Abstract
Study Objectives:
Obstructive sleep apnea (OSA) was recently shown to be associated with quantifiable retinal vascular changes, which correlate with disease severity. This follow-up study examines the response of retinal vascular changes in patients with OSA receiving continuous positive airway pressure (CPAP) treatment.
Methods:
This prospective cohort study recruited adult patients undergoing diagnostic polysomnography at a tertiary sleep clinic in Sydney, Australia, stratified into 4 groups by the apnea-hypopnea index; control patients and patients with mild, moderate, and severe OSA. At baseline and follow-up approximately 24 months later, static retinal vascular calibers were derived from fundus photographs, and dynamic vascular pulsation amplitudes were measured on video fundoscopy. A proportion of patients started CPAP therapy after baseline assessment.
Results:
Seventy-nine patients participated in this follow-up study: 9 control patients and 18 patients with mild OSA, 21 patients with moderate OSA, and 31 patients with severe OSA. Twenty-five patients started CPAP after baseline. In the severe group, patients not on treatment showed progressive narrowing of retinal arteries from baseline, whereas those on CPAP showed a slight improvement (mean, 171.3–165.1 and 171.2–174.0 μm, respectively; P = .012). Arterio-venous ratio was also significantly reduced in the nontreatment group compared to the treatment group in those with severe OSA (0.836–0.821 and 0.837–0.855, respectively; P = .031). CPAP did not seem to have a significant impact on venous caliber or vascular pulsatility.
Conclusions:
This study shows that patients with severe untreated OSA demonstrate progressive retinal arterial narrowing, whereas CPAP treatment may be protective.
Citation:
Wong B, Tong JY, Schulz AM, Graham SL, Farah CS, Fraser CL. The impact of continuous positive airway pressure treatment on retinal vascular changes in obstructive sleep apnea. J Clin Sleep Med. 2021;17(5):983–991.
Keywords: obstructive sleep apnea, retinal vasculature, continuous positive airway pressure therapy, cardiovascular disease, cerebrovascular disease
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea causes systemic vascular dysregulation which is associated with quantifiable changes to retinal vessel diameter with increasing disease severity. Retinal vascular changes such as arterial narrowing have been associated with an increased risk of major cardiovascular events including stroke.
Study Impact: This is the first study to examine structural retinal vascular changes in patients with obstructive sleep apnea in a longitudinal fashion, showing for the first time that these changes are modified by continuous positive airway pressure treatment. Retinal vascular changes could potentially be used as a surrogate biomarker of systemic cardiovascular risk in patients with obstructive sleep apnea.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition characterized by repetitive episodes of partial or complete collapse of the pharynx during sleep. Epidemiologic studies estimate prevalence of OSA between 9% and 37% for men and 4% and 19% for women.1–3 OSA is associated with systemic vascular dysregulation through recurrent exposure to hypoxia and sympathetic nervous system activation.4,5 There is accumulating evidence that OSA is an independent risk factor for hypertension, cardiovascular disease, and stroke.6–9
The retinal vasculature is vulnerable to the pathologic effects of OSA while being one of the few sites that allows direct noninvasive observation. A previous study found that patients with severe OSA were more likely to have retinal arterial changes resembling hypertensive retinopathy, independent of systemic hypertension (odds ratio: 1.09 per 5-unit increase in apnea-hypopnea index [AHI], 95% confidence interval [CI]: 1.02–1.16; P = .01).10 More recently, Tong et al11 reported an association between increasing AHI and decreased retinal arterial caliber and decreased retinal arterio-venous ratio (AVR). Increased AHI was also associated with reduced retinal arterial and venous pulsation amplitude.
Long-term changes in the retinal vasculature of adult patients with OSA are unknown. In addition, the potential for reversibility of these changes in patients treated with continuous positive airway pressure (CPAP) therapy has not been defined. Hence, this study aims to investigate the impact of CPAP therapy on longitudinal changes in static and dynamic retinal vessel caliber in patients with OSA.
METHODS
A prospective cohort study was performed of adult patients referred for diagnostic polysomnography at Macquarie University Hospital, Sydney, Australia, between April 2015 and December 2016, which recruited 115 patients.11 All patients who participated in this original cohort were invited to attend a follow-up ophthalmic examination approximately 24 months after their first visit. Written consent was obtained from all patients. The study was approved by the Macquarie University and Concord Hospital Human Research Ethics Committee (CH62/6/2015-209) and followed the Tenets of Helsinki.
Demographics
Basic demographic information was collected for each patient at baseline and follow-up including age, sex, body mass index (BMI), medical history, and current medications. Blood pressure and heart rate were recorded at each visit. Mean arterial pressure was calculated for each patient.
Exclusion criteria included glaucoma, prior history of any retinopathy or optic neuropathy, cataract causing reduced visual acuity less than 6/12, opacities obstructing clear imaging of the retina, and patients who started CPAP therapy before the baseline examination.
OSA stratification
Before their baseline visit, patients underwent standard in-laboratory diagnostic polysomnography (Embla Systems Inc., Kanata, ON, Canada). Apneas were defined as >90% reduction in upper respiratory airflow for at least 10 seconds, and hypopneas were defined as ≥50% reduction in airflow associated with decreased oxygen saturation of at least 4%. The frequency of AHI events per hour was used to stratify patients into 4 groups: normal (<5 events/h), mild OSA (5–14.9 events/h), moderate (15–29.9 events/h), and severe (≥30 events/h).12
CPAP therapy
After the original diagnostic sleep study, a proportion of patients were offered CPAP therapy at the discretion of their sleep medicine physician, who was blinded to the results of the ophthalmic assessment. At follow-up, information was collected on duration of CPAP treatment. Adherence data were extracted from individual patients’ machine memory cards over the 90-day period leading up to the follow-up visit. These data included information on the number of nights with device use, duration of nightly use, and AHI. CPAP adherence was defined as device use for at least 4 hours per night on at least 70% of nights because this is the most widely accepted criterion in sleep medicine.13
Ocular assessment
Ocular assessment performed at baseline and follow-up visits included visual acuity and intraocular pressure. Fifteen minutes before ocular imaging, the right eye of each patient was dilated using tropicamide and phenylephrine.
Fundus photographs were taken of the right retina centered on the optic disc using a mydriatic fundus camera (Zeiss Visucam, Oberkochen, Germany). Retinal vessel pulsations in the right eye were captured using video fundoscopy (Imedos Systems, Jena, Germany).
Measurement of retinal vessel caliber and pulsatility
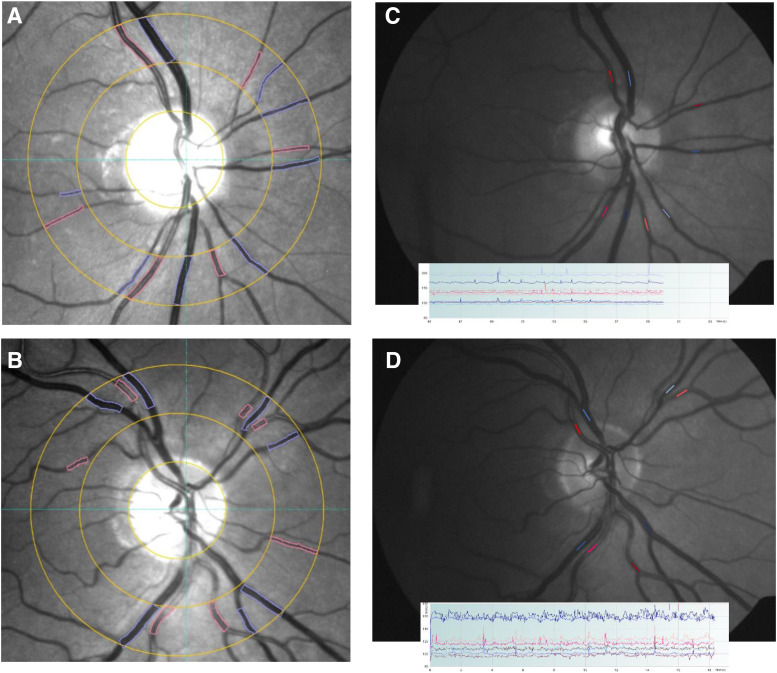
Retinal vessel analysis was performed as previously described by Tong et al.11 In brief, measurement of static vessel caliber was derived from fundus photographs using a specialized vessel analysis program (Figure 1) (VesselMap2, Imedos Systems). The cross-sectional diameter for retinal arteries and veins was calculated by the software and used to derive the central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE). The ratio between these values was defined as the AVR. The CRAE and CRVE summarized the general caliber of retinal arteries and veins, respectively, as a single value. The method used by the VesselMap2 program to calculate these values was as described by Hubbard.14 Analysis of retinal vessel pulsation amplitudes was derived from video fundoscopy using a specialized instrument called a dynamic vessel analyzer (Figure 1). The average waveform height for arteries and veins was used to define spontaneous retinal artery pulsation and spontaneous retinal venous pulsation, respectively. Quadrant-specific measurements were then combined into a global average. For both static and dynamic vessel analysis, measurements were taken from the same location on each retinal vessel at baseline and follow-up.
Figure 1. Measurement of static and dynamic retinal vessel diameter.
(A) and (B) Semiautomated measurement of retinal vessel caliber from fundus photos using the program VesselMap2. Measurements are taken from vessels passing through an annular zone 0.5 to 1 disc diameter away from the margin. (C) and (D) Measurement of retinal vessel pulsation amplitudes from video fundoscopy using the DVA.
Statistical analysis
All data analysis was conducted using the SPSS statistical software version 23.0 (SPSS Inc., Chicago, IL). Comparison of demographic data across the OSA severity groups was conducted using 1-way analysis of variance tests. Pearson’s χ2 test was used to analyze the categorical data. Mixed analysis of variance tests were used to evaluate the change in retinal vascular parameters (CRAE, CRVE, AVR, spontaneous retinal artery pulsation, and spontaneous retinal venous pulsation) between baseline and follow-up examinations in patients using CPAP and patients not using CPAP. The mild OSA and moderate OSA groups were combined into a single group for the purpose of this analysis because of the low number of participants on CPAP in the mild group. The role of vascular confounding factors such as age, BMI, mean arterial pressure, hypertension, smoking (current or past), and dyslipidemia was analyzed by including each as covariates and examining their respective interaction effects. When examining the effect of hypertension, patients were stratified into 3 groups based on whether they had hypertension, and if so, whether they were on antihypertensive therapy.
Independent-sample t tests were used to determine whether CPAP had a significant impact on retinal vasculature, regardless of the severity of OSA. For each of the 5 retinal vascular parameters, the magnitude of the change from baseline to follow-up was calculated (eg, follow-up CRAE minus baseline CRAE) and compared between CPAP users and non-CPAP users. Normality was assessed using the Shapiro-Wilks test.
Multivariate regression was used to assess the ability of CPAP use and the vascular confounding factors to predict change in vessel caliber between baseline and follow-up. Mean arterial pressure was also included in the multivariate analysis. Statistical significance was set at P < .05 for all tests.
RESULTS
Patient characteristics
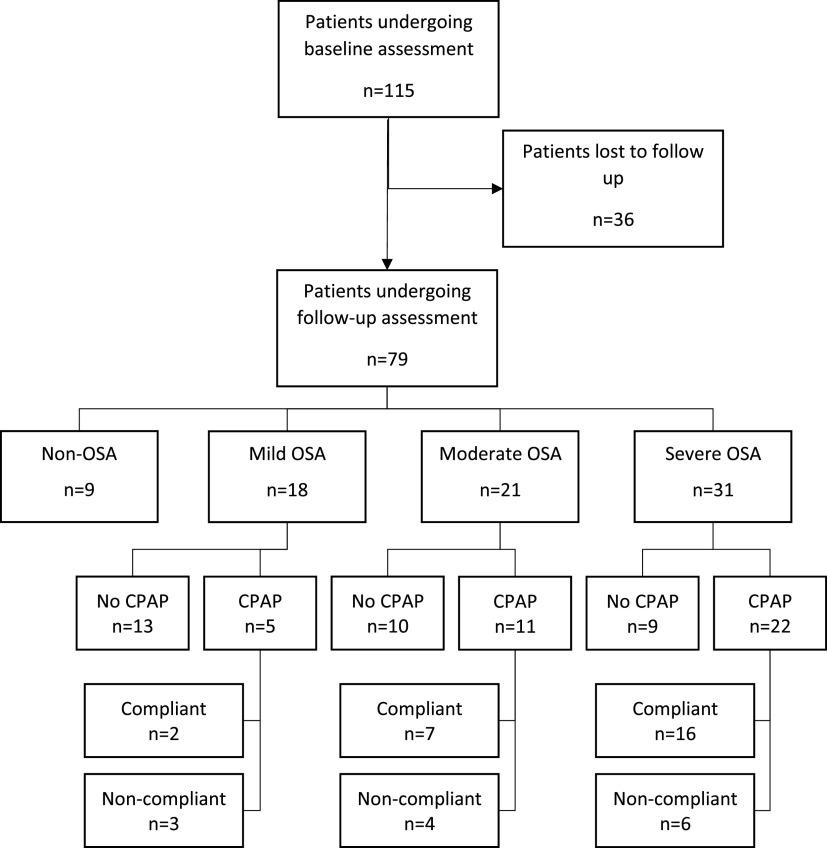
Of 115 patients recruited in the original study, 79 agreed to attend follow-up assessment as follows: 9 without OSA, 18 with mild OSA, 21 with moderate OSA, and 31 with severe OSA (Figure 2). The remaining 36 patients were either noncontactable or declined to participate in the follow-up study. Of these patients, 20 of 36 (56%) were male, and there were 6 without OSA, 8 with mild OSA, 14 with moderate OSA, and 8 with severe OSA.
Figure 2. Patient flow diagram.
For the 79 participants, mean follow-up time was 28.0 ± 3.9 months (range, 14.8–38.0 months). There were 53 males (67%) and 26 females. Mean age was 62.5 ± 10.2 years (range, 38–85 years). Mean BMI was 29.1 ± 4.7 kg/m2 (range, 21.0–41.4 kg/m2). Table 1 summarizes their baseline demographic data. There were no statistically significant differences in age or sex between the 4 severity groups. Increasing severity of OSA was associated with higher BMI (P = .007). All participants had normal intraocular pressure regardless of OSA severity, with no significant differences between groups. The overall prevalence of self-reported physician-diagnosed hypertension was 58.2% (46 of 79). All but 2 patients were on antihypertensives.
Table 1.
Baseline characteristics of all patients in this study.
| Controls | Mild | Moderate | Severe | P | |
|---|---|---|---|---|---|
| Sample size | 9 | 18 | 21 | 31 | |
| Sex (male: female) | 4: 5 | 12: 6 | 12: 9 | 25: 6 | .132 |
| Age at follow-up (y) | 59.8 ± 10.7 | 61.8 ± 12.1 | 63.2 ± 10.3 | 63.1 ± 9.2 | .825 |
| BMI (kg/m2) | |||||
| Baseline | 26.5 ± 3.0 | 27.7 ± 3.8 | 29.0 ± 4.7 | 31.2 ± 5.2 | .027 |
| Follow-up | 26.6 ± 2.5 | 27.2 ± 3.3 | 29.0 ± 4.6 | 31.2 ± 5.2 | .007 |
| Systolic BP (mm Hg) | |||||
| Baseline | 123.7 ± 31.7 | 126.7 ± 19.0 | 121.8 ± 12.6 | 129.5 ± 16.0 | .522 |
| Follow-up | 131.1 ± 16.1 | 127.1 ± 18.4 | 125.4 ± 16.0 | 131.8 ± 14.8 | .515 |
| Diastolic BP (mm Hg) | |||||
| Baseline | 77.0 ± 9.0 | 72.1 ± 11.3 | 71.4 ± 10.0 | 77.8 ± 12.6 | .176 |
| Follow-up | 85.9 ± 8.6 | 80.3 ± 12.8 | 78.6 ± 7.0 | 84.4 ± 11.5 | .152 |
| AHI on dPSG (events/h) | 3.2 ± 1.3 | 9.9 ± 3.4 | 20.8 ± 3.4 | 50.1 ± 19.8 | <.001 |
| Right eye IOP (mm Hg) | 15.8 ± 3.1 | 14.3 ± 3.3 | 16.0 ± 3.1 | 14.3 ± 3.9 | .283 |
| Prevalence of hypertension | 3/9 (33.3%) | 10/18 (55.5%) | 15/21 (71.4%) | 18/31 (58.1%) | .278 |
| Treated: untreated | 2: 1 | 10: 0 | 15: 0 | 17: 1 | |
| Smoking | 2/9 (22.2%) | 7/18 (38.9%) | 7/21 (33.3%) | 12/31 (38.7%) | .609 |
| Current: past | 0: 2 | 2: 5 | 0: 7 | 0: 12 | |
| Dyslipidemia | 4/9 (44.4%) | 6/18 (33.3%) | 13/21 (61.9%) | 16/31 (51.6%) | .453 |
| Treatment with diet: medication | 0: 4 | 1: 5 | 2: 11 | 0: 16 | |
| CPAP use with adequate adherence | 0 (0%) | 2/18 (11.1%) | 7/21 (33.3%) | 16/31 (51.6%) | .004 |
AHI = apnea-hypopnea index, BMI = body mass index, BP = blood pressure, CPAP = continuous positive airway pressure, dPSG = diagnostic polysomnography, IOP = intraocular pressure.
CPAP therapy
Thirty-eight patients were started on CPAP treatment after polysomnography; 5 with mild OSA, 11 with moderate OSA, and 22 with severe OSA (Figure 2). Of this group, 25 were found to be adequately adherent with treatment over the 90-day period leading up to the follow-up visit and almost all (24 of 25) had used CPAP for more than 1 year. The remaining patient had used CPAP treatment for only 3 months before review. Nearly one third of the cohort (13 of 38) were either nonadherent or had discontinued treatment. Of the 25 patients adequately adherent with CPAP, 20 had additional adherence data dating back up to 2 years. For those in whom the additional data were not available, 2 were from the moderate group and 3 were from the severe group. These additional data showed that all 20 patients still met the criteria for adequate CPAP use (greater than 4 hours per night on more than 70% of nights) over at least a 12-month period. In the treatment group, CPAP was highly effective for reducing AHI events. Mean AHI on CPAP was 1.18 ± 0.46, 3.96 ± 3.18, and 2.99 ± 2.77 events/h for the mild, moderate, and severe OSA groups, respectively, compared with a mean AHI on diagnostic polysomnography of 9.9 ± 3.4, 20.8 ± 3.4, and 50.1 ± 19.8 events/h for mild, moderate, and severe OSA, respectively.
Changes in static retinal vessel caliber
Table 2 shows a comparison of the effect of CPAP therapy vs no CPAP therapy on retinal vessel caliber. In patients with severe OSA, CPAP had a significant effect on CRAE and AVR but not CRVE. Patients not on treatment showed a decrease in CRAE, whereas those on CPAP showed a mild increase (171.3–165.1 vs 171.2–174.0 μm, respectively; P for interaction effect = .012). Age, BMI, mean arterial pressure, hypertension, smoking status, and dyslipidemia had no effect on CRAE (P for interaction effect > .05; see Table S1 (54.7KB, pdf) in the supplemental material). There was a decrease in CRVE for both groups with no significant differences (205.9–201.9 vs 206.8–205.2 μm; P for interaction effect = .475). AVR decreased for those not on CPAP and increased for those on CPAP (0.836–0.821 vs 0.837–0.855, respectively; P for interaction effect = .031). The P for the interaction effect of the confounding factors was >.05 (Table S1 (54.7KB, pdf) ).
Table 2.
Results of static vessel analysis.
| Baseline | Follow-Up | P | |||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| CRAE | |||||
| Controls | 187.6 | 178.0–197.2 | 187.9 | 178.2–197.6 | .882 |
| Mild-moderate | .464 | ||||
| CPAP | 177.6 | 165.8–189.4 | 179.1 | 166.9–191.4 | |
| Non-CPAP | 178.0 | 171.4–184.7 | 177.6 | 170.6–184.5 | |
| Severe | .012 | ||||
| CPAP | 171.2 | 164.7–177.7 | 174.0 | 166.6–181.3 | |
| Non-CPAP | 171.3 | 165.1–177.6 | 165.1 | 158.0–172.2 | |
| CRVE | |||||
| Controls | 223.2 | 204.7–241.7 | 219.6 | 200.0–239.2 | .200 |
| Mild-Moderate | .492 | ||||
| CPAP | 208.7 | 197.5–219.9 | 206.7 | 195.5–217.9 | |
| Non-CPAP | 209.8 | 203.4–216.1 | 210.4 | 204.1–216.8 | |
| Severe | .475 | ||||
| CPAP | 206.8 | 194.9–218.8 | 205.2 | 193.9–216.5 | |
| Non-CPAP | 205.9 | 194.4–217.4 | 201.9 | 191.1–212.8 | |
| AVR | |||||
| Controls | 0.812 | 0.773–0.851 | 0.840 | 0.784–0.896 | .053 |
| Mild-Moderate | .374 | ||||
| CPAP | 0.859 | 0.810–0.908 | 0.869 | 0.824–0.914 | |
| Non-CPAP | 0.848 | 0.820–0.875 | 0.844 | 0.818–0.869 | |
| Severe | .031 | ||||
| CPAP | 0.837 | 0.787–0.886 | 0.855 | 0.810–0.899 | |
| Non-CPAP | 0.836 | 0.789–0.884 | 0.821 | 0.778–0.864 | |
Two-way mixed analysis of variance model comparing the effect of CPAP vs no CPAP on static retinal vascular parameters. Values not adjusted for confounding factors. All results in micrometers. AVR = arterio-venous ratio, CI = confidence interval, CPAP = continuous positive airway pressure, CRAE = central retinal arterial equivalent, CRVE = central retinal venous equivalent.
In patients with mild and moderate OSA, CPAP did not demonstrate any statistically significant effect on CRAE, CRVE, or AVR (Table 2).
Overall, CPAP users across all severity groups showed an increase in CRAE of +2.25 μm (95% CI, −1.04 to 5.54 μm), whereas non-CPAP users showed a decrease of −2.57 μm (95% CI, −5.09 to −0.06 μm). The difference between groups was significant (P = .021). Both CPAP users and non-CPAP users showed a decrease in mean CRVE from baseline to follow-up, with change values of −1.81 μm (95% CI, −5.79 to 2.17 μm) and −1.41 μm (95% CI, −4.19 to 1.36 μm), respectively. The difference between groups was not statistically significant (P = .867; Table S2 (54.7KB, pdf) ). Multivariate regression showed that CPAP use was able to significantly predict change in CRAE between baseline and follow-up for the whole cohort (P = .040), whereas mean arterial pressure, hypertension, age, BMI, smoking, and dyslipidemia did not (all P > .05). There was no correlation between other retinal vascular parameters and CPAP use (Table S3 (54.7KB, pdf) ).
Changes in retinal vessel pulsatility
Table 3 shows a comparison of the effect of CPAP therapy vs no CPAP therapy on vessel pulsation amplitudes. Both groups produced generally stable results for spontaneous retinal artery pulsation and spontaneous retinal venous pulsation between baseline and follow-up.
Table 3.
Results of vessel pulsation analysis.
| Baseline | Follow-Up | P | |||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| SRAP | |||||
| Controls | 5.00 | 3.67–6.33 | 5.01 | 4.16–5.86 | .970 |
| Mild-moderate | .981 | ||||
| CPAP | 4.08 | 3.30–4.87 | 4.02 | 3.43–4.61 | |
| Non-CPAP | 3.99 | 3.56–4.41 | 3.93 | 3.61–4.25 | |
| Severe | .878 | ||||
| CPAP | 3.52 | 3.09–3.96 | 3.77 | 3.28–4.26 | |
| Non-CPAP | 3.52 | 3.08–3.95 | 3.33 | 2.84–3.81 | |
| SRVP | |||||
| Controls | 5.17 | 3.76–6.58 | 5.22 | 3.89–6.55 | .898 |
| Mild-moderate | .628 | ||||
| CPAP | 5.04 | 3.83–6.24 | 5.10 | 4.36–5.83 | |
| Non-CPAP | 4.42 | 3.77–5.08 | 4.75 | 4.35–5.15 | |
| Severe | .788 | ||||
| CPAP | 4.52 | 3.79–5.25 | 4.62 | 4.04–5.20 | |
| Non-CPAP | 4.41 | 3.68–5.14 | 4.65 | 4.07–5.22 | |
Two-way mixed analysis of variance model comparing the effect of CPAP vs no CPAP on spontaneous retinal arterial and venous pulsation amplitudes. Values not adjusted for confounding factors. All results in micrometers. CI = confidence interval, CPAP = continuous positive airway pressure, SRAP = spontaneous retinal artery pulsation, SRVP = spontaneous retinal venous pulsation.
DISCUSSION
In this study, we examined the impact of CPAP therapy on quantitative changes in retinal vasculature in patients with OSA. In patients with severe OSA, progressive retinal arterial narrowing was seen in those nonadherent with or not using CPAP. These changes were significantly reduced and even reversed by CPAP therapy. The beneficial effect of CPAP remained statistically significant when accounting for vascular confounding factors such as age, BMI, and hypertension.
In this study, we used CRAE and AVR as markers of retinal arterial caliber. CPAP users, regardless of severity of OSA, showed a statistically significant increase in CRAE from baseline, compared with a decrease for the nontreatment group. Generalized retinal arterial narrowing is shown to be associated with an increased risk of stroke.15–17 There is also evidence for an association between retinal arterial narrowing and coronary heart disease in certain populations. One study found that in women, but not men, each standard deviation decrease in AVR was associated with an increased risk of any incident coronary heart disease and acute myocardial infarction.18 Another study found that generalized retinal arterial narrowing was associated with greater cardiovascular mortality risk but only in a younger population aged 43–74 years.19
Given the association between changes to retinal vascular parameters and major cardiovascular events, stroke in particular, the measurement of retinal vessels could be a surrogate marker of systemic cardiovascular risk in patients with OSA. Our study demonstrates slowing and even reversal of such retinal vascular changes after adequate CPAP therapy in those with severe disease, which could correlate with a reduction their overall cardiovascular risk. Various studies have found that CPAP has a favorable impact on cardiovascular risk parameters including lowering blood pressure,20 improving lipid profile,21 and decreasing serum biochemical markers such as homocysteine and high-sensitivity C-reactive protein.22 The effect of CPAP use on major cardiovascular events is still being investigated, with mixed results being reported in the literature. Many observational studies report that CPAP therapy reduces the risk of nonfatal cardiovascular events (myocardial infarction, stroke, acute coronary syndrome requiring revascularization) and fatal events (deaths from myocardial infarction or stroke) in patients with OSA.23–27 Evidence from randomized control trials is mixed with some reporting that CPAP has no significant impact on the risk of major cardiovascular events.28,29 Others report that CPAP does have a significant impact.30 A meta-analysis of randomized trials found that CPAP was associated with a trend of decreased risk of cardiovascular events in participants with OSA, but the findings did not reach statistical significance.31 Thus, further studies are required to evaluate whether CPAP may influence the incidence of cardiovascular events and mortality. However, we have shown that structural vascular changes do reverse with CPAP therapy.
Significance of changes to retinal vascular pulsatility
Tong et al11 found that increased severity of OSA was associated with decreased retinal vessel pulsation amplitudes. Because retinal vascular pulsations are considered to reflect the state of perfusion of the optic nerve head and retina, these changes were thought to represent microvascular stasis retinopathy in the setting of generalized cerebrovascular ischemia. In this study, we did not find that CPAP had a significant impact on retinal vascular pulsations. One possible explanation for this finding is that vessel pulsation is a dynamic process that responds to changes in ambient conditions such as nocturnal intraocular pressure and apnea-induced hypoxia. The differences between CPAP users and non-CPAP users may only be evident during sleep conditions.
Proposed mechanisms of changes to retinal vasculature
The recurrent apneas and hypopneas that occur in OSA are thought to cause perturbations to microvascular function through 2 specific mechanisms: intermittent hypoxia and sympathetic nervous system activation. Acute hypoxia is hypothesized to stimulate peripheral chemoreceptor activity, leading to elevated sympathetic nervous system activity, episodic systemic hypertension, and long-term structural changes in retinal and systemic vasculature.32,33 Furthermore, these acute surges in mean arterial pressure are followed by precipitous falls in blood pressure and cerebral blood flow velocity at the termination of apneic episodes.34,35 These events have been likened to an ischemic-perfusion injury, which activates the inflammatory cascade and inhibits nitric oxide release, leading to ischemic damage and endothelial dysfunction.36–38
There is evidence that CPAP therapy improves nitric oxide release from vessel walls and endothelial function.39,40 CPAP may also decrease basal sympathetic nervous system activity and the responses of sympathetic nerves to hypoxic episodes.41 Further investigation of the therapeutic effects of CPAP on retinal vasculature is needed.
Study limitations and strengths
In this study, we investigated the potential impact of CPAP therapy on retinal vascular parameters through a cohort study design. CPAP was offered to patients at the discretion of their sleep physician, and as a result, there was no age or sex matching between groups. We attempted to account for potential differences between these groups in the statistical analysis. This study does incorporate a non-OSA patient group as a comparison to the groups with OSA, although no patients in this group were offered CPAP therapy.
There was a discrepancy in the proportion of patients receiving CPAP between disease severity groups. In the mild group, only 2 patients received CPAP because it is not routinely offered as first-line treatment to such patients. To increase statistical power, the mild and moderate severity groups were combined for data analysis. This pooling may potentially obscure any differences in treatment response between these 2 groups. Larger studies are required for greater statistical power.
Additional adherence data were available for 20 of 25 (80%) of the patients in the CPAP group, although this information generally only dated back to a maximum of 12 months. It was therefore not possible to query adherence over the entire interval between initial and follow-up assessments for these patients.
Most participants in this study who had hypertension were on antihypertensive treatment. Although retinal vascular changes in OSA have been shown to be independent of systemic blood pressure, it is possible that stricter control of hypertension could contribute to improvement of retinal arterial narrowing.10,42 Because treatment of OSA is integral to blood pressure management in most patients, the beneficial effect of CPAP therapy vs antihypertensives may be difficult to distinguish and warrants further investigation.
This study only used quantitative parameters to assess retinal microvasculature, which avoided potential interobserver variability related to historic indicators of microvascular pathology such as arterio-venous nicking and focal arteriolar narrowing. VesselMap2, the software used for the static vessel analysis, provides a semiautomated method used for assessing vessel caliber where measurement location is the only factor at the discretion of the examiner. It has shown good repeatability, with an intergrader reliability κ of 0.85 and 0.90 and an intragrader κ of 0.80 and 0.93 for arteries and veins, respectively.43 This study is the first to report on changes to retinal vascular pulsatility in a longitudinal fashion. The dynamic vessel analyzer is an instrument with high reproducibility of measurements, with an intraclass correlation κ of 0.98 for both arteries and veins.44
CONCLUSIONS
In this study, we found evidence of progressive retinal arterial narrowing and a decreased arterio-venous ratio in patients with untreated OSA. These changes were stabilized or partially reversed with CPAP therapy in those with severe OSA. Changes in retinal vessel pulsatility are likely to be a dynamic process and may not be evident during daytime testing conditions. Quantitative changes to retinal vasculature could be a surrogate indicator of systemic cardiovascular risk in patients with OSA, and reversibility of these changes with CPAP therapy could represent a decrease in overall risk.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This work was performed at the Department of Ophthalmology and Vision Science, Macquarie University, Sydney, Australia. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank the Sydney Informatics Hub, a Core Research Facility of the University of Sydney, and the staff of Macquarie University Ophthalmology Clinic for their support in this research.
Author contributions: BW contributed to patient recruitment data preparation, data analysis, data interpretation, and writing this manuscript. JYT and AMS contributed to patient recruitment data preparation, data analysis, and data interpretation. SLG, CSF, and CLF contributed to the formulation of the idea, study design, data interpretation, and review of the manuscript. CLF is the study guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing: Additional data available on reasonable request.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AVR
arterio-venous ratio
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CRAE
central retinal arterial equivalent
- CRVE
central retinal venous equivalent
- OSA
obstructive sleep apnea
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin H-M, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. 10.1164/ajrccm.163.3.9911064 [DOI] [PubMed] [Google Scholar]
- 3.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–776. 10.1161/01.CIR.98.8.772 [DOI] [PubMed] [Google Scholar]
- 5.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103(6):1763–1768. 10.1378/chest.103.6.1763 [DOI] [PubMed] [Google Scholar]
- 6.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. 10.1136/bmj.320.7233.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. 10.1001/archinte.1997.00440360178019 [DOI] [PubMed] [Google Scholar]
- 8.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169(3):207–214. 10.1016/j.ijcard.2013.08.088 [DOI] [PubMed] [Google Scholar]
- 10.Fraser CL, Bliwise DL, Newman NJ, et al. A prospective photographic study of the ocular fundus in obstructive sleep apnea. J Neuroophthalmol. 2013;33(3):241–246. 10.1097/WNO.0b013e318290194f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong JY, Golzan M, Georgevsky D, et al. Quantitative retinal vascular changes in obstructive sleep apnea. Am J Ophthalmol. 2017;182:72–80. 10.1016/j.ajo.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 12.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. 10.1016/j.smrv.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. 10.1016/S0161-6420(99)90525-0 [DOI] [PubMed] [Google Scholar]
- 15.Longstreth W Jr, Larsen EKM, Klein R, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):78–84. 10.1093/aje/kwj350 [DOI] [PubMed] [Google Scholar]
- 16.Cooper LS, Wong TY, Klein R, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006;37(1):82–86. 10.1161/01.STR.0000195134.04355.e5 [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. 10.1016/S0140-6736(01)06253-5 [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287(9):1153–1159. 10.1001/jama.287.9.1153 [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. 10.1016/S0161-6420(03)00084-8 [DOI] [PubMed] [Google Scholar]
- 20.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365(24):2277–2286. 10.1056/NEJMoa1103944 [DOI] [PubMed] [Google Scholar]
- 21.Nadeem R, Singh M, Nida M, et al. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10(12):1295–1302. 10.5664/jcsm.4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiropoulos P, Tsara V, Nena E, et al. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2007;132(3):843–851. 10.1378/chest.07-0074 [DOI] [PubMed] [Google Scholar]
- 23.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 24.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. 10.1378/chest.127.6.2076 [DOI] [PubMed] [Google Scholar]
- 25.Martínez-García MÁ, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36–41. 10.1164/rccm.200808-1341OC [DOI] [PubMed] [Google Scholar]
- 26.Martínez-García MA, Campos-Rodríguez F, Soler-Cataluña JJ, Catalán-Serra P, Román-Sánchez P, Montserrat JM. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J. 2012;39(4):906–912. 10.1183/09031936.00011311 [DOI] [PubMed] [Google Scholar]
- 27.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–1280. 10.1164/rccm.200611-1588OC [DOI] [PubMed] [Google Scholar]
- 28.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al.Spanish Sleep and Breathing Network . Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- 29.McEvoy RD, Antic NA, Heeley E, et al.SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 30.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 31.Guo J, Sun Y, Xue L-J, et al. Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2016;20(3):965–974. 10.1007/s11325-016-1319-y [DOI] [PubMed] [Google Scholar]
- 32.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15(12 Pt 2):1593–1603. [DOI] [PubMed] [Google Scholar]
- 33.Katragadda S, Xie A, Puleo D, Skatrud JB, Morgan BJ. Neural mechanism of the pressor response to obstructive and nonobstructive apnea. J Appl Physiol 1985. 1997;83(6):2048–2054. 10.1152/jappl.1997.83.6.2048 [DOI] [PubMed] [Google Scholar]
- 34.Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol. 2007;92(1):51–65. 10.1113/expphysiol.2006.035204 [DOI] [PubMed] [Google Scholar]
- 35.Bålfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1587–1591. 10.1164/ajrccm.150.6.7952619 [DOI] [PubMed] [Google Scholar]
- 36.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2004;287(5):H2054–H2060. 10.1152/ajpheart.00377.2004 [DOI] [PubMed] [Google Scholar]
- 37.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. 10.1053/smrv.2002.0261 [DOI] [PubMed] [Google Scholar]
- 38.Lavie L, Hefetz A, Luboshitzky R, Lavie P. Plasma levels of nitric oxide and L-arginine in sleep apnea patients: effects of nCPAP treatment. J Mol Neurosci. 2003;21(1):57–63. 10.1385/JMN:21:1:57 [DOI] [PubMed] [Google Scholar]
- 39.Trzepizur W, Gagnadoux F, Abraham P, et al. Microvascular endothelial function in obstructive sleep apnea: impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10(7):746–752. 10.1016/j.sleep.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 40.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61(6):491–495. 10.1136/thx.2004.039164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imadojemu VA, Gleeson K, Quraishi SA, Kunselman AR, Sinoway LI, Leuenberger UA. Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2002;165(7):950–953. 10.1164/ajrccm.165.7.2102003 [DOI] [PubMed] [Google Scholar]
- 42.Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez-Perez ME, McG Thom SA. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens. 2008;26(8):1703–1707. 10.1097/HJH.0b013e328304b072 [DOI] [PubMed] [Google Scholar]
- 43.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1(2):156–161. 10.1161/CIRCIMAGING.108.784876 [DOI] [PubMed] [Google Scholar]
- 44.Pache M, Nagel E, Flammer J. Reproducibility of measurements with the retinal vessel analyzer under optimal conditions. Article in German. Klin Monatsbl Augenheilkd. 2002;219(7):523–527. 10.1055/s-2002-33589 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.