Abstract
Cyclopentenone prostaglandins (cyPGs) are biologically active lipid mediators, including PGA2, PGA1, PGJ2, and its metabolites. cyPGs are essential regulators of inflammation, cell proliferation, apoptosis, angiogenesis, cell migration, and stem cell activity. cyPGs biologically act on multiple cellular targets, including transcription factors and signal transduction pathways. cyPGs regulate the inflammatory response by interfering with NF-κB, AP-1, MAPK, and JAK/STAT signaling pathways via both a group of nuclear receptor peroxisome proliferator-activated receptor-gamma (PPAR-γ) dependent and PPAR-γ independent mechanisms. cyPGs promote the resolution of chronic inflammation associated with cancers and pathogen (bacterial, viral, and parasitic) infection. cyPGs exhibit potent effects on viral infections by repressing viral protein synthesis, altering viral protein glycosylation, inhibiting virus transmission, and reducing virus-induced inflammation. We summarize their anti-proliferative, pro-apoptotic, cytoprotective, antioxidant, anti-angiogenic, anti-inflammatory, pro-resolution, and anti-metastatic potential. These properties render them unique therapeutic value, especially in resolving inflammation and could be used in adjunct with other existing therapies. We also discuss other α, β -unsaturated carbonyl lipids and cyPGs like isoprostanes (IsoPs) compounds.
Keywords: prostaglandins, PPAR-γ, viral (or virus), inflammation, antiviral
Introduction
Prostaglandins (PGs) are a group of lipids or oxygenated derivatives of arachidonic acid (AA) that sustain homeostatic functions and mediate the inflammatory response (Aoki and Narumiya, 2012). There are two types of PGs: conventional or classic PGs and cyclopentenone PGs (cyPGs). Examples of traditional PGs are PGD2, PGE2, prostacyclin (PGI2), PGF2α, and thromboxane A2 (TXA2), while the members of cyPGs include PGA1, PGA2, PGJ2, and metabolites of PGJ2, such as 15-Deoxy-Δ-12,14-Prostaglandin J2 (15d-PGJ2) and Δ12-PGJ2. As the name implies, cyPGs contain a cyclopentenone ring structure with a highly reactive α, β-unsaturated carbonyl group, which can alter many proteins and their functional properties covalent attachments with thiol groups of the proteins (Straus and Glass, 2001). cyPGs are potent bioactive molecules and have a wide range of functions (Burstein, 2020). cyPGs can repress inflammatory responses, inhibit cell growth, angiogenesis, and increase apoptosis. cyPGs can interfere with virus infections and cancer development, indicating their potential to serve as therapeutic agents. This review discusses cyPGs biosynthesis, mechanism of action, functions, and their effects on virus infection and cancer development. Despite the existing knowledge, the resolving, antiviral, anti-inflammatory, and anticancer potential of cyPGs have been minimally explored and warrant further attention.
Biosynthesis of Cyclopentenone Prostaglandins (PGA1, PGA2, and PGJ2 and Its Metabolites)
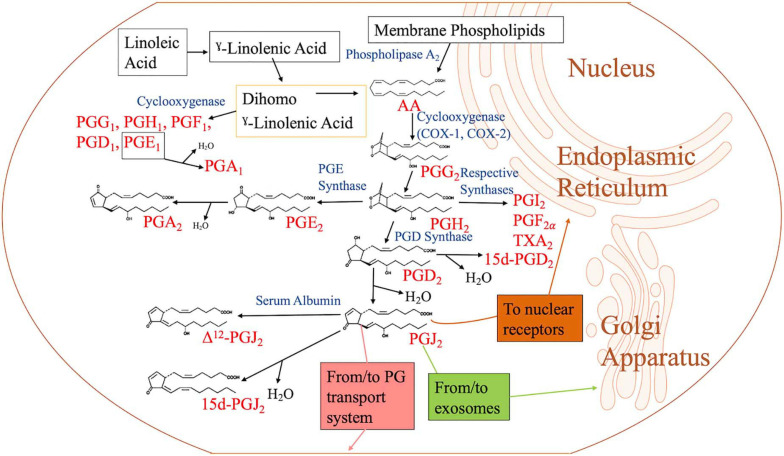
AA is liberated from membrane phospholipids by the enzyme phospholipase A2 (PLA2) (Vane and Botting, 1990). Myosin, an actin-binding protein, is phosphorylated when there is an increase in intracellular calcium levels, causing PLA2 to translocate from the cytoplasm to the intracellular membrane to access the phospholipids. Arachidonate is metabolized to PGG2 by cyclooxygenase (COX) 1 and 2 (COX-1 and COX-2), which are contained in the endoplasmic reticulum (ER) and nuclear membranes (Vane and Botting, 1990; Hanna and Hafez, 2018) (Figure 1). PGG2 is converted into PGH2 by hydroxyperoxidase. Unstable PGH2 diffuses from the ER lumen to the cytoplasm through the ER membrane. Due to its unstable nature, PGH2 is enzymatically converted into different PGs, including PGI2, PGF2, and TXA2, through the action of specific PG synthases (Figure 1). When PGH2 is acted upon by PGD2 synthase, PGD2 is created. PGD2 is unstable and spontaneously undergoes non-enzymatic dehydration to yield either 15d-PGD2 or PGJ2 (Figure 1). Further dehydration and a 13, 14 double bond rearrangement of PGJ2 yield 15-Deoxy-Δ-12,14-prostaglandin J2 (15d-PGJ2) in an albumin-independent manner, while PGJ2 dependent on serum albumin results in Δ12-PGJ2 (Figueiredo-Pereira et al., 2014). PGs of the J series are synthesized in vivo as Δ12-PGJ2 is a natural component of human body fluids. Its synthesis is inhibited by treatment with COX inhibitors (Hirata et al., 1988). When PGH2 is acted upon by PGE2 synthase, PGE2 is formed. Dehydration of PGE2 leads to PGA2 (Hamberg and Samuelsson, 1966; Nugteren et al., 1966) (Figure 1). 15d-PGJ2 could function in both an autocrine and paracrine manner and can be produced intracellularly and extracellularly via non-enzymatic conversion of PGD2 (Shibata et al., 2002).
FIGURE 1.
Biosynthesis of cyclopentenone prostaglandins. When the cell is activated by stressful stimuli, such as mechanical trauma, interferon, interleukin, or growth factors, the enzyme phospholipase A2 moves from the cytoplasm to intracellular membranes to liberate arachidonic acid (AA) from the nuclear envelope or endoplasmic reticulum. AA is converted by cyclooxygenase-1 (COX-1) or cyclooxygenase-2 (COX-2) to prostaglandin G2 (PGG2), followed by hydroperoxidation of PGG2 to PGH2. PGH2 is converted to other PGH2 metabolites such as PGD2, PGE2, PGF2, PGI2, and thromboxane A2 (TXA2) by their respective synthases. Of the metabolites, PGD2 is dehydrated to form J2 PGs. PGJ2 may be located in exosomes, transport systems, or nuclear receptors to execute its function. PGE2 is dehydrated to form PGA2. PGA1 is a metabolite of linoleic acid, which is obtained through diet.
The formation of the cyclopentenone PGA1 has a different genesis pathway compared to the other members of its family (PGA2 and PGJ2). The formation of PGA1 begins with linoleic acid (LA). In the human diet, linoleic acid is the most consumed polyunsaturated fatty acid (PUFA) (Whelan and Fritsche, 2013). Linoleic acid, an essential omega 6 (n = 6) fatty acid, is converted to γ-linoleic acid (GLA; GLA, 18:3-6) through the membrane-bound enzyme 6-desaturase (Δ-6-desaturase). GLA is then metabolized to dihomo γ-linolenic acid (DGLA, 20:3-6) by a Δ6 elongase. From this point, DGLA can be converted into AA by the enzyme 5-Desaturase, or PGE1 by the enzyme COX. PGE1 undergoes dehydration to become PGA1 (Kapoor and Huang, 2006; Kapoor et al., 2007).
15d-PGJ2 acts via G-protein-coupled seven-transmembrane PGD2 receptors (D prostanoid; DP1 and DP2) and through interaction with intracellular targets (Kato et al., 1986; Kim et al., 1993; Negishi and Katoh, 2002). DP2 (chemoattractant receptor-homologous molecule or GPR44 or CD294) is expressed on Th2 cells, eosinophils, activated mast cells, and basophils (Negishi and Katoh, 2002; Nagata et al., 2017). PGE1/PGA1 is native/endogenous ligands of orphan nuclear receptor-related 1 protein (Nurr1; NR4A2) and activates its transcriptional function (Negishi and Katoh, 2002; Pearen and Muscat, 2010; Kurakula et al., 2014).
Cyclopentenone Prostaglandins and Inflammation
Cyclopentenone Prostaglandins in Various Diseases
15d-PGJ2 is an immune regulator to modulate human autoimmune diseases as multiple sclerosis (MS), experimental allergic encephalomyelitis (EAE), polymyositis, Bechet’s diseases, rheumatoid arthritis (RA), atopic dermatitis, systemic lupus erythematosus (SLE) (Li and Pauza, 2009), and age-related neurodegenerative diseases, including Alzheimer’s (AD) and Parkinson’s disease (PD) (Koharudin et al., 2011). γΔT cells have been studied in context with autoimmune diseases in humans. γΔT cells possess the cytotoxic activity and produce IFN-γ, tumor necrosis factor-alpha; TNF-α, and chemokines involved in recruiting monocytes and macrophages. The induction of cytokines and secretion of interleukin-17 (IL-17) contributes to inflammatory processes and promotes autoimmunity. 15d-PGJ2, along with rosiglitazone (Avandia), suppressed γΔT cell proliferation in response and downregulated cytokine production (Li and Pauza, 2009). 15d-PGJ2 also plays an essential regulatory role in osteosarcoma, bone metastases, and bone metabolism (Kitz et al., 2011; Kim et al., 2015).
Cyclopentenone Prostaglandins Elicit Anti-inflammatory Responses via Regulating Transcription Factors Crucial for Inflammatory Response
15d-PGJ2 directly inhibits multiple steps in the NF-κB signaling pathway and NF-κB-dependent gene expression (Straus et al., 2000). NF-κB represents a family of structurally related inducible transcription factors (NF-κB1; p50, NF-κB2; p52, RelA; p65, RelB, and c-Rel) located in the cytoplasm, which activates genes responsible for inflammation and innate and adaptive immunity (Senftleben et al., 2001). The NF-κB proteins are typically sequestered in the cytoplasm by a family of inhibitory proteins, including IκB family members, which sterically block the nuclear localization sequence (NLS) of NF-κB (Senftleben et al., 2001; Sun, 2017). The IκB kinase (IKK) complex is crucial for the activation of NF-κB, as it can degrade the NF-κB inhibitor IκB through phosphorylation, subsequently freeing NF-κB (Senftleben et al., 2001). NF-κB is involved in the pathogenesis of inflammatory diseases, including RA, inflammatory bowel disease (IBD), MS, atherosclerosis, SLE, type 1 diabetes, chronic obstructive pulmonary disease (COPD), and asthma (Pai and Thomas, 2008). NF-κB activation induces proinflammatory cytokines (IL-1β, IL-1, IL-2, IL-6, IL-8, and TNF-α) (Lawrence, 2009; Wang et al., 2014) and regulates inflammasome function (Guo et al., 2015) in both innate and adaptive immune cells. PGA1, another cyPG, is also a potent inhibitor of NF-κB activation in human cells by inhibiting phosphorylation and preventing degradation of the NF-κB inhibitor IκB-α (Rossi et al., 1997). The α, β-unsaturated carbonyl group in the cyPGs, when reactive, can undergo a Michael reaction with the cysteine nucleophile at position 179 on the IKKβ subunit of the IKK complex. This cysteine is located in the activation loop of the enzyme, and the alkylation of the cysteine inhibits the phosphorylation of the activation loop. Therefore, cyPGs inhibit IKK complex activity by directly modifying the IKKβ subunit (Rossi et al., 2000). By doing so, the degradation IκB is inhibited, and NF-κB is unable to enter the nucleus.
15d-PGJ2 inhibits transcription factor activity of activating protein-1 (AP-1) (Perez-Sala et al., 2003). AP-1 is composed of dimeric complexes, which included members of four families of DNA-binding proteins such as Jun, Fos, ATF/cyclic AMP-responsive element-binding (CREB), and musculoaponeurotic fibrosarcoma (Maf) (Milde-Langosch, 2005; Hernandez et al., 2008). 15d-PGJ2 covalently modifies c-Jun and directly inhibits the DNA binding activity of AP-1 (Perez-Sala et al., 2003). AP-1 plays critical roles in inflammation, proliferation, innate immune response and stimulates growth factors and proinflammatory cytokines mediated by serine/threonine kinases as c-Jun NH2-terminal kinases (JNK), p38, extracellular signal-regulated kinases (ERK), and c-Fos-regulating kinases (FRK) MAP kinase pathways (Lin et al., 1995; Minden et al., 1995).
15d-PGJ2 non-specifically inhibits Signal transducer and activator of transcription (STAT) (Ji et al., 2005) and Janus kinase (JAK)-STAT signaling pathway in lymphocytes (Kim et al., 2005). STAT1 can be activated upon tyrosine phosphorylation by JAK1 tyrosine kinase (Mowen and David, 2000). Upon activation, STAT/STAT interactions occur immediately, and dimerized STATs can then enter the nucleus and regulate the transcription of inflammatory genes of cytokine and interferon signaling (Seif et al., 2017).
Anti-inflammatory, Anti-tumorigenic, Anti-angiogenic, Anti-metastatic, Anti-fibrotic, Resolving, and Antioxidant Modes of Action of Cyclopentenone Prostaglandins
cyPGs, such as 15d-PGJ2, PGJ2, PGA1, and PGA2, can activate peroxisome proliferator-activated receptor-gamma (PPAR-γ), and many of their biological functions are either PPAR-γ dependent or independent (Mukherjee et al., 1994; Ricote et al., 1998b; Yagami et al., 2018). PPAR-γ is one of the members (PPAR-α, PPAR-δ, and PPAR-γ) of the nuclear receptor superfamily and is a ligand-dependent transcription factor. The ligand 15d-PGJ2 activates PPAR-γ, and PPAR-γ then forms a heterodimer with retinoid X receptor (RXR) in the cytoplasm. Complex enters the nucleus (Scher and Pillinger, 2005; Li et al., 2019). This complex binds to specific PPAR response element (PPRE) regions in the DNA to activate different target genes (Forman et al., 1996).
Anti-inflammatory Actions
Peroxisome proliferator-activated receptor-gamma inhibits TNF-α, IL-6, inducible NO synthase (iNOS), gelatinase B, and COX-2 by acting as an antagonist to AP-1 and NF-κB (Welch et al., 2003). This inhibition mode was observed in activated macrophages expressing high levels of PPAR-γ (Ricote et al., 1998a,b; Straus et al., 2000). In general, when IFN-γ stimulated peritoneal macrophages were treated with 15d-PGJ2, instead of observing activated macrophages, morphological features classic of resting cells were seen (Ricote et al., 1998a,b). 15d-PGJ2 treatment inhibited the induction of iNOS transcription by inhibiting the binding of AP-1 and NF-κB on iNOS promoter (Ricote et al., 1998a,b). Usually, iNOS is upregulated in activated macrophages accompanied by the overproduction of nitric oxide (NO), which causes inflammation (Sharma and Staels, 2007). Excess NO also induces s-nitrosylation of Sirt1, an inhibitor of p65 NF-κB, which inactivates Sirt1 and enhances pro-inflammatory response (Nakazawa et al., 2017). 15d-PGJ2 treatment inhibits matrix metalloproteinase (MMP-9) or also called Gelatinase B in activated macrophages (Ricote et al., 1998a,b) at the transcription level. Inhibition by 15d-PGJ2 is mediated at the level of AP-1 binding as MMP-9 transcriptional activation is dependent on AP-1 (Saarialho-Kere et al., 1993). 15d-PGJ2 and TZDs reduced dendritic cells (DCs) stimulation with toll-like receptor (TLR) ligands via the MAP kinase and NF-κB pathways (Appel et al., 2005). In RAW264.7 cells, monocyte/macrophage-like cell lineage stimulated with LPS, a similar outcome to that of Jurkat cells was observed when treated with cyPGs (Straus et al., 2000). A different result was observed in HeLa cells, strengthening the fact that cyPGs’ effect is cell type specific. Instead of inhibiting IKK complex activity, cyPGs impede the binding of NF-κB to DNA since p50 and p65 have cysteine residues at C62 and C38, respectively. Alkylation of these cysteines via the Michael reaction results in the inhibition of the binding of NF-κB to DNA (Straus et al., 2000).
In human astrocytes treated with 15d-PGJ2, NF-κB was inhibited from binding to the COX-2 promoter on DNA (Janabi, 2002). In glial cells, 15d-PGJ2 induces the transcription of suppressor of cytokine signaling 1 and 3 (SOCS1 and SOCS3) can inhibit JAK, eventually inhibiting the transcription of inflammatory genes (Park E. J. et al., 2003; Park S. H. et al., 2003). 15d-PGJ2 inhibited the JAK/STAT1 mediated interferon regulatory factor-1 (IRF-1) expression, thereby decreasing the IFN-γ-induced costimulatory molecule B7-H1 expression needed by tumors to evade the host immune response (Seo et al., 2014). 15d-PGJ2 inhibits lethal anthrax toxin (LT) activation of the NLRP1 and nigericin-mediated activation of the NLRP3 inflammasome and associated IL-1β release (Maier et al., 2015). 15d-PGJ2 mitigates the macrophage hyperinflammatory response (Monroy et al., 2007).
PGD2 and the J2-series PGJ2 and Δ12-PGJ2 are critical components of the inflammatory response within adipose tissue during obesity thus producing inflammation-related adipokines implicated in insulin sensitivity (Peeraully et al., 2006). 15d-PGJ2 is the most potent inducer of fat cell (adipocyte) differentiation in vitro (Forman et al., 1995; Bell-Parikh et al., 2003). PGD2, PGJ2, and Δ12-PGJ2 treatment strongly down-regulates the production of leptin, a hormone secreted by adipocytes (Peeraully et al., 2006).
Anti-tumorigenic Actions
15d-PGJ2 exerts antitumor activity by regulating the Myc/Mad/max transcription factors to promote cell apoptosis, tubulin binding activity, inhibiting the expression of human telomerase reverse transcriptase (hTERT), enhancing TRAIL-induced apoptosis by downregulating AKT phosphorylation, reactive oxygen species (ROS)-dependent cell death pathway, ROS-dependent AKT activation, inhibition of COX-2, STAT-3, cell cycle (G2/M or G1) blockade, inhibition of vascular endothelial factor (VEGF), growth and expansion of tumor stem cells in gastric cancer (Inoue et al., 2000; Sato et al., 2000; Takashima et al., 2001; Yuan et al., 2005; Chearwae and Bright, 2008; Dionne et al., 2010; Li et al., 2017), oral squamous cell carcinoma (Nikitakis et al., 2002), leukemia (Han et al., 2007), lymphoma (Inoue et al., 2000; Sato et al., 2000; Takashima et al., 2001; Yuan et al., 2005; Chearwae and Bright, 2008; Dionne et al., 2010; Li et al., 2017), oesophageal cancer (Takashima et al., 2001), endometrial cancer (Li and Narahara, 2013), breast cancer (Cocca et al., 2009), osteosarcoma (Yen et al., 2014), and brain tumors (Inoue et al., 2000; Sato et al., 2000; Takashima et al., 2001; Yuan et al., 2005; Chearwae and Bright, 2008; Dionne et al., 2010; Li et al., 2017) (Table 1). Transforming growth factor-β (TGF-β) induces cell growth, cell migration, and epithelial to mesenchymal transition (EMT) and promotes HCC progression (Giannelli et al., 2014). Interestingly, TZDs and 15d-PGJ2 display antitumor effects on HCC (Hsu and Chi, 2014). PPAR-γ activation inhibits TGF-β expression via dephosphorylation of zinc finger transcription factor-9 (Zf9) (Lee et al., 2006). Zf9 is crucial for TGFβ1 gene regulation, and a phosphorylated form of Zf9 transactivates the TGFβ1 promoter (Kim et al., 1998).
TABLE 1.
Biological effects of cyclopentenone prostaglandins.
| Anti-inflammatory | Specific function | Site of action | References |
| 15d-PGJ2 | Inhibition of iNOS promoter containing binding sites for AP-1 and NF-κB | Macrophages | Ricote et al., 1998a,b |
| 15d-PGJ2 | Gelatinase B or MMP-9 | Macrophages | Ricote et al., 1998a,b |
| 15d-PGJ2 and TZDs | MAPK and NF-κB signaling | Dendritic cells (DCs) | Appel et al., 2005 |
| 15d-PGJ2, other cyPGs | Inhibition of NF-κB binding to DNA | RAW264.7 cells, monocyte/macrophage-like cell lineage | Straus et al., 2000 |
| 15d-PGJ2 | Inhibition of NF-κB binding to the COX-2 promoter STAT-1 and c-Jun expression | Human astrocytes, microglia | Janabi, 2002 |
| 15d-PGJ2 | Transcription of SOCS1 and SOCS3 | Brain inflammation | Park E. J. et al., 2003; Park S. H. et al., 2003 |
| 15d-PGJ2 | Inhibition of the JAK/STAT1 mediated IRF-1 expression decreasing cytokine production | B16F10 melanoma cells | Seo et al., 2014 |
| 15d-PGJ2 | Inhibition of caspase-1 activation by NLRP1 and NLRP3 inflammasomes prevents the autoproteolytic activation of caspase-1 and the maturation of IL-1β | NLRP3-dependent peritonitis model | Maier et al., 2015 |
| 15d-PGJ2 | Mitigates the macrophage hyperinflammatory response and inflammatory cytokines | Macrophages | Monroy et al., 2007 |
| PGD2, PGJ2, and Δ12-PGJ2 | Down-regulate the production of leptin | 3T3-L1 adipocytes | Peeraully et al., 2006 |
| 15d-PGJ2 | Inhibition of NF-κB signaling and at PI3K/Akt pathway | Primary astrocytes | Giri et al., 2004 |
| PGA1, PGJ2, PGD and 15d-PGJ2 | Direct inhibition, and modification of the IKKβ subunit, improve the utility of COX2 inhibitors. | Jurkat cells (immortalized line of human T lymphocyte cells) | Rossi et al., 2000 |
| Anti-tumorigenic | |||
| 15d-PGJ2 | Myc/Mad/max transcription factors | Gastric cancer, Oral Squamous cell carcinoma, Leukemia, Lymphoma, Oesophageal cancer, Endometrial cancer, Breast cancer, and Brain tumors | Inoue et al., 2000; Sato et al., 2000; Takashima et al., 2001; Nikitakis et al., 2002; Yuan et al., 2005; Han et al., 2007; Chearwae and Bright, 2008; Cocca et al., 2009; Dionne et al., 2010; Li and Narahara, 2013; Li et al., 2017 |
| 15d-PGJ2 | Enhancing TRAIL-induced apoptosis by downregulating AKT expression and phosphorylation | Leukemia | Han et al., 2007 |
| 15d-PGJ2 | ROS-dependent AKT activation, cell cycle inhibition | Osteosarcoma | Yen et al., 2014 |
| 15d-PGJ2 | A tubulin-binding agent that destabilizes microtubules and induces mitotic arrest | Breast cancer | Cocca et al., 2009 |
| 15d-PGJ2 | Cell cycle blockade | Oesophageal cancer | Takashima et al., 2001 |
| 15d-PGJ2 and TZDs | Tumor cell growth, migration, and invasion | Hepatocellular carcinoma (HCC) | Hsu and Chi, 2014 |
| 15d-PGJ2 and its derivatives (J11-C1) | Expression of genes associated with cell cycle arrest, apoptosis, and autophagy, decreased expression of the anti-apoptotic Bcl-2 | Ovarian cancer SKOV3 cells | Tae et al., 2018 |
| 15d-PGJ2 | Inhibition of STAT-3 | Oral Squamous cell carcinoma | Nikitakis et al., 2002 |
| 15d-PGJ2 | Apoptosis rate, Apoptosis-promoting protein, and reduced apoptosis-inhibiting protein expression | Hepatitis B virus (HBV) × protein (HBx)-positive HL7702-HBx and HL7702 liver cells | Chen et al., 2014 |
| Anti-angiogenic Anti-metastatic | |||
| 15d-PGJ2 Pioglitazone | Inhibiting VEGF | Renal cell carcinoma (RCC) | Yuan et al., 2005 |
| 15d-PGJ2 | Inhibiting angiopoietin-1 (Ang-1) | Gastric cancer | Fu et al., 2006 |
| 15d-PGJ2 | Reduced VEGF receptor 1 (Flt-1) and 2 (Flk/KDR), urokinase plasminogen activator (uPA), and increased plasminogen activator inhibitor-1 (PAI-1) mRNA | Human umbilical vein endothelial cells (HUVEC) | Xin et al., 1999; Funovics et al., 2006 |
| 15d-PGJ2 (PPAR-γ dependent), BRL49653, Ciglitizone | Block angiogenesis | Rat cornea | Xin et al., 1999 |
| 15d-PGJ2 HO-1-dependent mechanism | NF-κB and AP-1 mediated MMP-9 expression and invasion | MCF-7 breast cancer cells | Jang et al., 2020 |
| 15d-PGJ2 | Disassembled focal adhesions, downregulation of FAK signaling | Renal cell carcinoma (RCC) metastasis | Yamamoto et al., 2020 |
| Antioxidant | |||
| 15d-PGJ2 | Nrf2-Keap1 signaling pathway | Atherosclerosis | Itoh et al., 2004; Levonen et al., 2004; Mochizuki et al., 2005 |
| 15d-PGJ2 | HO-1, SOD, catalase, NAD(P)H dehydrogenase quinone 1 (NQO1), c-glutamylcysteine synthetase (GCS), glutathione reductase (GR), glutathione peroxidase 1 (GPx) | Pleurisy, atherosclerosis | Diers et al., 2010; Itoh et al., 2004; Kansanen et al., 2009; Magesh et al., 2012 |
| 15d-PGJ2 | 15-PGDH gene expression, protein level, and its activity, AP-1 and HO-1 | Human colon cancer cell line HCT-116 | Park and Na, 2019a,b; Tauber and Parker, 2019 |
| 15d-PGJ2 | eIF2α phosphorylation, Activation of Integrated stress response (ISR) | Neurodegenerative diseases | Park and Na, 2019a,b; Tauber and Parker, 2019 |
| Resolving inflammation | |||
| 15d-PGJ2 | Cytoprotective, Shifting PG production from PGE2 to PGD2 and 15d-PGJ2 | Dextran sodium sulfate-induced colitis in the rat and TNF-α-induced activation of PG production and PG synthase expression in cultured human peripheral blood monocytes (hPBMC) | Niro et al., 2010 |
| 15d-PGJ2 | DP1 receptor activation checkpoint controller of cytokine/chemokine synthesis as well as leukocyte influx and efflux | Self-resolving peritonitis | Rajakariar et al., 2007 |
| 15d-PGJ2 | PPAR-γ and CD36 expression | Enhance hematoma resolution | Flores et al., 2016 |
| 15d-PGJ2 | Inhibition of pro-inflammatory cytokines, such as IL-5, IL-13, IL-17, TNF-α Inhibition of NF-κB phosphorylation | Peribronchial accumulation of eosinophils and neutrophils, subepithelial fibrosis, and also mucus exacerbation | Coutinho et al., 2017 |
| Prostanylation and protein modification | |||
| PGE1 and PGA1 | Interact with the ligand-binding domain (LBD) of orphan nuclear receptor Nurr1, neuroprotective, enhanced expression of Nurr1 target genes in midbrain dopaminergic (mDA) neurons and improved motor deficits | Mouse models of Parkinson’s disease | Rajan et al., 2020 |
| 15d-PGJ2 and PGA1 | IKKα and β, NF-κB P65 and P50 subunits cysteine modification at various positions | Inhibition of NF-κB pathway | Castrillo et al., 2000; Rossi et al., 2000; Cernuda-Morollon et al., 2001 |
| 15d-PGJ2 and PGA1 | H-Ras modification at various cysteines | Activation of H-Ras | Oliva et al., 2003 |
| 15d-PGJ2 | c-Jun and c-Fos modification at various cysteines | Inhibition | Perez-Sala et al., 2003 |
| PGA1 | Thioredoxin, thioredoxin reductase, and Keap1 | Inhibition | Levonen et al., 2001, 2004; Shibata et al., 2003a; Itoh et al., 2004 |
| 15d-PGJ2 | Proteasome | Inhibition | Shibata et al., 2003b |
15d-PGJ2 and its derivatives exert antitumor activity by selectively modulating the expression of genes associated with cell cycle arrest, apoptosis, and autophagy (Inoue et al., 2000; Sato et al., 2000; Takashima et al., 2001; Yuan et al., 2005; Chearwae and Bright, 2008; Dionne et al., 2010; Li et al., 2017). Notably, J11-C1 is a novel candidate of class III histone deacetylases (HDACs) called Sirtuin SIRT1 inhibitor with anticancer activity. SIRTs are involved in biological functions, including aging, energy mobilization, and stress responses. SIRTs regulate cancer cell apoptosis and are potential targets for novel anticancer drugs that regulate the levels of deacetylated histone proteins, p53, and several transcriptional factors (Table 1) (Tae et al., 2018). 15d-PGJ2 treatment significantly induced apoptosis rate, apoptosis-promoting protein expression, and reduced apoptosis-inhibiting protein expression in the hepatitis B virus (HBV) × protein (HBx)-positive HL7702-HBx and HL7702 liver cells (Chen et al., 2014).
Anti-angiogenic/Anti-metastatic Actions
15d-PGJ2 exerts anti-angiogenic activity by inhibiting VEGF and angiopoietin-1 (Ang-1) in renal cancer (Yuan et al., 2005) and gastric cancer (Fu et al., 2006), respectively. Treatment of human umbilical vein endothelial cells (HUVEC) with 15d-PGJ2 reduced mRNA levels of VEGF receptors 1 (Flt-1) and 2 (Flk/KDR) and urokinase plasminogen activator (uPA) and increased plasminogen activator inhibitor-1 (PAI-1) mRNA (Funovics et al., 2006). Administration of 15d-PGJ2 could inhibit VEGF-induced angiogenesis in the rat cornea in a PPAR-γ dependent manner (Xin et al., 1999) (Table 1). Rosiglitazone (Avandia) and troglitazone (TGZ) inhibit cell migration via the upregulation of E-cadherin expression in HepG2 cells (Lee et al., 2009). 15d-PGJ2 inhibits NF-κB and AP-1-mediated MMP-9 expression and invasion of MCF-7 breast cancer cells employing a heme oxygenase-1 (HO-1)-dependent mechanism (Jang et al., 2020). Treatment with a low concentration of 15d-PGJ2 disassembled focal adhesions, reduced focal adhesion kinase (FAK) phosphorylation, and caused extensive filamentous actin reorganization (Yamamoto et al., 2020). PPAR-γ did not mediate the inhibitory effect of 15d-PGJ2 on the migration of Caki-2 cells and did not affect RCC metastasis (Yamamoto et al., 2020).
Antioxidant and Resolving Actions
Inflammation is accompanied by the production of ROS, and 15d-PGJ2 has antioxidant properties (Itoh et al., 2004; Levonen et al., 2004; Mochizuki et al., 2005) (Table 1). 15d-PGJ2 and structurally related isoprostanoids alkylate Kelch-like ECH-associated protein 1 (Keap1) to induce the NF-E2-related nuclear factor erythroid-2 (Nrf2-) dependent antioxidant bioactivity (Levonen et al., 2004; Kansanen et al., 2009; Diers et al., 2010; Mills et al., 2018). 15d-PGJ2 activates Nrf2-Keap1 signaling and induces gene transcription of antioxidant enzymes including HO-1, superoxide dismutase (SOD), catalase, NAD(P)H dehydrogenase quinone 1 (NQO1), c-glutamylcysteine synthetase (GCS), glutathione reductase (GR), and glutathione peroxidase 1 (GPx) (Itoh et al., 2004; Kansanen et al., 2009; Diers et al., 2010; Magesh et al., 2012). 15d-PGJ2 upregulates 15−hydroxyprostaglandin dehydrogenase (15-PGDH) gene expression, protein level, and its activity in human colon cancer cell line HCT-116 through AP-1 activation (Park and Na, 2019a,b). 15d-PGJ2 treatment-induces eIF2α phosphorylation and activation of the integrated stress response (ISR), also leading to bulk translation repression and preferential translation of stress response mRNAs (Tauber and Parker, 2019). 15d-PGJ2 is pro-resolving signaling and a neuroprotective (Rajan et al., 2020) molecule (Table 1) (Rajakariar et al., 2007; Niro et al., 2010; Flores et al., 2016; Coutinho et al., 2017).
Pro-metastatic Properties of the Cyclopenenone Prostaglandins
cyPGs also exhibit pro-metastatic properties such as 15d-PGJ2 significantly enhanced the rate of formation, the size, and the vascularization of papillomas in a murine carcinogenesis model (Millan et al., 2006). 15d-PGJ2 and PGJ2 induced the proliferation of COX-2 depleted colorectal cancer (HCA-7) cells at a nanomolar concentration (Chinery et al., 1999). However, the precise mechanisms responsible for tumor proliferative effects of 15d-PGJ2 remain incompletely clarified. VEGF is well known as a master regulator of angiogenic switch (Bussolati and Mason, 2006). Interestingly, VEGF upregulates HO-1 in vascular endothelial cells, while HO-1 may also regulate the synthesis and activity of VEGF, thus constituting a positive feedback loop (Bussolati and Mason, 2006). 15d-PGJ2 could stimulate VEGF expression in endothelial cells, human androgen-independent PC3 prostate cancer cells, and the 5,637 urinary bladder carcinoma cell line (Yamakawa et al., 2000; Haslmayer et al., 2002). The upregulation of VEGF by 15d-PGJ2 was accompanied by activation of PPAR-γ (Jozkowicz et al., 2002). However, the VEGF promoter does not harbor PPRE (Inoue et al., 2001; Jozkowicz et al., 2004). Interestingly, VEGF upregulation by 15d-PGJ2 could be mimicked by the induction of HO-1 expression (Jozkowicz et al., 2004). 15d-PGJ2 induced HO-1 expression in MCF-7 human breast cancer cells (Kim et al., 2004).
Nrf2, a transcription factor is responsible for maintenance of cellular redox balance (Loboda et al., 2016). HO-1 is a prototypic Nrf2 target gene, and the aberrant hyperactivation of Nrf2/HO-1 axis contributes to tumor progression, aggressiveness, chemoresistance, and poor prognosis (Zimta et al., 2019). 15d-PGJ2 induces VEGF expression and angiogenesis in human breast cancer cells through upregulation of HO-1 (Kim et al., 2006; Kweider et al., 2011).
Role of Cyclopentenone Prostaglandins During Viral Infections
Cyclopentenone Prostaglandins as Inhibitor of Viral Replication
cyPGs are potent inhibitors of viral replication (Table 2) and are effective against a wide range of viruses. These include negative-strand RNA viruses such as influenza A (Pica et al., 1993, 2000; Conti et al., 2001), Sendai virus (Amici and Santoro, 1991; Amici et al., 2001), and vesicular stomatitis virus (VSV) (Santoro et al., 1987; Pica et al., 1993); positive-strand RNA viruses such as Sindbis virus (Mastromarino et al., 1993), Poliovirus (Conti et al., 1996), and Human immunodeficiency virus-1 (Rozera et al., 1996) and DNA viruses such as herpes simplex virus (HSV) type 1 and 2 (Yamamoto et al., 1987; Amici et al., 2001). The ability of cyPGs to suppress virus production is very dramatic. In the African green monkey kidney (AGMK) cell line, replication of the Sendai virus is almost completely inhibited by 4 mg/ml of PGA1 (Santoro et al., 1987) and by 4 mg/ml of PGJ2 (Santoro et al., 1987) without being toxic to uninfected AGMK cells. Treatment of 6 mg/ml of Δ12-PGJ2 in Madin–Darby canine kidney cells (MDCK) infected with influenza A H1N1 (PR8) virus drastically suppressed the viral production by 95%. Simultaneously, a higher dose of Δ12-PGJ2 produced an undetectable virus yield (Pica et al., 1993). PGA1 treatment also strongly inhibits the viral production of Ulster 73 (H7N1 influenza A) in LLC-monkey kidney epithelial cells (LLC-MK2), African green monkey kidney-37RC cells (AGMK-37RC), and MDCK cells (Conti et al., 2001), suggesting that cyPGs are effective against various subtypes of influenza A virus in multiple host cells. Similarly, in vivo studies have shown that PGA1 and 16, 16-dimethyl-PGA2 (dmPGA2), a long-acting synthetic analog of PGA, in mice infected with a lethal dose of PR8 virus significantly decreases the virus titers in the lung and increases the survival rate (Santoro et al., 1987; Pica et al., 1993). In another study, the antiviral activity of the synthetic dmPGA1 in HSV-1 and human immunodeficiency virus (HIV)- infected cells was investigated (Hughes-Fulford et al., 1992). dmPGA1 affected HIV-1 replication in acutely infected T cells and chronically infected macrophages as assessed by a quantitative decrease in HIV-1 antigen p24 concentration (Hughes-Fulford et al., 1992). This study highlighted the unusual broad-spectrum antiviral activity of dmPGA1 against HSV and HIV-1 and its therapeutic potential for in vivo use (Hughes-Fulford et al., 1992).
TABLE 2.
Effects of cyclopentenone prostaglandins in viral infections.
| Anti-viral Activity | Virus | CyPGs | Mechanism | References |
| Inhibition of virus replication by altering viral gene/protein expression (transcription/translation level alteration) | Influenza A | Δ12-PGJ2 | Decrease synthesis of hemagglutinin (HA), nucleoprotein (NP), and membrane protein M1; induction of 70 kDa host HSP70 | Pica et al., 1993 |
| PGA1 | Delayed synthesis of HA, membrane protein M1, structural protein M2, and non-structural protein NS2; induction of 70 kDa host HSP70 | Conti et al., 2001 | ||
| Vesicular Stomatitis Virus (VSV) | Δ12-PGJ2, PGA1 | Inhibit VSV RNA polymerase | Bader and Ankel, 1990; Pica et al., 1993; Parker, 1995 | |
| Herpes Simplex Virus Type 1 (HSV-1) | PGA1 | Suppress NF-κB activation by inhibiting IKK complex (independent of the PPAR-γ pathway) | Amici et al., 2001 | |
| Herpes Simplex Virus Type 2 (HSV-2) | Δ7-PGA1, Δ12-PGJ2 | Inhibited the primary transcription of HSV-2 | Yamamoto et al., 1987 | |
| Human Immunodeficiency Virus-1 (HIV-1) | PGJ2 | Suppress NF-κB activation by inhibiting IKK complex (independent of the PPAR-γ pathway) | Rozera et al., 1996; Boisvert et al., 2008 | |
| 15d-PGJ2 | Covalently modify HIV-1 transactivating protein, Tat to inhibit virus transcriptional elongation | Kalantari et al., 2009 | ||
| Inhibition of virus replication by altering viral glycoprotein glycosylation (post-translational level alteration) | Vesicular Stomatitis Virus (VSV) | Δ12-PGJ2 | Inhibit glycosylation of virus glycoprotein G | Pica et al., 1993 |
| Sendai Virus | PGA1, Δ12-PGJ2 | Inhibit glycosylation of virus glycoproteins hemagglutinin-neuraminidase (HN) and fusion protein (F) | Santoro et al., 1987; Amici et al., 2001 | |
| Inhibition of virus cell-to-cell transmission | Human T-cell Leukemia Virus Type I (HTLV-1) | PGA1, PGJ2 | Inhibit host cell proliferation by inducing cell arrest at the G1/S interface | D’Onofrio et al., 1992; Lacal et al., 1994a,b |
| Inhibition of virus-induced inflammation | Influenza | 15d-PGJ2 | Decrease virus-induced release of proinflammatory cytokines (IL-6, TNF-α) and chemokines (CCL2, CCL3, CCL4, and CXCL10) via PPAR-γ pathway | Cloutier et al., 2012 |
| Respiratory Syncytial Virus (RSV) | 15d-PGJ2 | Decrease virus-induced release of cytokines (TNF-α, GMCSF, IL-1α, IL-6), and the chemokines (CXCL8 (IL-8) and CCL5) via PPAR-γ pathway. Reduce immune cells adhesion by inhibiting virus-induced up-regulation of intercellular adhesion molecule-1 (ICAM1). Reduce activity of inflammatory pathway, NF-κB. | Arnold et al., 2007 | |
| Human Immunodeficiency Virus-1 (HIV-1) | 15d-PGJ2 | Suppress NF-κB activation by inhibiting IKK complex | Boisvert et al., 2008 | |
| Zika virus (ZIKV) | 15d-PGJ2 | Control brain inflammation by downregulating microglial activation and by inducing apoptosis of activated microglia | Bernardo and Minghetti, 2006 |
Depending on the virus, cyPGs utilize various mechanisms and act on different viral cycle events to interfere with virus production. In HIV-1 infection and avian influenza, A virus infection, cyPGs prevent very early virus infection phases such as viral adsorption and penetration into target cells (Rozera et al., 1996; Carta et al., 2014). Even though antiviral action mechanisms differ between various viruses and host cell systems, the inhibition of virus replication by cyPGs is often associated with (1) alteration in viral protein synthesis and (2) alteration in viral glycoprotein glycosylation (Table 2). PGA1 treatment inhibited replication of Mayaro virus (MAYV) (an arbovirus endemic to certain humid forests of tropical South America) by 95% at 24 h post-infection in human epithelial type 2 (Hep-2) cells (Caldas et al., 2018). PGA1 treatment inhibited viral structural protein synthesis by 15%, possibly via heat shock protein70 (HSP70) induction (Caldas et al., 2018).
Cyclopentenone Prostaglandins Alter Viral Protein Synthesis
Inhibition of individual virus replication by cyPGs is marked by dysregulation of viral protein synthesis (Table 2). In influenza, A PR8 virus (a mouse-adapted H1N1 influenza virus causing severe infection in mice)-infected cells, treatment of Δ12-PGJ2 substantially decreased the synthesis of PR8 proteins such as hemagglutinin (HA), nucleoprotein (NP), and membrane protein M1 (Pica et al., 1993). PGA1 could cause a significant delay in the synthesis of late viral polypeptides: HA, membrane protein M1, structural protein M2, and non-structural protein NS2 (Conti et al., 2001). Furthermore, both studies showed that inhibition or delay of viral protein synthesis is accompanied by induction of a 70 kDa host polypeptide identified as HSP70 by immunoblot analysis (Pica et al., 1993; Conti et al., 2001). Because viral protein synthesis is repressed as long as HSP70 is present in the host cell, HSP70 seems to play an essential role in cyPGs antiviral activity.
In VSV infection, Δ12-PGJ2 can affect two distinct stages (an early stage and a late-stage) of the virus replication cycle in epithelial monkey cell lines (Pica et al., 1993). The inhibition of the virus at the initial stage is associated with altered viral protein synthesis. When the cells are treated with 8 mg/ml of Δ12-PGJ2 soon after virus infection, there is a dramatic decrease in VSV protein synthesis. Similar to the effect on influenza A virus replication, inhibition of VSV protein synthesis by Δ12-PGJ2 is also associated with the induction of a 74 kDa polypeptide belonging to the group of heat shock protein HSP70 (Pica et al., 1993). In another study, PGA1 treatment decreased VSV proteins’ production and the amount of respective viral mRNA (Bader and Ankel, 1990). This study found that PGA1 exerts its antiviral activity at the VSV genes’ primary transcription level, which leads to a reduction in viral mRNA synthesis, viral protein synthesis, and, ultimately, viral replication. To further investigate the antiviral activity of cyPGs, another study performed an RNA polymerase assay and reported that cyPGs potently inhibit VSV RNA polymerase (Parker, 1995). This inhibition correlates with the decrease in VSV replication in infected cells, indicating that cyPGs antiviral activity is due to VSV RNA polymerase inhibition.
In addition to VSV, cyPGs also exert a transcriptional block in the replication of herpes simplex virus type 1 (HSV-1) (Amici et al., 2001), HSV-2 (Yamamoto et al., 1987), and HIV-1 (Rozera et al., 1996). In HSV-1 infected human laryngeal carcinoma cells and neuroblastoma cells and HIV-1 infected colonic epithelial cells (caco-2 cells), cyPGs inhibit viral gene expression by suppressing NF-κB activation, independent of the PPAR-γ pathway (Amici et al., 2001; Boisvert et al., 2008). NF-κB is essential for many processes, including viral gene expression and, consequently, replication of viruses that contain NF-κB binding sites in their genomes. In its inactivated cytosolic form, NF-κB is bound to inhibitory IκB proteins such as IκBα. Stimuli like bacterial and viral infections increase the activity of the IKK complex, which phosphorylates IκBα, leading to ubiquitination and degradation of IκBα by proteasomes. Once NF-κB is free from IκBα, it translocates into the cell nucleus, activating the transcription of many genes, including the viral genes of HSV-1 and HIV-1 (Amici et al., 2001; Boisvert et al., 2008). Amici et al. (2001) showed that PGA1 significantly decreases the NF-κB induction in HSV-1 infected cells by inhibiting the IKK complex.
Similarly, another study reported that the administration of PGJ2 reduces IKK activity in HIV-1 infected cells (Boisvert et al., 2008). In both cases, suppression of IKK activity by cyPGs prevents IκBα degradation and NF-κB translocation to the nucleus. As a result, viral gene transcription and protein synthesis were repressed, leading to a significant reduction in virus production. In addition to interfering with NF-κB induction, cyPGs also target another pathway independent of NF-κB to inhibit HIV-1 replication. Kalantari et al. (2009) reported that 15d-PGJ2 represses HIV-1 transcription by inhibiting HIV-1 transactivating protein, Tat. While the host transcriptional factor NF-κB binds to the 5′ long terminal repeat (LTR) of HIV-1 to initiate transcription, viral Tat protein is recruited to an RNA stem-loop structure called transactivation response element (TAR) and is necessary for transcriptional elongation. Tat then recruits transcription elongation factor p-TEFb, which transactivates HIV LTR and allows the RNA polymerase II to continue the transcription with high processivity. 15d-PGJ2 interferes with Tat-dependent transcriptional elongation by covalently modifying the thiol groups of Tat’s cysteine residues (Kalantari et al., 2009). The resulting altered Tat protein is unable to transactivate HIV LTR in U937 human macrophages, inhibiting the transcription and replication of the virus.
Cyclopentenone Prostaglandins Alter Viral Glycoprotein Glycosylation
cyPGs can also inhibit viral replication at the post-translational level by altering the glycosylation of viral glycoproteins. This is seen in the VSV and Sendai virus (Table 2). As mentioned earlier, Δ12-PGJ2 inhibits the VSV replication in the epithelial monkey cell line at two stages of the virus replication cycle. The inhibition at the early stage is due to a block in viral protein synthesis. Administration of Δ12-PGJ2 at a later stage (6–8 h post-infection) also leads to a decrease in virus production even though viral protein synthesis should have been completed by that time (Pica et al., 1993). Δ12-PGJ2 treatment started at a later stage does not affect viral protein synthesis, but it drastically decreases the glucosamine incorporation into the virus glycoprotein G without altering most cellular proteins.
Similarly, PGA1 treatment in AGMK cells infected with the Sendai virus results in inhibition of glycosylation of viral glycoproteins hemagglutinin-neuraminidase (HN) and fusion protein (F), as indicated by the decrease in glucosamine incorporation (Santoro et al., 1987). The synthesis of non-glycosylated viral polypeptides of RNA transcriptase complex, including proteins P, NP, and matrix protein (M), are not affected by PGA1 treatment. Likewise, Δ12-PGJ2 also markedly reduces the incorporation of glucosamine into HN and F viral glycoproteins without inhibiting the synthesis of cellular or viral proteins (Amici et al., 2001). The altered HN glycoprotein cannot insert into the cell membrane, which leads to an inhibition of virus maturation and production.
The Effect of Cyclopentenone Prostaglandins on Viral Transmission
cyPGs can interfere with virus transmission via their antiproliferative activity. When PGA1 and PGJ2 are given to human T-cell leukemia virus type-I (HTLV-1) producing MT-2 cell line, they inhibit the growth of the cells in a dose-dependent manner (D’Onofrio et al., 1992). These cyPGs cause the cells to be arrested at the G1/S interface without detectable cellular toxicity. Another study showed that PGA1 and PGJ2 inhibit the proliferation of myeloid cells (K562 pluripotent stem cells, HL60 promyelocytic cells, and U937 monoblastoid cells) during early infection of HTLV-1, also in a dose-dependent manner (Lacal et al., 1994a,b). Furthermore, out of the three myeloid cell lines used in the study, the effect of growth inhibition is highest in U937 monoblastoid cells, followed by HL60 promyelocytic cells, and then K562 pluripotent stem cells. This suggests that cyPGs have a more significant antiproliferative effect on differentiated cells.
The primary mode of infection of HTLV-1 is cell-to-cell transmission (Yoshida and Seiki, 1987). Furthermore, for retrovirus-like HTLV-1, integration of proviral DNA occurs after the initiation of cellular DNA synthesis in dividing cells (Varmus et al., 1979). Thus, alterations in cell proliferation and cell cycle can affect the permissiveness of recipient cells to HTLV-1. Indeed, in U937 monoblastoid cells co-cultured with virus-donor cells, PGA1 and PGJ2 treatments reduce the transmission of HTLV-1 (Lacal et al., 1994a,b). However, in less differentiated K562 pluripotent stem cells and HL60 promyelocytic cells, infection of recipient cells increased after cyPGs treatment antiproliferative activity is observed in these cells. This suggests that the effect of cyPGs on virus transmission is affected by cell differentiation.
The Effect of Cyclopentenone Prostaglandins on Viral Infection Induced Inflammation
Viral infections such as influenza virus, HIV-1, and respiratory syncytial virus (RSV) are characterized by excessive inflammation with the upregulation of proinflammatory cytokines and chemokines. The amount of these proinflammatory molecules correlates with the severity of illness (Griffin et al., 1994; Wesselingh et al., 1994; Hornsleth et al., 2001; Welliver et al., 2002). Given the anti-inflammatory effects of cyPGs, studies have been done to explore the possibility of utilizing cyPGs as a therapeutic agent for viral infections. In mice infected with lethal influenza infection, administration of 15d-PGJ2 1 day after infection resulted in reduced influenza morbidity and mortality, accompanied by substantially decreased gene expression of proinflammatory cytokines (IL-6 and TNF-α) and chemokines (CCL2, CCL3, CCL4, and CXCL10) via activation of PPAR-γ pathway (Cloutier et al., 2012). Similarly, 15d-PGJ2 and other PPAR-γ agonists (ciglitazone and TGZ) can inhibit the RSV-induced release of cytokines TNF-α, GMCSF, IL-1α, IL-6, and the chemokines CXCL8 (IL-8) and CCL5 (Arnold et al., 2007). Moreover, RSV infection of the human airway epithelial cells causes an increase in expression of intercellular adhesion molecule-1 (ICAM1) on the cell surface, which enhances the adhesion of recruited immune effector cells, contributing to an intense inflammatory response and increased cytotoxicity (Wang et al., 2000; Arnold et al., 2007). Treatment of 15d-PGJ2 and other PPAR-γ agonists results in inhibition of the up-regulation of ICAM1, with the reduced cellular amount of ICAM1 mRNA (Arnold et al., 2007). This leads to a significant reduction in the adhesion of immune cells to RSV-infected cells. Also, the 15d-PGJ2 treatment in RSV-infected cells is associated with reduced activity of NF-κB, a transcription factor essential for inflammatory responses. In HIV-infected intestinal epithelial cells, 15d-PGJ2 also reduces the nuclear translocation of NF-κB and represses HIV-1 transcription by decreasing the activity of IKK (Boisvert et al., 2008). Overall, cyPGs can reduce the exaggerated inflammatory response associated with viral infections and great therapeutic value. PGD2/DP1 axis and 15d-PGJ2 signaling contributes to the regulation of the CNS-specific response to pathogens such as neurotropic coronavirus (CoV) (Vijay et al., 2017) and acute encephalitis (Rosenberger et al., 2004), chronic demyelinating encephalomyelitis causing neurotropic virus called “MHV” (mouse hepatitis virus strain JHM) (Zheng et al., 2020).
Zika virus (ZIKV), one of the most medically relevant viral infections, affects the developing brain during pregnancy, and its connection with congenital malformations/microcephaly is well documented (de Oliveira et al., 2019). Neuroinflammation is one of the critical factors contributing to ZIKV-related microcephaly, inflammatory processes mediated by glial cells (Wen et al., 2017; Huan et al., 2018). PGD2, PGE1, PGE2, and PGI2 have been correlated with neuroinflammation, protecting the CNS, and physiological responses to minimize further damage to neural tissue. Their anti-inflammatory reaction has been demonstrated in neuronal injuries (Shi et al., 2010) and neuroprotection during acute brain injury (Liang et al., 2005; An et al., 2014) 15d-PGJ2 activates PPAR-γ by downregulating microglial activation despite the proinflammatory environment because of the neural damage (Bernardo and Minghetti, 2006).
15d-PGJ2 has demonstrated beneficial effects in the severe diseases arising from bacterial infections of Staphylococcus aureus (Phulwani et al., 2006), Salmonella enterica Typhimurium (Buckner et al., 2013), leading to brain abscess, typhoid fever, gastroenteritis, and protozoan hemoflagellate Trypanosoma brucei infection-causing sleeping sickness in humans (Figarella et al., 2006).
Other Alpha, Beta-Unsaturated Carbonyl Lipids and Cyclopentenone Isoprostanes
There is another category of highly reactive electrophilic molecules, which react and modify both proteins and DNA resulting in toxicity, protein dysfunction (Sayre et al., 2006) or tissue damage and disease progression (Lee and Park, 2013). These are α, β-unsaturated aldehydes such as acrolein (ACR), 4-hydroxy-2-non-enal (4-HNE), and crotonaldehyde (CRA) are the most reactive and toxic α, β-unsaturated aldehydes (Lee and Park, 2013). These induce toxicity because of depletion of cellular GSH and inactivation of antioxidant enzymes (GPx and thioredoxin; TRx) subsequently leading to ROS production, reactive nitrogen species (RNS), and free radicals (Stocker and Keaney, 2004; Lee and Park, 2013). Lipid peroxidation (LPO)-derived α, β-unsaturated aldehydes play an important pathophysiological role in vascular diseases by inducing the production of various atherogenic factors, inflammatory mediators, activation of NF-κB signaling pathway, redox signaling mediators leading to cellular and tissue injury (Lee and Park, 2013).
Isoprostanes (IsoPs) are PG-like compounds that are produced in vivo independently of COX enzymes, primarily by ROS-mediated or free radical-induced peroxidation of arachidonic acid (Stamatakis and Perez-Sala, 2006). IsoPs along with cyPGs are reactive electrophilic eicosanoids that can form covalent adducts with thiol-containing molecules, cysteine residues in proteins through Michael addition (Stamatakis and Perez-Sala, 2006). Oxidation of DHA in the central nervous system, results in the formation of IsoP-like compounds, termed neuroprostanes and are uniquely valuable to understanding the clinical pharmacology of antioxidants (Montuschi et al., 2007). Cyclopentenone IsoPs are formed abundantly in brain tissue under conditions of oxidative stress (glutathione depletion, ROS generation, activation of redox-sensitive signaling pathways) and may contribute to neuronal death causing neurodegeneration and should be addressed when designing neuroprotective therapies (Musiek et al., 2006, 2007; Porta et al., 2013). IsoPs are measured in the plasma, urine, or cerebral spinal fluid (CSF) and their increase has been observed in obese adults (Morrow, 2005; Basu, 2008), ischemia-reperfusion (Sakamoto et al., 2002; Rossi et al., 2004), Alzheimer’s disease (AD) (Montine et al., 1998, 1999a; Pratico et al., 1998, 2000), Huntington’s disease (Montine et al., 1999b), Parkinson’s disease (Fessel et al., 2003; Seet et al., 2010), and amyotrophic lateral sclerosis (ALS) (D’Amico et al., 2013). Few studies have investigated the associations between levels of F2-IsoPs and risk of breast cancer (Rossner et al., 2006), hepatocellular carcinoma (Wu et al., 2008), prostate cancer (Barocas et al., 2011; Brys et al., 2013) gastric cancer (Asombang et al., 2013). IsoPs are increased in patients with genetic disorders such as autism-spectrum disorders (Ming et al., 2005; Gorrindo et al., 2013), Smith–Lemli–Opitz Syndrome (SLOS) (Korade et al., 2013), sickle cell anemia (Akohoue et al., 2007), cystic fibrosis (Collins et al., 1999; Ciabattoni et al., 2000; Montuschi et al., 2000), Rett syndrome (RTT) (De Felice et al., 2009, 2011; Signorini et al., 2011; Durand et al., 2013), and in various inborn errors of metabolism (Mc Guire et al., 2009).
Summary and Future Directions
There is significant evidence that cyPGs (PGA1, PGA2, and PGJ2), and metabolites of PGJ2 (15d-PGJ2 and Δ12- PGJ2) can induce anti-inflammatory and antiviral effects through covalent modification reactions with their α, β-unsaturated carbonyl group. cyPGs can exert anti-inflammatory and antiviral effects in various ways depending on the host cell and pathogen type. Cell type is not the only influencer on the anti-inflammatory effects of cyPGs. The concentration of cyPGs and the length/time of exposure to cyPGs have varying anti-inflammatory and antiviral effects. Based on these factors, cyPGs can show biphasic targeting of inflammation (Garzon et al., 2011). At high doses, 15d-PGJ2 has a dual action of stimulating anti-inflammation and anti-proliferation. Still, it can be toxic and induce both inflammation and cell proliferation at lower doses, and the biphasic pharmacodynamics has to be controlled carefully (Abbasi et al., 2016). Dose-related efficacy and safety of oral DP2 receptor antagonists fevipiprant (QAW039), timapriprant (OC000459), and BI 671800 have been tested in patients with allergic asthma and COPD, and PGD2 has shown anticancer effects in NSCLC (non-small cell lung carcinoma), kidney and lung fibrosis, and gastric cancer (Bateman Guerreros et al., 2017; Jandl and Heinemann, 2017; Pearson et al., 2017; Sandham et al., 2017a,b; Murillo et al., 2018; Brightling et al., 2020). Further research on outcomes based on specific concentrations is warranted. PPAR-γ antagonist (GW9662) and PPAR-γ ligands are new therapeutic targets in sepsis, hemorrhagic shock, and inflammation (Kaplan et al., 2005, 2010; Zingarelli and Cook, 2005; Chima et al., 2011). Synthetic PPAR-γ ligands rosiglitazone (Avandia) and pioglitazone have exhibited anti-inflammatory and antiviral effects in an EcoHIV mouse model that could decrease neurodegeneration. These drugs prove promising in treating HIV-1 associated neurocognitive disorders (Omeragic et al., 2020). This knowledge could significantly impact how viruses and inflammation can be treated.
The outcome of the 15d-PGJ2 treatment depends upon its exogenously administered dose as it stimulates anti-inflammation and anti-proliferation at high doses while can have toxic effects at a lower dose (Abbasi et al., 2016). Many strategies have been developed to deal with the biphasic pharmacodynamics of 15d-PGJ2 and one of them is using a nanoemulsion (NE) composed of triolein/distearoyl phosphatidylcholine/Tween 80 at a high encapsulation ratio (>83%) allowing slow-release kinetics (Abbasi et al., 2016). NE retained a high proportion of 15d-PGJ2 and directly delivered it to the cytosol, where proapoptotic targets are located, and could bypass cell membrane-associated targets involved in cell proliferation (Abbasi et al., 2016). NE could deliver 15d-PGJ2 to its desired site of action, excluding undesired sites, on a subcellular level (Abbasi et al., 2016) and could be used as one of the strategies for treatment. Since the use of solid lipid nanoparticles (SLN) can improve therapeutic properties by increasing drug efficiency and availability, 15d-PGJ2-SLN was developed and tested for its immunomodulatory potential. The 15d-PGJ2-SLN formulation showed good colloidal parameters, encapsulation efficiency (96%), and stability (up to 120 days) with low hemolytic effects as compared to unloaded SLN in in vivo experiments. The 15d-PGJ2-SLN formulation using low concentrations reduced neutrophil migration in three inflammation models tested. 15d-PGJ2-SLN increased IL-10 levels and reduced IL-1β as well as IL-17 in peritoneal fluid thus highlighting the perspectives of a potent anti-inflammatory system (de Melo et al., 2016). cyPGs have a wide spectrum of intracellular targets ranging from nuclear factors to mitochondria. Introduction of cyclopentenone moiety into molecules (jasmonates and chalcones) boosts their anticancer potential (Conti, 2006). Despite advancements made in the pharmacodynamics of cyPGs, a significant effort is needed to explore their unique therapeutic properties and tailor them to be used as leading anti-inflammatory, anticancer, and antiviral drugs.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All authors contributed equally to the article and approved the submitted version. NS-W apologizes to all the colleagues whose work could not be cited in this manuscript.
Abbreviations
- AD
Alzheimer’s disease
- AP-1
activating protein-1
- ALS
amyotrophic lateral sclerosis
- AGMK
African green monkey kidney
- COX
cyclooxygenase
- CCR
chemokine receptors
- CTL
cytotoxic T lymphocytes
- CREB
cyclic AMP-responsive element-binding
- cyPGs
cyclopentenone PGs
- COPD
chronic obstructive pulmonary disease
- DCs
dendritic cells
- DGLA
dihomo g-linolenic acid
- EAE
experimental allergic encephalomyelitis
- EBV
Epstein–Barr virus
- ERK
extracellular signal-regulated kinases
- EMT
epithelial to mesenchymal transition
- FRK
c-Fos -regulating kinases
- GMCSF
granulocyte-macrophage colony-stimulating factor
- GR
glutathione reductase
- GPx
glutathione peroxidase 1
- GCS
c-glutamylcysteine synthetase
- HCMV
human cytomegalovirus
- HDACs
histone deacetylases
- HO-1
heme oxygenase-1
- HSV
herpes simplex virus
- Hep-2
human epithelial type 2
- HSP70
heat shock protein70
- HTLV-1
human T-cell leukemia virus type-I
- hTERT
human telomerase reverse transcriptase
- ICAM-1
intercellular adhesion molecule 1
- IBD
inflammatory bowel disease
- IsoPs
isoprostanes
- IKK
I κ B kinase
- JAK
Janus kinase
- Keap1
Kelch-like ECH-associated protein 1
- KSHV
Kaposi’s sarcoma herpesvirus
- LBD
ligand-binding domain
- mTOR
mammalian target of rapamycin
- MMP-9
matrix metalloproteinase
- Nrf2
NF-E2-related nuclear factor erythroid-2
- NSCLC
non-small cell lung carcinoma
- NE
nanoemulsion
- NGS
next-generation sequencing
- NLS
nuclear localization sequence
- NSAIDs
non-steroidal anti-inflammatory drugs
- NQO1
NAD(P)H dehydrogenase quinone 1
- PAI-1
plasminogen activator inhibitor-1
- PD-1
programmed cell death protein-1
- PDL-1
programmed cell death ligand-1
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- 15-PGDH
15 − hydroxyprostaglandin dehydrogenase
- PLA2
phospholipase A2
- PPAR- γ
peroxisome proliferator-activated receptor-gamma
- ROS
reactive oxygen species
- RTT
Rett syndrome
- SOCS
suppressor of cytokine signaling
- SLN
solid lipid nanoparticles
- SOD
superoxide dismutase
- SLOS
Smith–Lemli–Opitz syndrome
- TAR
transactivation response element
- TGF- β
transforming growth factor- β
- TGZ
troglitazone
- TXA2
thromboxane A2
- uPA
urokinase plasminogen activator
- VEGF
vascular endothelial growth factor
- VSV
vesicular stomatitis virus
- VZV
varicella zoster virus.
Footnotes
Funding. We are grateful for funding support from the Center for Cancer Cell Biology, Immunology and Infection and NIH-funded grant R01CA 192970 to NS-W. The funders had no role in the design, decision to publish, or preparation of the manuscript.
References
- Abbasi S., Kajimoto K., Harashima H. (2016). Elimination of the biphasic pharmacodynamics of 15d-PGJ2 by controlling its release from a nanoemulsion. Int. J. Nanomed. 11 2685–2694. 10.2147/ijn.s106297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akohoue S. A., Shankar S., Milne G. L., Morrow J., Chen K. Y., Ajayi W. U., et al. (2007). Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr. Res. 61 233–238. 10.1203/pdr.0b013e31802d7754 [DOI] [PubMed] [Google Scholar]
- Amici C., Santoro M. G. (1991). Suppression of virus replication by prostaglandin A is associated with heat shock protein synthesis. J. Gen. Virol. 72(Pt 8), 1877–1885. 10.1099/0022-1317-72-8-1877 [DOI] [PubMed] [Google Scholar]
- Amici C., Belardo G., Rossi A., Santoro M. G. (2001). Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276 28759–28766. [DOI] [PubMed] [Google Scholar]
- An Y., Belevych N., Wang Y., Zhang H., Nasse J. S., Herschman H., et al. (2014). Prostacyclin mediates endothelial COX-2-dependent neuroprotective effects during excitotoxic brain injury. J. Inflamm. Res. 7 57–67. 10.2147/jir.s63205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Narumiya S. (2012). Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 33 304–311. [DOI] [PubMed] [Google Scholar]
- Appel S., Mirakaj V., Bringmann A., Weck M. M., Grunebach F., Brossart P. P. P. A. R. - (2005). gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood 106 3888–3894. 10.1182/blood-2004-12-4709 [DOI] [PubMed] [Google Scholar]
- Arnold R., Neumann M., Konig W. (2007). Peroxisome proliferator-activated receptor-gamma agonists inhibit respiratory syncytial virus-induced expression of intercellular adhesion molecule-1 in human lung epithelial cells. Immunology 121 71–81. 10.1111/j.1365-2567.2006.02539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asombang A. W., Kayamba V., Mwanza-Lisulo M., Colditz G., Mudenda V., Yarasheski K., et al. (2013). Gastric cancer in Zambian adults: a prospective case-control study that assessed dietary intake and antioxidant status by using urinary isoprostane excretion. Am. J. Clin. Nutr. 97 1029–1035. 10.3945/ajcn.112.051284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader T., Ankel H. (1990). Inhibition of primary transcription of vesicular stomatitis virus by prostaglandin A1. J. Gen. Virol. 71(Pt 12), 2823–2832. 10.1099/0022-1317-71-12-2823 [DOI] [PubMed] [Google Scholar]
- Barocas D. A., Motley S., Cookson M. S., Chang S. S., Penson D. F., Dai Q., et al. (2011). Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J. Urol. 185 2102–2107. 10.1016/j.juro.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. (2008). F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 10 1405–1434. 10.1089/ars.2007.1956 [DOI] [PubMed] [Google Scholar]
- Bateman Guerreros A. G., Brockhaus F., Holzhauer B., Pethe A., Kay R. A.. (2017). Fevipiprant, an oral prostaglandin DP2 receptor (CRTh2) antagonist, in allergic asthma uncontrolled on low-dose inhaled corticosteroids. Eur. Respir. J. 50:2. [DOI] [PubMed] [Google Scholar]
- Bell-Parikh L. C., Ide T., Lawson J. A., McNamara P., Reilly M., FitzGerald G. A. (2003). Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J. Clin. Invest. 112 945–955. 10.1172/jci200318012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A., Minghetti L. P. P. A. R. - (2006). gamma agonists as regulators of microglial activation and brain inflammation. Curr. Pharm. Des. 12 93–109. 10.2174/138161206780574579 [DOI] [PubMed] [Google Scholar]
- Boisvert M., Cote S., Vargas A., Pasvanis S., Bounou S., Barbeau B., et al. (2008). PGJ2 antagonizes NF-kappaB-induced HIV-1 LTR activation in colonic epithelial cells. Virology 380 1–11. 10.1016/j.virol.2008.07.023 [DOI] [PubMed] [Google Scholar]
- Brightling C. E., Brusselle G., Altman P. (2020). The impact of the prostaglandin D2 receptor 2 and its downstream effects on the pathophysiology of asthma. Allergy 75 761–768. 10.1111/all.14001 [DOI] [PubMed] [Google Scholar]
- Brys M., Morel A., Forma E., Krzeslak A., Wilkosz J., Rozanski W., et al. (2013). Relationship of urinary isoprostanes to prostate cancer occurrence. Mol. Cell Biochem. 372 149–153. 10.1007/s11010-012-1455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner M. M., Antunes L. C., Gill N., Russell S. L., Shames S. R., Finlay B. B. (2013). 15-Deoxy-Delta12,14-prostaglandin J2 inhibits macrophage colonization by Salmonella enterica serovar Typhimurium. PLoS One 8:e69759. 10.1371/journal.pone.0069759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S. H. (2020). The chemistry, biology and pharmacology of the cyclopentenone prostaglandins. Prostaglandins. Lipid. Mediat. 148:106408. 10.1016/j.prostaglandins.2020.106408 [DOI] [PubMed] [Google Scholar]
- Bussolati B., Mason J. C. (2006). Dual role of VEGF-induced heme-oxygenase-1 in angiogenesis. Antioxid. Redox Signal. 8 1153–1163. 10.1089/ars.2006.8.1153 [DOI] [PubMed] [Google Scholar]
- Caldas L. A., Ferreira D. F., Freitas T. R. P. (2018). Prostaglandin A1 triggers Mayaro virus inhibition and heat shock protein 70 expression in an epithelial cell model. Rev. Soc. Bras. Med. Trop. 51 584–590. 10.1590/0037-8682-0235-2018 [DOI] [PubMed] [Google Scholar]
- Carta S., La Frazia S., Donatelli I., Puzelli S., Rossi A., Santoro M. G. (2014). Prostaglandin A1 inhibits avian influenza virus replication at a postentry level: Effect on virus protein synthesis and NF-kappaB activity. Prostaglandins Leukot. Essent Fatty Acids. 91 311–323. 10.1016/j.plefa.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Castrillo A., Diaz-Guerra M. J., Hortelano S., Martin-Sanz P., Bosca L. (2000). Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol. Cell Biol. 20 1692–1698. 10.1128/mcb.20.5.1692-1698.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernuda-Morollon E., Pineda-Molina E., Canada F. J., Perez-Sala D. (2001). 15-Deoxy-Delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 276 35530–35536. 10.1074/jbc.m104518200 [DOI] [PubMed] [Google Scholar]
- Chearwae W., Bright J. J. (2008). PPARgamma agonists inhibit growth and expansion of CD133+ brain tumour stem cells. Br. J. Cancer 99 2044–2053. 10.1038/sj.bjc.6604786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Liu C., Wang X., Li X., Chen Y., Tang N. (2014). 15-Deoxy-Delta(12,14)-prostaglandin J2 (15d-PGJ2) promotes apoptosis of HBx-positive liver cells. Chem. Biol. Interact. 214 26–32. 10.1016/j.cbi.2014.02.009 [DOI] [PubMed] [Google Scholar]
- Chima R. S., LaMontagne T., Piraino G., Hake P. W., Denenberg A., Zingarelli B. (2011). C-peptide, a novel inhibitor of lung inflammation following hemorrhagic shock. Am. J. Physiol. Lung Cell. Mole. Physiol. 300 L730–L739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinery R., Coffey R. J., Graves-Deal R., Kirkland S. C., Sanchez S. C., Zackert W. E., et al. (1999). Prostaglandin J2 and 15-deoxy-delta12,14-prostaglandin J2 induce proliferation of cyclooxygenase-depleted colorectal cancer cells. Cancer Res. 59 2739–2746. [PubMed] [Google Scholar]
- Ciabattoni G., Davi G., Collura M., Iapichino L., Pardo F., Ganci A., et al. (2000). In vivo lipid peroxidation and platelet activation in cystic fibrosis. Am. J. Respir. Crit. Care Med. 162(4 Pt 1), 1195–1201. [DOI] [PubMed] [Google Scholar]
- Cloutier A., Marois I., Cloutier D., Verreault C., Cantin A. M., Richter M. V. (2012). The prostanoid 15-deoxy-Delta12,14-prostaglandin-j2 reduces lung inflammation and protects mice against lethal influenza infection. J. Infect. Dis. 205 621–630. 10.1093/infdis/jir804 [DOI] [PubMed] [Google Scholar]
- Cocca C., Dorado J., Calvo E., Lopez J. A., Santos A., Perez-Castillo A. (2009). 15-Deoxi-Delta(12,14)-prostaglandin J2 is a tubulin-binding agent that destabilizes microtubules and induces mitotic arrest. Biochem. Pharmacol. 78 1330–1339. 10.1016/j.bcp.2009.06.100 [DOI] [PubMed] [Google Scholar]
- Collins C. E., Quaggiotto P., Wood L., O’Loughlin E. V., Henry R. L., Garg M. L. (1999). Elevated plasma levels of F2 alpha isoprostane in cystic fibrosis. Lipids 34 551–556. 10.1007/s11745-999-0397-1 [DOI] [PubMed] [Google Scholar]
- Conti C., Mastromarino P., Tomao P., De Marco A., Pica F., Santoro M. G. (1996). Inhibition of poliovirus replication by prostaglandins A and J in human cells. Antimicrob. Agents Chemother. 40 367–372. 10.1128/aac.40.2.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G., Portincasa P., Visalli S., Chezzi C. (2001). Inhibition by prostaglandin PGA1 on the multiplication of influenza virus is a dose-dependent effect. Virus Res. 75 43–57. 10.1016/s0168-1702(01)00221-0 [DOI] [PubMed] [Google Scholar]
- Conti M. (2006). Cyclopentenone: a special moiety for anticancer drug design. Anticancer Drugs 17 1017–1022. 10.1097/01.cad.0000231471.54288.00 [DOI] [PubMed] [Google Scholar]
- Coutinho D. S., Anjos-Valotta E. A., do Nascimento C., Pires A. L. A., Napimoga M. H., Carvalho V. F., et al. (2017). 15-Deoxy-Delta-12,14-Prostaglandin J2 Inhibits Lung Inflammation and Remodeling in Distinct Murine Models of Asthma. Front. Immunol. 8:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico E., Factor-Litvak P., Santella R. M., Mitsumoto H. (2013). Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 65 509–527. 10.1016/j.freeradbiomed.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice C., Ciccoli L., Leoncini S., Signorini C., Rossi M., Vannuccini L., et al. (2009). Systemic oxidative stress in classic Rett syndrome. Free Radic. Biol. Med. 47 440–448. 10.1016/j.freeradbiomed.2009.05.016 [DOI] [PubMed] [Google Scholar]
- De Felice C., Signorini C., Durand T., Oger C., Guy A., Bultel-Ponce V., et al. (2011). F2-dihomo-isoprostanes as potential early biomarkers of lipid oxidative damage in Rett syndrome. J. Lipid. Res. 52 2287–2297. 10.1194/jlr.p017798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo N. F., de Macedo C. G., Bonfante R., Abdalla H. B., da Silva C. M., Pasquoto T., et al. (2016). 15d-PGJ2-Loaded Solid Lipid Nanoparticles: Physicochemical Characterization and Evaluation of Pharmacological Effects on Inflammation. PLoS One 11:e0161796. 10.1371/journal.pone.0161796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira D. N., Lima E. O., Melo C., Delafiori J., Guerreiro T. M., Rodrigues R. G. M., et al. (2019). Inflammation markers in the saliva of infants born from Zika-infected mothers: exploring potential mechanisms of microcephaly during fetal development. Sci. Rep. 9:13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers A. R., Higdon A. N., Ricart K. C., Johnson M. S., Agarwal A., Kalyanaraman B., et al. (2010). Mitochondrial targeting of the electrophilic lipid 15-deoxy-Delta12,14-prostaglandin J2 increases apoptotic efficacy via redox cell signalling mechanisms. Biochem. J. 426 31–41. 10.1042/bj20091293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne S., Levy E., Levesque D., Seidman E. G. (2010). PPARgamma ligand 15-deoxy-delta 12,14-prostaglandin J2 sensitizes human colon carcinoma cells to TWEAK-induced apoptosis. Anticancer Res. 30 157–166. [PubMed] [Google Scholar]
- D’Onofrio C., Amici C., Puglianiello A., Faraoni I., Lanzilli G., Santoro M. G., et al. (1992). Comparative anti-viral and anti-proliferative activity of PGA1 and PGJ2 against HTLV-I-infected MT-2 cells. Int. J. Cancer. 51 481–488. 10.1002/ijc.2910510324 [DOI] [PubMed] [Google Scholar]
- Durand T., De Felice C., Signorini C., Oger C., Bultel-Ponce V., Guy A., et al. (2013). F(2)-Dihomo-isoprostanes and brain white matter damage in stage 1 Rett syndrome. Biochimie 95 86–90. 10.1016/j.biochi.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Fessel J. P., Hulette C., Powell S., Roberts L. J., II, Zhang J. (2003). Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. J. Neurochem. 85 645–650. 10.1046/j.1471-4159.2003.01709.x [DOI] [PubMed] [Google Scholar]
- Figarella K., Uzcategui N. L., Beck A., Schoenfeld C., Kubata B. K., Lang F., et al. (2006). Prostaglandin-induced programmed cell death in Trypanosoma brucei involves oxidative stress. Cell Death Differ. 13 1802–1814. 10.1038/sj.cdd.4401862 [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira M. E., Rockwell P., Schmidt-Glenewinkel T., Serrano P. (2014). Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front. Mol. Neurosci. 7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J. J., Klebe D., Rolland W. B., Lekic T., Krafft P. R., Zhang J. H. (2016). PPARgamma-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol. Dis. 87 124–133. 10.1016/j.nbd.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Chen J., Evans R. M. (1996). The peroxisome proliferator-activated receptors: ligands and activators. Ann. N. Y. Acad. Sci. 804 266–275. 10.1111/j.1749-6632.1996.tb18621.x [DOI] [PubMed] [Google Scholar]
- Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., Evans R. M. (1995). 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83 803–812. 10.1016/0092-8674(95)90193-0 [DOI] [PubMed] [Google Scholar]
- Fu Y. G., Sung J. J., Wu K. C., Bai A. H., Chan M. C., Yu J., et al. (2006). Inhibition of gastric cancer cells associated angiogenesis by 15d-prostaglandin J2 through the downregulation of angiopoietin-1. Cancer Lett. 243 246–254. 10.1016/j.canlet.2005.11.039 [DOI] [PubMed] [Google Scholar]
- Funovics P., Brostjan C., Nigisch A., Fila A., Grochot A., Mleczko K., et al. (2006). Effects of 15d-PGJ(2) on VEGF-induced angiogenic activities and expression of VEGF receptors in endothelial cells. Prostaglandins Lipid. Mediat. 79 230–244. 10.1016/j.prostaglandins.2006.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon B., Oeste C. L., Diez-Dacal B., Perez-Sala D. (2011). Proteomic studies on protein modification by cyclopentenone prostaglandins: expanding our view on electrophile actions. J. Proteomics. 74 2243–2263. 10.1016/j.jprot.2011.03.028 [DOI] [PubMed] [Google Scholar]
- Giannelli G., Villa E., Lahn M. (2014). Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res. 74 1890–1894. 10.1158/0008-5472.can-14-0243 [DOI] [PubMed] [Google Scholar]
- Giri S., Rattan R., Singh A. K., Singh I. (2004). The 15-deoxy-delta12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-Akt-NF-kappaB-p300 pathway independent of peroxisome proliferator-activated receptor gamma. J. Immunol. 173 5196–5208. 10.4049/jimmunol.173.8.5196 [DOI] [PubMed] [Google Scholar]
- Gorrindo P., Lane C. J., Lee E. B., McLaughlin B., Levitt P. (2013). Enrichment of elevated plasma F2t-isoprostane levels in individuals with autism who are stratified by presence of gastrointestinal dysfunction. PLoS One 8:e68444. 10.1371/journal.pone.0068444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., Wesselingh S. L., McArthur J. C. (1994). Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann. Neurol. 35 592–597. 10.1002/ana.410350513 [DOI] [PubMed] [Google Scholar]
- Guo H., Callaway J. B., Ting J. P. (2015). Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21 677–687. 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. (1966). Prostaglandins in human seminal plasma. Prostaglandins and related factors 46. J. Biol. Chem. 241 257–263. [PubMed] [Google Scholar]
- Han H., Shin S. W., Seo C. Y., Kwon H. C., Han J. Y., Kim I. H., et al. (2007). 15-Deoxy-delta 12,14-prostaglandin J2 (15d-PGJ 2) sensitizes human leukemic HL-60 cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through Akt downregulation. Apoptosis 12 2101–2114. 10.1007/s10495-007-0124-2 [DOI] [PubMed] [Google Scholar]
- Hanna V. S., Hafez E. A. A. (2018). Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 11 23–32. 10.1016/j.jare.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslmayer P., Thalhammer T., Jager W., Aust S., Steiner G., Ensinger C., et al. (2002). The peroxisome proliferator-activated receptor gamma ligand 15-deoxy-Delta12,14-prostaglandin J2 induces vascular endothelial growth factor in the hormone-independent prostate cancer cell line PC 3 and the urinary bladder carcinoma cell line 5637. Int. J. Oncol. 21 915–920. [PubMed] [Google Scholar]
- Hernandez J. M., Floyd D. H., Weilbaecher K. N., Green P. L., Boris-Lawrie K. (2008). Multiple facets of junD gene expression are atypical among AP-1 family members. Oncogene 27 4757–4767. 10.1038/onc.2008.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y., Hayashi H., Ito S., Kikawa Y., Ishibashi M., Sudo M., et al. (1988). Occurrence of 9-deoxy-delta 9,delta 12-13,14-dihydroprostaglandin D2 in human urine. J. Biol. Chem. 263 16619–16625. 10.1016/s0021-9258(18)37435-0 [DOI] [PubMed] [Google Scholar]
- Hornsleth A., Loland L., Larsen L. B. (2001). Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J. Clin. Virol. 21 163–170. 10.1016/s1386-6532(01)00159-7 [DOI] [PubMed] [Google Scholar]
- Hsu H. T., Chi C. W. (2014). Emerging role of the peroxisome proliferator-activated receptor-gamma in hepatocellular carcinoma. J. Hepatocell Carcinoma. 1 127–135. 10.2147/jhc.s48512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan T., Tran T., Zheng J., Sapkota S., MacDonald S. W., Camicioli R., et al. (2018). Metabolomics Analyses of Saliva Detect Novel Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 65 1401–1416. 10.3233/jad-180711 [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M., McGrath M. S., Hanks D., Erickson S., Pulliam L. (1992). Effects of dimethyl prostaglandin A1 on herpes simplex virus and human immunodeficiency virus replication. Antimicrob. Agents Chemother. 36 2253–2258. 10.1128/aac.36.10.2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Tanabe T., Umesono K. (2000). Feedback control of cyclooxygenase-2 expression through PPARgamma. J. Biol. Chem. 275 28028–28032. 10.1074/jbc.m001387200 [DOI] [PubMed] [Google Scholar]
- Inoue M., Itoh H., Tanaka T., Chun T. H., Doi K., Fukunaga Y., et al. (2001). Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arterioscler. Thromb. Vasc. Biol. 21 560–566. 10.1161/01.atv.21.4.560 [DOI] [PubMed] [Google Scholar]
- Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., et al. (2004). Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol. Cell Biol. 24 36–45. 10.1128/mcb.24.1.36-45.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janabi N. (2002). Selective inhibition of cyclooxygenase-2 expression by 15-deoxy-Delta(12,14)(12,14)-prostaglandin J(2) in activated human astrocytes, but not in human brain macrophages. J. Immunol. 168 4747–4755. 10.4049/jimmunol.168.9.4747 [DOI] [PubMed] [Google Scholar]
- Jandl K., Heinemann A. (2017). The therapeutic potential of CRTH2/DP2 beyond allergy and asthma. Prostaglandins Lipid. Mediat. 133 42–48. 10.1016/j.prostaglandins.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H. Y., Hong O. Y., Youn H. J., Kim M. G., Kim C. H., Jung S. H., et al. (2020). 15d-PGJ2 inhibits NF-kappaB and AP-1-mediated MMP-9 expression and invasion of breast cancer cell by means of a heme oxygenase-1-dependent mechanism. BMB Rep. 53 212–217. 10.5483/bmbrep.2020.53.4.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J. D., Kim H. J., Rho Y. H., Choi S. J., Lee Y. H., Cheon H. J., et al. (2005). Inhibition of IL-10-induced STAT3 activation by 15-deoxy-Delta12,14-prostaglandin J2. Rheumatology 44 983–988. 10.1093/rheumatology/keh657 [DOI] [PubMed] [Google Scholar]
- Jozkowicz A., Huk I., Nigisch A., Weigel G., Weidinger F., Dulak J. (2002). Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antioxid Redox Signal. 4 577–585. 10.1089/15230860260220076 [DOI] [PubMed] [Google Scholar]
- Jozkowicz A., Nigisch A., Wegrzyn J., Weigel G., Huk I., Dulak J. (2004). Opposite effects of prostaglandin-J2 on VEGF in normoxia and hypoxia: role of HIF-1. Biochem. Biophys. Res. Commun. 314 31–38. 10.1016/j.bbrc.2003.12.059 [DOI] [PubMed] [Google Scholar]
- Kalantari P., Narayan V., Henderson A. J., Prabhu K. S. (2009). 15-Deoxy-Delta12,14-prostaglandin J2 inhibits HIV-1 transactivating protein, Tat, through covalent modification. FASEB J. 23 2366–2373. 10.1096/fj.08-124982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansanen E., Kivela A. M., Levonen A. L. (2009). Regulation of Nrf2-dependent gene expression by 15-deoxy-Delta12,14-prostaglandin J2. Free Radic. Biol. Med. 47 1310–1317. 10.1016/j.freeradbiomed.2009.06.030 [DOI] [PubMed] [Google Scholar]
- Kaplan J. M., Cook J. A., Hake P. W., O’Connor M., Burroughs T. J., Zingarelli B. (2005). 15-Deoxy-delta(12,14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock 24 59–65. 10.1097/01.shk.0000167108.88376.f2 [DOI] [PubMed] [Google Scholar]
- Kaplan J. M., Denenberg A., Monaco M., Nowell M., Wong H., Zingarelli B. (2010). Changes in peroxisome proliferator-activated receptor-gamma activity in children with septic shock. Intens. Care Med. 36 123–130. 10.1007/s00134-009-1654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M., Kojima F., Qian M., Yang L., Crofford L. J. (2007). Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator-activated receptor gamma: regulation by prostaglandin E2 via the phosphatidylinositol 3-kinase and Akt pathway. J. Biol. Chem. 282 5356–5366. 10.1074/jbc.m610153200 [DOI] [PubMed] [Google Scholar]
- Kapoor R., Huang Y. S. (2006). Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 7 531–534. 10.2174/138920106779116874 [DOI] [PubMed] [Google Scholar]
- Kato T., Fukushima M., Kurozumi S., Noyori R. (1986). Antitumor activity of delta 7-prostaglandin A1 and delta 12-prostaglandin J2 in vitro and in vivo. Cancer Res. 46 3538–3542. [PubMed] [Google Scholar]
- Kim E. H., Kim D. H., Na H. K., Surh Y. J. (2004). Effects of cyclopentenone prostaglandins on the expression of heme oxygenase-1 in MCF-7 cells. Ann. N. Y. Acad. Sci. 1030 493–500. 10.1196/annals.1329.061 [DOI] [PubMed] [Google Scholar]
- Kim E. H., Na H. K., Surh Y. J. (2006). Upregulation of VEGF by 15-deoxy-Delta12,14-prostaglandin J2 via heme oxygenase-1 and ERK1/2 signaling in MCF-7 cells. Ann. N. Y. Acad. Sci. 1090 375–384. 10.1196/annals.1378.041 [DOI] [PubMed] [Google Scholar]
- Kim H. J., Rho Y. H., Choi S. J., Lee Y. H., Cheon H., Um J. W., et al. (2005). 15-Deoxy-delta12,14-PGJ2 inhibits IL-6-induced Stat3 phosphorylation in lymphocytes. Exp. Mol. Med. 37 179–185. 10.1038/emm.2005.24 [DOI] [PubMed] [Google Scholar]
- Kim I. K., Lee J. H., Sohn H. W., Kim H. S., Kim S. H. (1993). Prostaglandin A2 and delta 12-prostaglandin J2 induce apoptosis in L1210 cells. FEBS Lett. 321 209–214. 10.1016/0014-5793(93)80110-g [DOI] [PubMed] [Google Scholar]
- Kim K. R., Kim H. J., Lee S. K., Ma G. T., Park K. K., Chung W. Y. (2015). 15-deoxy-delta12,14-prostaglandin j2 inhibits osteolytic breast cancer bone metastasis and estrogen deficiency-induced bone loss. PLoS One 10:e0122764. 10.1371/journal.pone.0122764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Ratziu V., Choi S. G., Lalazar A., Theiss G., Dang Q., et al. (1998). Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J. Biol. Chem. 273 33750–33758. 10.1074/jbc.273.50.33750 [DOI] [PubMed] [Google Scholar]
- Kitz K., Windischhofer W., Leis H. J., Huber E., Kollroser M., Malle E. (2011). 15-Deoxy-Delta12,14-prostaglandin J2 induces Cox-2 expression in human osteosarcoma cells through MAPK and EGFR activation involving reactive oxygen species. Free Radic Biol. Med. 50 854–865. 10.1016/j.freeradbiomed.2010.12.039 [DOI] [PubMed] [Google Scholar]
- Koharudin L. M., Furey W., Gronenborn A. M. (2011). Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J. Biol. Chem. 286 1588–1597. 10.1074/jbc.m110.173278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z., Xu L., Mirnics K., Porter N. A. (2013). Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J. Inherit. Metab. Dis. 36 113–122. 10.1007/s10545-012-9504-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakula K., Koenis D. S., van Tiel C. M., de Vries C. J. (2014). NR4A nuclear receptors are orphans but not lonesome. Biochim. Biophys. Acta 1843 2543–2555. 10.1016/j.bbamcr.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Kweider N., Fragoulis A., Rosen C., Pecks U., Rath W., Pufe T., et al. (2011). Interplay between vascular endothelial growth factor (VEGF) and nuclear factor erythroid 2-related factor-2 (Nrf2): implications for preeclampsia. J. Biol. Chem. 286 42863–42872. 10.1074/jbc.m111.286880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal P. M., Amici C., Bonmassar E., D’Onofrio C. (1994a). Effects of cyclopentenone prostaglandins on myeloid cells during early infection with HTLV-I. II. Regulation of synthesis of inducible p72 heat shock protein. J. Pharmacol. Exp. Ther. 271 1096–1102. [PubMed] [Google Scholar]
- Lacal P. M., Puglianiello A., Bonmassar E., D’Onofrio C. (1994b). Effects of cyclopentenone prostaglandins on myeloid cells during early infection with HTLV-I. I. Cell differentiation determines sensitivity to prostaglandins and virus infection. J. Pharmacol. Exp. Ther. 271 1086–1095. [PubMed] [Google Scholar]
- Lawrence T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spr. Harb. Perspect. Biol. 1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Su Y., Yin P. H., Lee H. C., Chi C. W. (2009). PPAR(gamma)/PGC-1(alpha) pathway in E-cadherin expression and motility of HepG2 cells. Anticancer Res. 29 5057–5063. [PubMed] [Google Scholar]
- Lee S. E., Park Y. S. (2013). Role of lipid peroxidation-derived alpha, beta-unsaturated aldehydes in vascular dysfunction. Oxid. Med. Cell Longev. 2013:629028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Yang E. K., Kim S. G. (2006). Peroxisome proliferator-activated receptor-gamma and retinoic acid X receptor alpha represses the TGFbeta1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: role for Zf9 dephosphorylation. Mol. Pharmacol. 70 415–425. 10.1124/mol.106.022954 [DOI] [PubMed] [Google Scholar]
- Levonen A. L., Dickinson D. A., Moellering D. R., Mulcahy R. T., Forman H. J., Darley-Usmar V. M. (2001). Biphasic effects of 15-deoxy-delta(12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 21 1846–1851. 10.1161/hq1101.098488 [DOI] [PubMed] [Google Scholar]
- Levonen A. L., Landar A., Ramachandran A., Ceaser E. K., Dickinson D. A., Zanoni G., et al. (2004). Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 378(Pt 2), 373–382. 10.1042/bj20031049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Narahara H. (2013). 15-Deoxy-Delta(12,14)-prostaglandin J(2) induces growth inhibition, cell cycle arrest and apoptosis in human endometrial cancer cell lines. Int. J. Mol. Med. 31 778–788. 10.3892/ijmm.2013.1268 [DOI] [PubMed] [Google Scholar]
- Li H., Pauza C. D. (2009). Effects of 15-deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) and rosiglitazone on human gammadelta2 T cells. PLoS One 4:e7726. 10.1371/journal.pone.0007726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Guo C., Wu J. (2019). 15-Deoxy–(12,14)-Prostaglandin J2 (15d-PGJ2), an Endogenous Ligand of PPAR-gamma: Function and Mechanism. PPAR Res. 2019:7242030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Atkinson K., Zhang T. (2017). Combination of chemotherapy and cancer stem cell targeting agents: Preclinical and clinical studies. Cancer Lett. 396 103–109. 10.1016/j.canlet.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Liang X., Wu L., Hand T., Andreasson K. (2005). Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J. Neurochem. 92 477–486. 10.1111/j.1471-4159.2004.02870.x [DOI] [PubMed] [Google Scholar]
- Lin A., Minden A., Martinetto H., Claret F. X., Lange-Carter C., Mercurio F., et al. (1995). Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268 286–290. 10.1126/science.7716521 [DOI] [PubMed] [Google Scholar]
- Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol. Life Sci. 73 3221–3247. 10.1007/s00018-016-2223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magesh S., Chen Y., Hu L. (2012). Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 32 687–726. 10.1002/med.21257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N. K., Leppla S. H., Moayeri M. (2015). The cyclopentenone prostaglandin 15d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes. J. Immunol. 194 2776–2785. 10.4049/jimmunol.1401611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromarino P., Conti C., Petruzziello R., De Marco A., Pica F., Santoro M. G. (1993). Inhibition of Sindbis virus replication by cyclopentenone prostaglandins: a cell-mediated event associated with heat-shock protein synthesis. Antiviral. Res. 20 209–222. 10.1016/0166-3542(93)90021-a [DOI] [PubMed] [Google Scholar]
- Mc Guire P. J., Parikh A., Diaz G. A. (2009). Profiling of oxidative stress in patients with inborn errors of metabolism. Mol. Genet. Metab. 98 173–180. 10.1016/j.ymgme.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde-Langosch K. (2005). The Fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 41 2449–2461. 10.1016/j.ejca.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Millan O., Rico D., Peinado H., Zarich N., Stamatakis K., Perez-Sala D., et al. (2006). Potentiation of tumor formation by topical administration of 15-deoxy-delta12,14-prostaglandin J2 in a model of skin carcinogenesis. Carcinogenesis 27 328–336. 10.1093/carcin/bgi213 [DOI] [PubMed] [Google Scholar]
- Mills E. L., Ryan D. G., Prag H. A., Dikovskaya D., Menon D., Zaslona Z., et al. (2018). Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A., Lin A., Claret F. X., Abo A., Karin M. (1995). Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81 1147–1157. 10.1016/s0092-8674(05)80019-4 [DOI] [PubMed] [Google Scholar]
- Ming X., Stein T. P., Brimacombe M., Johnson W. G., Lambert G. H., Wagner G. C. (2005). Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot. Essent Fatty Acids 73 379–384. 10.1016/j.plefa.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Ishii Y., Itoh K., Iizuka T., Morishima Y., Kimura T., et al. (2005). Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2 pathways in protection against acute lung injury. Am. J. Respir. Crit. Care Med. 171 1260–1266. [DOI] [PubMed] [Google Scholar]
- Monroy M. A., Opperman K. K., Pucciarelli M., Yerrum S., Berg D. A., Daly J. M. T. H. E. (2007). PPARgamma ligand 15d-PGJ2 modulates macrophage activation after injury in a murine trauma model. Shock 28 186–191. 10.1097/shk.0b013e3180310982 [DOI] [PubMed] [Google Scholar]
- Montine T. J., Beal M. F., Cudkowicz M. E., O’Donnell H., Margolin R. A., McFarland L., et al. (1999a). Increased CSF F2-isoprostane concentration in probable AD. Neurology 52 562–565. 10.1212/wnl.52.3.562 [DOI] [PubMed] [Google Scholar]
- Montine T. J., Beal M. F., Robertson D., Cudkowicz M. E., Biaggioni I., O’Donnell H., et al. (1999b). Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology 52 1104–1105. 10.1212/wnl.52.5.1104 [DOI] [PubMed] [Google Scholar]
- Montine T. J., Markesbery W. R., Morrow J. D., Roberts L. J., II (1998). Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann. Neurol. 44 410–413. 10.1002/ana.410440322 [DOI] [PubMed] [Google Scholar]
- Montuschi P., Barnes P., Roberts L. J., II (2007). Insights into oxidative stress: the isoprostanes. Curr. Med. Chem. 14 703–717. 10.2174/092986707780059607 [DOI] [PubMed] [Google Scholar]
- Montuschi P., Kharitonov S. A., Ciabattoni G., Corradi M., van Rensen L., Geddes D. M., et al. (2000). Exhaled 8-isoprostane as a new non-invasive biomarker of oxidative stress in cystic fibrosis. Thorax 55 205–209. 10.1136/thorax.55.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D. (2005). Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 25 279–286. 10.1161/01.atv.0000152605.64964.c0 [DOI] [PubMed] [Google Scholar]
- Mowen K., David M. (2000). Regulation of STAT1 nuclear export by Jak1. Mol. Cell Biol. 20 7273–7281. 10.1128/mcb.20.19.7273-7281.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R., Jow L., Noonan D., McDonnell D. P. (1994). Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J. Steroid Biochem. Mol. Biol. 51 157–166. 10.1016/0960-0760(94)90089-2 [DOI] [PubMed] [Google Scholar]
- Murillo J. C., Dimov V., Gonzalez-Estrada A. (2018). An evaluation of fevipiprant for the treatment of asthma: a promising new therapy? Exp. Opin. Pharmacother. 19 2087–2093. 10.1080/14656566.2018.1540589 [DOI] [PubMed] [Google Scholar]
- Musiek E. S., Breeding R. S., Milne G. L., Zanoni G., Morrow J. D., McLaughlin B. (2006). Cyclopentenone isoprostanes are novel bioactive products of lipid oxidation which enhance neurodegeneration. J. Neurochem. 97 1301–1313. 10.1111/j.1471-4159.2006.03797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek E. S., McLaughlin B., Morrow J. D. (2007). Electrophilic cyclopentenone isoprostanes in neurodegeneration. J. Mol. Neurosci. 33 80–86. 10.1007/s12031-007-0042-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N., Iwanari H., Kumagai H., Kusano-Arai O., Ikeda Y., Aritake K., et al. (2017). Generation and characterization of an antagonistic monoclonal antibody against an extracellular domain of mouse DP2 (CRTH2/GPR44) receptors for prostaglandin D2. PLoS One 12:e0175452. 10.1371/journal.pone.0175452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa H., Chang K., Shinozaki S., Yasukawa T., Ishimaru K., Yasuhara S., et al. (2017). iNOS as a Driver of Inflammation and Apoptosis in Mouse Skeletal Muscle after Burn Injury: Possible Involvement of Sirt1 S-Nitrosylation-Mediated Acetylation of p65 NF-kappaB and p53. PLoS One 12:e0170391. 10.1371/journal.pone.0170391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M., Katoh H. (2002). Cyclopentenone prostaglandin receptors. Prostaglandins. Lipid. Mediat. 6 611–617. 10.1016/s0090-6980(02)00059-x [DOI] [PubMed] [Google Scholar]
- Nikitakis N. G., Siavash H., Hebert C., Reynolds M. A., Hamburger A. W., Sauk J. J. (2002). 15-PGJ2, but not thiazolidinediones, inhibits cell growth, induces apoptosis, and causes downregulation of Stat3 in human oral SCCa cells. Br. J. Cancer. 87 1396–1403. 10.1038/sj.bjc.6600618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niro S., Hennebert O., Morfin R. A. (2010). native steroid hormone derivative triggers the resolution of inflammation. Horm Mol. Biol. Clin. Investig. 1 11–19. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H., Van Dorp D. A., Bergstrom S., Hamberg M., Samuelsson B. (1966). Absolute configuration of the prostaglandins. Nature 212 38–39. [DOI] [PubMed] [Google Scholar]
- Oliva J. L., Perez-Sala D., Castrillo A., Martinez N., Canada F. J., Bosca L., et al. (2003). The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J2 binds to and activates H-Ras. Proc. Natl. Acad. Sci. U S A. 100 4772–4777. 10.1073/pnas.0735842100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omeragic A., Saikali M. F., Currier S., Volsky D. J., Cummins C. L., Bendayan R. (2020). Selective peroxisome proliferator-activated receptor-gamma modulator, INT131 exhibits anti-inflammatory effects in an EcoHIV mouse model. FASEB J. 34 1996–2010. 10.1096/fj.201901874r [DOI] [PubMed] [Google Scholar]
- Pai S., Thomas R. (2008). Immune deficiency or hyperactivity-Nf-kappab illuminates autoimmunity. J. Autoimmun. 31 245–251. 10.1016/j.jaut.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Park E. J., Park S. Y., Joe E. H., Jou I. (2003). 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J. Biol. Chem. 278 14747–14752. 10.1074/jbc.m210819200 [DOI] [PubMed] [Google Scholar]
- Park J. M., Na H. K. (2019a). 15-Deoxy-Delta(12,14)-prostaglandin J2 Upregulates the Expression of 15-Hydroxyprostaglandin Dehydrogenase by Inducing AP-1 Activation and Heme Oxygenase-1 Expression in Human Colon Cancer Cells. J. Cancer Prev. 24 183–191. 10.15430/jcp.2019.24.3.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. M., Na H. K. (2019b). Erratum: 15-Deoxy-Delta(12,14)-prostaglandin J2 Upregulates the Expression of 15-Hydroxyprostaglandin Dehydrogenase by Inducing AP-1 Activation and Heme Oxygenase-1 Expression in Human Colon Cancer Cells. J. Cancer Prev. 24:245. 10.15430/jcp.2019.24.4.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. H., Kim K. E., Hwang H. Y., Kim T. Y. (2003). Regulatory effect of SOCS on NF-kappaB activity in murine monocytes/macrophages. DNA Cell Biol. 22 131–139. 10.1089/104454903321515931 [DOI] [PubMed] [Google Scholar]
- Parker J. (1995). Prostaglandin A2 protein interactions and inhibition of cellular proliferation. Prostaglandins 50 359–375. 10.1016/0090-6980(95)00136-0 [DOI] [PubMed] [Google Scholar]
- Pearen M. A., Muscat G. E. (2010). Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol. Endocrinol. 24 1891–1903. 10.1210/me.2010-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D., Weiss H. M., Jin Y., Jaap van Lier J., Erpenbeck V. J., Glaenzel U., et al. (2017). Absorption, Distribution, Metabolism, and Excretion of the Oral Prostaglandin D2 Receptor 2 Antagonist Fevipiprant (QAW039) in Healthy Volunteers and In Vitro. Drug Metab. Dispos. 45 817–825. 10.1124/dmd.117.075358 [DOI] [PubMed] [Google Scholar]
- Peeraully M. R., Sievert H., Bullo M., Wang B., Trayhurn P. (2006). Prostaglandin D2 and J2-series (PGJ2, Delta12-PGJ2) prostaglandins stimulate IL-6 and MCP-1, but inhibit leptin, expression and secretion by 3T3-L1 adipocytes. Pflugers Arch. 453 177–187. 10.1007/s00424-006-0118-x [DOI] [PubMed] [Google Scholar]
- Perez-Sala D., Cernuda-Morollon E., Canada F. J. (2003). Molecular basis for the direct inhibition of AP-1 DNA binding by 15-deoxy-Delta 12,14-prostaglandin J2. J. Biol. Chem. 278 51251–51260. 10.1074/jbc.m309409200 [DOI] [PubMed] [Google Scholar]
- Phulwani N. K., Feinstein D. L., Gavrilyuk V., Akar C., Kielian T. (2006). 15-deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) and ciglitazone modulate Staphylococcus aureus-dependent astrocyte activation primarily through a PPAR-gamma-independent pathway. J. Neurochem. 99 1389–1402. 10.1111/j.1471-4159.2006.04183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica F., De Marco A., De Cesare F., Santoro M. G. (1993). Inhibition of vesicular stomatitis virus replication by delta 12-prostaglandin J2 is regulated at two separate levels and is associated with induction of stress protein synthesis. Antiviral. Res. 20 193–208. 10.1016/0166-3542(93)90020-j [DOI] [PubMed] [Google Scholar]
- Pica F., Palamara A. T., Rossi A., De Marco A., Amici C., Santoro M. G. (2000). Delta(12)-prostaglandin J(2) is a potent inhibitor of influenza A virus replication. Antimicrob. Agents Chemother. 44 200–204. 10.1128/aac.44.1.200-204.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta A., Pasi M., Brunoldi E., Zanoni G., Vidari G. (2013). Biology and chemistry of neuroprostanes. First total synthesis of 17-A4-NeuroP: validation of a convergent strategy to a number of cyclopentenone neuroprostanes. Chem. Phys. Lipids. 174 64–74. 10.1016/j.chemphyslip.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Pratico D., Clark C. M., Lee V. M., Trojanowski J. Q., Rokach J., FitzGerald G. A. (2000). Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 48 809–812. [DOI] [PubMed] [Google Scholar]
- Pratico D., Myl V., Trojanowski J. Q., Rokach J., Fitzgerald G. A. (1998). Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 12 1777–1783. 10.1096/fasebj.12.15.1777 [DOI] [PubMed] [Google Scholar]
- Rajakariar R., Hilliard M., Lawrence T., Trivedi S., Colville-Nash P., Bellingan G., et al. (2007). Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc. Natl. Acad. Sci. U S A. 104 20979–20984. 10.1073/pnas.0707394104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Jang Y., Kim C. H., Kim W., Toh H. T., Jeon J., et al. (2020). PGE1 and PGA1 bind to Nurr1 and activate its transcriptional function. Nat. Chem. Biol. 16 876–886. 10.1038/s41589-020-0553-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M., Huang J., Fajas L., Li A., Welch J., Najib J., et al. (1998a). Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U S A. 95 7614–7619. 10.1073/pnas.95.13.7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. (1998b). The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391 79–82. 10.1038/34178 [DOI] [PubMed] [Google Scholar]
- Rosenberger T. A., Villacreses N. E., Hovda J. T., Bosetti F., Weerasinghe G., Wine R. N., et al. (2004). Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J. Neurochem. 88 1168–1178. 10.1046/j.1471-4159.2003.02246.x [DOI] [PubMed] [Google Scholar]
- Rossi A., Elia G., Santoro M. G. (1997). Inhibition of nuclear factor kappa B by prostaglandin A1: an effect associated with heat shock transcription factor activation. Proc. Natl. Acad. Sci. U S A. 94 746–750. 10.1073/pnas.94.2.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., et al. (2000). Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 403 103–108. 10.1038/47520 [DOI] [PubMed] [Google Scholar]
- Rossi P., Riutta A., Kuukasjarvi P., Vehmas T., Mucha I., Salenius J. P. (2004). Revascularization decreases 8-isoprostaglandin F2alpha excretion in chronic lower limb ischemia. Prostaglandins Leukot. Essent Fatty Acids 71 97–101. 10.1016/j.plefa.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Rossner P., Jr., Gammon M. D., Terry M. B., Agrawal M., Zhang F. F., Teitelbaum S. L., et al. (2006). Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 15 639–644. 10.1158/1055-9965.epi-05-0554 [DOI] [PubMed] [Google Scholar]
- Rozera C., Carattoli A., De Marco A., Amici C., Giorgi C., Santoro M. G. (1996). Inhibition of HIV-1 replication by cyclopentenone prostaglandins in acutely infected human cells. Evidence for a transcriptional block. J. Clin. Invest. 97 1795–1803. 10.1172/jci118609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. (1993). Expression of interstitial collagenase, 92-kDa gelatinase, and tissue inhibitor of metalloproteinases-1 in granuloma annulare and necrobiosis lipoidica diabeticorum. J. Invest. Dermatol. 100 335–342. 10.1111/1523-1747.ep12470032 [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Corcoran T. B., Laffey J. G., Shorten G. D. (2002). Isoprostanes–markers of ischaemia reperfusion injury. Eur. J. Anaesthesiol. 19 550–559. 10.1017/s0265021502000893 [DOI] [PubMed] [Google Scholar]
- Sandham D. A., Barker L., Brown L., Brown Z., Budd D., Charlton S. J., et al. (2017a). Correction to “Discovery of Fevipiprant (NVP-QAW039), a Potent and Selective DP2 Receptor Antagonist for Treatment of Asthma”. ACS Med. Chem. Lett. 8:987. 10.1021/acsmedchemlett.7b00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandham D. A., Barker L., Brown L., Brown Z., Budd D., Charlton S. J., et al. (2017b). Discovery of Fevipiprant (NVP-QAW039), a Potent and Selective DP2 Receptor Antagonist for Treatment of Asthma. ACS Med. Chem. Lett. 8 582–586. 10.1021/acsmedchemlett.7b00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Fukushima M., Benedetto A., Amici C. (1987). PGJ2, a new antiviral prostaglandin: inhibition of Sendai virus replication and alteration of virus protein synthesis. J. Gen. Virol. 68(Pt 4), 1153–1158. 10.1099/0022-1317-68-4-1153 [DOI] [PubMed] [Google Scholar]
- Sato H., Ishihara S., Kawashima K., Moriyama N., Suetsugu H., Kazumori H., et al. (2000). Expression of peroxisome proliferator-activated receptor (PPAR)gamma in gastric cancer and inhibitory effects of PPARgamma agonists. Br. J. Cancer 83 1394–1400. 10.1054/bjoc.2000.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre L. M., Lin D., Yuan Q., Zhu X., Tang X. (2006). Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug. Metab. Rev. 38 651–675. 10.1080/03602530600959508 [DOI] [PubMed] [Google Scholar]
- Scher J. U., Pillinger M. H. (2005). 15d-PGJ2: the anti-inflammatory prostaglandin? Clin. Immunol. 114 100–109. 10.1016/j.clim.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Seet R. C., Lee C. Y., Lim E. C., Tan J. J., Quek A. M., Chong W. L., et al. (2010). Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 48 560–566. 10.1016/j.freeradbiomed.2009.11.026 [DOI] [PubMed] [Google Scholar]
- Seif F., Khoshmirsafa M., Aazami H., Mohsenzadegan M., Sedighi G., Bahar M. (2017). The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 15 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U., Cao Y., Xiao G., Greten F. R., Krahn G., Bonizzi G., et al. (2001). Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293 1495–1499. 10.1126/science.1062677 [DOI] [PubMed] [Google Scholar]
- Seo S. K., Seo D. I., Park W. S., Jung W. K., Lee D. S., Park S. G., et al. (2014). Attenuation of IFN-gamma-induced B7-H1 expression by 15-deoxy-delta(12,14)-prostaglandin J2 via downregulation of the Jak/STAT/IRF-1 signaling pathway. Life Sci. 112 82–89. 10.1016/j.lfs.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Sharma A. M., Staels B. (2007). Review: Peroxisome proliferator-activated receptor gamma and adipose tissue–understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 92 386–395. 10.1210/jc.2006-1268 [DOI] [PubMed] [Google Scholar]
- Shi J., Johansson J., Woodling N. S., Wang Q., Montine T. J., Andreasson K. (2010). The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J. Immunol. 184 7207–7218. 10.4049/jimmunol.0903487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Kondo M., Osawa T., Shibata N., Kobayashi M., Uchida K. (2002). 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 277 10459–10466. [DOI] [PubMed] [Google Scholar]
- Shibata T., Yamada T., Ishii T., Kumazawa S., Nakamura H., Masutani H., et al. (2003a). Thioredoxin as a molecular target of cyclopentenone prostaglandins. J. Biol. Chem. 278 26046–26054. 10.1074/jbc.m303690200 [DOI] [PubMed] [Google Scholar]
- Shibata T., Yamada T., Kondo M., Tanahashi N., Tanaka K., Nakamura H., et al. (2003b). An endogenous electrophile that modulates the regulatory mechanism of protein turnover: inhibitory effects of 15-deoxy-Delta 12,14-prostaglandin J2 on proteasome. Biochemistry 42 13960–13968. 10.1021/bi035215a [DOI] [PubMed] [Google Scholar]
- Signorini C., De Felice C., Leoncini S., Giardini A., D’Esposito M., Filosa S., et al. (2011). F(4)-neuroprostanes mediate neurological severity in Rett syndrome. Clin. Chim. Acta. 412 1399–1406. 10.1016/j.cca.2011.04.016 [DOI] [PubMed] [Google Scholar]
- Stamatakis K., Perez-Sala D. (2006). Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann. N. Y. Acad. Sci. 1091 548–570. 10.1196/annals.1378.096 [DOI] [PubMed] [Google Scholar]
- Stocker R., Keaney J. F., Jr. (2004). Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84 1381–1478. 10.1152/physrev.00047.2003 [DOI] [PubMed] [Google Scholar]
- Straus D. S., Glass C. K. (2001). Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med. Res. Rev. 21 185–210. 10.1002/med.1006 [DOI] [PubMed] [Google Scholar]
- Straus D. S., Pascual G., Li M., Welch J. S., Ricote M., Hsiang C. H., et al. (2000). 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc. Natl. Acad. Sci. U S A. 97 4844–4849. 10.1073/pnas.97.9.4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C. (2017). The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 17 545–558. 10.1038/nri.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae I. H., Park E. Y., Dey P., Son J. Y., Lee S. Y., Jung J. H., et al. (2018). Novel SIRT1 inhibitor 15-deoxy-Delta12,14-prostaglandin J2 and its derivatives exhibit anticancer activity through apoptotic or autophagic cell death pathways in SKOV3 cells. Int. J. Oncol. 53 2518–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima T., Fujiwara Y., Higuchi K., Arakawa T., Yano Y., Hasuma T., et al. (2001). PPAR-gamma ligands inhibit growth of human esophageal adenocarcinoma cells through induction of apoptosis, cell cycle arrest and reduction of ornithine decarboxylase activity. Int. J. Oncol. 19 465–471. [PubMed] [Google Scholar]
- Tauber D., Parker R. (2019). 15-Deoxy-Delta(12,14)-prostaglandin J2 promotes phosphorylation of eukaryotic initiation factor 2alpha and activates the integrated stress response. J. Biol. Chem. 294 6344–6352. 10.1074/jbc.ra118.007138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R., Botting R. M. (1990). The mode of action of anti-inflammatory drugs. Postgrad. Med. J. 66 (Suppl. 4), S2–S17. [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R., Hughes S. E., Kung H. J., Heasley S., Majors J., et al. (1979). Synthesis, structure, and integration of the DNA of RNA tumor viruses. Cold Spr. Harb. Symp. Quant. Biol. 43(Pt 2), 851–864. 10.1101/sqb.1979.043.01.091 [DOI] [PubMed] [Google Scholar]
- Vijay R., Fehr A. R., Janowski A. M., Athmer J., Wheeler D. L., Grunewald M., et al. (2017). Virus-induced inflammasome activation is suppressed by prostaglandin D2/DP1 signaling. Proc. Natl. Acad. Sci. U S A. 114 E5444–E5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Liang H., Zen K. (2014). Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Hallsworth P. G., Dowling K. D., Alpers J. H., Bowden J. J., Forsyth K. D. (2000). Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur. Respir. J. 15 358–366. 10.1034/j.1399-3003.2000.15b23.x [DOI] [PubMed] [Google Scholar]
- Welch J. S., Ricote M., Akiyama T. E., Gonzalez F. J., Glass C. K. (2003). PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc. Natl. Acad. Sci. U S A. 100 6712–6717. 10.1073/pnas.1031789100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver R. C., Garofalo R. P., Ogra P. L. (2002). Beta-chemokines, but neither T helper type 1 nor T helper type 2 cytokines, correlate with severity of illness during respiratory syncytial virus infection. Pediatr. Infect Dis. J. 21 457–461. 10.1097/00006454-200205000-00033 [DOI] [PubMed] [Google Scholar]
- Wen Z., Song H., Ming G. L. (2017). How does Zika virus cause microcephaly? Genes Dev. 31 849–861. 10.1101/gad.298216.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh S. L., Glass J., McArthur J. C., Griffin J. W., Griffin D. E. (1994). Cytokine dysregulation in HIV-associated neurological disease. Adv. Neuroimmunol. 4 199–206. 10.1016/s0960-5428(06)80258-5 [DOI] [PubMed] [Google Scholar]
- Whelan J., Fritsche K. (2013). Linoleic acid. Adv. Nutr. 4 311–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Wang Q., Yang H. I., Ahsan H., Tsai W. Y., Wang L. Y., et al. (2008). Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis 29 971–976. 10.1093/carcin/bgn057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X., Yang S., Kowalski J., Gerritsen M. E. (1999). Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 274 9116–9121. 10.1074/jbc.274.13.9116 [DOI] [PubMed] [Google Scholar]
- Yagami T., Yamamoto Y., Koma H. (2018). Physiological and Pathological Roles of 15-Deoxy-Delta(12,14)-Prostaglandin J2 in the Central Nervous System and Neurological Diseases. Mol. Neurobiol. 55 2227–2248. 10.1007/s12035-017-0435-4 [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Hosoi M., Koyama H., Tanaka S., Fukumoto S., Morii H., et al. (2000). Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 271 571–574. 10.1006/bbrc.2000.2665 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Fukushima M., Tsurumi T., Maeno K., Nishiyama Y. (1987). Mechanism of inhibition of herpes simplex virus replication by delta 7-prostaglandin A1 and delta 12-prostaglandin J2. Biochem. Biophys. Res. Commun. 146 1425–1431. 10.1016/0006-291x(87)90809-6 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Koma H., Yagami T. (2020). 15-Deoxy-Delta(12,14)-prostaglandin J2 Inhibits Cell Migration on Renal Cell Carcinoma via Down-Regulation of Focal Adhesion Kinase Signaling. Biol. Pharm. Bull. 43 153–157. 10.1248/bpb.b19-00748 [DOI] [PubMed] [Google Scholar]
- Yen C. C., Hsiao C. D., Chen W. M., Wen Y. S., Lin Y. C., Chang T. W., et al. (2014). Cytotoxic effects of 15d-PGJ2 against osteosarcoma through ROS-mediated AKT and cell cycle inhibition. Oncotarget 5 716–725. 10.18632/oncotarget.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Seiki M. (1987). Recent advances in the molecular biology of HTLV-1: trans-activation of viral and cellular genes. Annu Rev. Immunol. 5 541–559. 10.1146/annurev.iy.05.040187.002545 [DOI] [PubMed] [Google Scholar]
- Yuan J., Takahashi A., Masumori N., Uchida K., Hisasue S., Kitamura H., et al. (2005). Ligands for peroxisome proliferator-activated receptor gamma have potent antitumor effect against human renal cell carcinoma. Urology 65 594–599. 10.1016/j.urology.2004.10.019 [DOI] [PubMed] [Google Scholar]
- Zheng J., Sariol A., Meyerholz D., Zhang Q., Abrahante Llorens J. E., Narumiya S., et al. (2020). Prostaglandin D2 signaling in dendritic cells is critical for the development of EAE. J. Autoimmun. 114:102508. 10.1016/j.jaut.2020.102508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimta A. A., Cenariu D., Irimie A., Magdo L., Nabavi S. M., Atanasov A. G., et al. (2019). The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B., Cook J. A. (2005). Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock 23 393–399. 10.1097/01.shk.0000160521.91363.88 [DOI] [PubMed] [Google Scholar]