Abstract
Pure water will become a golden resource in the context of the rising pollution, climate change and the recycling economy, calling for advanced purification methods such as the use of nanostructured adsorbents. However, coming up with an ideal nanoadsorbent for micropollutant removal is a real challenge because nanoadsorbents, which demonstrate very good performances at laboratory scale, do not necessarily have suitable properties in in full-scale water purification and wastewater treatment systems. Here, magnetic nanoadsorbents appear promising because they can be easily separated from the slurry phase into a denser sludge phase by applying a magnetic field. Yet, there are only few examples of large-scale use of magnetic adsorbents for water purification and wastewater treatment. Here, we review magnetic nanoadsorbents for the removal of micropollutants, and we explain the integration of magnetic separation in the existing treatment plants. We found that the use of magnetic nanoadsorbents is an effective option in water treatment, but lacks maturity in full-scale water treatment facilities. The concentrations of magnetic nanoadsorbents in final effluents can be controlled by using magnetic separation, thus minimizing the ecotoxicicological impact. Academia and the water industry should better collaborate to integrate magnetic separation in full-scale water purification and wastewater treatment plants.
Keywords: Magnetic nanoadsorbents, Water purification, Wastewater treatment, Magnetic separation
Introduction
Water is needed for umpteen day-to-day domestic, commercial and industrial activities. Yet, over the years, pollution of water has kept increasing to such an extent where matters have worsened into water stress and water scarcity conditions in many regions of the world. The release of untreated wastewater poses two major global ecological problems. One which encompasses the entire set of the potential damaging and irreversible impacts on the different components of the food web and ecosystems (Tijani et al. 2016; Arslan et al. 2017; Shao et al. 2019; Xu et al. 2019; Gautam and Anbumani 2020; Varjani and Sudha 2020; Rogowska et al. 2020; Golovko et al. 2020). Second, a much useful resource, which is in the form of untreated wastewater, is lost. This poses additional stress on rural and urban clean water supply chains. As a consequence, to sustain development within a circular economy, more clean water has to be tapped from the existing freshwater reserves to meet growing water demands. Circular economy is a resource recovery strategy which has been recently used in brine (saline wastewater) treatment as well (Panagopoulos and Haralambous 2020a, b). There is hence an absolute need to capture untreated wastewaters as much as possible to then treat them using the best-in-class treatment systems for eventually meeting all sanitary norms, effluent discharge standards and regulations.
During the last two decades, there has been a growing thrust in harnessing nanoscience and nanotechnology for designing myriad nanostructured materials which can potentially serve as more effective adsorbents for water purification and wastewater treatment (Santhosh et al. 2016; Mohammed et al. 2018; Villaseñor and Ríos 2018; Alvarez et al. 2018; Madhura et al. 2019; Bahadori et al. 2020; Soares et al. 2020; Scaria et al. 2020; Borji et al. 2020; Jain et al. 2020; Siddeeg et al. 2020). Nanoadsorbents can effectively deliver ultrahigh adsorption capabilities, fast removal kinetics, high removal efficiencies and selectivities for a very broad spectrum of micropollutants (such as pesticides residues in water (Valenzuela et al. 2020), the pharmaceutical drug diclofenac (Zhao et al. 2021a), the antidiabetic pharmaceutical agent metformin hydrochloride (Çavuşoğlu et al. 2021), and heavy metals (Singh et al. 2021)), and even be potential candidates for the selective and reversible adsorption of coronaviruses from contaminated waters (Ciejka et al. 2017; Carvalho and Conte‐Junior 2021). However, these excellent adsorption characteristics and performances for micropollutants removal are largely reported for controlled experimental conditions. Despite the limitations of laboratory scale adsorption analysis, a vast body of scientific insights has been garnered in the literature with regard to the synthesis, characterization, and examination of nanoadsorbents for their respective capability to sequester micropollutants. Subsequently, there is substantial potential for the scientific, engineering and technology development communities to further tune in their efforts and harness the ‘gold mine’ of nanoadsorbents for micropollutants removal in full-scale water purification and wastewater treatment facilities.
At present, the preferred commercial adsorbent wastewater treatment at the industrial scale is activated carbon. However, its widespread use is limited by its high cost (Crini et al. 2019). The current quest is in producing an ideal nanoadsorbent. There are a number of key features sought in an ideal nanoadsorbent (e.g., mesoporous nanoparticles, hydrogel, polymeric nanoparticles, aerogel or carbon nanotube-type materials) intended for scavenging different target micropollutants from contaminated waters and wastewaters under variable chemical, physical, biological and microbiological conditions. These are, inter alia:
-
(i)
High adsorption and removal capacities
-
(ii)
Chemical stability, thermal stability and adequate selectivity
-
(iii)
High recovery rate of spent adsorbents, regeneration and recyclability
-
(iv)
Adequate tunability of porosity
-
(v)
Scope for modification of surface chemistry by specific types of functionalization
-
(vi)
High mechanical strength, structural integrity and shape recovery potential
-
(vii)
Self-healing (Perera and Ayres 2020) and self-cleaning properties (Shen et al. 2019; Xiong et al. 2020)
-
(viii)
Amenability for being produced in bulk through green synthetic routes
-
(ix)
Ability for being integrated in large-scale water/wastewater treatment processes
-
(x)
Low-cost bulk production and regeneration
An ideal nanoadsorbent would competitively solve a reasonable part of the core technical, economic and secondary pollution issues related to existing conventional water purification and wastewater treatment methods and conventional adsorbents. For example, a novel iron oxide–hydrotalcite modified with dodecylsulfate and β-cyclodextrin magnetic adsorbent gave maximum adsorption capacities significantly superior to those reported for certain activated carbon-type and activated char adsorbents in the removal of phenol (216.08 mg g−1) and p-cresol (272.48 mg g−1) present in pulp and paper industry wastewater (Balbino et al. 2020). The latter maximum adsorption capacities are higher than the following ones: 144.93 mg g−1 for phenol by activated carbon (Zhang et al. 2016a), 129.24 mg g−1 for p-cresol by composite alginate beads-MnO2 activated carbon (Shim et al. 2019), and 32.77 mg g−1 for p-cresol by coconut shell-activated char (Zhu and Kolar 2014). More recently, the maximum removal capacity of Pb2+ and methylene blue on novel MoO3 nanobelts was 684.93 and 1408 mg g–1, respectively, while that of Au3+ and methylene blue on novel MoS2 nanoarrays was 1280.2 and 768 mg g–1, respectively (Zhou et al. 2022). MoO3 nanobelts and MoS2 nanoarrays could be easily synthesized, were high scalable, had good chemical stability, gave high repeatability, and these characteristics made them promising candidates for wastewater treatment (Zhou et al. 2022).
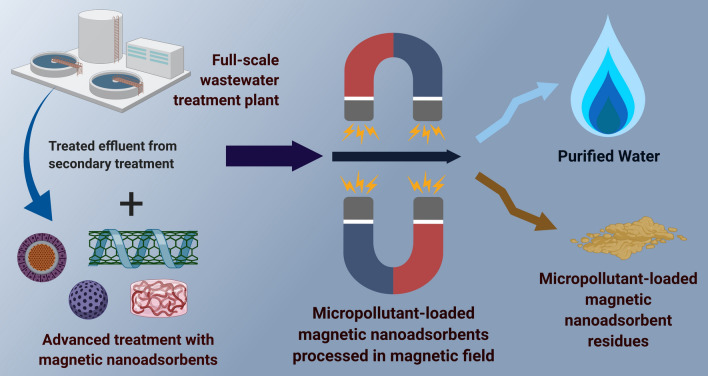
Accordingly, more research efforts have been deployed in formulating green schemes for the synthesis of novel nanoadsorbents which could compete with activated carbon. Nanoadsorbents have relatively very large specific surface areas (Mashile et al. 2020; He et al. 2021), and their surface chemistry and functionality can be engineered to augment their adsorption capacities in comparison with conventional and commercially used adsorbents (Vikrant and Kim 2019). Nanoadsorbents used for scavenging micropollutants are capable of exhibiting higher adsorption capacities (Wadhawan et al. 2020), strong reactivity (Lu et al. 2016), and specific affinity toward the targeted micropollutants (Zhang et al. 2016b). These nanomaterials can also have multiple active sorption sites and tuneable porosity (El-sayed 2020). One specific class of nanoadsorbents is magnetic nanoadsorbents (Mahamadi 2019; Franzreb 2020; Vicente-Martínez et al. 2020; He et al. 2021; Jiang et al. 2021; Peralta et al. 2021; Mohammadi et al. 2021; Nithya et al. 2021; Álvarez-Manzaneda et al. 2021; Plohl et al. 2021). According to a recent review, research on the preparation and use of magnetic adsorbents has been progressing fast, and has yielded more than eightfold rise in the number of publications in the period from 2010 to 2020 (Reshadi et al. 2020). In this review, we discuss a few research and development perspectives with respect to the potential use of novel high-performance magnetic nanoadsorbents for micropollutant removal and the integration of magnetic separation in the existing water purification and wastewater treatment plants (Fig. 1).
Fig. 1.
Conceptual representation of the use of magnetic nanoadsorbents and integration of magnetic separation in existing wastewater treatment facilities for micropollutant removal. This concept is envisioned in three major phases. First, the effluent from the secondary treatment stage is treated with selected magnetic nanoadsorbent. This phase will be an advanced treatment. Second, the treated effluent from the advanced treatment phase is processed in an integrated magnetic separation system, where the micropollutant-laden magnetic nanoadsorbents are decoupled from the purified wastewater. Third, the purified water and micropollutant-loaded magnetic nanoadsorbents are separated in two different streams for further use and processing. The micropollutant-loaded magnetic nanoadsorbents are then regenerated. Created with BioRender.com.
Magnetic nanoadsorbents
Magnetic nanoadsorbents are emerging as significantly effective functional materials with exceptional micropollutant sequestration capabilities and fast adsorption kinetics at the laboratory scale (Abdel Maksoud et al. 2020; D’Cruz et al. 2020; Hu et al. 2020; Mittal et al. 2020; Ahmad et al. 2020a; Wang et al. 2020b, d; Jafari et al. 2020; Keykhaee et al. 2020; Icten and Ozer 2021; Xin et al. 2021). Magnetic nanoadsorbents are generally characterized with high specific surface areas (e.g., 1188 m2 g–1 for magnetic coal-based activated carbon (Liu et al. 2021)), high pore volumes (Gupta et al. 2017; Yeap et al. 2017; Masunga et al. 2019; Li et al. 2020; Azam et al. 2020; Pan et al. 2021), robust structures (Lingamdinne et al. 2019a), and extensively interconnected porous networks (Tan et al. 2020; Fan et al. 2021) which collectively promote ultrahigh adsorption capacities for micropollutants.
Besides the redox activity and surface charge properties (Abdel Maksoud et al. 2020), low-cost synthesis and non-toxicity (Leone et al. 2018), high selectivity (Song et al. 2018; Asadi et al. 2020; Nisola et al. 2020; Wang et al. 2020c, 2021; He et al. 2021; Luan et al. 2021), binding specificity (Vishnu and Dhandapani 2021), and excellent reusability (D’Cruz et al. 2020; Hu et al. 2020; Li et al. 2020; Ahmad et al. 2020b; Vu and Wu 2020; Wang et al. 2020c; Nkinahamira et al. 2020; Tabatabaiee Bafrooee et al. 2021), a key feature of magnetic nanoadsorbents is that they can be separated in situ from adsorption-remediated waters in the form of a magnetic nanoadsorbent(s)–adsorbate(s) sludge by applying a strong enough magnetic field (Ambashta and Sillanpää 2010; Zaidi et al. 2014; Simeonidis et al. 2015; Moharramzadeh and Baghdadi 2016; Wanna et al. 2016; Tripathy et al. 2017; Mirshahghassemi et al. 2017; Yeap et al. 2017; Augusto et al. 2019; Kheshti et al. 2019a; Mashile et al. 2020; Brião et al. 2020; Balbino et al. 2020).
The opportunity to separate the micropollutant(s)-loaded spent magnetic nanoadsorbents from the purified water/wastewater to produce clean water is an enormous prospect for Research and Development in the area of water science and technology. The latter concepts motivate the following discussions which are focused on the potential of using magnetic nanoadsorbents effectively in full-scale water purification and wastewater treatment systems, and on the prospect of integrating magnetic separation in such systems to recuperate spent magnetic nanoadsorbents. Magnetic separation has some attractive advantages in comparison with the conventional processes. These merits are broadly related to: (i) the possibility of carrying out an integrated one-step capture and purification of specific species, (ii) the processing of high throughputs, and (iii) the low energy requirements and associated costs entailed by semi-continuous or continuous processes ran at relatively low pressure (Schwaminger et al. 2019).
The use of magnetic nanoadsorbents and the integration of magnetic separation for water purification and wastewater treatment can be envisaged at the tertiary effluent treatment level whereby effluent from the upstream secondary treatment units is polished through selective adsorptive sequestration of the target micropollutant(s). Yet, the mode of seeding of magnetic nanoadsorbents and the incorporation of magnetic separation at other possible points/locations within the wastewater treatment plants will surely require more investigation, scenario formulation and system analysis. This is because each wastewater treatment plant has its own sets of specific processes and type of wastewaters.
Ecotoxicity assessments of the sludge and purified water after the magnetic separation should also be part of an overall environmental safety-environmental impact monitoring plan. Based on the results obtained thereof, there can be the scope to reengineer the synthesis of magnetic nanoadsorbents into more benign schemes. Pristine magnetic nanoadsorbents can be functionalized with diverse moieties to bring out their favorable adsorption characteristics .(Augusto et al. 2019; Manyangadze et al. 2020; Wu et al. 2020; Dai et al. 2020; Nnadozie and Ajibade 2020; Safari et al. 2020; Bi et al. 2021; You et al. 2021; Aryee et al. 2021), and also increase their stability relative to oxidation with improved selectivity for one specific metal ion (Wadhawan et al. 2020). However, functionalized magnetic nanoadsorbents can be very expensive, and this economic feature limits their use in water purification and wastewater treatment processes at the industrial scale (Augusto et al. 2019).
Developments with magnetic nanoadsorbents and magnetic separation
In this section, the discussions are focused on the examination of magnetic nanoadsorbents at laboratory scale, pilot-type magnetic separation systems and their respective configuration, inventions and patents for magnetic separator systems, magnetic separation processes in large-scale water purification, and finally on the related gaps and research and development opportunities.
Magnetic nanoadsorbents at laboratory scale
Empirical investigations reported in the literature provide interesting scientific insights into the significantly diverse aspects of the adsorption dynamics of different adsorbate–magnetic nanoadsorbent combinations (Sivashankar et al. 2014; Mehta et al. 2015; Tamjidi et al. 2019; Kumar et al. 2020; Hassan et al. 2020; Mashkoor and Nasar 2020; Bharti et al. 2020; You et al. 2021). For example, doping Ag ions onto Fe3O4 nanoparticles had decreased particle sizes, but enhanced the magnetic characteristics of the as-prepared nanocomposites (Najafpoor et al. 2020). The Ag-magnetic nanoparticles had considerably higher efficacy for disinfecting effluent and in advanced treatment through an increased removal of chemical oxygen demand as well (Najafpoor et al. 2020). The switching from magnetic nanoparticles to Ag-loaded magnetic nanoparticles led to a 0.06 increase in total coliforms, fecal coliforms, and heterotrophic bacteria log reductions, and a 6.16% rise in the removal of chemical oxygen demand (Najafpoor et al. 2020).
In another study, a Fe3+-stabilized magnetic polydopamine composite (specific surface area=32.7 m2 g–1 and total pore volume =0.1943 cm3 g–1) demonstrated excellent adsorption capability for methylene blue in single adsorbate aqueous solutions (maximum adsorption capacity=608.8 mg g–1) for pH ranging 3–10 and at 45 °C (Chen et al. 2020). Encouragingly, the nanocomposite could selectively capture methylene blue from mixed dye aqueous systems (methylene blue/methyl orange, methylene blue/carmine, and methylene blue/Rhodamine B) and complex aqueous solutions having ionic strengths as high as 0.5 mol L–1 sodium chloride as well (Chen et al. 2020). The enhanced and selective adsorption of methylene blue occurred as a result of the synergistic effects of multiple mechanisms (Chen et al. 2020). In the case of the methylene blue/methyl orange mixed dye system, the faster and selective uptake of methylene blue was attributed to the strong electrostatic interactions between the negatively charged adsorbent and the cationic methylene blue molecules (Chen et al. 2020). In the case of methylene blue/Rhodamine B, the poor adsorption of Rhodamine B was set on account of mainly steric hindrance generated by the longer lateral alkyl chain connected to the N+ center, which in turn considerably weakened π–π stacking interactions and electrostatic attractions between Fe3O4/polydopamine-Fe3+ and the Rhodamine B molecules (Chen et al. 2020). Besides maintaining a four-cycle adsorption–desorption adsorptive efficiency greater than 80% of its initial uptake performance for methylene blue in simulated textile effluent, the nanocomposite could yield a superior adsorption performance than commercial powder-activated carbon in column adsorption setup (Chen et al. 2020).
The application of magnetite particles for treating real wastewater samples was investigated, and the variation of removal performances was assessed for samples withdrawn from three different points of a wastewater treatment facility (Castelo-Grande et al. 2021). Results, in general, indicated that magnetite particles had a very good behavior with regard to reduction in detergents and chemical oxygen demand, whereas removals of total nitrogen and phosphates, and those of most heavy metals examined (which included chromium, zinc, lead, copper and cobalt), were high to moderate (Castelo-Grande et al. 2021). The type of wastewater varied significantly among the sampling points in terms of the phosphates, total nitrogen, chemical oxygen demand, and detergents’ concentrations. Interestingly, the results provided preliminary insights which wastewater treatment plant managers may consider when selecting which contaminants to remove using magnetite-based adsorption, and when choosing an optimal point for integrating magnetic seeding in the overall plant process operations (Castelo-Grande et al. 2021).
Some recent high-performance supermagnetic nanoadsorbents examined for scavenging heavy metals and/or organic micropollutants are Fe3+-stabilized magnetic polydopamine composite (Chen et al. 2020), comb polymer-functionalized magnetic nanoparticles (Liu et al. 2020a), magnetic porous NiLa-layered double oxides (Vu and Wu 2020), magnetic β-cyclodextrin polymer (Hu et al. 2020; Nkinahamira et al. 2020), magnetic activated carbon-Fe3O4 (D’Cruz et al. 2020), cyanopropylsilane-functionalized TiO2 magnetic nanoparticles (Mousavi et al. 2019), magnetic graphene oxide modified by β-cyclodextrin (Wang et al. 2020a), hexadecyltrimethylammonium bromide-surface-functionalized magnetic UiO-66@UiO-67 composite adsorbent (Li et al. 2020), magnetic core-shell MnFe2O4@TiO2 nanoparticles loaded on reduced graphene oxide (Chang et al. 2021), magnetic graphene oxide decorated with persimmon tannins (Gao et al. 2019), magnetic montmorillonite nanocomposite (Fatimah et al. 2021), magnetic Fe3O4 nanocubes coated by SiO2 and TiO2 (Khalaf et al. 2019), ferrihydrite-loaded magnetic sugar cane bagasse charcoal adsorbent (Xin et al. 2021), ethylenediamine-functionalized magnetic graphene oxide for arsenic(III) removal from aqueous solutions (Tabatabaiee Bafrooee et al. 2021), and last but not least MnFe2O4/multiwalled carbon nanotubes (Zhao et al. 2021b). The list of recent magnetic nanoadsorbents is very long indeed. Hence, there is a vast body of findings in the literature reporting excellent micropollutant adsorption performances of different magnetic nanoadsorbents exhibiting high adsorption capacities, very fast adsorption kinetics, selectivity and good reusability (Table 1) (Xu et al. 2017; Yang et al. 2017a, 2019; Ul-Islam et al. 2017; Surendhiran et al. 2017; Ma et al. 2018; Biehl et al. 2018; Wang et al. 2018; Chen et al. 2018; Yao et al. 2019; Chavan et al. 2019; Sarkar et al. 2019; Fu et al. 2021; Li et al. 2021a).
Table 1.
Highlights of laboratory-scale adsorption performance of selected magnetic nanoadsorbents for micropollutants
| Adsorbent | Micropollutant | Highlights of adsorption behavior | References |
|---|---|---|---|
| Magnetic CrFe2O4 nanocomposite prepared sonochemically using a nonionic surfactant | Mo6+ | Thermodynamic data indicated that adsorption of Mo6+ ions was spontaneous and endothermic | Gamal et al. (2021) |
| The adsorbent could be regenerated through the desorption of more than 98% of Mo6+ with 1.0 mol L−1 sodium hydroxide | |||
| Magnetic nanocomposite Co-multiwalled carbon nanotubes | Methylene blue | Maximum adsorption capacity=324.34 mg g−1 | Çalımlı (2021) |
| Adsorption was endothermic and followed pseudo-second-order kinetic model | |||
| Fe3O4-MnO2-EDTA composite | Cu2+ ions from binary or ternary metal adsorbate system | As-synthesized adsorbents yielded high Cu2+ selective adsorption (both in binary and ternary systems) | Chen and Xie (2020) |
| In comparison with Fe3O4-MnO2, the magnetic Fe3O4-MnO2-EDTA nanoparticles resulted in rapid magnetic separation with high selectivity for Cu2+ | |||
| Magnetic CoFe2O4/graphene oxide adsorbents | Methylene blue, methyl orange and Rhodamine B | Adsorption of organic dyes for CoFe2O4/graphene oxide composite mainly attributable to contribution of graphene oxide | Chang et al. (2020) |
| Superior adsorption capacity qe(max) for methylene blue and Rhodamine B at 355.9 mg g−1 and 284.9 mg g−1, respectively (Langmuir adsorption model). | |||
| Selective adsorption with order of adsorption capacity as follows: Methylene blue > Rhodamine B > methyl orange | |||
| Hydroxypropyl-β- cyclodextrin-polyurethane/graphene oxide magnetic nanoconjugates | Cr6+ and Pb2+ | Adsorption capacity of adsorbents for Cr6+ and Pb2+ at 987 mg g−1 and 1399 mg g−1, respectively, and adsorption followed pseudo-second-order kinetics | Nasiri and Alizadeh (2021) |
| Reusability of adsorbent makes it a promising candidate for Pb2+ removal from aqueous solutions | |||
| This magnetic composite was endowed with a high adsorption performance and good reusability for heavy metal ions | |||
| Magnetic molecular imprint polymer networks synthesized from vinyl-functionalized magnetic nanoparticles | Antibiotics (ciprofloxacin and erythromycin) | Networks exhibited high binding capacity toward erythromycin and ciprofloxacin at 70 mg g−1 and 32 mg g−1, respectively. | Kuhn et al. (2020) |
| Networks were recyclable and retained their binding capacity after 4 cycles | |||
| Results demonstrated that the networks developed had high binding capacity, selectivity and recyclability | |||
| The networks can be utilized both for monitoring and removal of hazardous antibiotic pollutants potentially present in different samples and food products | |||
| Phosphoramide-functionalized magnetic nanoparticles | Uranium | High maximum adsorption capacity=95.2 mg U g−1 sorbent | Singhal et al. (2020) |
| 80% adsorption achieved for pH 4–8 with maximum adsorption observed at pH 6 | |||
| Higher than 90% uranium extraction was recorded during adsorption studies conducted using drinking water, tap water and seawater | |||
| Inferences were made in the study as follows: high adsorption capacity, low cost, less equilibration time, easy separation from matrix and non-toxicity of the adsorbent constitute some key merits sought when envisioning the process at an industrial scale | |||
| Magnetic tubular carbon nanofibers | Cu2+ | Maximum adsorption capacity of nanofibers for Cu2+=375.93 mg g−1 | Ahmad et al. (2020b) |
| Porous morphology, large surface area and tubular structure of the nanofibers contributed to the rapid and highest adsorption of Cu2+ ions | |||
| Langmuir adsorption isotherm model best described adsorption data | |||
| The nanofibers developed have exhibited excellent regenerability when treated with EDTA | |||
| Magnesium–zinc ferrites | Cr6+ and Ni2+ | Mg0·2Zn0·8Fe2O4 yielded best adsorption capacity (30.49 mg g−1) | Tatarchuk et al. (2021) |
| Mg0·4Zn0·6Fe2O4 was observed to be the most effective adsorbent for removing Ni2+ (93.2%) | |||
| Adjustment of magnesium content to an optimal value can enhance mixed ferrites’ ability to remove heavy metals from aqueous solutions | |||
| Sulfur-functionalized polyamidoamine dendrimer/magnetic Fe3O4 hybrid materials | Hg2+ and Ag+ | Maximum adsorption capacity for Hg2+ and Ag+ was 0.8 mmol g−1 and 1.29 mmol g−1, respectively | Luan et al. (2021) |
| Good adsorption selectivity (100% selective adsorption of Hg2+ in the presence of Ni2+, Zn2+ and Mn2+) | |||
| Excellent regeneration characteristics, and reuse repeatedly over four use cycles | |||
| Magnetic sodium alginate (SA)-based Fe3O4@SA-Ca gel beads | Direct Orange 26 in aqueous solutions | Gel had ultrahigh adsorption capacity of 1252 mg g−1 | Li and Lin (2021) |
| Dye removal efficiency=96.2 % (298 K, 50 mg polymer dosage, 2.6 g L−1 initial dye concentration, pH 2.0, 90 min adsorption time) | |||
| Adsorption was spontaneous and exothermic | |||
| Gel was easily separated and recuperated from aqueous solutions without secondary pollution |
EDTA Ethylenediaminetetraacetic acid, SA Sodium alginate
Many reviews have discussed the adsorption performances of many magnetic nanoadsorbents under different experimental conditions using aqueous solutions containing one or more micropollutant(s). Reviews have also been performed on the synthetic methods of magnetic nanoadsorbents and chemical reagents/reactants used, the functionalization and surface chemistry modifications of pristine magnetic nanoparticles, regeneration methods and reusability of magnetic nanoadsorbents, and the concerns around the commercialization of industry-ready magnetic separation equipment.
Although promising findings have been extensively compiled based on laboratory-scale investigations with magnetic nanoadsorbents in recent reviews with regard to excellent adsorption capacities, rapid adsorption kinetics, good selectivity and recyclability (Sivashankar et al. 2014; Mehta et al. 2015; Tamjidi et al. 2019; Kumar et al. 2020; Hassan et al. 2020; Mashkoor and Nasar 2020; Bharti et al. 2020; You et al. 2021; Faraji et al. 2021; Jain et al. 2021), there are still a number of hurdles which tend to retard the use of magnetic nanoadsorbents at the commercial scale for water purification and wastewater treatment systems. These limitations are related to their mechanical properties, chemical stability, scale-up and optimization of synthetic processes, possible downstream toxicity levels, and efficacy of regeneration methods and reusability (You et al. 2021). In addition, the estimation of the costs involved in the scaling-up of synthetic schemes for magnetic nanoadsorbents’ production and the development of customized magnetic separation systems is challenging.
Magnetic nanoadsorbents have been observed to lose their adsorptive capacity after multiple reuse cycles (Meng et al. 2018; Wanjeri et al. 2018; Aliannejadi et al. 2019; Ma et al. 2019; Baig et al. 2020; Masjedi et al. 2020; Rezaei et al. 2020; Peralta et al. 2021). For example, ibuprofen uptake by an as-prepared hybrid silica-based magnetic nanoadsorbent experienced a drastic 42% decline in the second cycle, implying that the regeneration reagent used (ethanol) had not extracted all of the ibuprofen adsorbed in the previous adsorption step (Peralta et al. 2021). Naphthalene removal efficiency by a highly branched dendrimeric magnetic nanoadsorbent decreased in the last use cycles to reach 54% by the tenth cycle (Aliannejadi et al. 2019). Fe3+ removal efficiency by a magnetic core-shell Fe3O4@mSiO2-NH2 adsorbent was reduced by about 8% after cycle 1, followed by a decrease of less than 2.5% in the next three cycles (Meng et al. 2018). The removal efficiency of Cr6+ ions by a corn straw-derived porous carbon adsorbent from aqueous solutions was 91.57% at the end of a first adsorption–desorption cycle, and remained above 70.65% after three cycles (Ma et al. 2019). However, Cr6+ ion removal efficiency declined to 52.39% in the fourth adsorption–desorption cycle (Ma et al. 2019). Hence, it becomes significantly relevant to reinstate, and if required to significantly reengineer possibly through functionalization (Sahoo and Hota 2018; Manyangadze et al. 2020; Peralta et al. 2020, 2021), the physical and chemical characteristics of the magnetic nanoadsorbents to sustain their effective reuse. Thus, regeneration potential, regeneration method and recovery efficiency for reuse are three critical factors, among others, which will guide the selection of a magnetic nanoadsorbent for a specific industrial-scale water purification and wastewater treatment process. These aspects are particularly crucial from the economic dimension given the high costs which can be involved (Neha et al. 2021).
There are many spent magnetic nanoadsorbent regeneration methods among which the chemical method appears to be popular (Meng et al. 2018; Campos et al. 2019; Gagliano et al. 2020; Sahoo et al. 2020; Bakhshi Nejad and Mohammadi 2020; Biata et al. 2020; Jain et al. 2021; Peralta et al. 2021). Other adsorbent regeneration methods are thermal (Aguedal et al. 2019), supercritical extraction (Momina et al. 2018), microbial regeneration (Momina et al. 2018), solvent extraction (Dutta et al. 2019), and microwave and ultraviolet irradiation (Sun et al. 2017). Accordingly, the utilization of regenerated magnetic nanoadsorbents can have an impact on the efficiency of the water purification and wastewater treatment processes where they are put to use. This is because the quality of the exhausted nanoadsorbent regeneration process is influenced by pH (Momina et al. 2018; Wen et al. 2020), molecular structure of adsorbate (Gagliano et al. 2020), functional groups present (Meng et al. 2018), temperature (Aguedal et al. 2019; Jiang et al. 2019) and surface charge (Meng et al. 2018). Thus, an optimization of the regeneration method for a specific exhausted magnetic nanoadsorbent becomes necessary. Such an optimization will be vital for ensuring a maximum possible stability, selectivity and improved adsorption efficiency of the regenerated magnetic nanoadsorbent during its next set of multiple adsorptive interactions with the target micropollutant(s).
Pilot-scale magnetic separation systems
As compared to the number of laboratory-scale studies which have examined the performance of magnetically separable adsorbents, there are relatively fewer studies which have reported the pilot-scale behaviors of novel magnetic adsorbents utilized in micropollutant removal. The following discussions revisit some salient aspects of these studies, and highlight a number of favorable findings and system-specific limitations. For example, an open-gradient magnetic separator consisting of identical electromagnets operating as the capture elements was designed, optimized, and experimentally examined for water purification under turbulent water flow regimes (Belounis et al. 2015). The optimization was based on the assessment of capture efficiencies of different separator configurations, and took into consideration the following parameters: capture element sizing, particle radius, particle mass density, particle magnetic permeability, channel diameter, water mass density and water dynamic viscosity, and average flow velocity (Belounis et al. 2015).
Recently, a laboratory-scale magnetic separator (μ-Jones) simulating large-scale wet magnetic separator systems was designed to demonstrate that magnetic extraction of vivianite from sludge was achievable (Prot et al. 2019). A number of interesting findings were reported in the latter work, and they demonstrated proof-of-concept of magnetic separation to some reasonable extent. Among the results obtained, magnetic separation was able to concentrate vivianite by a factor 2–3 and could also decrease organic content from 40 to 20% (Prot et al. 2019). Besides allowing recovery of total phosphorus as vivianite, implementation of magnetic separation at wastewater treatment plants could decrease the amount of waste sludge, and also augment its heating value by lowering its mineral content (Prot et al. 2019). Encouragingly, preliminary cost analysis indicate that these advantages (particularly the projected decrease in waste sludge volume) are in balance with putting into place a magnetic separator when the associated investment and operation costs are accounted for (Prot et al. 2019).
In a study which dealt with the removal and recovery of dissolved phosphate from wastewater in a pilot-scale system using ZnFeZr@Fe3O4/SiO2 adsorbent with magnetic harvesting, some operational limitations were observed (Drenkova-Tuhtan et al. 2017). Thus, besides the favorable removal performance observed on the whole in the pilot-scale tests (viz. an effective 50-time upscaling of the proposed technology by remediating 1.5 m3 wastewater in twenty cycles), some of the limitations were:
A decline in adsorption efficiency because of a consistent loss of adsorbent particles as cycle 10 was reached,
The high-gradient magnetic separation was confronted with discontinuous operation because of the need to effect regular flushing, which in turn induced the dilution of particle concentrate, and
Desorption efficiency varied more than in the laboratory-scale tests, possibly because of the higher mass of adsorbent particles per unit volume of desorption solution, which led to incomplete regeneration of the adsorbent in some cycles (Drenkova-Tuhtan et al. 2017).
An accurate estimation of running costs was not workable at that stage of the process development (Drenkova-Tuhtan et al. 2017). However, the pilot-scale findings pointed toward the principal operating costs being those for the replacement of lost or exhausted adsorbent particles, followed by those for energy and chemicals consumption (Drenkova-Tuhtan et al. 2017).
A preliminary assessment of a pilot-scale magnetic separator demonstrated that magnetizable clays could be effectively used for the treatment of textile dyeing wastewater on magnetic drum separators (Salinas et al. 2018). The magnetic drum separator had a rotating drum (external diameter=20 cm, depth=12.5 cm, and with an arrangement of fifty neodymium magnets of 5 × 2 × 0.5 cm on its inner side) mounted on a cylindrical plastic container by a metal shaft (Salinas et al. 2018). The magnetic clay was separated from the drum by a plastic blade and recuperated in a plastic container (Salinas et al. 2018). With the magnetic drum separator operated at a flow rate of 0.08 L min–1, 62% dye removal could be obtained, and the outlet effluent dye concentration was 92 ppm for a 10 min residence time on the separator (Salinas et al. 2018). In another study, the separation efficiency for magnetic hydrogel adsorbing Cr(VI) was more than 97% throughout the twenty cycles of treatment in an industrial wastewater treatment prototype (Tang et al. 2014). The prototype had a 5-L magnetic separation unit comprising an electromagnetic system at the bottom for generating a magnetic field of strength ~200 mT (Tang et al. 2014). This unit generated a magnetic field that had zigzag pathways for maximizing the magnetic hydrogel’s capture (Tang et al. 2014).
In a recent insightful work which highlights the merits of cooperative magnetophoresis, an in-line, wastewater-cooled electromagnetic collection system has been developed (Hutchins and Downey 2020). This new system could produce collections at very high efficiencies consistently more than 98% (with a magnetic core of 200 wires (Core I)) when paired with magnetite nanoparticles because of the intimate contact induced when placing the coil directly in the copper(II)-containing wastewater flow (Hutchins and Downey 2020). The water cooling feature of the electromagnetic collection system enabled the onset of a much more powerful magnetic field that, in turn, tends to allow the use of pipes with larger diameters and accommodate flows at higher fluid velocities (Hutchins and Downey 2020). The latter are two important requisites for an effective industrial-scale application of a magnetic separation system. Interestingly, flows of up to 8.1 L min–1 with up to 80 gram-particles could produce the target benchmark collection efficiency of 98% (Hutchins and Downey 2020). However, the decrease in collection efficiencies for particles of greater masses was attributed to the excess build-up of particles on the core wires, and at a specific point in this fluid-velocity-dependent build-up, the fluid drag force becomes greater than the magnetophoretic force, and the magnetite particles are carried into the flow (Hutchins and Downey 2020).
Recently, an innovative, scalable and optimized permanent magnetic nanoparticle recovery apparatus (called “MagNERD” having a maximum fluid volume of 1110 mL) has been developed (Powell et al. 2020). This device was examined using experimental investigations and computational fluid dynamics modeling approaches for its performance in separating, capturing and reusing superparamagnetic Fe3O4 nanoparticles from treated water in-line for continuous flows (Powell et al. 2020). Results indicated that the efficiency of the novel MagNERD system in recovering the magnetic nanoadsorbents was dependent on the configuration of the device and hydraulic flow conditions, and magnetic nanoadsorbents uptake (Powell et al. 2020). The MagNERD system had successfully removed more than 94% of As-bound Fe3O4, after mixing simulated drinking water consisting of arsenic with the magnetic nanoadsorbents used (Powell et al. 2020). In addition, this device was able in removing Fe3O4 in nanopowder form for as high as more than 95% at elevated concentrations of 500 ppm at 1 L min–1, and from different types of water (e.g., brackish water and ultrapure water) (Powell et al. 2020).
Magnetic separator inventions and patents
There are also some patents which describe interesting magnetic separator inventions having different geometries and different operating principles for prospective applications in water purification and wastewater treatment (Lombardi and Morley 2017; Liu et al. 2020b, WATER ONLINE 2008). One of these inventions reports the design of devices and development of procedures for undertaking in-line water treatment through the application of strong magnetic fields, which in turn exert an influence on corrosion, separation of toxins, suppressing of bacteria and bio-fouling, and prevention or considerable decrease in mineral scaling arising from fluid flow in or around the components in the equipment (Lombardi and Morley 2017).
There have been commercial applications of magnetic seeding for the treatment of drinking water with (e.g., ‘Comag’ process) and without (e.g., ‘Sirofloc’ technology) magnetic separation (Cort 2008, 2010). Interestingly, there is also an invention which is a ‘hybrid’ treatment system combining magnetic separation with activated sludge treatment designed to remove dissolved aqueous pollutants from a wide range of contaminated waters (municipal wastewaters, industrial wastewaters, combined sewer overflows, potable waters, any other waters containing dissolved inorganic or organic contaminants) (Cort 2009). In another example, the invention is particularly relevant for high flow water treatment applications which have to be efficient and simple; and for specific operational requirements, this invention can also combine vortex separation with magnetic separation to improve magnetic seed material cleaning and lower solids load on the final magnetic collector system (Cort 2007).
Magnetic separation in large-scale water purification
We have also come across a few full-scale case studies which have reported the application of magnetic separation in water purification. For example, a high-gradient magnetic separation system equipped with superconducting magnet (3 T, 0.68 m long and 0.4 m bore NbTi solenoid) was designed to purify paper mill wastewater continuously (Nishijima and Takeda 2006). The main features and performances to be achieved by this magnetic separation system were: (1) reducing the chemical oxygen demand of the purified effluent to less than 40 ppm and to be recyclable, and (2) processing wastewater flows above 2000 tons on a daily basis (Nishijima and Takeda 2006). In another example, one supermagnetic separation system was used by the Shandong New Dragon Energy Limited Liability Company (design treatment capacity = 34,000 m3 day–1) in March 2010 for treating underground mine water (Zhang et al. 2020).
In another investigation, a high-gradient magnetic separation (employing a 6-T cryo-cooled Nb-Ti superconducting magnet) was used to remove impurities from the condenser water (containing mostly hematite and maghemite) in a thermal power plant (Lee et al. 2011). In the test runs, the condenser water turbidity was decreased up to 99.6%, and more of the iron oxides could be scavenged at higher magnetic field strengths (1-6T) (Lee et al. 2011). Back in 1978, a report (EPA600/2-78/209, and under the Contract No. 68-03-2218) described the preliminary on-site stage testing of magnetic separation for seeded water treatment involving magnetite (Allen 1978). The investigations were conducted with a SALA high-gradient magnetic separator pilot unit on combined sewer overflows and raw sewage at SALA Magnetics, Inc. in Cambridge, Massachusetts, and at on-site places in the Boston area (Allen 1978). Although the on-site findings reported did not match those recorded with uniform batch samples in house, they were still good enough in demonstrating that high-gradient magnetic filtration was effective on fresh combined sewer overflows and raw sewage (Allen 1978). In addition, the on-site results indicated that the magnetic filtration-based treatment system could easily adapt to flow rate conditions and dynamic solids loading usually observed with storm water and integrated wet and dry treatment systems (Allen 1978).
A water treatment system in a thermal power plant was equipped with a high-gradient magnetic separation system utilizing a solenoidal superconducting magnet (model number JMTD-10T100E3, bore diameter=10 cm, height=46 cm) and magnetite for enhancing the efficiency of operations (Shibatani et al. 2016). The flow velocity was 0.6 ms–1 and the magnetic flux density applied was 2.0 T. In the high-gradient magnetic separation investigations which could be run at high-pressure and high-temperature, a reduction in the separation rate and an increase in pressure loss had been warded off, and the total amount of captured scale had augmented by reason of an appropriate filter design (Shibatani et al. 2016). The standard deviation of magnetite capture rate was 3.4 when the filter material was galvanized iron (16.3 g of magnetite captured in this case), whereas the capture rate was significantly higher at 29 when the filter material used was stainless steel 430 (11.2 g of magnetite captured) (Shibatani et al. 2016). At 10 ppm of magnetite, blockage of the magnetic filters occurred. In the former magnetic filter design, the starting separation rate was 89% which remained quasi-constant for the first 10 minutes, but then decreased to 64% over the next 10 minutes (Shibatani et al. 2016). For this same filter system, pressure loss gradually rose from 9.5 to 10.5 kPa and remained practically constant after 15 minutes. Based on the findings, the galvanized iron magnetic filter system (with a diameter of 51 mm) was thence deemed convenient for extended continuous operation for scale removal in the feed-water system of the plant (Shibatani et al. 2016).
Gaps and development openings
Based on our analysis of the literature so far, we infer there is reasonable ground for developing a large-scale (industrial) usage of magnetic nanoadsorbents for water purification and wastewater treatment together with the incorporation of magnetic separation operating downstream for recovering the spent magnetic nanoadsorbents (Lee et al. 2011; Liu et al. 2013; Simeonidis et al. 2015; Roy et al. 2017; Mirshahghassemi et al. 2017; Lompe et al. 2018; Lingamdinne et al. 2019b, a; Augusto et al. 2019; Huang et al. 2019; Prot et al. 2019; Cui et al. 2020; Ghernaout and Elboughdiri 2020; Abdel Maksoud et al. 2020; Kheshti et al. 2020; Powell et al. 2020; Salehin et al. 2020; Khan et al. 2020; Hussen Shadi et al. 2020; Acosta et al. 2020; Rais et al. 2021; Leonel et al. 2021).
Yet, there appears to be a major lacuna in the development and implementation of a mature combined magnetic nanoadsorbent-based adsorption–magnetic separation in water purification and wastewater treatment processes that are intended to operate at high capacity and under continuous flows at the industrial scale (Augusto et al. 2019; Powell et al. 2020). This gap gives way to substantial hope for more research and development and progress in the area of water science and water treatment technology using magnetic nanoadsorbents and magnetic separation downstream the unit operations housing the magnetic nanoadsorbents-based adsorption processes.
Three major interconnected components will require substantial research and development efforts toward the potential integration of magnetic nanoadsorbents’ use and magnetic separation in real-scale/industrial-scale water and wastewater depuration systems. These are:
Maximizing the capture of untreated wastewaters and channeling them to the large-scale water and wastewater treatment facilities
Selecting intelligent magnetic nanoadsorbent(s) for industrial application
System modeling, simulation and process optimization of real water/wastewater remediation systems using magnetic nanoadsorbents and magnetic separation
Further research and development can generate more real-world investigations of pilot-scale ‘intelligent’ magnetic nanoadsorbents-based adsorption system for their system design and optimization on a case-to-case basis. A case-to-case basis approach seems much plausible because the research and development investigations will need to consider the existing water purification and wastewater treatment processes, and then factor in the significant variabilities that can occur in physicochemical and biological characteristics of contaminated waters (e.g., groundwaters (Subba Rao et al. 2017; Yetiş et al. 2019; Ferrer et al. 2020; Gnanachandrasamy et al. 2020) and drinking water (Navab-Daneshmand et al. 2018; Kumar et al. 2019; Jehan et al. 2019)) and wastewaters (e.g., landfill leachates (Augusto et al. 2019) and complex textile wastewaters containing dyes (Bhatia et al. 2017; Huang et al. 2020)) being treated. The findings can then be used to formulate appropriate engineering project opportunities that enable the use magnetic nanoadsorbents and integration of magnetic separation in existing water purification and wastewater treatment units. Hence, we equally envision that innovative magnetic nanoadsorbent-based adsorption units and magnetic separation systems are retrofitted in the existing tertiary (de Andrade et al. 2018), or quarternary water/wastewater treatment units (Gawel 2015).
The word ‘intelligent’ has been used above to bring in the notion of a system using magnetic nanoadsorbents which can adequately self-modulate their properties and adsorption performances in response to external biological, chemical and/or physical stimuli normally encountered in real contaminated waters/wastewaters. The ‘intelligent sensing’ can be a response of the intelligent magnetic nanoadsorbent toward a single stimulus or more. The stimuli can be:
-
(i)
Physical such as exposure to variations in light intensity (Xu et al. 2020), temperature (Ebadollahzadeh and Zabihi 2020; Li et al. 2021b), magnetic field strength (Flores López et al. 2018) and hydrodynamic mechanical shear forces which can get onset during continuous turbulently mixed reactor-type (Xie et al. 2017; Jun et al. 2020) or bed-type adsorption processes (Niksefat Abatari et al. 2017);
-
(ii)
Chemical because of fluctuations in pH (Reguyal and Sarmah 2018), variations in ionic strength (Zhang et al. 2019), and due to variable concentrations of competing/coexisting species such as ammonium (Mazloomi and Jalali 2017), phosphate, sulfate, nitrate (Tuutijärvi et al. 2012; Rashid et al. 2017), multiple organic pollutants, e.g., dyes, pharmaceuticals and agrochemicals (Hlongwane et al. 2019), natural organic matter such as humic substances (Reguyal and Sarmah 2018; He et al. 2018), and alkali and alkali-earth metal ions (e.g., K+, Mg2+, Ca2+) (Quiroga-Flores et al. 2020), transition (e.g., Co2+, Cd2+, Ni2+) metal ions (Quiroga-Flores et al. 2020) and/or ions with a radioactive character (e.g., Sr2+ (Vivas et al. 2020), Cs+ (Işık et al. 2021) or uranyl ion () (Yang et al. 2017b)); and
-
(iii)
Microbiological due to potential interactions of magnetic nanoadsorbents with a multitude of microorganisms to form microbial aggregates which in turn can protect them (Tang et al. 2018).
Though relatively novel, there are already such intelligent magnetic materials which have been examined for their adsorption performance in water and wastewater remediation (Yu et al. 2020; Ciğeroğlu et al. 2021; Leonel et al. 2021; Yang et al. 2021). Therefore, we think it is opportune to borrow insights from these repositories of scientific data to design and scale-up intelligent magnetic nanoadsorbents-based adsorption units for application in full-scale water purification and wastewater treatment systems. These units will have to be stable, robust and adequately effective in producing final effluents which comply with the prevailing effluent discharge limits and regulatory standards of the target micropollutants.
System modeling, numerical simulation, and process optimization (Liu et al. 2019; Powell et al. 2020) will be integral components in the design of these units. This is because a balance will need to be constantly maintained amidst the interplay of the key process and design parameters. Some of these main parameters/features are: particle size of magnetic nanoadsorbents (Hutchins and Downey 2020), the geometry and configuration of the adsorption units, the dispersion or immobilization of magnetic nanoadsorbents, the spatial distribution of magnetic nanoadsorbents within the adsorption unit(s), the tendency for magnetic nanoadsorbents to aggregate or get leached, the susceptibility of magnetic nanoadsorbents to be biodegraded by indigenous or survivor microbes, and the overall adsorption behavior of magnetic nanoadsorbents in real water purification and wastewater treatment conditions. Controlling and minimizing agglomeration and precipitation of magnetic nanoadsorbents are important as well.
Moreover, the production of magnetic nanoadsorbents at the kilogram scale (and hopefully at the ton scale) under optimized operating conditions will have to be established as mature processes (Cheong and Moh 2018; Lorignon et al. 2020). In addition, it will be critical to ensure that the magnetic nanoadsorbents being produced in bulk have preserved enough of those outstanding properties and are effective in delivering those adsorption performances observed at laboratory scale for the target micropollutant(s). These requirements, when fulfilled, can assist in paving the way to the commercial use of magnetic nanoadsorbents in full-scale water purification and wastewater treatment facilities.
In addition, the development of optimized high-gradient magnetic separators (Kakihara et al. 2004; Baik et al. 2013; Simeonidis et al. 2015; Tripathy et al. 2017; Mirshahghassemi et al. 2017; Han et al. 2017; Ebeler et al. 2018; Kheshti et al. 2019a, 2020; Powell et al. 2020) occupies a core segment of the research and development efforts needed to mature the use of magnetic nanoadsorbents for application in water purification and wastewater treatment at the industrial scale. It is critical to design energy-efficient magnetic separation systems which respond favorably to the energy requirements of retrofitting such systems in the water purification and wastewater treatment industry.
The design of the magnetic separator system will have to be properly tuned for the following parameters on a case-to-case basis as well: its optimal geometry in relation to aqueous stream flow patterns (Kheshti et al. 2019b), potential flocculation and coagulation behaviors (Lv et al. 2019, 2021; Sun et al. 2021), flow paths (Kakihara et al. 2004; Tang et al. 2014) and effects of turbulences and variable fluid shear forces; concentrations and mass loading of magnetic nanoadsorbents (Powell et al. 2020); the magnetic field strength distributions, flow velocity profiles and liquid streamlines being developed during the magnetic separation; the exposure intensity; effects of any residual magnetization arising from presence of mechanical components (Powell et al. 2020); colloidal stability and magnetic separability (Hutchins and Downey 2020); and residence time distributions with or without effluent recirculation.
Research directions
Based on the findings of this review, we are of the mind the above points carry reasonable weight for warranting comprehensive pilot-scale and in situ experimentation, and system design and optimization of magnetic nanoadsorbent-based adsorption units and magnetic separation systems for enabling their integration in existing full-scale water purification and wastewater treatment facilities. Retrofitting existing water treatment facilities with optimized magnetic nanoadsorbent-based adsorption units and magnetic separation systems can potentially yield higher purification efficiencies. In addition, the recovery of micropollutant-saturated magnetic nanoadsorbents can be achieved at potentially higher capture efficiencies. In addition, by optimizing the operational parameter and design settings of the magnetic separation systems, the residual concentration of magnetic nanoadsorbents in the final-treated effluent can be brought to a safe minimum, and possibly to trace levels. Accordingly, we identify the following key research avenues:
Active involvement and contribution of interdisciplinary expertise, namely from physics, materials science and engineering, environmental chemistry, chemical process design and engineering, control system engineering, toxicology, environmental economics, and plausibly policy making as well for materializing the commercial production and use of magnetic nanoadsorbents.
It will be a significant research and development challenge to tailor make optimized and economic magnetic nanoadsorbents’ regeneration routes when planning their use in large-scale water purification and wastewater treatment systems. Thus, defining the finite frequency at which the regenerated magnetic nanoadsorbents can be economically replaced in a process becomes important.
It is of the utmost importance to keep on demonstrating the ‘proof-of-concept’ of yet more innovative magnetic separation systems capable of treating high flow rates continuously and in-line in existing full-scale water purification and wastewater treatment facilities on a case-to-case basis.
The lifecycle environmental impacts of the use of magnetic nanoadsorbents and magnetic separation systems in large-scale water purification and wastewater treatment systems have to be comprehensively elucidated.
More collaboration of key industry partners and the research community will be equally crucial in research and development activities related to the design and pilot-scale testing of effective magnetic separation system in the existing water treatment facilities.
Conclusion
Demonstration of the aforementioned ‘proof-of-concept’ can hopefully help in dispelling doubts and reducing risk-related reluctance (Kiparsky et al. 2016; Trapp et al. 2017; Sherman et al. 2020) of the water treatment industry toward retrofitting of the existing installations. Accordingly, stronger ‘university-utility’ collaborations (Brown et al. 2020) have to be developed for harnessing the potential of selected ‘super’ magnetic nanoadsorbents in large-scale water purification and wastewater treatment systems. We look forward to a mature utilization of magnetic nanoadsorbents for target micropollutant removal coupled with a viable integration of magnetic separation in the existing full-scale water purification and wastewater treatment facilities gradually becoming a “disruptive innovation” (Si and Chen 2020) in the water treatment sector.
Acknowledgements
The contents of this article have been crosschecked for similarity in the Turnitin software multiple times. Figure 1 was created with BioRender.com, and exported under the paid plan having Receipt #2116-9809.
Author contributions:
AM involved in conceptualization, data curation, writing–original draft, review & editing and revision. MS involved in review of original draft & editing and revision
Funding
This work received no funding.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ackmez Mudhoo, Email: a.mudhoo@uom.ac.mu.
Mika Sillanpää, Email: mika.sillanpaa@tdtu.edu.vn.
References
- Abdel Maksoud MIA, Elgarahy AM, Farrell C, et al. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord Chem Rev. 2020;403:213096. doi: 10.1016/j.ccr.2019.213096. [DOI] [Google Scholar]
- Acosta L, Galeano-Caro D, Medina OE, et al. Nano-intermediate of magnetite nanoparticles supported on activated carbon from spent coffee grounds for treatment of wastewater from oil industry and energy production. Processes. 2020;9:63. doi: 10.3390/pr9010063. [DOI] [Google Scholar]
- Aguedal H, Iddou A, Aziz A, et al. Effect of thermal regeneration of diatomite adsorbent on its efficacy for removal of dye from water. Int J Environ Sci Technol. 2019;16:113–124. doi: 10.1007/s13762-018-1647-5. [DOI] [Google Scholar]
- Ahmad M, Wang J, Xu J, et al. Novel synthetic method for magnetic sulphonated tubular trap for efficient mercury removal from wastewater. J Colloid Interface Sci. 2020;565:523–535. doi: 10.1016/j.jcis.2020.01.024. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Wang J, Xu J, et al. Magnetic tubular carbon nanofibers as efficient Cu(II) ion adsorbent from wastewater. J Clean Prod. 2020;252:119825. doi: 10.1016/j.jclepro.2019.119825. [DOI] [Google Scholar]
- Aliannejadi S, Hassani AH, Panahi HA, Borghei SM. Fabrication and characterization of high-branched recyclable PAMAM dendrimer polymers on the modified magnetic nanoparticles for removing naphthalene from aqueous solutions. Microchem J. 2019;145:767–777. doi: 10.1016/j.microc.2018.11.043. [DOI] [Google Scholar]
- Allen DM (1978) Treatment of combined sewer overflows by high gradient magnetic separation. On-site testing with mobile pilot plant trailer. U.S. Environmental Protection Agency, Washington, D.C., EPA/600/2-78/209. https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=ORD&dirEntryID=49771 and https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=9100SR4K.TXT. (accessed 12 February 2021)
- Alvarez PJJ, Chan CK, Elimelech M, et al. Emerging opportunities for nanotechnology to enhance water security. Nat Nanotechnol. 2018;13:634–641. doi: 10.1038/s41565-018-0203-2. [DOI] [PubMed] [Google Scholar]
- Álvarez-Manzaneda I, Guerrero F, Cruz-Pizarro L, et al. Magnetic particles as new adsorbents for the reduction of phosphate inputs from a wastewater treatment plant to a Mediterranean Ramsar wetland (Southern Spain) Chemosphere. 2021;270:128640. doi: 10.1016/j.chemosphere.2020.128640. [DOI] [PubMed] [Google Scholar]
- Ambashta RD, Sillanpää M. Water purification using magnetic assistance: A review. J Hazard Mater. 2010;180:38–49. doi: 10.1016/j.jhazmat.2010.04.105. [DOI] [PubMed] [Google Scholar]
- Arslan M, Ullah I, Müller JA, et al. Enhancing cleanup of environmental pollutants. Cham: Springer International Publishing; 2017. Organic Micropollutants in the environment: ecotoxicity potential and methods for remediation; pp. 65–99. [Google Scholar]
- Aryee AA, Dovi E, Shi X, et al. Zirconium and iminodiacetic acid modified magnetic peanut husk as a novel adsorbent for the sequestration of phosphates from solution: Characterization, equilibrium and kinetic study. Colloids Surfaces A Physicochem Eng Asp. 2021;615:126260. doi: 10.1016/j.colsurfa.2021.126260. [DOI] [Google Scholar]
- Asadi M, Sereshti H, Rashidi Nodeh H. Development of magnetic dispersive microsolid-phase extraction using lanthanum phosphate nanoparticles doped on magnetic graphene oxide as a highly selective adsorbent for pesticide residues analysis in water and fruit samples. Res Chem Intermed. 2020;46:2789–2803. doi: 10.1007/s11164-020-04121-y. [DOI] [Google Scholar]
- Augusto PA, Castelo-Grande T, Merchan L, et al. Landfill leachate treatment by sorption in magnetic particles: preliminary study. Sci Total Environ. 2019;648:636–668. doi: 10.1016/j.scitotenv.2018.08.056. [DOI] [PubMed] [Google Scholar]
- Azam K, Raza R, Shezad N, et al. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J Environ Chem Eng. 2020;8:104220. doi: 10.1016/j.jece.2020.104220. [DOI] [Google Scholar]
- Bahadori E, Ramis G, Rossetti I (2020) Matching nanotechnologies with reactor scale-up and industrial exploitation. In: Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier, pp 407–442
- Baig U, Uddin MK, Gondal MA. Removal of hazardous azo dye from water using synthetic nano adsorbent: Facile synthesis, characterization, adsorption, regeneration and design of experiments. Colloids Surfaces A Physicochem Eng Asp. 2020;584:124031. doi: 10.1016/j.colsurfa.2019.124031. [DOI] [Google Scholar]
- Baik SK, Ha DW, Kwon JM, et al. Magnetic force on a magnetic particle within a high gradient magnetic separator. Phys C Supercond. 2013;484:333–337. doi: 10.1016/j.physc.2012.03.033. [DOI] [Google Scholar]
- Bakhshi Nejad S, Mohammadi A. Epoxy-triazinetrione-functionalized magnetic nanoparticles as an efficient magnetic nanoadsorbent for the removal of malachite green and Pb(II) from aqueous solutions. J Chem Eng Data. 2020;65:2731–2742. doi: 10.1021/acs.jced.0c00063. [DOI] [Google Scholar]
- Balbino TAC, Bellato CR, da Silva AD, et al. Preparation and evaluation of iron oxide/hydrotalcite intercalated with dodecylsulfate/β-cyclodextrin magnetic organocomposite for phenolic compounds removal. Appl Clay Sci. 2020;193:105659. doi: 10.1016/j.clay.2020.105659. [DOI] [Google Scholar]
- Belounis A, Mehasni R, Ouil M, et al. Design With Optimization of a magnetic separator for turbulent flowing liquid purifying applications. IEEE Trans Magn. 2015;51:1–8. doi: 10.1109/TMAG.2015.2424401. [DOI] [Google Scholar]
- Bharti MK, Gupta S, Chalia S, et al. Potential of magnetic nanoferrites in removal of heavy metals from contaminated water: mini review. J Supercond Nov Magn. 2020;33:3651–3665. doi: 10.1007/s10948-020-05657-1. [DOI] [Google Scholar]
- Bhatia D, Sharma NR, Singh J, Kanwar RS. Biological methods for textile dye removal from wastewater: A review. Crit Rev Environ Sci Technol. 2017;47:1836–1876. doi: 10.1080/10643389.2017.1393263. [DOI] [Google Scholar]
- Bi R, Li F, Chao J, et al. Magnetic solid-phase extraction for speciation of mercury based on thiol and thioether-functionalized magnetic covalent organic frameworks nanocomposite synthesized at room temperature. J Chromatogr A. 2021;1635:461712. doi: 10.1016/j.chroma.2020.461712. [DOI] [PubMed] [Google Scholar]
- Biata NR, Jakavula S, Mashile GP, et al. Recovery of gold(III) and iridium(IV) using magnetic layered double hydroxide (Fe3O4/Mg-Al-LDH) nanocomposite: equilibrium studies and application to real samples. Hydrometallurgy. 2020;197:105447. doi: 10.1016/j.hydromet.2020.105447. [DOI] [Google Scholar]
- Biehl P, von der Lühe M, Schacher FH. Reversible adsorption of methylene blue as cationic model cargo onto polyzwitterionic magnetic nanoparticles. Macromol Rapid Commun. 2018;39:1800017. doi: 10.1002/marc.201800017. [DOI] [PubMed] [Google Scholar]
- Borji H, Ayoub GM, Al-Hindi M, et al. Nanotechnology to remove polychlorinated biphenyls and polycyclic aromatic hydrocarbons from water: a review. Environ Chem Lett. 2020;18:729–746. doi: 10.1007/s10311-020-00979-x. [DOI] [Google Scholar]
- Brião G de V, de Andrade JR, da Silva MGC, Vieira MGA (2020) Removal of toxic metals from water using chitosan-based magnetic adsorbents. A review. Environ Chem Lett 18:1145–1168. doi: 10.1007/s10311-020-01003
- Brown M, Karimova F, Love N, et al. University–utility partnerships: Best practices for water innovation and collaboration. Water Environ Res. 2020;92:314–319. doi: 10.1002/wer.1252. [DOI] [PubMed] [Google Scholar]
- Çalımlı MH. Magnetic nanocomposite cobalt-multiwalled carbon nanotube and adsorption kinetics of methylene blue using an ultrasonic batch. Int J Environ Sci Technol. 2021;18:723–740. doi: 10.1007/s13762-020-02855-1. [DOI] [Google Scholar]
- Campos AFC, de Oliveira HAL, da Silva FN, et al. Core-shell bimagnetic nanoadsorbents for hexavalent chromium removal from aqueous solutions. J Hazard Mater. 2019;362:82–91. doi: 10.1016/j.jhazmat.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Carvalho APA, Conte-Junior CA. Recent advances on nanomaterials to COVID-19 management: a systematic review on antiviral/virucidal agents and mechanisms of SARS-CoV-2 inhibition/inactivation. Glob Challenges. 2021 doi: 10.1002/gch2.202000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Grande T, Augusto PA, Rico J, et al. Magnetic water treatment in a wastewater treatment plant: Part I - sorption and magnetic particles. J Environ Manage. 2021;281:111872. doi: 10.1016/j.jenvman.2020.111872. [DOI] [PubMed] [Google Scholar]
- Çavuşoğlu FC, Bayazit ŞS, Secula MS, Cagnon B. Magnetic carbon composites as regenerable and fully recoverable adsorbents: Performance on the removal of antidiabetic agent metformin hydrochloride. Chem Eng Res Des. 2021;168:443–452. doi: 10.1016/j.cherd.2021.01.034. [DOI] [Google Scholar]
- Chang S, Zhang Q, Lu Y, et al. High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Sep Purif Technol. 2020;238:116400. doi: 10.1016/j.seppur.2019.116400. [DOI] [Google Scholar]
- Chang L, Pu Y, Jing P, et al. Magnetic core-shell MnFe2O4@TiO2 nanoparticles decorated on reduced graphene oxide as a novel adsorbent for the removal of ciprofloxacin and Cu(II) from water. Appl Surf Sci. 2021;541:148400. doi: 10.1016/j.apsusc.2020.148400. [DOI] [Google Scholar]
- Chavan VD, Kothavale VP, Sahoo SC, et al. Adsorption and kinetic behavior of Cu(II) ions from aqueous solution on DMSA functionalized magnetic nanoparticles. Phys B Condens Matter. 2019;571:273–279. doi: 10.1016/j.physb.2019.07.026. [DOI] [Google Scholar]
- Chen S, Xie F. Selective adsorption of Copper (II) ions in mixed solution by Fe3O4-MnO2-EDTA magnetic nanoparticles. Appl Surf Sci. 2020;507:145090. doi: 10.1016/j.apsusc.2019.145090. [DOI] [Google Scholar]
- Chen R, Wang P, Li M, et al. Removal of Cr(VI) by magnetic Fe/C crosslinked nanoparticle for water purification: rapid contaminant removal property and mechanism of action. Water Sci Technol. 2018;78:2171–2182. doi: 10.2166/wst.2018.497. [DOI] [PubMed] [Google Scholar]
- Chen B, Cao Y, Zhao H, et al. A novel Fe3+-stabilized magnetic polydopamine composite for enhanced selective adsorption and separation of Methylene blue from complex wastewater. J Hazard Mater. 2020;392:122263. doi: 10.1016/j.jhazmat.2020.122263. [DOI] [PubMed] [Google Scholar]
- Cheong VF, Moh PY. Recent advancement in metal–organic framework: synthesis, activation, functionalisation, and bulk production. Mater Sci Technol. 2018;34:1025–1045. doi: 10.1080/02670836.2018.1468653. [DOI] [Google Scholar]
- Ciejka J, Wolski K, Nowakowska M, et al. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater Sci Eng C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciğeroğlu Z, Küçükyıldız G, Erim B, Alp E. Easy preparation of magnetic nanoparticles-rGO-chitosan composite beads: Optimization study on cefixime removal based on RSM and ANN by using genetic algorithm approach. J Mol Struct. 2021;1224:129182. doi: 10.1016/j.molstruc.2020.129182. [DOI] [Google Scholar]
- Cort S (inventor) (2007) Assignee: Cort SL. Water treatment using magnetic and other field separation technologies. United States patent application US 11/503,951 (22 February 2007). https://patents.google.com/patent/US20070039894A1/en. (accessed 29 January 2021 to 10 February 2021)
- Cort SL (inventor) (2008) Assignee: Cort SL. Magnetic Separator for Water Treatment System. United States patent application US 11/862,767 (27 March 2008). https://patents.google.com/patent/US20080073283A1/en. (accessed 29 January 2021 to 10 February 2021)
- Cort SL (inventor) (2009) Assignee: Cort SL. Use of a magnetic separator to biologically clean water. United States patent US 7,625,490 (1 December 2009). https://patents.google.com/patent/US7625490B2/en. (accessed 29 January 2021 to 10 February 2021)
- Cort SL (inventor) (2010) Assignee: Cort CJ. Magnetic separation and seeding to improve ballasted clarification of water. United States patent US 7,820,053 (26 October 2010). https://patents.google.com/patent/US7820053B2/en. (accessed 29 January 2021 to 10 February 2021)
- Crini G, Lichtfouse E, Wilson LD, Morin-Crini N. Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett. 2019 doi: 10.1007/s10311-018-0786-8. [DOI] [Google Scholar]
- Cui Y, Kang W, Qin L, et al. Magnetic surface molecularly imprinted polymer for selective adsorption of quinoline from coking wastewater. Chem Eng J. 2020;397:125480. doi: 10.1016/j.cej.2020.125480. [DOI] [Google Scholar]
- D’Cruz B, Madkour M, Amin MO, Al-Hetlani E. Efficient and recoverable magnetic AC-Fe3O4 nanocomposite for rapid removal of promazine from wastewater. Mater Chem Phys. 2020;240:122109. doi: 10.1016/j.matchemphys.2019.122109. [DOI] [Google Scholar]
- Dai K, Liu G, Xu W, et al. Judicious fabrication of bifunctionalized graphene oxide/MnFe2O4 magnetic nanohybrids for enhanced removal of Pb(II) from water. J Colloid Interface Sci. 2020;579:815–822. doi: 10.1016/j.jcis.2020.06.085. [DOI] [PubMed] [Google Scholar]
- de Andrade JR, Oliveira MF, da Silva MGC, Vieira MGA. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind Eng Chem Res. 2018;57:3103–3127. doi: 10.1021/acs.iecr.7b05137. [DOI] [Google Scholar]
- Drenkova-Tuhtan A, Schneider M, Franzreb M, et al. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent @ Fe3O4/SiO2 particles with magnetic harvesting. Water Res. 2017;109:77–87. doi: 10.1016/j.watres.2016.11.039. [DOI] [PubMed] [Google Scholar]
- Dutta T, Kim T, Vellingiri K, et al. Recycling and regeneration of carbonaceous and porous materials through thermal or solvent treatment. Chem Eng J. 2019;364:514–529. doi: 10.1016/j.cej.2019.01.049. [DOI] [Google Scholar]
- Ebadollahzadeh H, Zabihi M. Competitive adsorption of methylene blue and Pb (II) ions on the nano-magnetic activated carbon and alumina. Mater Chem Phys. 2020;248:122893. doi: 10.1016/j.matchemphys.2020.122893. [DOI] [Google Scholar]
- Ebeler M, Pilgram F, Wolz K, et al. Magnetic separation on a new level: characterization and performance prediction of a cGMP scompliant “rotor-stator” high-gradient magnetic separator. Biotechnol J. 2018;13:1700448. doi: 10.1002/biot.201700448. [DOI] [PubMed] [Google Scholar]
- El-sayed MEA. Nanoadsorbents for water and wastewater remediation. Sci Total Environ. 2020;739:139903. doi: 10.1016/j.scitotenv.2020.139903. [DOI] [PubMed] [Google Scholar]
- Fan S, Qu Y, Yao L, et al. MOF-derived cluster-shaped magnetic nanocomposite with hierarchical pores as an efficient and regenerative adsorbent for chlortetracycline removal. J Colloid Interface Sci. 2021;586:433–444. doi: 10.1016/j.jcis.2020.10.107. [DOI] [PubMed] [Google Scholar]
- Faraji M, Shirani M, Rashidi-Nodeh H. The recent advances in magnetic sorbents and their applications. TrAC Trends Anal Chem. 2021;141:116302. doi: 10.1016/j.trac.2021.116302. [DOI] [Google Scholar]
- Fatimah I, Citradewi PW, Fadillah G, et al. Enhanced performance of magnetic montmorillonite nanocomposite as adsorbent for Cu(II) by hydrothermal synthesis. J Environ Chem Eng. 2021;9:104968. doi: 10.1016/j.jece.2020.104968. [DOI] [Google Scholar]
- Ferrer N, Folch A, Masó G, et al. What are the main factors influencing the presence of faecal bacteria pollution in groundwater systems in developing countries? J Contam Hydrol. 2020;228:103556. doi: 10.1016/j.jconhyd.2019.103556. [DOI] [PubMed] [Google Scholar]
- Flores López SL, Moreno Virgen MR, Hernández Montoya V, et al. Effect of an external magnetic field applied in batch adsorption systems: Removal of dyes and heavy metals in binary solutions. J Mol Liq. 2018;269:450–460. doi: 10.1016/j.molliq.2018.08.063. [DOI] [Google Scholar]
- Franzreb M. New classes of selective separations exploiting magnetic adsorbents. Curr Opin Colloid Interface Sci. 2020;46:65–76. doi: 10.1016/j.cocis.2020.03.012. [DOI] [Google Scholar]
- Fu H, He H, Zhu R, et al. Phosphate modified magnetite@ferrihydrite as an magnetic adsorbent for Cd(II) removal from water, soil, and sediment. Sci Total Environ. 2021;764:142846. doi: 10.1016/j.scitotenv.2020.142846. [DOI] [PubMed] [Google Scholar]
- Gagliano E, Sgroi M, Falciglia PP, et al. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020;171:115381. doi: 10.1016/j.watres.2019.115381. [DOI] [PubMed] [Google Scholar]
- Gamal R, Rizk SE, El-Hefny NE. The adsorptive removal of Mo(VI) from aqueous solution by a synthetic magnetic chromium ferrite nanocomposite using a nonionic surfactant. J Alloys Compd. 2021;853:157039. doi: 10.1016/j.jallcom.2020.157039. [DOI] [Google Scholar]
- Gao M, Wang Z, Yang C, et al. Novel magnetic graphene oxide decorated with persimmon tannins for efficient adsorption of malachite green from aqueous solutions. Colloids Surfaces A Physicochem Eng Asp. 2019;566:48–57. doi: 10.1016/j.colsurfa.2019.01.016. [DOI] [Google Scholar]
- Gautam K, Anbumani S (2020) Ecotoxicological effects of organic micro-pollutants on the environment. In: Current Developments in Biotechnology and Bioengineering. Elsevier, pp 481–501
- Gawel E. Fighting micropollutants: comparing the leipzig and the swiss model of funding quarternary wastewater treatment. GAIA - Ecol Perspect Sci Soc. 2015;24:254–260. doi: 10.14512/gaia.24.4.11. [DOI] [Google Scholar]
- Ghernaout D, Elboughdiri N. Magnetic field application: an underappreciated outstanding technology. OALib. 2020;07:1–12. doi: 10.4236/oalib.1106000. [DOI] [Google Scholar]
- Gnanachandrasamy G, Dushiyanthan C, Jeyavel Rajakumar T, Zhou Y. Assessment of hydrogeochemical characteristics of groundwater in the lower Vellar river basin: using Geographical Information System (GIS) and Water Quality Index (WQI) Environ Dev Sustain. 2020;22:759–789. doi: 10.1007/s10668-018-0219-7. [DOI] [Google Scholar]
- Golovko O, Rehrl A-L, Köhler S, Ahrens L. Organic micropollutants in water and sediment from Lake Mälaren. Sweden. Chemosphere. 2020;258:127293. doi: 10.1016/j.chemosphere.2020.127293. [DOI] [PubMed] [Google Scholar]
- Gupta N, Pant P, Gupta C, et al. Engineered magnetic nanoparticles as efficient sorbents for wastewater treatment: a review. Mater Res Innov. 2017 doi: 10.1080/14328917.2017.1334846. [DOI] [Google Scholar]
- Han J, Xiao J, Qin W, et al. Copper recovery from yulong complex copper oxide ore by flotation and magnetic separation. JOM. 2017;69:1563–1569. doi: 10.1007/s11837-017-2383-x. [DOI] [Google Scholar]
- Hassan M, Naidu R, Du J, et al. Critical review of magnetic biosorbents: their preparation, application, and regeneration for wastewater treatment. Sci Total Environ. 2020;702:134893. doi: 10.1016/j.scitotenv.2019.134893. [DOI] [PubMed] [Google Scholar]
- He S, Li Y, Weng L, et al. Competitive adsorption of Cd2+, Pb2+ and Ni2+ onto Fe3+-modified argillaceous limestone: Influence of pH, ionic strength and natural organic matters. Sci Total Environ. 2018;637–638:69–78. doi: 10.1016/j.scitotenv.2018.04.300. [DOI] [PubMed] [Google Scholar]
- He H, Meng X, Yue Q, et al. Thiol-ene click chemistry synthesis of a novel magnetic mesoporous silica/chitosan composite for selective Hg(II) capture and high catalytic activity of spent Hg(II) adsorbent. Chem Eng J. 2021;405:126743. doi: 10.1016/j.cej.2020.126743. [DOI] [Google Scholar]
- Hlongwane GN, Sekoai PT, Meyyappan M, Moothi K. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci Total Environ. 2019;656:808–833. doi: 10.1016/j.scitotenv.2018.11.257. [DOI] [PubMed] [Google Scholar]
- Hu X, Hu Y, Xu G, et al. Green synthesis of a magnetic β-cyclodextrin polymer for rapid removal of organic micro-pollutants and heavy metals from dyeing wastewater. Environ Res. 2020;180:108796. doi: 10.1016/j.envres.2019.108796. [DOI] [PubMed] [Google Scholar]
- Huang D, Wu J, Wang L, et al. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem Eng J. 2019;358:1399–1409. doi: 10.1016/j.cej.2018.10.138. [DOI] [Google Scholar]
- Huang X, Wan Y, Shi B, et al. Characterization and application of poly-ferric-titanium-silicate-sulfate in disperse and reactive dye wastewaters treatment. Chemosphere. 2020;249:126129. doi: 10.1016/j.chemosphere.2020.126129. [DOI] [PubMed] [Google Scholar]
- Hussen Shadi AM, Kamaruddin MA, Niza NM, et al. Efficient treatment of raw leachate using magnetic ore iron oxide nanoparticles Fe2O3 as nanoadsorbents. J Water Process Eng. 2020;38:101637. doi: 10.1016/j.jwpe.2020.101637. [DOI] [Google Scholar]
- Hutchins DL, Downey JP. Effective separation of magnetite nanoparticles within an industrial-scale pipeline reactor. Sep Sci Technol. 2020;55:2822–2829. doi: 10.1080/01496395.2019.1646762. [DOI] [Google Scholar]
- Icten O, Ozer D. Magnetite doped metal–organic framework nanocomposites: an efficient adsorbent for removal of bisphenol-A pollutant. New J Chem. 2021;45:2157–2166. doi: 10.1039/D0NJ05622G. [DOI] [Google Scholar]
- Işık B, Kurtoğlu AE, Gürdağ G, Keçeli G. Radioactive cesium ion removal from wastewater using polymer metal oxide composites. J Hazard Mater. 2021;403:123652. doi: 10.1016/j.jhazmat.2020.123652. [DOI] [PubMed] [Google Scholar]
- Jafari Z, Avargani VM, Rahimi MR, Mosleh S. Magnetic nanoparticles-embedded nitrogen-doped carbon nanotube/porous carbon hybrid derived from a metal-organic framework as a highly efficient adsorbent for selective removal of Pb(II) ions from aqueous solution. J Mol Liq. 2020;318:113987. doi: 10.1016/j.molliq.2020.113987. [DOI] [Google Scholar]
- Jain M, Mudhoo A, Ramasamy DL, et al. Adsorption, degradation, and mineralization of emerging pollutants (pharmaceuticals and agrochemicals) by nanostructures: a comprehensive review. Environ Sci Pollut Res. 2020;27:34862–34905. doi: 10.1007/s11356-020-09635-x. [DOI] [PubMed] [Google Scholar]
- Jain A, Kumari S, Agarwal S, Khan S. Water purification via novel nano-adsorbents and their regeneration strategies. Process Safety Environ Protection. 2021;152:441–454. doi: 10.1016/j.psep.2021.06.031. [DOI] [Google Scholar]
- Jehan S, Khan S, Khattak SA, et al. Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur agency, Pakistan. Measurement. 2019;139:249–257. doi: 10.1016/j.measurement.2019.02.090. [DOI] [Google Scholar]
- Jiang J, Zhang Q, Zhan X, Chen F. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem Eng J. 2019;358:1539–1551. doi: 10.1016/j.cej.2018.10.144. [DOI] [Google Scholar]
- Jiang R, Zhu H-Y, Fu Y-Q, et al. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr Polym. 2021;252:117158. doi: 10.1016/j.carbpol.2020.117158. [DOI] [PubMed] [Google Scholar]
- Jun B-M, Kim S, Rho H, et al. Ultrasound-assisted Ti3C2Tx MXene adsorption of dyes: Removal performance and mechanism analyses via dynamic light scattering. Chemosphere. 2020;254:126827. doi: 10.1016/j.chemosphere.2020.126827. [DOI] [PubMed] [Google Scholar]
- Kakihara Y, Fukunishi T, Takeda S, et al. Superconducting High Gradient Magnetic Separation for Purification of Wastewater From Paper Factory. IEEE Trans Appiled Supercond. 2004;14:1565–1567. doi: 10.1109/TASC.2004.830709. [DOI] [Google Scholar]
- Keykhaee M, Razaghi M, Dalvand A, et al. Magnetic carnosine-based metal-organic framework nanoparticles: fabrication, characterization and application as arsenic adsorbent. J Environ Heal Sci Eng. 2020;18:1163–1174. doi: 10.1007/s40201-020-00535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf MM, Al-Amer K, Abd El-lateef HM. Magnetic Fe3O4 nanocubes coated by SiO2 and TiO2 layers as nanocomposites for Cr (VI) up taking from wastewater. Ceram Int. 2019;45:23548–23560. doi: 10.1016/j.ceramint.2019.08.064. [DOI] [Google Scholar]
- Khan FSA, Mubarak NM, Khalid M, et al. Magnetic nanoadsorbents’ potential route for heavy metals removal—a review. Environ Sci Pollut Res. 2020;27:24342–24356. doi: 10.1007/s11356-020-08711-6. [DOI] [PubMed] [Google Scholar]
- Kheshti Z, Azodi Ghajar K, Altaee A, Kheshti MR. High-Gradient Magnetic Separator (HGMS) combined with adsorption for nitrate removal from aqueous solution. Sep Purif Technol. 2019;212:650–659. doi: 10.1016/j.seppur.2018.11.080. [DOI] [Google Scholar]
- Kheshti Z, Hassanajili S, Ghajar KA. Study and optimization of a high-gradient magnetic separator using flat and lattice plates. IEEE Trans Magn. 2019;55:1–8. doi: 10.1109/TMAG.2018.2883624. [DOI] [Google Scholar]
- Kheshti Z, Ghajar KA, Moreno-Atanasio R, et al. Investigating the high gradient magnetic separator function for highly efficient adsorption of lead salt onto magnetic mesoporous silica microspheres and adsorbent recycling. Chem Eng Process - Process Intensif. 2020;148:107770. doi: 10.1016/j.cep.2019.107770. [DOI] [Google Scholar]
- Kiparsky M, Thompson BH, Binz C, et al. Barriers to innovation in urban wastewater utilities: attitudes of managers in California. Environ Manage. 2016;57:1204–1216. doi: 10.1007/s00267-016-0685-3. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Aylaz G, Sari E, et al. Selective binding of antibiotics using magnetic molecular imprint polymer (MMIP) networks prepared from vinyl-functionalized magnetic nanoparticles. J Hazard Mater. 2020;387:121709. doi: 10.1016/j.jhazmat.2019.121709. [DOI] [PubMed] [Google Scholar]
- Kumar M, Nagdev R, Tripathi R, et al. Geospatial and multivariate analysis of trace metals in tubewell water using for drinking purpose in the upper Gangetic basin, India: Heavy metal pollution index. Groundw Sustain Dev. 2019;8:122–133. doi: 10.1016/j.gsd.2018.10.001. [DOI] [Google Scholar]
- Kumar M, Singh Dosanjh H, Sonika, , et al. Review on magnetic nanoferrites and their composites as alternatives in waste water treatment: synthesis, modifications and applications. Environ Sci Water Res Technol. 2020;6:491–514. doi: 10.1039/C9EW00858F. [DOI] [Google Scholar]
- Lee Y-J, Kwon J-M, Baik S-K, et al. Application of superconducting magnetic separation for condenser water treatment in thermal power plant. Prog Supercond Cryog. 2011;13:21–24. doi: 10.9714/psac.2011.13.2.021. [DOI] [Google Scholar]
- Leone VO, Pereira MC, Aquino SF, et al. Adsorption of diclofenac on a magnetic adsorbent based on maghemite: experimental and theoretical studies. New J Chem. 2018;42:437–449. doi: 10.1039/C7NJ03214E. [DOI] [Google Scholar]
- Leonel AG, Mansur AAP, Mansur HS. Advanced functional nanostructures based on magnetic iron oxide nanomaterials for water remediation: a review. Water Res. 2021;190:116693. doi: 10.1016/j.watres.2020.116693. [DOI] [PubMed] [Google Scholar]
- Li BG, Lin WJ. Preparation of magnetic alginate-based biogel composite cross-linked by calcium ions and its super efficient adsorption for direct dyes. Mater Sci Forum. 2021;1035:1022–1029. doi: 10.4028/www.scientific.net/MSF.1035.1022. [DOI] [Google Scholar]
- Li L, Xu Y, Zhong D, Zhong N. CTAB-surface-functionalized magnetic MOF@MOF composite adsorbent for Cr(VI) efficient removal from aqueous solution. Colloids Surfaces A Physicochem Eng Asp. 2020;586:124255. doi: 10.1016/j.colsurfa.2019.124255. [DOI] [Google Scholar]
- Li L, Liu C, Ma R, et al. Rapid removal of thallium from water by a new magnetic nano-composite using graphene oxide for efficient separation. Int Biodeterior Biodegradation. 2021;161:105245. doi: 10.1016/j.ibiod.2021.105245. [DOI] [Google Scholar]
- Li Q, Zhu S, Hao G, et al. Fabrication of thermoresponsive metal–organic nanotube sponge and its application on the adsorption of endocrine-disrupting compounds and pharmaceuticals/personal care products: Experiment and molecular simulation study. Environ Pollut. 2021;273:116466. doi: 10.1016/j.envpol.2021.116466. [DOI] [PubMed] [Google Scholar]
- Lingamdinne LP, Koduru JR, Karri RR. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J Environ Manage. 2019;231:622–634. doi: 10.1016/j.jenvman.2018.10.063. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liang Z, Wu S, Liu F. Treatment of municipal wastewater by a magnetic activated sludge device. Desalin Water Treat. 2013 doi: 10.1080/19443994.2013.848416. [DOI] [Google Scholar]
- Liu L, Zhao L, Yang X, et al. Innovative design and study of an oil-water coupling separation magnetic hydrocyclone. Sep Purif Technol. 2019;213:389–400. doi: 10.1016/j.seppur.2018.12.051. [DOI] [Google Scholar]
- Liu X, Guan J, Lai G, et al. Stimuli-responsive adsorption behavior toward heavy metal ions based on comb polymer functionalized magnetic nanoparticles. J Clean Prod. 2020;253:119915. doi: 10.1016/j.jclepro.2019.119915. [DOI] [Google Scholar]
- Liu Y, Zhu Z, Cheng Q, et al. One-step preparation of environment-oriented magnetic coal-based activated carbon with high adsorption and magnetic separation performance. J Magn Magn Mater. 2021;521:167517. doi: 10.1016/j.jmmm.2020.167517. [DOI] [Google Scholar]
- Liu R, Zenglu QI, Zhu L, Liu H, Huachun LAN, Qu J (2020b) Research Center for Eco Environmental Sciences of CAS. Magnetic adsorbent for removing arsenic and antimony by means of adsorption-superconducting magnetic separation and preparation method therefor. U.S. Patent Application 16/724,371 (7 May 2020). https://patents.google.com/patent/US20200139341A1/en. (accessed 29 January 2021 to 10 February 2021)
- Lombardi MR, Morley NB (inventors) (2017) Assignee: Hydroflux Technology LLC. Apparatus and method for applying magnetic fields to fluid flows. United States patent US 9,719,738 (1 August 2017). https://patents.google.com/patent/US9719738B2/en. (accessed 29 January 2021 to 10 February 2021)
- Lompe KM, Vo Duy S, Peldszus S, et al. Removal of micropollutants by fresh and colonized magnetic powdered activated carbon. J Hazard Mater. 2018;360:349–355. doi: 10.1016/j.jhazmat.2018.07.072. [DOI] [PubMed] [Google Scholar]
- Lorignon F, Gossard A, Carboni M. Hierarchically porous monolithic MOFs: an ongoing challenge for industrial-scale effluent treatment. Chem Eng J. 2020;393:124765. doi: 10.1016/j.cej.2020.124765. [DOI] [Google Scholar]
- Lu H, Wang J, Stoller M, et al. An Overview of Nanomaterials for Water and Wastewater Treatment. Adv Mater Sci Eng. 2016;2016:1–10. doi: 10.1155/2016/4964828. [DOI] [Google Scholar]
- Luan L, Tang B, Liu Y, et al. Selective capture of Hg(II) and Ag(I) from water by sulfur-functionalized polyamidoamine dendrimer/magnetic Fe3O4 hybrid materials. Sep Purif Technol. 2021;257:117902. doi: 10.1016/j.seppur.2020.117902. [DOI] [Google Scholar]
- Lv M, Zhang Z, Zeng J, et al. Roles of magnetic particles in magnetic seeding coagulation-flocculation process for surface water treatment. Sep Purif Technol. 2019;212:337–343. doi: 10.1016/j.seppur.2018.11.011. [DOI] [Google Scholar]
- Lv M, Li D, Zhang Z, et al. Magnetic seeding coagulation: Effect of Al species and magnetic particles on coagulation efficiency, residual Al, and floc properties. Chemosphere. 2021;268:129363. doi: 10.1016/j.chemosphere.2020.129363. [DOI] [PubMed] [Google Scholar]
- Ma Z, Shan C, Liang J, Tong M. Efficient adsorption of Selenium(IV) from water by hematite modified magnetic nanoparticles. Chemosphere. 2018;193:134–141. doi: 10.1016/j.chemosphere.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Ma H, Yang J, Gao X, et al. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J Hazard Mater. 2019;369:550–560. doi: 10.1016/j.jhazmat.2019.02.063. [DOI] [PubMed] [Google Scholar]
- Madhura L, Singh S, Kanchi S, et al. Nanotechnology-based water quality management for wastewater treatment. Environ Chem Lett. 2019;17:65–121. doi: 10.1007/s10311-018-0778-8. [DOI] [Google Scholar]
- Mahamadi C. Will nano-biosorbents break the Achilles’ heel of biosorption technology? Environ Chem Lett. 2019;17:1753–1768. doi: 10.1007/s10311-019-00909-6. [DOI] [Google Scholar]
- Manyangadze M, Chikuruwo NHM, Narsaiah TB, et al. Enhancing adsorption capacity of nano-adsorbents via surface modification: A review. South African J Chem Eng. 2020;31:25–32. doi: 10.1016/j.sajce.2019.11.003. [DOI] [Google Scholar]
- Mashile GP, Mpupa A, Nqombolo A, et al. Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. J Water Process Eng. 2020;33:101011. doi: 10.1016/j.jwpe.2019.101011. [DOI] [Google Scholar]
- Mashkoor F, Nasar A. Magsorbents: Potential candidates in wastewater treatment technology – A review on the removal of methylene blue dye. J Magn Magn Mater. 2020;500:166408. doi: 10.1016/j.jmmm.2020.166408. [DOI] [Google Scholar]
- Masjedi A, Askarizadeh E, Baniyaghoob S. Magnetic nanoparticles surface-modified with tridentate ligands for removal of heavy metal ions from water. Mater Chem Phys. 2020;249:122917. doi: 10.1016/j.matchemphys.2020.122917. [DOI] [Google Scholar]
- Masunga N, Mmelesi OK, Kefeni KK, Mamba BB. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review. J Environ Chem Eng. 2019;7:103179. doi: 10.1016/j.jece.2019.103179. [DOI] [Google Scholar]
- Mazloomi F, Jalali M. Adsorption of ammonium from simulated wastewater by montmorillonite nanoclay and natural vermiculite: experimental study and simulation. Environ Monit Assess. 2017;189:415. doi: 10.1007/s10661-017-6080-6. [DOI] [PubMed] [Google Scholar]
- Mehta D, Mazumdar S, Singh SK. Magnetic adsorbents for the treatment of water/wastewater—A review. J Water Process Eng. 2015;7:244–265. doi: 10.1016/j.jwpe.2015.07.001. [DOI] [Google Scholar]
- Meng C, Zhikun W, Qiang L, et al. Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J Hazard Mater. 2018;341:198–206. doi: 10.1016/j.jhazmat.2017.07.062. [DOI] [PubMed] [Google Scholar]
- Mirshahghassemi S, Ebner AD, Cai B, Lead JR. Application of high gradient magnetic separation for oil remediation using polymer-coated magnetic nanoparticles. Sep Purif Technol. 2017;179:328–334. doi: 10.1016/j.seppur.2017.01.067. [DOI] [Google Scholar]
- Mittal H, Babu R, Dabbawala AA, Alhassan SM. Low-temperature synthesis of magnetic carbonaceous materials coated with nanosilica for rapid adsorption of methylene blue. ACS Omega. 2020;5:6100–6112. doi: 10.1021/acsomega.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Z, Kelishami AR, Ashrafi A. Application of Ni0.5Zn0.5Fe2O4 magnetic nanoparticles for diclofenac adsorption: isotherm, kinetic and thermodynamic investigation. Water Sci Technol. 2021;83:1265–1277. doi: 10.2166/wst.2021.049. [DOI] [PubMed] [Google Scholar]
- Mohammed N, Grishkewich N, Tam KC. Cellulose nanomaterials: promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ Sci Nano. 2018;5:623–658. doi: 10.1039/C7EN01029J. [DOI] [Google Scholar]
- Moharramzadeh S, Baghdadi M. In situ sludge magnetic impregnation (ISSMI) as an efficient technology for enhancement of sludge sedimentation: Removal of methylene blue using nitric acid treated graphene oxide as a test process. J Environ Chem Eng. 2016;4:2090–2102. doi: 10.1016/j.jece.2016.03.039. [DOI] [Google Scholar]
- Momina Shahadat M, Isamil S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: a review. RSC Adv. 2018;8:24571–24587. doi: 10.1039/C8RA04290J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SV, Bozorgian A, Mokhtari N, et al. A novel cyanopropylsilane-functionalized titanium oxide magnetic nanoparticle for the adsorption of nickel and lead ions from industrial wastewater: Equilibrium, kinetic and thermodynamic studies. Microchem J. 2019;145:914–920. doi: 10.1016/j.microc.2018.11.048. [DOI] [Google Scholar]
- Najafpoor A, Norouzian-Ostad R, Alidadi H, et al. Effect of magnetic nanoparticles and silver-loaded magnetic nanoparticles on advanced wastewater treatment and disinfection. J Mol Liq. 2020;303:112640. doi: 10.1016/j.molliq.2020.112640. [DOI] [Google Scholar]
- Nasiri S, Alizadeh N. Hydroxypropyl-β-cyclodextrin-polyurethane/graphene oxide magnetic nanoconjugates as effective adsorbent for chromium and lead ions. Carbohydr Polym. 2021 doi: 10.1016/j.carbpol.2021.117731. [DOI] [PubMed] [Google Scholar]
- Navab-Daneshmand T, Friedrich MND, Gächter M, et al. Escherichia coli contamination across Multiple environmental compartments (Soil, Hands, Drinking Water, and Handwashing Water) in urban harare: correlations and risk factors. Am J Trop Med Hyg. 2018;98:803–813. doi: 10.4269/ajtmh.17-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neha R, Adithya S, Jayaraman RS, et al. Nano-adsorbents an effective candidate for removal of toxic pharmaceutical compounds from aqueous environment: A critical review on emerging trends. Chemosphere. 2021;272:129852. doi: 10.1016/j.chemosphere.2021.129852. [DOI] [PubMed] [Google Scholar]
- Niksefat Abatari M, Sarmasti Emami MR, Jahanshahi M, Shahavi MH. Superporous pellicular κ-Carrageenan–Nickel composite beads; morphological, physical and hydrodynamics evaluation for expanded bed adsorption application. Chem Eng Res Des. 2017;125:291–305. doi: 10.1016/j.cherd.2017.07.012. [DOI] [Google Scholar]
- Nishijima S, Takeda S. Superconducting high gradient magnetic separation for purification of wastewater from paper factory. IEEE Trans Appl Supercond. 2006;16:1142–1145. doi: 10.1109/TASC.2006.871346. [DOI] [Google Scholar]
- Nisola GM, Parohinog KJ, Cho MK, et al. Covalently decorated crown ethers on magnetic graphene oxides as bi-functional adsorbents with tailorable ion recognition properties for selective metal ion capture in water. Chem Eng J. 2020;389:123421. doi: 10.1016/j.cej.2019.123421. [DOI] [Google Scholar]
- Nithya R, Thirunavukkarasu A, Sathya AB, Sivashankar R. Magnetic materials and magnetic separation of dyes from aqueous solutions: a review. Environ Chem Lett. 2021;19:1275–1294. doi: 10.1007/s10311-020-01149-9. [DOI] [Google Scholar]
- Nkinahamira F, Alsbaiee A, Zeng Q, et al. Selective and fast recovery of rare earth elements from industrial wastewater by porous β-cyclodextrin and magnetic β-cyclodextrin polymers. Water Res. 2020;181:115857. doi: 10.1016/j.watres.2020.115857. [DOI] [PubMed] [Google Scholar]
- Nnadozie EC, Ajibade PA. Adsorption, kinetic and mechanistic studies of Pb(II) and Cr(VI) ions using APTES functionalized magnetic biochar. Microporous Mesoporous Mater. 2020;309:110573. doi: 10.1016/j.micromeso.2020.110573. [DOI] [Google Scholar]
- Pan Y, Ding Q, Li B, et al. Self-adjusted bimetallic zeolitic-imidazolate framework-derived hierarchical magnetic carbon composites as efficient adsorbent for optimizing drug contaminant removal. Chemosphere. 2021;263:128101. doi: 10.1016/j.chemosphere.2020.128101. [DOI] [PubMed] [Google Scholar]
- Panagopoulos A, Haralambous K-J. Minimal liquid discharge (MLD) and zero liquid discharge (ZLD) strategies for wastewater management and resource recovery – Analysis, challenges and prospects. J Environ Chem Eng. 2020;8:104418. doi: 10.1016/j.jece.2020.104418. [DOI] [Google Scholar]
- Panagopoulos A, Haralambous K-J. Environmental impacts of desalination and brine treatment - Challenges and mitigation measures. Mar Pollut Bull. 2020;161:111773. doi: 10.1016/j.marpolbul.2020.111773. [DOI] [PubMed] [Google Scholar]
- Peralta ME, Ocampo S, Funes IG, et al. Nanomaterials with tailored magnetic properties as adsorbents of organic pollutants from wastewaters. Inorganics. 2020;8:24. doi: 10.3390/inorganics8040024. [DOI] [Google Scholar]
- Peralta ME, Mártire DO, Moreno MS, et al. Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J Environ Chem Eng. 2021;9:104841. doi: 10.1016/j.jece.2020.104841. [DOI] [Google Scholar]
- Perera MM, Ayres N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym Chem. 2020;11:1410–1423. doi: 10.1039/C9PY01694E. [DOI] [Google Scholar]
- Plohl O, Simonič M, Kolar K, et al. Magnetic nanostructures functionalized with a derived lysine coating applied to simultaneously remove heavy metal pollutants from environmental systems. Sci Technol Adv Mater. 2021;22:55–71. doi: 10.1080/14686996.2020.1865114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CD, Atkinson AJ, Ma Y, et al. Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J Nanoparticle Res. 2020;22:48. doi: 10.1007/s11051-020-4770-4. [DOI] [Google Scholar]
- Prot T, Nguyen VH, Wilfert P, et al. Magnetic separation and characterization of vivianite from digested sewage sludge. Sep Purif Technol. 2019;224:564–579. doi: 10.1016/j.seppur.2019.05.057. [DOI] [Google Scholar]
- Quiroga-Flores R, Noshad A, Wallenberg R, Önnby L. Adsorption of cadmium by a high-capacity adsorbent composed of silicate-titanate nanotubes embedded in hydrogel chitosan beads. Environ Technol. 2020;41:3043–3054. doi: 10.1080/09593330.2019.1596167. [DOI] [PubMed] [Google Scholar]
- Rais S, Islam A, Ahmad I, et al. Preparation of a new magnetic ion-imprinted polymer and optimization using Box-Behnken design for selective removal and determination of Cu(II) in food and wastewater samples. Food Chem. 2021;334:127563. doi: 10.1016/j.foodchem.2020.127563. [DOI] [PubMed] [Google Scholar]
- Rashid M, Price NT, Gracia Pinilla MÁ, O’Shea KE. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017;123:353–360. doi: 10.1016/j.watres.2017.06.085. [DOI] [PubMed] [Google Scholar]
- Reguyal F, Sarmah AK. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol. Sci Total Environ. 2018;628–629:722–730. doi: 10.1016/j.scitotenv.2018.01.323. [DOI] [PubMed] [Google Scholar]
- Reshadi MAM, Bazargan A, McKay G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci Total Environ. 2020;731:138863. doi: 10.1016/j.scitotenv.2020.138863. [DOI] [PubMed] [Google Scholar]
- Rezaei H, Shahbazi K, Behbahani M. Application of Amine Modified Magnetic Nanoparticles as an Efficient and Reusable Nanofluid for Removal of Ba2+ in High Saline Waters. Silicon. 2020 doi: 10.1007/s12633-020-00743-4. [DOI] [Google Scholar]
- Rogowska J, Cieszynska-Semenowicz M, Ratajczyk W, Wolska L. Micropollutants in treated wastewater. Ambio. 2020;49:487–503. doi: 10.1007/s13280-019-01219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy E, Patra S, Karfa P, et al. Complex Magnetic Nanostructures. Cham: Springer International Publishing; 2017. Role of magnetic nanoparticles in providing safe and clean water to each individual; pp. 281–316. [Google Scholar]
- Safari N, Ghanemi K, Buazar F. Selenium functionalized magnetic nanocomposite as an effective mercury (II) ion scavenger from environmental water and industrial wastewater samples. J Environ Manage. 2020;276:111263. doi: 10.1016/j.jenvman.2020.111263. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Hota G. Surface functionalization of GO with MgO/MgFe2O4 binary oxides: A novel magnetic nanoadsorbent for removal of fluoride ions. J Environ Chem Eng. 2018;6:2918–2931. doi: 10.1016/j.jece.2018.04.054. [DOI] [Google Scholar]
- Sahoo SK, Padhiari S, Biswal SK, et al. Fe3O4 nanoparticles functionalized GO/g-C3N4 nanocomposite: An efficient magnetic nanoadsorbent for adsorptive removal of organic pollutants. Mater Chem Phys. 2020;244:122710. doi: 10.1016/j.matchemphys.2020.122710. [DOI] [Google Scholar]
- Salehin S, Rebosura M, Keller J, et al. Recovery of in-sewer dosed iron from digested sludge at downstream treatment plants and its reuse potential. Water Res. 2020;174:115627. doi: 10.1016/j.watres.2020.115627. [DOI] [PubMed] [Google Scholar]
- Salinas T, Durruty I, Arciniegas L, et al. Design and testing of a pilot scale magnetic separator for the treatment of textile dyeing wastewater. J Environ Manage. 2018;218:562–568. doi: 10.1016/j.jenvman.2018.04.096. [DOI] [PubMed] [Google Scholar]
- Santhosh C, Velmurugan V, Jacob G, et al. Role of nanomaterials in water treatment applications: A review. Chem Eng J. 2016;306:1116–1137. doi: 10.1016/j.cej.2016.08.053. [DOI] [Google Scholar]
- Sarkar AK, Bediako JK, Choi J-W, Yun Y-S. Functionalized magnetic biopolymeric graphene oxide with outstanding performance in water purification. NPG Asia Mater. 2019;11:4. doi: 10.1038/s41427-018-0104-8. [DOI] [Google Scholar]
- Scaria J, Nidheesh PV, Kumar MS. Synthesis and applications of various bimetallic nanomaterials in water and wastewater treatment. J Environ Manage. 2020;259:110011. doi: 10.1016/j.jenvman.2019.110011. [DOI] [PubMed] [Google Scholar]
- Schwaminger SP, Fraga-García P, Eigenfeld M, et al. Magnetic separation in bioprocessing beyond the analytical scale: from biotechnology to the food industry. Front Bioeng Biotechnol. 2019;7:1–12. doi: 10.3389/fbioe.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Chen Z, Hollert H, et al. Toxicity of 10 organic micropollutants and their mixture: implications for aquatic risk assessment. Sci Total Environ. 2019;666:1273–1282. doi: 10.1016/j.scitotenv.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhu C, Song S, et al. Defect-abundant covalent triazine frameworks as sunlight-driven self-cleaning adsorbents for volatile aromatic pollutants in water. Environ Sci Technol. 2019;53:9091–9101. doi: 10.1021/acs.est.9b02222. [DOI] [PubMed] [Google Scholar]
- Sherman L, Cantor A, Milman A, Kiparsky M. Examining the complex relationship between innovation and regulation through a survey of wastewater utility managers. J Environ Manage. 2020;260:110025. doi: 10.1016/j.jenvman.2019.110025. [DOI] [PubMed] [Google Scholar]
- Shibatani S, Nakanishi M, Mizuno N, et al. Study on magnetic separation device for scale removal from feed-water in thermal power plant. IEEE Trans Appl Supercond. 2016;26:1–4. doi: 10.1109/TASC.2016.2523433. [DOI] [Google Scholar]
- Shim J, Kumar M, Goswami R, et al. Removal of p-cresol and tylosin from water using a novel composite of alginate, recycled MnO2 and activated carbon. J Hazard Mater. 2019;364:419–428. doi: 10.1016/j.jhazmat.2018.09.065. [DOI] [PubMed] [Google Scholar]
- Si S, Chen H. A literature review of disruptive innovation: What it is, how it works and where it goes. J Eng Technol Manag. 2020;56:101568. doi: 10.1016/j.jengtecman.2020.101568. [DOI] [Google Scholar]
- Siddeeg SM, Tahoon MA, Alsaiari NS, et al. Application of functionalized nanomaterials as effective adsorbents for the removal of heavy metals from wastewater: a review. Curr Anal Chem. 2020;17:4–22. doi: 10.2174/1573411016999200719231712. [DOI] [Google Scholar]
- Simeonidis K, Kaprara E, Samaras T, et al. Optimizing magnetic nanoparticles for drinking water technology: the case of Cr(VI) Sci Total Environ. 2015;535:61–68. doi: 10.1016/j.scitotenv.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Singh A, Chaudhary S, Dehiya BS. Fast removal of heavy metals from water and soil samples using magnetic Fe3O4 nanoparticles. Environ Sci Pollut Res. 2021;28:3942–3952. doi: 10.1007/s11356-020-10737-9. [DOI] [PubMed] [Google Scholar]
- Singhal P, Vats BG, Yadav A, Pulhani V. Efficient extraction of uranium from environmental samples using phosphoramide functionalized magnetic nanoparticles: Understanding adsorption and binding mechanisms. J Hazard Mater. 2020;384:121353. doi: 10.1016/j.jhazmat.2019.121353. [DOI] [PubMed] [Google Scholar]
- Sivashankar R, Sathya AB, Vasantharaj K, Sivasubramanian V. Magnetic composite an environmental super adsorbent for dye sequestration – A review. Environ Nanotechnol Monit Manag. 2014;1–2:36–49. doi: 10.1016/j.enmm.2014.06.001. [DOI] [Google Scholar]
- Soares SF, Fernandes T, Trindade T, Daniel-da-Silva AL. Recent advances on magnetic biosorbents and their applications for water treatment. Environ Chem Lett. 2020;18:151–164. doi: 10.1007/s10311-019-00931-8. [DOI] [Google Scholar]
- Song Y, Lu M, Huang B, et al. Decoration of defective MoS2 nanosheets with Fe3O4 nanoparticles as superior magnetic adsorbent for highly selective and efficient mercury ions (Hg2+) removal. J Alloys Compd. 2018;737:113–121. doi: 10.1016/j.jallcom.2017.12.087. [DOI] [Google Scholar]
- Subba Rao N, Marghade D, Dinakar A, et al. Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh. India. Environ Earth Sci. 2017;76:747. doi: 10.1007/s12665-017-7093-8. [DOI] [Google Scholar]
- Sun Y, Zhang B, Zheng T, Wang P. Regeneration of activated carbon saturated with chloramphenicol by microwave and ultraviolet irradiation. Chem Eng J. 2017;320:264–270. doi: 10.1016/j.cej.2017.03.007. [DOI] [Google Scholar]
- Sun Y, Yu Y, Zheng X, et al. Magnetic flocculation of Cu(II) wastewater by chitosan-based magnetic composite flocculants with recyclable properties. Carbohydr Polym. 2021;261:117891. doi: 10.1016/j.carbpol.2021.117891. [DOI] [PubMed] [Google Scholar]
- Surendhiran D, Sirajunnisa A, Tamilselvam K. Silver–magnetic nanocomposites for water purification. Environ Chem Lett. 2017;15:367–386. doi: 10.1007/s10311-017-0635-1. [DOI] [Google Scholar]
- Tabatabaiee Bafrooee AA, Moniri E, Ahmad Panahi H, et al. Ethylenediamine functionalized magnetic graphene oxide (Fe3O4@GO-EDA) as an efficient adsorbent in Arsenic(III) decontamination from aqueous solution. Res Chem Intermed. 2021;47:1397–1428. doi: 10.1007/s11164-020-04368-5. [DOI] [Google Scholar]
- Tamjidi S, Esmaeili H, Kamyab Moghadas B. Application of magnetic adsorbents for removal of heavy metals from wastewater: a review study. Mater Res Express. 2019;6:102004. doi: 10.1088/2053-1591/ab3ffb. [DOI] [Google Scholar]
- Tan Z, Gao M, Dai J, et al. Magnetic Interconnected macroporous imprinted foams for selective recognition and adsorptive removal of phenolic pollution from water. Fibers Polym. 2020;21:762–774. doi: 10.1007/s12221-020-8695-4. [DOI] [Google Scholar]
- Tang SCN, Yan DYS, Lo IMC. Sustainable wastewater treatment using microsized magnetic hydrogel with magnetic separation technology. Ind Eng Chem Res. 2014;53:15718–15724. doi: 10.1021/ie502512h. [DOI] [Google Scholar]
- Tang J, Wu Y, Esquivel-Elizondo S, et al. How microbial aggregates protect against nanoparticle toxicity. Trends Biotechnol. 2018;36:1171–1182. doi: 10.1016/j.tibtech.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Tatarchuk T, Myslin M, Lapchuk I, et al. Magnesium-zinc ferrites as magnetic adsorbents for Cr(VI) and Ni(II) ions removal: Cation distribution and antistructure modeling. Chemosphere. 2021 doi: 10.1016/j.chemosphere.2020.129414. [DOI] [PubMed] [Google Scholar]
- Tijani JO, Fatoba OO, Babajide OO, Petrik LF. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: a review. Environ Chem Lett. 2016;14:27–49. doi: 10.1007/s10311-015-0537-z. [DOI] [Google Scholar]
- Trapp JH, Kerber H, Schramm E. Implementation and diffusion of innovative water infrastructures: obstacles, stakeholder networks and strategic opportunities for utilities. Environ Earth Sci. 2017;76:154. doi: 10.1007/s12665-017-6461-8. [DOI] [Google Scholar]
- Tripathy SK, Singh V, Rama Murthy Y, et al. Influence of process parameters of dry high intensity magnetic separators on separation of hematite. Int J Miner Process. 2017;160:16–31. doi: 10.1016/j.minpro.2017.01.007. [DOI] [Google Scholar]
- Tuutijärvi T, Repo E, Vahala R, et al. Effect of Competing anions on arsenate adsorption onto maghemite nanoparticles. Chinese J Chem Eng. 2012;20:505–514. doi: 10.1016/S1004-9541(11)60212-7. [DOI] [Google Scholar]
- Ul-Islam M, Ullah MW, Khan S, et al. Current advancements of magnetic nanoparticles in adsorption and degradation of organic pollutants. Environ Sci Pollut Res. 2017;24:12713–12722. doi: 10.1007/s11356-017-8765-3. [DOI] [PubMed] [Google Scholar]
- Valenzuela EF, Menezes HC, Cardeal ZL. Passive and grab sampling methods to assess pesticide residues in water. A review. Environ Chem Lett. 2020;18:1019–1048. doi: 10.1007/s10311-020-00998-8. [DOI] [Google Scholar]
- Varjani S, Sudha MC (2020) Occurrence and human health risk of micro-pollutants—A special focus on endocrine disruptor chemicals. In: Current Developments in Biotechnology and Bioengineering. Elsevier, pp 23–39
- Vicente-Martínez Y, Caravaca M, Soto-Meca A. Total removal of Hg (II) from wastewater using magnetic nanoparticles coated with nanometric Ag and functionalized with sodium 2-mercaptoethane sulfonate. Environ Chem Lett. 2020;18:975–981. doi: 10.1007/s10311-020-00987-x. [DOI] [Google Scholar]
- Vikrant K, Kim K-H. Nanomaterials for the adsorptive treatment of Hg(II) ions from water. Chem Eng J. 2019;358:264–282. doi: 10.1016/j.cej.2018.10.022. [DOI] [Google Scholar]
- Villaseñor MJ, Ríos Á. Nanomaterials for water cleaning and desalination, energy production, disinfection, agriculture and green chemistry. Environ Chem Lett. 2018;16:11–34. doi: 10.1007/s10311-017-0656-9. [DOI] [Google Scholar]
- Vishnu D, Dhandapani B (2021) Evaluation of column studies using Cynodon dactylon plant‐mediated amino‐grouped silica‐layered magnetic nanoadsorbent to remove noxious hexavalent chromium metal ions. IET Nanobiotechnology nbt2.12029. doi: 10.1049/nbt2.12029 [DOI] [PMC free article] [PubMed]
- Vivas EL, Lee S, Cho K. Brushite-infused polyacrylonitrile nanofiber adsorbent for strontium removal from water. J Environ Manage. 2020;270:110837. doi: 10.1016/j.jenvman.2020.110837. [DOI] [PubMed] [Google Scholar]
- Vu CT, Wu T. Magnetic porous NiLa-Layered double oxides (LDOs) with improved phosphate adsorption and antibacterial activity for treatment of secondary effluent. Water Res. 2020;175:115679. doi: 10.1016/j.watres.2020.115679. [DOI] [PubMed] [Google Scholar]
- Wadhawan S, Jain A, Nayyar J, Mehta SK. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: a review. J Water Process Eng. 2020;33:101038. doi: 10.1016/j.jwpe.2019.101038. [DOI] [Google Scholar]
- Wang W, Xu Z, Zhang X, et al. Rapid and efficient removal of organic micropollutants from environmental water using a magnetic nanoparticles-attached fluorographene-based sorbent. Chem Eng J. 2018;343:61–68. doi: 10.1016/j.cej.2018.02.101. [DOI] [Google Scholar]
- Wang G, Luo Q, Dai J, Deng N. Adsorption of dichromate ions from aqueous solution onto magnetic graphene oxide modified by β-cyclodextrin. Environ Sci Pollut Res. 2020;27:30778–30788. doi: 10.1007/s11356-020-09389-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Tong X, Chen Y, et al. Enhanced removal of Cr(III) in high salt organic wastewater by EDTA modified magnetic mesoporous silica. Microporous Mesoporous Mater. 2020;303:110262. doi: 10.1016/j.micromeso.2020.110262. [DOI] [Google Scholar]
- Wang Z, Zhang J, Wu Q, et al. Magnetic supramolecular polymer: Ultrahigh and highly selective Pb(II) capture from aqueous solution and battery wastewater. Chemosphere. 2020;248:126042. doi: 10.1016/j.chemosphere.2020.126042. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhao D, Wu C, et al. Magnetic metal organic frameworks/graphene oxide adsorbent for the removal of U(VI) from aqueous solution. Appl Radiat Isot. 2020;162:109160. doi: 10.1016/j.apradiso.2020.109160. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang G, Qiao S, Zhou J. Magnetic Fe0/iron oxide-coated diatomite as a highly efficient adsorbent for recovering phosphorus from water. Chem Eng J. 2021;412:128696. doi: 10.1016/j.cej.2021.128696. [DOI] [Google Scholar]
- Wanjeri VWO, Sheppard CJ, Prinsloo ARE, et al. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J Environ Chem Eng. 2018;6:1333–1346. doi: 10.1016/j.jece.2018.01.064. [DOI] [Google Scholar]
- Wanna Y, Chindaduang A, Tumcharern G, et al. Efficiency of SPIONs functionalized with polyethylene glycol bis(amine) for heavy metal removal. J Magn Magn Mater. 2016;414:32–37. doi: 10.1016/j.jmmm.2016.04.064. [DOI] [Google Scholar]
- WATER ONLINE (2008) Magnetic separator for industrial waste-water treatment. https://www.wateronline.com/doc/magnetic-separator-for-industrial-wastewater-0001. (accessed 12 February 2021)
- Wen L, Zhang Y, Liu C, Tang Y. All-Biomass Double Network Gel: Highly Efficient Removal of Pb2+ and Cd2+ in Wastewater and Utilization of Spent Adsorbents. J Polym Environ. 2020;28:2669–2680. doi: 10.1007/s10924-020-01806-8. [DOI] [Google Scholar]
- Wu Y, Zhou Q, Yuan Y, et al. Enrichment and sensitive determination of phthalate esters in environmental water samples: A novel approach of MSPE-HPLC based on PAMAM dendrimers-functionalized magnetic-nanoparticles. Talanta. 2020;206:120213. doi: 10.1016/j.talanta.2019.120213. [DOI] [PubMed] [Google Scholar]
- Xie X, Deng R, Pang Y, et al. Adsorption of copper(II) by sulfur microparticles. Chem Eng J. 2017;314:434–442. doi: 10.1016/j.cej.2016.11.163. [DOI] [Google Scholar]
- Xin Y, Gu P, Long H, et al. Fabrication of ferrihydrite-loaded magnetic sugar cane bagasse charcoal adsorbent for the adsorptive removal of selenite from aqueous solution. Colloids Surfaces A Physicochem Eng Asp. 2021;614:126131. doi: 10.1016/j.colsurfa.2020.126131. [DOI] [Google Scholar]
- Xiong Y, Xu L, Jin C, Sun Q. Cellulose hydrogel functionalized titanate microspheres with self-cleaning for efficient purification of heavy metals in oily wastewater. Cellulose. 2020;27:7751–7763. doi: 10.1007/s10570-020-03329-w. [DOI] [Google Scholar]
- Xu M, Han X, Hua D. Polyoxime-functionalized magnetic nanoparticles for uranium adsorption with high selectivity over vanadium. J Mater Chem A. 2017;5:12278–12284. doi: 10.1039/C7TA02684F. [DOI] [Google Scholar]
- Xu S, Zhou S, Xing L, et al. Fate of organic micropollutants and their biological effects in a drinking water source treated by a field-scale constructed wetland. Sci Total Environ. 2019;682:756–764. doi: 10.1016/j.scitotenv.2019.05.151. [DOI] [PubMed] [Google Scholar]
- Xu W, Gao M, Yin X, et al. Photo-stimulated “turn-on/off” molecularly imprinted polymers based on magnetic mesoporous silicon surface for efficient detection of sulfamerazine. J Sep Sci. 2020;43:2550–2557. doi: 10.1002/jssc.202000043. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang H, Peng J, et al. Smart magnetic ionic liquid-based Pickering emulsions stabilized by amphiphilic Fe3O4 nanoparticles: Highly efficient extraction systems for water purification. J Colloid Interface Sci. 2017;485:213–222. doi: 10.1016/j.jcis.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Yang S, Qian J, Kuang L, Hua D. Ion-imprinted mesoporous silica for selective removal of uranium from highly acidic and radioactive effluent. ACS Appl Mater Interfaces. 2017;9:29337–29344. doi: 10.1021/acsami.7b09419. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang J, Liu Y, et al. Rapid removal of anionic dye from water by poly(ionic liquid)-modified magnetic nanoparticles. J Mol Liq. 2019;284:383–392. doi: 10.1016/j.molliq.2019.04.029. [DOI] [Google Scholar]
- Yang W, Hu W, Zhang J, et al. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem Eng J. 2021;405:126629. doi: 10.1016/j.cej.2020.126629. [DOI] [Google Scholar]
- Yao Z, Jiao W, Shao F, et al. Fabrication and characterization of amphiphilic magnetic water purification materials for efficient PPCPs removal. Chem Eng J. 2019;360:511–518. doi: 10.1016/j.cej.2018.12.016. [DOI] [Google Scholar]
- Yeap SP, Lim J, Ooi BS, Ahmad AL. Agglomeration, colloidal stability, and magnetic separation of magnetic nanoparticles: collective influences on environmental engineering applications. J Nanoparticle Res. 2017;19:368. doi: 10.1007/s11051-017-4065-6. [DOI] [Google Scholar]
- Yetiş R, Atasoy AD, Demir Yetiş A, Yeşilnacar Mİ. Hydrogeochemical characteristics and quality assessment of groundwater in Balikligol Basin, Sanliurfa. Turkey. Environ Earth Sci. 2019;78:331. doi: 10.1007/s12665-019-8330-0. [DOI] [Google Scholar]
- You J, Wang L, Zhao Y, Bao W. A review of amino-functionalized magnetic nanoparticles for water treatment: Features and prospects. J Clean Prod. 2021;281:124668. doi: 10.1016/j.jclepro.2020.124668. [DOI] [Google Scholar]
- Yu C-X, Wang K-Z, Li X-J, et al. Highly efficient and facile removal of Pb2+ from water by using a negatively charged azoxy-functionalized metal-organic framework. Cryst Growth Des. 2020;20:5251–5260. doi: 10.1021/acs.cgd.0c00437. [DOI] [Google Scholar]
- Zaidi NS, Sohaili J, Muda K, Sillanpää M. Magnetic field application and its potential in water and wastewater treatment systems. Sep Purif Rev. 2014;43:206–240. doi: 10.1080/15422119.2013.794148. [DOI] [Google Scholar]
- Zhang D, Huo P, Liu W. Behavior of phenol adsorption on thermal modified activated carbon. Chinese J Chem Eng. 2016;24:446–452. doi: 10.1016/j.cjche.2015.11.022. [DOI] [Google Scholar]
- Zhang Y, Wu B, Xu H, et al. Nanomaterials-enabled water and wastewater treatment. NanoImpact. 2016;3–4:22–39. doi: 10.1016/j.impact.2016.09.004. [DOI] [Google Scholar]
- Zhang Y, Zhu C, Liu F, et al. Effects of ionic strength on removal of toxic pollutants from aqueous media with multifarious adsorbents: a review. Sci Total Environ. 2019;646:265–279. doi: 10.1016/j.scitotenv.2018.07.279. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang H, He X, et al. Research progress, problems and prospects of mine water treatment technology and resource utilization in China. Crit Rev Environ Sci Technol. 2020;50:331–383. doi: 10.1080/10643389.2019.1629798. [DOI] [Google Scholar]
- Zhao R, Zheng H, Zhong Z, et al. Efficient removal of diclofenac from surface water by the functionalized multilayer magnetic adsorbent: Kinetics and mechanism. Sci Total Environ. 2021;760:144307. doi: 10.1016/j.scitotenv.2020.144307. [DOI] [PubMed] [Google Scholar]
- Zhao W, Tian Y, Chu X, et al. Preparation and characteristics of a magnetic carbon nanotube adsorbent: Its efficient adsorption and recoverable performances. Sep Purif Technol. 2021;257:117917. doi: 10.1016/j.seppur.2020.117917. [DOI] [Google Scholar]
- Zhou W, Deng J, Qin Z, et al. Construction of MoS2 nanoarrays and MoO3 nanobelts: Two efficient adsorbents for removal of Pb(II), Au(III) and Methylene Blue. J Environ Sci. 2022;111:38–50. doi: 10.1016/j.jes.2021.02.031. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kolar P. Adsorptive removal of p-cresol using coconut shell-activated char. J Environ Chem Eng. 2014;2:2050–2058. doi: 10.1016/j.jece.2014.08.022. [DOI] [Google Scholar]