Abstract
A prospective cohort study was conducted to evaluate long-term safety of tedizolid as suppressive antimicrobial treatment in patients with implant-associated bone and joint infection caused by multidrug-resistant gram-positive pathogens. Seventeen patients received tedizolid with a median duration of treatment of 6 months. No patients developed a serious adverse event.
Keywords: bone and joint infection, linezolid, periprosthetic joint infection, suppressive antimicrobial therapy, tedizolid
Infections are one of the most dramatic complications after an arthroplasty or osteosynthesis following a fracture [1]. These infections are difficult to treat, requiring prolonged treatment courses, with frequent relapses, despite a dedicated medico-surgical management. In cases when complete microbiological eradication is not possible, suppressive antimicrobial therapy (SAT) may be required, especially if further surgery is not feasible in patients with significant comorbidities or advanced age [2].
Staphylococcus aureus is the cause in the majority of orthopedic-implant infections, but less virulent organisms, such as coagulase-negative Staphylococcus (CoNS), are also frequently implicated. The prevalence of multidrug-resistant (MDR) gram-positive organisms, such as methicillin-resistant S. aureus and vancomycin-resistant enterococci (VRE), has been steadily increasing, with methicillin-resistant Staphylococcus epidermidis identified more frequently through the years [1, 3].
Linezolid (LZD), an oxazolidinone antibiotic, remains active against these MDR gram-positive pathogens in most cases. Nevertheless, the risk of myelotoxicity and polyneuropathy, predominately associated with long-term use, and drug–drug interactions with serotoninergic agents are common issues in patients with chronic complex bone and joint infections (BJIs) [4]. Tedizolid (TZD), a newer oxazolidinone agent, has been associated with lower rates of myelotoxicity and drug–drug interactions, and therefore may be a viable alternative [4, 5]. Indeed, TZD is thought to be less active on mitochondrial protein synthesis than LZD, and consequently fewer side effects are expected [6]. Tedizolid has only been only validated for the treatment of acute bacterial skin and skin-structure infections, and treatment duration in those approved indications is short (6 days) [7]. To our knowledge, the longest treatment duration with TZD in BJI reported was 12 weeks, with several other case reports of longer treatment durations for variable types of infections [8–10]. The aim of this study was to describe our experience with TZD as SAT in patients with complex implant-associated BJI due to MDR gram-positive pathogens.
METHODS
A prospective monocentric cohort study was conducted between 2017 and 2020 at our referral center for the management of complex BJI (Centre de Référence des Infections Ostéo‐Articulaires complexes [CRIOAc]) in Lyon, France (http://www.crioac-lyon.fr).
Periprosthetic joint infections and osteosynthesis-associated infections (OAIs) were defined according to the MusculoSkeletal Infection Society 2018 criteria [11] and Fracture-Related Infection Consensus Group [12], respectively. The clinical situation of every adult patient referred to our reference center with PJI or OAI was discussed during multidisciplinary meetings (infectious disease specialist, microbiologist, orthopedic and plastic surgeons). In all cases, use of tedizolid was validated as the last oral treatment option for SAT, due to intolerance, allergy, or resistant pathogen to other available oral antibiotics. Notably, the choice of TZD was driven by antimicrobial susceptibility results of the current and previous infectious episodes, if considered as not cured.
SAT was defined as an indefinite administration of antibiotics without curative intention in the context of a chronic infection that would normally require implant removal; the aim of SAT was to prevent infection progression and thus, the loss of limb function. There were no exclusion criteria.
The primary objective of this study was to evaluate the safety of TZD as SAT. Any adverse event (AE), defined as an untoward medical event possibly associated with the use of TZD and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, any reason for discontinuation, and standard biological data were prospectively collected. Failure of SAT was defined as the presence of clinical signs suggestive of uncontrolled infection and the need for a new surgical procedure. Anemia was defined as a decrease in hemoglobin level of ≥2 g/dL, thrombocytopenia as a platelet count <150 x 109/L, leukopenia as a white blood cell count <4 x 109/L, and neutropenia as an absolute neutrophil count <1.5 x 109/L.
Data were analyzed with Stata 16.1 software (StataCorp, College Station, Texas). Categorical variables were described by counts and percentages, while mean and standard deviation or median and interquartile range (IQR) were used to summarize continuous variables. Biological variables were compared at baseline and 12 months using paired t test. Significance was set at P < .05.
Patient Consent Statement
This study was subject to declaration with the local Commission for Data Protection and Liberties under the number 20_232 and was recorded on ClinicalTrials.gov under the number NCT04662736.
This study was part of the Lyon BJI cohort study, and patients’ consent was obtained for each inclusion.
RESULTS
Between 2017 and 2020, 17 patients received TZD orally at 200 mg once daily (no adjustment for liver or renal function was made) as SAT for late complex BJI, with a median duration of TZD treatment of 6 months (range, 1–31 months; IQR, 2–15 months). Only 1 patient was considered to have failed TZD treatment at 1 month, but 2 others had also received a short course of treatment at the time of data collection, explaining the IQR from 2 to 15 months. Patients were predominantly male (n = 13 [76%]), with a median age of 73 years (IQR, 69–81 years), a mean body mass index of 28.1 ± 5.1 kg/m2 (range, 19.5–36.7 kg/m2), and a mean American Society of Anesthesiologists score of 2.2 ± 0.6 (range, 1–3). Knee PJIs were the most frequent infections (n = 10 [59%]), followed by hip (n = 5 [29%]) and shoulder PJI (n = 1 [6%]). There was 1 femoral intramedullary nail infection (6%) (Table 1).
Table 1.
Patient Demographics and Outcomes of Late Chronic Infections Under Suppressive Antimicrobial Therapy With Tedizolid
| Patient ID | Sex | Age at Infection, y | BMI, kg/m2 | ASA Score | Concomitant Drug Administrationa | Site of Infection | Type of Surgery | Pathogens | LZD AE | Days of LZD Prior to Switch to TZD | Duration of TZD, mo (d) | Reason for Stopping TZD | TZD AE | Follow-up duration, mo (d) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 71 | 27.8 | 1 | Amitriptyline, tramadol | Knee PJI | DAIR | MRSE | NA | NA | 31 (960) | NA | None | 38 (1168) | Infection controlled |
| 2 | M | 86 | 22.0 | 3 | None | Knee PJI | No surgery (inextirpable implant) | History of various pathogens infection (Helcococcus kunzii, Enterococcus faecalis, MSSA, Corynebacterium striatum, Proteus mirabilis, Escherichia coli) | NA | NA | 6 (210) | Persistent discharge, uncontrolled infection. TZD stopped as a consequence of a serious AE (seizure) due to ertapenem (concomitant administration) | None | 9 (281) | Failure |
| 3 | M | 79 | 26.8 | 2 | Tramadol | Knee PJI | DAIR | No growth (history of MRSE infection) | Diarrhea Asthenia |
29 | 16 (516) | Persistent discharge, pain, positive culture despite SAT | None | 17 (545) | Failure |
| 4 | M | 82 | 28.9 | 3 | Tramadol | Knee PJI | DAIR | MRSE | Thrombopenia (63 x 109/L) | 19 | 17 (540) | NA | None | 18 (559) | Infection controlled |
| 5 | M | 74 | 26.6 | 2 | None | Hip PJI | DAIR with ceramic head exchange | MRSE | Anemia (6.9 g/dL) | 28 | 2 (61) | New PJI | None | 5 (153) | Failure |
| 6 | M | 64 | 29.4 | 2 | None | Knee PJI | DAIR | MSSE Staphylococcus hominis (MS) (history of MRSE infection) |
NA | NA | 14 (441) | NA | None | 17 (524) | Infection controlled |
| 7 | M | 71 | 28.4 | 2 | None | Knee PJI | Arthrodesis | MRSE | None | 28 | 6 (190) | NA | None | 7 (243) | Infection controlled |
| 8 | M | 69 | 32.0 | 2 | None | Hip PJI | DAIR | MRSE Pseudomonas aeruginosa |
None | 35 | 5 (175) | NA | None | 8 (256) | Infection controlled |
| 9 | F | 89 | 20.3 | 2 | None | Knee PJI | DAIR | MRSE | Neutropenia | 14 | 21 (645) | NA | None | 21 (659) | Small fistulab |
| 10 | F | 87 | 34.2 | 3 | None | Knee PJI | DAIR | MRSE | Thrombopenia (16 x 109/L) | 24 | 8 (244) | NA | None | 8 (269) | Small fistulab |
| 11 | M | 81 | 36.1 | 3 | None | Shoulder PJI | DAIR | MSSA (history of MRSE infection) |
Anemia (8.6 g/dL) Thrombopenia (103 x 109/L) |
5 | 15 (483) | NA | None | 15 (488) | Infection controlled |
| 12 | F | 24 | 19.5 | 2 | None | Femoral intramedullary nail | Nail exchange |
Staphylococcus capitis Enterococcus faecium (VR) |
Anemia (6.9 g/dL) | 56 | 13 (423) | NA | None | 16 (498) | Infection controlled |
| 13 | F | 70 | 28.4 | 2 | Amitriptyline | Hip PJI | DAIR | MSSE C striatum (PCR) |
Anemia (8.1 g/dL) Thrombopenia (131 x 109/L) |
20 | 6 (212) | NA | None | 7 (236) | Infection controlled |
| 14 | M | 67 | 22.5 | 2 | None | Hip PJI | 1-stage exchange | MRSE MSSA P mirabillis |
Thrombopenia (75 x 109/L) | 28 | 2 (87) | NA | None | 5 (157) | Infection controlled |
| 15 | M | 77 | 31.1 | 2 | None | Knee PJI | DAIR | Staphylococcus caprae (MR) (history of MSSA, S capitis [MR] infection) | None | 14 | 1 (35) | Persistent discharge, uncontrolled infection | None | 2 (78) | Failure |
| 16 | M | 54 | 36.7 | 2 | None | Hip PJI | DAIR | MRSE (history of MRSA) | None | 14 | 1 (36) | NA | None | 3 (93) | Infection controlled |
| 17 | M | 73 | 27.1 | 3 | Tramadol | Knee PJI | DAIR | C striatum | NA | NA | 1 (50) | NA | None | 3 (97) | Infection controlled |
Abbreviations: AE, adverse event; ASA, American Society of Anesthesiologists; BMI, body mass index; DAIR, debridement, antibiotic, implant retention; F, female; ID, identifier; LZD, linezolid; M, male; MR, methicillin-resistant; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; MS, methicillin-susceptible; MSSA, methicillin-susceptible Staphylococcus aureus; MSSE, methicillin-susceptible Staphylococcus epidermidis; NA, not applicable; PCR, polymerase chain reaction; PJI, periprosthetic joint infection; SAT, suppressive antimicrobial therapy; TZD, tedizolid; VR, vancomycin-resistant.
aOnly considered drugs with potential drug–drug interactions with oxazolidinones: tricyclic antidepressant, selective serotonin reuptake inhibitors, and tramadol. No other drugs with potential interaction were prescribed to patients.
bThese 2 patients were not considered as having failed treatment (see text).
Pathogens included MDR CoNS (n = 16 [76.2%]), followed by Corynebacterium striatum (n = 2), vancomycin-resistant Enterococcus faecium (VRE; n = 1), and/or methicillin-sensitive S aureus (n = 2). Coinfections with gram-negative pathogens (Pseudomonas aeruginosa, Serratia marcescens, and Proteus mirabilis) were observed in 3 patients. All antimicrobial susceptibilities are found in Supplementary Table 1.
An empirical intravenous (IV) antibiotic therapy followed each surgical procedure (mainly debridement and implant retention; n = 13 [76.5%]), with subsequent adjustment based on bacterial susceptibility. Median duration of this primary IV treatment was 47 days (IQR, 35–79 days; range, 5–168 days), followed by LZD in 13 (76.5%) patients. Antibiotic treatment was then changed to tedizolid, including 9 (75.0%) patients who experienced linezolid-induced serious AE: myelotoxicity in 8 (66.7%) patients and severe gastrointestinal intolerance in 1 patient (Table 1). All events were reversible after transition to TDZ. In 4 other patients, TZD was introduced in first intention for SAT after discussion during a multidisciplinary meeting.
The only reason for discontinuation of TZD was failure of the conservative strategy in 4 patients (23.5%), mostly (n = 3 [17%]) due to persistence of sinus tract infection. Two patients presented with a small and intermittent sinus tract; it was considered beneficial to continue TZD and they were not considered as failure. One patient presented a new infection of his arthroplasty, with a new gram-negative pathogen (Citrobacter koseri). No serious AE or discontinuation of TZD due to an AE was observed. There were no clinically significant drug–drug interactions despite coadministration with serotoninergic agents: tricyclic antidepressants (n = 2) and tramadol (n = 4).
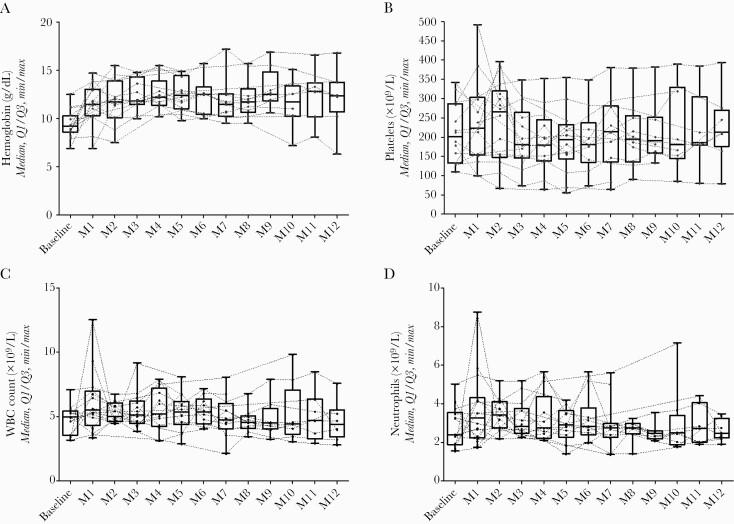
At 12 months after surgery, there was no difference from baseline in platelet count (P = .55), white blood cell count (P = .75), or neutrophil count (P = .93) (Figure 1, Supplementary Table 2). Hemoglobin level increased during the first year of treatment (mean difference of 2.95 ± 3.55 g/dL from baseline), but it was not statistically significant (P = .051). Anemia was observed in 2 patients, in whom an alternate etiology was already known (chronic leukemia and esophageal varices). One case of thrombocytopenia, already observed before introduction of TZD in a cirrhotic patient (platelet count of 80 [160–400] x 109/L), remained stable during a treatment course of 12 months. Transient mild neutropenia (1.4 x 109/L, WBC count of 2.1 x 109) was observed in 1 patient. No neurological or gastrointestinal AEs were observed.
Figure 1.
Evolution of hemoglobin (A), platelet count (B), white blood cell (WBC) count (C), and neutrophil count (D) during the first 12 months of suppressive antimicrobial therapy with tedizolid.
Median duration of follow-up was 8 months (IQR, 5–17 months). One patient died during the study period, with death not considered associated with TZD, AE, or uncontrolled infection. Thus, at the end of the study, except for the 4 patients considered as having failed treatment and the 1 patient who died, TZD was continued in all remaining patients with control of their infection.
DISCUSSION
To our knowledge, this is the longest cohort study of patients who received TZD as an SAT for complex orthopedic implant–associated infections. In this study, TZD was well-tolerated, without any significant hematology- and neurology-related AE, despite the fact that it was initiated because of intolerance to LZD in 9 patients. This suggests that the safety profile of TZD may be more favorable than that of LZD during prolonged treatment courses.
In a clinical trial assessing noninferiority of TZD vs LZD in acute skin and soft tissue infections, a lower incidence of myelotoxicity was observed at the end of treatment with TZD (4.9%) when compared to LZD (10.8%; P = .0003) [13]. Furthermore, several case reports and case series have suggested the safety of TZD treatment in patients with LZD-associated myelotoxicity, with resolution of hematologic troubles following the switch to TZD [5].
Recently, 2 other studies on osteoarticular infection treatment by prolonged courses of TZD have been published [8, 9]. In a study on PJI treatment with TZD, patients were treated with a mean duration of TZD of 8.0 ± 3.27 weeks (range, 6–12 weeks). More than 80% of the patients completed their treatment, and only a few experienced hematological complications, with none leading to discontinuation of TZD [8]. As in our study, their results showed an increase in hemoglobin values, which appears to be related to a restoration process after blood depletion secondary to surgical procedures.
In our cohort study, clinical failure of SAT occurred in 4 patients (23.5%), a rate quite similar to previous studies [2]. It is arguable that 2 patients presenting sinus tract were not considered to have failed treatment. Given the intermittent nature of these small fistula, however, treatment with TZD was considered beneficial to control the infection and to avoid another surgery.
The main limitation of this study is the small size of the population, which precludes definitive safety conclusions, especially regarding rare AEs. Yet, no patient developed AEs leading to treatment discontinuation despite the long duration of the follow-up, and no clinically significant drug–drug interaction was reported. Further clinical investigation is required to confirm the efficacy and safety of TZD as a therapy for BJI.
TZD treatment remains expensive (currently approximately €200 per tablet in France [approximately US$400]), compared with LZD, which is available as a generic drug and costs approximately €14 per day in France (approximately US$5). This could be a barrier for TDZ administration as SAT, particularly when considering long treatment courses. In our study, TZD was not provided by the company ; in France, this drug is attributed to and delivered by hospitals. However, TDZ is not available in all countries.
It is possible that chronic low-grade implant-associated infections may not always require the administration of long-term suppressive antibiotic treatment. However, considering the high risk of infection progression and devastating consequences on the function of the limb and the patient’s life, we believe that SAT is indicated after conservative surgical procedures in those patients without curative alternatives. In addition to TZD, dalbavancin remains an alternative choice in these cases of MDR gram-positive infections. However, this treatment requires IV administration every 1–2 weeks [14], and it has been decided not to use this option at our center.
In conclusion, TZD seems to be a safe option as SAT in patients with complex implant-associated BJI due to MDR gram-positive pathogens, if no other safe oral antibiotic treatment option is available.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Potential conflicts of interest. T. F. received speaker’s fees from MSD in 2021. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fang C, Wong TM, Lau TW, et al. Infection after fracture osteosynthesis—part I. J Orthop Surg (Hong Kong) 2017; 25:2309499017692712. [DOI] [PubMed] [Google Scholar]
- 2. Prendki V, Ferry T, Sergent P, et al. Prolonged suppressive antibiotic therapy for prosthetic joint infection in the elderly: a national multicentre cohort study. Eur J Clin Microbiol Infect Dis 2017; 36:1577–85. [DOI] [PubMed] [Google Scholar]
- 3. Titécat M, Senneville E, Wallet F, et al. Bacterial epidemiology of osteoarticular infections in a referent center: 10-year study. Orthop Traumatol Surg Res 2013; 99:653–8. [DOI] [PubMed] [Google Scholar]
- 4. Douros A, Grabowski K, Stahlmann R. Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin Drug Metab Toxicol 2015; 11:1849–59. [DOI] [PubMed] [Google Scholar]
- 5. Ferry T, Batailler C, Conrad A, et al. Correction of linezolid-induced myelotoxicity after switch to tedizolid in a patient requiring suppressive antimicrobial therapy for multidrug-resistant Staphylococcus epidermidis prosthetic-joint infection. Open Forum Infect Dis 2018; 5:ofy246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flanagan S, McKee EE, Das D, et al. Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function. Antimicrob Agents Chemother 2015; 59:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan SH, Lin WT, Chang SP, et al. Tedizolid versus linezolid for the treatment of acute bacterial skin and skin structure infection: a systematic review and meta-analysis. Antibiotics (Basel) 2019; 8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senneville E, Dinh A, Ferry T, et al. Tolerance of prolonged oral tedizolid for prosthetic joint infections: results of a multicentre prospective study. Antibiotics (Basel) 2020; 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benavent E, Morata L, Escrihuela-Vidal F, et al. Long-term use of tedizolid in osteoarticular infections: benefits among oxazolidinone drugs. Antibiotics (Basel) 2021; 10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matin A, Sharma S, Mathur P, Apewokin SK. Myelosuppression-sparing treatment of central nervous system nocardiosis in a multiple myeloma patient utilizing a tedizolid-based regimen: a case report. Int J Antimicrob Agents 2017; 49:488–92. [DOI] [PubMed] [Google Scholar]
- 11. Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018; 33:1309–14.e2. [DOI] [PubMed] [Google Scholar]
- 12. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury 2018; 49:505–10. [DOI] [PubMed] [Google Scholar]
- 13. Shorr AF, Lodise TP, Corey GR, et al. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinh A, Duran C, Pavese P, et al. ; Dalbavancin French Study Group . French national cohort of first use of dalbavancin: a high proportion of off-label use. Int J Antimicrob Agents 2019; 54:668–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.