Abstract
Introduction:
While depression generally improves after bariatric surgery, less is known regarding heterogeneity in long-term symptom change. Given that depressive symptoms have been associated with weight change following bariatric surgery, identifying and characterizing subgroups with more severe depressive symptoms may have prognostic utility for understanding post-surgical weight loss. This study sought to characterize patterns of change in depressive symptoms and evaluate associations with weight loss in the seven years following bariatric surgery.
Methods:
Participants were 2,308 patients who underwent bariatric surgery as part of the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Depressive symptoms (measured by the Beck Depression Inventory) and weight were assessed annually following surgery.
Results:
A group-based trajectory model identified six subgroups that evidenced distinct patterns of change in depressive symptoms, with the majority (87.0%) exhibiting stable low to average levels. Generalized linear mixed models indicated trajectory groups differed in percent total weight loss (%TWL), with trajectories characterized by initial decreases in depressive symptoms over the first two years (5.2% of participants) experiencing the highest %TWL (20.7% vs. 14.9–18.4% in the other trajectories at 7 years).
Conclusions:
Findings demonstrate meaningful heterogeneity in the pattern of changes in depressive symptoms after surgery. While most patients experience relatively low stable levels of depressive symptoms, those who have initial symptom improvement demonstrate the greatest magnitude of weight loss. Further research is necessary to explore the directionality of this association and the time-varying mechanisms by which depression and weight may mutually influence each other.
Keywords: bariatric surgery, depression, follow-up, weight loss outcomes
Introduction
Although bariatric surgery is an efficacious intervention for severe obesity, relationships between psychological factors and long-term surgery outcomes are not completely understood. Existing evidence indicates that depression is the most common psychiatric illness among those who undergo bariatric surgery (i.e., current prevalence of 19%), and that levels of depression are higher among bariatric surgery candidates compared to the general population.(1) However, the nature of long term post-surgical changes in depressive symptoms, and the extent to which such changes may be associated with weight loss, remains unclear.
With respect to postsurgical outcomes, most studies have found improvement in depressive symptomatology.(1) A three-year follow-up of patients in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study found that depressive symptoms significantly decreased in the year following surgery.(2) However, levels increased between the one- and three-year follow-up, albeit remaining below pre-surgical levels, which suggests that the trajectory of depression symptom change may become increasingly heterogeneous over time.(2)
Similarly, weight outcomes after bariatric surgery evidence significant variability.(3) While psychological symptoms, including depression, have been shown to be related to preoperative obesity severity (4), a 2016 review of five studies found that preoperative depression did not emerge as a consistent predictor of post-surgical weight outcomes (1). In four studies, preoperative depression was not significantly related to weight outcomes six months to two years following surgery, though one study found patients with preoperative depression or anxiety had less weight loss at four years.(5)
Despite more inconsistent findings from studies examining preoperative levels of depression as predictors of weight loss, other studies have shown levels of depression following surgery may correspond with weight loss. One study found that depression levels at six months post-surgery was predictive of concurrent weight loss as well as weight loss at the 12-month (but not 24-month) follow-up.(6) Within the LABS-2 study, improvement in depressive symptoms was related to weight change three years later (2). Among a smaller sample of patients in the LABS-3 cohort, there was also a trend for the presence of postoperative, but not preoperative, mood disorders to be associated with greater weight regain over the seven years after surgery.(7)
In sum, depression is a salient issue among bariatric surgery patients, and while there appears to be short-term improvement in depressive symptoms following surgery, symptoms may re-emerge by two years after surgery, which is also the time when patients tend to experience weight regain.(8) Although extant evidence suggests heterogeneity in the long-term course of depressive symptoms and weight loss, there remains a dearth of studies examining the extent to which changes in post-surgical depressive symptoms are related to long-term weight loss and other relevant demographic and clinical characteristics. Characterizing subgroups with less improvement in depressive symptoms may have prognostic utility for understanding post-surgical weight loss, and potentially identify key periods for intervention.
Therefore, the present study aimed to characterize patterns of changes in depressive symptoms during the seven years following surgery, thereby providing a longer-term follow-up to the previously published data (2), and to examine the extent to which different trajectories of depressive symptoms are related to weight loss and demographic and clinical characteristics (i.e., age, gender, surgery type, depression treatment). Based on previous findings, it was expected that depression trajectories characterized by higher stable or increasing depressive symptoms would be related to less weight loss over time.
Methods
Participants
Participants were 2,308 adults (78.7% female; Mage=45.5±11.4 years) who underwent Roux-en-Y gastric bypass (RYGB) or laparoscopic adjustable gastric banding (LAGB) in the LABS-2 study at 10 hospitals throughout the United States (2006–2009). Participants who underwent more than one bariatric surgery during follow-up were excluded, leaving n=1,769 RYGB and n=539 LAGB. Data collection procedures and seven-year weight outcomes have been published previously (3). Following surgery participants returned for a 6-month and then annual follow-ups with the exception of year 6, which only involved a brief assessment done by telephone or mail. Informed consent was obtained from all participants.
Measures
Depressive symptoms.
Depressive symptoms over the past week were assessed using the Beck Depression Inventory-1 (BDI; (9), which was administered prior to surgery (year 0), 6 months, and years 1, 2, 3, 4, 5, and 7. Given that weight loss in this population can be rapid and was likely to be intentional, no points were assigned to the BDI item, “I have lost more than 5 pounds.” The following ranges were used to interpret the BDI total score: 0–9, 10–18, 19–29 and 30–60, reflecting no/minimal, mild, moderate and severe depression symptomatology, respectively.(7)
Treatment for Depression.
At each visit, participants reported hospital admissions and/or counseling for depression within the past 12 months and if they were currently taking antidepressant medication.1 Treatments for depression was operationalized as the total number of endorsements of hospitalizations for depression, counseling for depression, and antidepressant use over the seven years.
Statistical Analyses
Descriptive statistics characterized depressive symptoms in the sample; t-tests examined changes in BDI total scores between pre-surgery and the seven-year follow-up, as well as changes in the proportion of participants exhibiting minimal symptoms. To explore the degree of attrition and related factors, the compliance rate for each participant was also calculated as the number of completed assessments out of the total possible assessments. Participant age at enrollment, presurgical BDI and BMI, race, gender, and surgery type were then explored as predictors of compliance using a generalized linear model (specifying a gamma distribution).
A group-based trajectory model was used to identify subgroups of participants whose depressive symptoms (BDI total z-score) followed a similar pattern of change. The BDI total z-score was calculated as (x-μ)/σ, where x is the raw score, μ is the population mean, and σ is the population standard deviation. The model was estimated using maximum likelihood procedures with PROC TRAJ for SAS 9.4.(10) The optimal number of groups was selected based on the Bayesian information criterion (BIC) and Akaike information criterion (AIC). Group membership was determined using the posterior predicted probabilities for each individual being a member of each of the trajectories, and assignment was based on the largest posterior membership probability. Model fit was evaluated based on the recommended average posterior probability of assignment for each group (≥.70).(11)
Differences between groups were first examined with respect to percent total weight loss (%TWL). %TWL was calculated as 100*([weight at seven-year follow-up]–[pre-surgery weight])/pre-surgery weight, such that more negative values indicate greater %TWL. To examine differences between trajectories in %TWL at each assessment, a linear mixed model was conducted that included trajectory group as independent variables. Covariates were also included, which were based on those found to be related to percent weight loss in recent studies of the LABS-2 cohort: race, age, pre-surgery diabetes status and pre-surgery self-reported acceptable percent weight loss, status of self-weighing at least weekly, cutting out between meal snacking, loss of control eating and binge eating disorder.(12–14)
Additional generalized linear models examined the degree to which trajectory groups differed in age and total endorsements of each type of depression treatment using Wald chi-square (χ2) test, whereas Pearson’s χ2 tests examined differences in surgical procedure and gender by trajectory group. Negative binomial distributions were used to account for skewed distributions of depression treatment variables. Effects were followed up with pairwise comparisons using Fisher’s least significance difference.
Results
Descriptive information is shown in Table 1. Age at consent and participant gender were significantly associated with compliance (age: χ2=58.87, p<.001; gender: χ2=4.56, p=.003), such that older individuals and individuals who identified as female compared to male had higher compliance. Other variables (i.e., race, presurgical BDI and BMI, surgery type) were not significantly associated with compliance (ps>.05). Compared to pre-surgical BDI total scores, there were significant decreases in scores by year 7 (t=12.34, [1007], p<.001). The proportion of the sample categorized as having minimal depressive symptoms at year 7 (74.8%) relative to more severe categories was significantly higher at year 7 compared to pre-surgery (58.0%), (t(3)=9.47, p<.001).
Table 1.
Mean Beck Depression Inventory (BDI) score, depressive symptom severity, and treatment for depression by year
| BDI Severity categories | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDI total | Minimal (0–9) | Mild (10–18) | Moderate (19–29) | Severe (30–63) | Antidepressant | Counseling | Hospitalization | |||||
| Time | Total N | M (SD) | N (%) | N (%) | N (%) | N (%) | Total N | N (%) | Total N | N (%) | Total N | N (%) |
| Pre-surgery | 2248 | 9.7 (7.3) | 1303(58.0) | 674 (30.0) | 229 (10.2) | 42 (1.9) | 2257 | 701 (31.1) | 2211 | 317 (14.3) | 2248 | 126 (5.6) |
| 6-month | 1909 | 6.3 (5.9) | 1565 (82.0) | 263 (13.8) | 67 (3.5) | 14 (0.7) | 1855 | 475 (25.6) | 1841 | 217 (11.8) | 1862 | 19 (1.0) |
| Year 1 | 1846 | 5.5 (6.4) | 1539 (83.4) | 219 (11.9) | 61 (3.3) | 26 (1.4) | 1648 | 428 (26.0) | 1647 | 215 (13.1) | 1669 | 22 (1.3) |
| Year 2 | 1576 | 5.5 (7.0) | 1292 (82.0) | 193 (12.2) | 69 (4.4) | 22 (1.4) | 1566 | 419 (26.8) | 1562 | 201 (12.9) | 1584 | 29 (1.8) |
| Year 3 | 1520 | 6.1 (7.3) | 1193 (78.5) | 225 (14.8) | 78 (5.1) | 24 (1.6) | 1546 | 424 (27.4) | 1533 | 220 (14.4) | 1554 | 18 (1.2) |
| Year 4 | 1486 | 6.7 (7.9) | 1123 (75.6) | 227 (15.3) | 105 (7.1) | 31 (2.1) | 1572 | 437 (27.8) | 1563 | 236 (15.1) | 1585 | 21 (1.3) |
| Year 5 | 1523 | 6.7 (7.9) | 1151 (75.6) | 240 (15.8) | 99 (6.5) | 33 (2.2) | 1104 | 301 (27.3) | 1102 | 165 (15.0) | 1109 | 16 (1.4) |
| Year 7 | 1034 | 6.6 (7.4) | 775 (74.8) | 182 (17.6) | 57 (5.5) | 20 (1.9) | 2257 | 701 (31.1) | 2211 | 317 (14.3) | 2248 | 126 (5.6) |
Note. BDI=Beck Depression Inventory. Total N reflects the number of participants who responded to the assessment at each assessment.
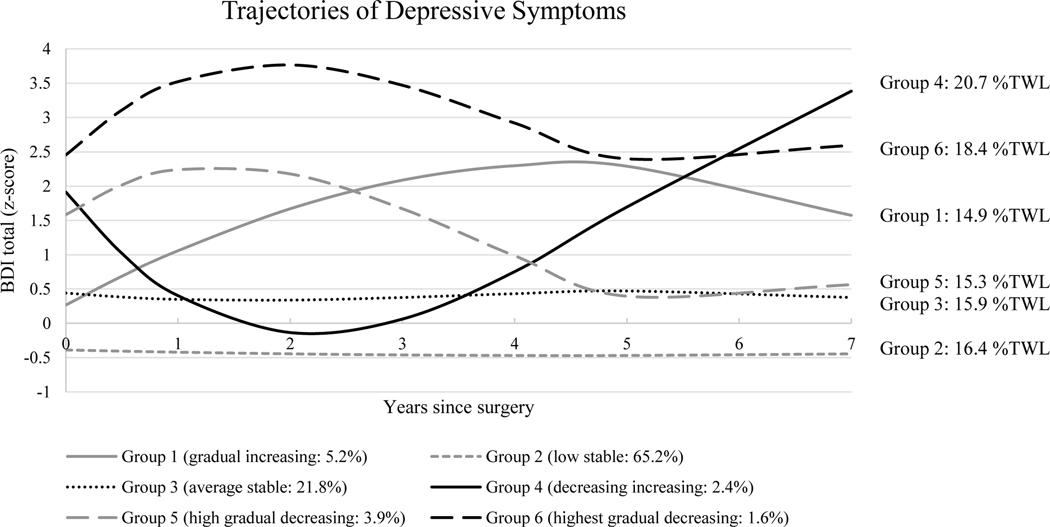
The group-based trajectory model identified six trajectories of depressive symptoms based on the AIC and BIC (Figure 1; see Table 2 with fit indices). Group 1 (“gradual increasing”: 5.2% of participants) were within 1 SD of the sample mean prior to surgery but showed gradual increases to >2 SDs above the sample mean by year 5, followed by gradual declines. The majority of participants exhibited a “low stable” (Group 2: 65.2% of participants) or “average stable” (Group 3: 21.8% of participants) trajectory of depressive symptoms, with BDI scores remaining within 1 SD below or above the sample mean over the seven years, respectively. The next trajectory (Group 4: 2.4% of participants) showed a “decreasing increasing” pattern of symptom severity, which started over 1.5 SD above the sample mean, decreased during the first two years to within 1 SD of the sample mean, then increased steadily through year 7 to >3 SD above the sample mean. The final 2 groups showed parallel trajectories that differed in severity: Groups 5 (“high gradual decreasing”: 3.9% of participants) and 6 (“highest gradual decreasing”: 1.6% of participants) were characterized by initial elevations in symptoms (i.e., >1.5 SD above sample mean) over the first two years, followed by gradual declines until year 5, and subsequent stabilization.
Figure 1.
Six identified trajectories of depressive symptoms (Beck Depression Inventory [BDI] z-scores) following Roux-en-Y gastric bypass (RYGB) or laparoscopic adjustable gastric banding (LAGB) surgery. Group percentages reflect percent of total sample. %TWL reflects percent total weight loss from pre-surgery to year 7.
Table 2.
Fit indices
| Number of Groups | Bayesian Information Criterion (BIC) | Akaike Information Criterion (AIC) |
|---|---|---|
| 2 | −16455.50 | −16426.52 |
| 3 | −15877.90 | −15834.42 |
| 4 | −15622.10 | −15564.05 |
| 5 | −15430.90 | −15358.42 |
| 6 | −15292.70 | −15205.66 |
| 7 | −15305.60 | −15206.11 |
BDI trajectory groups did not differ in surgical procedure (χ2[5]=5.32, p=.378) or age (χ2[5]=4.74, p=.449), but significant gender differences were observed (χ2[5] =19.58, p=.002). As shown in Table 3, Group 1 (“gradual increasing”) was associated with a higher proportion of women (93.4%) relative to the other trajectory groups, in which the proportion of women ranged from 72.7–83.7%.
Table 3.
Treatment, gender, and percent total weight loss (%TWL) across BDI trajectory groups
| BDI Trajectory Group | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | 1. Gradual increasing | 2. Low stable | 3. Average stable | 4. Decreasing increasing | 5. High gradual decreasing | 6. Highest gradual decreasing | Pairwise comparisons |
| Antidepressant | |||||||
| M | 2.57 | 1.19 | 2.22 | 3.18 | 2.34 | 2.02 | 2<1,3,4,5,6 |
| SD | 2.54 | 2.08 | 2.56 | 2.56 | 2.31 | 1.86 | |
| Hospitalization | |||||||
| M | 0.49 | 0.05 | 0.14 | 0.49 | 0.34 | 0.36 | 2<1,3,4,5,6 3<1,4,5,6 |
| SD | 1.17 | 0.25 | 0.44 | 1.02 | 0.73 | 0.84 | |
| Counseling | |||||||
| M | 1.98 | 0.39 | 1.22 | 2.46 | 1.81 | 1.44 | 2<1,3,4,5,6 3<1,5,6 |
| SD | 2.20 | 1.01 | 1.96 | 2.64 | 2.15 | 1.86 | |
| Gender | |||||||
| Men | 6.6% | 23.0% | 19.4% | 23.1% | 16.3% | 27.3% | |
| Women | 93.4% | 77.0% | 80.6% | 76.9% | 83.7% | 72.7% | |
| %TWL | |||||||
| Mean | −14.9 | −16.4 | −15.9 | −20.7 | −15.3 | −18.4 | 4 < 1,2,3,5 6<1,5 |
| SE | 1.3 | 1.1 | 1.1 | 1.5 | 1.3 | 1.7 | |
| Lower CI | −17.4 | −18.5 | −18.1 | −23.6 | −17.8 | −21.6 | 2<1 |
| Upper CI | −12.4 | −14.3 | −13.8 | −17.7 | −12.7 | −15.1 | |
Note. BDI=Beck Depression Inventory. Treatment means reflect total number of assessments with the specified treatment endorsement across the 7 years of data collections; pairwise differences are significant at p<.05.
Generalized linear models indicated effects of BDI trajectory group for the number of follow-up assessments with endorsement of antidepressant medication use (Wald χ2 [5]=155.58, p<.001), hospitalizations for depression (Wald χ2 [5]=191.12, p<.001), and reports of counseling for depression (Wald χ2 [5]=427.06, p<.001). The number of follow-up assessments with endorsements of each type of treatment across BDI trajectories are displayed in Table 3. Group 2 (“low stable”) had significantly fewer endorsements of antidepressant medication use, hospitalizations for depression, and counseling for depression compared to all other groups (ps<.05). Group 3 (“average stable”) had significantly fewer hospitalizations compared to all other groups except Group 2 (ps<.05) and significantly fewer reports of counseling compared to Groups 1 (“gradual increasing”), 5 (“high gradual decreasing”), and 6 (“highest gradual decreasing”) (ps<.05).
Results of mixed model analysis examining the association between BDI trajectory group and %TWL indicated a main effect of trajectory group (F [5, 9735])=5.50, p<.001), after adjusting for effects of covariates (i.e., race, age, pre-surgery diabetes status, status of self-weighing at least weekly, cutting out between meal snacking, loss of control eating and binge eating disorder).2 The total estimated %TWL over time in each trajectory group and pairwise comparisons are shown in Table 3, with estimated %TWL by year 7 depicted in Figure 1. With respect to 7 year %TWL, Group 6 (“highest gradual decreasing”) and Group 4 (“decreasing increasing”) had the greatest %TWL (18.4% and 20.7%, respectively). Pairwise comparisons of %TWL at year 7 indicated Group 4 had more %TWL compared to Groups 1 (“gradual increasing”: 14.9%), 2 (“low stable”: 16.4%), 3 (“average stable”: 15.9%), and 5 (“high gradual decreasing”: 15.3%), and Group 6 also evidenced greater %TWL than Groups 1 and 5, and Group 2 showed greater %TWL than Group 1. Group 6 (“highest gradual decreasing”) was not significantly different from other groups in %TWL.
Discussion
This study investigated heterogeneity in trajectories of depressive symptoms following bariatric surgery across seven years of follow-up, as well as the degree to which different trajectories of depressive symptoms were associated with weight loss and demographic and clinical characteristics. There was a significant decrease in the severity of depressive symptoms over the seven years, with a greater proportion of individuals (74.8%) evidencing minimal symptoms at year 7 compared to the proportion experiencing minimal symptoms prior to surgery (58%). Six depression symptom trajectories were identified, with 87% of the sample having low-to-average stable levels of depressive symptoms across the seven years (i.e., Groups 2 and 3). This suggests that for the majority of bariatric surgery patients, there is little variability in overall depression symptom levels. However, the remaining participants followed four distinct trajectories of symptom change across the follow-up.
Three groups showed either initial (Group 4) or delayed (Groups 5–6) declines in symptoms, which may reflect surgery-related increases in quality of life and body satisfaction.(15) However, 5.6% reported gradual increases in depressive symptoms (Group 1), and 2.4% (Group 4) showed a rebound in symptoms following initial declines. This underscores the need for continued monitoring of psychological symptoms well beyond the first postoperative year.
Heterogeneity in depressive symptoms was also related to weight loss. Trajectories characterized by initial decreases in depressive symptoms (Group 4) or initial very high symptoms that later decreased (Group 6) were associated with the greatest weight loss, with Group 4 showing more weight loss compared to all other groups except for Group 6. Reductions in depressive symptoms may enhance factors related to early behavior change and weight maintenance, such as self-efficacy and adaptive coping.(16) Consequently, such individuals may be better equipped to adhere to post-surgical lifestyle recommendations. This pattern of results for Group 4 is also consistent with the literature on rapid response indicating early symptom improvements during treatment for depression predict better long-term outcomes (17). The current results extend such findings, in that rapid improvement in depressive symptoms could be related to long-term weight loss in bariatric surgery patients. The first postoperative year may be a key intervention point for those with higher initial levels.
It was somewhat unexpected that those who initially decreased in depressive symptoms but later reported steady increases (Group 4) had more weight loss despite worsening depressive symptoms. It could be that this rebound in depressive symptoms in conjunction with greater weight loss reflected the development of concerns related to body image and disordered eating behaviors, given the association between eating disorder symptoms and depression.(18) In addition, loss of appetite may be a salient manifestation of depression in this group, which could contribute to weight loss.
There were also differences in demographic and clinical characteristics across trajectories. The finding that Group 1 (“gradual increasing”) included a higher proportion of women is consistent with research indicating higher rates of depression in women, especially women with obesity.(19, 20) It is not surprising that those with low stable levels of depressive symptoms (Group 2) reported less treatment for depression compared to other groups, followed by those with average stable symptoms (Group 3). Given that over one-fourth of participants were on antidepressant medication throughout follow-up, this may in part account for the low symptom levels. Further, the use of antidepressant medication could possibly be an indicator that a substantial portion of patients are being appropriately targeted for treatment. Additional research is warranted to better understand the type, timing, and intensity of treatments that most effectively target depressive symptoms remaining groups characterized by more variable patterns of depressive symptoms.
It is important to note limitations of the study. Participants were recruited from centers with research-oriented bariatric surgery programs, which may reduce the generalizability. A multi-method approach would be useful to elucidate bio-behavioral mechanisms underlying a range of depressive symptoms (e.g., anhedonia, suicidal thoughts, appetite), as well as more frequent assessments.(21) In addition, due to the timing of study recruitment, this study did not include patients who underwent sleeve gastrectomy. Participants who underwent multiple bariatric surgeries were also excluded, and thus there may be risk of bias in the present results given that these patients may have a more severe clinical profile and present with greater depressive symptoms.
These results demonstrate meaningful heterogeneity in depressive symptoms following bariatric surgery that is linked to weight outcomes. Such findings highlight the need for continued assessment of psychological functioning after initial weight loss and emphasize the importance of early reductions in depressive symptoms as possible prognostic markers of weight outcome. Further research is necessary to examine potential bi-directional mechanistic pathways by which depression and weight loss may influence each other, which span behavioral, neurocognitive, physiological, and social domains.(22) For instance, the improvements in body image and quality of life that have been shown to follow surgery may facilitate reductions in depressive symptoms.(15) Examining such questions may inform the development of more targeted prevention and intervention efforts in the years following bariatric surgery.
Acknowledgments
Funding: LABS-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants U01 DK066557 (University of Pittsburgh, Data Coordinating Center); U01-DK66667 and UL1-RR024996 (Columbia- Presbyterian in collaboration with Cornell University Medical Center Clinical and Translational Research Center [CTRC]); U01-DK66568 and M01RR-00037 (University of Washington in collaboration with Cornell University Medical Center CTRC); U01-DK66471 (Neuropsychiatric Research Institute); U01-DK66526 (East Carolina University); U01-DK66585 and UL1- RR024153 (University of Pittsburgh Medical Center in collaboration with Cornell University Medical Center CTRC); and U01-DK66555 (Oregon Health & Science University). This research was registered at ClinicalTrials.gov (Identifier: NCT00465829)
Footnotes
The authors declare that they have no conflict of interest.
The self-report measure of antidepressant medication used in the present study differs from that used by Mitchell and colleagues (2015).
Fixed effects of covariates were as follows: race: F(5)=1.11, p=.353, age: F(1)=47.60, p<.001, pre-surgery diabetes status: F(2)=16.43, p<.001, status of self-weighing at least weekly: F(1)=152.97, p<.001, cutting out between meal snacking: F(3)=628.40, p<.001, loss of control eating: F(3)=10.48, p<.001, and binge eating disorder: F(1)=81.27, p<.001.
References
- 1.Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM, et al. Mental Health Conditions Among Patients Seeking and Undergoing Bariatric Surgery: A Meta-analysis. JAMA. 2016;315(2):150–63. Epub 2016/01/13. doi: 10.1001/jama.2015.18118. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JE, King WC, Chen JY, Devlin MJ, Flum D, Garcia L, et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity (Silver Spring). 2014;22(8):1799–806. Epub 2014/03/19. doi: 10.1002/oby.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427–34. Epub 2017/12/08. doi: 10.1001/jamasurg.2017.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalarchian MA, Marcus MD, Levine MD, Courcoulas AP, Pilkonis PA, Ringham RM, et al. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry. 2007;164(2):328–34; quiz 74. Epub 2007/02/03. doi: 10.1176/ajp.2007.164.2.328. [DOI] [PubMed] [Google Scholar]
- 5.Legenbauer T, De Zwaan M, Benecke A, Muhlhans B, Petrak F, Herpertz S. Depression and anxiety: their predictive function for weight loss in obese individuals. Obes Facts. 2009;2(4):227–34. Epub 2010/01/08. doi: 10.1159/000226278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White MA, Kalarchian MA, Levine MD, Masheb RM, Marcus MD, Grilo CM. Prognostic Significance of Depressive Symptoms on Weight Loss and Psychosocial Outcomes Following Gastric Bypass Surgery: A Prospective 24-Month Follow-Up Study. Obes Surg. 2015;25(10):1909–16. Epub 2015/02/28. doi: 10.1007/s11695-015-1631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalarchian MA, King WC, Devlin MJ, Hinerman A, Marcus MD, Yanovski SZ, et al. Mental disorders and weight change in a prospective study of bariatric surgery patients: 7 years of follow-up. Surg Obes Relat Dis. 2019;15(5):739–48. Epub 2019/03/04. doi: 10.1016/j.soard.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the Performance of Common Measures of Weight Regain After Bariatric Surgery for Association With Clinical Outcomes. JAMA. 2018;320(15):1560–9. Epub 2018/10/17. doi: 10.1001/jama.2018.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. Epub 1961/06/01. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 10.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS procedure for estimating them. Sociological Methods & Research. 2007;35:542–71. [Google Scholar]
- 11.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 12.King W, Hinerman A, White G, et al. Associations between physical activity and changes in weight across 7 years following Roux-en-Y gastric bypass surgery: a multicenter prospective cohort study. Annals of Surgery. Under review. [DOI] [PubMed] [Google Scholar]
- 13.Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis. 2015;11(5):1109–18. Epub 2015/04/01. doi: 10.1016/j.soard.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell JE, Christian NJ, Flum DR, Pomp A, Pories WJ, Wolfe BM, et al. Postoperative Behavioral Variables and Weight Change 3 Years After Bariatric Surgery. JAMA Surg. 2016;151(8):752–7. Epub 2016/04/21. doi: 10.1001/jamasurg.2016.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarwer DB, Steffen KJ. Quality of Life, Body Image and Sexual Functioning in Bariatric Surgery Patients. Eur Eat Disord Rev. 2015;23(6):504–8. Epub 2015/11/27. doi: 10.1002/erv.2412. [DOI] [PubMed] [Google Scholar]
- 16.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. Epub 2005/01/19. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 17.Renaud J, Brent DA, Baugher M, Birmaher B, Kolko DJ, Bridge J. Rapid response to psychosocial treatment for adolescent depression: a two-year follow-up. J Am Acad Child Adolesc Psychiatry. 1998;37(11):1184–90. Epub 1998/11/11. doi: 10.1097/00004583-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry. 2007;61(3):348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolen-Hoeksema S. Gender differences in depression. Current directions in psychological science. 2001;10(5):173–6. [Google Scholar]
- 20.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000;90(2):251–7. Epub 2000/02/10. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oquendo MA, Barrera A, Ellis SP, Li S, Burke AK, Grunebaum M, et al. Instability of symptoms in recurrent major depression: a prospective study. Am J Psychiatry. 2004;161(2):255–61. Epub 2004/02/03. doi: 10.1176/appi.ajp.161.2.255. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz S, Friedman MA, Arent SM Understanding the relation between obesity and depression: causal mechanisms and implications for treatment. Clinical Psychology: Science and Practice. 2008;15(1):1–20. [Google Scholar]