Abstract
Background and Aims
Canada has one of the highest inflammatory bowel disease (IBD) incidence rates worldwide. Higher IBD incidence rates have been identified among urban regions compared to rural regions. The study objectives were to (i) estimate IBD incidence rates in Saskatchewan from 1999 to 2016 and (ii) test for differences in IBD incidence rates for rural and urban regions of Saskatchewan.
Methods
A population-based study was conducted using provincial administrative health databases. Individuals aged 18+ years with newly diagnosed Crohn’s disease or ulcerative colitis were identified using a validated case definition. Generalized linear models with a negative binomial distribution were used to estimate incidence rates and incidence rate ratios (IRRs) adjusted for age group, sex and rurality with 95% confidence intervals (CIs).
Results
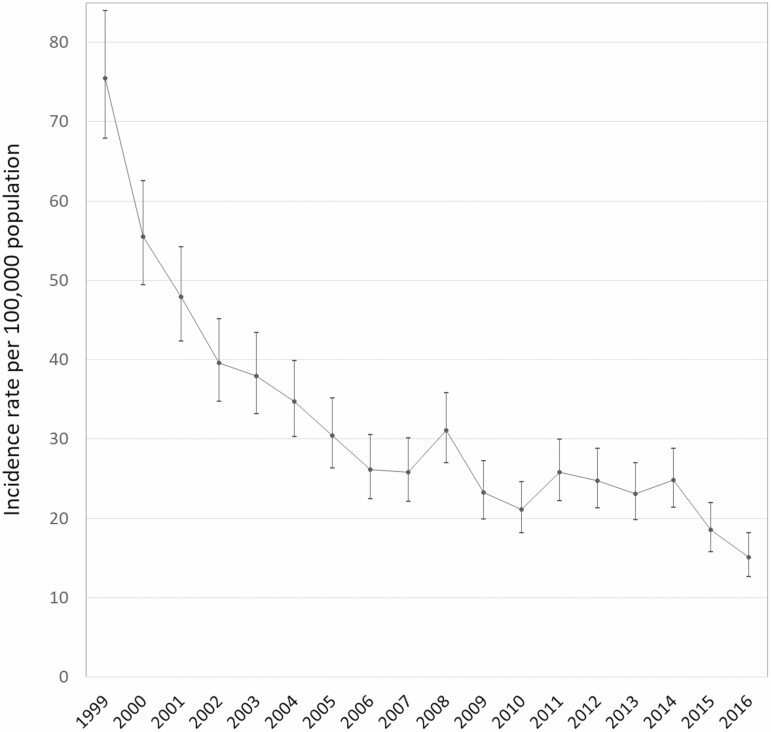
The average annual incidence rate of IBD among adults in Saskatchewan decreased from 75/100,000 (95% CI 67 to 84) in 1999 to 15/100,000 (95% CI 12 to 18) population in 2016. The average annual incidence of IBD declined significantly by 6.9% (95% CI −7.6 to −6.2) per year. Urban residents had a greater overall risk of IBD (IRR = 1.19, 95% CI 1.11 to 1.27) than rural residents. This risk difference was statistically significant for Crohn’s disease (IRR = 1.25, 95% CI 1.14 to 1.36), but not for ulcerative colitis (IRR = 1.08, 95% CI 0.97 to 1.19).
Conclusions
The incidence of IBD in Saskatchewan dropped significantly from 1999 to 2016 with urban dwellers having a 19% higher risk of IBD onset compared to their rural counterparts. Health care providers and decision-makers should plan IBD-specific health care programs considering these specific IBD rates.
Keywords: Crohn’s disease, Epidemiology, Incidence, Inflammatory bowel disease, Ulcerative colitis, Urban population
INTRODUCTION
Inflammatory bowel disease (IBD), comprising of ulcerative colitis (UC) and Crohn’s disease (CD), is a long-term disorder causing inflammation in the gastrointestinal system (1). As a worldwide disease with predominance in developed countries, IBD is estimated to affect as many as 1.5 million individuals in North America and 2.5 to 3 million people in Europe (2,3). The incidence of IBD varies considerably geographically. The highest incidence of IBD has been described in westernized countries (4). However, recent epidemiological studies have also described increasing incidence trends of IBD in areas that previously reported low rates of the disease, including Asia, South America and Middle East (5–10). Thus, countries including, China, India, Turkey and Brazil are reporting increasing incidence rate of IBD as opposed to European and North American countries who have demonstrated variable patterns of decreasing, plateauing and increasing rates of the disease (4–11). As a result, the differences in the incidence rates between developed and developing countries have been reducing (4,12–14).
Canada is among countries with the highest incidence rate of IBD (15). In 2018, it was estimated that 270,000 people were living with IBD in Canada (16). Population-based studies assessing the incidence of IBD in different Canadian provinces have provided varying results in the trends of the disease. Some provinces have described decreasing incidence rates of IBD, while others have reported stable trends over time or increasing incidence rates among specific age groups (15,17,18).
Furthermore, over the past two decades, numerous studies assessing differences between location of residence and IBD in places such as Europe, North and South America have found a higher risk of IBD (both CD and UC) in urban areas compared to rural locations (8,19–21). Most of these studies have pinpointed westernized lifestyle and urbanization as the key reasons behind this risk. Likewise, in Canada, extensive population-based studies regarding IBD epidemiology and location of residence have been assessed. All these studies revealed rurality as a protective factor for IBD (19–21).
Epidemiologic studies on the incidence trends of IBD are essential to provide researchers clues to the etiology of IBD. Information on this topic will provide evidence on how rapidly the disease is decreasing or increasing in a given region. Moreover, understanding the differences between IBD and location of residence is crucial for designing effective strategies needed to reduce, prevent and treat individuals living with the disease. Data on the incidence in Western Canada are lacking since year 2000. Therefore, this study aimed to (i) estimate the incidence rates and trends of IBD in Saskatchewan and (ii) determine if the incidence rates of IBD in urban settings are higher than those in rural areas.
METHODS
Study Design, Participants, and Setting
A population-based cohort study was conducted using administrative databases for the province of Saskatchewan. This western Canadian province has approximately 1.2 million people, most residing in one of the two largest cities, Regina or Saskatoon (22,23), and around 35% of the population is dispersed in rural and remote areas (23). Saskatchewan has a universal health care system (24) stewarded by the provincial Ministry of Health which routinely collects data of health care utilization on almost the entire covered population. In this study, we included data from all individuals 18 years and older newly diagnosis of IBD, including CD and UC, between fiscal years April 1st, 1999, to March 31st, 2016. The data source spans from 1990 to 2018. However, the study population was restricted for 1999 and 2016 to allow for backward and forward washout period. We excluded individuals less than 18 years of age due to data unavailability and because there is no validated pediatric IBD case definition for Saskatchewan administrative health data. For several years, no pediatric gastroenterologists were available in Saskatchewan and patients were required to access colonoscopies and pediatric IBD care in neighbouring provinces.
Data Source
Three de-identified databases were linked and used in this study (i.e., hospital discharge abstracts, Person Health Registration System [PHRS] and physician services claims). The Discharge Abstract Database (DAD) contains information on hospitalizations completed when patients are discharged from an acute-care facility, and includes inpatient hospitalizations, diagnostic procedures (including endoscopies) and day surgeries (22,25). All data were accessed through the Saskatchewan Health Quality Council (HQC). The PHRS captures individual demographic characteristics including the year of birth, sex, date of health care coverage (initiation and termination), the status of health insurance coverage and location of residence (22). The Medical Services Billing database captures information submitted by physicians to claim reimbursement from the provincial government for services delivered to patients (22,25). Salaried physicians are required to submit claims for the care they provided, a process known as ‘shadow-billing’. Although shadow-billing could lead to under-reporting (22), the case definition used in this study required multiple inpatient and outpatient health care contacts, so this is unlikely to affect our results.
Case Definition
Diagnosed incident IBD cases were identified by applying a case definition that was validated in Manitoba (26). This algorithm has been used in other population-based studies in Saskatchewan (16,19,25) and has been proven to be one of the most accurate case definitions to identify adults with the diagnosis of IBD (27). For this case definition, the International Classification of Disease version 9 (ICD-10) and 10 (ICD-10) codes were used. This case definition requires five or more physician claims or hospitalizations with the diagnosis of IBD, either CD (ICD-9 555.x and ICD-10-CA K50.x) or UC (ICD-9 556.x and ICD-10-CA K51.x), within at least 2 years of continuous health care coverage, or a minimum of three health care contacts with these diagnostic codes if individuals had less than 2 years of continuous health care coverage (25,26). We classified eligible cases as CD or UC based on the most prevalent diagnosis (25). After identifying eligible IBD cases, an 8-year washout period was used to distinguish between the incident and prevalent diagnosed IBD cases; specifically, 8 years of continuous health care coverage without IBD health care contacts (i.e., either hospitalizations or physician billing claims) were required prior to the date of diagnosis (i.e., the first eligible health care contact of the case definition). This 8-year washout period has been previously used in IBD studies based on administrative health data (18,28).
Definition of Rural/Urban Status
All diagnosed incident IBD cases were assigned to rural or urban residence location at the date of the diagnosis of IBD. There is no standard definition of rurality in Canada. Various definitions have been proposed by Statistics Canada to define rural and urban residence. These definitions include the ‘population size, population density, or the economic and social influence of a city on neighbouring regions’ (29). A 2017 Canadian study conducted by Benchimol et al. (20) validated the rural/urban definition and outlined the Census Metropolitan Areas (CMA) definition as the best option. In this study, we used an approach adopted by the Saskatchewan HQC. Individuals with a residential postal code within a CMA or Census Agglomeration with a population of 15,000 or more inhabitants were classified as living in an urban residence. This definition has been used in previous population-based studies in the province (25).
Statistical Analysis
Generalized linear models with a negative binomial distribution were used, considering a good model fit when observing a Pearson χ 2 / residual of degree of freedom ratio closer to 1 (30,31). A negative binomial distribution was chosen due to its ability to accommodate over-dispersed count data (30). Incidence was modelled considering as dependent variable the observed number of IBD incidence cases and as independent variables sex, rural/urban place of residence, fiscal year and age group stratum (i.e., 18 to 29 [reference], 30 to 39, 40 to 49, 50 to 59, 60+ years old). The natural logarithm of the Saskatchewan population at risk in each age group stratum stratified by sex and location of residence (i.e., rural or urban) was used as an offset variable in the models. Information about the population at risk derived from the PHRS database.
The models were used to estimate incidence rates and incidence rate ratios (IRRs) with their corresponding 95% confidence intervals (95% CIs). A linear trend test across the years was estimated. Also, an interaction term between year and location of residence was tested in the model. A nominal α = 0.05 was adopted for all tests of statistical significance. SAS version 9.4 (SAS Institute, Cary, NC, USA) with the GENMOD procedure was used for all analyses. The Saskatchewan Biomedical Research Ethics Board (REB) approved this study as BIO 91.
Results
In total, 4908 individuals with the diagnosis of IBD were included in the study. In this group, 1447 (29.9%) people were living in rural areas and 3392 (70.1%) in urban locations. Table 1 describes the demographics of the study cohort.
Table 1.
Descriptive characteristics of the incident cohort of inflammatory bowel disease in Saskatchewan between 1999 and 2016 (n = 4908)
| Variable | n (%) |
|---|---|
| Age group, years | |
| 18–29 | 1148 (23.4) |
| 30–39 | 934 (19.0) |
| 40–49 | 1052 (21.4) |
| 50–59 | 838 (17.1) |
| 60+ | 936 (19.1) |
| Disease type | |
| Crohn’s disease | 2612 (53.2) |
| Ulcerative colitis | 2296 (46.8) |
| Sex | |
| Male | 2285 (46.6) |
| Female | 2623 (53.4) |
| Residence location | |
| Urban | 3392 (70.1) |
| Rural | 1447 (29.9) |
Incidence of IBD
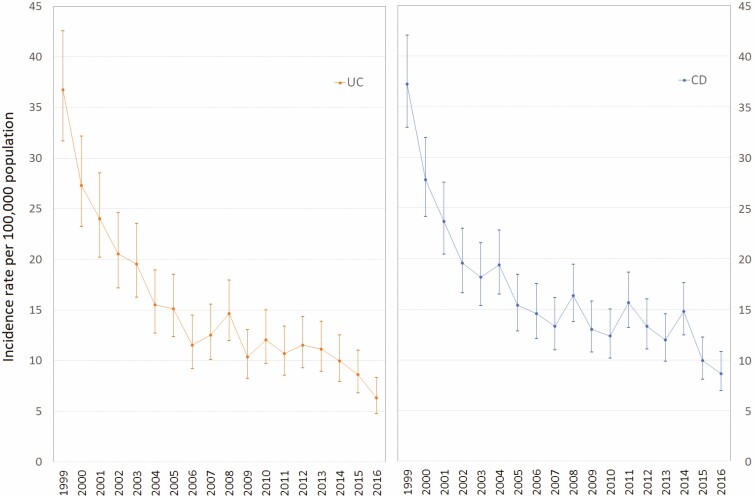
The observed number of individuals diagnosed with IBD decreased from 608 in 1999 to 141 in 2016; in other words, we recognized a reduction of about 76% in the number of incident cases during the study period. The overall annual average incidence rate of IBD decreased from 75 (95% CI 67 to 84) per 100,000 population in 1999 to 15 (95% CI 12 to 18) per 100,000 in 2016. This decrease was evident for both diagnosed UC and CD cases. The average incidence rate of diagnosed UC decreased from 36 (95% CI 31 to 42) to 6 (95% CI 4 to 8) per 100,000 population, and the incidence rate of diagnosed CD decreased from 37 (95% CI 32 to 42) to 8 (95% CI 6 to 10) per 100,000 people from 1999 to 2016 respectively.
An average annual decline of 6.9% (95% CI −7.6 to −6.2) in the incidence rate of diagnosed IBD was identified from 1999 to 2016 (Figure 1). Similarly, we observed a decrease of 7.7% (95% CI −8.6 to −6.8) and 6.0% (95% CI −6.8 to −5.2) in the incidence rate of diagnosed UC and CD (Figure 2).
Figure 1.
Adjusted annual incidence trends of inflammatory bowel disease in Saskatchewan, Canada.
Figure 2.
Adjusted annual incidence trends of ulcerative colitis (UC) and Crohn’s disease (CD) in Saskatchewan, Canada.
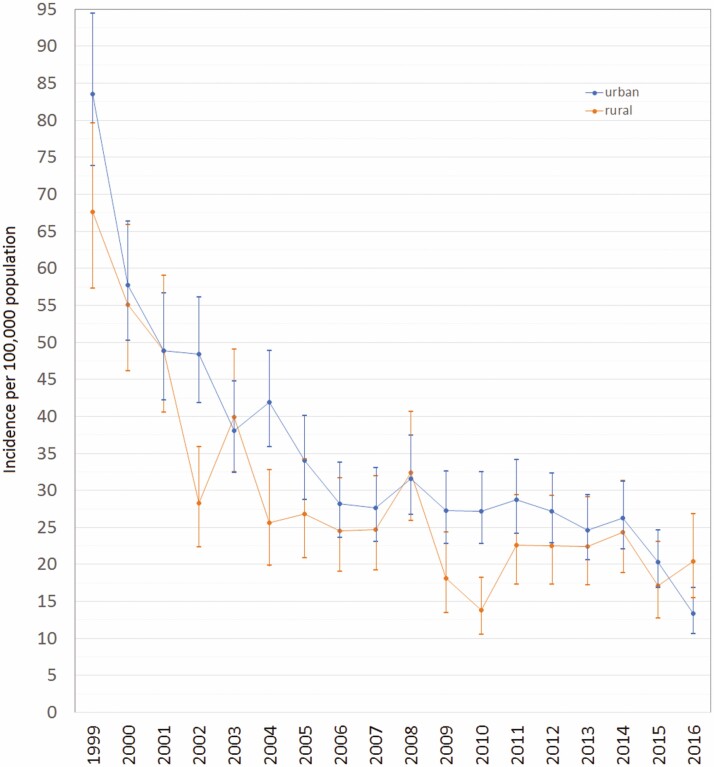
By rural/urban location of residence, an average annual decrease of 7.1% (95% CI −7.9 to −6.3) and 6.5% (95% CI −7.7 to −5.3) was observed, respectively, among urban and rural dweller (Figure 3). The interaction between year and location of residence was not statistically significant (P = 0.61).
Figure 3.
Adjusted annual incidence trends of inflammatory bowel disease by urban–rural location in Saskatchewan, Canada.
Incidence Rate Ratios
Urban Saskatchewan residents had a 19% higher risk (IRR = 1.19; 95% CI 1.11 to 1.27) of diagnosed IBD in comparison to those living in rural areas. Similarly, individuals living in urban areas had a 25% higher risk of diagnosed CD (IRR = 1.25; 95% CI 1.14 to 1.36) than those in rural settings; however, this association was not significant among those with diagnosed UC (IRR = 1.08, 95% CI 0.97 to 1.19).
Additionally, males had a lower risk of diagnosed IBD (IRR = 0.89, 95% CI 0.84 to 0.96) than females. This difference was observed in the diagnosed CD group (IRR = 0.77, 95% CI 0.71 to 0.83), but not statistically significant in the diagnosed UC group (IRR = 1.02, 95% CI 0.93 to 1.12). Similarly, a lower risk of diagnosed IBD was observed among those aged 60+ (IRR = 0.72, 95% CI 0.65 to 0.80) in comparison to those in the18–29 age group; this finding was also significant by type of disease (UC IRR = 0.77 [95% CI 0.66 to 0.89] and CD IRR = 0.69 [95% CI 0.61 to 0.78]; Table 2).
Table 2.
Incidence rate ratios with corresponding 95% confidence intervals (95% CIs) for inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn’s disease (CD) for demographic characteristics
| IBD | UC | CD | |
|---|---|---|---|
| Sex | |||
| Female | Ref. | Ref. | Ref. |
| Male | 0.89 (0.84–0.96) | 1.02 (0.93–1.12) | 0.77 (0.71–0.83) |
| Age group | |||
| 18–29 | Ref. | Ref. | Ref. |
| 30–39 | 1.02 (0.92–1.13) | 1.07 (0.92–1.24) | 1.00 (0.88–1.13) |
| 40–49 | 1.08 (0.97–1.19) | 1.19 (1.03–1.38) | 1.01 (0.90–1.15) |
| 50–59 | 1.02 (0.92–1.14) | 1.05 (0.90–1.22) | 0.99 (0.87–1.12) |
| 60+ | 0.72 (0.65–0.80) | 0.77 (0.66–0.89) | 0.69 (0.61–0.78) |
| Place of residence | |||
| Rural | Ref. | Ref | Ref. |
| Urban | 1.19 (1.11–1.27) | 1.08 (0.97–1.19) | 1.25 (1.14–1.36) |
Statistically significant values at alpha = 0.05 are bolded.
Discussion
We conducted a population-based cohort study using administrative health databases to estimate the incidence of diagnosed IBD in the province of Saskatchewan. In Canada, the highest and lowest incidence rates of IBD were previously reported, respectively in Nova Scotia (45.7/100,000 population) and British Columbia (18.7/100,000 population) (17,19). Our study contributes to these data with a significantly decreasing rate of IBD incidence cases in the province of Saskatchewan.
Similar decreasing IBD incidence rates have also been reported in some Canadian provinces. For instance, researchers in Québec observed between 2001 and 2008 a decrease in the rates of both CD (from 18.1 to 16.8/100,000 person-years) and UC (from 12.5 to 9.8/100,000 person-years) (32). A decrease in the incidence of IBD was also reported in Manitoba between 1990 and 2013 (15), while data from Alberta showed a stable trend in IBD rates between 2010 and 2015 (33). A recent retrospective population-based cohort study from Ontario observed an increasing incidence trend in the past 9 years for ages 30 to 60, and a stable trend in other age groups (18). Clearly, these studies indicate variations in IBD rates across Canada which may be attributed to regional differences in the incidence of the disease and potentially to the discrepancies in the algorithms used to capture the cases of UC and CD. A population-based national study estimating IBD rates using homogenous case definition and methodology, like the model-base estimation used in this provincial study, could provide a clearer picture of the actual trends of the disease across provinces and in Canada as a whole.
Nevertheless, we observed a noticeable decline in the incidence of IBD among adults in Saskatchewan, a pattern previously described in Nova Scotia (17), Quebec (32) and Manitoba (15). The complementing trend of increasing incidence rates of IBD among children has been identified in Canada (18,34,35) and other countries (36,37). This could be one reason behind the observed decline, relative to that of the children, in the incidence rates of CD and UC among adults in Saskatchewan and other Canadian provinces. In other words, the declining incidence trends of IBD among adults might reflect that the disease is starting to be diagnosed in the early stages of life. As presented by Coward et al. (33), the incidence rates of IBD appear to be stable over time in Alberta when considering all age groups (33); although, decreasing incidence rates of UC and CD could be observed in the adult population when stratifying the trends by age groups (33). Thus, the decision to include or exclude individuals under 18 years in epidemiologic studies about IBD could have an impact on the observed trends. Furthermore, in our study, the declining trend in the incidence rates of IBD was consistently observed across age groups; although, two age groups (30 to 39 and 40 to 49 years) had steeper decreasing trends than those observed in the younger and older age groups. Future provincial and national studies could explore incidence rate trends by age groups, including individuals under 18 years and considering birth cohort study designs.
Turning to global epidemiological studies, a 2015 review on the incidence of IBD in Europe reported a rise in the disease. Specifically, an increase in the incidence rate of UC was observed from 6.0 to 9.8/100,000 person-years from 1962 to 2010, while CD increased from 1.0 to 6.3/100,000 person-years for the same period (38,39). Data from South America have shown a similar pattern. In 2004, Appleyard et al. (40) reported a rise in IBD incidence rates for Puerto Rico from 3.07 to 7.74/100,000 population from 1996 to 2000. In Brazil, researchers also observed a hike in CD rates over a period of 27 years from 0.08 to 0.68/100,000 person-years to 5.5/100,000 (41). Conversely, a 2017 systematic review in the United States by Ng et al. (2) reported the lowest incidence estimates for CD in California (6.3/100,000 person-years) and for UC in Olmsted County (8.8/100,000 person-years). Not surprisingly, these studies also revealed that the incidence rate of IBD varies across the globe. Some researchers have attributed these variations to environmental risk factors and rapid socio-economic development in certain locations (42–44).
Overall, our population-based study showed a higher risk of developing IBD for people living in urban places in Saskatchewan compared to their rural counterparts. Specifically, urban dwellers have a 25% higher risk of CD onset compared to their rural counterparts. Population-based studies conducted in other Canadian provinces have shown similar patterns. Manitoba, for example, reported a 23% lower risk for rural residents, while Alberta showed a 13% higher rate for CD and a 44% higher rate for UC in urban populations (19,20). A 2017 study conducted by Benchimol et al. (20) in four Canadian provinces reported a 10% lower risk of IBD for people living in rural areas in comparison to those in urban areas.
Our results support evidence from studies conducted around the world assessing urban−rural differences in the incidence rates of IBD. Between 1935 and 1975, the incidence rate of CD in Minnesota, United States, was found to be higher in people located in urban locations than in rural areas (45). Between 1986 and 1987, rates for both CD and UC were higher in northern parts of the United States than in southern ones, as well as in urban than in rural areas (46). Moreover, European studies reported a similar pattern. For instance, in Poland, a higher incidence rate of IBD was found among urban people compared to their rural counterparts (47). Spanish data from 1981 to 1988 also revealed a higher CD rate in the cities, namely 1.87/100,000 compared with a rate of 0.86/100,000 for rural residents (48). Victoria et al. (8) also observed the same pattern in the Midwest of São Paulo State, Brazil. A recent systematic review that included more than 40 countries reported higher IRRs of IBD for urban locations (21). Suggested explanations of this difference include diet variations, vitamin D exposure, air pollution and physical inactivity (20,21). In addition, in a 2006 population-based Canadian study, Bernstein et al. (19) underlined access to care as a potential by-product of this rural–urban differences. Further research in this field should aim to explain the increased risk of CD in urban Saskatchewan areas.
The strengths of the study include the sample size (n = 4,908 diagnosed IBD incidence cases), with 18-year study period. These study characteristics made it possible to assess the trends in the incidence of the disease over time. Also, our model-based approach enabled us to control our estimates and analyses by rural/urban differences. However, we acknowledge the limitations of using administrative health databases, including the presence of potential misclassification bias in assessing disease surveillance for individuals with chronic diseases (49). To address this, patients with diagnosed IBD were identified utilizing a validated algorithm that captured patients only if they had physician claims or hospitalizations related to ICD-9 or ICD-10 diagnosed codes of IBD. This reduced the effect of misclassifying non-diagnosed IBD patients as diagnosed. Second, since IBD is a disease with relapse and remission of symptoms, there could be difficulties in differentiating incident from prevalent cases. Prevalent cases are more likely to be misclassified in earlier years where washout periods are shorter, which could result in a declining trend in incidence over time (50). To reduce this effect, a washout period of 8 years was incorporated in the study to avoid the overestimation of incidence cases.
Health administrative databases contain rich sources of information that are suitable for population-based epidemiological studies (51,52). Given that the quality of the Saskatchewan health administrative data has evolved in the last few decades with data holdings for 2001 onwards ascertained to have better quality compared to previous years, as a sensitivity analysis, we conducted our statistical analyses with data from 2001 to 2016 fiscal years only. The results of this analysis did not differ significantly from our initially estimated incidence rates, trend tests, and IRR.
In conclusion, our study provides up-to-date epidemiological data on the incidence as well as rural/urban differences of IBD on the province of Saskatchewan. The evidence from this population-based study confirmed that the incidence rates of both CD and UC among adults are declining in the province. Urban Saskatchewan dwellers were described to be at higher risk of IBD than rural residents. A comprehensive understanding of the burden of IBD on the province of Saskatchewan, in terms of its prevalence and cost, could help with resource allocation and planning, and should be the focus of future studies. We emphasize that health care providers and decision-makers plan IBD-specific health care programs taking into account the presented evidence.
What is already known on this topic?
Previous epidemiological studies in developed countries have reported decreasing, plateauing or increasing incidence rates of IBD.
Urban dwellers are more likely to develop IBD compared to rural residents.
What this study adds?
Urban dwellers in Saskatchewan, Canada, have a 25% higher risk of developing CD than rural dwellers.
In 2016, the incidence rate of IBD in Saskatchewan appears to be one of the lowest reported across Canadian provinces.
Acknowledgments
The authors would like to thank the College of Medicine (University of Saskatchewan) and the Canadian Institutes of Health Research (CIHR) Project Grant for funding this study. In addition, the authors thank the staff of the Saskatchewan Health Quality Council, specially Meric Osman, Jacqueline Quail, Nirmal S. Sidhu and Wenbin Li, who helped in the development of this study. GGK is a CIHR Embedded Clinician Research Chair. This study is based in part on de-identified data provided by the Saskatchewan Ministry of Health and eHealth Saskatchewan. The interpretation and conclusions contained herein do not necessarily represent those of the Government of Saskatchewan, the Saskatchewan Ministry of Health or eHealth Saskatchewan. This study is part of a master’s thesis.
Conflicts of Interest: The authors disclose no conflicts of interest.
Author Contributions: JAO initiated and designed the study, performed data analysis, drafted the manuscript and critically revised the study for important intellectual content. JNP-S, SF and LML designed the study, drafted the manuscript and critically revised the study for important intellectual content. NM and GGK drafted the manuscript and critically revised the study for important intellectual content. All authors contributed to data interpretation and approval of the final manuscript.
Funding
This work was supported by the College of Medicine Graduate Student Award - CoMGRAD (University of Saskatchewan) and the Canadian Institutes of Health Research (CIHR) Project Grant (funding reference number PJT-162393).
References
- 1. Benchimol EI, Bernstein CN, Bitton A, et al. The impact of inflammatory bowel disease in Canada 2018: A scientific report from the Canadian Gastro-Intestinal Epidemiology Consortium to Crohn’s and Colitis Canada. J Can Assoc Gastroenterol 2019;2(Suppl. 1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018;390(10114):2769–78. [DOI] [PubMed] [Google Scholar]
- 3. Burisch J, Jess T, Martinato M, et al. ; ECCO -EpiCom . The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7(4):322–37. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan GG. The global burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12(12):720–7. [DOI] [PubMed] [Google Scholar]
- 5. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology 2013;145(1):158–65.e2. [DOI] [PubMed] [Google Scholar]
- 6. Sood A, Midha V, Sood N, et al. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut 2003;52(11):1587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tozun N, Atug O, Imeryuz N, et al. ; Members of the Turkish IBD Study Group . Clinical characteristics of inflammatory bowel disease in Turkey: A multicenter epidemiologic survey. J Clin Gastroenterol 2009;43(1):51–7. [DOI] [PubMed] [Google Scholar]
- 8. Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol 2009;46(1):20–5. [DOI] [PubMed] [Google Scholar]
- 9. Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: A comparison of Eastern and Western perspectives. World J Gastroenterol 2014;20(33):11525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng SC. Emerging leadership lecture: Inflammatory bowel disease in Asia: Emergence of a “Western” disease. J Gastroenterol Hepatol 2015;30(3):440–5. [DOI] [PubMed] [Google Scholar]
- 11. King JA, Underwood FE, Panaccione N, et al. Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: A population-based study of countries in the Organisation for Economic Co-operation and Development. Lancet Gastroenterol Hepatol 2019;4(4):287–95. [DOI] [PubMed] [Google Scholar]
- 12. Zheng JJ, Zhu XS, Huangfu Z, et al. Crohn’s disease in mainland China: A systematic analysis of 50 years of research. Chin J Dig Dis 2005;6(4):175–81. [DOI] [PubMed] [Google Scholar]
- 13. Thia KT, Loftus EV Jr, Sandborn WJ, et al. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 2008;103(12):3167–82. [DOI] [PubMed] [Google Scholar]
- 14. Foster A, Jacobson K. Changing incidence of inflammatory bowel disease: Environmental influences and lessons learnt from the South Asian population. Front Pediatr 2013;1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan GG, Bernstein CN, Coward S, et al. The impact of inflammatory bowel disease in Canada 2018: Epidemiology. J Can Assoc Gastroenterol 2019;2(Suppl. 1):S6–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019;156(5):1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 17. Leddin D, Tamim H, Levy AR. Decreasing incidence of inflammatory bowel disease in Eastern Canada: A population database study. BMC Gastroenterol 2014;14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: A population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20(10):1761–9. [DOI] [PubMed] [Google Scholar]
- 19. Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am J Gastroenterol 2006;101(7):1559–68. [DOI] [PubMed] [Google Scholar]
- 20. Benchimol EI, Kaplan GG, Otley AR, et al. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: A population-based inception and birth cohort study. Am J Gastroenterol 2017;112(9):1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soon IS, Molodecky NA, Rabi DM, et al. The relationship between urban environment and the inflammatory bowel diseases: A systematic review and meta-analysis. BMC Gastroenterol 2012;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson M, Revie CW, Quail JM, et al. The effect of socio-demographic factors on mental health and addiction high-cost use: A retrospective, population-based study in Saskatchewan. Can J Public Health 2018;109(5-6):810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saskatchewan Bureau of Statistics. Demography and Census Reports and Statistics. 2016 Census Reports. <https://www.saskatchewan.ca/government/government-data/bureau-of-statistics/population-and-census> (Accessed March 10, 2020).
- 24. Marchildon GP. Canada health system review. Health Syst Transition 2013;15:1 – 179. [PubMed] [Google Scholar]
- 25. Peña-Sánchez JN, Lix LM, Teare GF, et al. Impact of an integrated model of care on outcomes of patients with inflammatory bowel diseases: Evidence from a population-based study. J Crohns Colitis 2017;11(12):1471–9. [DOI] [PubMed] [Google Scholar]
- 26. Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: A population-based study. Am J Epidemiol 1999;149(10):916–24. [DOI] [PubMed] [Google Scholar]
- 27. Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol 2014;67(8):887–96. [DOI] [PubMed] [Google Scholar]
- 28. Targownik LE, Benchimol EI, Witt J, et al. The effect of initiation of anti-TNF therapy on the subsequent direct health care costs of inflammatory bowel disease. Inflamm Bowel Dis 2019;25(10):1718–28. [DOI] [PubMed] [Google Scholar]
- 29. Du Plessis V, Beshiri R, Bollman RD, Clemenson H. Definitions of Rural. Rural and Small Town Canada Analysis Bulletin, Vol. 3, no. 3. Ottawa: Statistics Canada, 2001. <https://www150.statcan.gc.ca/n1/en/pub/21-006-x/21-006-x2001003-eng.pdf?st=KPt9S4Us> (Accessed March 10, 2020). [Google Scholar]
- 30. McCullagh P, Nelder JA. Generalized Linear Models, 2nd edn. London: Chapman and Hall, 1989. [Google Scholar]
- 31. Lix LM, Hobson DE, Azimaee M, et al. Socioeconomic variations in the prevalence and incidence of Parkinson’s disease: A population-based analysis. J Epidemiol Community Health 2010;64(4):335–40. [DOI] [PubMed] [Google Scholar]
- 32. Bitton A, Vutcovici M, Patenaude V, et al. Epidemiology of inflammatory bowel disease in Québec: Recent trends. Inflamm Bowel Dis 2014;20(10):1770–6. [DOI] [PubMed] [Google Scholar]
- 33. Coward S, Benchimol E, Clement F, et al. P104 the incidence of inflammatory bowel disease: Analyzing historical trends to predict the future. Inflamm Bowel Dis 2019;25(Suppl. 1):S50–1. [Google Scholar]
- 34. Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: Evidence from health administrative data. Gut 2009;58(11):1490–7. [DOI] [PubMed] [Google Scholar]
- 35. El-Matary W, Moroz SP, Bernstein CN. Inflammatory bowel disease in children of Manitoba: 30 years’ experience of a tertiary center. J Pediatr Gastroenterol Nutr 2014;59(6):763–6. [DOI] [PubMed] [Google Scholar]
- 36. Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm Bowel Dis 2011;17(1):423–39. [DOI] [PubMed] [Google Scholar]
- 37. Sýkora J, Pomahačová R, Kreslová M, et al. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol 2018;24(25):2741–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaaro Benallal D, Guerra Veloz MF, Argüelles-Arias F, et al. Evolution of the incidence of inflammatory bowel disease in Southern Spain. Rev Esp Enferm Dig 2017;109(11):757–60. [DOI] [PubMed] [Google Scholar]
- 39. Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol 2015;50(8):942–51. [DOI] [PubMed] [Google Scholar]
- 40. Appleyard CB, Hernández G, Rios-Bedoya CF. Basic epidemiology of inflammatory bowel disease in Puerto Rico. Inflamm Bowel Dis 2004;10(2):106–11. [DOI] [PubMed] [Google Scholar]
- 41. Kotze PG, Underwood FE, Damião AOMC, et al. Progression of inflammatory bowel diseases throughout Latin America and the Caribbean: A systematic review. Clin Gastroenterol Hepatol 2020;18(2):304–12. [DOI] [PubMed] [Google Scholar]
- 42. Ng SC, Bernstein CN, Vatn MH, et al. ; Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD) . Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013;62(4):630–49. [DOI] [PubMed] [Google Scholar]
- 43. Buenavida G, Casañias A, Vásquez C, et al. ; National Inflammatory Bowel Disease Registry . Incidence of inflammatory bowel disease in five geographical areas of Uruguay in the biennial 2007-2008. Acta Gastroenterol Latinoam 2011;41(4):281–7. [PubMed] [Google Scholar]
- 44. Lakatos L, Lakatos PL. Is the incidence and prevalence of inflammatory bowel diseases increasing in Eastern Europe? Postgrad Med J 2006;82(967):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sedlack RE, Whisnant J, Elveback LR, et al. Incidence of Crohn’s disease in Olmsted County, Minnesota, 1935-1975. Am J Epidemiol 1980;112(6):759–63. [DOI] [PubMed] [Google Scholar]
- 46. Sonnenberg A, McCarty DJ, Jacobsen SJ. Geographic variation of inflammatory bowel disease within the United States. Gastroenterology 1991;100(1):143–9. [DOI] [PubMed] [Google Scholar]
- 47. Wiercinska-Drapalo A, Jaroszewicz J, Flisiak R, et al. Epidemiological characteristics of inflammatory bowel disease in North-Eastern Poland. World J Gastroenterol 2005;11(17):2630–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maté-Jimenez J, Muñoz S, Vicent D, et al. Incidence and prevalence of ulcerative colitis and Crohn’s disease in urban and rural areas of Spain from 1981 to 1988. J Clin Gastroenterol 1994;18(1):27–31. [DOI] [PubMed] [Google Scholar]
- 49. Manuel DG, Rosella LC, Stukel TA. Importance of accurately identifying disease in studies using electronic health records. Br Med J 2010;341:c4226. [DOI] [PubMed] [Google Scholar]
- 50. Kaplan GG. Pitfalls and perils of using administrative databases to evaluate the incidence of inflammatory bowel disease overtime. Inflamm Bowel Dis 2014;20(10):1777–9. [DOI] [PubMed] [Google Scholar]
- 51. Rezaie A, Quan H, Fedorak RN, et al. Development and validation of an administrative case definition for inflammatory bowel diseases. Can J Gastroenterol 2012;26(10):711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cadarette SM, Wong L. An introduction to health care administrative data. Can J Hosp Pharm 2015;68(3):232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]