Abstract
COVID-19 is the respiratory illness caused by the novel coronavirus, SARS-CoV-2. Cytokine storm appears to be a factor in COVID-19 mortality. Echinacea species have been used historically for immune modulation. A previous rapid review suggested that Echinacea supplementation may decrease the levels of pro-inflammatory cytokines involved in cytokine storm. The objective of the present systematic review was to identify all research that has assessed changes in levels of cytokines relevant to cytokine storm in response to administration of Echinacea supplementation. The following databases were searched: Medline (Ovid), AMED (Ovid), CINAHL (EBSCO), EMBASE (Ovid). Title and abstract screening, full text screening, and data extraction were completed in duplicate using a piloted extraction template. Risk of bias assessment was completed. Qualitative analysis was used to assess for trends in cytokine level changes. The search identified 279 unique publications. After full text screening, 105 studies met criteria for inclusion including 13 human studies, 24 animal studies, and 71 in vitro or ex vivo studies. The data suggest that Echinacea supplementation may be associated with a decrease in the pro-inflammatory cytokines IL-6, IL-8, and TNF, as well as an increase in the anti-inflammatory cytokine IL-10. The risk of bias in the included studies was generally high. While there is currently no substantive research on the therapeutic effects of Echinacea in the management of either cytokine storm or COVID-19, the present evidence related to the herb's impact on cytokine levels suggests that further research may be warranted in the form of a clinical trial involving patients with COVID-19.
Keywords: Echinacea, Herbal medicine, Cytokine, Cytokine storm, Cytokine release syndrome, COVID-19
Abbreviations: ARDS, acute respiratory distress syndrome; CCL, C–C motif ligand chemokine; COVID-19, coronavirus disease 2019; CSF, Colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; SARS, Severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TFN, tumor necrosis factor
Highlights
-
•
Modulation of the immune system has been identified as a possible management strategy in severe COVID-19.
-
•
A systematic review of all studies assessing changes in cytokine levels following Echinacea supplementation was undertaken.
-
•
Echinacea supplementation may decrease the pro-inflammatory cytokines IL-6, IL-8, and TNF.
-
•
Echinacea supplementation may increase the anti-inflammatory cytokine IL-10.
-
•
Clinical trials assessing the effectiveness of Echinacea in the treatment of cytokine storm in COVID-19 may be warranted.
1. Introduction
In early January of 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the agent responsible for coronavirus disease 2019 (COVID-19) [1]. As of June 2021, the global spread of this virus has led to a pandemic with approximately 176 million confirmed cases, including over 3.8 million deaths worldwide [2]. While the majority of COVID-19 patients experience mild to moderate flu-like symptoms (including fever, myalgia or fatigue, and dry cough), severe cases may lead to the development of complications such as acute respiratory distress syndrome (ARDS) and multiple-organ failure [3]. Current scientific literature suggests that “cytokine storm”’ is the main cause of ARDS and multiple organ failure in COVID-19 patients [4] through a pathologic process involving excessive inflammation and interference with coagulation leading to clot formation, organ tissue damage (notably in the lungs), multiple organ dysfunction syndrome, septic shock and ultimately death [1,5].
Cytokine storm, also known as cytokine release syndrome, is a phenomenon observed in response to a number of viral infections and is characterized by a rapid release of pro-inflammatory cytokines [6]. A recent literature review proposed a unified characterization of cytokine storm based on three criteria: “elevated cytokine levels, acute systemic inflammatory symptoms and secondary organ dysfunction beyond that which could be attributed to a normal response to a pathogen, if a pathogen is present'' [7]. Cytokines involved in cytokine storm include proinflammatory interleukin (IL)-6, IL-8, IL-1β, IL-12 and tumor necrosis factor (TNF), while other cytokines such as IL-10 inhibit the process through an anti-inflammatory effect [6]. When considering the role of cytokines in COVID-19 specifically, it has been observed that higher levels of IL-6, IL-8 and TNF, at the time of admission, were associated with significantly lower rates of survival after adjusting for demographics and comorbidities as confounding variables [8]. An association between higher IL-6 and IL-8 levels and increasing disease severity was also observed [8]. In another cohort of COVID-19 patients, highly impaired Interferon (IFN) type 1 response was consistent among severe and critically ill patients [9]. Decreased levels of INF-α and IFN-β were associated with ongoing elevation in blood viral load and an over-active response of pro-inflammatory modulators TNF and IL-6(9).
Given the central role of cytokine storm in the progression of severe COVID-19 cases, suppressing this immune response may be an opportunity to intervene. As such, several immunomodulatory treatments (including corticosteroids, Janus kinase (JAK) inhibitors, hydroxychloroquine, Tocilizumab and Colchicine) as well as antivirals like remdesivir and lopinavir/ritonavir have been proposed, but results have been mixed [[10], [11], [12], [13], [14]]. To date, only tocilizumab and dexamethasone have been shown to reduce mortality in severe COVID-19, while baricitinibe (a JAK inhibitor) is combination with remdesivir reduces recovery time [[15], [16], [17]]. Despite advances in treatment approach, severe COVID-19 remains challenging to treat and additional effective interventions are needed [[10], [11], [12], [13], [14]].
Herbal medicines, including species of Echinacea, have been used historically to modulate the immune system. The genus Echinacea has nine different species, with Echinacea angustifolia, Echinacea pallida and Echinacea purpurea commonly employed for medicinal purposes, notably as a treatment for various upper respiratory tract infections and inflammatory ailments [18]. Although the active constituents of the Echinacea genus are well known (e.g., polysaccharides, glycoproteins, caffeic acid derivative and alkamides), their exact mechanism of action is not well understood [[19], [20], [21]]. Nonetheless, this herbal therapy seems to be well tolerated with few adverse reactions reported [20].
Previous research indicates that the use of Echinacea may decrease the duration and severity of respiratory tract infections [18], making it a potential candidate to mitigate the symptoms of COVID-19. However, given its ability to stimulate the immune system, there are concerns that using this herb to treat COVID-19 could contribute to or exacerbate the potential for cytokine storm. Interestingly, a recent rapid literature review of clinical trials suggests that Echinacea may have the opposite effect, decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines, which may provide a therapeutic benefit in the management of COVID-19(22). As such, the objective of the present systematic review is to identify all research that has assessed changes in levels of cytokines relevant to cytokine storm in response to administration of Echinacea supplementation.
2. Methods
2.1. Search strategy and databases
The following search terms were used: (Echinacea OR Echinacea angustifolia OR Echinacea purpurea OR coneflower) AND (Cytokine* OR cytokine storm OR cytokine release syndrome OR chemokine* OR interferon* OR interleukin* OR tumour necrosis factor* OR colony-stimulating factor*). The databases searched included Medline (Ovid), AMED (Ovid), CINAHL (EBSCO), EMBASE (Ovid). The search strategy was informed by an earlier rapid review [22] and conducted on July 14, 2020. An update of the search was conducted on April 12, 2021.
2.2. Study selection
Inclusion criteria: 1) administered Echinacea, 2) reported changes in levels of cytokine relevant to cytokine storm (at least one of the following: interferon, interleukin, chemokine, tumor necrosis factor, colony-stimulating factor) and 3) experimental or observational study design, including humans or animals, in vitro/ex vivo studies, and case reports. Exclusion criteria: 1) administration of echinacea in combination with other herbal, medical or nutritional supplements, 2) Reviews, systematic reviews, commentaries, and historical articles. Abstract and full text screening was completed independently in duplicate with any disagreement resolved by consensus.
2.3. Data extraction
Data extraction was completed using piloted extraction templates for human, animal, and cell culture studies. Complete study data was extracted by one reviewer. A second reviewer independently extracted outcome data and completed risk of bias assessment in duplicate; any disagreement was resolved by consensus. Predefined outcomes of interest included: changes in chemokines, interferon, interleukin, tumor necrosis factors, and colony stimulating factors, as well as the incidence of cytokine storm. The change in cytokine level reported in each study was extracted (i.e., increase, decrease or no change in cytokine production). The predefined study characteristics that were extracted from the human studies included: author, sponsorship, study design, study population, Echinacea species, Echinacea dose and duration, control or placebo, number of participants, inclusion/exclusion criteria, change in cytokine levels and incidence of cytokine storm. The characteristics extracted from the animal studies included: author, sponsorship, animal model, infection or method immune stimulation, Echinacea species, Echinacea dose, from and standardization, control or placebo, number of subjects, change in cytokine levels, and incidence of cytokine storm. The characteristics extracted from the cell culture studies included: author, sponsorship, cell or tissue culture, infection or method immune stimulation, Echinacea species, Echinacea dose, form and standardization, duration, control or placebo, change in cytokine levels, and incidence of cytokine storm.
2.4. Risk of bias assessment
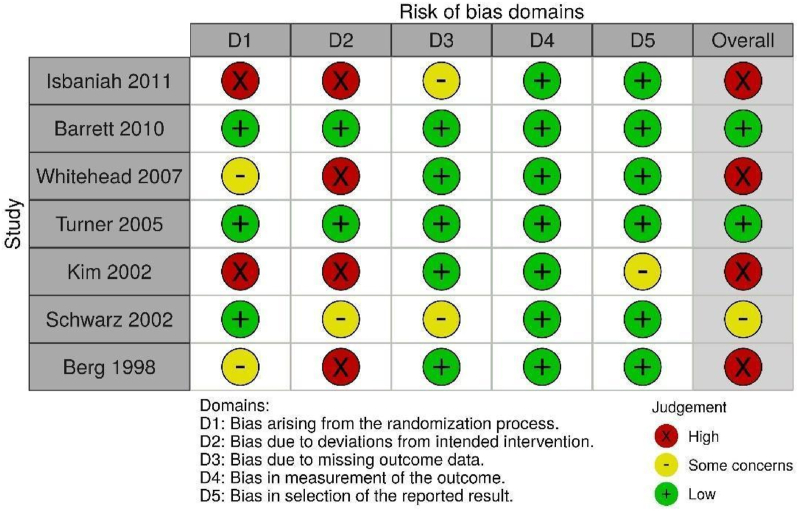
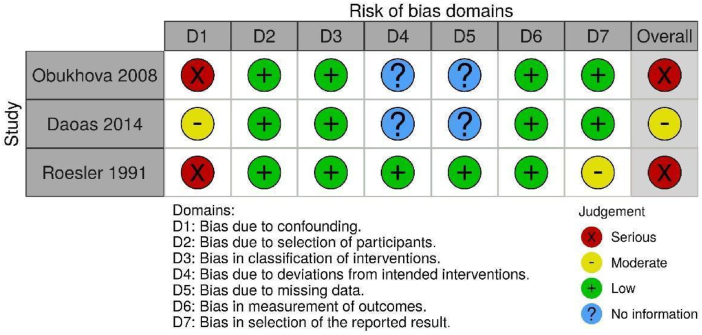
Risk of bias assessment was completed using the following tools: Cochrane Risk of Bias 2.0 (randomized clinical trials) [23], ROBINS-I (non-randomized trials) [24], NIH Quality Assessment Tool (pre-post studies with no control group) [25], OHAT (animal studies) [26], and ToxRtool (in vitro studies) [27].
2.5. Data analysis
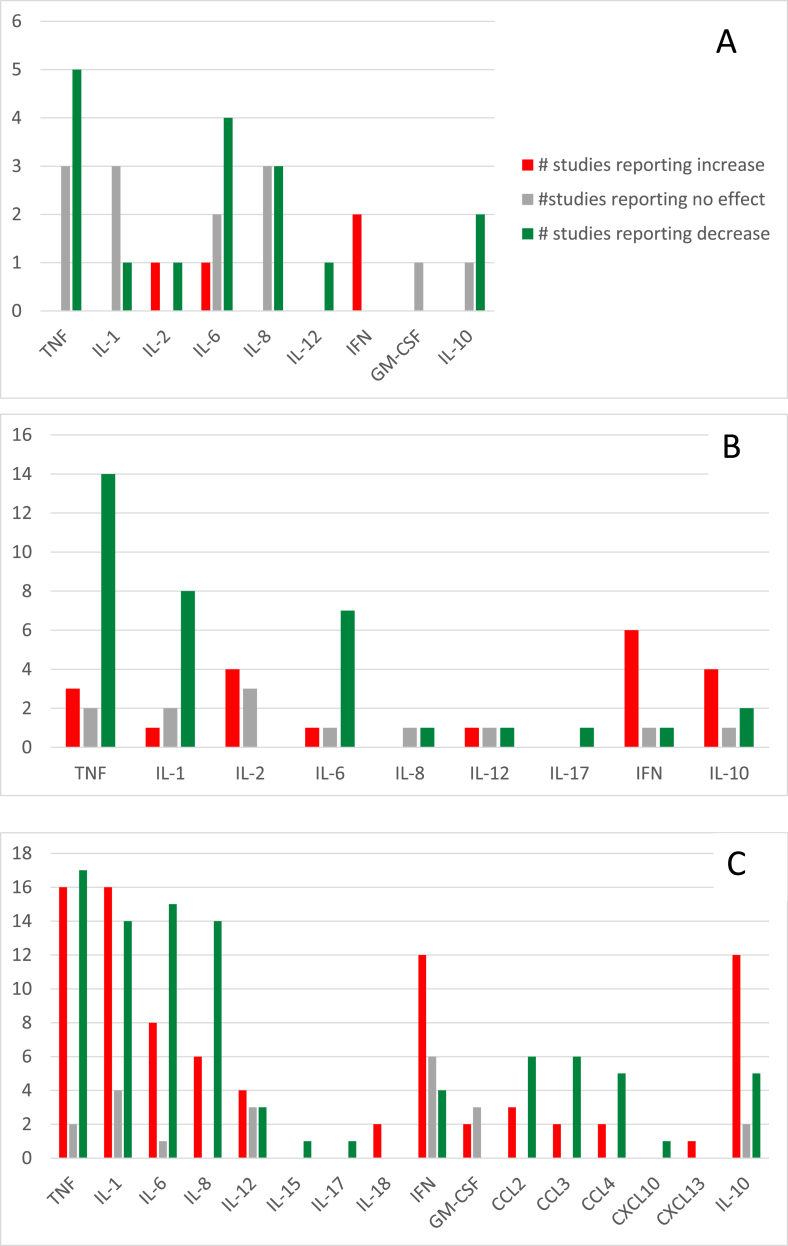
Studies were grouped based on methodology. The number of studies reporting increases, decreases or no change in each cytokine were counted and presented in figures to assess for trends visually. Statistical pooling was not feasible due to a qualitative assessment of heterogeneity made by the author team.
3. Results
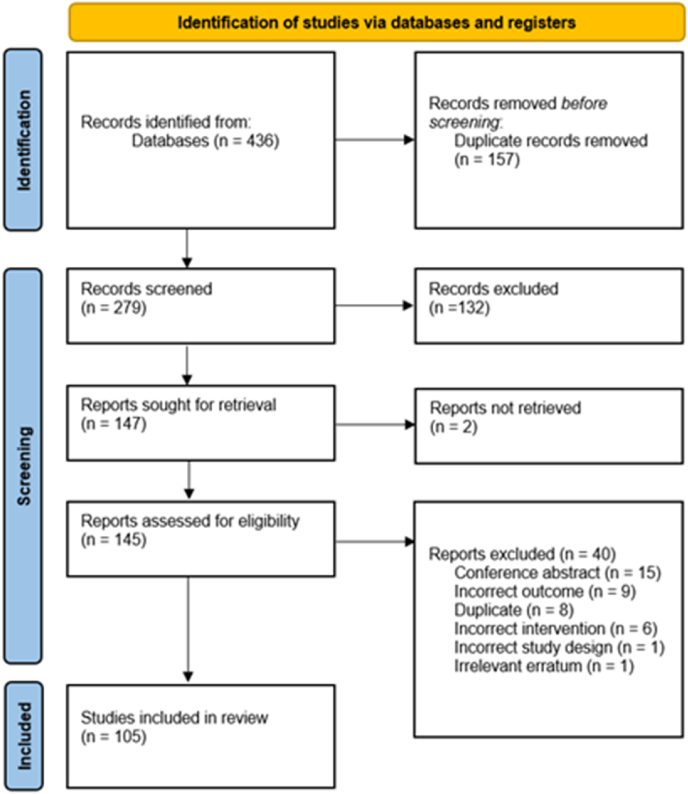
Of the 436 records identified, 105 studies met criteria for inclusion in the present systematic review (Fig. 1). Excluded studies are listed in Supplemental File 1. Of the 13 studies involving human participants, seven were randomized clinical trials [[28], [29], [30], [31], [32], [33], [34]], three were non-randomized trials [[35], [36], [37]] and three were pre/post uncontrolled trials [[38], [39], [40]]. Twenty-four studies reported outcomes related to animal experiments [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]] and 69 studies reported outcomes related to in vitro or ex vivo studies [39,[64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131]]. Table 1, Table 2, Table 3 present the characteristics and results of the human, animal and in vitro/ex vivo studies respectfully.
Fig. 1.
PRISMA flow diagram of included studies.
Table 1.
Characteristics of the human studies included.
| Author | Sponsorship | Design | Study Population | Echinacea Spp | Dose and Duration of Treatment | Control or Placebo | Number of participants in analysis | Inclusion/Exclusion criteria | Change in Cytokine Levels |
|---|---|---|---|---|---|---|---|---|---|
| Barrett 2010 [26] | National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (NIH). | Placebo controlled RCT (4 arms) | People 12–80 years of age, with new-onset common cold | E. purpurea and E. angustifolia root extracts | Four doses of 2 tablets within 24 h of enrollment (10.2 g of dried echinacea root). Followed by one tablet four times per day (5.1 g per day) for 4 days. 1 tablet = 675 mg of E. purpurea and 600 mg E.angustifolia, each standardized to 2.1 mg of alkamides. DURATION: 5 days |
Visually matched placebo containing identical amounts of excipients (calcium acid phosphate, cellulose, silica, sodium starch glycollate, Hypromellose and magnesium stearate) | TOTAL: 713 INTERVENTION: 183 blinded & 181 unblinded PLACEBO: 173 unblinded & 176 blinded |
INCLUSION: At least 1 of 4 common cold symptoms (nasal discharge, nasal obstruction, sneezing, or sore throat) and a score of 2 or higher on Jackson criteria. EXCLUSION: Use of antibiotics, antivirals, nasal steroids, decongestants, antihistamines, combination cold formulas, echinacea, zinc or vitamin C. History of allergic rhinitis and/or asthma. People with autoimmune/immune deficiency disease and pregnant women. |
-Non statistically significant rise in mean nasal rinse IL-8 levels in both echinacea groups compared to placebo. |
| Isbaniah, 2011 [27] | Frutarom Switzerland Ltd. |
Double-blind, placebo controlled RCT (3 arms) | COPD outpatients 40–81 years of age (mean age of 65.8) | E. purpurea from dried pressed juice of the aerial parts of the plant | 500 mg of ciprofloxacin twice a day for 7 days and either tablets with 1) 500 mg E. purpurea or 2) 500 mg of E. purpurea with 10 mg zinc, 15 μg selenium and 50 mg ascorbic acid (EP+) once a day. DURATION: 14 days |
Composition not stated | TOTAL: 108 INTERVENTION: 36 Echinacea only & 37 Echinacea with zinc, selenium and ascorbic acid PLACEBO: 35 |
INCLUSION: COPD outpatients 40+ years of age with an acute exacerbation episode (non-gradual increase in at least one major symptom: dyspnoea, sputum production and sputum purulence). EXCLUSION: History of asthma, severe immune system disorder, malignancy or haematologic disorder, obstructive pulmonary disease caused by other reasons or any other disease with known impact on COPD recovery. Increase of >/ = 12% of the pulmonary function after using a bronchodilator; severe clinical symptoms in addition to cor pulmonale and heart failure, utilization of extra respiratory muscles, and oxygen dependence (scale IV); requirement for treatment anti-inflammatory drugs; pregnancy or lactation; hypersensitivity to Echinacea or ciprofloxacin. |
-No statistically significant change in IL, IL-10 or TNF-α serum concentration for echinacea only group compared to placebo. -IL1-β serum concentration significantly increased in both the echinacea only and placebo group (no difference between groups). |
| Turner, 2005 [28] | Supported by a grant (R01 AT001146) from the National Center for Complimentary and Alternative Medicine of the NIH | Double-blind, placebo controlled RCT (7 arms) | Healthy young adult (age 20.8 ± 3.3) volunteers exposed to rhinovirus experimentally |
E. angustifolia root extract tincture extracted with either 1) supercritical CO2, 2) 60% ethanol or, 3) 20% ethanol |
Dose: 1.5 mL of tincture containing 300 mg of echinacea extract three times a day. Two phases: 1) Prophylaxis - 7 days before viral challenge 2) Treatment- 5 days after viral challenge. Seven interventions: 1) One of three echinacea preparations during both prophylaxis and treatment 2) Placebo during prophylaxis and an echinacea preparation during treatment 3) Placebo during both prophylaxis and treatment. DURATION: 12 days |
Mixture of alcoholic beverages, denatonium benzoate and tap water | TOTAL: 399 INTERVENTION: 48-52 per arm PLACEBO: 103 |
INCLUSION: Healthy young adults, susceptible to rhinovirus type 39 (based on antibody testing). EXCLUSION: Existing antibodies to test virus at screening or at day zero. |
-Prophylaxis and/or treatment with three different echinacea preparations did not have a statistically significant effect on IL-8 in nasal lavage in response to infection when compared to placebo. |
| Kim, 2002 [29] | Celestial Seasonings inc, Larex inc, Lee Dexter and associates | Double-blind, placebo controlled RCT (6 intervention arms) | Healthy female volunteers 22–51 years of age (mean age 36.7) | E. purpurea whole herb extract (4% phenols), ultra-refined E. purpurea whole herb, E. angustifolia root, E. purpurea whole herb | Two capsules twice per day for a daily total of either: 1) 1500 mg of E. purpurea with 4% phenols (EP); 2) 780 mg of E. purpurea (4% phenols) and 680 mg of ultra-refined E. purpurea and E. angustifolia (urEPA); 3) 908 mg of E. purpurea (4% phenols), 464 mg of E. purpurea, and 36 mg of E. angustifolia (EPA); 4) 908 mg of E. purpurea (4% phenols), 464 mg of E. purpurea, 46 mg of E. angustifolia and 1500 mg of larch arabinogalactan; 5) 1500 g of larch arabinogalactan. DURATION: 28 days |
Alfalfa and rice capsules matching in colour, size and taste. | TOTAL: 46 INTERVENTION: 8 per arm PLACEBO: 8 |
INCLUSION: Healthy adult females EXCLUSION: Major illness: cancer, diabetes, cardiovascular, autoimmune/immune diseases. Acute illness at enrollment/during study period including upper respiratory tract infections and sinusitis. Taking immune enhancing/altering supplements or medications. |
-Statistically significant (p = 0.040) decrease in TNF-α serum concentration after 4-weeks of intervention in urEPA group. -No significant (p>0.05) decreases in TNF-α levels in groups taking EP, EPA or placebo. |
| Whitehead, 2007 [30] | Unlear | Double-blind, placebo controlled Randomized/matched trial | Healthy male volunteers, 24.9 ± 4.2 years of age, with 19.3% ± 6.5% body fat | E. purpurea extract from the aerial parts of the plant - Puritan's Pride® | Five 400 mg E. purpurea capsules four times per day for a total daily intake of 8 g per day. Daily multivitamin. DURATION: 28 days |
Wheat flour and a multivitamin | TOTAL: 24 INTERVENTION: 12 PLACEBO: 12 |
INCLUSION: Healthy male students, age 18–30, deemed recreationally active (i.e., ≥30 min of physical activity 3 days/week). EXCLUSION: Taking medications, using dietary supplements or any form of tobacco, any sign/symptom of cardiovascular or metabolic diseases. |
-IL-3 serum concentration increased significantly (p = 0.011) at day 14 (65% increase from baseline) and 21 (73% increase from baseline) in the Echinacea group compared to placebo group. -No significant changes in Granulocyte-macrophage CSF levels between echinacea and placebo groups. |
| Schwartz, 2002 [31] | Grants from Shaper & Bruemmer and two of the authors (C. Bode and J. C. Bode) | Double-blind, placebo controlled crossover RCT | Healthy male volunteers 28 ± 5.8 years of age, with a body mass index of 22.9 ± 2.1 | E. purpurea, freshly expressed juice; identical to commercially available ESBERITOX™ mono | Unspecified amount of either juice or placebo two times per day for 14 days; 4-week washout period followed by 14 days of opposite intervention. DURATION: 14 days |
Ethanol, water solution with artificial color and flavour mimicking Echinacea juice. | TOTAL: 40 INTERVENTION: 40 PLACEBO: 40 |
INCLUSION: Healthy men, 20–40 years old. EXCLUSION: Acute or chronic disease, known atopic diathesis, acute infection one month prior to the study, obesity (BMI >28), immunomodulating drugs (NSAIDs, smoking, excessive alcohol consumption). |
-No statistically significant change in production of IL-1β from isolated blood monocytes. -TNF-α production of monocytes cultured with LPS did not differ between intervention and control groups (40 pg/mL detection limit). |
| Berg 1998 | Unclear | Double-blind, placebo controlled RCT (3 intervention arms) | Healthy male triathletes 27.5 ± 5.3 years of age, with VO2 max>52mL/kg/min, undergoing regular training for triathlon sprint competition (mean 4.3 years) | E. purpurea pressed juice (Echniacin) | The following medications were taken daily, in three divided doses at meal times: 1) 8 mL of pressed echinacea juice (final concentration of 80 g in 22% ethanol) plus 12 flavoured placebo tablets or; 2)12 Magnesium tablets and 8 mL of flavoured 22% ethanol or; 3)12 flavoured tablets and 8 mL of flavoured 22% ethanol. DURATION: 28 days (prior to triathlon sprint competition) |
Flavoured tablets and 120 drops (8 mL) flavoured 22% ethanol. Note: Magnesium group served as “a reference for supplementation with a nutrient required for optimal muscular function”. Each tablet contained 265 mg Mg (HPO4) 2*3H2O and 6 g Mg (hydrogen citrate) 2*3H2O |
TOTAL: 40 INTERVENTION: 14 Echinacin 13 Magnesium PLACEBO: 13 |
INCLUSION: Male triathletes, 18–47 years old, free from any infection 2 weeks prior to the start of the study. EXCLUSION: Treatment with vitamin E (>200 mg/day) or other antioxidants, fish oil products, regular laxatives, tonics, corticosteroids, immunosuppressants, lipid lowering agents or anticoagulant drugs, and excessive alcohol use. |
-All groups experienced a decrease in urine and serum sIL-2R and IL-6 1 h after the competition. After 24 h sIL-2R concentration remained low while IL-6 concentration returned to baseline. -Statistically significant (p < 0.05) decrease in serum IL-2R 1 h and 20 h after the competition in the Echinacin group compared to placebo. -Treatment with Echinacin resulted in a significantly more pronounced increase in urine IL-6 1 h after the competition, compared to placebo. |
| Obukhova, 2008 [32] | Unclear | Non-randomized, controlled, intervention study | Patients with clinical remission of chronic herpes infection, 17–52 years of age | Plant preparation of 60% E. purpurea and 40% E. pallida extracts (phytomicropheres). | Two echinacea capsules (unspecified amount) during day one (morning and evening). Then one capsule per day for four days. DURATION: 5 days |
Patients with clinical remission of chronic herpes infection that did not receive Echinacea immune-corrective therapy. | TOTAL: 52 INTERVENTION: 38 CONTROL: 14 |
INCLUSION: Patients with clinical remission of chronic herpes infection (defined as absence of chronic inflammation at least one month before the trial). EXCLUSION: none included. |
-IFN-γ, IL-1β and IL-6 plasma concentrations at baseline were above normal in the intervention and control groups (p < 0.05). -IFN-γ concentration in the intervention group increased significantly (p < 0.05) on day 7 post-treatment and continued to increase progressively on days 14 and 21 exceeding levels before and 7 days after therapy (p < 0.01 and p < 0.05, respectively). There were no statistically significant changes in IFN-γ plasma concentration in the control group. -IL-1β plasma concentration in the intervention group decreased significantly (p < 0.05) on day 7 post-treatment, then increased slightly (without exceeding pre-treatment levels) on days 14 and 21 post-treatment. There were no statistically significant changes in IL-1β plasma concentration in the control group. -IL-6 plasma concentration in patients of the treatment group decreased significantly (p < 0.05) on day 7 post-treatment, then increased back to baseline levels on day 14, and increased further on day 21 post treatment (p < 0.05). There were no statistically significant changes in IL-6 plasma concentration in the control group. |
| Roesler, 1991 [33] | Unclear | Non-randomized, controlled intervention study | Healthy volunteers 20–45 years of age | E. purpurea polysaccharides purified from large-scale cell cultures | Injection containing 5 mg of E. purpurea polysaccharides (2:1 xyloglucanes, arabinogalactane mixture). DURATION: Single dose |
0.9% NaCL | TOTAL: 10 INTERVENTION: 5 CONTROL: 5 |
INCLUSION: negative history of allergies, autoimmune diseases, and severe diseases. EXCLUSION: none included. |
-No statistically significant changes in IL1-β, IL-6, TNF-α or neopterin concentrations in serum and plasma between the echinacea and placebo groups. |
| Dapas, 2014 [34] | Italian Minister of Instruction, University and Research (MIUR), PRIN 2010, number 20109PLMH2. | Interrupted time series study (before-after study with control baseline). | Healthy adults (age 26–53) of both genders | E. angustifolia dry root extract (triple standardized extract syrup Polinacea®) | 10 mL of syrup once a day (between meals) containing 100 mg of Polinacea (4.7 mg of echinacoside and 8.0 mg of high molecular weight polysaccharides). DURATION: 28 days |
N/A | TOTAL: 10 INTERVENTION: 10 CONTROL: N/A |
INCLUSION: Healthy individuals with normal liver function. No medicines taken one week before or during the study. Fasting at baseline. EXCLUSION: Smoking, dietary restrictions, allergy to Compositae or Grossulariacee plants. |
-Statistically significant (p < 0.05) increase in IL-2 and decrease in IL-6 plasma concentrations post intervention. Non-statistically significant change in IL-8 (p = 0.08) and TNF-α (p = 0.58) plasma concentrations post intervention compared to baseline. -Statistically significant (p < 0.05) downregulation of TNF-α mRNA in circulating lymphocytes post intervention. |
| Guiotto, 2008 [35] | DALCO s.r.l. and the Region Friuli Venezia Giulia |
Single blind crossover study (3 arms, no control group) | Healthy individuals of both genders | E. purpurea dry root extract | One lozenge (3 g) after overnight fasting containing glucose syrup, crystalline sugar and 100 mg of dry E. purpurea extract with either 0.7 mg, 0.21 mg or 0.9 mg of dodeca-2E,4E,8Z,10E/Z-tetraenoic isobutylamides. Doses were administered in increasing order with a 2-week washout period between them. DURATION: Single dose |
N/A | TOTAL: 6 INTERVENTION: 6 CONTROL: N/A |
INCLUSION: Healthy individuals. Abstinence from smoking, eating and drinking (only water allowed) starting 12 h before treatment and culminating 2 h post treatment. No medicine to be taken from one week before to the end of the study except for oral contraceptives. EXCLUSION: Dietary restrictions |
-All three dose quantities led to a statistically significant (p < 0.05) decrease in IL-12p70, IL-8 and IL-6 plasma concentration 24 h post-intervention compared to baseline. The two larger doses also led to statistically significant decreases in IL 10 and TNF-α (p < 0.05), however the smallest dose did not (p = 0.059). 24 h after intervention the level of TNF-α was approximately 61% of the pre-treatment value, 68% for IL-6, 64% for IL-8, 73% for IL-10 and 76% for IL1-2p70. |
| Dall'Acqua, 2015 [36] | Farmaderbe, Pradamano (Udine) and Indena S.p.A. (Milan, Italy) | Single blind, before-after study without control group | Healthy adults (age 26–53) of both genders | E. angustifolia lipophilic root extract -Echinamid ® | One soft gel capsule (10 mg) after overnight fasting containing 1 mg of dodeca-2E,4E,8Z,10E/Z-tetraenoic isobutylamides, gelatin, glycerin, titanium dioxide, and iron oxide yellow. DURATION: Single dose |
N/A | TOTAL: 10 INTERVENTION: 10 CONTROL: N/A |
INCLUSION: Healthy individuals with normal liver function. Abstinence from smoking, eating and drinking (only water allowed) starting 12 h before treatment. No medicines to be taken during the study. EXCLUSION: Dietary restrictions, allergy or sensitivity to Compositae or Grossulariacee plants. |
-Statistically significant (p < 0.05) decrease in IL-2, IL-6, IL-8, IL-10 and TNF-α plasma concentration 24 h post-intervention. -Statistically significant (p < 0.05) decrease in IL-2, IL-6, IL-8 and TNF-α mRNA/28S levels (measured via real time PCR). -Statistically significant (p < 0.05) increase in IL-10 mRNA levels. |
| Randolph, 2003 [37] | Unclear | Open label, before-after study without control group | Healthy adults (age 18–65) of both genders, weighing 55–79 kg. | E. purpurea (root and aerial parts) and E. angustifolia root extracts (NUTRILITE Triple Guard® Echinacea tablets) | Three tablets, three times daily (1518 mg/day) for two days, plus three tablets on day three (506 mg/day). 1 tablet = 252 mg of E. purpurea (aerial parts), 16 mg of E. purpurea (root), 12 mg of E. angustifolia (root) and 33 mg of Citrus Bioflavonoid (Citrus limon, C. paradisi, C. reticulate x, C. sinesis) DURATION: 2.5 days |
N/A | TOTAL: 6 INTERVENTION: 6 CONTROL: N/A |
INCLUSION: Adults (age 18–65), non-smoking, normally active, good health based on interview and physical examination. EXCLUSION: Smoking. |
-Gene expression of IFN-α2 increased steadily through day 12 post-intervention in all subjects achieving statistical significance (p = 0.02) on day 12 (compared to baseline). -Small (non-statistically significant) down-regulation of IL-1β and IL-8 gene expression in some but not all subjects. -Small down-regulation in TNF-α gene expression in some but not all subjects. The magnitude of this downregulation achieved statistical significance (p = 0.04) on day 5 post-intervention but reverted toward baseline levels by day 12. |
COPD: Chronic Obstructive Pulmonary Disease; EP; Echinacea purpurea; g: Grams; IFN: Interferon; IL: Interleukin; kg: Kilograms; mg: Milligrams; ml: Millilitres; NaCl: Sodium Chloride; NSAID: Nonsteroidal Anti-Inflammatory Drugs; RCT: Randomized controlled trial; TNF: Tumour Necrosis Factor; ug: Microgram.
Table 2.
Characteristics of the animal studies included.
| Author | Sponsorship | Animal Model | Infection or immune stimulation | Echinacea Spp or individual constituent | Dose, form, standardization | Control or Placebo formula used | Total Number of Subjects | Change cytokine levels |
|---|---|---|---|---|---|---|---|---|
| Abdelmonem, 2015 [38] | No financial support | Male Wistar rats, weighing 170 ± 20 g | Subcutaneous injection of isoprenaline (85 mg/kg) for 2 successive days (infarct-like myocardial lesion) |
E. purpurea |
E. purpurea (130 mg/kg) DURATION: 28 days |
saline with no treatment; Isopropaline with no treatment | TOTAL: 84 INTERVENTION: 12 PLACEBO: 24 |
-no statistically significant change in IL-8 levels |
| Abdallah, 2015 [39] | Unspecified | Adult Sprague-Dawley rats, weighing 125–150 g | 3 days of cyclophosphamide injection of 50 mg/kg/day | E. purpurea suspension cultures | Either 100 mg/kg or 200mg/kg oral dose of E. purpurea suspension cultures DURATION: 21 days |
10 mg/kg of normal saline orally | TOTAL: 24 INTERVENTION: 6 per group (12 total) CONTROL: 6 saline only; 6 cyclophosphamide |
-IL-1 statistically significant decrease in 200 mg/kg group -Statistically significant dose-dependent decrease in TNF-α |
| Abdel Rahman, 2018 [40] | No financial support | Nile Tilapia, 65–91 g | None | Dry extract of E.purpurea | 500 mg E.purpurea /kg twice daily DURATION: 28 days |
Basal diet | TOTAL 120 INTERVENTION: 30 in E.purpurea group (remaining animals received other herbs) PLACEBO: 10 |
-No difference in IL-1β expression -Statistically significant decrease in TNF-α expression in head kidney but not intestine |
| Cundell, 2003 [41] | Philadelphia University | Male Sprague- Dawley rats, 12 months of age | None | E. purpurea extract from aerial parts | 1.05 g E. purpurea, 10.5 mg cichoric acid combined with gelatin and water for a total daily intake of 50 mg/kg of Echinacea and 0.5 mg/kg cichoric acid). DURATION: 8 weeks |
Peanut butter | TOTAL: 16 INTERVENTION: 8 PLACEBO: 8 |
-increase in circulating IL-2 levels during weeks 4–5 |
| Dogan, 2014 [42] | No financial support | Male Wistar-Albino rats, weighing 200–250 g | Acute colitis induced by 4% acetic acid | 100 mg E. angustifolia & 400 mg E. purpurea | 50 mg/kg of Echinacea per day using a catheter to rats DURATION: 14 days |
Either acetic acid and saline or no acetic acid and no treatment | TOTAL: 20 INTERVENTION: 5 per group (colitis; no colitis) PLACEBO: 5 per group (colitis; no colitis) |
-significantly decreased IL-1β (p < 0.007) -significantly decreased TNF-α p < 0.001) |
| Fusco, 2010 [43] |
Weill Cornell Medical College Clinical and Translational Science Center (NIH), Stony-Wold Herbert Fund, National Center for Complementary & Alternative Medicine | Female C57BL6 mice, 6–8 weeks of age, 15–20 g | Influenza A/WSN/33 (H1N1) strain | E. purpurea Ethanol extracts freeze-dried to powder form | 10 mg (100 μl of stock solution) administered to mice daily by gavage DURATION: 5 days |
PBS | TOTAL: 59 INTERVENTION: 15 PLACEBO: 34 |
-Statistically significantly lower IFN-γ in serum (p-0.01), not lung (p = 0.3) -Statistically significantly lower IL-10 in serum and lung, decreased IL-5 and IL-12 on day 3, no statistically significant diff in IL-1β, IL-2, IL-4 -TNF-α No statistically significant diff |
| Ghaemi, 2009 [44] | Unspecified | Female BALB/c mice, 4–5 weeks of age, with an average weight of 20 g. | Live KOS strain of HSV-1 on Day 0 and 21 | E. purpurea extract, concentration of 20 mg/mL | 100 g of E. purpurea extract E. purpurea extract E. purpurea extract E. purpurea extract DURATION: 28 days |
PBS inoculation or HSV-1 only | TOTAL: 30 INTERVENTION: 10 PLACEBO: 20 |
-increased IFN-γ (p-value not reported) |
| Goel, 2002 [45] | Unspecified | Male Sprague Dawley rats weighing 425–475 g | LPS | Cichoric acid, polysaccharide and alkylamide fractions | Group B: 40mcg/kg/day of Cichoric acid, 1000mcg/kg/day polysaccharide and 4mcg/kg/day alkylamide as oral gavage twice a day. Groups C, D & E got 3, 20 & 50 times this amount. DURATION: 4 days |
50% ethanol | TOTAL: 30 INTERVENTION: 24 PLACEBO: 6 |
-Statistically significant increase IFN-γ (p < 0.05) at highest dose (50 times the extract level) -No effect on IL2- release -Statistically significant increase in TNF-α production at higher doses (50 times the extract level) (p < 0.05). |
| Goel, 2002 [46] | Unspecified | Male Sprague–Dawley rats, weighing 225–275 g | LPS | Cichoric acid, polysaccharide and alkylamide fractions | Oral gavage twice a day for 4 days of either: 1) cichoric acid (5–120mg/kg/day); 2) polysaccharides (125–3000mg/kg/day); or 3) alkylamides (0.5–12mg/kg/day) DURATION: 4 days |
50% ethanol | TOTAL: 60 INTERVENTION: 54 PLACEBO: 6 |
-No Statistically significant effect on the release of IFN-γ by the rat splenocytes was observed -No statistically significant effect from any extract on IL-2 -Statistically significant increase in TNF-α production after exposure to polysaccharide and alkylamide (p < 0.05) but not cichoric acid |
| Hayashi, 2001 [47] | No financial support. The E. purpurea preparation was donated by API Companey, Gifu, Japan. | Female AKR/J mice, 3–4 weeks of age | Thymic injection of recombinant Leukemia Viruses from thymuses inducing leukemia | 70% ethanol extract from partially purified powder from the leaves of E. purpurea | Oral 0.25 mg/ml EP suspended in PBS 3 times per week for 8 weeks amounting to 75mg/kg/week. DURATION: 24 weeks |
Oral PBS | TOTAL: 20 INTERVENTION: 10 PLACEBO: 10 |
-Production of IFN-γ in the peritoneal exudate increased. No p-value reported -Modest production of IL-12, no p-value reported -Modest production of TNF-α, no p-value reported |
| Jiang, 2014 [48] | Key Nature Science Foundation for Colleges and Universities of Anhui Province of China and Anhui Agricultural University | Male Sprague Dawley rats, 160–200 g | Collagen-induced arthritis | Cichoric acid extract | Either 8, 16, or 32 mg/kg/day orally DURATION: 28 days |
Tripterygium glycosides tablet (10 mg/kg/day) | TOTAL: 60 INTERVENTION: 10 per group (30 total) PLACEBO: 30 |
-Statistically significant reduction in IL-1β in serum (p < 0.01) -Statistically significant reduction of TNF-α in serum for all doses, only 32 mg/kg reduced in synovium |
| Liu, 2012 [49] | National Science Foundation of China, China National “863″ program | Kunming mice (weighing 14–16 g) and dogs (weighing 5–8 kg, 3–4 months of age) | Rabies vaccine | Echinacea polysaccharide containing 80% glucose | Injection of polysaccharides added to vaccine at 2 mg/mL for mice and 10mg/mL for dogs DURATION: 14 days for mice, 6 months for dogs |
vaccine without polysaccharides | TOTAL: 250 mice and 30 dogs INTERVENTION: 50 mice per group (150 total), 6 dogs per group (24 total) PLACEBO: 50 control mice, 6 control dogs |
-Statistically significant increase in IFN-γ response. Statistically significant increase in IFN-α (p < 0.05). -Enhanced release of cytokines within 1 day after inoculation. Includes IL-1β, IL-5 and IL-6. Statistically significantly higher than those in the control group (p < 0.05). |
| Liu, 2017 [50] | National Key Research and Development Program of China, National Natural Science Foundation of China, Scientific Startup Funds for Doctors of Northwest Agriculture and Forestry University | C57BL/6J mice, 3 months of age | 0.25mg/kg/day LPS injection | Chicoric acid | 0.05% Chicoric acid in drinking water DURATION: 54 days |
Healthy control or LPS-induced | TOTAL: 30 INTERVENTION: 10 PLACEBO: 10 per group (20 total) |
-serum IL-1β inhibited, and suppressed upregulation of L-6, IL-1β mRNA, but promoted IL-10 mRNA expression -serum TNF-α inhibited and suppressed upregulation of its mRNA expression |
| Li, 2020 [51] | Key Research and Discovery Program of Shandong Province, National Natural Science Foundation of China, High-Level Talent Research Foundation of Qingdao, Agricultural University, China, Chinese Herbal Medicine Industry Innovation Team of Shandong Province, Agricultural Technology System. | Male BALB/C mice (6–8 weeks old) | LPS induced Immune stimulation | E. purpurea aerial parts | 50 mg per g IP injection of polysaccharides (30 min before LPS injection). DURATION: 8 h |
Saline | TOTAL: 18 INTERVENTION: 6 CONTROL: 6 LPS only, 6 saline only |
-Statistically significant decreased secretion of IL-6 and TNF-α (p < 0.05) -Statistically significant increased secretion of IL-10 (p < 0.05) |
| Park, 2018 [52] | Frutarom, Switzerland; Novarex, Republic of Korea; and Program for Industrial Needs - Matched Education (PRIME), Ewha Womans University funded by the Ministry of Education of Korea | Male BALB/c mice, 6 weeks of age, weighing 18–20 g | Restraint-induced immunosuppression | Cold pressed E. purpurea juice with extract ratio of 40–50:1 |
E. purpurea at doses of 10, 30, and 100 mg/kg of body weight DURATION: 2 weeks |
0.9% saline | TOTAL: 70 INTERVENTION: 14 per group (42 total) CONTROL: 0.9% saline |
-Statistically significant reduction of IL-6, IL-10, and IL-17 and downregulated their mRNA expression (p < 0.05, p < 0.01, and p < 0.01, respectively) |
| Sgorlon, 2016 [53] | Nutrigene S.r.l. from the University of Udine, Italy | Medium to large sized dogs >2 years of age | None | E. angustifolia | 2% extract at 5 mg/kg daily DURATION: 60 days |
Food without nutraceuticals | TOTAL: 74 INTERVENTION: 14 in Echinacea group CONTROL: 21 |
-Statistically significant up regulation of CXCL8 expression (p < 0.01) -Statistically significant down regulation of TNF-α (p < 0.05) |
| Shi, 2020 [54] | National Natural Science Foundation of China, Third Batch of Giant Project of Hebei Province, Top Talent Project for Youths of Hebei Province, Doctoral Startup Foundation of Hebei Normal University of Science and Technology, High School Hundred Excellent Innovation Talent Program of Hebei Province, Natural Science Foundation of Hebei Province, Project of Department of Science and Technology of Hebei Province | Male c57BL/6 mice (8-week-old, 20 g) | LPS induced Immune stimulation | E. purpurea (90.26% purity) | 5 or 10 mg per kg, with or without LPS DURATION: 1 day |
No treatment | TOTAL: 30 INTERVENTION: 18 CONTROL: 6 no treatment, 6 LPS only |
-Statistically significant downregulation of IL-1β, IL-6, and TNF-α |
| Sutovska, 2015 [55] | BioMed, Slovak GrantAgency VEGA, APVV agency, MZ | Adult male Trik strain guinea pigs, weighing 200–350 g | Ovalbumin exposure causing allergic airway inflammation | E. purpurea extract | Oral Echinacea complex (50 mg/kg) DURATION: 14 days |
Either 1) saline, 2) salbutamol, 3) budesonide, or 4) healthy controls | TOTAL: 50 INTERVENTION: 10 PLACEBO: 40 |
-Statistically significant decrease in IL-4, IL-5, IL-13 in both bronchoalveolar lavage fluid and serum -Statistically significant decrease in TNF-α in both bronchoalveolar lavage fluid and serum (p < 0.001) |
| Turkistani, 2019 [56] | Unspecified | Male rats Sprague Dawley (180–210 g) | CISP induced renal toxicity | E. purpurea root liquid extract | Oral E. purpurea with 500 mg/kg/day for four weeks, on the day 21st received a single IP injection of CISP DURATION: 4 weeks |
No treatment or CISP only | TOTAL: 40 INTERVENTION: 10 EP only, 10 EP + CISP CONTROL: 20 |
-Statistically significant increase in IL-10 (p < 0.001) -Statistically significant decrease in TNF-α (p < 0.001) |
| Uluisik, 2012 [57] | The Scientific Research Projects Coordination Unit of Selcuk University | Male Fisher rats, 6 weeks of age | None | E. purpurea root powder | Pellets with 0.75 g/kg of E. purpurea root powder DURATION: 40 days |
Standard rat pellets | TOTAL: 48 INTERVENTION: 16 echinacea echinacea CONTROL: 16 control |
-No Statistically significant diff in IL-10 mRNA expression -TNF-α mRNA expression Statistically significant higher than control on 20th day but not 40th day |
| Yamada, 2011 [58] | Unspecified | Male Sprague Dawley rats, 4 weeks of age | ConA mitogen | Ethanol extracts of E. purpurea | 10 g of Echinacea, per kg of rat feed DURATION: 4 weeks 4 weeks |
Experimental diet without herb | TOTAL: 40 INTERVENTION: 30 PLACEBO: 10 |
-Statistically significant increase in IFN- γ secretion -IL-2: Statistically significantly increased production; IL-4 Statistically significantly increased production (with ConA immune stimulation only); IL-6 Statistically significantly decreased (with ConA immune stimulation only) -Significant decrease in TNF-α production |
| Yu, 2013 [59] | Key National Sciences Foundation of Colleges and Universities, Anhui Province | Male Kunming mice weighing 18–22 g, male Wistar rats weighing 180–220 g | Xylene induced ear edema on mice, or egg albumin induced paw edema on rats, or cotton-induced granuloma on rats | E. purpurea essential oil | 2.5 g, 5 g or 10 g of crude drug/kg/kgg/kg DURATION: 7 days |
33 mg aspirin or saline | TOTAL: 120 rats (60 per type of infection) and 60 mice INTERVENTION: 10 per dosage group (90 total) CONTROL: 10 normal control, 10 model control, 10 aspirin (90 total) |
-IL-6 levels were Statistically significantly reduced in the low dose group (p < 0.05). In the high dose group, IL-2 levels were increased (p < 0.05). -TNF-α statistically significant reduced at high dose (p < 0.05). |
| Zhai, 2007 [60] | National Institute of Environmental Health Sciences, Office of Dietary Supplements, National Institutes of Health | Male BALB/c mice, 8 weeks of age | Mitogen stimulation | Ethanol extracts from the dried roots of E. angustifolia, E. pallida, and E. purpurea | Oral gavage of 130 mg/kg of body weight once daily DURATION: 7 days |
5% ethanol gavage | TOTAL: Not reported INTERVENTION: Not reported CONTROL: Not reported |
-Statistically significantly increased IFN-γ production (p < 0.035) -All 3 preparations inhibited the release of IL-1β (p = 0.007). Only E. angustifolia and E. pallida-treated mice demonstrated statistically significantly higher production of IL-4 (p = 0.046) and increased IL-10 production (p = 0.057) -no effect on IL-6 by any of the preparation -Statistically significantly increased IL-2 (p < 0.035) -no effect on IL-12 production -Statistically significant inhibition of TNF-α production from splenocytes from all 3 preparations. (p = 0.004) |
| Zhang, 2020 [61] | National Natural Science Foundation of China, Third Batch of Giant Project of Hebei Province, Top Talent Project for Youths of Hebei Province, Doctoral Startup Foundation of Hebei Normal University of Science and Technology, High School Hundred Excellent Innovation Talent Program of Hebei Province, Central Committee Guides Local Science and Technology Development Project, Natural Science Foundation of Hebei Province | Male C57BL/6 mice 8 weeks old, 18–22 g | LPS induced immune stimulation | E. purpurea | 5 or 10 mg per kg DURATION: 24 h |
Saline | TOTAL: 30 INTERVENTION: 6 LPS + EP 5 mg/kg, 6 LPS + EP 10 mg/kg CONTROL: 6 LPS only, 6 EP 10 mg/kg only, 6 saline only |
-Statistically significant dose-dependent decrease in IL-1β, IL-6, and TNF-α (all p < 0.01) |
CISP: Cisplatin; ConA: Concanavalin A; CXCL: Chemokine Ligand; EP: Echinacea Purpurea; g: Grams; HSV-1: Herpes Simplex Virus-1; IFN; Interferon; IL: Interleukin; IP: Intraperitoneal; kg: Kilogram; LPS: Lipopolysaccharide; mcg: Microgram; mg: Milligram; mL: Millilitres; PBS: Phosphate-buffered Saline; TNF-α; Tumour Necrosis Factor alpha; μl: Microlitres.
Table 3.
Characteristics of the in vitro and ex vivo studies included.
| Author | Sponsorship source/association | Cells or tissue culture | Infection or immune stimulation | Echinacea Spp or individual constituent | Dose, form, standardization, Duration of treatment |
Control or Placebo formula used | Change in cytokines | Risk of Biasa |
|---|---|---|---|---|---|---|---|---|
| Altamirano-Dimas, 2007 [62] | Not stated | The tracheo-bronchial line BEAS-2B and the rhinovirus-sensitive H-1 derivative of HeLa cells | Human rhinovirus type 14 | E. purpurea | Two extracts: E1: an expressed juice extract of the aerial parts of E. purpurea E2: a 55% EtOH tincture, prepared with E. purpurea roots (1:9 w/v) Dose: 100 μg/mL of E1 or 50 μg/mL of E2 DURATION: 18 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Increased genetic expression: IL-8, IL-1RN, CSF2 Decreased genetic expression: TNF-α |
3 |
| Altamirano-Dimas, 2009 [63] | Not stated | The tracheo-bronchial line BEAS-2B and the rhinovirus-sensitive H-1 derivative of HeLa cells | Rhinovirus type 14 | E. purpurea | Two extracts: E1: an aqueous expressed juice extract of the aerial parts of E. purpurea E2: a 50% EtOH tincture, prepared with E. purpurea roots (1:9 w/v) Dose: 100 μg/mL of E1 or 50 μg/mL of E2 DURATION: 18 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Increased gene transcription: IL-1β, IL-13, IL-6, CXCL5, CXCL1, CXCL2, CXCL12, CXCL13, CXCL14, CXCL5, CXCL4, CXCL8, CCL4, CCL2, GM-CSF Decreased gene transcription: IL-1α, IL-4, IL-10, IL-12, IL-16, CXCL9, CXCL1, CXCL2, CXCL11, CXCL5, CXCL4, CXCL8, CXCL17, CXCL12, CXCL18, CXCL4, CCL5, CCL7, CCL8, CCL2, CCL4, TNF-α |
3 |
| Benson, 2010 [64] | This project was supported by grants from NSF-EPSCoR (EPS-0091995) and NCRR (P20RR17670). NCRR is a component of the NIH. | Bone marrow-derived dendritic cells from C57BI/6 mice | OVA-FITC (10 μg/mL) | E. purpurea | 2 extracts were prepared using the leaf and root with 75% EtOH as the solvent. Root extract doses: 150 μg/mL and 450 μg/mL Leaf extract doses: 50 μg/mL and 150 μg/mL DURATION: 48 h |
Negative control: 0.5% EtOH | Increased: IL-6 and TNF-α |
3 |
| Brovelli, 2005 [65] | Not stated | TPH-1 cells | LPS (500 ng/mL) | E. purpurea |
E. purpurea was harvested at various stages of plant development, aerial parts were dried, and extracts were created from dried parts and the solvent 50% DMSO/30% EtOH/20% water. Dose: 100 μg/mL DURATION: 6 h |
Negative control: no treatment Positive control: LPS (500 ng/mL) |
Increased production: IFN-γ, IL-1α, IL-1β, IL-8, MIP-α and TNF-α Decreased production: IL-10 |
3 |
| Burger, 1997 [66] | Not stated | Human peripheral blood macrophages (isolated from a 50-year-old female) | LPS (5 μg/mL) | E. purpurea | Two 20% EtOH commercial preparations: echinacea fresh pressed juice and echinacea dried juice Fresh pressed juice doses: 10, 3.0, 1.2, 0.2, and 0.05 μg/mL Dried juice doses: 10, 1.0, 0.I, 0.03, and 0.01 μg/mL DURATION: 18, 36, or 72 h |
Negative control: no treatment Positive control: LPS (5 μg/mL) |
Increased secretion: IL-1, IL-6, IL-10 and TNF-α |
1 |
| Cadiz, 2019 [67] | University of Minnesota Undergraduate Research Opportunity Program and the Office of the Vice President for Research of the University of Minnesota (UMM Faculty Enhancement Research Fund). | Splenocytes from C57BL/6J wild-type mice | ConA (5 μg/mL for full dose, 5×10^-3 μg/mL for suboptimal dose) | E. purpurea |
E. purpurea root extract Doses: 0, 0.1, 1, and 10 mg/mL DURATION: 24 or 48 h |
Negative control: No treatment on ConA-stimulated cells | Increased levels: TNF-α No change in levels: IFN-γ and IL-2 |
3 |
| Canlas, 2010 [68] | Not funded | BEAS-2B and Human skin fibroblasts | Leishmania donovani Rhinovirus type 1A |
E. purpurea | Standardized commercial extract: Echinaforce, A. Vogel/Bioforce Dose used not specified DURATION: 48 h |
Positive control: LPS (10 μg/mL) | Decreased concentration: IL-6 and IL-8 | 1 |
| Cech, 2006 [69] | NIH NCCAM (Grant No. K01 AT00065–01, T32-AT00815, and R15 AT001466-01) and Research Corporation (grant No. CC5972). | Leukemic human T-lymphocytic cells (Jurkat E6.1 clone) | PHA and PMA | E. purpurea and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutyl- amide | EtOH extract was prepared from E. purpurea roots. Dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutyl- amide was obtained from Chromadex; Santa Ana, CA, USA. Two E. purpurea doses containing 4 or 0.9 μg/mL of dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutyl- amide Two dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutyl- amide doses: 1.8 or 0.19 μg/mL DURATION: 2 h |
Controls included cells with media alone, stimuli alone, and microsome reagents both with and without NADPH. | Decreased concentration: IL-2 | 1 |
| Cech, 2010 [70] | UNC Research Competitiveness Fund | Murine RAW 264.7 macrophage-like cells | Influenza strain A/PR8/34 | E. purpurea and alkylamides 4 (undeca-2E,4Z-diene-8,10-diynoic acid isobutylamide), 11a/b (dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamide), 15 (dodeca-2E,4E-dienoic acid isobutylamide), and 16 (undeca-2E-ene-8,10-diynoic acid isobutylamide) | 17 extracts: E. purpurea roots were harvested from 17 cultivation sites across North Carolina, pulverized into a fine powder, macerated for seven days in 75% EtOH at a ratio of 1:5 (g plant material: mL solvent), pressed, and filtered. Dose of extract #7 used in general cytokine and chemokine experiments: a dilution of 85% EtOH (precipitated) extract was used to produce a final concentration of 22 μm dodecatetraenoic acid isobutylamide (11a/b). Dose of extracts used in TNF-α experiments: 6.7 μL of 75% EtOH extracts and 5.8 μL of 85% EtOH (precipitated) extracts Doses of alkylamides: 0, 6.25, 12.5, 25, and 50 μg/mL DURATION: 24 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on infected cells |
Increased production: IL-12p70 Decreased production: 1L-13, CXCL5, CCL2, CCL3, CCL5, CCL9, TNF-α No change in production: IL-4 and CCL1 |
1 |
| Chicca, 2009 [71] | Not stated | Human peripheral blood mononuclear cells | LPS (350 ng/mL) | E. purpurea | Three extracts obtained from A. Vogel Bioforce AG, Switzerland: herba, root, and combo herba + root in a ratio of 95:5 Doses: herba extract (9.5 μg/mL), radix extract (0.5 μg/mL), and comb herba + radix extract (10 μg/mL) DURATION: 18 h |
Positive control: LPS alone | Increased levels: IL-10 and TNF-α |
1 |
| Chiu, 2010 [72] | Genomics and Proteomics Program, Academia Sinica (AS94F002); National Science Council (96-2320-B-001-008), Taiwan, Republic of China; China Medical University and Hospital (DMR-97-143); Taiwan Department of Health Clinical Trial; Research Center of Excellence (DOH99-TD-B-111- 004) | Human myelogenic leukemia cell line THP-1 | LPS (1 μg/mL) | E. purpurea | Extract: Butanol partitioned fraction of the stem + leaf of the E. purpurea Dose: 100 μg/mL DURATION: 0.5, 4 or 12 h |
Positive control: LPS alone | Increased genetic expression: IL-5, IL-IR2, CXCR4, CCR1 and CCR8 Decreased genetic expression: IL-1β, IL-4, IL-13, IL, TNF-α, CCR2,CCR3,CCR4, CCL2, CCL4, CCL8, CCL22 and CXCR4 |
3 |
| Classen, 2006 [73] | Not stated | Alveolar mouse macrophages | LPS (30 μg/mL) | E. purpurea | Seeds from E.purpurea were treated with absolute EtOH and a 1:10 dilution of deomestos Dose not stated. DURATION: 24 h |
Negative control: no treatment Positive control: LPS (10 μg/mL) |
Increased production: IL-6 |
3 |
| Codorean, 2010 [74] | National Institute of Pathology, Bucharest | Human peripheral whole blood | 5 mg/mL PHA, 2,5 mg/mL ConA, 50 ng/mL LPS | E. purpurea | 15 mg/mL standardized extract DURATION: 48 h |
Ech was the positive control. Exposure to a cytotoxic compound used as a negative control | Increased production: IL-2 No change production: IL-1β |
3 |
| Dong, 2006 [75] | Grant from the National Science Council of Taiwan (NSC91-3112-P-001-035-Y). | Jurkat leukemic T-cells | Anti-CD3 plus anti-CD28 (CD28-dependent stimulation) and PMA plus ionomycin (CD28− independent stimulation) | E. purpurea and cynarin | Crude water extract of E. purpurea. Cynarin was extract from the crude extract using high performance liquid chromatography Dose for both: 100 μg/mL DURATION: 24 h |
Negative control: PMA and ionomycin or anti-CD3 and anti-CD28 Positive control: FK506 (1 μg/mL) |
Decreased production: IL-2 | 1 |
| Fan, 2021 [76] | Grants the Jilin Scientific and Technological Development Program for the financial support and the National Natural Science Foundation of China | Mouse macrophages | LPS (0.1 μg/mL) | E. pallida and E. purpurea | Advantagoues roots of E.pallida (11.4 g) and E.purpurea (8.6 g) were cut into approx 1 cm length DURATION: 24 h |
Negative control: No treatment | Decreased production: IL-6 and IL-1β |
1 |
| Farinacci, 2009 [77] | PRIN2005, Research Unit Bruno Stefanon | Ovine neutrophils | PMA | E. angustifolia | Standardized hydroethanolic extract called Polinacea that was prepared by the authors using a patent Extracts doses used: 0, 20, and 60 μg/mL DURATION: 1 or 22 h |
Negative control: no treatment | Increased gene expression: IL-8 |
1 |
| Fonseca, 2012 [78] | Integrative Medicine Service, Memorial Sloan-Kettering Cancer Centre | Jurkat T-cells | PMA plus ionomycin and Ionomycin | E. purpurea | Various concentrations Extract doses used: 0,10,25, 100 and 250 μg/mL DURATION: 40 min and 24 h |
Untreated cells | Increased production: IFN-γ and IL-2 | 1 |
| Fonseca, 2014 [79] | NIH NCCAM and ODS:1-P50-AT02779 Botanical Research Center for Botanical Immunomodulators, NIH NCI Cancer Education and Career Development R25 CA105012: Nutrition and Cancer Prevention and the Children's Cancer and Blood Foundation | Human Jurkat T-cells (cell line e6-1) | PMA and/or ionomycin | E. purpurea | Extract: fresh aerial parts were extracted with water, ethanolic precipitation, and size-exclusion chromatography Extract doses used: 0, 10, 25, 100 and 250 μg/mL DURATION: 40 min and 24 h |
Negative control: FK506 (1 μg/mL in DMSO) | Increased concentration: IFN-γ and IL-2 | 1 |
| Fu, 2017 [80] | National Natural Science Foundation of China (No. 31472128). | Murine bone marrow-derived macrophages | LPS (10 ng/mL) | E. purpurea | Extract obtained from Shandong Qilu Animal Health Co., Ltd. Chemical composition of extract: cichoric acid (3.045%), caftaric acid (1.575%), chlorogenic acid(0.065%), Nndeca-2Z,4E-diene-8,10-diynoic acid isobutylamide (1.635%). Dose: 100 μg/mL DURATION: 12 or 24 h |
Negative control: no treatment Positive control: IFN-γ (10 ng/mL) + LPS (10 ng/mL) or IL-4 (20 ng/mL) |
Increased secretion: IFN-γ, IL-1α, IL-6 and TNF-α | 1 |
| Groom, 2007 [81] | Charles River Laboratories Preclinical Services Montreal Inc. | Macrophages (cell line J774A.1) and NK cells (IL-2-dependent NK-92 cell line) | LPS (3 μg/mL) | E. purpurea | Standardized extract of echinacea (4% total phenolics) obtained from Stryka Botanics Co., Inc., Hillsborough, NJ. Dose: 0.128, 0.385, and 1.28 mg/mL DURATION: exact duration not stated |
Positive control: LPS (3 μg/mL) for macrophages and IL-12 (3 U/ml) for NK cells | Increased synthesis: IFN-γ No change in synthesis: IL-12 |
3 |
| Guidetti, 2016 [82] | Not stated | Human peripheral blood mononuclear cells [from 10 healthy volunteers] and canine peripheral blood mononuclear cells [from 10 healthy dogs] | PMA and ionomycin | E. purpurea |
E. purpurea dried extract, polyphenols content min 4%, dissolved in EtOH and water. Dose not specified DURATION: 10–12 h |
Positive control: stimulation with no treatment | Decreased production: IFN-γ No change in production: IL-4 |
3 |
| Gulledge, 2018 [83] | Grants from the National Center for Complementary and Integrative Health, a component of the National Institutes of Health (1R15AT007259), the National Institutes of Health (R01 HD072968 to AJM), the Research and Innovation Seed Fund at North Carolina State University, the Departments of Biological Sciences and Chemistry at North Carolina State University, and the Comparative Medicine Institute at North Carolina State University. | RBL-2H3 cells, a basophilic leukemia cell line | Calcium ionophore A23187 | E. purpurea root extract and alkylamide dodeca-2E,4E-dienoic acid isobutylamide (A15) | Alkylamide dodeca-2E,4E-dienoic acid isobutylamide was synthesized and used in doses of 25, 50 and 100 μM DURATION: 8 h |
Stimulation with A23187 without A15 | Decreased production: TNF-α | 1 |
| Hou, 2010 [84] | Institutional grant of Academia Sinica and national research program for genomic medicine (NSC 97-3112-B-001-020) of National Science Council of Taiwan, R.O.C. | Murine macrophage RAW 264.7 cells | LPS (1.0 μg/mL) | E. purpurea, dodeca-2E,4E,8Z,10Z(E)-tetraenoic acid isobutylamide, and cichoric acid | A series of isolations from a methanolic extraction of E. purpurea were carried out to yield [1] a fraction containing an alkamides mixture [2], dodeca-2E,4E,8Z,10Z(E)-tetraenoic acid isobutylamide, and [3] cichoric acid. Alkamide mixture dose: 5 and 25 μg/mL Dodeca-2E,4E,8Z,10Z(E)-tetraenoic acid isobutylamide dose: 5 and 100 μM Cichoric acid dose: 50 and 100 μM DURATION: 4 and 20 h |
Negative control: no treatment and no stimulation Positive control: stimulation with no treatment |
Decreased secretion IL- 1β, IL-6, IL-10, IL-12p70, IL-13, IL-1α and IL-2, MCP-1, MIP-1β9, RANTES and GM-CSF |
1 |
| Hwang, 2004 [85] | Presented in part during receipt of the ‘‘Paul E. Strandjord Young Investigator Award for 2003″, at the 38th annual meeting of the Academy of Clinical Laboratory Physicians and Scientists (ACLPS), Tucson, AZ (June 2003). | Female BALB/c mouse splenocytes, further sub fractionated to adherent and non-adherent cell populations | N/A | E. purpurea | Liquid extract: fresh Echinacea root juice, mature seed, fresh leaf juice and fresh fruit juice extracted in 44–50% alcohol Solid extract: solid extract (dried Echinacea root and leaf) dissolved in either in distilled water or absolute alcohol in the ratio of 25 mg of solid extract per ml of solvent Dose of Echinacea preparation: 1 mg/mL DURATION: 48 h |
None | Increased production: IL-6, IL-10, MIP-1α and TNF-α No change in production: IFN-γ, IL-1β, IL-2 and IL-12 |
3 |
| Kapai, 2011 [86] | N.N. Blokhin Russian Oncological Research Center, the Russian Academy of Medical Sciences, Moscow | MNL isolated from heprin-stabilized periphereal blood | N/A | E. purpurea tincture |
E. purpurea tincture in a series of 10-fold dilutions. the active concentration was D1-D17. DURATION: 48 h |
Saline containing EtOH | Increased production: IL-1, IL-8, IL-1β, IL-10 and IL-14 |
3 |
| Lee, 2015 [87] | National Research Foundation of Korea (NRF)funded by the Ministry of Education (NRF-2014R1A1A2008663). | HMC-1 | PMACI A23187 |
Chicoric acid | ≥95% purity Dose: 12.5, 25, or 50 μM DURATION: 24 h |
Negative control: no treatment and no PMACI stimulation Positive control: no treatment and PMACI stimulation |
Decreased mRNA expression: IL-6, IL-1β and TNF-α | 1 |
| Li, 2017 [88] | Grants from the National Natural Science Foundation of China (No. 31472128). | Bone marrow-derived dendritic cells from C57BL/6 mice | LPS (50 ng/mL) | E. purpurea | Extract purchased from Shandong Qilu Animal Health Co., Ltd. Chemical composition of extract: cichoric acid (3.045%), caftaric acid (1.575%), chlorogenic acid(0.065%), dodeca-2E, 4E, 8Z, 10E/Z-tetraenoic acid isobutylamide(1.635%). Dose: 400 μg/mL DURATION: 24 h |
Negative control: no treatment | Increased secretion: IFN-γ, IL-10 and IL-12 | 1 |
| Luettig, 1989 [89] | Not stated | Spleen T cells, thioglycolate-induced peritoneal macrophages, bone marrow macrophages, and resident peritoneal macrophages from C57BL/6 mice | T Cells - ConA at 1 and 5 μg/mL B cells - LPS 50 μg/mL Macrophages in virto - LPS 100 μg/mL |
Arabinogalactan from E. purpurea | Varied per experiment, but ranged from 3.7 to 500 μg/mL DURATION: 18–48 h |
Negative control: no treatment Positive control: LPS (10 or 20 μg/mL) |
Increased production: IFN-β2, IL-1 and TNF-α No change in production: IL-2 |
3 |
| Matthias, 2007 [90] | MediHerb Research Laboratories, Queensland, Australia | Mouse macrophage cell line | LPS (0.1 μg/mL) or PMA (2 nM) | Alkylamide 1. (2E)-N-isobutylundeca-2-ene-8,10-diynamide; Alkylamide 2. (2E,4E,8Z,10Z)-N-isobutyldodeca-2,4,8,10-tetraenamide.; An ethanolic extract (Echinacea Premium Liquid; EPL) of E. purpurea (300 mg/mL), E. angustifolia (200 mg/mL) roots and EPL alkylamide fraction (EPL AA) was separated from caffeic acid fraction and cichoric acid |
Alkylamides concentration 0.2 ng/mL; cichoric acid concentration 0.8 ng/mL DURATION: 4 and 20 h |
Unstimulated cells | Decreased production: TNF-α | 3 |
| McCann, 2007 [91] | Grant P01ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH. | Human peripheral blood mononuclear cells (isolated from 19 subjects between the ages of 19 and 36 who donated blood 8 h pre- and 4 weeks post- receiving the 2005/2006 trivalent influenza Fluzone vaccine) | Influenza type A H1N1 virus (A/New Caledonia/20/99) | E. angustifolia, E. pallida, E. paradoxa, E. purpurea, E. sanguinea, E. simulata, and E. tennesseensis | Root tinctures of each species extracted in 50% EtOH/50% water at a ratio of 1 part plant/9 part solvent. Tinctures were stored at −20 °C for 24 months. Dose: 1:12.5 dilution DURATION: 24 or 48 h |
Experiment 1: Negative control: no treatment Experiment 2: Negative control: no treatment on uninfected cells Positive control: no treatment on infected cells |
Increased levels: IL-10 Decreased levels: IL-2 No change in levels: IFN-γ, IL-12 and TNF-α |
1 |
| Mishima, 2004 [92] | NAGARAGAWA Research Center, Suxuka University of Medical Science Graduate School of Health Science | Peripheral blood cells and T lymphocytes | Radiation | E. purpurea | 360 mg/kg; mice administered treatment every other day every other day DURATION: 3 weeks |
Blood from; Mice + saline/no E.Purpurea + radiation, Mice + E.Purpurea + no radiation, Mice + radiation only | Increased production: IFN-γ | 1 |
| Moazami, 2015 [93] | Partially funded by NC State's Office of Research, Innovation, and Economic Development, in partnership with the Kenan Institute for Engineering, Technology and Science and the Center for Comparative Medicine and Translational Research. | Murine RAW 264.7 macrophage-like cells | LPS (10 ng/mL) | Fatty acid amide dodeca-2E,4E-dienoic acid isobutylamide, a constituent of E. purpurea, and a series of analogs that varied by unsaturation, alkyl chain length, and amide head group | Fatty acid amide was chemically synthesized de novo, and analogs were created by altering the double bonds and/or the alkyl chain length in the fatty acid unit. Dose: 100 μM DURATION: 18 h |
Negative control: treatment without LPS stimulation Positive control: LPS stimulation without treatment |
Decreased production: TNF-α | 1 |
| Morazzoni, 2005 [94] | Dipartimento di Scienze Cliniche e Biologiche, Università degli Studi di Torino, Torino, Italy | J774. a murine macrophage cell | LPS (1 μg/mL) | E. angustifolia | The roots were exhaustively treated with 90% EtOH for echinacoside extraction and then counter- extracted with n-hexane for isobutylamides elimination. Wet roots were extracted with 15% aq. DURATION: 7 days |
Negative control: no treatment | Increased production: IFN-γ | 1 |
| Olah, 2017 [95] | Bundesministerium für Wirtschaft und Energie (BMWi), Germany (ZIM-KOOP, grant number: KF2611301MD0; Dr. August Wolff GmbH & Co. KG Arzneimittel (Bielefeld, Germany); Hungarian research grants (NRDIO 121360, NRDIO 120552). | Human immortalized HaCaT keratinocytes | Polyinosinic-polycytidylic acid | E. purpurea root extract | Extract is prepared by supercritical CO2-extraction of E. purpurea roots. Dose: 20 μg/mL DURATION: 3 and 24 h |
Negative control: no treatment and no stimulation Positive control: stimulation with no treatment |
Decreased mRNA expression: IL-6 and IL-8 | 1 |
| Pomari, 2014 [96] | Progetto Nutriheart POR FESR 2007–2013 Friuli Venezia Giulia, Italy. | RAW264.7 murine macrophages | H2O2 (200 μM) | E. angustifolia | Commercial ethanolic root extract standardized to ≥4% echinacoside Dose: 10 μg/mL DURATION: 24 h |
Negative control: no treatment and no stimulation Positive control: stimulation with no treatment |
Increased mRNA expression: TNF-α Decreased mRNA expression: IL-1β |
1 |
| Pugh, 2004 [97] | National Center for Natural Products Research, University of Mississippi, University, | THP-1 human monocyte cell line | LPS (10 μg/mL) | E. angustifolia, E. pallida and E. purpurea - specifically melanin extracted from the latter plants | 0.1, 0.4 and 1.0 μg/mL DURATION: 4 days |
Negative control: no treatment | Increased secretion: IL-1β |
1 |
| Raduner, 2006 [98] | Initial financial support provided by Prof. Dr. Jorg Heilmann | Human peripheral whole blood [from healthy volunteers] | LPS (313 ng/mL) | 3 alkylamides from E. purpurea: A1 (dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide), A2 (dodeca-2E,4E-dienoic acid isobutylamide), and A3 (undeca-2E-en-8,10-diynoic acid isobutylamide). | A2 was isolated from E. purpurea. A1 and A3 were gifted by MediHerb, Australia. Dose: 5 nM, 50 nM, 500 nM, and 5000 nM DURATION: 18 h |
Negative control: treatment without stimulation Positive control: stimulation without treatment |
Decreased expression: IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α |
1 |
| Randolph, 2003 [37] | Nutrilite Health Institute, Access Business Group, LLC, Buena Park, California and Source Precision Medicine, Boulderm Colorado | THP-1 human monocyte cell line | 18S mRNA | E. angustifolia root, E. purpurea root and herb | 10 μg/mL, 50 μg/mL, 250 μg/mL DURATION: 6 h |
Untreated cells | Increased gene expression: IL-1α, IL1β, IL-8, IL-10 and TNF-α |
3 |
| Rininger, 2000 [99] | Paracelsian, Incorporated, Ithaca, New York | RAW264.7 macrophage cells | LPS 0.1 μg/mL | E. purpurea | 5 μg/mL, 20 μg/mL, 80 μg/mL, 320 μg/mL DURATION: 48 h |
Medium alone and LPS + medium | Increased production: IL-1α, IL-1β, IL-6, IL-10 and TNF-α |
1 |
| Ritchie, 2011 [100] | Founded by A. Vogel Bioforce AG, Switzerland; Funded by Bioforce, Switzerland. | Blood samples | Zymosan (333 μg/mL) or LPS (from E.Coli at 100 ng/mL)/super-antigen SEB at 25 ng/mL) | E. purpurea | Echinaforce - patient took 4 1 mL doses for 5 days, then 10 1 mL doses for 3 days. Blood sample taken each day for analysis; Echinaforce phytochemical profile: 264.4 μg/mL caftaric acid, 40.2 μg/mL chlorogenic acid, 313.8 μg/L cichoric acid, 6.9 μg/mL echinacoside, 35.9 μg/mL dodeca tetraene; Echinaforce made from freshly harvested herbs and roots of E. purpurea in a 95:5 ratio. DURATION: 8 days of supplementation, blood cells stimulated for 24 h |
Baseline - blood samples prior to Echinaforce supplementation | Increased production: IFN-γ, IL-8 and IL-10 Decreased production: IL1-β and TNF-α |
3 |
| Sasagawa, 2006 [101] | Bastyr Univerisity, Department of Basic Sciences, Kenmore, United States | Jurakat cells | PHA and PMA; Treatments: PHA; 10 ng/mL PMA; or 1 μg/mL PHA+1 ng/mL PMA |
E.purpurea extract, Alkylamides (1. Dodeca-2(E),4(E),8(Z),10(Z)-tetraenoic acid isobutylamide; 2. Dodeca-2(E),4(E)-dienoic acid isobutylamide in 05% EtOH) and caffeic acid derivatives (3. Caftaric acid 47.5% EtOH; 4. Cichoric acid in 95% EtOH; 5. Chlorogenic acid 47.5% EtOH) |
E.purpurea extract; 0.1 μg/mL, 1 μg/mL, 10 μg/mL, 50 μg/mL and 100 μg/mL in 95:5, 75:25, 50:50, 25:75 EtOH:water mixtures.//Echinacea consitituents; stock concentration of 5 mg/mL diluted to final concentration of 0.625–25 μg/mL DURATION: 24 h |
0.5% EtOH vehicle | Decreased production: IL-2 | 1 |
| Senchina, 2005 [102] | Grant number P01ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH. | Human monocytes [isolated from blood from 5 healthy human donors] | N/A | E. angustifolia var. angustifolia, E. pallida, E. purpurea, E. sanguinea, and E. tennesseensis | 3 extracts for each Echinacea species: 50% EtOH, cold water infusion, and hot water infusion [1 part plant to 9 parts solvent]. Extracts were stored at 4 °C and tested at 1 and 4 days post-extraction. Dose not stated. DURATION: 24 h |
Negative control: no treatment | Increased production: IL-10 (immediately), IL-12, TNF-α Decreased production: IL-10 (later time point) |
3 |
| Senchina, 2006 [103] | Grant number P01ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH. | Human peripheral blood mononuclear cells (from 15 healthy human young adult donors) | N/A | E. angustifolia, E. pallida, E. paradoxa, E. purpurea, E. sanguinea, E. simulata, and E. tennesseensis | Method of extraction not stated. Extracts were stored at −20 °C for 1 month before beginning experiments. Dose not stated. DURATION: 24 h |
Negative control: no treatment | Increased production: IL-1β and TNF-α No change in production: IL-2 |
3 |
| Senchina, 2006 [104] | Grant number P01ES012020 from the National Institute of Environmental Health Sciences(NIEHS) and the Office of Dietary Supplements (ODS), NIH | Human peripheral blood mononuclear cells (isolated from older adults 6 months post receiving trivalent influenza vaccine) | Influenza A/New Caledonia/20/99 (H1N1) virus or the Influenza A/Wyoming/03/2003 (H3N2) virus | E. angustifolia, E. pallida, E. paradoxa, E. purpurea, E. sanguinea, E. simulata, and E. tennesseensis | 50% ethanolic tinctures of roots from each species [1 part plant, 9 parts solvent]. Dose: 1:12.5 dilution DURATION: 48 h |
Negative control: no treatment on infected cells | Increased levels: IL-10 Decreased levels: IL-2 and IFN-γ |
1 |
| Senchina, 2009 [105] | Grant Number P01ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH. | Human peripheral blood mononuclear cells (from 16 subjects between the ages of 19 and 36 who donated blood) | N/A | E. tennesseensis | Separate 50% EtOH tinctures prepared from roots, stems, leaves, and flower. Tincture aliquots were stored at three different temperatures (4, −20, and −80 °C) for 21 h before testing. The −20 °C aliquots were saved and tested again 1 month later. Dose: 1:12.5 dilution DURATION: 24 h |
Negative control: no treatment | Increased production: IL-1β, IL-10 and TNF-α No change in production: IL-2 |
1 |
| Senchina, 2009 [106] | faculty start-up funds allocated to DSS at Drake University. | Human blood mononuclear cells (from 12 healthy young men) | 2 separate exercise bouts [1]: VO2max test and [2] 90 min of cycling at 85% of ventilatory threshold | E. tennesseensis | Separate 50% EtOH tinctures prepared from roots and flowers. Extracts were stored at−80 °C undisturbed for 3 years before the study took place. Dose: 50 μL DURATION: 24, 48 and 72 h |
Negative control: no exercise stimulation and no treatment Positive control: exercise stimulation with no treatment |
No change: IL-1β, IL-10 and TNF-α | 1 |
| Senchina, 2010 [107] | grant number P01Es012020 from NIEHS and the Office of Dietary Supplements. | RAW264.7 murine macrophage cells | HSV-1 virus | E. angustifolia var. strigosa, E. purpurea, and E. tennesseensis | 3 separate tinctures of dried root samples of the three species made with 50% EtOH/50% water at a ratio of 1:9 parts plant material:solvent. E. purpurea roots were also made into a 4th extract with 95% EtOH and using the Soxhlet apparatus. Dose: 1:12.5 dilution DURATION: 24 h |
Negative control: EtOH at the same concentration (<0.2%) Positive control: Poly I:C |
Decreased levels: IFN-α No Change in levels: IFN-β |
1 |
| Senchina, 2011 [108] | faculty start-up funds given to DSS at Drake University. | Human peripheral blood mononuclear cells [from 16 subjects (9 males, 7 females, age 23.5 ± 3.8 years) who donated blood] | LPS and PHA antigen | E. laevigata, E. angustifolia, E. pallida, and E. purpurea | Root tinctures of each species extracted in 50% EtOH/50% cell culture water at a ratio of 1:9 parts plant material:solvent. Dose: 50 μL/well DURATION: 24, 48 or 72 h |
Negative control: no treatment Positive control: LPS and PMA antigen |
Increased levels: IL-10 and TNF-α No change in levels: IL-2 |
1 |
| Sharma, 2006 [109] | Not stated | The tracheo-bronchial line BEAS-2B and the rhinovirus-sensitive H-1 derivative of HeLa cells | Rhinovirus type 14 | E. purpurea | Two extracts: E1: an expressed juice extract of the aerial parts of E. purpurea E2: a 50% alcoholic tincture, derived from E. purpurea roots (1:9 w/v) Dose: 100 μg/mL of E1 or 50 μg/mL of E2 DURATION: 24–96 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Increased secretion: IL-1β, IL-2, IL-3, and IL-7 Decreased secretion: IFN-γ, IL-1⍺, IL-1β, IL-2, IL-3, IL-5, IL-6, IL-7, IL-8, IL-15, IL-17, TNF-α, GM-CSF, CCL8, CCL10, CCL11, MIP-1α, MIP1β and MIP-4 |
3 |
| Sharma, 2009 [110] | Not stated | The tracheo-bronchial line BEAS-2B, H-1 sub clone of HeLa cells, the lung-derived epithelial cell line A549, and human skin fibroblasts | Rhinovirus types 1A and 14 | E. purpurea | Echinaforce by A. Vogel Bioforce AG, Switzerland: a 65% ethanol extract of freshly harvested aerial parts supplemented with 5% roots. Dose: dilutions of 1:20, 1:100, 1:200, and 1:400 DURATION: 48 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Decreased secretion: IL-6 and IL-8 | 3 |
| Sharma, 2009 [111] | Not stated | Two human epithelial cell lines: the tracheo-bronchial line BEAS-2B and the lung-derived epithelial cell line A549 as well as human skin fibroblasts | Viruses: RV1A, RV14, influenza, RSV, adenovirus types 3 and 11, and HSV | E. purpurea | Echinaforce obtained from A. Vogel Bioforce AG, Roggwil, Switzerland, batch no.: 018451: standardized preparation derived by EtOH extraction of freshly harvested E. purpurea herb and roots(95:5) Dose: 1:100 dilution of Echinacea in DMEM without serum, corresponding to a final concentration of 160 μg/mL (dry mass/vol) DURATION: 24 and 48 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Decreased levels: IL1-α, IL-1β, IL-5, IL-6, IL-8, MIP-1α, MIP-1β, GRO-α, MCP-1, CCL5 and TNF-α | 3 |
| Sharma, 2010 [112] | Not stated | A total of three, separate, normal human airway epithelial tissues (code AIR-100), from three different donors | Rhinovirus type 1A | E. purpurea | Echinaforce by A. Vogel Bioforce AG, Switzerland: a 65% EtOH extract of freshly harvested aerial parts supplemented with 5% roots. Dose: 1:100 dilution of Echinaforce DURATION: 24 and 48 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Decreased secretion: IL-6 and IL-8 | 1 |
| Sharma, 2010 [113] | Not stated | Two human epithelial cell lines: the tracheo-bronchial line BEAS-2B and the lung-derived epithelial cell line A549 as well as human skin fibroblasts |
H. influenzae L. pneumophila MSSA MRSA S. pyogenes |
E. purpurea | Echinaforce by A. Vogel Bioforce AG, Switzerland: a 65% EtOH extract of freshly harvested aerial parts supplemented with 5% roots. Dose: 1:100 dilution of Echinacea in DMEM without serum, corresponding to a final concentration of 160 μg/mL (dry mass/vol) DURATION: 48 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on virally infected cells |
Decreased secretion: IL-4, IL-6 and IL-8, MIP-1α, GRO-α, MCP-1 and GM-CSF | 3 |
| Sharma, 2011 [114] | Not stated | Two human epithelial cell lines: the tracheo-bronchial line BEAS-2B and the lung-derived epithelial cell line A549 as well as human skin fibroblasts | Propionibacterium acnes | E. purpurea | Echinaforce by A. Vogel Bioforce AG, Switzerland: a 65% EtOH extract of freshly harvested aerial parts (drug extract ratio 1:12) supplemented with 5% roots (drug extract ratio 1:11). Dose: 1:100 dilution of Echinacea in DMEM without serum, corresponding to a final concentration of 160 μg/mL (dry mass/vol) DURATION: 48 h |
Negative control: no treatment on uninfected cells Positive control: no treatment on infected cells |
Decreased secretion: IL-6, IL-8 and TNF-α | 3 |
| Spelman, 2009 [115] | University of North Carolina Greensboro, Department of Chemistry and Biochemistry, Greensboro, United States | Jurakat T cells | PMA (1.25 ng/mL) or PHA (0.25 ng/mL) | E. angustifolia-derived alkylamide undeca-2E-ene-8,10-diyonic acid isobutylamide (This chemical constituent binds to PPAR-γ receptor to inhibit IL-2 production thus researchers explored this). | 0.033 μg/mL, 0.1 μg/mL, 0.33 μg/mL, 1 μg/mL, 3.3 μg/mL DURATION: 18 h |
EtOH/DMSO vehicle | Decreased secretion: IL-2 | 1 |
| Stimpel, 1984 [116] | Not stated | Bone marrow macrophages from C57BL/10 mice | 100 μg of LPS or μg of EPS | Purified polysaccharides from E. purpurea | Polysaccharides were purified by chromatography from alkaline-water extracts of E. purpurea. Dose: 100 μg DURATION: 8–24 h |
Negative control: unstimulated macrophages Positive control: LPS (10 μg) |
Increased production: IL-1 |
3 |
| Sullivan, 2008 [117] | Natural Sciences and Engineering Research Council of Canada and the Nova Scotia Health Research Foundation, Halifax, Nova Scotia, Canada. | Murine peritoneal macrophages | LPS | E. purpurea; IL-6 2400, 1200, 600, 300 and 150 μg/mL//IL-12, IL-1B 500 μg/mL | IL-6 48 h//IL-12, IL-1B 24 h. DURATION: 24 or 48 h |
IL-6 LPS positive control and media and negative control//IL-12, IL1B media control | Increased production: IL-6 and IL-12, TNF-α No change: IL-1β |
|
| Todd, 2015 [118] | Grant #1R15AT007259 from the National Centre for Complementary and Alternative Medicine, Maryland, United States. | RAW 264.7 macrophage-like cells | LPS 100 μg/mL | 75% Echinacea extract (ground root), various liquid partitions, EE, HL, ML, WL and CL (Each of these fall under one of the fractions 1–13, see Fig. 1) | TNF 50 μg/mL, 100 mg/mL//Chemokines - varying degrees of alkylamides for fractions 1–13 and CL (precise concentrations and chemical structures in paper, Table 1 and Fig. 3) DURATION: 16–18 h |
Medium | Decreased production: CCL3, CCL5 and TNF-α | 3 |
| Vimalanathan, 2009 [119] | Not stated | BEAS-2B | Rhinovirus type 14 (RV 14) (infection at 1 virus/cell (1 pfu/cell)) | Root, leaf and flower extracts of E. purpurea (L.) Moench, Root extracts of E. angustifolia (D.C.) and E. pallida (Nutt.) Nutt. | 250 μg/mL DURATION: 48 h |
Cells with no virus + treatment | Decreased production: IL-6 and IL-8 | 3 |
| Vimalanathan, 2017 [120] | A.Vogel Bioforce AG, Roggwill(TG), Switzerland | BEAS-2B | Influenza (H3N2) and bacterial LPS | Echinaforce (E. purpurea) | CFU assay - 1:200 (50 μg/mL), 1:400 (40 μg/mL), 1:800 (20 μg/mL)//Cytokine assay - 1:100, 1:200, 1:400//NFκB p65 expression assay - 1:200, 1:400 DURATION: 24 and 48 h |
CFU assay, cytokine assay, NFκB expression assay - vehicle alone, no treatment | Decreased production: IL-6 and IL-8 | 1 |
| Wang, 2006 [121] | Agricultural Biotechnology Research Center, Academia Sinica, Nankang, Taipei 115, Taiwan, Republic of China | Human DCs | LPS (1 μg/mL) | E. purpurea - stem + leaf (0.10% alkylamide) and root (3.01% alkylamide) | Used 100 μg/mL for data presented DURATION: 4 and 16 h |
Vehicle control | Increased gene expression: IL-7, CCL2 and CCL4 Decreased gene expression: IL-1β, CCL3 and CCL8 |
1 |
| Wang, 2008 [122] | Agricultural Biotechnology Research Center, Taiwan | Human immature dendritic cells | LPS (100 ng/mL) | E.Purpurea - Stem and leaf fractions in n-butanol (BF/S + L/Ep) or cichoric acid | Concentration of cichoric acid 8.4% w/w and rutin 22.3% w/w DURATION: 4 and 24 h |
0.1% DMSO as vehicle control | Increased gene expression: IL-1β, IL-8, IL-18, CXCL1, CCL2 and CCL5 Decreased gene expression: IFN-α |
1 |
| Wilasrusmee, 2002 [123] | Not stated | Human peripheral blood mononuclear cells | 5000-rad γ -irradiated stimulator cells | E. purpurea | Dried and ground fresh herb homogenized in RPMI and filtered. Dose not specified. DURATION: 5 days |
Negative control: no treatment | No change in production: IL-2 and IL-10 | 3 |
| Woelkart, 2006 [124] | Institute of pharmaceutical sciences, department of pharmacognosy | Blood samples | LPS 100 pg mL + E51:F51 | E.purpurea tincture (Echinaforce) or tablet |
E. purpurea tincture containing 0.018 mg/mL of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides and 1 E.purpurea tablet is 0.006 mg DURATION: 23 h |
Alcohol or lactose | Decreased production: IL-8 and TNF-α No change in production: IL-6 |
3 |
| Wu, 2009 [125] | PolinaceaTM was donated by Indena s.p.a.; MiUR (PRIN 05) and Università degli Studi della Tuscia, and the Asia Link Project ‘‘Organic Farming: ethical, economic, technical and scientific aspects in a global perspective | Peripheral blood mononuclear cells (from six healthy Holstein heifers) | ConA (1 μg/mL) | E. angustifolia | Hydroethanolic root extract called Polinacea donated by Indena s.p.a. (Settala, Milan, Italy). Doses: 0, 6.3, 20, 60, and 180 μg/mL DURATION: 72 h |
Negative control: no stimulation and no treatment | No change in secretion: IFN-γ | 3 |
| Yang, 2018 [126] | State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University | Spleen lymphocytes | ConA (100 μg/mL) | Tetraploid (CPE4) (85.51% crude polysaccharide) and diploid (CPE2) E. purpurea (44.65% crude polysaccharide) | 0.5–0.0039 mg/mL DURATION: 48 h |
10 μg/mL ConA | Increased production: IFN-γ, IL-2, TNF-α | 3 |
| Yao, 2019 [127] | College of Veterinary Medicine, South China Agricultural University | Chicken bone marrow-derived dendritic cells | 5 μg/mL LPS | E. purpurea polysaccharide (EPP) and sulfated EPP (sEPP) | EPP (2−2, 2–3, 2–4 mg/mL, marked as EPPH, EPPM, EPPL, respectively) or sEPP (2–7, 2–8, 2–9 mg/mL, marked as sEPPH, sEPPM, sEPPL, respectively) DURATION: 48 h |
Serum-free DMEM and only LPS stimulation | Increased production: IFN-γ, IL-2 Decreased production: IL-4 and IL-10 |
3 |
| Zhai, 2007 [128] | the National Institute of Environmental Health Sciences (grant P01ESO12020) and the Office of Dietary Supplements, National Institutes of Health. | Splenocytes | ConA of 1 and 3 μg/mL and LPS (10 μg/mL) | E.angustifolia, E.pallida, and E.purpurea | 130 mg/kg delivered orally DURATION: 7 days |
Vehicle control: 5% EtOH | Decreased secretion: TNF-α No change in secretion: IL-1β and IL-10 |
1 |
| Zhang, 2012 [129] | grant number 9P50AT004155-06 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), National Institutes of Health (NIH). | RAW264.7 mouse macrophage cells | LPS (1 μg/mL) |
E. angustifolia, E. pallida, E. paradoxa, E. paradoxa var. paradoxa, and E. purpurea Bauer ketones 22, 23 and 24 |
E. paradoxa var. paradoxa was fractionated into 5 fractions by semipreparative HPLC system. Doses: 184 μg/mL (fraction 1), 75 μg/mL (fraction 2), 101 and 20 μg/mL (fraction 3), 20 and 3.2 μg/mL (fraction 4), 36 and 20 μg/mL (fraction 5), 187 and 20 μg/mL (fraction 6). Bauer ketones 22, 23 and 24 (present in fraction 5) where chemically synthesized. Doses: 3.1 μM (#22), 1.6 μM (#23), and 9.7 μM (#24). DURATION: 24 h |
Negative control: stimulation with no treatment Positive control: quercetin |
Decreased production: IL-1β, IL-6 and TNF-α | 1 |
BEAS-2B: Human Bronchial Epithelial Cell Line; ConA: Concanavalin A; CXCL/CCL: Chemokine Ligand; CL: Chloroform Layer; DC: Dendritic Cells; DMEM: Dulbecco's Modified Eagle Medium; DMSO: Dimethylsulfoxide; EE: Ethanol Extract; EPP: E. purpurea Polysaccharide; EPS: Extracellular Polymeric Substances; EtOH: Ethanol; g: Gram; GM-CSF: Granulocyte-macrophage Colony-stimulating Factor; GRO: Growth Regulated Oncogene; HaCaT cells: Human Keratinocyte Cells; HL: Hexane Layer; HMC-1: Human Mast Cells; H2O2: Hydrogen Peroxide; IFN: Interferon; Il: Interleukin; kg: Kilogram; LPS: Lipopolysaccharide; MCP: Monocyte Chemoattractant Protein; MIP: Macrophage Inflammatory Protein; ml: Millilitre; ML: Methane Layer; MNL: Mononuclear Leukocyte; MRSA: Methicillin-resistant Staphylococcus Aureus; MSSA: Methicillin-susceptible Staphylococcus Aureus; NADPH: Nicotinamide adenine dinucleotide phosphate; NFκB: Nuclear Factor kappa B; ng: Nanogram; NK: Natural Killer; nM: Nanomolar; OVA-FITC: Ovalbumin Fluorescein Conjugate; PHA: Phytohemagglutinin; PMA: Phorbol 12-myristate 13- acetate; PMACI: Phorbol-12-myristate 13-acetate plus calcium ionophore; PPAR-γ: Peroxisome Proliferator-activated Receptor gamma; RANTES: Regulated on Activation Normal T Expressed and Secreted; RBL: Rat Basophilic Leukemia cells; RPMI: Roswell Park Memorial Institute Medium; SEB: Staphylococcal enterotoxin B; sEPP: Sulfated E. purpurea Polysaccharide; TNF: Tumour Necrosis Factor; TPH-1: Tryptophan hydroxylase-1; μg: Microgram; μM: Micrometre; WL: Water Layer.
1 = reliable without restrictions, 3 = unreliable.
The most commonly studied Echinacea species in human, animal and in vitro/ex vivo studies alike was E. purpurea. Approximately 66% of all studies used E. purpurea alone and another 19% used E. purpurea in combination with other species. The second most commonly studied species was E. angustifolia; with approximately 8% of studies using it on its own and 18% using it in combination with other species.
Human studies were conducted primarily in the USA (38%, n = 5), followed by Italy and Germany (23%, n = 3 each), Indonesia (8%, n = 1) and Ukraine (8%, n = 1). Of the 13 human studies, eight (61%) examined the effects of Echinacea on healthy adults. The remaining five studies examined the effects of Echinacea on: healthy male triathletes training for competition [34], healthy adults exposed to rhinovirus [30], teenagers and adults with new inset of the common cold [28], adults in clinical remission of chronic herpes [35], and COPD outpatients [29]. The largest human study was a clinical trial with 713 participants [28] and the smallest were two non-randomized studies without a control group [39,40] with six participants each. The average number of participants in human studies was 112 (SD = 208) and the median was 40. The Echinacea dosage and duration of treatment employed also varied widely, ranging from a one-time injection containing 5 mg of Echinacea polysaccharides [36] to a daily dose of 8000 mg of Echinacea capsules for 28 consecutive days [32]. A total of four studies [31,32,34,37] implemented 28-day interventions and three employed a one-time dose [36,38,40]. Concerningly, two studies [33,35] did not specify the dosage of Echinacea used. Moreover, Echinacea tablets or soft gel capsules were the most common type of intervention. Additional interventions included Echinacea lozenges, syrup, juice and tinctures. All of the human studies except for one [31] assessed changes in interleukins, with IL-6 being the most common, closely followed by IL-8, IL-1B, then IL-10, IL-2, IL-12 and IL-3. The second most commonly studied cytokine was TNF (61%, n = 8). Lastly, three studies (23%) assessed changes in INF and only one (8%) assessed changes in GM-CSF. None of the human studies included assessed changes in chemokines.
Animal studies were conducted in mouse or rat models, although studies also included dogs [54], tilapia [45], and guinea pigs [55]. Sixteen trials had a duration of at least two weeks while five lasted four to seven days [41,43,50,57,131] and three lasted one day or less [60,61,63]. The daily dose of Echinacea varied widely from 5 to 500 mg/kg per day.
The cell culture studies used a variety of immune cells. Immune stimulation was achieved through a variety of methods; the most common where exposure to LPS (n = 29), viruses (n = 14) and phytohemagglutinin and/or phorbol 12-myristate 13- acetate (n = 10). Studies assessed changes in the amount of cytokines produced or changes in genetic expression following exposure to Echinacea.
3.1. Change in cytokine levels
The changes in cytokine levels that followed Echinacea supplementation are presented in Fig. 2. Results are presented for the cytokines relevant to the progression of cytokine storm. Among the human studies, decreased levels of the pro-inflammatory cytokine IL-6, IL-8, and TNF were reported by 57, 50, and 62% of studies that measured these cytokines, respectively. Among the animal studies decreased levels of pro-inflammatory cytokines IL-1, IL-6, and TNF, were reported by 73, 78, 74% of studies that measured these cytokines, respectively. However, increased levels of the pro-inflammatory cytokine IL-2 were reported by 57% of animal studies. In addition, an increase in levels of the anti-inflammatory cytokine IL-10 were reported by 57% of animal studies that measured this cytokine. Among the cell culture studies, decreased levels of pro-inflammatory cytokines IL-6, IL-8, CCL2, CCCL3, and CCL4 were reported by 63, 70, 67, 75, 71% of studies that measured these cytokines, respectively. Moreover, nearly two thirds of the cell culture studies that measured levels of the anti-inflammatory cytokine IL-10 reported an increase. IFN levels were increased in the majority of human, animal, and cell culture studies; while this cytokine is considered to be pro-inflammatory, decreased levels of IFN have been detected among COVID-19 patients. None of the studies reported cases of cytokine storm.
Fig. 2.
Change in cytokine levels following Echinacea exposure. A: Human studies, B: animal Studies, C: Cell culture studies.
3.2. Risk of bias assessment
The results of the risk of bias assessments for the human RCT and non-RCT studies are presented in Fig. 3, Fig. 4. In total, six of these studies had a “high risk of bias”, two studies had “some concerns” or “moderate risk of bias” and two studies had “low risk of bias”. Among the pre-post human studies, two received a rating of “fair” and one received a rating of “poor”. Among the animal studies, each one received a rating of “probably high risk of bias” in at least one category. Three received a rating of “definitely high risk of bias” in one category. Additional information on the risk of bias assessment for the pre-post and animal studies is found in Supplemental File 2. Among the cell culture studies, thirty-eight (55%) received as score of 1 corresponding to “reliable without restrictions”. Thirty-one (45%) received a score of 3 corresponding to “unreliable”.
Fig. 3.
Risk of Bias 2.0 for human randomized controlled trials.
Fig. 4.
ROBINS-I Assessment of bias for non-randomized human studies with a comparison.
4. Discussion
The present systematic review identified all human, animal, and cell culture data reporting the impact of Echinacea supplementation on cytokine levels. The data suggest that Echinacea supplementation may be associated with a decrease in the pro-inflammatory cytokines IL-6, IL-8 and TNF as well as an increase in the anti-inflammatory cytokine IL-10. In addition, it may be associated with an increase in IFN, a pro-inflammatory cytokine reported to be low in patients with COVID-19. Overall, the findings of the human and animal studies were more likely to report primarily anti-inflammatory effects. Ex vivo and in vitro studies demonstrated more of a mixture of pro- and anti-inflammatory effects; however, given that they were conducted in the isolation of cell culture rather than in the context of a highly complex, functioning immune system, the results may be less relevant to use in humans. The findings suggest that the use of Echinacea supplementation may be useful in the prevention or management of COVID-19-related cytokine storm in humans, however further targeted studies are needed.
Levels of IL-6 and TNF both independently predict COVID-19 disease severity and mortality [8] and may be important therapeutic targets. Therapies aimed at inhibiting these cytokines have demonstrated improvements in the clinical course of severely ill COVID-19 patients. A meta-analysis of studies administering the IL-6 receptor monoclonal antibody tocilizumab to patients with severe COVID-19 revealed a reduction in mortality and the need for mechanical ventilation [132]. The effects of other immunomodulatory agents including anakinra, an inhibitor of IL-1, and sarilumab and siltuximab, inhibitors of IL-6, were inconclusive [133]. Observational registry data from patients with inflammatory bowel disease who contracted COVID-19 suggest a possible benefit from taking anti-TNF medication in terms of a composite outcome of death or hospital admission, however not with either outcome alone [134]. A call to prioritize the study of anti-TNF therapy has been made [134]. Because IL-6 and TNF are independently associated with clinical outcomes, it has been hypothesized that therapy targeted at the inhibition of both cytokines simultaneously may yield additional benefit and warrant study [8]. Echinacea may decrease production of these two cytokines.
Among the studies identified in the present review, more studies reported an increase in IFN production than a decrease following Echinacea supplementation. While IFN-α and β are considered proinflammatory in nature, they also play a critical role in exerting an antiviral effect. Observation of depressed levels of IFN-α and β among COVID-19 patients has occurred [9]. While the trial reporting this finding was primarily cross-sectional, sequential assessment found that the depressed levels of IFN-α preceded worsening of disease severity and transfer to more intensive care [9]. The virus SARS-CoV, the causative agent of severe acute respiratory syndrome (SARS), inhibits production of IFNs in order to diminish the innate immune response of the host [135]. A need to explore therapeutic approaches to increase IFN in the treatment of COVID-19 has been proposed [9].
Additional evidence that may be considered regarding the potential usefulness of Echinacea in the management of COVID-19 include the herb's ability to decrease the severity and duration of acute respiratory tract infections [22] and in vitro data demonstrating direct antiviral effect of Echinacea against several coronaviruses including SARS-CoV-2([136]).
The present review has several strengths and limitations. Strengths of the review include a rigorous search strategy that was conducted in multiple databases, as well as duplicate screening and data extraction. The review process is limited by a high level of heterogeneity among the included studies and subsequently, the inability to complete meta-analysis. The findings are limited by the high risk of bias found in many of the included studies. They are also limited by the fact that none of the studies assessed the impact of Echinacea on cytokine changes in patients or models of COVID-19. Many of the human studies involved healthy participants or participants with relatively mild infections such as the common cold. The animal and cell culture studies used a variety of immune stimulating agents such as lipopolysaccharide (LPS), bacterial and viral infections. While animal models of cytokine storm exist [137], none were used by the studies included in the present review. These factors may decrease the generalizability of the findings to the treatment of COVID-19.
Similarly, the studies did not assess the changes in cytokine levels in models of cytokine storm. Cytokine storm is a complex syndrome involving cascades of interdependent inflammatory mediators which changes over the course of clinical progression. Defining this condition has been challenging due to the difficulty of differentiating a dysregulated immune response from a physiologic response to a severe infection [7]. Cytokines play an important role in the host response to an infection but at the same time, may cause harm to the host when released in excess. It has been hypothesized that inhibition of cytokine signaling could impair clearance of SARS-CoV-2, and result in worse outcomes such as secondary infections; this has been previously observed in the treatment of influenza [138] and subsequent to the use of IL-6 inhibitors in COVID-19 patients [133]. These findings may suggest that immune modulation may be appropriate for only a subgroup of COVID-19 patients. Additionally, cytokine production varies over the course of the response to the pathogen. Ideally, the immune response should be proportionate to the severity of the infection and result in a return to homeostasis following clearance of the pathogen [7]. The importance of timing may be relevant to interpreting the findings of the present review. The included studies measured cytokine levels at a variety of timepoints in the course of an infection; the impact of timing may account for some of the heterogeneity in the results presented. It has been hypothesized that the cytokine storm seen in COVID-19 occurs in two stages. The first stage is an underactive initial immune response which fails to adequately clear the virus. Subsequently, in response to the failed clearance, there is an overactive immune response [139]. Changes in the immune response at different time points in the course of disease progression suggest that the timing of different immunomodulatory therapies may be highly important [139].
5. Conclusion
The findings of the present systematic review suggest that the effect of Echinacea supplementation on cytokines may be predominantly anti-inflammatory, including the inhibition of cytokines that play a key role in the progression of severe COVID-19. Investigation of the potential therapeutic role of Echinacea supplementation in the prevention or treatment of cytokine storm due to COVID-19 may be warranted.
Funding
No funding was received for the conduct of this research.
Author contributions
The project was conceived by MA, KC and VC. MA, KC and VC developed the study protocol. The search strategy was conducted by VC. Data extraction was completed by all authors. Preliminary data analysis was completed by MA. All authors contributed to manuscript preparation and approved the final manuscript draft.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
The authors wish to acknowledge the long history of use of Echinacea by the Indigenous peoples of North America.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2021.100115.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2021. WHO (COVID-19) homepage.https://covid19.who.int/ [Available from: [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19) StatPearls. Treasure Island (FL) 2021 [PubMed] [Google Scholar]
- 6.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoletti M., Marconi L., Scudeller L., Pancaldi L., Tedeschi S., Giannella M. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect. 2021;27(1):105–111. doi: 10.1016/j.cmi.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariyanto T.I., Kristine E., Jillian hardi C., Kurniawan A. Efficacy of Lopinavir/Ritonavir compared with standard care for treatment of coronavirus disease 2019 (COVID-19): a systematic review. Infect Disord Drug Targets. 2020 doi: 10.2174/1871526520666201029125725. PMID: 33121422. [DOI] [PubMed] [Google Scholar]
- 13.Hariyanto T.I., Halim D.A., Jodhinata C., Yanto T.A., Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823–830. doi: 10.1111/1440-1681.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin H.X.J., Cho S., Meyyur Aravamudan V., Sanda H.Y., Palraj R., Molton J.S. Remdesivir in Coronavirus Disease 2019 (COVID-19) treatment: a review of evidence. Infection. 2021;49(3):401–410. doi: 10.1007/s15010-020-01557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group Rc Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percival S.S. Use of echinacea in medicine. Biochem Pharmacol. 2000;60(2):155–158. doi: 10.1016/s0006-2952(99)00413-x. [DOI] [PubMed] [Google Scholar]
- 19.Bauer R. [Echinacea drugs–effects and active ingredients] Z Arztl Fortbild. 1996;90(2):111–115. [PubMed] [Google Scholar]
- 20.Barnes J., Anderson L.A., Gibbons S., Phillipson J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt.,Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57(8):929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 21.Manayi A., Vazirian M., Saeidnia S. Echinacea purpurea: pharmacology, phytochemistry and analysis methods. Pharm Rev. 2015;9(17):63–72. doi: 10.4103/0973-7847.156353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aucoin M., Cooley K., Saunders P.R., Care J., Anheyer D., Medina D.N. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv Integr Med. 2020;7(4):203–217. doi: 10.1016/j.aimed.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Sterne J.A., Hernan M.A., Reeves B.C., Savovic J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIH Quality assessment tool for before-after (Pre-Post) studies with No control group [available from. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 26.Rooney A.A., Boyles A.L., Wolfe M.S., Bucher J.R., Thayer K.A. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122(7):711–718. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider K., Schwarz M., Burkholder I., Kopp-Schneider A., Edler L., Kinsner-Ovaskainen A. ToxRTool", a new tool to assess the reliability of toxicological data. Toxicol Lett. 2009;189(2):138–144. doi: 10.1016/j.toxlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Barrett B., Brown R., Rakel D., Mundt M., Bone K., Barlow S. Echinacea for treating the common cold: a randomized trial. Ann Intern Med. 2010;153(12):769–777. doi: 10.7326/0003-4819-153-12-201012210-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isbaniah F., Wiyono W.H., Yunus F., Setiawati A., Totzke U., Verbruggen M.A. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: results from a randomized controlled trial. J Clin Pharm Therapeut. 2011;36(5):568–576. doi: 10.1111/j.1365-2710.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 30.Turner R.B., Bauer R., Woelkart K., Hulsey T.C., Gangemi J.D. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353(4):341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 31.Kim L.S., Waters R.F., Burkholder P.M. Immunological activity of larch arabinogalactan and Echinacea: a preliminary, randomized, double-blind, placebo-controlled trial. Alternative Med Rev. 2002;7(2):138–149. [PubMed] [Google Scholar]
- 32.Whitehead M.T., Martin T.D., Scheett T.P., Webster M.J. The effect of 4 wk of oral echinacea supplementation on serum erythropoietin and indices of erythropoietic status. Int J Sport Nutr Exerc Metabol. 2007;17(4):378–390. doi: 10.1123/ijsnem.17.4.378. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz E., Metzler J., Diedrich J.P., Freudenstein J., Bode C., Bode J.C. Oral administration of freshly expressed juice of Echinacea purpurea herbs fail to stimulate the nonspecific immune response in healthy young men: results of a double-blind, placebo-controlled crossover study. Journal of immunotherapy (Hagerstown, Md. 1997) 2002;25(5):413–420. doi: 10.1097/00002371-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Berg A., Northoff H., Konig D., Weinstock C., Grathwohl D., Parnham M.J. Influence of Echinacin (EC31) treatment on the exercise-induced immune response in athletes. J Clin Res. 1998;1(367–380):367–380. [Google Scholar]
- 35.Obukhova O.O., Shvayuk A.P., Gorbenko O.M., Trunov A.N., Trunova L.A. Content of proinflammatory cytokine in patients with clinical remission of chronic herpes infection during immunocorrection. Bull Exp Biol Med. 2008;146(6):803–805. doi: 10.1007/s10517-009-0418-1. [DOI] [PubMed] [Google Scholar]
- 36.Roesler J., Emmendorffer A., Steinmuller C., Luettig B., Wagner H., Lohmann-Matthes M.L. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to test subjects mediates activation of the phagocyte system. Int J Immunopharm. 1991;13(7):931–941. doi: 10.1016/0192-0561(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 37.Dapas B., Dall'Acqua S., Bulla R., Agostinis C., Perissutti B., Invernizzi S. Immunomodulation mediated by a herbal syrup containing a standardized Echinacea root extract: a pilot study in healthy human subjects on cytokine gene expression. Phytomedicine. international journal of phytotherapy and phytopharmacology. 2014;21(11):1406–1410. doi: 10.1016/j.phymed.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 38.Dall'Acqua S., Perissutti B., Grabnar I., Farra R., Comar M., Agostinis C. Pharmacokinetics and immunomodulatory effect of lipophilic Echinacea extract formulated in softgel capsules. European journal of pharmaceutics and biopharmaceutics. official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2015;97(Pt A):8–14. doi: 10.1016/j.ejpb.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Randolph R.K., Gellenbeck K., Stonebrook K., Brovelli E., Qian Y., Bankaitis-Davis D. Regulation of human immune gene expression as influenced by a commercial blended Echinacea product: preliminary studies. Exp Biol Med. 2003;228(9):1051–1056. doi: 10.1177/153537020322800910. [DOI] [PubMed] [Google Scholar]
- 40.Guiotto P., Woelkart K., Grabnar I., Voinovich D., Perissutti B., Invernizzi S. Pharmacokinetics and immunomodulatory effects of phytotherapeutic lozenges (bonbons) with Echinacea purpurea extract. Phytomedicine. international journal of phytotherapy and phytopharmacology. 2008;15(8):547–554. doi: 10.1016/j.phymed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Goel V., Chang C., Slama J., Barton R., Bauer R., Gahler R. Echinacea stimulates macrophage function in the lung and spleen of normal rats. J Nutr Biochem. 2002;13(8):487. doi: 10.1016/s0955-2863(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 42.Yamada K., Hung P., Park T.K., Park P.J., Lim B.O. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J Ethnopharmacol. 2011;137(1):231–235. doi: 10.1016/j.jep.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Fusco D., Liu X., Savage C., Taur Y., Xiao W., Kennelly E. Echinacea purpurea aerial extract alters course of influenza infection in mice. Vaccine. 2010;28(23):3956–3962. doi: 10.1016/j.vaccine.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang L., Li W., Wang Y., Zhang X., Yu D., Yin Y. Effects of cichoric acid extract from Echinacea purpurea on collagen-induced arthritis in rats. Am J Chin Med. 2014;42(3):679–692. doi: 10.1142/S0192415X1450044X. [DOI] [PubMed] [Google Scholar]
- 45.Abdel Rahman A.N., Khalil A.A., Abdallah H.M., ElHady M. The effects of the dietary supplementation of Echinacea purpurea extract and/or vitamin C on the intestinal histomorphology, phagocytic activity, and gene expression of the Nile tilapia. Fish Shellfish Immunol. 2018;82(dr8, 9505220):312–318. doi: 10.1016/j.fsi.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 46.Abdelmonem M., Kassem S.H., Gabr H., Shaheen A.A., Aboushousha T. Avemar and Echinacea extracts enhance mobilization and homing of CD34(+) stem cells in rats with acute myocardial infarction. Stem Cell Res Ther. 2015;6(101527581):172. doi: 10.1186/s13287-015-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cundell D.R., Matrone M.A., Ratajczak P., Pierce J.D., Jr. The effect of aerial parts of Echinacea on the circulating white cell levels and selected immune functions of the aging male Sprague-Dawley rat. Int Immunopharm. 2003;3(7):1041–1048. doi: 10.1016/S1567-5769(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 48.Dogan Z., Ergul B., Sarikaya M., Filik L., Gonultas M.A., Hucumenoglu S. The protective effect of Echinacea spp. (Echinacea angustifolia and Echinacea purpurea) in a rat colitis model induced by acetic acid. Pak J Pharm Sci. 2014;27(6):1827–1835. [PubMed] [Google Scholar]
- 49.Ghaemi A., Soleimanjahi H., Gill P., Arefian E., Soudi S., Hassan Z. Echinacea purpurea polysaccharide reduces the latency rate in herpes simplex virus type-1 infections. Intervirology. 2009;52(1):29–34. doi: 10.1159/000212988. [DOI] [PubMed] [Google Scholar]
- 50.Goel V., Chang C., Slama J.V., Barton R., Bauer R., Gahler R. Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. Int Immunopharm. 2002;2(2–3):381–387. doi: 10.1016/s1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi I., Ohotsuki M., Suzuki I., Watanabe T. Effects of oral administration of Echinacea purpurea (American herb) on incidence of spontaneous leukemia caused by recombinant leukemia viruses in AKR/J mice. Nihon Rinsho Men'eki Gakkai kaishi = Japanese journal of clinical immunology. 2001;24(1):10–20. doi: 10.2177/jsci.24.10. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., Zhang S., Zhang F., Hu R. Adjuvant activity of Chinese herbal polysaccharides in inactivated veterinary rabies vaccines. Int J Biol Macromol. 2012;50(3):598–602. doi: 10.1016/j.ijbiomac.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 53.Park S., Lee M.-S., Jung S., Lee S., Kwon O., Kreuter M.H. Echinacea purpurea protects against restraint stress-induced immunosuppression in BALB/c mice. J Med Food. 2018;21(3):261–268. doi: 10.1089/jmf.2017.4073. [DOI] [PubMed] [Google Scholar]
- 54.Sgorlon S., Stefanon B., Sandri M., Colitti M. Nutrigenomic activity of plant derived compounds in health and disease: results of a dietary intervention study in dog. Res Vet Sci. 2016:109. doi: 10.1016/j.rvsc.2016.10.005. ((Sgorlon, Stefanon, Sandri, Colitti) Department of Agricultural, Food, Environmental and Animal Sciences, University of Udine, Via delle Scienze, 208, Udine 33100, Italy):142-8. [DOI] [PubMed] [Google Scholar]
- 55.Sutovska M., Capek P., Kazimierova I., Pappova L., Joskova M., Matulova M. Echinacea complex–chemical view and anti-asthmatic profile. J Ethnopharmacol. 2015;175(7903310, k8t):163–171. doi: 10.1016/j.jep.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Uluisik D., Keskin E. Effects of ginseng and echinacea on cytokine mRNA expression in rats. TheScientificWorldJOURNAL. 2012;2012(101131163) doi: 10.1100/2012/942025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu D., Yuan Y., Jiang L., Tai Y., Yang X., Hu F. Anti-inflammatory effects of essential oil in Echinacea purpurea L. Pak J Pharm Sci. 2013;26(2):403–408. [PubMed] [Google Scholar]
- 58.Zhai Z., Liu Y., Wu L., Senchina D.S., Wurtele E.S., Murphy P.A. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J Med Food. 2007;10(3):423–434. doi: 10.1089/jmf.2006.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdallah H.M.I., Asaad G.F.M., Nada S.A., Taha H.S., El-Nasr M.M.S. Influence of extract derived in-vitro cell suspension cultures of Echinacea purpurea against some immunosuppressive effects. Research Journal of Pharmaceutical. Biological and Chemical Sciences. 2015;6(1):1136–1143. [Google Scholar]
- 60.Li Q., Yang F., Hou R., Huang T., Hao Z. Post-screening characterization of an acidic polysaccharide from Echinacea purpurea with potent anti-inflammatory properties in vivo. Food & function. 2020;11(9):7576–7583. doi: 10.1039/d0fo01367f. [DOI] [PubMed] [Google Scholar]
- 61.Shi Q., Lang W., Wang S., Li G., Bai X., Yan X. Echinacea polysaccharide attenuates lipopolysaccharide-induced acute kidney injury via inhibiting inflammation, oxidative stress and the MAPK signaling pathway. Int J Mol Med. 2021;47(1):243–255. doi: 10.3892/ijmm.2020.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turkistani A.M. Modulatory effect of echinacea purpurea root extract on cisplatin-induced renal toxicity in rats: antioxidant and anti-inflammatory pathways. Int J Pharm Phytopharmacol Res. 2019;9(5):88–96. [Google Scholar]
- 63.Zhang H., Lang W., Wang S., Li B., Li G., Shi Q. 2020. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-kappaB signal pathway. International Immunopharmacology; p. 88. ((Zhang, Shi) College of Animal Science and Technology, Hebei Normal University of Science and Technology, Qinhuangdao, Hebei 066004, China(Lang) College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China(Wang) Institu):106974. [DOI] [PubMed] [Google Scholar]
- 64.Altamirano-Dimas M., Hudson J.B., Cochrane D., Nelson C., Arnason J.T. Modulation of immune response gene expression by echinacea extracts: results of a gene array analysis. Can J Physiol Pharmacol. 2007;85(11):1091–1098. doi: 10.1139/Y07-110. [DOI] [PubMed] [Google Scholar]
- 65.Altamirano-Dimas M., Sharma M., Hudson J.B. Echinacea and anti-inflammatory cytokine responses: results of a gene and protein array analysis. Pharmaceut Biol. 2009;47(6):500–508. [Google Scholar]
- 66.Benson J.M., Pokorny A.J., Rhule A., Wenner C.A., Kandhi V., Cech N.B. Echinacea purpurea extracts modulate murine dendritic cell fate and function. Food and chemical toxicology. an international journal published for the British Industrial Biological Research Association. 2010;48(5):1170–1177. doi: 10.1016/j.fct.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brovelli E.A., Rua D., Roh-Schmidt H., Chandra A., Lamont E., Noratto G.D. Human gene expression as a tool to determine horticultural maturity in a bioactive plant (Echinacea purpurea L. Moench) J Agric Food Chem. 2005;53(21):8156–8161. doi: 10.1021/jf0505372. [DOI] [PubMed] [Google Scholar]
- 68.Burger R.A., Torres A.R., Warren R.P., Caldwell V.D., Hughes B.G. Echinacea-induced cytokine production by human macrophages. Int J Immunopharm. 1997;19(7):371–379. doi: 10.1016/s0192-0561(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 69.Cadiz M.P., Schara M.R., Kemp B.H. Gibbons johnson RM. Echinacea purpurea root extract increases tumor necrosis factor production by Concanavalin A-activated murine splenocytes. J Med Food. 2019;22(11):1146–1150. doi: 10.1089/jmf.2019.0065. [DOI] [PubMed] [Google Scholar]
- 70.Canlas J., Hudson J.B., Sharma M., Nandan D. Echinacea and trypanasomatid parasite interactions: growth-inhibitory and anti-inflammatory effects of Echinacea. Pharmaceut Biol. 2010;48(9):1047–1052. doi: 10.3109/13880200903483468. [DOI] [PubMed] [Google Scholar]
- 71.Cech N.B., Kandhi V., Davis J.M., Hamilton A., Eads D., Laster S.M. Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE2 from RAW 264.7 macrophage-like cells. Int Immunopharm. 2010;10(10):1268–1278. doi: 10.1016/j.intimp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Cech N.B., Tutor K., Doty B.A., Spelman K., Sasagawa M., Raner G.M. Liver enzyme-mediated oxidation of Echinacea purpurea alkylamides: production of novel metabolites and changes in immunomodulatory activity. Planta Med. 2006;72(15):1372–1377. doi: 10.1055/s-2006-951718. [DOI] [PubMed] [Google Scholar]
- 73.Chicca A., Raduner S., Pellati F., Strompen T., Altmann K.-H., Schoop R. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharm. 2009;9(7–8):850–858. doi: 10.1016/j.intimp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Chiu S.-C., Tsao S.-W., Hwang P.-I., Vanisree S., Chen Y.-A., Yang N.-S. Differential functional genomic effects of anti-inflammatory phytocompounds on immune signaling. BMC Genom. 2010;11(100965258):513. doi: 10.1186/1471-2164-11-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Classen B., Thude S., Blaschek W., Wack M., Bodinet C. Immunomodulatory effects of arabinogalactan-proteins from baptisia and echinacea. Phytomedicine. international journal of phytotherapy and phytopharmacology. 2006;13(9–10):688–694. doi: 10.1016/j.phymed.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Dong G.-C., Chuang P.-H., Forrest M.D., Lin Y.-C., Chen H.M. Immuno-suppressive effect of blocking the CD28 signaling pathway in T-cells by an active component of Echinacea found by a novel pharmaceutical screening method. J Med Chem. 2006;49(6):1845–1854. doi: 10.1021/jm0509039. [DOI] [PubMed] [Google Scholar]
- 77.Farinacci M., Colitti M., Stefanon B. Modulation of ovine neutrophil function and apoptosis by standardized extracts of Echinacea angustifolia, Butea frondosa and Curcuma longa. Vet Immunol Immunopathol. 2009;128(4):366–373. doi: 10.1016/j.vetimm.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 78.Fonseca F.N., Papanicolaou G., Lin H., Lau C.B.S., Kennelly E.J., Cassileth B.R. Echinacea purpurea (L.) Moench modulates human T-cell cytokine response. Int Immunopharm. 2014;19(1):94–102. doi: 10.1016/j.intimp.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu A., Wang Y., Wu Y., Chen H., Zheng S., Li Y. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J Cell Biochem. 2017;118(9):2664–2671. doi: 10.1002/jcb.25875. [DOI] [PubMed] [Google Scholar]
- 80.Groom S.N., Johns T., Oldfield P.R. The potency of immunomodulatory herbs may be primarily dependent upon macrophage activation. J Med Food. 2007;10(1):73–79. doi: 10.1089/jmf.2006.233. [DOI] [PubMed] [Google Scholar]
- 81.Guidetti G., Di Cerbo A., Giovazzino A., Rubino V., Palatucci A.T., Centenaro S. 2016. Vitro effects of some botanicals with anti-inflammatory and antitoxic activity. Journal of immunology research; p. 2016. ((Guidetti, Centenaro, Fraccaroli, Canello) Division of Research and Development, SANYpet SpA, Bagnoli di Sopra 35023, Italy(Di Cerbo) School of Specialization in Clinical Biochemistry, G. d'Annunzio University, Chieti 66100, Italy(Giovazzino, Rubino, Rugg):5457010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gulledge T.V., Collette N.M., Mackey E., Johnstone S.E., Moazami Y., Todd D.A. Mast cell degranulation and calcium influx are inhibited by an Echinacea purpurea extract and the alkylamide dodeca-2E,4E-dienoic acid isobutylamide. J Ethnopharmacol. 2018;212(7903310, k8t):166–174. doi: 10.1016/j.jep.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hou C.-C., Chen C.-H., Yang N.-S., Chen Y.-P., Lo C.-P., Wang S.-Y. Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J Nutr Biochem. 2010;21(11):1045–1059. doi: 10.1016/j.jnutbio.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 84.Hwang S.-A., Dasgupta A., Actor J.K. Cytokine production by non-adherent mouse splenocyte cultures to Echinacea extracts. Clinica chimica acta. international journal of clinical chemistry. 2004;343(1–2):161–166. doi: 10.1016/j.cccn.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Lee N.Y., Chung K.-S., Jin J.S., Bang K.S., Eom Y.-J., Hong C.-H. Effect of chicoric acid on Mast cell-mediated allergic inflammation in vitro and in vivo. J Nat Prod. 2015;78(12):2956–2962. doi: 10.1021/acs.jnatprod.5b00668. [DOI] [PubMed] [Google Scholar]
- 86.Li Y., Wang Y., Wu Y., Wang B., Chen X., Chen H. 2017. Echinacea pupurea extracts promote murine dendritic cell maturation by activation of JNK, p38 MAPK and NF-kappaB pathways. Developmental and Comparative Immunology; p. 73. ((Li, Wang, Wu, Wang, Xu, Li, Xu) Key Laboratory of Molecular Animal Nutrition and Feed Sciences, College of Animal Science, Zhejiang University, Hangzhou 310058, China(Li) Animal Nutrition and Human Health Laboratory, School of Life Sciences, Hunan Normal):21-6. [DOI] [PubMed] [Google Scholar]
- 87.Luettig B., Steinmuller C., Gifford G.E., Wagner H., Lohmann-Matthes M.L. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst. 1989;81(9):669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 88.McCann D.A., Solco A., Liu Y., Macaluso F., Murphy P.A., Kohut M.L. Cytokine- and interferon-modulating properties of Echinacea spp. root tinctures stored at -20degreeC for 2 years. J Interferon Cytokine Res. 2007;27(5):425–436. doi: 10.1089/jir.2006.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moazami Y., Gulledge T.V., Laster S.M., Pierce J.G. Synthesis and biological evaluation of a series of fatty acid amides from Echinacea. Bioorg Med Chem Lett. 2015;25(16):3091–3094. doi: 10.1016/j.bmcl.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 90.Olah A., Szabo-Papp J., Soeberdt M., Knie U., Dahnhardt-Pfeiffer S., Abels C. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J Dermatol Sci. 2017;88(1):67–77. doi: 10.1016/j.jdermsci.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Pomari E., Stefanon B., Colitti M. Effect of plant extracts on H2O2-induced inflammatory gene expression in macrophages. J Inflamm Res. 2014;7(1):103–112. doi: 10.2147/JIR.S61471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raduner S., Majewska A., Chen J.-Z., Xie X.-Q., Hamon J., Faller B. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J Biol Chem. 2006;281(20):14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- 93.Senchina D.S., Flagel L.E., Wendel J.F., Kohut M.L. PHENETIC COMPARISON OF SEVEN echinacea SPECIES BASED ON IMMUNOMODULATORY CHARACTERISTICS. Econ Bot. 2006;60(3):205–211. doi: 10.1663/0013-0001(2006)60[205:pcoses]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Senchina D.S., Hallam J.E., Dias A.S., Perera M.A. Human blood mononuclear cell in vitro cytokine response before and after two different strenuous exercise bouts in the presence of bloodroot and Echinacea extracts. Blood Cells Mol Dis. 2009;43(3):298–303. doi: 10.1016/j.bcmd.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Senchina D.S., Martin A.E., Buss J.E., Kohut M.L. Effects of Echinacea extracts on macrophage antiviral activities. Phytotherapy research. PT. 2010;24(6):810–816. doi: 10.1002/ptr.2991. [DOI] [PubMed] [Google Scholar]
- 96.Senchina D.S., McCann D.A., Asp J.M., Johnson J.A., Cunnick J.E., Kaiser M.S. Changes in immunomodulatory properties of Echinacea spp. root infusions and tinctures stored at 4degreeC for four days. Clin Chim Acta. 2005;355(1–2):67–82. doi: 10.1016/j.cccn.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 97.Senchina D.S., McCann D.A., Flinn G.N., Wu L., Zhai Z., Cunnick J.E. Echinacea tennesseensis ethanol tinctures harbor cytokine- and proliferation-enhancing capacities. Cytokine. 2009;46(2):267–272. doi: 10.1016/j.cyto.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Senchina D.S., Strauch J.H., Hoffmann G.B., Shah N.B., Laflen B.K., Dumke B.L. Phytochemical and immunomodulatory properties of an Echinacea laevigata (Asteraceae) tincture. J Alternative Compl Med. 2011;17(4):375–377. doi: 10.1089/acm.2010.0373. [DOI] [PubMed] [Google Scholar]
- 99.Senchina D.S., Wu L., Flinn G.N., Konopka D.N., McCoy J.-A., Widrlechner M.P. Year-and-a-half old, dried Echinacea roots retain cytokine-modulating capabilities in an in vitro human older adult model of influenza vaccination. Planta Med. 2006;72(13):1207–1215. doi: 10.1055/s-2006-947254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma M., Anderson S.A., Schoop R., Hudson J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir Res. 2009;83(2):165–170. doi: 10.1016/j.antiviral.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Sharma M., Arnason J.T., Burt A., Hudson J.B. Echinacea extracts modulate the pattern of chemokine and cytokine secretion in rhinovirus-infected and uninfected epithelial cells. Phytotherapy research. PT. 2006;20(2):147–152. doi: 10.1002/ptr.1824. [DOI] [PubMed] [Google Scholar]
- 102.Sharma M., Schoop R., Hudson J.B. Echinacea as an antiinflammatory agent: the influence of physiologically relevant parameters. Phytotherapy research. PT. 2009;23(6):863–867. doi: 10.1002/ptr.2714. [DOI] [PubMed] [Google Scholar]
- 103.Sharma M., Schoop R., Hudson J.B. The efficacy of Echinacea in a 3-D tissue model of human airway epithelium. Phytotherapy research : PT. 2010;24(6):900–904. doi: 10.1002/ptr.3051. [DOI] [PubMed] [Google Scholar]
- 104.Sharma M., Schoop R., Suter A., Hudson J.B. The potential use of Echinacea in acne: control of Propionibacterium acnes growth and inflammation. Phytotherapy research : PT. 2011;25(4):517–521. doi: 10.1002/ptr.3288. [DOI] [PubMed] [Google Scholar]
- 105.Sharma S.M., Anderson M., Schoop S.R., Hudson J.B. Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce): dual actions against respiratory bacteria. Phytomedicine. international journal of phytotherapy and phytopharmacology. 2010;17(8–9):563–568. doi: 10.1016/j.phymed.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 106.Stimpel M., Proksch A., Wagner H., Lohmann-Matthes M.L. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun. 1984;46(3):845–849. doi: 10.1128/iai.46.3.845-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilasrusmee C., Siddiqui J., Bruch D., Wilasrusmee S., Kittur S., Kittur D.S. In vitro immunomodulatory effects of herbal products. Am Surg. 2002;68(10):860–864. [PubMed] [Google Scholar]
- 108.Wu H., Nardone A., Lacetera N. Effects of a standardized purified dry extract from Echinacea angustifolia on proliferation and interferon gamma secretion of peripheral blood mononuclear cells in dairy heifers. Res Vet Sci. 2009;87(3):396–398. doi: 10.1016/j.rvsc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 109.Zhang X., Rizshsky L., Hauck C., Qu L., Widrlechner M.P., Nikolau B.J. Bauer ketones 23 and 24 from Echinacea paradoxa var. paradoxa inhibit lipopolysaccharide-induced nitric oxide, prostaglandin E2 and cytokines in RAW264.7 mouse macrophages. Phytochemistry. 2012;74(alb, 0151434):146–158. doi: 10.1016/j.phytochem.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kapai N.A., Anisimova N.Y., Kiselevskii M.V., Sitdikova S.M., Slavetskaya M.B. Selective cytokine-inducing effects of low dose Echinacea. Bull Exp Biol Med. 2011;150(6):711–713. doi: 10.1007/s10517-011-1230-2. [DOI] [PubMed] [Google Scholar]
- 111.Morazzoni P., Cristoni A., Di Pierro F., Avanzini C., Ravarino D., Stornello S. In vitro and in vivo immune stimulating effects of a new standardized Echinacea angustifolia root extract (PolinaceaTM) Fitoterapia. 2005;76(5):401–411. doi: 10.1016/j.fitote.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 112.Ritchie M.R., Gertsch J., Klein P., Schoop R. Effects of Echinaforce treatment on ex vivo-stimulated blood cells. Phytomedicine. 2011;18(10):826–831. doi: 10.1016/j.phymed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 113.Sasagawa M., Cech N.B., Gray D.E., Elmer G.W., Wenner C.A. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int Immunopharm. 2006;6(7):1214–1221. doi: 10.1016/j.intimp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Spelman K., Iiams-Hauser K., Cech N.B., Taylor E.W., Smirnoff N., Wenner C.A. Role for PPARgamma in IL-2 inhibition in T cells by Echinacea-derived undeca-2E-ene-8,10-diynoic acid isobutylamide. Int Immunopharm. 2009;9(11):1260–1264. doi: 10.1016/j.intimp.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 115.Vimalanathan S., Arnason J.T., Hudson J.B. Anti-inflammatory activities of Echinacea extracts do not correlate with traditional marker components. Pharmaceut Biol. 2009;47(5):430–435. [Google Scholar]
- 116.Codorean E., Nichita C., Albulescu L., Raducan E., Popescu I.D., Lonita A.C. Correlation of XMAP and ELISA cytokine profiles; development and validation for immunotoxicological studies in vitro. Roum Arch Microbiol Immunol. 2010;69(1):13–19. [PubMed] [Google Scholar]
- 117.Matthias A., Banbury L., Stevenson L.M., Bone K.M., Leach D.N., Lehmann R.P. Alkylamides from echinacea modulate induced immune responses in macrophages. Immunol Invest. 2007;36(2):117–130. doi: 10.1080/08820130600745786. [DOI] [PubMed] [Google Scholar]
- 118.Mishima S., Saito K., Maruyama H., Inoue M., Yamashita T., Ishida T. Antioxidant and immuno-enhancing effects of Echinacea purpurea. Biol Pharmaceut Bull. 2004;27(7):1004–1009. doi: 10.1248/bpb.27.1004. [DOI] [PubMed] [Google Scholar]
- 119.Pugh N.D., Balachandran P., Lata H., Dayan F.E., Joshi V., Bedir E. Melanin: dietary mucosal immune modulator from Echinacea and other botanical supplements. Int Immunopharm. 2005;5(4):637–647. doi: 10.1016/j.intimp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 120.Rininger J.A., Kickner S., Chigurupati P., McLean A., Franck Z. Immunopharmacological activity of Echinacea preparations following simulated digestion on murine macrophages and human peripheral blood mononuclear cells. J Leukoc Biol. 2000;68(4):503–510. [PubMed] [Google Scholar]
- 121.Sullivan A.M., Laba J.G., Moore J.A., Lee T.D.G. Echinacea-induced macrophage activation. Immunopharmacol Immunotoxicol. 2008;30(3):553–574. doi: 10.1080/08923970802135534. [DOI] [PubMed] [Google Scholar]
- 122.Todd D.A., Gulledge T.V., Britton E.R., Oberhofer M., Leyte-Lugo M., Moody A.N. Ethanolic echinacea purpurea extracts contain a mixture of cytokine-suppressive and cytokine-inducing compounds, including some that originate from endophytic bacteria. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0124276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vimalanathan S., Schoop R., Suter A., Hudson J. Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017;233(x98, 8410979):51–59. doi: 10.1016/j.virusres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Wang C.-Y., Chiao M.-T., Yen P.-J., Huang W.-C., Hou C.-C., Chien S.-C. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: a cell- and gene-based study. Genomics. 2006;88(6):801–808. doi: 10.1016/j.ygeno.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 125.Wang C.-Y., Staniforth V., Chiao M.-T., Hou C.-C., Wu H.-M., Yeh K.-C. Genomics and proteomics of immune modulatory effects of a butanol fraction of echinacea purpurea in human dendritic cells. BMC Genom. 2008;9(100965258):479. doi: 10.1186/1471-2164-9-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang G., Li K., Liu C., Peng P., Bai M., Sun J. A comparison of the immunostimulatory effects of polysaccharides from tetraploid and diploid echinacea purpurea. BioMed Res Int. 2018;2018(101600173) doi: 10.1155/2018/8628531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao L., Bai L., Tan Y., Sun J., Qu Q., Shi D. The immunoregulatory effect of sulfated Echinacea purpurea polysaccharide on chicken bone marrow-derived dendritic cells. Int J Biol Macromol. 2019;139(ay6, 7909578):1123–1132. doi: 10.1016/j.ijbiomac.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 128.Fan M.Z., Wu X.H., Li X.F., Piao X.C., Jiang J., Lian M.L. 2021. Co-cultured adventitious roots of Echinacea pallida and Echinacea purpurea inhibit lipopolysaccharide-induced inflammation via MAPK pathway in mouse peritoneal macrophages. Chinese Herbal Medicines. ((Fan, Wu, Li, Piao, Jiang, Lian) College of Agriculture, Yanbian University, Yanji 133002, China) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fonseca F.N., Papanicolaou G., Lin H., Lau C.B.S., Kennelly E., Cassileth B.R. Echinacea Purpurea L. modulates human t-cell cytokine response. Planta Med. 2012;(11):78. doi: 10.1016/j.intimp.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Woelkart K., Marth E., Suter A., Schoop R., Raggam R.B., Koidl C. Bioavailability and pharmacokinetics of Echinacea purpurea preparations and their interaction with the immune system. Int J Clin Pharmacol Therapeut. 2006;44(9):401–408. doi: 10.5414/cpp44401. [DOI] [PubMed] [Google Scholar]
- 131.Zhai Z., Haney D., Wu L., Solco A., Murphy P.A., Wurtele E.S. Alcohol extracts of Echinacea inhibit production of nitric oxide and tumor necrosis factor-alpha by macrophages in vitro. Food Agric Immunol. 2007;18(3–4):221–236. doi: 10.1080/09540100701797363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aziz M., Haghbin H., Abu Sitta E., Nawras Y., Fatima R., Sharma S. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 133.Khan F.A., Stewart I., Fabbri L., Moss S., Robinson K., Smyth A.R. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-215266. [DOI] [PubMed] [Google Scholar]
- 134.Robinson P.C., Richards D., Tanner H.L., Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2(11):e653–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Signer J., Jonsdottir H.R., Albrich W.C., Strasser M., Zust R., Ryter S. In vitro virucidal activity of Echinaforce(R), an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol J. 2020;17(1):136. doi: 10.1186/s12985-020-01401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J., Kaplan N., Wysocki J., Yang W., Lu K., Peng H. The ACE2-deficient mouse: a model for a cytokine storm-driven inflammation. Faseb J. 2020;34(8):10505–10515. doi: 10.1096/fj.202001020R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lauder S.N., Jones E., Smart K., Bloom A., Williams A.S., Hindley J.P. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013;43(10):2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.