Abstract
Study Objectives:
Obstructive sleep apnea and other sleep disorders overlap with comorbidities associated with poor outcomes related to severe acute respiratory syndrome coronavirus 2 infection. However, the prevalence of obstructive sleep apnea among patients hospitalized for COVID-19 and relationship to outcomes is poorly characterized, and the relevance of other sleep disorders remains unknown. The objective of this study was to identify the prevalence of pre-existing sleep disorders and association with outcomes related to severe COVID-19 illness.
Methods:
Patients with severe acute respiratory syndrome coronavirus 2 infection admitted to the University of Michigan Hospital System were included. Electronic medical records were queried for sleep disorders diagnostic codes. Data were extracted from polysomnography and home sleep testing in a subgroup with previous diagnostic testing at our center. Logistic regression was used to examine the association of sleep disorders with mechanical ventilation requirement, treatment with vasopressors, and death and Cox proportional hazards regression for time to discharge.
Results:
Among n = 572 adult patients hospitalized for COVID-19, 113 (19.8%) patients had obstructive sleep apnea, 4 patients had central sleep apnea (0.7%), 5 had hypoventilation (0.9%), 63 had insomnia (11.0%), and 22 had restless legs syndrome or periodic limb movements disorder (3.9%). After adjusting for age, sex, body mass index, and race, no significant relationship was apparent between sleep disorders diagnoses or indices of sleep-disordered breathing severity and outcomes.
Conclusions:
This is the first study to determine the prevalence of obstructive sleep apnea and other sleep disorders in a well-characterized cohort of patients hospitalized for COVID-19. Once hospitalized, a significant contribution of sleep disorders to outcomes was not identified. Therefore, future evaluations should focus on earlier outcomes, such as infection or clinical manifestations after exposure to severe acute respiratory syndrome coronavirus 2.
Citation:
Goldstein CA, Rizvydeen M, Conroy DA, et al. The prevalence and impact of pre-existing sleep disorder diagnoses and objective sleep parameters in patients hospitalized for COVID-19. J Clin Sleep Med. 2021;17(5):1039–1050.
Keywords: COVID-19, sleep disorders in hospitalized patients, mechanical ventilation, mortality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Many have hypothesized that sleep disorders, particularly obstructive sleep apnea, are relevant in patients infected with severe acute respiratory syndrome coronavirus 2 given the overlap of with comorbidities associated with worse outcomes related to COVID-19. However, objective data regarding the prevalence of sleep disorders and association with outcomes in patients hospitalized with COVID-19 are minimal.
Study Impact: Sleep disorders were common in individuals hospitalized for COVID-19 but did not contribute to mortality or other critical outcomes. Therefore, earlier outcomes such as the development of illness after exposure to severe acute respiratory syndrome coronavirus 2 or requirement of hospitalization warrant further investigation. The role of sleep disorders in COVID-19 is particularly important given the disproportionate impact of both sleep disorders and COVID-19 on individuals with unfavorable social determinants of health.
INTRODUCTION
In March of 2020, the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic.1 Although the majority of SARS-CoV-2 infections do not produce severe symptoms,2–9 individuals with illness that warrants hospitalization often require support in the intensive care unit, and the case fatality rate in the United States was 2% during the drafting of this manuscript.10
Comorbidities such as cardiovascular disease, diabetes, hypertension, chronic lung disease, chronic kidney disease, and tobacco use are associated with more severe manifestations of COVID-19 illness and increased mortality.9,11–15 Obstructive sleep apnea (OSA)16–22 and other sleep disorders23–28 are common in individuals with these chronic conditions and, therefore, may have relevance for the clinical course of those infected with SARS-CoV-2.
Notably, OSA has become increasingly recognized as prevalent in hospitalized patients and may worsen acute outcomes.29–35 For example, in individuals hospitalized for pneumonia, OSA was associated with increased intensive care unit transfers, intubation, and prolonged inpatient stay.36 Though the prevalence of other chronic sleep disorders such as insomnia and restless legs syndrome (RLS) in hospitalized patients is less clear, sleep disturbances from any source are associated with inpatient complications such as delirium,37–39 failure of noninvasive ventilation,40 prolonged ventilator weaning,41 and poor glucose control.42
However, at this time, the prevalence of sleep disorders among patients hospitalized with COVID-19 is poorly characterized. In 3 small studies (n = 21–104), OSA was identified in approximately 6–29% of patients43–45; however, impact on COVID-19 outcomes was not evaluated in these investigations. The Coronavirus SARS-CoV-2 & Diabetes Outcomes study (CORONADO) presented an inpatient cohort of 1,317 patients with diabetes and reported that patients treated for OSA had a higher risk of death from COVID-19 (adjusted odds ratio [OR], 2.65; 95% confidence interval: 1.36, 5.19), although body mass index (BMI) was not included as a covariate in this model and details on how the categorization of “treated OSA” was derived were not provided.46
In a large population (n = 9,409), not isolated to inpatients, OSA (as identified by International Classification of Diseases, Tenth Revision, [ICD] code) was present in 6.3% and was associated with increased risk of COVID-19 infection, hospitalization, and respiratory failure, even after models were adjusted for BMI, diabetes, and hypertension.47 However, another sample of patients with COVID-19 infection (n = 4,668) also used ICD codes and found OSA in 9.5% but did not observe a statistically significant association between OSA and hospitalization, death, or the composite outcome of death, mechanical ventilation, and critical care requirement after models were adjusted for BMI and other comorbidities.48
Therefore, the prevalence of OSA among patients with COVID-19 and, specifically, in individuals who require hospitalization for severe symptoms, and contribution to outcomes remain unclear. Furthermore, no information regarding other pre-existing sleep disorders among patients with COVID-19 is available and no studies have included objectively quantified data from diagnostic sleep studies. Thus, the objective of this study was to identify the prevalence of OSA (and other sleep-disordered breathing diagnoses), insomnia, and RLS in patients hospitalized with SARS-CoV-2 virus and explore relationships with COVID-related outcomes including the requirement for mechanical ventilation and vasopressors, length of stay, and death.
Two assessments of sleep disorders were conducted among patients admitted to the University of Michigan for care related to SARS-CoV-2 infection: (1) evaluation of diagnostic codes for sleep disorders extracted from the electronic medical record and (2) in patients who had undergone diagnostic sleep study prior to infection, evaluation of objective sleep measures from polysomnography or home sleep apnea test. With these approaches, the impact of pre-existing sleep disorders on COVID-19-related health outcomes could be examined.
METHODS
This observational study accessed the Michigan Institute for Clinical and Health Research’s COVID-19 Rapid Response Registry for the clinical characterization of persons with SARS-CoV-2 infection, further described elsewhere.49 All patients admitted to the University of Michigan Hospital System (Michigan Medicine) who tested positive for SARS-CoV-2 infection are included in this registry. Reverse-transcriptase polymerase chain reaction positive SARS-CoV-2 tests were used to confirm infection. The registry includes core items from the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Clinical Characterization Protocol50,51 and follows STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations.52 The Institutional Review Board of the University of Michigan approved the study (HUM00180621).
Patients included in the current study were adults, admitted between March 9 to November 4, 2020. Out of a total of 893 adult patients, 321 patients who had been transferred from other hospitals were excluded from the primary analysis, as data on their clinical course were incomplete. This left 572 patients to be examined for the main analyses, and their follow-up data continued through November 25, 2020. Data analyses on the entire population of 893 patients, inclusive of transfers, was also performed and is available in the supplemental material. Four COVID-related outcomes were examined in this study: the use of mechanical ventilation after hospital admission, treatment with vasopressors, length of stay, and the occurrence of death.
To investigate the potential influence of pre-existing sleep disorders on these medical outcomes, 2 approaches were taken. The first approach searched each patient’s electronic medical record for sleep disorder diagnostic codes to indicate a diagnosis of sleep-disordered breathing, insomnia, RLS, and periodic limb movement disorder (PLMD) (Table S1 (228.7KB, pdf) in the supplemental material contains all diagnostic codes queried). Codes were collapsed into categories of OSA, hypoventilation, central sleep apnea (CSA), insomnia, and RLS/PLMD to approximate the ICSD, third edition, disease categorizations.53 This electronic medical record search was conducted automatically with use of the University of Michigan’s Data Direct program.54 Specifically, we included diagnostic codes used for billing purposes in the ambulatory environment within 2 years prior to COVID-19 admission to best approximate the methodology used by Jolley and colleagues55 that validated billing codes against sleep clinic diagnoses.
The second approach consisted of identifying all patients who had completed a diagnostic sleep study at the University of Michigan’s Sleep Disorder Centers prior to COVID-19 infection and extracting relevant objective measures from the sleep study records (see details below). Whenever multiple diagnostic studies were available, the data from the most recent diagnostic sleep study were used.
Diagnostic sleep study data
Diagnostic sleep studies took place in laboratory or were conducted out of center with type III portable monitoring devices. In-laboratory polysomnography (PSG) included either full night diagnostic or split-night studies (diagnostic portion followed by positive airway pressure [PAP] titration if severity criteria met during the first 2 hours of recording). All in-laboratory data were recorded on the Compumedics acquisition system (Compumedics Limited, Victoria, Australia) and recorded signals from the following leads: frontal, central and occipital electroencephalogram, electrooculogram, submentalis electromyogram, oronasal thermocouple, nasal pressure transducer, electrocardiogram, thoracic and abdominal inductance plethysmography, right and left anterior tibialis electromyogram, snore microphone, and pulse oximetry. Out-of-center home sleep apnea tests (HSATs) were conducted with either the Somte (Compumedics Limited, Victoria, Australia), Alice PDx (Philips Respironics, Eindhoven, The Netherlands), Alice NightOne (Philips Respironics, Eindhoven, The Netherlands), or ApneaLink (ResMed, San Diego, CA) systems and recorded signal from nasal pressure transducer (airflow and snore), respiratory inductance plethysmography (respiratory effort), and pulse oximetry (oxygen saturation and heart rate).
Sleep studies were conducted in accordance with the technical specifications and scoring guidelines set for in The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications56,57 or prior to release, the recommendations published in 2001 by the American Academy of Sleep Medicine Clinical Practice Review Committee.58 Given the introduction of the scoring manual in 2007, all versions in publication through version 2.6 were used.56,57
Given the absence of electroencephalogram during HSATs and change in hypopnea scoring criteria over time, to harmonize the respiratory indices (apnea-hypopnea index [AHI] and respiratory effort index [REI]), the AHI and REI extracted used the 4% hypopnea scoring criteria. Therefore, hypopneas, regardless of derivation from PSG or HSAT, were scored in the presence of ≥ 30% decrement in airflow of at least 10 seconds in duration with resultant oxygen desaturation of 4%. Apneas are scored when airflow decreases by at least 90% for 10 seconds or more independent of oxygen desaturation. Apneas and hypopneas are designated as obstructive or central dependent on the presence or absence or respiratory effort, respectively. The AHI with use of the 4% oxygen desaturation criteria was not reported prior to 2004 in our laboratory; therefore, these studies were not considered for inclusion. In addition to the AHI (REI), the following were extracted from in laboratory PSGs and HSATs: minimum oxygen saturation (SpO2), mean SpO2, total sleep time with SpO2 ≤ 88%, and sleep study diagnosis.
Statistical analysis
Descriptive statistics were provided overall and by sleep disorder. Frequencies and percentages were used for categorical variables and medians and interquartile ranges used for continuous variables, given nonnormal distributions. Comparisons of characteristics with and without sleep disorders were made using chi-square and Kruskal-Wallis tests. Logistic regression was used to test the association between each sleep disorder and mechanical ventilation, vasopressor treatment, and death. Length of stay was modeled as time to discharged alive using Cox proportional hazards models (as of the final data review, 4 patients were still hospitalized; deaths were treated as nonevents). Models were also adjusted for age, race, and BMI. For the subset of patients with diagnostic sleep study data, continuous sleep study measures were compared by ventilator status and survival status using Kruskal-Wallis tests. Two-sided α = 0.05 was used to assess statistical significance. Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 572 adult patients hospitalized for COVID-19 were included. Of these, 109 patients were mechanically ventilated (19.1%), 102 required treatment with vasopressors (17.8%), and 72 patients died (12.6%). Clinical characteristics were compared between transferred (n = 321) and nontransferred (n = 572) patients and revealed that the transferred population had significantly worse makers of disease severity and worse outcomes (Table S3 (228.7KB, pdf) ).
Diagnostic codes in the electronic medical record indicated that 113 (19.8%) patients had OSA, 4 patients had CSA (0.7%), 5 had hypoventilation (0.9%), 63 had insomnia (11.0%), and 22 had RLS/PLMD (3.9%). Seventy-three (12.8%) of the patients had previously completed a diagnostic sleep study. The median follow-up period after admission was 224 days (range 121–239 days).
The baseline characteristic differences between those patients with and without diagnoses of OSA and insomnia are shown in Table 1. Baseline characteristics by sleep disorders with lower frequency (CSA, hypoventilation, and RLS/PLMD) can be found in the supplemental material (Table S2 (228.7KB, pdf) ). Patients with OSA, as expected, had a higher BMI and overall had poorer health, including a lower estimated glomerular filtration rate (kidney function) and a trend for increased comorbid chronic cardiac disease and asthma. Patients with insomnia were more likely to be white, were older (although age did not meet statistical significance), and had comorbid asthma. Additionally, alanine aminotransferase, aspartate aminotransferase, ferritin, and white blood cell count were lower among individuals with insomnia. Patients with CSA were more likely to be male and have chronic cardiac disease and lower lactate dehydrogenase levels. Patients with hypoventilation were more likely to be black, have a higher BMI and comorbid asthma, and demonstrated lower total bilirubin levels. Patients with RLS/PLMD were more likely to be white, and have lower lymphocyte count.
Table 1.
Descriptive characteristics of 572 hospitalized patients with COVID-19 by OSA and insomnia diagnoses.
| Characteristic | Overall (n = 572) | OSA | P | Insomnia | P | ||
|---|---|---|---|---|---|---|---|
| Yes (n = 113) | No (n = 459) | Yes (n = 63) | No (n = 509) | ||||
| Age (years), median (IQR) | 63.0 (50.0 to 73.5) (n = 572) | 62.0 (52.0 to 67.0) (n = 113) | 64.0 (49.0 to 74.0) (n = 459) | .17 | 65.0 (55.0 to 76.0) (n = 63) | 62.0 (49.0 to 72.0) (n = 509) | .07 |
| Female, n (%) | 252 (44) | 48 (42) | 204 (44) | .17 | 34 (54) | 218 (43) | .25 |
| Race, n (%) | .83 | .003 | |||||
| Black | 160 (28) | 32 (28) | 128 (28) | 15 (24) | 145 (28) | ||
| Other | 81 (14) | 14 (12) | 67 (15) | 1 (2) | 80 (16) | ||
| White | 331 (58) | 67 (59) | 264 (58) | 47 (75) | 284 (56) | ||
| BMI (kg/m2), median (IQR) | 31.0 (25.5 to 37.8) (n = 537) | 35.9 (29.7 to 41.5) (n = 108) | 29.4 (25.0 to 35.9) (n = 429) | < .001 | 31.4 (26.2 to 39.0) (n = 56) | 31.0 (25.5 to 37.8) (n = 481) | .83 |
| Temperature (°Fahrenheit), median (IQR) | 98.8 (98.2 to 99.9) (n = 569) | 98.7 (98.1 to 99.6) (n = 112) | 98.8 (98.2 to 99.9) (n = 457) | .42 | 98.6 (98.2 to 99.1) (n = 61) | 98.8 (98.2 to 100.0) (n = 508) | .27 |
| Absolute lymphocyte count (109/L) | 0.9 (0.6 to 1.3) (n = 551) | 0.9 (0.6 to 1.4) (n = 111) | 0.9 (0.6 to 1.3) (n = 440) | .62 | 1.0 (0.5 to 1.3) (n = 61) | 0.9 (0.6 to 1.3) (n = 490) | .72 |
| Alkaline phosphatase (IU/L), median (IQR) | 78.0 (60.0 to 104.0) (n = 539) | 81.0 (66.0 to 109.0) (n = 105) | 76.5 (59.0 to 103.0) (n = 434) | .14 | 78.0 (56.0 to 103.0) (n = 61) | 78.0 (60.0 to 104.0) (n = 478) | .85 |
| Alanine aminotransferase (IU/L), median (IQR) | 32.0 (21.0 to 54.0) (n = 542) | 35.0 (23.0 to 51.0) (n = 105) | 31.0 (21.0 to 54.0) (n = 437) | .48 | 29.0 (17.0 to 42.0) (n = 61) | 32.0 (22.0 to 56.0) (n = 481) | .01 |
| Aspartate aminotransferase (IU/L), median (IQR) | 43.5 (30.0 to 66.0) (n = 542) | 45.0 (33.0 to 65.0) (n = 105) | 43.0 (29.0 to 66.0) (n = 437) | .26 | 37.0 (24.0 to 54.0) (n = 61) | 44.0 (30.0 to 67.0) (n = 481) | .01 |
| Albumin (g/dL), median (IQR) | 3.7 (3.4 to 4.0) (n = 539) | 3.7 (3.4 to 4.0) (n = 105) | 3.7 (3.4 to 4.0) (n = 434) | .91 | 3.8 (3.5 to 4.1) (n = 61) | 3.7 (3.4 to 4.0) (n = 478) | .11 |
| eGFR (ml/min/1.73m2), median (IQR) | 70.0 (45.4 to 90.9) (n = 557) | 67.7 (35.2 to 84.8) (n = 107) | 71.1 (47.7 to 91.5) (n = 450) | .03 | 68.5 (47.6 to 85.4) (n = 63) | 70.1 (45.3 to 91.4) (n = 494) | .75 |
| Total bilirubin (mg/dl), median (IQR) | 0.6 (0.4 to 0.9) (n = 539) | 0.6 (0.4 to 0.9) (n = 105) | 0.6 (0.4 to 0.9) (n = 434) | .80 | 0.5 (0.4 to 0.9) (n = 61) | 0.6 (0.4 to 0.9) (n = 478) | .70 |
| C-reactive protein (mg/L), median (IQR) | 7.6 (3.8 to 15.1) (n = 438) | 6.4 (2.8 to 12.9) (n = 89) | 8.1 (3.8 to 15.6) (n = 349) | .10 | 5.9 (2.3 to 8.1) (n = 43) | 8.1 (3.8 to 15.5) (n = 395) | .01 |
| D-Dimer (mg/dL), median (IQR) | 1.0 (0.6 to 1.9) (n = 410) | 0.8 (0.6 to 1.6) (n = 81) | 1.0 (0.6 to 2.0) (n = 329) | .24 | 0.8 (0.6 to 1.5) (n = 38) | 1.0 (0.6 to 2.0) (n = 372) | .14 |
| Ferritin (ng/mL), median (IQR) | 611.1 (249.8 to 1350.2) (n = 450) | 502.4 (248.9 to 1006.5) (n = 90) | 636.5 (251.9 to 1417.7) (n = 360) | .18 | 350.7 (125.8 to 917.9) (n = 46) | 637.5 (275.9 to 1417.7) (n = 404) | .004 |
| Lactate dehydrogenase (IU/L), median (IQR) | 348.0 (258.0 to 486.0) (n = 401) | 374.0 (274.0 to 515.0) (n = 77) | 347.0 (253.0 to 476.5) (n = 324) | .30 | 276.0 (234.0 to 376.0) (n = 37) | 361.0 (262.0 to 497.5) (n = 364) | .003 |
| White blood cell count (109/L), median (IQR) | 7.0 (5.1 to 9.9) (n = 569) | 7.0 (5.2 to 9.8) (n = 113) | 7.0 (5.1 to 10.0) (n = 456) | .72 | 6.0 (4.7 to 8.8) (n = 63) | 7.1 (5.2 to 10.2) (n = 506) | .04 |
| Chronic pulmonary disease, n (%) | 88 (15) | 24 (21) | 64 (14) | .05 | 10 (16) | 78 (15) | .91 |
| Chronic cardiac disease, n (%) | 163 (28) | 44 (39) | 119 (26) | .01 | 25 (40) | 138 (27) | .04 |
| Chronic kidney disease, n (%) | 140 (24) | 36 (32) | 104 (23) | .04 | 14 (22) | 126 (25) | .66 |
| Asthma, n (%) | 88 (15) | 31 (27) | 57 (12) | < .001 | 18 (29) | 70 (14) | .002 |
| Hypertension, n (%) | 357 (62) | 82 (73) | 275 (60) | .01 | 46 (73) | 311 (61) | .07 |
| Diabetes, n (%) | 204 (36) | 49 (43) | 155 (34) | .06 | 22 (35) | 182 (36) | .90 |
BMI = body mass index, eGFR = estimated glomerular filtration rate, IQR = interquartile range, OSA = obstructive sleep apnea.
Unadjusted models did not find an association between sleep disorder diagnoses and outcomes of mechanical ventilation, vasopressor requirement, length of stay, or death (Table 2). Analysis of the population as a whole, with inclusion of transferred patients, also did not reveal an association between sleep disorder diagnoses and outcomes (Table S4 (228.7KB, pdf) ).
Table 2.
Sleep disorders among 572 hospitalized patients with COVID-19: prevalence and unadjusted association with mechanical ventilation, vasopressor requirement, death, and length of stay.
| Sleep Diagnosis | All* (n = 572) | Mechanical Ventilation* (n = 109) | OR [95% CI] | Vasopressor Requirement* (n = 102) | OR [95% CI] | Death* (n = 72) | OR [95% CI] | LOS† (n = 572) | HR to Discharge [95% CI] |
|---|---|---|---|---|---|---|---|---|---|
| OSA | 113 (19.8) | 28 (25.7) | 1.54 [0.94, 2.51] | 26 (25.5) | 1.51 [0.91, 2.49] | 16 (22.2) | 1.19 [0.65, 2.16] | 8 (3, 43) | 0.92 [0.74, 1.15] |
| Insomnia | 63 (11.0) | 14 (12.8) | 1.25 [0.66, 2.35] | 16 (15.7) | 1.67 [0.91, 3.01] | 12 (16.7) | 1.76 [0.89, 3.49] | 12 (4, 76) | 0.81 [0.61, 1.08] |
| RLS/PLMD | 22 (3.9) | 1 (0.9) | 0.20 [0.02, 1.47] | 2 (2.0) | 0.45 [0.10, 1.96] | 3 (4.2) | 1.10 [0.32, 3.82] | 10.5 (5, 42) | 0.86 [0.54, 1.35] |
Values are n (%). †Values are median (interquartile range). CI = confidence interval, HR = hazard ratio, LOS = length of stay, OR = odds ratio, OSA = obstructive sleep apnea, PLMD = periodic limb movement disorder, RLS = restless legs syndrome.
Multivariable models adjusting for age, sex, BMI, and race did not show a statistically significant impact of OSA, insomnia, or RLS/ PLMD on mechanical ventilation, vasopressor requirement, length of stay, or death. The presence of OSA and insomnia demonstrated a nearly statistically significant association with increased odds of death and vasopressor requirement respectively (OR [95% confidence interval], 1.83 [0.89, 3.75] and 1.86 [0.98, 3.56]). The small number of patients precluded inclusion of hypoventilation and CSA as predictors (Table 3). Analysis of the population as a whole, with inclusion of transferred patients, did not reveal an association between sleep disorder diagnoses and outcomes in the adjusted model (Table S5 (228.7KB, pdf) ).
Table 3.
Multivariable logistic and Cox proportional-hazards regression.
| Characteristic | Mechanical Ventilation, OR [95% CI] | Vasopressor Requirement, OR [95% CI] | Death, OR [95% CI] | HR to Discharge [95% CI] |
|---|---|---|---|---|
| OSA | 1.53 [0.90, 2.59] | 1.40 [0.81, 2.40] | 1.83 [0.89, 3.75] | 0.81 [0.64, 1.03] |
| Age* | 1.08 [0.94, 1.24] | 1.17 [1.01, 1.35] | 2.46 [1.89, 3.21] | 0.83 [0.79, 0.87] |
| Sex | 1.78 [1.13, 2.81] | 1.82 [1.14, 2.90] | 1.43 [0.80, 2.56] | 0.85 [0.71, 1.03] |
| Race (Black vs White) | 1.29 [0.89, 1.86] | 1.28 [0.88, 1.88] | 1.02 [0.64, 1.65] | 1.02 [0.82, 1.26] |
| Race (other vs White) | 0.77 [0.47, 1.28] | 0.75 [0.44, 1.28] | 0.99 [0.53, 1.83] | 1.27 [0.95, 1.70] |
| BMI** | 1.01 [0.90, 1.15] | 1.07 [0.94, 1.21] | 0.92 [0.75, 1.12] | 1.08 [1.03, 1.13] |
| Insomnia | 1.44 [0.74, 2.80] | 1.86 [0.98, 3.56] | 1.64 [0.75, 3.57] | 0.85 [0.62, 1.16] |
| Age* | 1.07 [0.94, 1.23] | 1.16 [1.00, 1.34] | 2.41 [1.86, 3.12] | 0.83 [0.79, 0.88] |
| Sex | 1.82 [1.15, 2.87] | 1.88 [1.18, 3.02] | 1.48 [0.83, 2.63] | 0.85 [0.70, 1.02] |
| Race (Black vs White) | 1.29 [0.89, 1.86] | 1.28 [0.88, 1.88] | 1.03 [0.64, 1.67] | 1.02 [0.82, 1.26] |
| Race (other vs White) | 0.77 [0.47, 1.28] | 0.77 [0.45, 1.31] | 0.99 [0.53, 1.82] | 1.28 [0.96, 1.72] |
| BMI** | 1.03 [0.92, 1.16] | 1.08 [0.96, 1.22] | 0.94 [0.78, 1.14] | 1.07 [1.02, 1.12] |
| RLS/PLMD | 0.20 [0.03, 1.55] | 0.46 [0.10, 2.07] | 1.23 [0.32, 4.77] | 0.81 [0.50, 1.32] |
| Age* | 1.08 [0.94, 1.24] | 1.17 [1.01, 1.35] | 2.42 [1.87, 3.14] | 0.83 [0.79, 0.87] |
| Sex | 1.77 [1.12, 2.79] | 1.81 [1.13, 2.89] | 1.46 [0.82, 2.60] | 0.85 [0.70, 1.02] |
| Race (Black vs White) | 1.29 [0.89, 1.85] | 1.29 [0.88, 1.88] | 1.04 [0.65, 1.67] | 1.01 [0.81, 1.25] |
| Race (other vs White) | 0.74 [0.45, 1.22] | 0.73 [0.43, 1.23] | 0.96 [0.52, 1.77] | 1.29 [0.96, 1.73] |
| BMI** | 1.03 [0.91, 1.16] | 1.08 [0.96, 1.22] | 0.94 [0.78, 1.15] | 1.07 [1.02, 1.12] |
Age per 10 years. **BMI in 5-kg/m2 increments. BMI = body mass index, CI = confidence interval, HR = hazard ratio, OR = odds ratio, OSA = obstructive sleep apnea, PLMD = periodic limb movement disorder, RLS = restless legs syndrome.
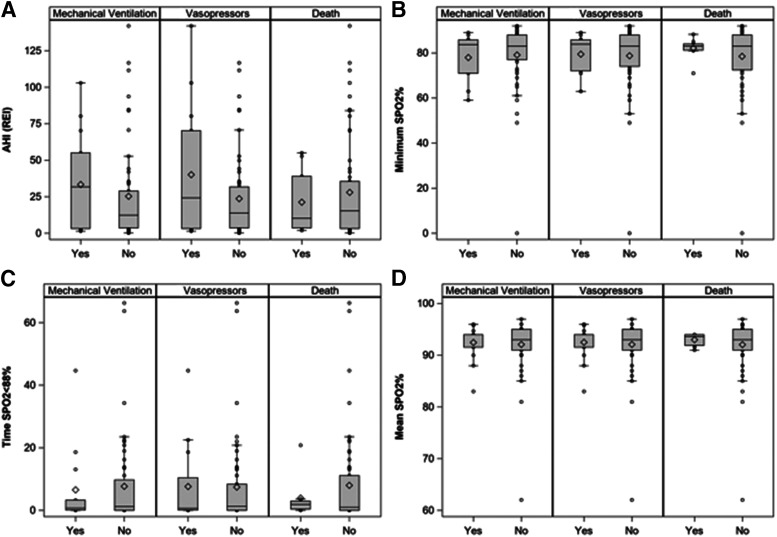
Objective sleep variables available in 73 patients were derived from HSAT (n = 4), full-night diagnostic PSG (n = 33), and split-night PSG (n = 6). Objective sleep variables (AHI/REI, minimum SpO2, mean SpO2, time SpO2 < 88%, and PLMI) were not significantly related to mechanical ventilation, vasopressor requirement, or death (Table 4 and Figure 1). Analysis of the entire population, with inclusion of transferred patients who had prior HSAT, full-night or split-night PSG within our system did not demonstrate a relationship between sleep study parameters and outcomes (Table S6 (228.7KB, pdf) and Figure S1 (228.7KB, pdf) ).
Table 4.
HSAT or PSG parameters in 73 patients with available diagnostic sleep study data.
| Sleep Parameter | Mechanical Ventilation | P | Vasopressor Requirement | P | Death | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||||
| AHI (REI) (events/h)* | .32 | .32 | .89 | ||||||
| n (missing) | 15 (0) | 53 (5) | 14 (0) | 54 (5) | 9 (0) | 59 (5) | |||
| Median (IQR) | 31.8 (3.2 to 55.1) | 12.4 (3.6 to 29.0) | 24.3 (3.2 to 70.3) | 13.8 (3.6 to 31.8) | 10.2 (3.7 to 39.1) | 15.3 (3.2 to 35.6) | |||
| Range | 1.5 to 103.0 | 0.2 to 142.0 | 1.5 to 142.0 | 0.2 to 116.7 | 1.9 to 55.1 | 0.2 to 142.0 | |||
| Mean SpO2 (%) | .97 | .81 | .89 | ||||||
| n (missing) | 15 (0) | 58 (0) | 14 (0) | 59 (0) | 9(0) | 64 (0) | |||
| Median (IQR) | 94.0 (91.6 to 94.0) | 93.0 (91.0 to 95.0) | 94.0 (91.6 to 94.0) | 93.0 (91.0 to 95.0) | 93.7 (91.9 to 94.0) | 93.0 (91.0 to 95.0) | |||
| Range | 83.0 to 96.0 | 62.0 to 97.0 | 83.0 to 96.0 | 62.0 to 97.0 | 91.0 to 94.0 | 62.0 to 97.0 | |||
| Minimum SpO2 (%) | .40 | .69 | .97 | ||||||
| n (missing) | 15 (0) | 58 (0) | 14 (0) | 59 (0) | 9 (0) | 64 (0) | |||
| Median (IQR) | 83.7 (71.0 to 85.8) | 83.0 (77.0 to 88.0) | 83.9 (72.0 to 85.8) | 83.0 (74.0 to 88.0) | 83.0 (81.1 to 84.0) | 83.0 (72.4 to 88.0) | |||
| Range | 59.0 to 89.0 | 0.0 to 92.0 | 63.0 to 89.0 | 0.0 to 92.0 | 71.0 to 88.2 | 0.0 to 92.0 | |||
| Time SpO2 < 88% (minutes) | .88 | .88 | .90 | ||||||
| n (missing) | 13 (2) | 52 (6) | 12 (2) | 53 (6) | 8 (1) | 57 (7) | |||
| Median (IQR) | 0.7 (0.1 to 3.3) | 1.2 (0.0 to 9.7) | 0.7 (0.1 to 10.4) | 1.2 (0.0 to 8.4) | 1.9 (0.4 to 2.9) | 1.0 (0.0 to 11.1) | |||
| Range | 0.0 to 44.7 | 0.0 to 66.3 | 0.0 to 44.7 | 0.0 to 66.3 | 0.0 to 20.8 | 0.0 to 66.3 | |||
| PLM index (events/h)** | .45 | .62 | .19 | ||||||
| n (missing) | 15(0) | 54 (4) | 14 (0) | 55 (4) | 9 (0) | 60 (4) | |||
| Median (IQR) | 0.0 (0.0 to 10.6) | 0.7 (0.0 to 9.6) | 0.0 (0.0 to 10.6) | 0.7 (0.0 to 9.6) | 10.2 (0.0 to 14.9) | 0.1 (0.0 to 8.0) | |||
| Range | 0.0 to 14.9 | 0.0 to 129.9 | 0.0 to 14.9 | 0.0 to 129.9 | 0.0 to 129.9 | 0.0 to 48.2 | |||
Both AHI and REI were derived with use of 4% hypopnea scoring criteria to harmonize between data extracted from HSAT and in-lab PSG. **Missing in participants with HSAT. AHI = apnea-hypopnea index, HSAT = home sleep apnea test, IQR = interquartile range, PLM = periodic limb movement, PSG = polysomnography, REI = respiratory event index, SpO2 = oxygen saturation.
Figure 1. Respiratory parameters from PSG and HSAT in the 73 individuals with diagnostic sleep study data.
(A) Both AHI and REI were derived with use of 4% hypopnea scoring criteria to harmonize between data extracted from HSAT and in-lab PSG. (B) Minimum SpO2 (%). (C) Time with SpO2 < 88% (minutes). (D) Mean SpO2 (%). AHI = apnea-hypopnea index, HSAT = home sleep apnea test, PSG = polysomnography, REI = respiratory event index, SpO2 = oxygen saturation.
DISCUSSION
To our knowledge, this is the first study to systematically evaluate the prevalence of sleep-disordered breathing (OSA, CSA, and hypoventilation), insomnia, and RLS/PLMD in a cohort of patients hospitalized for COVID-19. Additionally, this work evaluated the contribution of sleep diagnoses, as well objective parameters from diagnostic sleep studies (PSG and HSAT), to outcomes in hospitalized COVID-19 patients.
Prevalence
We found that OSA, CSA, and hypoventilation diagnoses were present in 19.8%, 0.7%, and 0.9% of patients, respectively. Insomnia was observed in 11% and RLS/PLMD in 3.9% of patients.
Currently, 3 of the 4 available small studies (n = 21–124) that assessed the presence of OSA diagnoses among patients hospitalized for COVID-19 found a prevalence of 20–30%43,44,59 with 1 outlier that identified 7.25%.45 In a much larger population, the CORONADO cohort,46 “treated obstructive sleep apnea” was present in 12.1% of inpatients with diabetes and COVID-19; however, no details regarding the derivation of the designation is available, so whether patients were prescribed treatment, self-reported treatment use, or were objectively confirmed to adhere to treatment (PAP-generated data) remains unclear.
Variable (and often unclear) methods of identifying which patients carried a diagnosis of OSA preclude direct comparison between our study and other investigations that cite OSA prevalence in COVID-19 inpatients. However, our predominately male (56%) population was characterized by a median age of 63 and median BMI in the obese range (31 kg/m2); therefore, our finding that 20% of individuals hospitalized for COVID-19 were diagnosed with OSA is not unexpected. Additionally, because the odds ratios for hypertension among patients with OSA are up to 2- to 3-fold60 and 15–30% of patients with OSA have diabetes,18 comorbid OSA is not unexpected in our cohort, which also had a high prevalence of diabetes (39%) and hypertension (68%).
Notably the proportion OSA in our hospitalized cohort was much greater than the prevalence in populations with COVID-19 that include outpatients (< 10%).47,48
In regards to sleep disturbances apart from OSA, we are unaware of available data regarding the prevalence of pre-existing CSA, hypoventilation, insomnia, or RLS/PLMD in hospitalized patients infected with COVID-19. However, insomnia symptoms increase with age, and chronic insomnia has been observed in more than 40% of individuals with underlying medical comorbidities such as hypertension and cardiovascular disease.61,62 Similarly, an increased burden of comorbid conditions (including diabetes, hypertension, myocardial infarction, obesity, stroke, cancer, and renal disease) has been associated with increased prevalent and incident RLS.63
Therefore, given significant overlap between chronic comorbidities and sleep disorders, the presence of OSA and insomnia diagnoses among patients hospitalized for COVID-19, who were older, overweight, and had high prevalence of hypertension and diabetes, was not surprising.
Contribution to outcomes
We did not identify any associations between sleep-disordered breathing, insomnia, or RLS/PLMD with outcomes among hospitalized COVID-19 patients. Among the subgroup of individuals with diagnostic sleep study data, AHI (REI), minimum SpO2, mean SpO2, time SpO2 < 88%, and PLMI, were also unrelated to outcomes.
The theory that disordered sleep could worsen outcomes related to COVID-19 has been postulated by multiple investigators and potential mechanisms include: increased expression of angiotensin converting enzyme 2 (the entry receptor of SARS-CoV-2) and dysregulation of renin-angiotensin system related to OSA,64,65 hypoxemia worsened by OSA (and, if present, obesity hypoventilation syndrome),66 and exaggeration of the cytokine storm seen in COVID-19 by the proinflammatory states of OSA and obesity.66
Additionally, sleep disruption is associated with proinflammatory cytokines (interleukin-6 and tumor necrosis factor-α),67 increased viral susceptibility, resistance to the anti-inflammatory effects of corticosteroids, and in animal models, pulmonary inflammation and increased mortality in the face of septic challenges.65,67 Therefore, sleep deprivation, independent of sleep-disordered breathing, is potentially relevant to COVID-19 pathogenesis.67
Despite these putative mechanisms, scant evidence is available to confirm or refute the impact of disturbed sleep on COVID-19 outcomes in hospitalized patients. In a group of 1,317 individuals with diabetes, Cariou and colleagues46 found that treated OSA was associated with increased mortality on day 7 of admission (OR = 2.65; 95% confidence interval: 1.36, 5.19) after adjustment for age, sex, hypertension, microvascular and macrovascular complications of diabetes, heart failure, cancer, and use of beta-blockers, metformin, insulin, loop diuretics, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid-receptor antagonist. However, BMI was not included as a confounder in this model. Furthermore, how patients with “treated obstructive sleep apnea”’ were identified is not detailed. Treated OSA could refer to treatment ordered, self-reported treatment use, or objectively confirmed treatment of OSA (by assessment of PAP-generated adherence data). Additionally, treatment of OSA could include PAP (CPAP or bilevel PAP), surgical interventions, hypoglossal nerve stimulation, or oral appliance use. Therefore, whether the relationship between treated OSA and death reflects a detriment of OSA itself or of the treatment for OSA remains unknown.46 No investigations are available that report the contribution of non-OSA diagnoses or objective sleep parameters to COVID-19 outcomes.
The absence of an impact of sleep disorder diagnoses or objective sleep parameters to inpatient outcomes is perhaps not surprising in this study, given that COVID-19 illness severe enough to result in hospitalization is associated with high risk of endotracheal intubation and mechanical ventilation and death. For example, among 2,741 patients hospitalized for COVID-19 in a US cohort, 647 (23.6%) required mechanical ventilation and 24.2% died or progressed to hospice care.11 Therefore, the role of pre-existing sleep disorders in COVID-19 may exert more of an impact in susceptibility to the virus or disease severity prior to hospitalization. In support of this hypothesis, among a large, 10 hospital integrated health care system, individuals diagnosed with OSA were more likely to become infected by SARS-CoV-2 and those with OSA and COVID-19 were more likely to progress to hospitalization (OR = 1.65; 95% confidence interval: 1.36, 2.02) and respiratory failure (OR = 1.98; 95% confidence interval: 1.65, 2.37), despite adjustment for BMI, diabetes, and hypertension. Another large health care system study found that OSA was a risk factor for hospitalization, death, critical care requirement, and mechanical ventilation, but this finding did not persist after adjusting for BMI, hypertension, diabetes, and chronic lung disease. Notably, this investigation demonstrated effect sizes (unadjusted odds of death: OR = 1.79; 95% confidence interval: 1.31, 2.45) and odds of death adjusted for BMI and demographics (OR = 1.39; 95% confidence interval: 0.97, 1.98) not dissimilar to those reported here.48
Strengths
This is the first study to evaluate OSA and other sleep disorders diagnoses in a large population of well-characterized patients hospitalized for COVID-19. The identification of patients with sleep disorders was systematically extracted by mining the electronic health record for sleep disorder diagnostic codes. The use of diagnostic codes from our electronic health record database allowed for a systemic evaluation of pre-existing sleep disorders that was not dependent on provider query or patient self-report at the time of admission, which may be vulnerable to recall bias. Although one other larger study (n = 1,317) evaluated the role of OSA in COVID-19 specifically in hospitalized patients, the population was limited to diabetic patients, the designation of “treated OSA” was not well-defined, and BMI was not included in the multivariable models assessing the relationship of treated OSA with outcomes.46 No published study to our knowledge has reported on other sleep disorders in patients hospitalized for COVID-19. Additionally, in a subgroup of patients, diagnostic sleep study data were available, which allowed us to explore objective severity measures (AHI [REI], oxygen measures, and PLMI) on COVID-19 related outcomes.
Limitations
Although novel, this study has a number of limitations that should be considered. Use of diagnosis codes may result in the coding for conditions that are suspected and not confirmed. However, an investigation of sleep disorders codes (compared to the gold standard of sleep disorder confirmed by a sleep specialist) demonstrated that ICD-9 sleep disorder codes had a positive predictive value of 89% for confirmed sleep disorders.55 The negative predictive value of the presence of an ICD-9 sleep disorders code was 16%; therefore, of greater concern is that use of diagnostic codes may not capture all patients with sleep disorders.55 This limitation takes place on the backdrop of a decreased reporting of sleep related symptoms in racial/ethnic minorities and the socioeconomically disadvantaged,68 groups that are disproportionately affected by COVID-19.14,69,70 Additionally, the time elapsed since the diagnosis was coded in the electronic health record was not appraised and could vary between individuals, although we restricted the use of the diagnosis codes to the 2 years prior to COVID-19 hospitalization.
The treatment of sleep disorders was unknown in our cohort. This may be of particular relevance in OSA, where the adherence to the most common therapeutic intervention, CPAP, is poor (nonadherence estimated in 29–83% of patients).71,72 Additionally, CPAP adherence may be worse in groups with disparate outcomes related to COVID-19, including racial/ethnic minorities68 and older adults with lower socioeconomic status.73 Interestingly, in individuals who use CPAP, adherence has remained relatively stable74 or marginally improved during shelter in place restrictions during the COVID-19 pandemic.75
Additionally, our sample size, although large, may have been insufficient. For example, we observed an adjusted OR of 1.83 for the impact of OSA on mechanical ventilation. If the true OR is indeed equal to 1.83, our sample of 113 OSA and 459 non-OSA patients only had 60% power to detect a statistically significant difference. This is based on the likelihood ratio test, with use of a 2-sided α = 0.05 and assuming an event rate of 0.12 in the non-OSA group. Therefore, a sample of 191 with OSA and 775 without OSA (total n = 996) would be required for 80% power to detect a statistically significant difference (assuming the same ratio of patients with OSA to those without OSA in a subsequent population). Regarding the signal seen in the association of insomnia and death, our sample of 63 patients with insomnia and 509 without insomnia had 32% power to detect a true OR of 1.64 (assuming an event rate of 0.12 in the non-insomnia group). Therefore, a sample of 270 with insomnia and 2,182 without insomnia (2,452 total) would be required for 80% power a statistically significant difference (assuming the same ratio of insomnia to patients without insomnia in a subsequent population).
Diagnostic sleep study data were only available on a limited number of our patients (13%), potentially because sleep studies were performed outside of our health system. Given the small number, the respiratory indices to determine sleep-disordered breathing were considered together whether data were acquired from PSG (AHI) or HSAT (REI). To best harmonize these data, AHI and REI extracted used the 4% hypopnea scoring criteria. However, despite use of the 4% hypopnea scoring criteria, the accuracy of HSAT in determining sleep-disordered breathing severity may be inferior to PSG, as the REI denominator is inherently less precise (total recording time or patient reported sleep duration) and technical issues may degrade the signals acquired by out-of-center testing. Additionally, at the University of Michigan, we do not routinely report the oxygen desaturation index, which may be a marker of greater utility in determining the contribution of OSA severity to other disease states.76,77 However, overall, the oxygen measures used in this study (minimum SpO2, mean SpO2, and time SpO2 < 88%) were actually more favorable in individuals with worse outcomes (though not reaching statistically significant difference); but this cannot be logically interpreted without information regarding outpatient treatment and may be irrelevant give the frequent use of supplemental oxygen in hospitalized patients.
A large proportion of our inpatient population is transferred from other hospitals and, therefore, does not have complete data regarding their disease course. However, analysis with inclusion of transfers was also conducted and did not change our conclusions based on nontransfers.
CONCLUSIONS
In this first investigation that evaluated the presence of pre-existing sleep disorders in patients hospitalized for COVID-19, a high prevalence of OSA (20%) and insomnia (11%) were identified. However, these values might be considered lower than expected compared to population studies in individuals with similar demographic characteristics and comorbidities, which may highlight the under recognition of sleep disorders in the same underserved patient groups who may be at increased risk for poor outcomes related to COVID-19 infection.
With adjustment for relevant confounders, pre-existing sleep disorders or objective markers of sleep-disordered breathing severity were not associated with mechanical ventilation, vasopressor use, length of stay, or death among patients hospitalized for COVID-19. Therefore, future evaluations on the role of earlier outcomes, such as infection or illness after exposure to SARS-CoV-2 or hospitalization, may be beneficial. Additionally, since myocardial injury is prevalent among critically ill patients with COVID-19,78 sleep disorders may be relevant for long-term cardiac outcomes, given their contribution to cardiovascular health. Furthermore, given the at-risk population, it remains an open question if new sleep disorders will be long-term sequelae in those who recover from severe COVID-19.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the University of Michigan Health System. This study was funded by Michigan Institute for Health and Research (MICHR) grant UL1TR002240. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors acknowledge the Michigan Institute of Clinical and Health Research (MICHR) infrastructure and staff for extraordinary support and teamwork in creation of the COVID-19 Rapid Response Registry. The authors thank Dr. Elizabeth LaPensee and the MICHR Interdisciplinary Research Initiatives team, Shari Sidener for database management, Jane Bugden for project management, and Janine Capsouras for administrative support. They also thank Aubrie Andrews, Brandon McCoy, Alyssa Nielsen, Peter Link, and Thomas Mobley for technical support, as well as Chiu-Mei Chen, MA MS, with support from the Michigan Medicine Department of Emergency Medicine and the Joyce and Don Massey Family Foundation. The authors also acknowledge the Michigan Genomic Initiative participants, the University of Michigan Medical School Research Data Warehouse/DataDirect, and Precision Health for providing data aggregation, management, and distribution services in support of the research reported in this publication.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CORONADO
Coronavirus SARS-CoV-2 & Diabetes Outcomes study
- CSA
central sleep apnea
- HSAT
home sleep apnea test
- ISARIC
International Severe Acute Respiratory and Emerging Infection Consortium
- OR
odds ratio
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PLMD
periodic limb movement disorder
- PSG
polysomnography
- REI
respiratory event index
- RLS
restless legs syndrome
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SpO2
oxygen saturation
REFERENCES
- 1.World Health Organization . Listings of WHO's response to COVID-19. https://www.who.int/news-room/detail/29-06-2020-covidtimeline. Accessed September 14, 2020.
- 2.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajema KL, Oster AM, McGovern OL, et al. ; 2019-CoV Persons Under Investigation Team . Persons evaluated for 2019 novel coronavirus—United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(6):166–170. 10.15585/mmwr.mm6906e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133(9):1025–1031. 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/. Published 2020. Accessed December 15, 2020.
- 11.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. 10.1001/jama.291.16.2013 [DOI] [PubMed] [Google Scholar]
- 18.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: A state of the art review. Chest. 2017;152(5):1070–1086. 10.1016/j.chest.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154(19):2219–2224. 10.1001/archinte.1994.00420190121014 [DOI] [PubMed] [Google Scholar]
- 20.Unruh ML, Sanders MH, Redline S, et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol. 2006;17(12):3503–3509. 10.1681/ASN.2006060659 [DOI] [PubMed] [Google Scholar]
- 21.Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138(3):543–550. 10.1378/chest.09-3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somers VK, White DP, Amin R, et al. ; American College of Cardiology Foundation . Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080–1111. 10.1161/CIRCULATIONAHA.107.189420 [DOI] [PubMed] [Google Scholar]
- 23.Htoo A, Talwar A, Feinsilver SH, Greenberg H. Smoking and sleep disorders. Med Clin North Am. 2004;88(6):1575–1591, xii. 10.1016/j.mcna.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 24.Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129–137. 10.1016/j.diabres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 25.Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. 10.1016/j.chest.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. 10.1212/01.wnl.0000287072.93277.c9 [DOI] [PubMed] [Google Scholar]
- 27.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259–270. 10.5664/jcsm.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker S, Fine A, Kryger MH. Sleep complaints are common in a dialysis unit. Am J Kidney Dis. 1995;26(5):751–756. 10.1016/0272-6386(95)90438-7 [DOI] [PubMed] [Google Scholar]
- 29.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15(9):739–746. 10.1016/j.cardfail.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–1469. 10.1093/eurheartj/ehu522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7(6):521–528. 10.1016/j.sleep.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 32.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137. 10.5664/jcsm.27760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correia LC, Souza AC, Garcia G, et al. Obstructive sleep apnea affects hospital outcomes of patients with non-ST-elevation acute coronary syndromes. Sleep. 2012;35(9):1241–1245. 10.5665/sleep.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144(3):903–914. 10.1378/chest.12-2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallowell PT, Stellato TA, Schuster M, et al. Potentially life-threatening sleep apnea is unrecognized without aggressive evaluation. Am J Surg. 2007;193(3):364–367, discussion 367. 10.1016/j.amjsurg.2006.09.022 [DOI] [PubMed] [Google Scholar]
- 36.Lindenauer PK, Stefan MS, Johnson KG, Priya A, Pekow PS, Rothberg MB. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest. 2014;145(5):1032–1038. 10.1378/chest.13-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ángeles-Castellanos M, Ramírez-Gonzalez F, Ubaldo-Reyes L, Rodriguez-Mayoral O, Escobar C. Loss of melatonin daily rhythmicity is associated with delirium development in hospitalized older adults. Sleep Sci. 2016;9(4):285–288. 10.1016/j.slsci.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48(6):679–684. 10.1111/j.0001-5172.2004.00401.x [DOI] [PubMed] [Google Scholar]
- 39.Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9(3):464–468. [PubMed] [Google Scholar]
- 40.Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):477–485. 10.1097/CCM.0b013e3181bc8243 [DOI] [PubMed] [Google Scholar]
- 41.Thille AW, Reynaud F, Marie D, et al. Impact of sleep alterations on weaning duration in mechanically ventilated patients: a prospective study. Eur Respir J. 2018;51(4):1702465. 10.1183/13993003.02465-2017 [DOI] [PubMed] [Google Scholar]
- 42.DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188–193. 10.2337/dc16-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arentz M, Yim E, Klaff L, et al. . Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Memtsoudis SG, Ivascu NS, Pryor KO, Goldstein PA. Obesity as a risk factor for poor outcome in COVID-19-induced lung injury: the potential role of undiagnosed obstructive sleep apnoea. Br J Anaesth. 2020;125(2):e262–e263. 10.1016/j.bja.2020.04.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cariou B, Hadjadj S, Wargny M, et al. ; CORONADO Investigators . Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. 10.1007/s00125-020-05180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maas MB, Kim M, Malkani RG, Abbott SM, Zee PC. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2020:1–3. 10.1007/s11325-020-02203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cade BE, Dashti HS, Hassan SM, Redline S, Karlson EW. Sleep apnea and COVID-19 mortality and hospitalization. Am J Respir Crit Care Med. 2020;202(10):1462–1464. 10.1164/rccm.202006-2252LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19 [published online ahead of print, 11 Jul 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed]
- 50.Dunning JW, Merson L, Rohde GGU, et al. ; ISARIC Working Group 3, ISARIC Council . Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14(1):8–9. 10.1016/S1473-3099(13)70327-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ISARIC Clinical Characterisation Group . Global outbreak research: harmony not hegemony. Lancet Infect Dis. 2020;20(7):770–772. 10.1016/S1473-3099(20)30440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 53.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 54.DataDirect: a self-serve tool for data retrieval [computer program]. Ann Arbor, MI: University of Michigan; 2015.
- 55.Jolley RJ, Liang Z, Peng M, et al. Identifying cases of sleep disorders through international classification of diseases (ICD) codes in administrative data. Int J Popul Data Sci. 2018;3(1):448. 10.23889/ijpds.v3i1.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grigg-Damberger MM. The AASM Scoring Manual four years later. J Clin Sleep Med. 2012;8(3):323–332. 10.5664/jcsm.1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berry RB, Quan SF, Abreu AR, et al. ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 58.Meoli AL, Casey KR, Clark RW, et al.; Clinical Practice Review Committee . Hypopnea in sleep-disordered breathing in adults. Sleep. 2001;24(4):469–470. [PubMed] [Google Scholar]
- 59.Gupta N, Agrawal S, Ish P, et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020;90(1). 10.4081/monaldi.2020.1294 [DOI] [PubMed] [Google Scholar]
- 60.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 61.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 62.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. 10.1093/sleep/30.2.213 [DOI] [PubMed] [Google Scholar]
- 63.Szentkirályi A, Völzke H, Hoffmann W, Trenkwalder C, Berger K. Multimorbidity and the risk of restless legs syndrome in 2 prospective cohort studies. Neurology. 2014;82(22):2026–2033. 10.1212/WNL.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 64.Pazarlı AC, Ekiz T, İlik F. Coronavirus disease 2019 and obstructive sleep apnea syndrome [published online ahead of print, 28 Apr 2020]. Sleep Breath. doi: 10.1007/s11325-020-02087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McSharry D, Lam MT, Malhotra A. OSA as a probable risk factor for severe COVID-19. J Clin Sleep Med. 2020;16(9):1649. 10.5664/jcsm.8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McSharry D, Malhotra A. Potential influences of obstructive sleep apnea and obesity on COVID-19 severity. J Clin Sleep Med. 2020;16(9):1645. 10.5664/jcsm.8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salles C, Mascarenhas Barbosa H. COVID-19 and obstructive sleep apnea. J Clin Sleep Med. 2020;16(9):1647. 10.5664/jcsm.8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. 10.1016/j.annepidem.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. 10.1016/j.smrv.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. 10.1513/pats.200708-119MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wickwire EM, Jobe SL, Oldstone LM, Scharf SM, Johnson AM, Albrecht JS. Lower socioeconomic status and co-morbid conditions are associated with reduced continuous positive airway pressure adherence among older adult Medicare beneficiaries with obstructive sleep apnea. Sleep. 2020;43(12):zsaa122. 10.1093/sleep/zsaa122 [DOI] [PubMed] [Google Scholar]
- 74.Batool-Anwar S, Omobomi OS, Quan SF. Impact of the novel coronavirus disease on treatment adherence and sleep duration in patients with obstructive sleep apnea treated with positive airway pressure. J Clin Sleep Med. 2020;16(11):1917–1920. 10.5664/jcsm.8746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Attias D, Pepin JL, Pathak A. Impact of COVID-19 lockdown on adherence to continuous positive airway pressure by obstructive sleep apnoea patients. Eur Respir J. 2020;56(1):2001607. 10.1183/13993003.01607-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29(6):777–783. 10.1093/sleep/29.6.777 [DOI] [PubMed] [Google Scholar]
- 77.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.