Abstract
Background
New therapies to achieve hepatitis B surface antigen (HBsAg) clearance are under development. However, gaps in knowledge exist in understanding the incidence and predictors of HBsAg clearance in a racially diverse HIV population.
Methods
We examined the incidence and risk of HBsAg clearance in a retrospective cohort of people with HIV/hepatitis B virus (HBV). Included patients had sufficient data to establish chronic infection based on Centers for Disease Control and Prevention guidelines. We examined the incident rate for HBsAg loss and hazard rate ratios to evaluate predictors for HBsAg clearance in a multivariable model.
Results
Among 571 HIV/HBV patients, 87% were male, 61% were Black, 45% had AIDS, 48% were HBeAg positive, and the median follow-up was 88 months. Incident HBsAg clearance was 1.5 per 100 person-years. In the multivariate model, those with AIDS at baseline (adjusted hazard ratio [aHR], 2.43; 95% CI, 1.37–4.32), Hispanics (aHR, 3.57; 95% CI, 1.33–9.58), and those with injection drug use as an HIV risk factor (aHR, 3.35; 95% CI, 1.26–8.89) were more likely to lose HBsAg, whereas those who were HBeAg positive (aHR, 0.34; 95% CI, 0.19–0.63) were less likely to lose HBsAg. The median change in CD4 cell count during the observation period was 231 cells/mm3 in those with HBsAg loss vs 112 cells/mm3 in those with HBsAg persistence (P = .004).
Conclusions
HBsAg loss occurs in about 10% of those with chronic HBV infection. Being Hispanic, having AIDS at baseline, having an injection drug use history, and having HBeAg-negative status at baseline predicted the likelihood of HBsAg loss. Immune restoration may be a mechanism through which HBsAg loss occurs in HIV patients.
Keywords: hepatitis B surface antigen loss, HIV, immune reconstitution, injection drug use, race
The World Health Organization (WHO) has set a goal of eradicating viral hepatitis B and C by 2030 [1]. Efforts to achieve this are ongoing for hepatitis C. For hepatitis B, implementation of programs to improve hepatitis B vaccination in unvaccinated and at-risk adults is occurring [2, 3]. Furthermore, drugs are under development that focus on hepatitis B surface antigen (HBsAg) clearance with or without hepatitis B surface antibody (HBsAb) gain, defined as a “functional” cure. However, understanding the rates of HBsAg clearance with or without nucleoside therapies can help us to evaluate the context in which HBsAg loss is likely to occur with new therapeutics.
A meta-analysis of HBsAg clearance from 34 studies involving >42 500 patients and >303 000 person-years of follow-up found a pooled annual incidence rate of 1.02% (95% CI, 0.79%–1.27%) for HBsAg clearance. A higher proportion of those who were hepatitis B e antigen (HBeAg) negative at baseline had HBsAg loss (1.33%; 95% CI, 0.76%–2.05%). HBsAg loss was also associated with lower baseline hepatitis B virus (HBV) DNA and lower HBsAg level at baseline [4].These studies were mostly from Asia and Europe and did not include those with HIV infection.
People with HIV (PWH) have an 8%–10% prevalence of hepatitis B due to shared routes of transmission, which in the United States are predominately sexual [5]. Chronic HBV in adulthood likely occurs due to immune suppression or the inability to clear the infection [6]. However, PWH are on antiviral therapy that is active against HBV, which may lead to higher rates of HBsAg clearance. One single-center study of 99 patients in the United States reported 18% HBsAg loss, with 3 having seroreversion, meaning HBsAg re-appeared [7]. A study from Brazil found that 15% had HBsAg clearance but 50% had HBsAg seroreversion [8]. In Sub-Saharan Africa, a study reported a cumulative proportion of HBsAg loss of 6.6% in both HBeAg-positive and -negative patients [9], and a French study reported cumulative HBsAg clearance of 7.4% after a median of 4.6 years [10]. Thus far, studies done in the United States, Europe, Africa, South America, and Thailand have had small sample sizes and have found variable rates of HBsAg clearance, from 6% to 29% [7–13]. It is unclear if some of the studies could have included those with acute HBV, which may have led to higher rates of HBsAg clearance. Given the variability of the data that exist, there remains uncertainty about the rate of HBsAg clearance in HIV patients with chronic hepatitis B. We therefore conducted a retrospective study based on 3 HIV clinics in the United States to understand HBsAg clearance in HIV patients and the predictors that lead to HBsAg loss.
METHODS
Inclusion and Exclusion Criteria
From the initial cohort, excluded patients were those who (1) lacked sufficient HBV serology to determine viral status, such as only having 1 HBsAg test and no additional antigen, antibody, or HBV DNA tests; (2) did not appear to have hepatitis B, as determined by a failed HBsAg confirmatory test or only 1 positive HBsAg test, with further negative tests and no antibodies to support an actual infection; or (3) had successfully cleared their HBV infection before documentation of HIV infection.
Other inclusion criteria included being HIV infected and being an adult age >18 years.
Definition of Chronic Hepatitis B
The Centers for Disease Control and Prevention case definition used for this study included patients who either (1) had 2 tests showing evidence of an HBV infection 6 months apart (these tests could include a positive HBsAg, a detectable HBV DNA, or positive hepatitis B e antigen [HBeAg]) or (2) had a negative HBV core IgM antigen test at baseline while being HBsAg positive.
Study Design and Clinical Variables
A patient was noted as entering our cohort at the time of their first positive HBsAg test or first detectable HBV viral load if that predated an HBsAg test that was available in the electronic medical record. For each patient, we collected demographic information, body mass index, insurance status, HIV risk factors—which included being a man who has sex with men (MSM), being heterosexual, having a history of injection drug use (IDU), or other risks—and alcohol use. We collected comorbid conditions including cirrhosis and hepatitis C. A patient had evidence of cirrhosis if they had documentation of cirrhosis on clinic note or computed tomography, magnetic resonance imaging, or ultrasound.
We recorded the patients’ first HBV DNA, HBsAb, HBeAg, and hepatitis B e antibody (HBeAb) test results with dates. We followed the patient’s HBV status through the last follow-up time, including markers of HBV immunological changes such as HBsAg loss, HBsAb development, HBeAg loss, HBeAb development, and HBV DNA suppression. At each of these changes in HBV immunological status, baseline, and the last visit in the electronic health record, we collected the patient’s CD4 cell count, HIV RNA, and liver function tests (LFTs) within 3 months of the date of serologic data or baseline/last visit. We collected data on the HBV-active ART used to treat these patients, including start and stop dates of the first and last HIV treatment regimens given to the patient as recorded in the electronic health record. A description of the clinical sites and population is available in the Supplementary Data.
Patient Consent Statement
The design of the study was approved by each site’s local institutional review board. Consent was deemed to not be needed due to the retrospective nature of the study.
Statistical Analysis
Baseline date was the first HBsAg or HBV DNA in the electronic health record. Follow-up continued until the last HIV viral load date or the date of HBsAg loss recorded in clinic records. Comparison of HBsAg loss to HBsAg persistence using the chi-square test for categorical variables and t test for continuous variables occurred. Data are presented as the mean +/- SD for continuous variables and as percentages for categorical variables. The incidence rate of HBsAg loss was calculated using a generalized linear regression model. Baseline clinical characteristics examined by univariate and multivariate Cox proportional hazard regression models include factors that may predict loss of HBsAg, including age, gender, race, AIDS, cirrhosis, HBeAg, HIV RNA suppression, hepatitis C virus (HCV), HIV risk factors, insurance, HBV viral load, and alcohol. We only included variables present at baseline or assumed to be present at baseline (ie, HCV status, alcohol use). We did not include variables that were time-dependent such as HBV DNA suppression or CD4 change because the aim of the study was to examine predictors of HBsAg loss based on baseline information. A 2-sided P value of <.05 was considered statistically significant. We performed statistical analysis with SAS, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
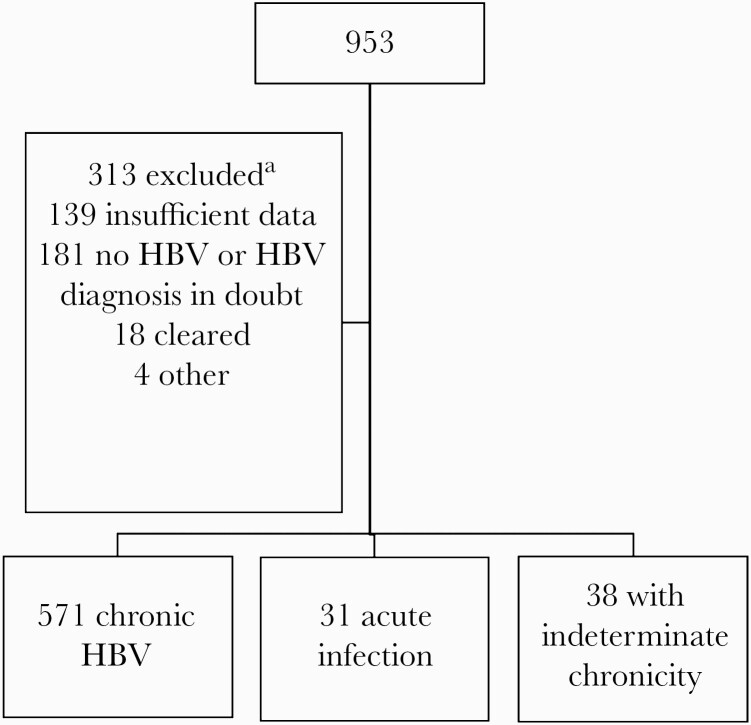
We identified 953 PWH with HBsAg-positive or detectable HBV DNA. From this, we excluded 313 (Figure 1) for insufficient serology, inability to confirm hepatitis B, or clearance of HBV before HIV diagnosis. We excluded 31 due to acute infection and 38 due to undetermined chronicity, to arrive at a cohort of 571 with chronic HBV infection. Our cohort was predominately male, Black, and uninsured; almost half reported being a man who has sex with men as their HIV risk factor; a little over half did not have AIDS at baseline; and a little less than half were HBeAg positive at baseline at the first recorded lab result. Among 269 HBeAg-positive individuals at baseline, 40 lost HBeAg during follow-up and 9/40 lost HBsAg. There were 141 who were HBV DNA suppressed at baseline, of whom 24 (17%) were HBeAg positive and 117 (83%) were HBeAg negative. Baseline median CD4 (interquartile range [IQR]) for HBeAg-positive and HBeAg-negative individuals was 204 (52–376) cells/μL and 265 (91–494) cells/μL (P = .006), respectively. This cohort had a median (IQR) of 88 (43–128) months of follow-up. Approximately 10.9% of the HBV cohort lost HBsAg. As seen in Table 1, those who lost HBsAg were similar to those with HBsAg persistence by gender, race, insurance, and HIV risk factor. A trend did exist for those who lost HBsAg to have AIDS at baseline (43% vs 57%; P = .03). Among those who lost HBsAg, a higher proportion were HBeAg negative at baseline (50% vs 71%; P = .001).
Figure 1.
Consort diagram of the cohort of HIV/HBV patients from 3 HIV clinics in Texas. Patients were excluded if insufficient data existed to categorize them as having acute infection or if HIV or HBV diagnosis was not clear. Clear acute infection and chronicity (ie, infection >6 months) could not be established. aNot exclusive. Abbreviation: HBV, hepatitis B virus.
Table 1.
Baseline Characteristics of Those who Lost Hepatitis B Surface Antigen Compared With Those who Did Not
| Overall (n = 571), No. (%) | HBsAg Persistent (n = 509), No. (%) | HBsAg Loss (n = 62), No. (%) | P Value | |
|---|---|---|---|---|
| Gender | .80 | |||
| Female | 77 (13.49) | 68 (13.36) | 9 (14.52) | |
| Male | 494 (86.51) | 441 (86.64) | 53 (85.48) | |
| Race/ethnicity | .17 | |||
| Non-Hispanic White | 114 (19.96) | 107 (21.02) | 7 (11.29) | |
| Black | 347 (60.77) | 308 (60.51) | 39 (62.90) | |
| Hispanic | 88 (15.41) | 74 (14.54) | 14 (22.58) | |
| Other | 22 (3.85) | 20 (3.93) | 2 (3.23) | |
| Insurance | .08 | |||
| Commercial | 52 (9.11) | 44 (8.64) | 8 (12.90) | |
| Medicaid | 124 (21.72) | 111 (21.81) | 13 (20.97) | |
| Medicare | 162 (28.37) | 138 (27.11) | 24 (38.71) | |
| Ryan White/uninsured | 233 (40.81) | 216 (42.44) | 17 (27.42) | |
| BMI | 25.88 ± 5.95 | 25.82 ± 5.82 | 26.49 ± 7.00 | .41 |
| HIV risk factor | .82 | |||
| Heterosexual | 176 (30.82) | 159 (31.24) | 17 (27.42) | |
| MSM | 279 (48.86) | 249 (48.92) | 30 (48.39) | |
| IDU | 57 (9.98) | 49 (9.63) | 8 (12.90) | |
| Other | 59 (10.33) | 52 (10.22) | 7 (11.29) | |
| AIDS, baseline | .03 | |||
| no | 323 (56.57) | 296 (58.15) | 27 (43.55) | |
| Yes | 248 (43.43) | 213 (41.85) | 35 (56.45) | |
| Hepatitis B e antigen, baseline | .001 | |||
| Negative | 295 (51.66) | 251 (49.31) | 44 (70.97) | |
| Positive | 276 (48.34) | 258 (50.69) | 18 (29.03) | |
| cirrhosis | .29 | |||
| No | 480 (84.06) | 425 (83.50) | 55 (88.71) | |
| Yes | 91 (15.94) | 84 (16.50) | 7 (11.29) |
Abbreviations: BMI, body mass index; IDU, injection drug use; MSM, men who have sex with men.
The incidence rate of HBsAg loss was 1.50 per 100 person-years. The incidence rate for HBsAg loss was highest for HBeAg-negative individuals at baseline (2.16 per 100 person-years; 95% CI, 1.61–2.91) and lowest among non-Hispanic Whites (0.87 per 100 person-years; 95% CI, 0.41–1.82).
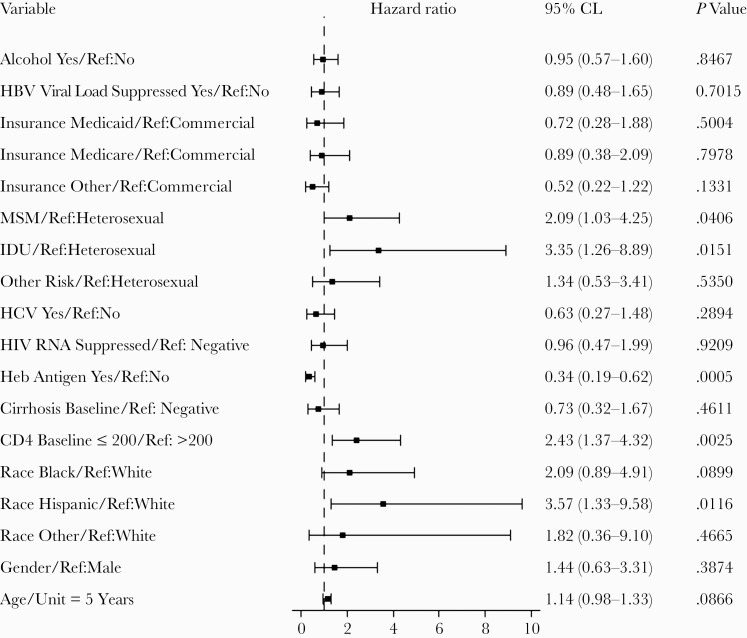
As seen in Table 2 in univariate analysis, positive predictors of HBsAg loss were being Hispanic compared with being non-Hispanic White, having AIDS at baseline, and being HBeAg negative at baseline. In a multivariable model (Figure 2), the independent hazard of HBsAg loss included being Hispanic vs being non-Hispanic White (adjusted HR [aHR], 3.57; 95% CI, 1.33–9.58), being HBeAg positive (aHR, 0.34; 95% CI, 0.19–0.63), and having AIDS at baseline (aHR, 2.43; 95% CI, 1.37–4.32), as well as IDU compared with being heterosexual (aHR, 3.35; 95% CI, 1.26–8.88). There was an interaction between AIDS at baseline and HBeAg status. Those who had AIDS at baseline and were HBeAg negative were more likely to lose HBsAg (HR, 4.24; 95% CI, 1.92–9.35) (data not shown). In stratified analysis by AIDS or no AIDS (Supplementary Table 1), in those without AIDS we found no difference in HBsAg loss by age, gender, race, HBeAg status at baseline, cirrhosis, HCV, insurance, HIV risk factor, alcohol use, or HBV DNA suppression at baseline. In those with AIDS, we found no difference in other categories except for being HBeAg positive (HR, 0.23; 95% CI, 0.10–0.50). Thus, HBeAg-negative individuals were significantly more likely to lose HBsAg compared with HBeAg-positive individuals with AIDS. We found no differences in those with AIDS or in different races but did find a difference in HBeAg status and a racial distribution wherein Blacks were less likely to be HBeAg positive compared with non-Hispanic Whites or Hispanics.
Table 2.
Univariate Cox Proportional Hazard Ratio of Hepatitis B Surface Antigen Loss Over Time by Baseline Characteristic
| Hazard Ratio | 95% CI | |
|---|---|---|
| Age, 5-y increments | 1.11 | 0.97–1.28 |
| Gender | ||
| Female | Reference | Reference |
| Male | 0.96 | 0.47–1.95 |
| Race/ethnicity | ||
| Non-Hispanic White | Reference | Reference |
| Black | 1.73 | 0.78–3.88 |
| Hispanic | 2.61 | 1.05–6.49 |
| Other | 1.81 | 0.38–8.75 |
| AIDS at baseline (CD4 <200 cells/μL) | ||
| No | Reference | Reference |
| Yes | 2.12 | 1.28–3.51 |
| Cirrhosis | ||
| No | Reference | Reference |
| Yes | 0.68 | 0.31–1.48 |
| Hepatitis B e antigen at baseline | ||
| Negative | Reference | Reference |
| Positive | 0.40 | 0.23–0.69 |
| HIV RNA suppressed at baseline (<200 copies/mL) | ||
| No | Reference | Reference |
| Yes | 0.75 | 0.40–1.38 |
| Hepatitis C coinfection | ||
| No | Reference | Reference |
| Yes | 1.01 | 0.48–2.13 |
| HIV risk factor | ||
| Heterosexual | Reference | Reference |
| MSM/bisexual | 1.28 | 0.70–2.32 |
| IDU | 1.81 | 0.78–4.24 |
| Other | 1.07 | 0.44–2.60 |
| Insurance | ||
| Commercial | Reference | Reference |
| Medicaid | 0.73 | 0.30–1.76 |
| Medicare | 0.91 | 0.41–2.02 |
| Ryan White/uninsured | 0.64 | 0.27–1.48 |
| Hepatitis B DNA suppressed at baseline | ||
| No | Reference | Reference |
| Yes | 1.30 | 0.76–2.21 |
| Alcohol use | ||
| No | Reference | Reference |
| Yes | 1.03 | 0.63–1.70 |
Abbreviations: BMI, body mass index; IDU, injection drug use; MSM, men who have sex with men.
Figure 2.
Multivariable analysis examining risk of hepatitis B surface antigen loss by baseline characteristics. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; IDU, injection drug use; MSM, men who have sex with men.
Follow-up Data
We examined cumulative HBsAg clearance over time; the 5-year cumulative incidence was 5.78%, and the 10-year cumulative incidence was 9.11%. Among those who had a loss of HBsAg, hepatitis B surface antibody was positive in 74% (n = 28) of those in whom it was measured. We examined change in CD4 count from baseline to last follow-up and found a significant difference in the median change in CD4 cell count in those with HBsAg clearance vs those without (230 CD4 cells/μL vs 112 CD4 cell/μL; P = .0004). During follow-up, we found that 98.42% were on active HBV treatment.
DISCUSSION
In our multicenter study, we found HBsAg clearance to occur in 10.9% of patients. We found the incidence rate of HBsAg clearance to be 1.5 per 100 person-years annually. In 1 study of 290 patients with a median of 7.4 years of follow-up, HBsAg clearance occurred in 1.2 per 100 person-years in the first 3 years and then dropped to 0.6/100 person-years [10]. Another study from France of 1419 HIV/HBV patients on lamivudine (3TC) or emtricitabine (FTC), tenofovir disoproxil fumarate (TDF) alone, or a combination of TDF and 3TC or FTC with a median of 89 months of follow-up found a rate of HBsAg clearance of 0.7 per 100-person years and a rate of development of hepatitis B surface antibody (HBsAb) of 0.5 per 100 person-years, with a higher likelihood of clearance in those on a TDF-based regimen [14]. We also found HBsAb positivity in 74% of those with loss of HBsAg, showing that there was a transition from immune clearance/inactive carrier stage to immune stage.
We found a 2 times higher likelihood of HBsAg clearance in those with AIDS at baseline. The mechanism of HBsAg loss may be due to immune reconstitution, as CD4 cell count was significantly higher at the end of follow-up in those who had HBsAg loss than those with HBsAg persistence, a finding seen in other studies [7]. We also found that those who were HBeAg positive were less likely to achieve HBsAg loss. A significant number of those who were HBeAg negative at baseline were those who lost HBsAg. These patients may have already been on the path to losing HBsAg, as they did not have evidence of replicating virus. We did find an interaction with AIDS and HBeAg-negative status. Those who were HBeAg positive and had AIDS were less likely to clear HBsAg. These observations raise the possibility that AIDS may have prevented the loss of HBeAg. The combination of AIDS and being HBeAg negative at baseline was an effect modifier concerning HBsAg loss. Thus, our data suggest that AIDS prevented natural progression from being HBeAg negative to HBsAg clearance until immune restoration occurred. Decreases in quantitative HBsAg levels, which have been found to predict HBsAg loss [9, 15], have been found to be higher in those who are HBeAg positive and lower in those who are HBeAg negative [16]. One study has also shown that changes in CD4 count lead to reduction in quantitative HBsAg levels, indicating that immune restoration acts as a mechanism for HBSAg clearance [17]. Another limitation of our study is that we were unable to measure HBsAg levels. Our observations need further confirmation in other cohorts.
Additionally, we observed that HBsAg clearance was 3 times more likely among those who reported IDU than heterosexuals as an HIV risk factor. High rates of HBV, either before or during infection, have been reported among IDU [18]. However, to our knowledge, it has not been reported that a higher rate of HBsAg loss occurs among this group. There are reports of occult HBV among IDU, but our study did not consider them to have lost HBsAg if HBsAg was not positive or was missing at baseline. Furthermore, we excluded those with acute infection. Further studies are needed to determine if HBsAg loss occurs more often in those with IDU.
Finally, we observed that Hispanics, compared with non-Hispanic Whites, were 3 times as likely to lose HBsAg, an association not previously reported. Hispanics may be different from Whites in diagnosis of HIV. Many studies have reported delayed diagnosis among Hispanics [19], which may explain why a higher proportion had AIDS at baseline. Hispanics, however, were not more likely to be HBeAg negative at baseline. Further studies are needed to understand these findings.
We observed intermittent hepatitis B (HBeAg, HBsAg) testing. Perhaps more HBsAg loss occurred in our cohort, but we were unable to detect this due to lack of testing. From our data, it would be useful for providers to check HBsAg in patients who are HBeAg negative and have improved CD4 cell counts. Loss of HBsAg will allow clinicians to have more flexibility in ART regimens if patients develop renal disease. Further data are still needed to determine the durability of HBsAg loss.
This study is a large cohort of PWH with chronic hepatitis B and shows that >10% of the cohort lost HBsAg over time. These findings will help to elucidate the baseline rate of HBsAg loss in PWH and can be used to measure effectiveness on new HBV drugs in development, which aim to produce HBsAg loss. There are, however, limitations with this study. First, this was a retrospective cohort. Data were not collected prospectively at timed intervals, and thus there may have been misclassification bias. We suspect that we may have underestimated the true prevalence of HBsAg loss because of this bias. We did not have longitudinal ART data to evaluate the impact of ART duration on HBsAg loss. We also did not have precise data on the timing of HBsAg loss and associated CD4 cell counts to determine if immune reconstitution requires a threshold CD4 count. The majority of our patients were on ART that was active for hepatitis B. It is unknown if hepatitis B therapy or change in CD4 count was principally responsible for HBsAg loss.
Regardless, this study is one of the largest cohorts of HIV/HBV in the United States to examine HBsAg loss. Our data raise a critical hypothesis regarding the role of immune reconstitution in HBsAg clearance. Furthermore, this study raises the possibility that race may impact HBsAg loss. Finally, it suggests that clinicians should consider rechecking HBsAg in patients who are HBeAg negative and have had immune reconstitution on ART.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. M.K.J. has received research funding from Gilead Sciences, Janssen Pharmaceuticals, Merck, GSK/ViiV, and VasGene. She has been a consultant to Gilead Sciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global hepatitis report, 2017. 2017. Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 3 April 2019.
- 2. Abara WE, Qaseem A, Schillie S, et al. ; High Value Care Task Force of the American College of Physicians and the Centers for Disease Control and Prevention . Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2017; 167:794–804. [DOI] [PubMed] [Google Scholar]
- 3. Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ 2017; 66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeo YH, Ho HJ, Yang HI, et al. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: a systematic review and meta-analysis. Gastroenterology 2019; 156:635–46.e9. [DOI] [PubMed] [Google Scholar]
- 5. Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003; 188:571–7. [DOI] [PubMed] [Google Scholar]
- 6. Morsica G, Galli L, Bossolasco S, et al. Brief report: outcome of acute hepatitis B virus infection in HIV-1-infected patients: possible factors associated with resolution or chronicity. J Acquir Immune Defic Syndr 2019; 82: 175–80. [DOI] [PubMed] [Google Scholar]
- 7. Huang AJ, Núñez M. Outcomes in HIV/HBV-coinfected patients in the tenofovir era are greatly affected by immune suppression. J Int Assoc Provid AIDS Care 2015; 14:360–8. [DOI] [PubMed] [Google Scholar]
- 8. Toscano AL, Corrêa MC. Evolution of hepatitis B serological markers in HIV coinfected patients: a case study. Rev Saude Publica 2017; 51:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyd A, Maylin S, Moh R, et al. ; ANRS 12240 VarBVA study . Hepatitis B surface antigen quantification as a predictor of seroclearance during treatment in HIV-hepatitis B virus coinfected patients from Sub-Saharan Africa. J Gastroenterol Hepatol 2016; 31:634–44. [DOI] [PubMed] [Google Scholar]
- 10. Boyd A, Gozlan J, Miailhes P, et al. Rates and determinants of hepatitis B ‘e’ antigen and hepatitis B surface antigen seroclearance during long-term follow-up of patients coinfected with HIV and hepatitis B virus. AIDS 2015; 29: 1963–73. [DOI] [PubMed] [Google Scholar]
- 11. Hamers RL, Zaaijer HL, Wallis CL, et al. ; PharmAccess African Studies to Evaluate Resistance (PASER) . HIV-HBV coinfection in Southern Africa and the effect of lamivudine- versus tenofovir-containing cART on HBV outcomes. J Acquir Immune Defic Syndr 2013; 64:174–82. [DOI] [PubMed] [Google Scholar]
- 12. Matthews JE, Stephenson R, Sullivan PS. Factors associated with self-reported HBV vaccination among HIV-negative MSM participating in an online sexual health survey: a cross-sectional study. PLoS One 2012; 7:e30609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosi L, Reiberger T, Payer BA, et al. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. J Viral Hepat 2012; 19:801–10. [DOI] [PubMed] [Google Scholar]
- 14. Gantner P, Cotte L, Allavena C, et al. ; Dat’AIDS Study Group . Higher rates of HBsAg clearance with tenofovir-containing therapy in HBV/HIV co-infection. PLoS One 2019; 14:e0215464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews GV, Ali RJ, Avihingsanon A, et al. Quantitative HBsAg and HBeAg predict hepatitis B seroconversion after initiation of HAART in HIV-HBV coinfected individuals. PLoS One 2013; 8:e61297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maylin S, Boyd A, Lavocat F, et al. Kinetics of hepatitis B surface and envelope antigen and prediction of treatment response to tenofovir in antiretroviral-experienced HIV-hepatitis B virus-infected patients. AIDS 2012; 26: 939–49. [DOI] [PubMed] [Google Scholar]
- 17. Jaroszewicz J, Reiberger T, Meyer-Olson D, et al. Hepatitis B surface antigen concentrations in patients with HIV/HBV co-infection. PLoS One 2012; 7:e43143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shing JZ, Ly KN, Xing J, et al. Prevalence of hepatitis B virus infection among US adults aged 20–59 years with a history of injection drug use: National Health and Nutrition Examination Survey, 2001–2016. Clin Infect Dis 2020; 70: 2619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen NE, Gallant JE, Page KR. A systematic review of HIV/AIDS survival and delayed diagnosis among Hispanics in the United States. J Immigr Minor Health 2012; 14:65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.