, Andersen H, Østergaard L, Calcutt NA, Jensen TS.. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017;13(3):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith AG, Singleton JR.. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18(1):60–84. [DOI] [PubMed] [Google Scholar]
- 15. Tesfaye S, Boulton AJM, Dyck PJ, et al. ; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA.. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. [DOI] [PubMed] [Google Scholar]
- 17. Dyck PJ, Albers JW, Andersen H, et al. ; on behalf of The Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: Update on research definition, diagnostic criteria and estimation of severity. Diabetes Care. 2011;27:620–628. [DOI] [PubMed] [Google Scholar]
- 18. Meijer JW, van Sonderen E, Blaauwwiekel EE, et al. Diabetic neuropathy examination: A hierarchical scoring system to diagnose distal polyneuropathy in diabetes. Diabetes Care. 2000;23(6):750–753. [DOI] [PubMed] [Google Scholar]
- 19. Bril V, Perkins BA.. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care. 2002;25(11):2048–2052. [DOI] [PubMed] [Google Scholar]
- 20. Bril V, Tomioka S, Buchanan RA, Perkins BA; mTCNS Study Group. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med. 2009;26(3):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lauria G, Hsieh ST, Johansson O, et al. ; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903–12.e44-9. [DOI] [PubMed] [Google Scholar]
- 22. Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36(11):3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. England JD, Asbury AK.. Peripheral neuropathy. Lancet. 2004;363(9427):2151–2161. [DOI] [PubMed] [Google Scholar]
- 24. Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes. 1997;46 (Suppl 2):S54–7. [DOI] [PubMed] [Google Scholar]
- 25. Boulton AJ, Malik RA, Arezzo JC, Sosenko JM.. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. [DOI] [PubMed] [Google Scholar]
- 26. Boulton AJ, Vinik AI, Arezzo JC, et al. ; American Diabetes Association. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. [DOI] [PubMed] [Google Scholar]
- 27. Callaghan BC, Kerber KA, Lisabeth LL, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol. 2014;71(9):1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenberg NR, Portegies P, de Visser M, Vermeulen M.. Diagnostic investigation of patients with chronic polyneuropathy: Evaluation of a clinical guideline. J Neurol Neurosurg Psychiatry. 2001;71(2):205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. England JD, Gronseth GS, Franklin G, et al. ; American Academy of Physical Medicine and Rehabilitation. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199–207. [DOI] [PubMed] [Google Scholar]
- 30. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sopacua M, Hoeijmakers JGJ, Merkies ISJ, Lauria G, Waxman SG, Faber CG.. Small-fiber neuropathy: Expanding the clinical pain universe. J Peripher Nerv Syst. 2019;24(1):19–33. [DOI] [PubMed] [Google Scholar]
- 32. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142(12):3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Truini A, Spallone V, Morganti R, et al. ; Neuropathic Pain Special Interest Group of the Italian Society of Neurology. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain. 2018;159(12):2658–2666. [DOI] [PubMed] [Google Scholar]
- 35. Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG.. Small-fibre neuropathies–advances in diagnosis, pathophysiology and management. Nat Rev Neurol. 2012;8(7):369–379. [DOI] [PubMed] [Google Scholar]
- 36. Jensen TS, Baron R, Haanpaa M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. [DOI] [PubMed] [Google Scholar]
- 37. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain - Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. [DOI] [PubMed] [Google Scholar]
- 38. Gylfadottir SS, Weeracharoenkul D, Andersen ST, Niruthisard S, Suwanwalaikorn S, Jensen TS.. Painful and non-painful diabetic polyneuropathy: Clinical characteristics and diagnostic issues. J Diabetes Investig. 2019;10:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 40. Jensen TS, Finnerup NB.. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. [DOI] [PubMed] [Google Scholar]
- 41. Boulton AJ, Armstrong DG, Albert SF, et al. ; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gewandter JS, Gibbons CH, Campagnolo M, et al. Clinician-rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology. 2019;93(8):346–360. [DOI] [PubMed] [Google Scholar]
- 43. Perkins BA, Olaleye D, Zinman B, Bril V.. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24(2):250–256. [DOI] [PubMed] [Google Scholar]
- 44. Dyck PJ, Overland CJ, Low PA, et al. ; Cl vs. NPhys Trial Investigators. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M.. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108–111. [DOI] [PubMed] [Google Scholar]
- 46. Määttä LL, Charles M, Witte DR, et al. Prospective study of neuropathic symptoms preceding clinically diagnosed diabetic polyneuropathy: ADDITION-Denmark. Diabetes Care. 2019;42(12):2282–2289. [DOI] [PubMed] [Google Scholar]
- 47. Pop-Busui R, Braffett BH, Zinman B, et al. ; DCCT/EDIC Research Group. Cardiovascular autonomic neuropathy and cardiovascular outcomes in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Care. 2017;40(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andersen H, Stålberg E, Gjerstad MD, Jakobsen J.. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve. 1998;21(12):1647–1654. [DOI] [PubMed] [Google Scholar]
- 49. Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H.. Accelerated atrophy of lower leg and foot muscles–a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia. 2009;52(6):1182–1191. [DOI] [PubMed] [Google Scholar]
- 50. Andreassen CS, Jakobsen J, Andersen H.. Muscle weakness: A progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes. 2006;55(3):806–812. [DOI] [PubMed] [Google Scholar]
- 51. Andersen H, Gjerstad MD, Jakobsen J.. Atrophy of foot muscles: A measure of diabetic neuropathy. Diabetes Care. 2004;27(10):2382–2385. [DOI] [PubMed] [Google Scholar]
- 52. Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. [DOI] [PubMed] [Google Scholar]
- 53. Tankisi H, Pugdahl K, Beniczky S, Andersen H, Fuglsang-Frederiksen A.. Evidence-based recommendations for examination and diagnostic strategies of polyneuropathy electrodiagnosis. Clin Neurophysiol Pract. 2019;4:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sasaki H, Kawamura N, Dyck PJ, Dyck PJB, Kihara M, Low PA.. Spectrum of diabetic neuropathies. Diabetol Int. 2020;11(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Monaco CMF, Perry CGR, Hawke TJ.. Diabetic myopathy: Current molecular understanding of this novel neuromuscular disorder. Curr Opin Neurol. 2017;30(5):545–552. [DOI] [PubMed] [Google Scholar]
- 56. Vas PR, Sharma S, Rayman G.. Distal sensorimotor neuropathy: Improvements in diagnosis. Rev Diabet Stud. 2015;12(1-2):29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kural MA, Pugdahl K, Fuglsang-Frederiksen A, Andersen H, Tankisi H.. Near-nerve needle technique versus surface electrode recordings in electrodiagnosis of diabetic polyneuropathy. J Clin Neurophysiol. 2016;33(4):346–349. [DOI] [PubMed] [Google Scholar]
- 58. Kural MA, Karlsson P, Pugdahl K, Isak B, Fuglsang-Frederiksen A, Tankisi H.. Diagnostic utility of distal nerve conduction studies and sural near-nerve needle recording in polyneuropathy. Clin Neurophysiol. 2017;128(9):1590–1595. [DOI] [PubMed] [Google Scholar]
- 59. Park KS, Lee SH, Lee KW, Oh SJ.. Interdigital nerve conduction study of the foot for an early detection of diabetic sensory polyneuropathy. Clin Neurophysiol. 2003;114(5):894–897. [DOI] [PubMed] [Google Scholar]
- 60. Uluc K, Isak B, Borucu D, et al. Medial plantar and dorsal sural nerve conduction studies increase the sensitivity in the detection of neuropathy in diabetic patients. Clin Neurophysiol. 2008;119(4):880–885. [DOI] [PubMed] [Google Scholar]
- 61. Dupuis JE, Li J, Callaghan BC, Reynolds EL, London ZN.. Bilateral nerve conduction studies in the evaluation of distal symmetric polyneuropathy. Muscle Nerve. 2019;60(3):305–307. [DOI] [PubMed] [Google Scholar]
- 62. Tankisi H, Pugdahl K, Fuglsang-Frederiksen A.. Electrodiagnostic testing of large fiber polyneuropathies: A review of existing guidelines. J Clin Neurophysiol. 2020;37(4):277–287. [DOI] [PubMed] [Google Scholar]
- 63. Fruhstorfer H, Lindblom U, Schmidt WC.. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39(11):1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL.. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43(8):1508–1512. [DOI] [PubMed] [Google Scholar]
- 65. Jensen TS, Bach FW, Kastrup J, Dejgaard A, Brennum J.. Vibratory and thermal thresholds in diabetics with and without clinical neuropathy. Acta Neurol Scand. 1991;84(4):326–333. [DOI] [PubMed] [Google Scholar]
- 66. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. [DOI] [PubMed] [Google Scholar]
- 67. Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C.. Reference data for quantitative sensory testing (QST): Refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. [DOI] [PubMed] [Google Scholar]
- 68. Jensen TS, Baron R.. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- 69. Baron R, Förster M, Binder A.. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: A first step to a stratified treatment approach. Lancet Neurol. 2012;11(11):999–1005. [DOI] [PubMed] [Google Scholar]
- 70. Finnerup NB, Kuner R, Jensen TS.. Neuropathic pain: From mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. [DOI] [PubMed] [Google Scholar]
- 71. Baron R, Maier C, Attal N, et al. ; German Neuropathic Pain Research Network (DFNS), and the EUROPAIN, and NEUROPAIN consortia. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gwathmey KG, Pearson KT.. Diagnosis and management of sensory polyneuropathy. BMJ. 2019;365:l1108. [DOI] [PubMed] [Google Scholar]
- 73. Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202–207. [DOI] [PubMed] [Google Scholar]
- 74. Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016;23(2):333–338. [DOI] [PubMed] [Google Scholar]
- 75. Zhou L. Small fiber neuropathy. Semin Neurol. 2019;39(5):570–577. [DOI] [PubMed] [Google Scholar]
- 76. Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy. Eur J Neurol. 2018;25(6):848–853. [DOI] [PubMed] [Google Scholar]
- 77. Karlsson P, Hincker AM, Jensen TS, Freeman R, Haroutounian S.. Structural, functional, and symptom relations in painful distal symmetric polyneuropathies: A systematic review. Pain. 2019;160(2):286–297. [DOI] [PubMed] [Google Scholar]
- 78. Gylfadottir SS, Itani M, Krøigård T, et al. Diagnosis and prevalence of diabetic polyneuropathy: A cross-sectional study of Danish patients with type 2 diabetes. Eur J Neurol. 2020;27:2575–2585. [DOI] [PubMed] [Google Scholar]
- 79. Karlsson P, Haroutounian S, Polydefkis M, Nyengaard JR, Jensen TS.. Structural and functional characterization of nerve fibres in polyneuropathy and healthy subjects. Scand J Pain. 2016;10:28–35. [DOI] [PubMed] [Google Scholar]
- 80. Galosi E, La Cesa S, Di Stefano G, et al. A pain in the skin. Regenerating nerve sprouts are distinctly associated with ongoing burning pain in patients with diabetes. Eur J Pain. 2018;22(10):1727–1734. [DOI] [PubMed] [Google Scholar]
- 81. Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–2154. [DOI] [PubMed] [Google Scholar]
- 82. Sloan G, Shillo P, Selvarajah D, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–191. [DOI] [PubMed] [Google Scholar]
- 83. Cheng HT, Dauch JR, Porzio MT, et al. Increased axonal regeneration and swellings in intraepidermal nerve fibers characterize painful phenotypes of diabetic neuropathy. J Pain. 2013;14(9):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cheung A, Podgorny P, Martinez JA, Chan C, Toth C.. Epidermal axonal swellings in painful and painless diabetic peripheral neuropathy. Muscle Nerve. 2015;51(4):505–513. [DOI] [PubMed] [Google Scholar]
- 85. Karlsson P, Gylfadottir S S, Kristensen A G, et al. Axonal swellings are related to type 2 diabetes, but not to distal diabetic sensorimotor polyneuropathy. Diabetologia. 2021;64(4):923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karlsson P, Provitera V, Caporaso G, et al. Increased peptidergic fibers as a potential cutaneous marker of pain in diabetic small fiber neuropathy. Pain. 2021;162:778–786. [DOI] [PubMed] [Google Scholar]
- 87. Shepherd AJ, Copits BA, Mickle AD, et al. Angiotensin II triggers peripheral macrophage-to-sensory neuron redox crosstalk to elicit pain. J Neurosci. 2018;38(32):7032–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abdo H, Calvo-Enrique L, Lopez JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365(6454):695–699. [DOI] [PubMed] [Google Scholar]
- 89. Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: A non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–688. [DOI] [PubMed] [Google Scholar]
- 90. Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One. 2017;12(7):e0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Andersen ST, Grosen K, Tankisi H, et al. Corneal confocal microscopy as a tool for detecting diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes: ADDITION-Denmark. J Diabetes Complications. 2018;32(12):1153–1159. [DOI] [PubMed] [Google Scholar]
- 92. Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: A pooled multinational consortium study. Diabetologia. 2018;61(8):1856–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: Comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schaldemose EL, Fontain FI, Karlsson P, Nyengaard JR.. Improved sampling and analysis of images in corneal confocal microscopy. J Microsc. 2017;268(1):3–12. [DOI] [PubMed] [Google Scholar]
- 95. Schaldemose EL, Hammer RE, Ferdousi M, Malik RA, Nyengaard JR, Karlsson P.. An unbiased stereological method for corneal confocal microscopy in patients with diabetic polyneuropathy. Sci Rep. 2020;10(1):12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tavakoli M, Ferdousi M, Petropoulos IN, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: A multinational normative data set. Diabetes Care. 2015;38(5):838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Callaghan BC, Gallagher G, Fridman V, Feldman EL.. Diabetic neuropathy: What does the future hold? Diabetologia. 2020;63(5):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sharma S, Vas PR, Rayman G.. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol. 2015;9(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA.. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PLoS One. 2014;9(1):e86515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kural MA, Andersen ST, Andersen NT, et al. The utility of a point-of-care sural nerve conduction device for detection of diabetic polyneuropathy: A cross-sectional study. Muscle Nerve. 2019;59(2):187–193. [DOI] [PubMed] [Google Scholar]
- 101. Ziegler D, Papanas N, Rathmann W, Heier M, Scheer M, Meisinger C.; KORA Study Group. Evaluation of the Neuropad sudomotor function test as a screening tool for polyneuropathy in the elderly population with diabetes and pre-diabetes: The KORA F4 survey. Diabetes Metab Res Rev. 2012;28(8):692–697. [DOI] [PubMed] [Google Scholar]
- 102. Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S.. One-stop microvascular screening service: An effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabet Med. 2018;35(7):887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: Validation and reliability study. Neurology. 1999;53(8):1660–1664. [DOI] [PubMed] [Google Scholar]
- 104. Dyck PJ, Davies JL, Litchy WJ, O'Brien PC.. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997;49(1):229–239. [DOI] [PubMed] [Google Scholar]
- 105. Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: A sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13(3):218–227. [DOI] [PubMed] [Google Scholar]
- 106. Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Raputova J, Srotova I, Vlckova E, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: A cross-sectional observational study. Pain. 2017;158(12):2340–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nawroth PP, Bendszus M, Pham M, et al. The quest for more research on painful diabetic neuropathy. Neuroscience. 2017;387:28–37. [DOI] [PubMed] [Google Scholar]
- 109. Gilron I, Jensen TS, Dickenson AH.. Combination pharmacotherapy for management of chronic pain: From bench to bedside. Lancet Neurol. 2013;12(11):1084–1095. [DOI] [PubMed] [Google Scholar]
- 110. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Finnerup NB, Haroutounian S, Baron R, et al. Neuropathic pain clinical trials: Factors associated with decreases in estimated drug efficacy. Pain. 2018;159(11):2339–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Attal N, Cruccu G, Baron R, et al. ; European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. [DOI] [PubMed] [Google Scholar]
- 113. Bril V, England J, Franklin GM, et al. ; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161(9):639–649. [DOI] [PubMed] [Google Scholar]
- 115. Waldfogel JM, Nesbit SA, Dy SM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology. 2017;88(20):1958–1967. [DOI] [PubMed] [Google Scholar]
- 116. Bönhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D.. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev. 2019;40(1):153–192. [DOI] [PubMed] [Google Scholar]
- 117. Demant DT, Lund K, Vollert J, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–2273. [DOI] [PubMed] [Google Scholar]
- 118. Han C, Themistocleous AC, Estacion M, et al. The novel activity of carbamazepine as an activation modulator extends from NaV1.7 mutations to the NaV1.8-S242T mutant channel from a patient with painful diabetic neuropathy. Mol Pharmacol. 2018;94(5):1256–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yang Y, Dib-Hajj SD, Zhang J, et al. Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a Na(V)1.7 mutant channel. Nat Commun. 2012;3:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ziegler D, Hidvégi T, Gurieva I, et al. ; Lacosamide SP743 Study Group. Efficacy and safety of lacosamide in painful diabetic neuropathy. Diabetes Care. 2010;33(4):839–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. de Greef BTA, Hoeijmakers JGJ, Geerts M, et al. Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: A randomized controlled trial. Brain. 2019;142(2):263–275. [DOI] [PubMed] [Google Scholar]
- 122. Dyck PJ. Detection, characterization, and staging of polyneuropathy: Assessed in diabetics. Muscle Nerve. 1988;11(1):21–32. [DOI] [PubMed] [Google Scholar]
- 123. Herman WH, Pop-Busui R, Braffett BH, et al. ; DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP.. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: The Diabetic Neuropathy Symptom score. Diabet Med. 2002;19(11):962–965. [DOI] [PubMed] [Google Scholar]
- 125. Bennett M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1-2):147–157. [DOI] [PubMed] [Google Scholar]
- 126. Baron R, Tolle TR, Gockel U, Brosz M, Freynhagen R.. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146(1-2):34–40. [DOI] [PubMed] [Google Scholar]
- 127. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1-2):29–36. [DOI] [PubMed] [Google Scholar]
- 128. Worster A, Carpenter C.. Incorporation bias in studies of diagnostic tests: How to avoid being biased about bias. CJEM. 2008;10(2):174–175. [DOI] [PubMed] [Google Scholar]