Abstract
Neuronal aggregates of misfolded alpha-synuclein protein are found in the brain and periphery of patients with Parkinson’s disease. Braak and colleagues have hypothesized that the initial formation of misfolded alpha-synuclein may start in the gut, and then spread to the brain via peripheral autonomic nerves hereby affecting several organs, including the heart and intestine. Age is considered the greatest risk factor for Parkinson’s disease, but the effect of age on the formation of pathology and its propagation has not been studied in detail. We aimed to investigate whether propagation of alpha-synuclein pathology from the gut to the brain is more efficient in old versus young wild-type rats, upon gastrointestinal injection of aggregated alpha-synuclein. Our results demonstrate a robust age-dependent gut-to-brain and brain-to-gut spread of alpha-synuclein pathology along the sympathetic and parasympathetic nerves, resulting in age-dependent dysfunction of the heart and stomach, as observed in patients with Parkinson’s disease. Moreover, alpha-synuclein pathology is more densely packed and resistant to enzymatic digestion in old rats, indicating an age-dependent maturation of alpha-synuclein aggregates. Our study is the first to provide a detailed investigation of alpha-synuclein pathology in several organs within one animal model, including the brain, skin, heart, intestine, spinal cord and autonomic ganglia. Taken together, our findings suggest that age is a crucial factor for alpha-synuclein aggregation and complete propagation to heart, stomach and skin, similar to patients. Given that age is the greatest risk factor for human Parkinson’s disease, it seems likely that older experimental animals will yield the most relevant and reliable findings. These results have important implications for future research to optimize diagnostics and therapeutics in Parkinson’s disease and other age-associated synucleinopathies. Increased emphasis should be placed on using aged animals in preclinical studies and to elucidate the nature of age-dependent interactions.
Keywords: alpha-synuclein, Parkinson’s disease, ageing, autonomic nervous system, Fischer 344 rat
Van DB et al. show that ageing facilitates the propagation of alpha-synuclein pathology from gut to brain in rats. In aged rats, alpha-synuclein pathology is also more resistant to enzymatic digestion, suggesting that older animals may provide superior models of neurodegenerative diseases such as Parkinson's disease.
Introduction
Neurodegeneration in Parkinson’s disease is associated with the presence of pathological aggregates of misfolded alpha-synuclein (α-syn) protein, the most important constituent of Lewy bodies and neurites. Up to 20 years before diagnosis, pathological α-syn inclusions are found not only in the brain, but also in several peripheral organs,1,2 including the gastrointestinal tract,3,4 heart5 and skin.6 Thus, non-motor autonomic symptoms such as constipation and orthostatic hypotension are common during prodromal Parkinson’s disease.7–9 Misfolded α-syn can transmit from cell-to-cell within the CNS, as well as from the peripheral nervous system (PNS) to the CNS.10 Braak and colleagues11 hypothesized that α-syn pathology may originate in the enteric nervous system (ENS) and spread to the brain via autonomic nerves. In support, truncal vagotomy in humans reduces the risk of Parkinson’s disease by 40–50% after 10–20 years of follow-up,12,13 and the autonomic PNS is known to degenerate years in advance of the nigrostriatal dopamine system in some patients.14
Worldwide, 96% of patients with Parkinson’s disease are above 50 years of age at the time of diagnosis, and Parkinson’s disease affects more than 4% of the population aged 85 years or older.15 Therefore, age is considered the greatest risk factor for Parkinson’s disease.16 Despite these facts, the propagation of misfolded α-syn has almost exclusively been investigated in young transgenic or wild-type animals. Misfolded α-syn protein, i.e. pathogenic seeds, is usually injected in the form of artificial preformed fibrils (PFFs) or Parkinson’s disease brain lysate, and α-syn pathology has been shown to propagate both retrogradely and anterogradely along the vagus nerve.17–19 In young transgenic animals, injection of α-syn seeds into the gastrointestinal tract leads to robust propagating pathology to the CNS.20–22 However, such transgenic animals have artificially increased susceptibility towards α-syn aggregation, which is probably not an accurate reflection of idiopathic Parkinson’s disease. In contrast, studies that used young wild-type animals generally failed to observe persistent PNS-to-CNS spreading of pathology.23,24
It is believed that initiation of α-syn pathology results from complex genetic–environmental interactions with ageing as the main risk factor.25 Ageing affects homeostatic processes that protect against protein misfolding and is associated with an increase in oxidative stress, neuroinflammation and mitochondrial-lysosomal dysfunction. The increased susceptibility to neurodegeneration caused by age-related cellular changes, together with failing compensatory clearance mechanisms contribute to the enhanced risk of Parkinson’s disease pathogenesis in older subjects.26 Thus, it is conceivable that intact proteostatic mechanisms may be preventing progression of α-syn pathology in young wild-type animals, including humans. Surprisingly, the role of ageing with respect to α-syn propagation and consequent symptom development in animal models of Parkinson’s disease has received almost no attention.
The aim of this study was to investigate the effects of age on α-syn aggregation and the efficiency of α-syn pathology propagation from the gut to the autonomic PNS and brain, hereby providing an animal model that better emulates human idiopathic Parkinson’s disease. We injected PFFs in the upper gastrointestinal tract of young adult, late adult, and old wild-type Fischer 344 rats. We then investigated the presence of α-syn pathology along with signs of synaptic dysfunction in the CNS, and several peripheral organs and tissues, including the gut, heart, autonomic ganglia, skin, muscle and kidney.
Materials and methods
Animals
Young adult (n = 15; 3 months old), late adult (n = 11; 10–12 months old), and old (n = 2; 18 months old) wild-type Fischer 344 rats were used (purchased from Envigo or Janvier). Here, the term ‘young’ is used to refer to young adult rats, the term ‘adult’ is used to refer to late adult rats, and the term ‘aged’ is used to refer to the combined group of late adult and old rats.
Gastrointestinal seeding with α-synuclein fibrils
The experimental procedures involving animals were approved by The Danish Animal Experiments Inspectorate (license 2016-15-0201-01004) and followed the Danish and European animal experimentation legislations (directive 2010/63/EU). Housing conditions, surgery, post-operative care and health monitoring of the animals were previously described.22 Recombinant PFFs (5 µl; 2 µg/µl) or PBS were injected into the gut wall of the pylorus and duodenum at six different sites spaced ∼0.5 cm apart using a 10 µl Hamilton syringe (25-gauge needle). The allocation of animals to the PFF and PBS groups was randomized. Two PFF types were used: wild-type mouse α-syn and human α-syn S129A mutant (non-phosphorylatable at Ser129 residue). Generation and validation of human α-syn fibrils (hPFFs) was previously described.22 The present fibrils belonged to the same batch. Wild-type mouse α-syn fibrils (mPFFs) were generated and validated with identical methodology. Animals were sacrificed 10 or 20 weeks post-injection. The term ‘seeding’ refers to the injection of fibrillar α-syn ‘seeds’.
Whole-gut transit time
We measured whole-gut transit time in young and late adult rats 10 and 20 weeks post-injection by recording the time between oral gavage with non-absorbable red dye (600 µl carmine red 6% and methylcellulose in PBS, Sigma-Aldrich) and output of a red faecal pellet.27,28 After gavage, faecal pellets were monitored every 3 h during the first 12 h, and every 30 min after that for the presence of carmine red.
Faecal output
Young and late adult rats were fasted overnight and water was withdrawn 3 h before the experiment. After fasting, the rats were transferred to a cage without bedding and were allowed food and water for 6 h. Following conclusion of the test, faecal pellets were collected and dabbed with paper towels to absorb urine released during the test. Pellets were weighed immediately to determine the wet weight, after which they were dried in an oven for 4 h at 80°C to determine the dry weight and water weight per pellet. Finally, weights were normalized to body weight as determined right before the test. Additionally, food and water intake produced in that 6-h timeframe were measured and normalized to rat body weight.
Tissue collection and processing
At 10 and 20 weeks post-injection, the rats were sedated and perfused transcardially with PBS and phosphate-buffered 4% formaldehyde. The brain, spinal cord, cervical and coeliac ganglia, heart, stomach, pylorus, rectum, kidney, skin and muscle were sampled to study the route of α-syn pathology propagation through the autonomic connectome from gut to brain. The tissue was processed and cut as previously described.22 Sections (4-µm thick, 10-µm thick if skin) from several subjects (three to six subjects depending on section size) were randomized and mounted on one tissue slide. For the gut tissue, a control section was mounted on each slide to evaluate staining variability.
Immunohistochemistry
Tissue sections were deparaffinized and stained as described previously.22 To investigate solubility of the α-syn aggregates, the sections were pretreated with proteinase K (Ab64220/1:1000, Abcam). Supplementary Table 1 provides a list of the primary antibodies used in this study, including details about the supplier, concentration, and specificity. A pons and colon sample from two patients with reported Lewy pathology served as validation of our staining protocol against phosphorylated α-syn antibodies (Supplementary Fig. 1). Vesicular acetylcholine transporter (VAChT) and choline acetyltransferase (ChAT) were used to identify cholinergic innervation in the gut and dorsal motor nucleus of the vagus (DMV) respectively. Tyrosine hydroxylase (TH) was used for identification of noradrenergic innervation in the heart, skin and locus coeruleus. Protein gene protein 9.5 (PGP9.5) was used for identification of nerve fibres and piloerector muscle in skin.
Quantification assessment of immunoreactivity
Images were collected from 4-μm thick sections using the Olympus VS120 automated slide-scanning microscope. We performed three types of quantification depending on the tissue-type: (i) quantification of immunoreactive density in brain tissue; (ii) quantification of immunoreactive area for brainstem, gut and heart tissue; and (iii) semi-quantification based on morphology and location in renal tissue.
For quantification of immunoreactive density, optical density values were calculated using ImageJ as described previously.22 Quantification of immunoreactive area in the brainstem, gut and heart was based on the percentage of immunoreactivity above a certain threshold to the total area of the DMV or locus coeruleus, the gut wall in a cross-section of the pylorus and stomach, or myocardium, respectively. The threshold is a minimum grey scale value applied as a cut-off for inclusion in the immunoreactive area, and calculated by averaging the individual thresholds that best represent the immunoreactivity in a positive control section (from the same animal) on each slide, using the Olympus cellSens threshold tool.
Quantification of α-syn pathology in renal tissue was based on assessment of morphology and location of phosphorylated α-syn deposits. Signal intensity (i.e. α-syn deposit maturity) in the renal medulla and cortex was evaluated separately on a semi-quantitative scale from 0 to 5: 1 (sparse granular deposits), 3 (dense granular deposits), and 5 (Lewy body-like deposits). In case of ambiguity, an intermediate score of 2 or 4 was attributed. When a sample contained different types of deposits, the score represents the most mature deposit. Quantification was performed in a blinded fashion.
Statistical analyses
Two-way ANOVAs were used to interrogate group differences, with post hoc Tukey test to adjust for multiple comparisons among groups. Results are presented using box and whisker plots containing minimum, maximum, interquartile interval and median value, and P < 0.05 was considered statistically significant. Spearman’s correlation was used to test for relationships between cholinergic or noradrenergic innervation and α-syn pathology, in the stomach and the heart respectively.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Age-dependent α-synuclein pathology in the CNS
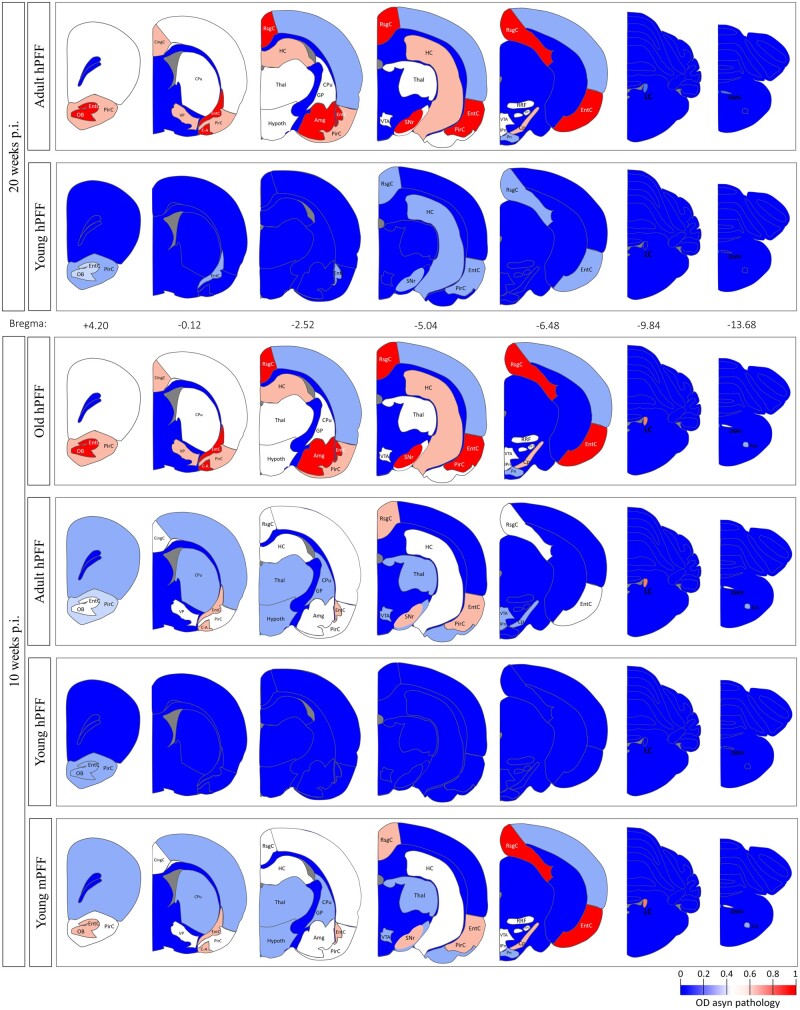
We injected groups of young (3 months old), adult (10–12 months old), and old rats (18 months old) intragastrically with either hPFFs, mPFFs or PBS and analysed subsequent appearance of α-syn pathology in their CNS at 10 and 20 weeks post-injection (Figs 1 and 2). Young rats did not display any well-defined CNS pathology. In contrast, adult and old rats showed deposits of phosphorylated α-syn throughout the brain at 10 weeks post injection The brain areas most heavily affected with α-syn pathology included the DMV, locus coeruleus, substantia nigra pars reticulata, amygdala, piriform cortex, entorhinal cortex, retrosplenial granular cortex, and olfactory bulb. However, pathology in the DMV and locus coeruleus was absent or minor at 20 weeks post-injection, suggesting that α-syn pathology in the lower brainstem is transient—at least in adult rats (Fig. 2B). Interestingly, we observed extensive α-syn pathology throughout the brains of young wild-type rats seeded with mPFFs but not hPFFs, indicating that mPFFs are more potent than hPFFs to induce initiation and propagation of α-syn pathology in wild-type rats. Additionally, our data indicate that wild-type Fischer 344 rats develop minor spontaneous age-dependent α-syn pathology in some brain areas but not in the lower brainstem. The spontaneous pathology in age-matched controls was significantly lower compared to seeded rats in all brain areas at 10 weeks post-injection and at all brain areas except the lower brainstem at 20 weeks post-injection (Figs 1, 2 and Supplementary Fig. 2).
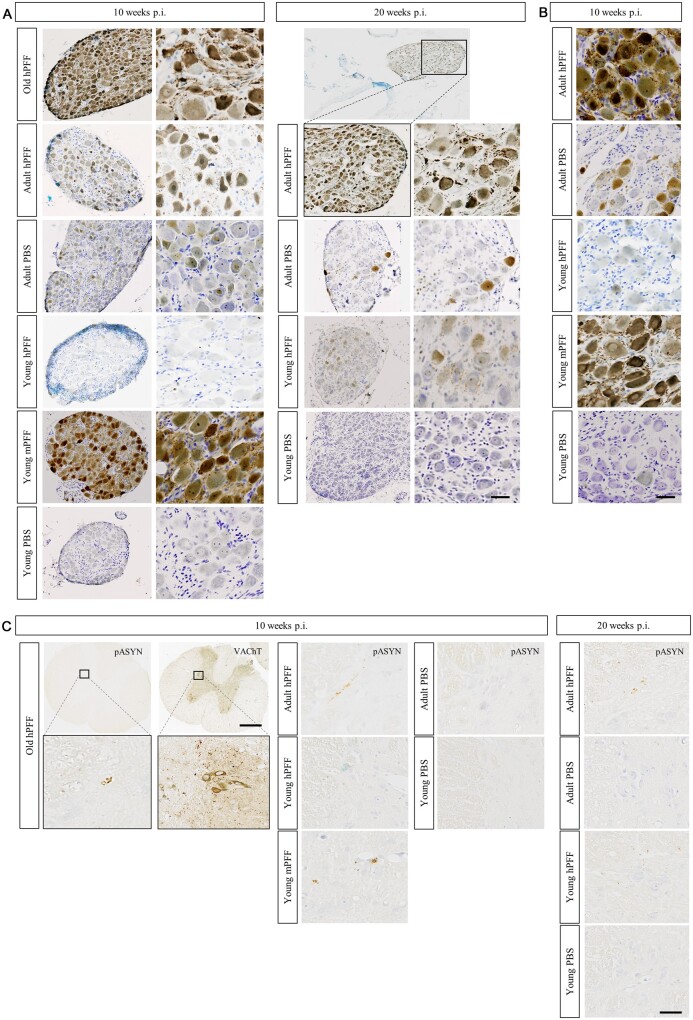
Figure 1.
Age-dependent whole-brain distribution of α-syn pathology in old (18 months), late adult (10–12 months) and young (3 months) rats seeded with α-syn aggregates in the gut. The Paxinos rat brain atlas is used for anatomical reference, bregma coordinates are in millimetres. The colour scale represents the optic density value of phosphorylated α-syn pathology in a certain brain area. Amg = Amygdala; C-A = cortical-amygdala transition area; CingC = cingulate cortex; Cp = cerebral peduncle; CPu = caudate putamen; EntC = entorhinal cortex; GP = globus pallidus; HC = hippocampus; Hypoth = hypothalamus; IPn = interpeduncular nuclei; LC = locus coeruleus; NA = nucleus ambiguus; OB = olfactory bulb; p.i. = post-injection; PirC = piriform cortex; Pn = pontine nuclei; PPT = pedunculopontine nucleus; RRF = retrorubral field; RsgC = retrosplenial granular cortex; SNr = substantia nigra pars reticulata; Thal = thalamus; VP = ventral pallidum; VTA = ventral tegmental area. See Supplementary Fig. 2 for data in control groups.
Figure 2.
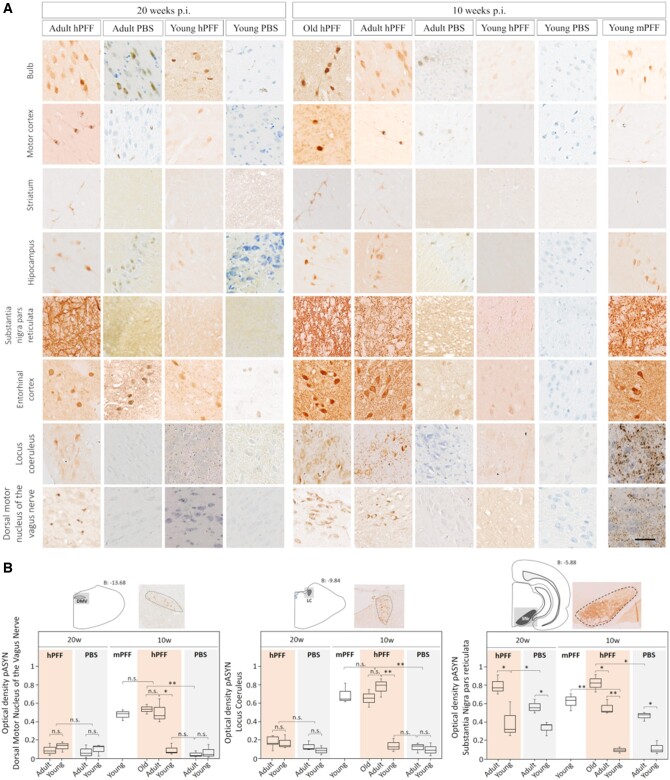
High magnification photomicrographs of age-dependent phosphorylated α-syn (pASYN, Ab51253) pathology in several brain areas of seeded and age-matched control rats. (A) The pathology is progressive and age-dependent in all brain areas, except in the brainstem where the pathology diminishes significantly at 20 weeks post-injection compared to 10 weeks post-injection. Scale bar = 50 µm. (B) Optical density measurements of pASYN pathology in the DMV, locus coeruleus and neuropil of the substantia nigra pars reticulata of rats injected with hPFF, mPFF or vehicle (PBS). *P < 0.05; **P < 0.01; n.s. = not significant. The regions of interest used for the analysis are indicated at the top of each graph.
Age-dependent α-synuclein pathology and neurodegeneration in the enteric nervous system
Old and adult rats seeded with hPFFs showed significantly more pathology at the injection site (pylorus) than seeded young rats and age-matched controls, indicating that the initiation and short-term persistence of misfolded α-syn formation is age-dependent (Figs 3B and 4A). Figure 3A shows the co-localization of α-syn pathology and cholinergic innervation (VAChT immunoreactivity) in the pylorus. VAChT immunoreactivity was similar across groups. In young rats the pathology at the injection site was much more severe after seeding with mPFFs compared to seeding with hPFFs, and resembled the pathology in old rats seeded with hPFFs (Figs 3B and 4A).
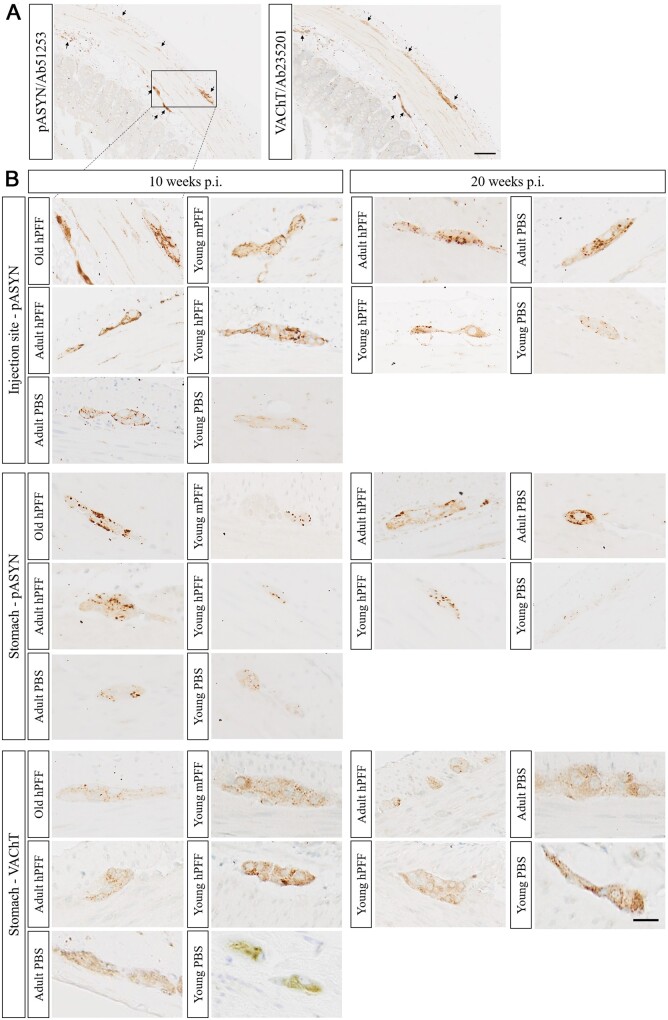
Figure 3.
Age-dependent enteric α-syn pathology and cholinergic denervation in seeded and age-matched control rats. (A) Representative micrograph of the distribution of phosphorylated α-syn (pASYN, left) and cholinergic innervation (VAChT, right) in neighbouring sections of the pylorus of an old rat at 10 weeks post-injection with hPFF. Clear co-localization was observed between staining for VAChT and pASYN. Scale bar = 100 µm. (B) High-magnification micrographs of α-syn pathology in the myenteric plexus at the injection site (top) or stomach (middle), and cholinergic innervation in the stomach (bottom) of young, adult and old rats at 10 or 20 weeks post-injection with hPFF or mPFF, and age-matched controls. Scale bar = 50 µm.
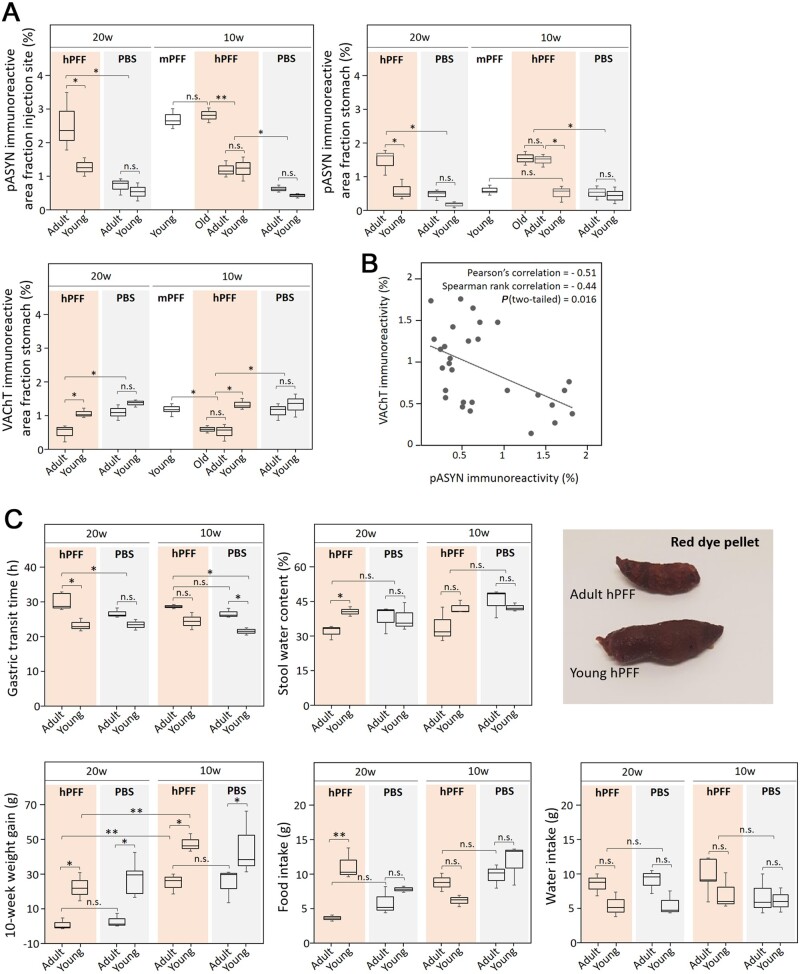
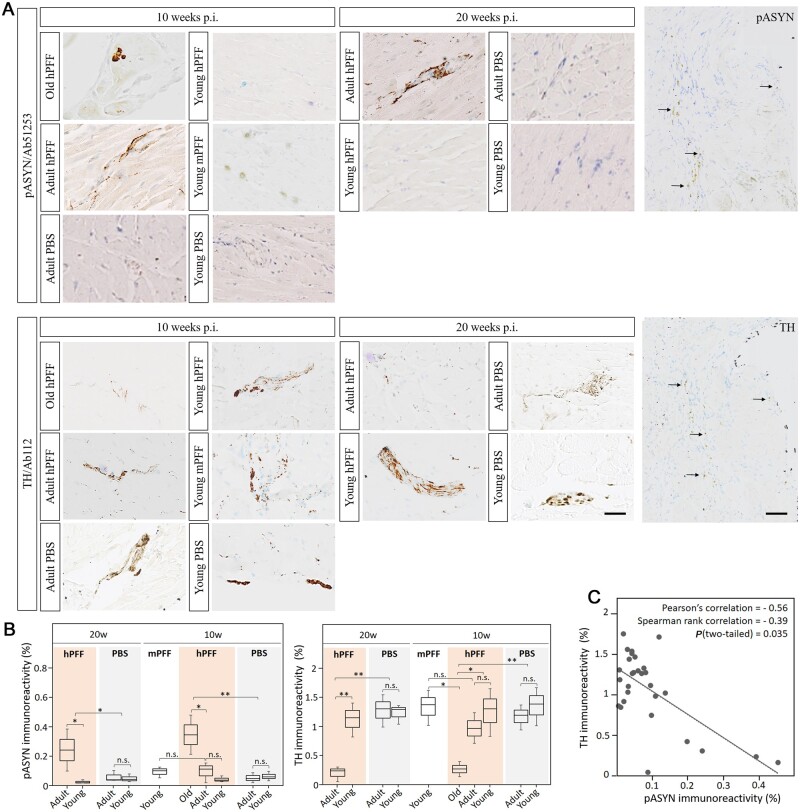
In the stomach wall, several centimetres from the injection site, we also observed significantly more phosphorylated α-syn pathology in old and adult compared to young hPFF-seeded rats and age-matched controls. However, in contrast to the pylorus, we did not observe increased levels of pathology in the stomach wall of young mPFF-seeded rats. Furthermore, VAChT immunoreactivity was decreased in the stomach wall in seeded adult and old rats at 10 and 20 weeks compared to seeded young and age-matched control rats (Figs 3B and 4A), supported by a negative correlation between VAChT and phosphorylated α-syn immunoreactivity in the stomach wall of seeded rats (Pearson’s ρ = −0.51; Fig. 4B). This apparent loss of cholinergic innervation might explain the delayed gastric emptying in adult rats at 20 weeks post-injection compared to young and age-matched control rats (Fig. 4C). A non-significant trend towards delayed gastric emptying was also observed at 10 weeks post-injection and in age-matched controls. Pellet size and stool water weight were also reduced in adult rats as opposed to young rats at 20 weeks post-injection. After overnight fasting, water intake remained similar across groups, but food intake was significantly reduced in adult rats at 20 weeks. Taken together, these results indicate an age-dependent anterograde brain-to-gut spread that is associated with cholinergic neurodegeneration and gut dysfunction.
Figure 4.
Quantification of age-dependent enteric α-syn pathology, cholinergic denervation and concomitant gut dysfunction in seeded and age-matched control rats. (A) Quantitative assessment of phosphorylated α-syn and cholinergic innervation in the intestinal wall of the pylorus and the stomach detected with immunohistochemistry. Adult and old PFF-injected rats showed more pathology than young PFF-injected rats, those adult and old PFF-injected rats also showed more pathology than the adult PBS-injected control rats at the injection site and stomach (P < 0.05). At the injection site, young rats seeded with mPFFs exhibit a similar level of pathology compared to old rats seeded with hPFFs. (B) Correlation plot of VAChT and pASYN immunoreactivity indicates a negative correlation between α-syn pathology and cholinergic innervation in the stomach. (C) Gastrointestinal assessment at 10 and 20 weeks post-injection with hPFF or PBS in young and late adult rats. Results on gastric transit time, stool water weight, food and water intake during 6 h post-fasting, and weight gain over a period of 10 weeks are shown and indicative of gastrointestinal dysfunction in aged rats. The gastric transit time of an orally administered red dye is increased and stool water weight and food intake are decreased in late adult rats compared to young rat at 20 weeks post injection The stool pellet of an aged rat is much smaller compared to a young rat at 20 weeks post-seeding. The gastric transit time in adult hPFF-injected rats is significantly longer than in adult PBS-injected rats at 20 weeks post-injection (P < 0.05). *P < 0.05; **P < 0.01; n.s. = not significant.
Finally, we observed slight spontaneous α-syn pathology in the pylorus and stomach of both young and adult control rats injected with PBS as depicted in Fig. 3B. The spontaneous pathology in controls was significantly lower than in age-matched seeded rats (Fig. 4B).
Age-dependent α-synuclein pathology and neurodegeneration in the sympathetic nervous system
After seeding with hPFFs, striking α-syn pathology was observed in coeliac ganglia and cervical ganglia of the sympathetic trunk in aged, but not in young rats (Fig. 5A and B). Remarkably, in the autonomic ganglia of young rats, no pathology was seen after seeding with hPFFs, but substantial pathology was seen after seeding with mPFFs, resembling the pathology in old and adult rats seeded with hPFFs.
Figure 5.
Age-dependent phosphorylated α-syn pathology (Ab51253) in the autonomic ganglia and spinal cord of seeded and age-matched control rats. High magnification micrographs of phosphorylated α-syn pathology in the coeliac ganglia (A) and cervical ganglia (B) at 10 and 20 weeks post injection (p.i.). Scale bar = 20 µm. α-Syn pathology is age-dependent and progressive. Aged rats seeded with hPFF show significantly more pathology than young and age-matched control rats. Young rats seeded with mPFF exhibit a similar level of pathology compared to old rats seeded with hPFF. (C) Co-localization of α-syn pathology and VAChT (Ab235201) staining in the intermediolateral nucleus of the spinal cord. α-Syn pathology is age-dependent and is absent in young rats seeded with hPFFs and age-matched controls, but not in young rats seeded with mPFFs. The VAChT distribution is similar across experimental groups. Scale bars = 50 µm and 500 µm.
Furthermore, we detected very localized pathology in the intermediolateral nucleus (IML) of aged, but not in young hPFF-seeded rats nor in age-matched controls (Fig. 5C). The pathology ranged from a dot-like pattern to single inclusion- or neurite-type assemblies. Phosphorylated α-syn deposits were also detected in the IML of young rats seeded with mPFFs; however, to a lesser extent compared to old hPFF-seeded rats.
In the heart, progressive α-syn pathology was detected in myocardial ganglia and sympathetic nerves of aged, but not in young hPFF-seeded rats nor in age-matched controls. As shown in Fig. 6, α-syn deposits co-localized with TH immunoreactivity. These results indicate age-dependent anterograde propagation from the cervical ganglia of the sympathetic trunk to the sympathetic ganglia of the myocardium. Moreover, quantitative assessment of cardiac α-syn and noradrenergic fibres showed a negative correlation (Pearson’s ρ = −0.56; Fig. 6C). Cardiac denervation was significant at 20 weeks post-injection in adult seeded rats (but not in young) compared to age-matched controls. We observed a trend towards cardiac denervation in adult rats at 10 weeks post-injection, and this trend was significant in old seeded rats.
Figure 6.
Age-dependent phosphorylated α-syn pathology (Ab51253) in the myocardium of seeded and age-matched control rats. (A) High magnification micrographs of α-syn pathology (pASYN, top) and tyrosine hydroxylase (TH, bottom) in myocardial ganglia of old, late adult and young rats at 10 and 20 weeks post-injection (p.i.) with hPFF or mPFF. α-Syn pathology seems to be age-dependent, progressive and co-localized with TH. Scale bars = 20 µm and 50 µm. (B) Quantitative assessment of phosphorylated α-syn (pASYN) and noradrenergic innervation (TH) in the heart detected with immunohistochemistry. Adult (at 20 weeks post-injection) and old (at 10 weeks post-injection) hPFF-injected rats show more pathology than young hPFF-injected and age-matched control rats in the heart (P < 0.05). Noradrenergic innervation is decreased in hPFF-seeded old rats (at 10 weeks post-injection) and late adult rats (at 20 weeks post-injection) (C) Correlation plot of TH and pASYN immunoreactivity indicates a negative correlation between α-syn pathology and noradrenergic innervation in the heart. (P < 0.05). *P < 0.05; **P < 0.01; n.s. = not significant.
Minor α-syn pathology was observed in the myocardium of young rats seeded with mPFFs. Of note, we detected low-level spontaneous α-syn pathology in autonomic ganglia of rats after PBS injection, whereas the IML and heart remained free of phosphorylated α-syn pathology (Fig. 6).
We then proceeded to investigate other relevant but understudied organs. We detected phosphorylated α-syn deposits in the skin, muscle, and kidney of adult rats seeded with hPFFs. In the skin, α-syn deposits were observed in sympathetic nerves, arterioles, arteries, and piloerector muscles of adult rats at 20 weeks, but not in young rats nor in age-matched controls (Supplementary Fig. 4A and B). A skin sample from a human patient with pure autonomic failure (PAF) served as validation of our protocol (PAF patients show copious amounts of α-syn pathology in the skin6). The immunoreactive patterns of α-syn pathology in arterioles and nerves were similar in the patient (Supplementary Fig. 4C) and our rat skin samples, although the level of pathology between rats was quite variable as indicated in Supplementary Fig. 4D. α-Syn pathology in muscle tissue was observed in one single adult rat seeded with hPFFs. In this rat, the pathology was more prominent in the thoracic spine muscle compared to the sciatic muscle (Supplementary Fig. 4E and F). In renal tissue, phosphorylated α-syn inclusions were detected in adult rats at 20 weeks with hPFFs, and co-localized with sympathetic nerves (Supplementary Fig. 5). Sparse α-syn deposits were detected in muscle and renal tissue of young wild-type rats seeded with mPFFs.
Neuropathological findings in young and aged hPFF-seeded rats are summarized in Fig. 7, which includes a schematic overview of the hypothesized trans-synaptic gut-to-brain propagation of α-syn pathology through the autonomic connectome, thereby suggesting a sequential involvement of different organs and structures. At stage 0, injected hPFFs can convert endogenous α-syn into misfolded α-syn in aged and young rats. The templating pathology propagates retrogradely along the parasympathetic pathway to the DMV (stage 1) in aged but not in young rats, and subsequent caudo-rostral propagation is seen in the brainstem with involvement of the locus coeruleus (stage 2) and substantia nigra pars reticulata (stage 3). In addition, α-syn pathology propagates through the sympathetic nervous system via the coeliac ganglia (stage 1) to the IML of the spinal cord and the sympathetic trunk including cervical ganglia (stage 2) in aged but not in young rats. From the sympathetic trunk, the pathology propagates anterogradely to sympathetic structures of the myocardium, skin, muscle, and kidney (stage 3). Moreover, pathology is detected in the myenteric ganglia of the stomach (stage 2) several centimetres from the injection site indicative of anterograde (brain-to-gut) propagation from the DMV or coeliac ganglia.
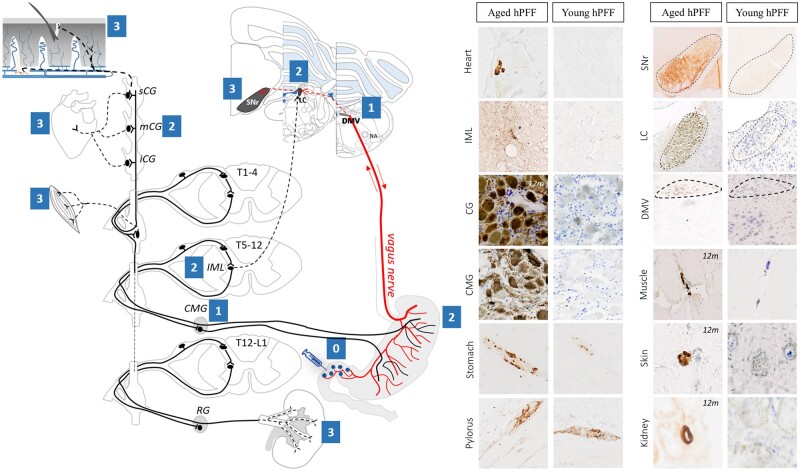
Figure 7.
Overview of hypothetical, sequential gut-to-brain propagation of α-syn pathology through the autonomic nervous system in aged Fischer 344 rats and neuropathological findings. Left: In the pylorus (stage 0), the injected α-syn fibrils induce the conversion of endogenous α-syn into α-syn pathology, which propagates retrogradely along the parasympathetic pathway to the DMV (stage 1). Subsequent caudo-rostral propagation is seen in the brainstem with involvement of the locus coeruleus (LC, stage 2) and the substantia nigra pars reticulata neuropil (SNr, stage 3). Furthermore, α-syn pathology propagates through the sympathetic nervous system via the coeliac ganglion (CMG, stage 1) to the IML of the spinal cord and sympathetic trunk including cervical ganglia (CG, stage 2), from where it spreads anterogradely to sympathetic structures of the myocardium (stage 3). In addition, pathology is detected in the myenteric ganglia of the stomach (stage 2) several centimetres from the injection site indicative of anterograde propagation from the DMV or CMG. Finally, pathology is detected in sympathetic nerves of several peripheral structures, including skin, muscle and kidney (stage 3). Right: Representative micrographs of α-syn pathology in several tissues of aged (12–18 months) and young rats upon seeding with human fibrils. There is no propagation of α-syn pathology beyond the pylorus (stage 0) in young rats seeded with human fibrils.
Mouse PFFs were considerably more potent than hPFFs with respect to initiating early (i.e. stage 0–2) gut-to-brain propagation in young wild-type rats. Strikingly, pathology was absent or minor in stage 3 structures such as the heart, skin, muscle and kidney, indicating that age is a crucial factor for complete propagation through the autonomic connectome. Further seeding studies in aged rats with mPFFs would be valuable to confirm our observations.
Age-dependent maturation of α-synuclein pathology
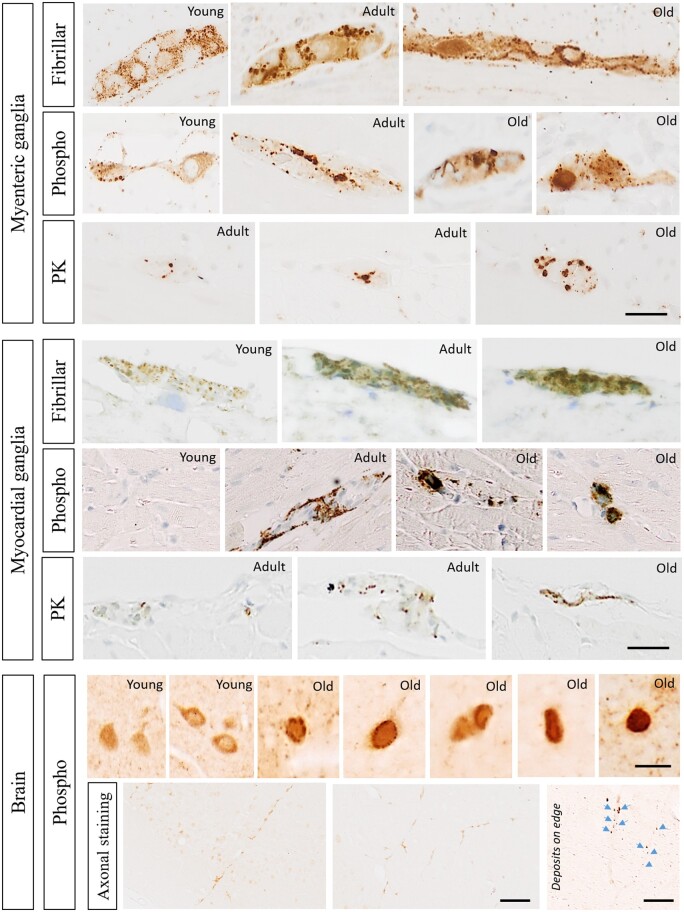
Figure 8 shows high magnification micrographs of α-syn pathology deposits in myenteric ganglia of the pylorus, myocardial ganglia and cortex of young, adult and old hPFF-seeded rats. In myenteric ganglia of young rats, α-syn immunoreactivity showed a solely granular appearance. The density and size of these granules appeared to increase with age, manifesting in Lewy-body like inclusions in older rats. Phosphorylated α-syn deposits were absent in the heart of young rats, and displayed a granular or neurite-like appearance in adult rats and larger inclusion bodies in old rats. Phosphorylated α-syn pathology throughout the brain exhibited mainly a diffuse appearance. In adult and old rats, occasional dense rings of deposits around cell nuclei were detected in addition to a more general diffuse immunoreactivity. Phosphorylated α-syn pathology was resistant against proteinase K in adult rats at 20 weeks (but not at 10 weeks) post-injection and also in old rats at 10 weeks post-injection, and showed a dot-like appearance in the gut and in the heart, but not in the brain.
Figure 8.
Age-dependent manifestation of enteric, cardiac and cortical α-syn aggregates. High-magnification micrographs of α-syn deposits in pyloric myenteric ganglia (top), myocardial ganglia (middle) and cortex (bottom) of young, adult (20 weeks post-injection) and old (10 weeks post-injection) rats seeded with human fibrils. Top: In myenteric ganglia of the pylorus and duodenum, aggregates detected with fibrillar α-syn antibody (Ab209538) or phosphorylated α-syn antibody (Ab51253) displayed a solely granular appearance in young subjects. The density and size of these granules increases with age, with detection of Lewy-body like inclusions in older subjects. Phosphorylated α-syn pathology (phospho) was resistant against pretreatment with proteinase K (PK) solely in adult rats at 20 weeks post-injection and old rats at 10 weeks post-injection, and had a dot-like appearance. Scale bar = 20 µm. Middle: In myocardial ganglia, fibrillary α-syn deposits displayed a similar granular appearance across all ages but increased in density and diameter with age. Phosphorylated α-syn deposits were absent in the heart of young rats, but showed a granular or neurite-like appearance in adult rats and inclusion-like appearance in old rats. Phosphorylated α-syn pathology was resistant against proteinase K pretreatment solely in adult rats at 20 weeks post-injection or old rats at 10 weeks post-injection, and had a dot-like appearance. In general, fibrillary α-syn immunoreactivity was stronger than phosphorylated α-syn immunoreactivity. Scale bar = 20 µm. Bottom: Phosphorylated α-syn pathology throughout the brain mainly showed a diffuse appearance (top row). In adult and old rats sporadic ring-like inclusions were detected surrounding the cell nucleus as well as extranuclear or extracellular (resembling Lewy pathology) in addition to diffuse immunoreactivity. Scale bar = 10 µm. In aged animals, prominent axonal staining was observed in the frontal cortex and striatum (bottom row). Scale bar = 10 µm (axonal stain detail) and 50 µm (axonal stain overview).
Staining against conformation-specific α-syn or fibrillar α-syn showed a higher signal in both myenteric and myocardial ganglia of all subjects compared to staining against phosphorylated α-syn, indicating that not all α-syn aggregates were phosphorylated.
Discussion
The key findings in this study are (i) age is a crucial factor for efficient stereotypic propagation of α-syn pathology along the gut-brain axis in wild-type hPPF-seeded rats; (ii) apparent vagal denervation in the stomach and sympathetic cardiac denervation are age-dependent and correlate with the amount of α-syn pathology; (iii) phosphorylated α-syn deposits are more dense and more proteinase K resistant in old animals; (iv) mPFFs are more potent than hPFFs to induce gut-to-brain propagation of α-syn pathology in young rats, which may reflect a species barrier; and (v) pathological findings in aged animals injected with hPFFs were similar or sometimes worse than those in young rats injected with mPFFs, suggesting that ageing lowers the species barrier.
Age-dependent CNS pathology
We and others have shown that upper gastrointestinal tract injections of α-syn pathology in rodents robustly induce stereotypical gut-to-brain propagation via peripheral nerves.22,29 Here we show that pathogenesis in the CNS appears to be heavily age-dependent as we observed phosphorylated α-syn pathology throughout the whole brain of adult and old wild-type rats, at 10 weeks post injection with hPFFs. In contrast, only minimal CNS involvement was seen in our young rats. Somewhat surprisingly, the α-syn pathology we observed in the DMV and locus coeruleus of adult rats appeared to be relatively transient, and showed markedly attenuated or absent staining intensity at 20 weeks post-injection with hPFFs, when compared to 10 weeks post-injection. This declining magnitude of α-syn pathology could not be ascribed to neuronal loss in the DMV and locus coeruleus, since the percentage immunoreactive area of noradrenergic and cholinergic markers remained similar across groups (Supplementary Fig. 3). A similar lack of persisting CNS pathology was noted in previous studies of young rodents and non-human primates following α-syn seeding in the gut.23,24 Notably, a recent study using mature monkeys showed no DMV pathology at 2 years post gastrointestinal seeding, supporting our finding of lacking α-syn pathology in the DMV in adult rats at later time points.30 In contrast to our findings, Kim et al.29 did observe progressive pathology in the brainstem upon seeding young wild-type mice with hPFFs up to 7 months post injection. Interestingly, they report a non-significant trend of decreasing pathology in the entire brainstem, but not in the rest of the brain, at 10 months compared to 7 months post-injection, indicating potential transient brainstem pathology at later time points. This observation in animal models is at odds with human data, since 90% of Parkinson’s disease patients have pathology in the DMV.11 It could be speculated that DMV pathology is reversible in some animals but permanent in most human patients. In more rostral brain structures, the pathology was clearly age-dependent and progressive in seeded rats.
One previous study investigated ageing effects on gut-to-brain transmission of α-syn pathology upon gut seeding,31 but the investigation of ageing effects was limited to the ENS with focus on disruptions of ENS network connectivity and the endoplasmic reticulum-Golgi-lysosome pathway. Furthermore, the detection of pathology was limited to the injection site and to the DMV with minor transient pathology. In contrast, we observed a striking trans-synaptic gut-to-brain propagation of α-syn pathology through the autonomic connectome, involving key structures known to be affected in human Parkinson’s disease, including the DMV, locus coeruleus and substantia nigra pars reticulata. In contrast to our findings, the cited study reported reduced levels of striatal dopamine in seeded aged mice but not young mice, which may suggest that age-related altered neurotransmission precedes α-syn aggregation.32,33
Furthermore, we detected localized pathology in the IML of adult and old, but not young rats seeded with hPFFs. This α-syn pathology in the IML of aged rats resembled the grain or dot-like pathology pattern detected in the IML of Parkinson’s disease patients older than 85 years old.34 Our results indicate a prominent age-dependent retrograde spread of α-syn pathology from the injection site to the coeliac ganglia and IML in wild-type rats seeded with hPFFs.
Taken together, our results indicate that old age is a crucial factor for effective gut-to-brain propagation of α-syn pathology in wild-type rats upon gut seeding. Age-matched control rats did show progressive levels of spontaneous α-syn pathology in the brain (except the brainstem), but considerably higher levels of α-syn pathology was seen in seeded rats. These results indicate that ageing itself may contribute to protein aggregation. Thus, an interplay of both the ageing and aggregation process may contribute to disease initiation and progression upon seeding. It is well-known that misfolded α-syn mimics the behaviour of prions, and the process of ageing has also been noted to increase vulnerability in the context of true prion disorders.35–37 It is conceivable that in Parkinson’s disease, the ageing process itself could also alter biochemical properties of physiological α-syn protein, making it more prone to convert into pathogenic misfolded α-syn. Besides biochemical changes to native α-syn protein, the ageing process also affects homeostatic processes that protect against protein misfolding, including mitochondrial-lysosomal dysfunction, increased oxidative stress and altered calcium homeostasis.26,38 Our data are in agreement with ageing studies in prion disease research, and we speculate that the combination of both age-related cellular changes and failing compensatory clearance mechanisms contribute to the enhanced risk of α-syn pathology progression in older subjects.
Age-dependent enteric nervous system pathology
While considerably higher levels of α-syn pathology were observed in seeded rats compared to age-matched controls, this particular rat strain does develop slight spontaneous gastrointestinal pathology with increasing age.39,40 We detected significantly more pathology in the stomach (away from the injection site) of adult and old seeded rats, which suggests anterograde DMV-to-gut or coeliac ganglion-to-gut propagation after initial retrograde spreading. Of note, bidirectional propagation of misfolded α-syn through the vagus has previously been documented.18,19,22 Bidirectional propagation through the vagus has important implications for the understanding of Parkinson’s disease and how to interpret spatial patterns and temporal sequences of α-syn propagation in histopathology studies of Parkinson’s disease patients.
We observed markedly higher levels of phosphorylated α-syn in the myenteric plexus than in the submucosal plexus of the gut wall. This pathology was mainly cytoplasmic with sporadic immunoreactivity in the nucleus of old rats. These results corroborate findings in aged bacterial artificial chromosome mice, where cell bodies of the myenteric plexus mostly contained α-syn pathology in aged mice, but not in young.41 Since phoshorylated α-syn showed preferential colocalization with VAChT immunoreactivity in the gut wall, it seems that specifically cholinergic neurons in the myenteric plexus are targeted. It has been reported that α-syn is preferentially expressed in the axons of cholinergic enteric neurons in the guinea-pig ileum and human colon, suggesting specific vulnerability of enteric cholinergic neurons to Parkinson’s disease.42,43
Interestingly, we observed decreased cholinergic innervation in the stomach wall of seeded adult and old rats, correlating with enteric α-syn pathology. These results indicate that α-syn pathology leads to progressive cholinergic synaptic dysfunction. Of note, Parkinson’s disease patients also show decreased small intestinal and colonic cholinergic signal on 11C-donepezil PET.14,44
Delayed gastric emptying and decreased stool water weight were observed in adult rats at 20 weeks but not at 10 weeks post-injection, suggesting that cholinergic dysfunction in the stomach precedes symptom development. Another study reported delayed gut transit time and decreased faecal water weight in wild-type mice from 2 months post-injection.31
Age-dependent sympathetic pathology
We observed significantly more α-syn pathology in autonomic ganglia and myocardium of old and adult rats seeded with hPFFs compared to young rats and age-matched controls. Thus, it seems that initial α-syn pathology originating in the gut rapidly propagates via the coeliac ganglia to the sympathetic trunk, and then anterogradely to cardiac sympathetic nerve terminals. A recent study using transgenic mice reported that direct PFF-seeding in autonomic ganglia lead to trans-synaptic α-syn propagation to the CNS, as well as to the heart and ENS, accompanied by orthostatic hypotension and constipation.45
The immunoreactive area of cardiac α-syn pathology was negatively correlated to the immunoreactive area of cardiac TH, suggesting that α-syn pathology in the cardiac sympathetic nerve fibres leads to synaptic dysfunction. In human Parkinson’s disease, these fibres are often affected in the premotor phase and nearly all patients show marked cardiac denervation by Hoehn and Yahr stage 3.46,47
In addition, we observed age-dependent α-syn pathology (without apparent neurodegeneration) in sympathetic nerves of other peripheral structures, including the skin, muscle and kidney. Focus on skin research in Parkinson’s disease has been intensified due to the possibility of using skin biopsies for diagnostics. To our knowledge, this is the first study to report α-syn pathology in the skin of a Parkinson’s disease rodent model. We observed α-syn deposits in the piloerector muscle, arterioles, arteries and sympathetic nerves in the cervical dermis of adult but not in young rats at 20 weeks post-injection. In patients with Parkinson’s disease, phosphorylated α-syn has been detected in dermal sympathetic nerve fibres of arterioles, piloerector muscle and sweat glands.48,49 Besides phosphorylated α-syn, cutaneous denervation is observed in Parkinson’s disease patients with a proximal‐to‐distal gradient,50,51 but we did not see such loss of small nerve fibres in our rats. Finally, we also detected α-syn pathology in muscle tissue of one aged rat at 20 weeks post-injection and in the renal medulla of aged but not young hPFF-seeded rats.
α-Synuclein aggregation is related to neuronal dysfunction
Our data indicate progressive aggregation of α-syn protein throughout the brain (except the lower brainstem where the pathology was transient), and in gut and heart of wild-type rats seeded with hPFFs. Phosphorylation at Ser129 and proteinase K resistance of α-syn are among the most common pathogenic modifications of α-syn and are commonly used for Parkinson’s disease diagnostics.52,53
In the myenteric and cardiac plexus, we observed dense aggregation in the cytosol of adult rats at 20 weeks, compared to 10 weeks post-injection which displayed a dot-like pattern of small immature aggregates. In addition, some Lewy-body like larger α-syn inclusions were detected almost exclusively in old rats. In support, previous studies reported punctate α-syn immunoreactivity in the brainstem, autonomic ganglia and heart of young transgenic rats that became more condense inclusions over time.45,54 A recent in vitro study showed that maturation into Lewy bodies is followed by synaptic dysfunction, indicating that Lewy body formation is a driver of neurodegeneration.55 Our data support these findings as we detect dense α-syn inclusions in the gut and heart of the animals with strongest cholinergic or noradrenergic denervation respectively, i.e. late adult rats at 20 weeks post-injection and old rats at 10 weeks post-injection.
Furthermore, we observed that phosphorylated α-syn pathology in the cardiac and myenteric plexus was more resistant to enzymatic proteinase K digestion in aged rats. CNS pathology was apparently not proteinase K resistant. Since α-syn pathology was initiated in the gut of our animals, it is possible that CNS α-syn aggregates had had less time to consolidate into mature, proteinase K-resistant inclusions. However, the resistance of phosphorylated α-syn to proteinase K digestion is limited and depends heavily on the proteinase K antigen retrieval protocol.32,55,56 Interestingly, we detected elongated axonal staining with tiny α-syn deposits and enhanced axonal outgrowth in the frontal cortex and striatum, specifically in some aged rats. It has been reported that α-syn induces enhanced axonal arborization in the cortex and striatal white matter tracts, and that this phenomenon is associated with axonal injury in early Parkinson’s disease.57 As we did not observe neurodegeneration in the CNS, these results might indicate that axonal degeneration precedes neuronal degeneration and concomitant motor symptoms.
Species barrier
α-Syn pathology was mostly absent in young rats seeded with hPFFs at 10 weeks post-injection, in contrast to young rats seeded with mPFFs that displayed high levels of phosphorylated α-syn in the CNS and autonomic ganglia. These results reinforce that mPFFs are more potent inducers of α-syn pathology than hPFFs as shown previously.58–60 Human and mouse α-syn show a 95% sequence homology.61 Nonetheless, even small sequence incompatibilities greatly reduce the interface between the pathogenic seed and its substrate, subsequently limiting the toxicity of the pathogenic seed.62,63 And small structural differences in fibrillar α-syn result in divergent histopathology.64–66 Here, the concept of the species barrier reflects the complex interplay of natural mechanisms behind reduced transmission of α-syn pathology in young rats seeded with hPFFs, compared to mPFFs. Interestingly, pathology in stage 3 sympathetic structures, including the heart, skin, muscle and kidney were absent or minor in young mPFF-seeded rats, indicating that old age is crucial for a complete propagation pattern affecting the entire autonomic nervous system. These results indicate that ageing lowers the species barrier.
Limitations
This study has several limitations. In theory, the observed pathology both in the heart and in the stomach could have been caused by hematogenic seeding. It has been shown previously that intravenous injection of 50 µg of mPFFs can lead to disease at 6–7 months post-injection in transgenic mice.67 If a small amount of PFFs were to escape into the bloodstream during seeding, that amount would most likely be too little to initiate pathology, as we injected only 60 µg and also used wild-type rats, which have a decreased propensity to develop pathology compared to transgenic rodent models. Moreover, it has been shown by Kim et al.29 that vagotomy prevents gut-to-brain propagation in wild-type mice, providing evidence that the vagus nerve serves as a gateway for bidirectional transmission of pathology between the gut and the brain. Considering the striking α-syn pathology in the brainstem and coeliac ganglion in late adult and old rats at 10 weeks post injection, we find alternative explanations less likely.
Furthermore, rats were only followed up to 20 weeks, which may have been insufficient time for developing a full clinical picture including neurodegeneration of the substantia nigra and other important CNS nuclei. Further studies with long-term follow-up and elaborate motor symptom testing are required to investigate neurodegeneration in the brain and subsequent motor deficits. Additionally, we have only investigated two time points in this study. Extra intermediate time points would be beneficial to map out the brain-to-gut and gut-to-brain propagation more meticulously.
Finally, we opted to use 10 different experimental groups to maximize control of experimental variables. This, together with the use of aged animals, resulted in rather small sample sizes. Because of the binary nature of our immunohistochemical data, i.e. there is a lack of pathology or extensive pathology, we were able to obtain statistically significant differences among groups.
Conclusion
Nearly all published reports about Parkinson’s disease wild-type models used young animals and often reported minimal propagation of α-syn pathology. Our results highlight that ageing in otherwise healthy animals substantially promotes the development of α-syn pathology, and is crucial for complete propagation to heart and skin like observed in human Parkinson’s disease. Given that age is the greatest risk factor for human Parkinson’s disease, it seems likely that older experimental animals will yield the most relevant and reliable findings, and that findings in young animals are probably not an accurate reflection of human Parkinson’s disease.
Although α-syn pathology was widespread in both the CNS and PNS, neuronal degeneration was restricted to peripheral cholinergic and noradrenergic neurons at the early time points studied here. This observation indicates that peripheral autonomic neurodegeneration precedes CNS neurodegeneration, when the pathology is initiated in the gut—which is compatible with the known sequence of neurodegeneration evidence in human ‘body-first’ Parkinson’s disease.
To our knowledge, our aged wild-type rodent model provides the most complete recapitulation of human ‘body-first’ Parkinson’s disease reported so far, which could be relevant clinically to optimize diagnostics and therapeutics in aged patients. Such models can also be used to elucidate the nature of age-dependent interactions with α-syn pathology.
Supplementary Material
Acknowledgements
We thank the Health Bioimaging core Facility at Aarhus University for the use of their microscope and imaging software and Christian Hasselbalch Garm for his valuable assistance with microscopic image acquisition and analysis. We are thankful to Karina Lassen Holm, animal caretaker, for her great assistance with behavioural testing and animal care.
Competing interests
The authors report no competing interests.
Funding
N.V.D.B. is funded by the Lundbeck Foundation (R322-2019-2544) and Danish Parkinson’s Association. P.B. is funded by the Lundbeck Foundation (R276-2018-294), Jascha Foundation, Danish Parkinson’s Association, and Novo Nordisk Foundation. The Center for Stochastic Geometry and Advanced Bioimaging is supported by Villum Foundation. A.T. is funded by Independent Research Fund Denmark. P.K. is part of the International Diabetic Neuropathy Consortium (IDNC) research programme, which is supported by a Novo Nordisk Foundation Challenge Programme grant (Grant number NNF14OC0011633) and is funded by the Novo Nordisk Foundation (Grant number NNF18OC0052301). P.H.J. is funded by Lundbeck Foundation grants R223-2015-4222 and R248-2016-2518 for Danish Research Institute of Translational Neuroscience-DANDRITE, Nordic-EMBL Partnership for Molecular Medicine, Aarhus University, Denmark, and N.F. by Postdoctoral Fellowship R171-2014-591.
Glossary
- α-syn
alpha-synuclein
- DMV
dorsal motor nucleus of the vagus
- h/mPFF
human/mouse preformed fibril
- IML
intermediolateral nucleus
- VAChT
vesicular acetylcholine transporter
References
- 1. Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gelpi E, Navarro-Otano J, Tolosa E, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–1101. [DOI] [PubMed] [Google Scholar]
- 3. Chiang H, Lin C.. Altered Gut microbiome and intestinal pathology in Parkinson’s disease. J Mov Disorder. 2019;12:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P.. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79:940–949. [DOI] [PubMed] [Google Scholar]
- 5. Orimo S, Uchihara T, Nakamura A, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131 (Pt):642–650. [DOI] [PubMed] [Google Scholar]
- 6. Donadio V, Incensi A, Piccinini C, et al. Skin nerve misfolded alpha‐synuclein in pure autonomic failure and Parkinson disease. Ann Neurol. 2016;79:306–316. [DOI] [PubMed] [Google Scholar]
- 7. Borghammer P. How does Parkinson's disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33:48–57. [DOI] [PubMed] [Google Scholar]
- 8. Cheon SM, Ha MS, Park MJ, Kim JW.. Nonmotor symptom s of Parkinson’s disease: Prevalence and awareness of patients and families. Parkinsonism Rel Disord. 2008;14:286–290. [DOI] [PubMed] [Google Scholar]
- 9. Palma JA, Kaufmann H.. Orthostatic hypotension in Parkinson disease. Clin Geriatr Med. 2020;36:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng C, Trojanowski JQ, Lee VM.. Protein transmission in neurodegenerative disease. Nat Rev Neurol. 2020;16:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braak H, Rüb U, Gai WP, Del Tredici K.. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. [DOI] [PubMed] [Google Scholar]
- 12. Liu B, Fang F, Pedersen NL, et al. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78:522–529. [DOI] [PubMed] [Google Scholar]
- 14. Knudsen K, Fedorova TD, Hansen AK, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–628. [DOI] [PubMed] [Google Scholar]
- 15. de Rijk MC, Breteler MM, Graveland GA, et al. Prevalence of Parkinson’s disease in the elderly: The Rotterdam study. Neurology. 1995;45:2143–2146. [DOI] [PubMed] [Google Scholar]
- 16.GBD 2016 Neurology Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. [DOI] [PubMed] [Google Scholar]
- 18. Ulusoy A, Phillips RJ, Helwig M, Klinkenberg M, Powley TL, Di Monte DA.. Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 2017;133:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ulusoy A, Rusconi R, Perez-Revuelta BI, et al. Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol Med. 2013;5:1119–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ayers JI, Brooks MM, Rutherford NJ, et al. Robust central nervous system pathology in transgenic mice following peripheral injection of alpha-synuclein fibrils. J Virol. 2017;91:e02095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breid S, Bernis ME, Babila JT, Garza MC, Wille H, Tamguney G.. Neuroinvasion of alpha-synuclein prionoids after intraperitoneal and intraglossal inoculation. J Virol. 2016;90:9182–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Den Berge N, Ferreira N, Gram H, et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019;138:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manfredsson FP, Luk KC, Benskey MJ, et al. Induction of alphasynuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol Dis. 2018;112:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uemura N, Yagi H, Uemura MT, Hatanaka Y, Yamakado H, Takahashi R.. Inoculation of alpha-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener. 2018;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannon JR, Greenamyre JT.. Gene-environment interactions in Parkinson’s disease: specific evidence in humans and mammalian models. Neurobiol Dis. 2013;57: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A Jr.. The Gut and Parkinson's Disease—a bidirectional pathway. Front Neurol. 2019;10:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimball ES, Palmer JM, D'Andrea MR, Hornby PJ, Wade PR.. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–G1273. [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic α-synuclein from the Gut to the brain models Parkinson's Disease. Neuron. 2019;103:627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arotcarena ML, Dovero S, Prigent A, et al. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain. 2020;143:1462–1475. [DOI] [PubMed] [Google Scholar]
- 31. Challis C, Hori A, Sampson TR, et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci. 2020;23:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Froula JM, Henderson BW, Gonzalez JC, et al. α-Synuclein fibril-induced paradoxical structural and functional defects in hippocampal neurons. Acta Neuropathol Commun. 2018;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oinas M, Paetau A, Myllykangas L, Notkola IL, Kalimo H, Polvikoski T.. alpha-Synuclein pathology in the spinal cord autonomic nuclei associates with alpha-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol. 2010;119:715–722. [DOI] [PubMed] [Google Scholar]
- 35. Gasperini L, Legname G.. Prion protein and aging. Front Cell Dev Biol. 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goedert M, Masuda-Suzukake M, Falcon B.. Like prions: The propagation of aggregated tau and alphasynuclein in neurodegeneration. Brain. 2017;140:266–278. [DOI] [PubMed] [Google Scholar]
- 37. Uchihara T, Giasson BI.. Propagation of alpha‐synuclein pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:49–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reeve A, Simcox E, Turnbull D.. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL.. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson's disease? Neuroscience. 2008;153:733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phillips RJ, Walter GC, Ringer BE, Higgs KM, Powley TL.. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp Neurol. 2009;220:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen QQ, Haikal C, Li W, Li MT, Wang ZY, Li JY.. Age-dependent alpha-synuclein accumulation and aggregation in the colon of a transgenic mouse model of Parkinson's disease. Transl Neurodegener. 2018;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharrad DF, Gai WP, Brookes SJ.. Selective coexpression of synaptic proteins, α-synuclein, cysteine string protein-α, synaptophysin, synaptotagmin-1, and synaptobrevin-2 in vesicular acetylcholine transporter-immunoreactive axons in the guinea pig ileum. J Comp Neurol. 2013;521:2523–2537. [DOI] [PubMed] [Google Scholar]
- 43. Sharrad DF, de Vries E, Brookes SJ.. Selective expression of α-synuclein-immunoreactivity in vesicular acetylcholine transporter-immunoreactive axons in the guinea pig rectum and human colon. J Comp Neurol. 2013;521:657–676. [DOI] [PubMed] [Google Scholar]
- 44. Fedorova TD, Seidelin LB, Knudsen K, et al. Decreased intestinal acetylcholinesterase in early Parkinson disease: An 11C-donepezil PET study. Neurology. 2017;88:775–781. [DOI] [PubMed] [Google Scholar]
- 45. Wang XJ, Ma MM, Zhou LB, et al. Autonomic ganglionic injection of α-synuclein fibrils as a model of pure autonomic failure α-synucleinopathy. Nat Commun. 2020;11:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kashihara K, Imamura T, Shinya T.. Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:252–255. [DOI] [PubMed] [Google Scholar]
- 47. Nagayama H, Hamamoto M, Ueda M, Nagashima J, Katayama Y.. Reliability of MIBG myocardial scintigraphy in the diagnosis of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson's disease. J Neural Transm. 2006;113:1169–1176. [DOI] [PubMed] [Google Scholar]
- 49. Ikemura M, Saito Y, Sengoku R, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67:945–953. [DOI] [PubMed] [Google Scholar]
- 50. Donadio V, Incensi A, Rizzo G, et al. Spine topographical distribution of skin alpha‐synuclein deposits in idiopathic Parkinson disease. J Neuropathol Exp Neurol. 2017;76:384–389. [DOI] [PubMed] [Google Scholar]
- 51. Melli G, Vacchi E, Biemmi V, et al. Cervical skin denervation associates with alpha-synuclein aggregates in Parkinson disease. Ann Clin Transl Neurol. 2018;5:1394–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fujiwara H, Hasegawa M, Dohmae N, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. [DOI] [PubMed] [Google Scholar]
- 53. Giasson BI, Murray IVJ, Trojanowski JQ, Lee VM-Y.. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. [DOI] [PubMed] [Google Scholar]
- 54. Wegrzynowicz M, Bar-On D, Calo L, et al. Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol. 2019;138:575–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mahul-Mellier AL, Burtscher J, Maharjan N, et al. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A. 2020;117:4971–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Resnikoff H, Metzger JM, Lopez M, et al. Colonic inflammation affects myenteric alpha-synuclein in nonhuman primates. J Inflamm Res. 2019; 12:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schechter M, Grigoletto J, Abd-Elhadi S, et al. A role for α-Synuclein in axon growth and its implications in corticostriatal glutamatergic plasticity in Parkinson's disease. Mol Neurodegener. 2020;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fares MB, Maco B, Oueslati A, et al. Induction of de novo α-synuclein fibrillization in a neuronal model for Parkinson’s disease. Proc Natl Acad Sci U S A. 2016;113:E912–E921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY.. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Masuda-Suzukake M, Nonaka T, Hosokawa M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lv G, Kumar A, Giller K, et al. Structural comparison of mouse and human α-synuclein amyloid fibrils by solid-state NMR. J Mol Biol. 2012;420:99–111. [DOI] [PubMed] [Google Scholar]
- 62. Angers RC, Kang HE, Napier D, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328:1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Luk KC, Covell DJ, Kehm VM, et al. Molecular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep. 2016;16:3373–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bousset L, Pieri L, Ruiz-Arlandis G, et al. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peelaerts W, Bousset L, Van der Perren A, et al. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. [DOI] [PubMed] [Google Scholar]
- 66. Woerman AL, Stohr J, Aoyagi A, et al. Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci U S A. 2015;112:E4949–E4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lohmann S, Bernis ME, Tachu BJ, Ziemski A, Grigoletto J, Tamgüney G.. Oral and intravenous transmission of α-synuclein fibrils to mice. Acta Neuropathol. 2019;138:515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.