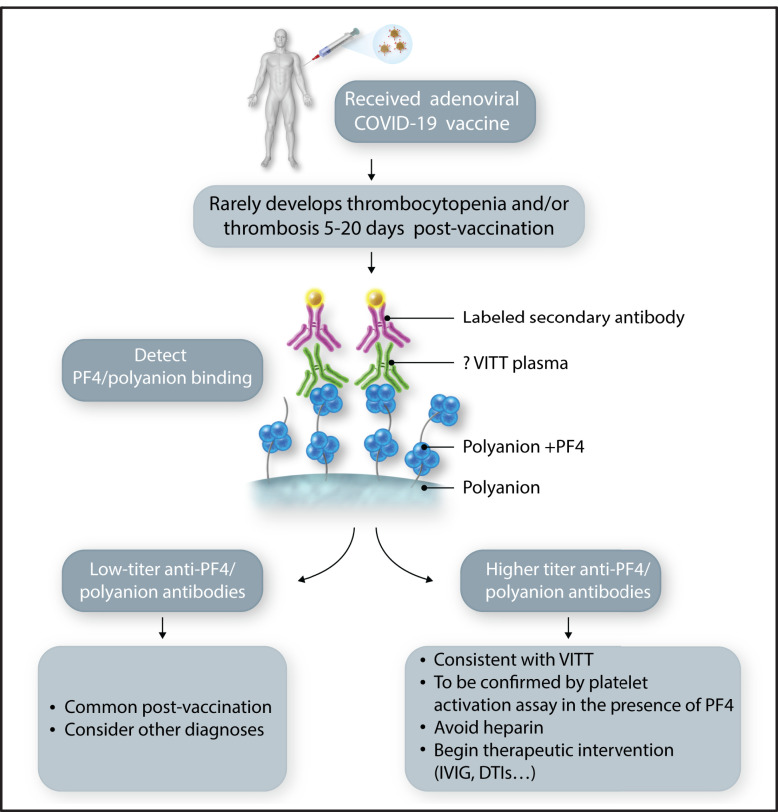
In this issue of Blood, Thiele et al1 describe postvaccination changes in anti-PF4/polyanion antibodies, which are a hallmark of laboratory abnormalities in a rare, but life-threatening, complication from COVID-19 vaccination that also impacts our understanding of this complication.
Presented with the challenge of a fast-spreading, deadly pandemic, modern medicine came up with a solution in the form of efficacious vaccines with unprecedented speed. Less than a year after identifying the SARS-CoV-2 virus, there were several clinical trials of different vaccines, including those based on lipid nanoparticle delivery of messenger RNA (mRNA) encoding the SARS-CoV-2 spike protein and replication-defective adenoviral vectors expressing modified DNA for this protein. Both vaccines were shown to be remarkably effective. A world-wide massive vaccination program has begun, but the adenoviral vaccines have presented physicians with a distinct challenge: the rare occurrence of a deadly complication with several different names, including vaccine-induced thrombocytopenia and thrombosis (VITT).2
VITT occurs typically 5 to 20 days after the first dose of adenovirus-based vaccines and is characterized by thrombocytopenia; thrombosis, often in atypical locations (cerebral and splanchnic veins); and an immune response to the endogenous protein, platelet factor 4 (PF4), resembling autoimmune heparin-induced thrombocytopenia (HIT).3 This emerging rare complication has raised many unanswered questions. Theile et al address some of the underlying pathophysiology by evaluating the immune response to PF4 before and after vaccination with the adenoviral vaccine ChAdOx1 nCoV-19 (AstraZeneca) as well as the mRNA vaccine BNT162b2 (Pfizer). They found low-titer anti-PF4/polyanion antibodies with comparable frequency in both vaccine groups (8.0% for ChAdOx1 nCoV-19 vs 6.8% for BNT162b2); however, of the individuals who had prevaccination samples available, more than half (7 of 11) had already tested low-titer positive before vaccination. Overall, the frequency of low-titer, anti-PF4/polyanion antibodies after vaccination appeared to be near that seen in healthy control subjects and may reflect the mild systemic inflammation that occurs with either type of COVID-19 vaccine.4 Importantly, none of these antibodies activated platelets in the presence of PF4, which is one of the distinguishing features of both HIT5 and VITT.2,6,7 In another study, the prevalence of anti-PF4/polyanion antibodies in a larger cohort of adenoviral vaccine–treated individuals was even lower (1.2%), with no thrombocytopenia observed.8 Thus, in aggregate, the data in these 2 studies suggest that a low PF4/polyanion titer can occur with mild inflammation alone and is not a reason to be concerned about an individual developing VITT (see figure); however, given the low incidence of VITT (∼2-10 individuals per million), a much larger study is warranted to define how many patients develop higher titer antibodies to PF4/polyanions, and then to learn the size of the subgroup that goes on to develop VITT.
Laboratory screening for VITT. An individual receiving a vaccination for COVID-19. Those receiving an adenoviral-based vaccine have a rare risk of developing VITT. If they develop symptomatology concerning for thrombosis and/or thrombocytopenia, especially 5 to 20 days after the first vaccination, laboratory testing should be performed beginning with an antigenic anti-PF4/polyanion assay. Absent-to-low titers should be reassuring, but elevated levels should lead to a platelet functional assay and consideration of therapeutic intervention. DTI, direct thrombin inhibitor; IVIG, IV immunoglobulin. Professional illustration by Somersault18:24.
The results of this study are important for VITT diagnosis and further our understanding of the natural course between COVID-19 vaccination and developing VITT. Levels of anti-PF4/polyanion antibodies in patients with VITT are usually remarkably high, accompanied by thrombocytopenia and the antibodies’ ability to activate healthy donor platelets in the presence of PF4. Low titers of anti-PF4/polyanion antibodies in vaccinated individuals, especially in the absence of a clinical picture of thrombosis should not be overestimated and are most probably induced by the inflammatory state after vaccination with either the adenoviral- or mRNA-based vaccine. Risk factors for developing the rare but more vigorous immune response to PF4 after vaccination with adenoviral-based vectors present a challenge similar to identifying risk factors for developing spontaneous or post–heparin exposure HIT.
Supplementary Material
Footnotes
Conflict-of-interest disclosure: M.P. is a consultant for both AstraZeneca and Johnson & Johnson on mechanistic insights into VITT. L.R. declares no competing financial interests.
REFERENCES
- 1.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138(4):299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22): 2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study [published online ahead of print 27 April 2021]. Lancet Infect Dis. doi:10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cines DB, Bussel JB.. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia [published online ahead of print 16 April 2021]. N Engl J Med. doi:10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amiral J, Bridey F, Dreyfus M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68(1):95-96. [PubMed] [Google Scholar]
- 6.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccine [published online ahead of print 28 April 2021]. Blood. doi:10.1182/blood.2021011958. [Google Scholar]
- 8.Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination [published online ahead of print 28 April 2021]. J Thromb Haemost. doi:10.111/jth.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.