Abstract
Study Objectives:
Self-reported perception of sleep often differs from objective sleep study measures, but factors predicting the discrepancy between self-reported and objective sleep parameters are controversial, and a comparison of laboratory vs ambulatory polysomnography (PSG) is lacking.
Methods:
We retrospectively analyzed PSGs conducted between 2012 and 2016. Linear regression was applied to predict the discrepancy between self-reported and objective sleep parameters (total sleep time, sleep efficiency, sleep latency, using age, sex, arousal index, type of sleep disorder, and PSG type [laboratory vs ambulatory] as regressors).
Results:
A total of 303 PSGs were analyzed (49% women, median age 48 years), comprising patients with insomnia (32%), sleep-related breathing disorders (27%), sleep-related movement disorders (15%), hypersomnia/narcolepsy (14%), and parasomnias (12%). Sleep disorder was the best predictor of discrepancy between self-reported and objective total sleep time, and patients with insomnia showed higher discrepancy values compared to all other patient groups (P < .001), independent of age and PSG type (P > .05). Contributory effects for higher discrepancy values were found for lower arousal index. Patients with insomnia underestimated both total sleep time (median discrepancy: 46 minutes, P < .001) and sleep efficiency (median discrepancy: 11%, P < .001). No significant predictor for discrepancy of sleep latency was found.
Conclusions:
Misperception of sleep duration and efficiency is common in sleep lab patients, but most prominent in insomnia, independent of age, sex, or laboratory vs ambulatory recording setting. This underlines the role of PSG in patients with a clinical diagnosis of insomnia and its use in cognitive behavioral therapy.
Citation:
Trimmel K, Eder HG, Böck M, Stefanic-Kejik A, Klösch G, Seidel S. The (mis)perception of sleep: factors influencing the discrepancy between self-reported and objective sleep parameters. J Clin Sleep Med. 2021;17(5):917–924.
Keywords: misperception, self-reported, objective, PSG, insomnia, ambulatory, arousal, sex
BRIEF SUMMARY
Current Knowledge/Study Rationale: A discrepancy of self-reported and objective sleep parameters is common in sleep laboratory patients, but predicting factors are poorly understood and a comparison of laboratory vs ambulatory polysomnography is lacking. We retrospectively analyzed 303 polysomnographies of patients with insomnia (32%), sleep-related breathing disorders (27%), sleep-related movement disorders (15%), hypersomnia/narcolepsy (14%), and parasomnias (12%).
Study Impact: Misperception of sleep duration and sleep efficiency is most prominent in patients with insomnia, but independent of age, sex, or laboratory vs ambulatory recording setting. Perception of sleep may substantially aid the behavioral treatment of insomnia, which supports the extended use of polysomnography in these patients.
INTRODUCTION
A discrepancy between self-reported and objective sleep measures is common in sleep laboratory patients and is particularly frequent in patients with insomnia. Patients with insomnia usually experience greater sleep difficulties and shorter sleep duration compared to the respective polysomnographic parameters.1–3
Sleep discrepancy is also observed in patients with sleep-related breathing disorders (SRBD), albeit with contradictory results, where both an overestimation as well as underestimation of total sleep time (TST) have been reported.4–7 Surprisingly few studies have looked at discrepancy between self-reported and objective sleep parameters in sleep-related movement disorders (SRMD), hypersomnia, or parasomnia, and results are also conflicting. For patients with SRMD, both an overestimation of sleep latency (SL)8 as well as an absence of relevant discrepancies between self-reported and objective TST and SL9 were reported. A relatively accurate sleep perception was observed for patients with idiopathic hypersomnia (including narcolepsy) or parasomnia.8
Discrepancy between self-reported and objective sleep parameters is usually reported from polysomnographies (PSGs) performed in laboratory settings. Given the high costs and personnel requirements associated with laboratory PSG, ambulatory recordings are increasingly applied. However, it remains unclear whether a discrepancy between sleep perception and PSG differs in regard to the recording setting.
In summary, a discrepancy between self-reported and objective sleep parameters seems to be present not only in patients with insomnia, but also in patients with SRMD and SRBD. Studies have reported controversial findings regarding discrepancies between self-reported and objective sleep parameters, and a comparison between laboratory and ambulatory PSG is lacking.
Therefore, the objective of the present study was to investigate the underlying factors contributing to sleep misperception in a routine sample of sleep laboratory patients, including a comparison between PSG recordings in an ambulatory vs laboratory setting.
METHODS
Patients
For this study, full-night PSG recordings of all consecutive patients who underwent full-night ambulatory or laboratory PSG between January 2012 and December 2016 at our sleep lab at the Department of Neurology were analyzed. Prior to every PSG, each patient had been seen by a sleep expert of the Department of Neurology and a comprehensive semistructured interview on the general and sleep history had been taken.
The final diagnosis of sleep disorders followed the International Classification of Sleep Disorders10 and was established by a sleep specialist (K.T., S.S.), assigning patients to the following diagnostic categories: insomnia, hypersomnia (including patients with narcolepsy), SRMD, SRBD, and parasomnia (including both non-rapid eye movement and rapid eye movement parasomnias).
From a total of 352 recordings, 45 were excluded from this study due to incomplete PSG and/or clinical data. Four patients were excluded because the final diagnosis revealed a relevant neurologic or psychiatric comorbidity (major depression, epilepsy, Danon disease, Paramyotonia congenita in 1 patient each). Hence, we included 303 recordings (78 ambulatory, 225 laboratory) of a total of 279 patients (51.1% male) in this study. Twenty-four patients had 2 consecutive PSG nights and 4 patients had 3 consecutive PSG recordings.
This study was approved by the local ethics committee of the Medical University of Vienna. Due to the retrospective nature of the study, no written informed consent was obtained from participants.
Polysomnography
For laboratory recordings, all patients underwent at least one night of full video PSG, which included (i) electroencephalography (EEG; C3 and C4 with M1 and M2 as reference electrodes until February 2016; F3, C3, and O1 with M2 as reference electrode thereafter); (ii) electrooculography (vertical and horizontal eye movements); (iii) electromyogram (EMG) (mental, both anterior tibialis muscles); (iv) cardiorespiratory recording (single-channel electrocardiography, pneumoflow, respiratory movements from induction plethysmography, and transcutaneous oxygen saturation); and (v) a body position sensor. For ambulatory PSG recordings, EEG, electrooculography, and mental EMG were equally applied without video documentation.
Sleep was scored according to Rechtschaffen and Kales11 and parameters TST, SL, and sleep efficiency (SE) were assessed. For scoring of EMG activity, bipolar surface EMG was recorded with the low-pass filter at 35 Hz, the high-pass filter at 10 Hz, and a sampling rate of 100 Hz. Amplification was set at 5 μV/mm for scoring of REM-related EMG activity, and at 10 μV/mm for scoring of isolated limb movements (LM), periodic LM, high frequency LM, and fragmentary myoclonus. Impedance of surface EMG electrodes had to be lower than 10 kΩ. LM and arousals were scored according to the Atlas Task Force of the American Sleep Disorders Association.12
Assessment of self-reported sleep parameters
Self-reported sleep parameters were assessed using a standardized self-rated clinical questionnaire.13 Self-reported ratings on times of falling asleep and waking up, number and duration of awakenings per night, and total duration of sleep were used to calculate self-reported TST, SL, and SE.
Statistical analysis
Statistical analyses were done in IBM SPSS 25.0 for Windows. Linear regression analyses were performed using difference values of objective vs self-reported sleep parameters (TST, SL, SE) as dependent variables and sex, age, arousal index, type of sleep disorder (insomnia, hypersomnia, parasomnia, SRBD, SRMD), and PSG type (laboratory vs ambulatory) as regressors. Non-normally distributed variables were naturally log-transformed for regression analyses. Kruskal-Wallis tests for independent samples were used for intergroup comparisons of age as well as discrepancy values of sleep parameters among the different sleep disorder groups. Wilcoxon signed rank test for paired samples was used for comparisons between self-reported and objective sleep parameters within patient groups. A significance level of P < .05 was applied to all analyses. Bonferroni correction of P values was applied to the three linear regression analyses (P < .017). Exploratory analyses for the significant predictors in the regression models were performed without P value correction.
RESULTS
Demographics and sleep disorders
Demographic characteristics of the whole patient population are shown in Table 1.
Table 1.
Descriptive characteristics.
| All | Insomnia | Hypersomnia | Parasomnia | SRMD | SRBD | |
|---|---|---|---|---|---|---|
| n (%) | 303 (100%) | 97 (32%) | 43 (14%) | 36 (12%) | 46 (15%) | 81 (27%) |
| Sex (F/M) | 147/156 | 64/33 | 22/21 | 19/17 | 23/23 | 19/62 |
| Age | 47.5 (25.0) | 41.0 (21.5) | 36.0 (24.0) | 33.5 (21.0) | 55.0 (20.3) | 54.0 (20.5) |
| Arousal index (TST) | 12.0 (11.4) | 10.2 (7.6) | 10.9 (13.1) | 9.5 (6.6) | 15.0 (12.8) | 19.1 (21.8) |
| PSG type (amb/lab) | 78/225 | 55/92 | 18/25 | 2/34 | 1/45 | 2/79 |
| Δ sTST-oTST (min) | 5.5 (103.3) | −46 (111.0) | 11.5 (84.0) | 24.5 (80.9) | 10.5 (113.5) | 24.5 (103.8) |
| Δ sSOL-oSOL (min) | 24.5 (23.8) | 8.5 (27.3) | 2 (18.0) | 3.3 (21.6) | 9 (28.5) | 1 (25.5) |
| Δ sSEI-oSEI (%) | −1.7 (21.6) | −11.1 (25.6) | 0.7 (18.2) | −0.5 (15.0) | −1.7 (23.3) | 2.8 (22.5) |
Values are presented as number of events or as median and interquartile range. Δ = difference value, amb = ambulatory, lab = laboratory, o = objective, PSG = polysomnography, s = self-reported, SEI = sleep efficiency index in percentage of total sleep time, SOL = sleep onset latency, SRBD = sleep-related breathing disorder, SRMD = sleep-related movement disorder, TST = total sleep time.
Chronic insomnia (32%) was the most prevalent sleep disorder, followed by SRBD (27%), SRMD (15%), hypersomnia/narcolepsy (14%), and parasomnia (12%; 8% non-rapid eye movement-related and 4% rapid eye movement-related parasomnias). Age significantly differed between the patient groups (H = 63.94; df = 4; P < .001), and post hoc pairwise comparisons indicated that patients with SRBD or SRMD were significantly older than patients with insomnia, hypersomnia, or parasomnia (all P < .001, Table 1).
Linear regression
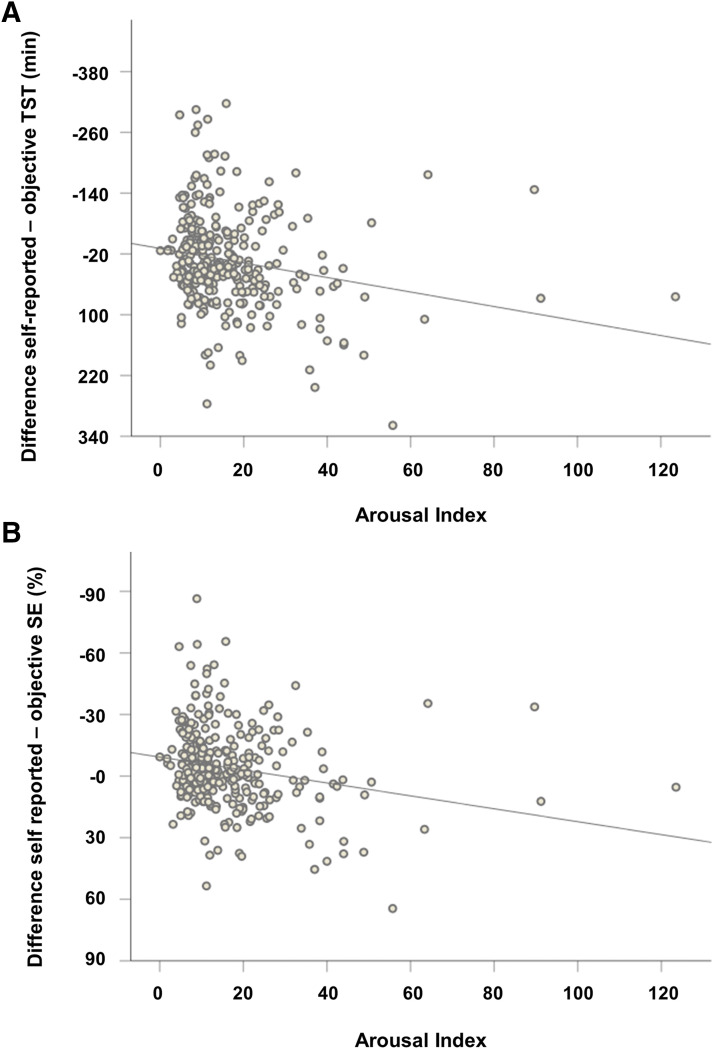
The linear regression model to predict a discrepancy of self-reported and objective sleep time was significant (F8,295 = 6.84; P < .001, adjusted R2 = .13; Figure 1), indicating that patients with insomnia showed higher discrepancy values compared to patients with SRBD (Beta = −0.31, P < .001), parasomnia and hypersomnia/narcolepsy (both Beta = −0.21, P = .001). An additional effect was observed for a lower arousal index predicting higher discrepancy (Beta = −0.17; P = .01; Figure 2). For predicting the discrepancy between self-reported and objective sleep efficiency (F8,295 = 6.79; P < .001, adjusted R2 = .13; Figure 1), again, sleep disorders were the best predictors, and patients with insomnia showed higher discrepancy values compared to patients with SRBD (Beta = −0.34, P < .001), parasomnia (Beta = −0.24, P < .001), hypersomnia/narcolepsy (Beta = −0.21, P = .001), and SRMD (Beta = −0.18, P = .008). A significant effect was also observed for lower arousal index (Beta = −0.16; P = .007; Figure 2). The regression model for the discrepancy between self-reported and objective sleep latency remained non-significant (F8,295 = 1.35; P = .22, adjusted R2 = .009).
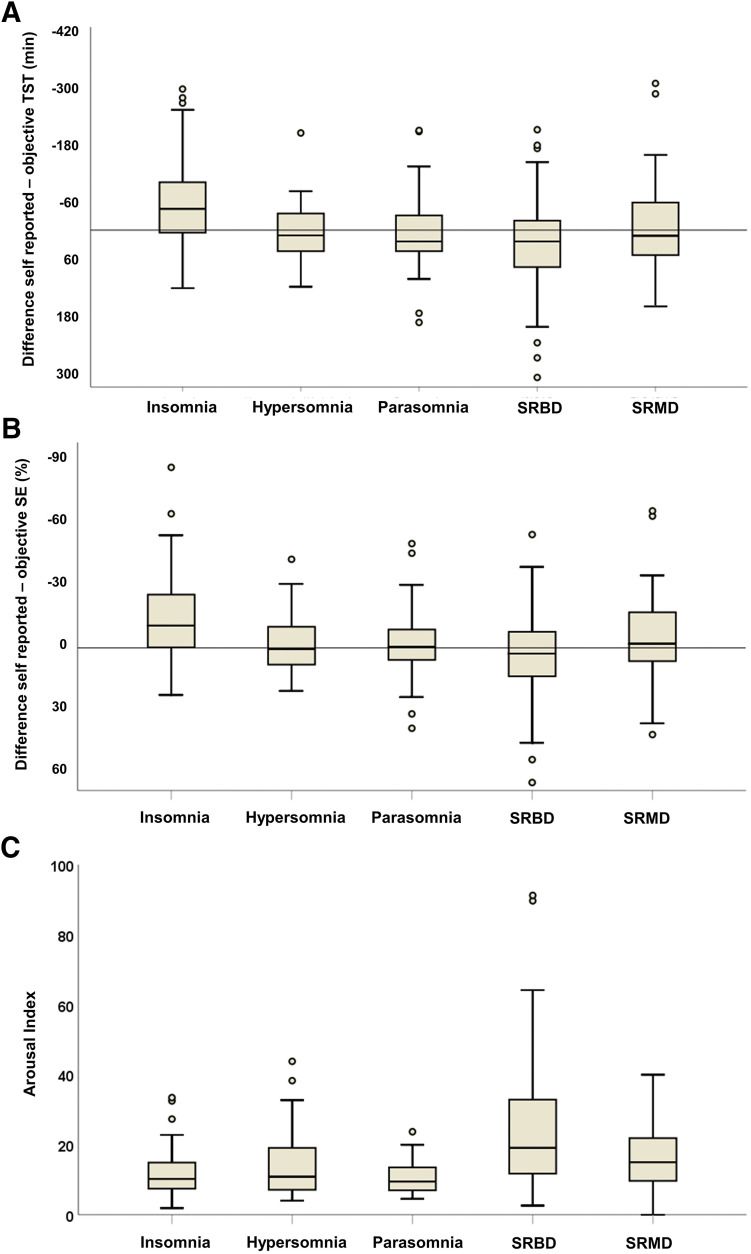
Figure 1. Difference of self-reported and objective sleep parameters.
(A) Difference values of self-reported minus objective total sleep time (TST). Patients with insomnia show highest discrepancy values and underestimate their TST, while patients with hypersomnia, parasomnia, sleep-related breathing disorder (SRBD), and sleep-related movement disorder (SRMD) overestimate TST. (B) Difference values of self-reported minus objective sleep efficiency (SE). Patients with insomnia underestimate their SE, while patients with hypersomnia, parasomnia, SRBD, and SRMD show good concordance of self-reported and objective SE. (C) Arousal indices among the different patient groups. Patients with SRBD show highest arousal indices. Boxes represent median and interquartile range, whiskers represent range, circles represent outliers.
Figure 2. Correlation of arousal index with difference values of self-reported minus objective total sleep time (TST) and sleep efficiency (SE) across all patients.
(A) Greater discrepancy of self-reported-objective TST correlates with lower arousal index (Spearman’s Rho = −0.24, P < .001). (B) Greater discrepancy of self-reported-objective SE correlates with lower arousal index (Spearman’s Rho = −0.23, P < .001).
Type of sleep disorder influences the discrepancy between self-reported and objective sleep parameters
Since regression analyses indicated that patients with insomnia showed higher discrepancy of self-reported and objective sleep parameters than other groups, we performed exploratory analyses to display detailed effects for the different sleep disorders. Within patient groups, those with insomnia significantly underestimated their TST by a median of 46 min (W = 975; P > .001), while patients with SRBD significantly overestimated their TST by a median of 11 min (W = 2.292; P = .003; Table 1; Figure 1). Patients with SRMD, hypersomnia, or parasomnia did not show significant differences between self-reported and objective TST (all P > .05; Figure 1).
Patients with insomnia significantly underestimated their SE by a median of 11% (W = 695; P < .001; Table 1; Figure 1). All other patient groups did not show significant differences between self-reported and objective SE, albeit those with SRBD showed a trend to overestimate their SE (W = 2.035; P = .077; Table 1; Figure 1).
Arousal index differs between sleep disorder patient groups
The arousal index significantly differed between patient groups (H = 51.61; df = 4; P < .001; Table 1; Figure 1), and post hoc analysis showed that patients with SRBD had significantly higher arousal indices compared to patients with insomnia, parasomnia, or hypersomnia (all P < .001; Figure 1) and that patients with SRMD had higher arousal indices than patients with insomnia (P = .04; Figure 1).
DISCUSSION
A discrepancy of self-reported vs objective sleep measures is common among sleep laboratory patients and represents a relevant concern for the treating physician, with resulting difficulties for both diagnosis and treatment of sleep disorders. Using multiple regression analyses, we could show that the type of sleep disorder is the main predictor of a discrepancy between self-reported and objective TST and SE, with patients with insomnia showing the most pronounced discrepancy between self-reported and objective TST and SE compared to all other sleep disorders. This effect was independent of the patients’ age, sex, or whether PSG was performed in an ambulatory or a laboratory setting. Apart from the type of sleep disorder, contributory effects for higher discrepancy of self-reported vs objective sleep discrepancy were found for lower arousal indices.
Sleep misperception in insomnia vs other sleep disorders
In line with previous findings, we found that sleep discrepancy frequently occurred in patients with insomnia, who significantly underestimated their TST and SE.2,3 Patients with insomnia underestimated their TST by a median of 46 minutes, which is in line with previous findings reporting an underestimation of sleep time in insomnia ranging from a mean of 17–47 minutes14 up to 70–110 minutes.3,8,15
The causal mechanisms of sleep misperception in insomnia are not yet fully understood. Certain personality traits, such as neuroticism or hypochondriasis, have been associated with sleep discrepancy, which may then escalate feelings of anxiety and arousal, particularly in patients with paradoxical insomnia, where objective PSG measures show normal or nearly normal results.16,17 Neurophysiological studies report altered EEG frequency bands and event-related potentials during non-rapid eye movement sleep in paradoxical insomnia,18,19 which may represent an indirect correlate of increased microarousals in these patients. This concept is further supported by altered glucose metabolism in brain regions associated with sensory processing and consciousness in patients with insomnia with sleep discrepancy.20
In contrast to findings in patients with insomnia, we found an overestimation of TST and a trend for overestimation of SE in patients with SRBD. This is in accordance with previous reports on a “positive sleep discrepancy”15 or “reverse sleep misperception”21 in patients with SRBD, while patients with hypersomnia or parasomnia seem to have a relatively accurate perception of sleep duration and efficiency.8 In regard to patients with SRMD, results of foregoing investigations are partly conflicting, where both an accurate perception of sleep parameters9 as well as an underestimation of SE8 have been reported. The discrepancies in findings were attributed to potential differences in sample characteristics (proportion of patients with RLS vs periodic limb movement disorder). Our results support the view that perception of TST and SE is relatively accurate in patients with SRMD.
Sleep misperception and arousal
Interestingly, we found a negative correlation of arousal indices with sleep discrepancy, indicating that patients with lower arousal indices tend to more profoundly underestimate their TST and SE. Although this might at first seem counterintuitive, it is well explained by the distribution of arousal indices among patient groups. Arousal indices were highest in SRBD patients from our sample, who generally overestimated their sleep time and sleep efficiency, and significantly lower in all other patient groups, including patients with insomnia, who profoundly underestimated their total sleep time and sleep efficiency. Similar effects were observed in a comparative study between patients with SRBD and insomnia, where patients with SRBD showed an overestimation of sleep time and efficiency despite generally higher arousal indices compared to patients with insomnia.15
The EEG frequency band changes that interfere with the perception of sleep quantity and quality and lead to hyperarousal in patients with insomnia seem to occur independent of “macro-architectural” phenomena22 like the “arousal” as defined from American Academy of Sleep Medicine standards.23 This suggests that sleep discrepancy in insomnia is not merely resulting from an increased number of (micro)arousals as measured from traditional PSG measures, but rather the outcome of complex biological, neurophysiological, and psychological interactions leading to a hypervigilant state.
Ambulatory vs laboratory PSG
To this point, it remains unclear whether sleep discrepancy is related to the setting of sleep recording. Standard laboratory PSG with monitoring of EEG, electrooculogram, EMG (chin, leg), electrocardiography, respiratory recording, and other parameters represents the most important diagnostic tool in sleep medicine.24 However, complimentary or alternative ambulatory diagnostic tools are warranted to mitigate high costs and personnel requirements associated with traditional PSG, with the additional advantage of the possibility to assess sleep in the patient’s routine environment as compared to the unfamiliar sleep laboratory setting.25 Furthermore, current events such as the COVID-19 pandemic will most likely increase the proportion of ambulatory investigations in the future to reduce hospital visits. Ambulatory recordings are suggested to better represent habitual sleep habits such as body posture, where patients are more likely to spend time in a supine position in a laboratory setting than at home.26 For the diagnosis of obstructive sleep apnea syndrome, there generally seems to be a high level of agreement between ambulatory and laboratory sleep studies,27–31 but comparative studies are generally scarce, particularly regarding sleep pathologies other than SRBD.
In our cohort, multiple regression analysis did not indicate a predictive effect of type of sleep study for sleep discrepancy. This demonstrates that misperception of sleep occurs independent of whether recordings are performed in an ambulatory or laboratory setting, which forms an important foundation for future diagnostic or interventional studies related to discrepancy of self-reported vs objective sleep parameters.
Strengths and limitations
While most previous studies focus on patients with insomnia only when investigating sleep discrepancy, we additionally included patients with SRBD, SRMD, hypersomnia, and parasomnia. To our knowledge, this is the first study to investigate misperception of sleep when comparing ambulatory vs laboratory setting of sleep studies.
There are several limitations to our work. Since our study sample included all patients attending our sleep lab at a certain time period, certain patient groups, particularly those with parasomnias, may be underrepresented in this study. In context with the clinically suspected type of sleep disorder, patient subgroups were unequally represented in ambulatory vs laboratory recording settings, and the vast majority of ambulatory recordings were performed in patients with insomnia or hypersomnia. Additionally, a potential bias resulting from first-night effects32,33 was not accounted for in our analyses, since 2 or more consecutive PSGs were only performed in a small subgroup of patients who had laboratory recordings and in none of the ambulatory recordings. It should be noted, however, that the type of recording setting did not influence our findings on discrepancy of self-reported and objective sleep parameters in the regression models. Formal mental health assessment with standardized questionnaires was not routinely performed in our patients, which should be addressed in future studies to take into account effects of distinct psychiatric comorbidities on perception of sleep. Data were acquired from a single center, and due to the retrospective nature of the study, control data were not collected, which limits generalizability. Furthermore, a detailed assessment of patient characteristics, including coexistent medical conditions, occupation, shift work, medication, or social drugs should be warranted in future prospective investigations.
Previous findings in healthy individuals suggest a high level of congruency between self-reported and objective sleep duration,34 although a potential underestimation of sleep duration in healthy sleepers has also been reported.35 Sleep deprivation leads to overestimation of sleep duration in healthy participants,35 while experimentally induced insomnia may elicit both an over- as well as underestimation of sleep duration, depending on length and time of day of sleep opportunity,34 which should be addressed in future comparative studies. Lastly, sleep was scored according to Rechtschaffen and Kales11 and not according to American Academy of Sleep Medicine scoring standards.23
Clinical implications
Evaluating sleep misperception has therapeutic implications, particularly for patients with insomnia. Cognitive behavioral therapy represents the most important form of treatment of insomnia.10,36 Drawing a patient’s attention to their misperception of sleep may aid to their understanding of symptoms of insomnia, which can be integrated into the therapeutic process. Ultimately, improving sleep perception by cognitive behavioral therapy can significantly improve insomnia.3,37,38 Based on current sleep medicine practice guidelines, the diagnosis of insomnia relies on clinical history and sleep quality questionnaires, and does not require objective investigations like PSG.10,36 Our findings, in line with previous research,15 suggest that performing PSG for the assessment of sleep perception in insomnia is highly relevant, with the potential to substantially improve therapeutic options.
CONCLUSIONS
Misperception of sleep quality is common in sleep disorders, but most prominent in patients with insomnia, who tend to underestimate their sleep duration and efficiency. Conversely, patients with SRBD, SRMD, hypersomnia, or parasomnia relatively accurately estimate their SE and tend to slightly overestimate their TST. These seem to be unrelated to patient age, sex, or to PSG setting (ambulatory vs laboratory). In patients with insomnia, a general state of hypervigilance is suspected to cause sleep misperception, albeit this seems unrelated to arousal indices as measured from PSG standards. Assessment of differences of self-reported vs objective sleep measures may form an integral part of the behavioral treatment of insomnia, which challenges the concept of sole clinical diagnosis of insomnia and supports the extended use of PSG in these patients.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the Medical University of Vienna. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all patients who participated in the study.
ABBREVIATIONS
- EEG
electroencephalography
- EMG
electromyogram
- LM
limb movements
- PSG
polysomnography
- SE
sleep efficiency
- SL
sleep latency
- SRBD
sleep-related breathing disorder
- SRMD
sleep-related movement disorder
- TST
total sleep time
REFERENCES
- 1.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–188. 10.1046/j.1365-2869.1997.00045.x [DOI] [PubMed] [Google Scholar]
- 2.Rezaie L, Fobian AD, McCall WV, Khazaie H. Paradoxical insomnia and subjective-objective sleep discrepancy: A review. Sleep Med Rev. 2018;40:196–202. 10.1016/j.smrv.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 3.Crönlein T, Lehner A, Schüssler P, Geisler P, Rupprecht R, Wetter TC. Changes in subjective-objective sleep discrepancy following inpatient cognitive behavior therapy for insomnia. Behav Ther. 2019;50(5):994–1001. 10.1016/j.beth.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18(4):232–239. 10.1093/sleep/18.4.232 [DOI] [PubMed] [Google Scholar]
- 5.McCall WV, Turpin E, Reboussin D, Edinger JD, Haponik EF. Subjective estimates of sleep differ from polysomnographic measurements in obstructive sleep apnea patients. Sleep. 1995;18(8):646–650. 10.1093/sleep/18.8.646 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi MT, Williams KL, McKinney S, Ellenbogen JM. The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res. 2013;22(5):557–568. 10.1111/jsr.12046 [DOI] [PubMed] [Google Scholar]
- 7.Castillo J, Goparaju B, Bianchi MT. Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography. J Psychosom Res. 2014;76(5):361–367. 10.1016/j.jpsychores.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reess T, Steinig J, Lanz M, Dempewolf S, Bunten S, Happe S. Perception of sleep: Subjective vs objective sleep parameters in patients with insomnia, hypersomnia, parasomnia, and sleep-related movement disorders. Somnologie (Berl). 2010;14(4):253–259. 10.1007/s11818-010-0491-8 [DOI] [Google Scholar]
- 9.Vanable PA, Aikens JE, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23(1):71–79. 10.1093/sleep/23.1.1d [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 11.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: US National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. [Google Scholar]
- 12.American Sleep Disorders Association . EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. 10.1093/sleep/15.2.173 [DOI] [PubMed] [Google Scholar]
- 13.Saletu B, Wessey P, Gruenberger J, Schultes M. Erste klinische Erfahrungen mit einem neuen schlafanstoßenden Benzodiazepin, Cinolazepam, mittels eines Selbstbeurteilungsbogens für Schlaf- und Aufwachqualität (SSA). Neuropsychiatrie (Deisenhof.). 1987;1:169–176. [Google Scholar]
- 14.Chan WS, Dautovich ND, McNamara JPH, et al. Sleep discrepancy in a randomized controlled trial of brief behavioral therapy for chronic insomnia in older adults. Behav Sleep Med. 2021;19(2):221–231. 10.1080/15402002.2020.1726750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto LR Jr, Pinto MCR, Goulart LI, et al. Sleep perception in insomniacs, sleep-disordered breathing patients, and healthy volunteers--an important biologic parameter of sleep. Sleep Med. 2009;10(8):865–868. 10.1016/j.sleep.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 16.Dorsey CM, Bootzin RR. Subjective and psychophysiologic insomnia: an examination of sleep tendency and personality. Biol Psychiatry. 1997;41(2):209–216. 10.1016/0006-3223(95)00659-1 [DOI] [PubMed] [Google Scholar]
- 17.Harvey AG, Tang NKY. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. 10.1037/a0025730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640. [PubMed] [Google Scholar]
- 19.Turcotte I, St-Jean G, Bastien CH. Are individuals with paradoxical insomnia more hyperaroused than individuals with psychophysiological insomnia? Event-related potentials measures at the peri-onset of sleep. Int J Psychophysiol. 2011;81(3):177–190. 10.1016/j.ijpsycho.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 20.Kay DB, Karim HT, Soehner AM, et al. Subjective-objective sleep discrepancy is associated with alterations in regional glucose metabolism in patients with insomnia and good sleeper controls. Sleep. 2017;40(11): zsx155. 10.1093/sleep/zsx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attarian HP, Duntley S, Brown KM. Reverse sleep state misperception. Sleep Med. 2004;5(3):269–272. 10.1016/j.sleep.2003.10.014 [DOI] [PubMed] [Google Scholar]
- 22.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–117. 10.1093/sleep/24.1.110 [DOI] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Hirshkowitz M. Polysomnography challenges. Sleep Med Clin. 2016;11(4):403–411. 10.1016/j.jsmc.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 25.Broughton R, Fleming J, Fleetham J. Home assessment of sleep disorders by portable monitoring. J Clin Neurophysiol. 1996;13(4):272–284. 10.1097/00004691-199607000-00002 [DOI] [PubMed] [Google Scholar]
- 26.Pereira EJ, Driver HS, Stewart SC, Fitzpatrick MF. Comparing a combination of validated questionnaires and level iii portable monitor with polysomnography to diagnose and exclude sleep apnea. J Clin Sleep Med. 2013;9(12):1259–1266 10.5664/jcsm.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portier F, Portmann A, Czernichow P, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162(3 Pt 1):814–818. 10.1164/ajrccm.162.3.9908002 [DOI] [PubMed] [Google Scholar]
- 28.Gagnadoux F, Pelletier-Fleury N, Philippe C, Rakotonanahary D, Fleury B. Home unattended vs hospital telemonitored polysomnography in suspected obstructive sleep apnea syndrome: a randomized crossover trial. Chest. 2002;121(3):753–758. 10.1378/chest.121.3.753 [DOI] [PubMed] [Google Scholar]
- 29.Andrade L, Paiva T. Ambulatory versus laboratory polysomnography in obstructive sleep apnea: comparative assessment of quality, clinical efficacy, treatment compliance, and quality of life. J Clin Sleep Med. 2018;14(8):1323–1331. 10.5664/jcsm.7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilgin C, Erkorkmaz Ü, Uçar MK, Akın N, Nalbant A, Annakkaya AN. A study on the use of a portable monitoring device (Somnocheck Micro) for the investigation and diagnosis of obstructive sleep apnoea in comparison with polysomnography. Pak J Med Sci. 2016;32(2):471–475 10.12669/pjms.322.9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driver HS, Pereira EJ, Bjerring K, et al. Validation of the MediByte® type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can Respir J. 2011;18(3):137–143. 10.1155/2011/760958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saletu B, Klösch G, Gruber G, Anderer P, Udomratn P, Frey R. First-night-effects on generalized anxiety disorder (GAD)-based insomnia: laboratory versus home sleep recordings. Sleep. 1996;19(9):691–697. [PubMed] [Google Scholar]
- 33.Veauthier C, Piper SK, Gaede G, Penzel T, Paul F. The first night effect in multiple sclerosis patients undergoing home-based polysomnography. Nat Sci Sleep. 2018;10:337–344. 10.2147/NSS.S176201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchi MT, Wang W, Klerman EB. Sleep misperception in healthy adults: implications for insomnia diagnosis. J Clin Sleep Med. 2012;8(5):547–554. 10.5664/jcsm.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maric A, Bürgi M, Werth E, Baumann CR, Poryazova R. Exploring the impact of experimental sleep restriction and sleep deprivation on subjectively perceived sleep parameters. J Sleep Res. 2019;28(3):e12706. 10.1111/jsr.12706 [DOI] [PubMed] [Google Scholar]
- 36.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. 10.5664/jcsm.27286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downey R 3rd, Bonnet MH. Training subjective insomniacs to accurately perceive sleep onset. Sleep. 1992;15(1):58–63. 10.1093/sleep/15.1.58 [DOI] [PubMed] [Google Scholar]
- 38.Janků K, Šmotek M, Fárková E, Kopřivová J. Subjective-objective sleep discrepancy in patients with insomnia during and after cognitive behavioural therapy: An actigraphy study. J Sleep Res. 2020;29(4):e13064. 10.1111/jsr.13064 [DOI] [PubMed] [Google Scholar]