Abstract
We measured severe acute respiratory syndrome coronavirus 2 immunoglobulin G responses in 67 patients with hematological malignancies after 2 messenger RNA vaccine doses. Forty-six percent were nonresponders; patients with B-cell chronic lymphocytic leukemia were at highest risk (77% nonresponders). Patients with hematological malignancies should continue wearing masks and socially distancing. Studies of revaccination, boosters, and humoral immune correlates of protection are needed.
Keywords: antibody, COVID, 19 vaccine, hematological malignancy, SARS, CoV, 2
Patients with hematologic malignancies are at high risk for coronavirus disease 2019 (COVID-19)–related complications, with mortality rates exceeding 30% [1–3]. These patients have also been shown to develop prolonged shedding of infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and are thought to be sources of novel SARS-CoV-2 variants of concern [4–8]. Such patients should therefore be prioritized for primary prevention of COVID-19 via vaccination [9]. Until recently, the performance of COVID-19 messenger RNA (mRNA) vaccines in patients with hematological malignancies has been unknown, as these individuals were excluded from COVID-19 vaccine clinical trials [10, 11].
Unfortunately, emerging data have demonstrated that immunocompromised patients, including those with cancer, are at risk for vaccine failure [12–20]. These data are not surprising given prior studies with influenza vaccines [21]. However, they highlight the continued risk of COVID-19 among immunocompromised patients, which may be increased as a result of the revised Centers for Disease Control and Prevention (CDC) masking guidance from May 2021, which allows vaccinated individuals to resume daily activities without wearing a mask or practicing social distancing in most settings [22]. To define the immunogenicity of mRNA COVID-19 vaccines in patients with hematological malignancies, we conducted a retrospective study of antibody responses after COVID-19 vaccination in this patient population. We hypothesized that compared to the 100% seroconversion rates seen in phase 1/2 mRNA vaccine trials [23, 24], a lower proportion of patients with hematological malignancies will develop SARS-CoV-2 antibodies after vaccination.
METHODS
We included patients with hematological malignancy at the University of Pittsburgh Medical Center (UPMC) Hillman Cancer Center who received 2 doses of either the mRNA-1273 (Moderna) or BNT162b2 (Pfizer) vaccine between December 2020 and March 2021 and who underwent SARS-CoV-2 immunoglobulin G (IgG) testing as part of routine care, prior to the wide dissemination of recommendations against routine postvaccine serological monitoring by various national and cancer societies [25–28]. Patients with prior known COVID-19 by report or a positive SARS-CoV-2 polymerase chain reaction test were excluded. Antibody assays were performed at the UPMC clinical laboratories using the Beckman Coulter SARS-CoV-2 platform, which detects IgG against the spike protein receptor-binding domain. These results are expressed as extinction coefficient (signal/cutoff) ratios or “levels” and are interpreted as positive (≥1.00), equivocal (>0.80 to <1.00), or nonreactive (≤0.80) [29]; semi-quantitative antibody levels were available for analysis. It should be noted that the cutoffs of this assay are designed to balance sensitivity of detection with specificity to avoid false positives; this means that some who may respond weakly to vaccines may fall into the “nonresponsive” category [30]. The semi-quantitative antibody levels using this assay have previously been shown to correlate strongly with virus neutralization titers [31]. Reactive results were defined as positive, and equivocal or nonreactive results were defined as negative. We calculated the proportion of patients with a positive vs negative result with 95% Clopper-Pearson exact confidence intervals (CIs) and used χ 2, Fisher exact, or Wilcoxon rank-sum testing for comparisons as appropriate. Analyses were performed using Stata version 16.1 (StataCorp) and GraphPad Prism 8.3.1.
Institutional review board approval was obtained with a waiver of consent for retrospective collection of data.
RESULTS
Sixty-seven patients were included. Median age was 71 years (interquartile range [IQR], 65–77 years); 47.8% (32/67) were female. Underlying malignancies were multiple myeloma (29/67 [43.3%]), lymphomas (21/67 [31.3%]), B-cell chronic lymphocytic leukemia (CLL; 13/67 [19.4%]), and other myeloid malignancies (4/68 [5.97%]); only 1 patient had undergone an allogeneic hematopoietic cell transplant and was not receiving immunosuppression (Table 1). Twenty-nine patients (43.3%) had received cancer-directed therapy within 3 months prior to vaccination (“treatment”), whereas 38 (56.7%) were under observation. Among those receiving treatment, regimens varied widely and included thalidomide derivatives (20.4%), dexamethasone (20.4%), proteasome inhibitors (18.4%), anti-CD38 monoclonal antibodies (18.4%), tyrosine kinase inhibitors (14.3%), anti-CD20 monoclonal antibodies (4.1%), and other therapies (4.1%); most patients were receiving >1 drug. Among the 62 patients whose vaccine type was known, 50.8% (34/67) and 41.8% (28/67) had received the BNT162b2 or mRNA-1273 vaccine, respectively. Median duration from the second vaccine dose to the antibody test was 23 days (IQR, 16–31 days).
Table 1.
Comparison of Patients With Hematological Malignancies With Positive Versus Negative Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Results After Administration of 2 Doses of a Messenger RNA Coronavirus Disease 2019 Vaccine
| SARS-CoV-2 Antibody Result | |||
|---|---|---|---|
| Positive | Negative | ||
| Characteristic | (n = 36) | (n = 31)a | P Value |
| Age, y, median (IQR) | 70 (62.5–73.5) | 74 (68–79) | .009 |
| Sex | |||
| Male | 19 (54.3) | 16 (45.7) | .92 |
| Female | 17 (53.1) | 15 (46.9) | |
| Vaccine type | |||
| BNT162b2 | 15 (44.1) | 19 (55.9) | .31 |
| mRNA-1273 | 16 (57.1) | 12 (42.9) | |
| Days between second dose of vaccine and antibody level, median (IQR) | 23 (14–33) | 25 (16–31) | .93 |
| IgG level, mg/dL, median (IQR)b | 723.5 (510–1045) | 549 (472–939) | .22 |
| Therapy within 90 d of vaccination | |||
| Observation | 21 (55.3) | 17 (44.7) | .58 |
| Active treatment | 15 (51.7) | 14 (48.3) | |
| TKI | 2 (3.8) | 5 (3.2) | .24 |
| No TKI | 34 (32.2) | 26 (27.8) | |
| Anti-CD20 monoclonal antibody within 12 mo of vaccination | |||
| Yes | 2 (22.2) | 7 (77.8) | .070 |
| No | 34 (58.6) | 24 (41.4) | |
| Cancer type | .01d | ||
| CLL | 3 (23.1) | 10 (76.9) | |
| Non-CLL | 33 (61.1) | 21 (38.9) | |
| Lymphomas | 11 (52.4) | 10 (47.6) | |
| Multiple myeloma | 19 (65.5) | 10 (34.5) | |
| Otherc | 3 (75.0) | 1 (25.0) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CLL, chronic lymphocytic leukemia; IgG, immunoglobulin G; IQR, interquartile range; mRNA, messenger RNA; TKI, tyrosine kinase inhibitor.
aIncludes 31 patients with nonreactive tests and 1 patient with an equivocal test.
bRepresents lowest IgG level obtained within 90 days of the severe acute respiratory syndrome coronavirus 2 antibody. IgG levels available for 55 patients. Only 2 patients had received intravenous immunoglobulin during this time period.
cIncludes 2 patients with acute myelogenous leukemia (1 of whom had undergone a hematopoietic cell transplant 10 years prior) and 2 with chronic myeloid leukemia.
dComparison between CLL vs non-CLL patients.
In total, 31 of 67 patients (46.3% [95% CI, 35.4%–60.3%]) had a negative antibody result after vaccination. Older patients were more likely to be vaccine nonresponders than younger patients (Table 1). Sex, IgG levels, number of days between the second vaccine dose and antibody measurement, lymphocyte count in non-CLL patients around the time of vaccination, and whether the patient was receiving treatment (including tyrosine kinase inhibitor therapy) did not differ among vaccine responders vs nonresponders. However, we found a biologically plausible association between administration of an anti-CD20 monoclonal antibody within 12 months prior to vaccination and lack of antibody response, though this did not reach statistical significance (Table 1). None of the 4 patients who received an anti-CD20 monoclonal antibody within 6 months of vaccination developed antibodies (0%), compared to 40% (2/5) of those who received an anti-CD20 monoclonal antibody between 6 and 12 months before vaccination (P = .44). Patients with CLL were significantly less likely to develop SARS-CoV-2 antibodies compared to patients with other hematological malignancies (23.1% [3/13] vs 61.1% [33/54], respectively; P = .01), even though 69.2% (9/13) of CLL patients were not actively undergoing treatment. There was no difference between age or IgG level between CLL and non-CLL patients.
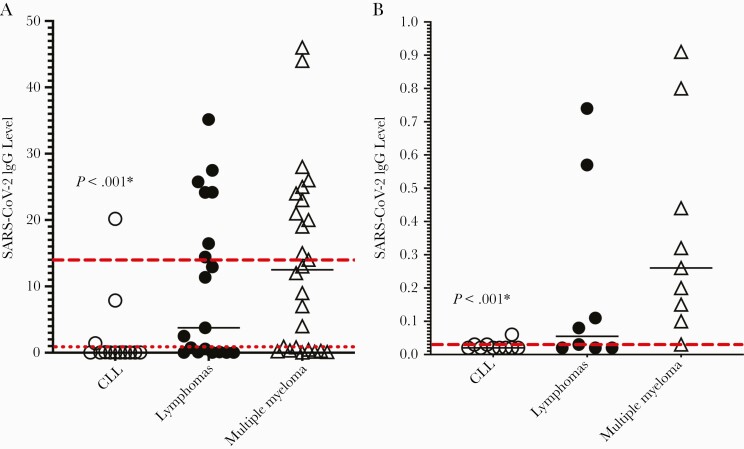
Figure 1 depicts antibody levels by cancer subtype. Among all patients with reactive antibody results, the median antibody level was 14.42 (IQR, 3.31–25.58; range, 1.05–38.6), with 35% of individuals exhibiting a level <10. These levels appear to be lower than those observed in a published report of elderly individuals who were tested for postvaccine antibodies using the same assay, all of whom were found to be seropositive with a mean level of 23.5, and with only 22.5% of individuals exhibiting a level <10 [31]. Overall, antibody levels were significantly lower among CLL vs non-CLL patients (median, 0.02 [IQR, 0.02–0.06] vs 2.89 [0.32–17.91], respectively; P < .001) (Figure 1A). We then analyzed antibody levels among nonreactive patients only and found that levels from CLL nonresponders were also significantly lower than those of non-CLL nonresponders: All nonreactive CLL patients had levels of 0.06 or lower, compared to patients without CLL, whose median nonreactive level was 0.15 (range, 0.02–0.91) (P < .001; Figure 1B).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike immunoglobulin G (IgG) levels for all patients (A) and those with negative antibody results (B). A, All patients. Dotted red line indicates threshold for “reactive” antibody result (level of (≥1.00). Dashed red line shows median antibody level (14.42) for patients with positive results. B, Patients with negative antibody results. Dashed red line shows median antibody level (0.03) for patients with negative results. *Comparison between chronic lymphocytic leukemia (CLL) vs non-CLL patients. There was no difference between lymphoma and myeloma patients. Levels for the 4 patients with other myeloid malignancies were not available. Solid lines indicate medians.
DISCUSSION
Our data show that nearly half of patients with hematological malignancies do not generate antibodies after completing their COVID-19 vaccine series, which is in stark contrast with the results of phase 1/2 mRNA vaccine immunogenicity trials, in which antibody responses were seen in essentially all participants [23, 24]. This lack of response was particularly pronounced among patients with CLL (77% nonresponders), a phenomenon demonstrated in 2 recent studies showing that 48%–60.5% of patients with CLL failed to produce antibodies to COVID-19 vaccines, likely because of the humoral defects that characterize CLL [13, 14]. These nonresponding CLL patients exhibited no detectable antibody levels (all levels ≤0.06), suggesting a near total failure of B-cell activation, likely because these patients’ circulating B-lymphocytes are dysfunctional CLL cells. While our chemotherapy regimens were too heterogenous to allow for proper comparisons, we did observe a biologically plausible albeit statistically nonsignificant association between anti-CD20 monoclonal antibody use within 12 months prior to vaccination and an absence of antibodies. Although the net state of immunosuppression is difficult to quantify, various immunosuppressive regimens in oncology patients, such as anti-CD20 monoclonal antibodies, venetoclax, and tyrosine kinase inhibitors, have been associated with absent SARS-CoV-2 IgG production after vaccination [12–14]. As we enter a postmasking era [22], our findings underscore the importance of adherence to nonpharmaceutical interventions such as masking and social distancing to prevent COVID-19 in patients with hematological malignancies, who should be counseled that the May 2021 CDC guidance permitting vaccinated people to forgo their masks in most settings does not apply to immunocompromised individuals [22].
The immune correlates of protection conferred by SARS-CoV-2 vaccines remain to be determined, and the influence of vaccine-induced T-cell and memory B-cell responses on the severity of COVID-19, even in the absence of antibodies, is unknown. Since thresholds of seroprotection (or antibody levels required to reduce the risk of COVID-19) are not defined, and since current vaccine regulation in the United States (US) does not allow clinicians to offer revaccination or boosters for seronegative patients, results of antibody tests may not currently have any actionable value in clinical practice. Indeed, multiple national societies, including the CDC [25], the National Comprehensive Cancer Network [26], the US Food and Drug Administration [27], the American Society of Hematology [28], and the American Society of Transplantation and Cellular Therapy [28], currently recommend against routine testing with SARS-CoV-2 serology outside of a research protocol, while still acknowledging that some providers may choose to offer serological monitoring. Thus, before serological monitoring becomes mainstream, prospective studies need to be conducted linking SARS-CoV-2 antibody titers with clinical effectiveness of vaccines.
An unprecedented challenge facing clinicians is how to counsel seronegative patients in light of news reports advocating for the routine use of boosters among immunocompromised vaccine nonresponders [32] despite the absence of both data and a mechanism to administer boosters in the US. Given that a recently published study of 30 transplant recipients found that 67% of those who failed to respond to their initial COVID-19 vaccine series also failed to respond to a booster [33], clinicians should advise patients to await the results of clinical trials of boosters (eg, NCT04885907) before pursuing revaccination. Furthermore, our data suggest a heterogeneity among the “nonresponders” (Figure 1B), calling for investigations into which groups or individuals would benefit from a third vaccine dose; preliminary data from transplant recipients suggest that humoral responses to boosters among nonresponders are linked to whether initial antibody responses were truly negative or simply low [33]. Thus, studies of boosters should be designed to examine the impact of antibody levels after completion of the initial vaccine series on the probability of success after a booster [33]. Another strategy that may potentially improve immune responses in hematological malignancy and other immunocompromised patients is delaying the second mRNA vaccine dose, which has been shown to result in an increase in SARS-CoV-2 antibody titers [34]. While our study was underpowered to show a difference in the immunogenicity of the 2 mRNA vaccines, a prior study in transplant recipients did suggest that mRNA-1273 was more likely to elicit an immune response than was BNT162b2 [12]; these findings, if validated, should be incorporated into vaccine guidelines for immunocompromised individuals. Whether primary prevention with monoclonal antibodies (ClinicalTrials.gov identifier NCT04625725), which were shown to be efficacious in the postexposure prophylaxis of COVID-19 [35], will effectively prevent SARS-CoV-2 infection remains to be determined.
Limitations of this study include a small sample size, cross-sectional design, and lack of an internal control group. In addition, we did not determine whether antibodies from vaccine responders are able to neutralize SARS-CoV-2; although antibody titers appear to correlate with viral neutralization titers in healthy individuals [31], whether the same will hold true for patients with hematological malignancies is not known. Finally, we did not measure cellular immune responses. Nonetheless, these early findings suggest that COVID-19 vaccine responses in patients with hematological malignancies are suboptimal, particularly in those with CLL. Future studies should focus on postvaccination antibody durability, T-cell and memory B-cell responses, and novel strategies of COVID-19 prevention (eg, revaccination or monoclonal antibody prophylaxis) in patients with hematological malignancies [8]. In the interim, and as parts of the world begin to enter a postmasking era, immunocompromised patients should continue wearing masks and practicing social distancing, and all those around them should be vaccinated, until additional strategies to protect immunocompromised patients are available.
Notes
Author contributions. M. A. and G. H. designed the research and wrote the first draft of the manuscript. M. A. and M. B. collected the data. G. H. analyzed the data. A. W. and C. C. performed the experiments. All authors have seen and approved the manuscript and contributed significantly to the work.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award number K23AI154546 to G. H.).
Potential conflicts of interest. G. H. receives research funds from Karius, Inc. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol 2021; 8:e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020; 136:2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis 2021; 72:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020; 183:1901–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020; 383:2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hensley MK, Bain WG, Jacobs J, et al. Intractable COVID-19 and prolonged SARS-CoV-2 replication in a CAR-T-cell therapy recipient: a case study [manuscript published online ahead of print 28 January 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kemp SA, Collier DA, Datir RP, et al. ; CITIID-NIHR BioResource COVID-19 Collaboration; COVID-19 Genomics UK (COG-UK) Consortium . SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021; 592:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haidar G, Mellors JW. Improving the outcomes of immunocompromised patients with COVID-19 [manuscript published online ahead of print 5 May 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ribas A, Sengupta R, Locke T, et al. ; AACR COVID-19 and Cancer Task Force . Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov 2021; 11:233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021; 137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia [manuscript published online ahead of print 13 May 2021]. Leukemia 2021. doi: 10.1038/s41375-021-01270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi SG, Knight RJ, Graviss EA, et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation 2021; 105:e72–73. [DOI] [PubMed] [Google Scholar]
- 16. Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data [manuscript published online ahead of print 17 May 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients [manuscript published online ahead of print 26 May 2021]. Am J Transplant 2021. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song CC, Christensen J, Kumar D, et al. Early experience with SARs-CoV-2 mRNA vaccine breakthrough among kidney transplant recipients [manuscript published online ahead of print 29 May 2021]. Transpl Infect Dis 2021. doi: 10.1111/tid.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer [manuscript published online ahead of print 29 May 2021]. JAMA Oncol 2021. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 2021; 22:765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Natori Y, Humar A, Lipton J, et al. A pilot randomized trial of adjuvanted influenza vaccine in adult allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2017; 52:1016–21. [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. When you’ve been fully vaccinated, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html. Accessed 16 May 2021.
- 23. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020; 586:589–93. [DOI] [PubMed] [Google Scholar]
- 24. Jackson LA, Anderson EJ, Rouphael NG, et al. ; mRNA-1273 Study Group . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing, 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed 19 May 2021.
- 26. National Comprehensive Cancer Network. Recommendations of the NCCN COVID-19 vaccination advisory committee, 2021. https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v2-0.pdf?sfvrsn=b483da2b_2. Accessed 19 May 2021.
- 27. US Food and Drug Administration. Antibody testing is not currently recommended to assess immunity after COVID-19 vaccination: FDA safety communication, 2021.https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety. Accessed 19 June 2021.
- 28. American Society for Transplantation and Cellular Therapy. ASH-ASTCT COVID-19 vaccination for HCT and CAR-T cell recipients: frequently asked questions (V2.0), 2021.https://www.astct.org/viewdocument/ash-astct-covid-19-vaccination-for-1?CommunityKey=d3949d84-3440-45f4-8142-90ea05adb0e5&tab=librarydocuments. Accessed 19 June 2021.
- 29. US Food and Drug Administration. Beckman Coulter Access immunoassay systems instructions for use. FDA emergency use authorization, 2021.https://www.fda.gov/media/139627/download. Accessed 2 April 2021.
- 30. Zilla M, Wheeler BJ, Keetch C, et al. Variable performance in 6 commercial SARS-CoV-2 antibody assays may affect convalescent plasma and seroprevalence screening. Am J Clin Pathol 2021; 155:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nace DA, Kip KE, Mellors JW, et al. Antibody responses after mRNA-based COVID-19 vaccination in residential older adults: implications for reopening [manuscript published online ahead of print 12 June 2021]. J Am Med Dir Assoc 2021. doi: 10.1016/j.jamda.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. CBS News. Millions taking immunosuppressive medications at risk for reduced response to COVID-19 vaccines.https://www.cbsnews.com/video/millions-taking-immunosuppressive-medications-at-risk-for-reduced-response-to-covid-19-vaccines/. Accessed 19 July 2021.
- 33. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series [manuscript published online ahead of print 15 June 2021]. Ann Intern Med 2021. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smouth S. Delayed second Pfizer COVID-19 shot produces more antibodies—study. https://www.reuters.com/business/healthcare-pharmaceuticals/delayed-second-pfizer-covid-19-shot-produces-more-antibodies-study-2021-05-13/. Accessed 19 May 2021.
- 35. Cohen MS, Nirula A, Mulligan MJ, et al. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial [manuscript published online ahead of print 3 June 2021]. JAMA 2021. doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]