Abstract
Study Objectives:
To evaluate the effects of a mind-body exercise, qigong Baduanjin, on sleep disturbances in women experiencing intimate partner violence and explore the mediating role of depressive symptoms, perceived stress, and inflammation in producing the effects.
Methods:
A subgroup of a parent randomized controlled trial was randomized for a 22-week Baduanjin intervention (n = 94) or wait-list control (n = 92). Questionnaires, including the General Sleep Disturbance Scale, Perceived Stress Scale, and Beck Depression Inventory version II, were administered at baseline, posttraining (6 weeks), and postintervention (22 weeks), and blood samples were collected to assess tumor necrosis factor and interleukin 6 levels at baseline and postintervention only.
Results:
Of the 186 participants, 170 completed the study. Results indicate that the total sleep disturbance scores for the intervention group were significantly lower than those for the wait-list control group at week 6 (difference = −7.96; 95% confidence interval [CI], −13.63 to −2.30; P = .006) and week 22 (difference = −7.17; 95% CI, −12.58 to −1.76; P = .01). Mediation analysis showed a statistically significant indirect effect of the intervention on sleep improvement through reducing depressive symptoms (β = 2.58, 95% CI, 0.69 to 5.09), while the mediating effects of perceived stress and inflammation were not significant.
Conclusions:
Qigong Baduanjin can be recommended for women who experience intimate partner violence and report sleep disturbances. More research is needed to understand the clinical significance of the observed sleep improvements.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Qigong Intervention Program for Abused Chinese Women; URL: https://clinicaltrials.gov/ct2/show/NCT02060123; Identifier: NCT02060123.
Citation:
Cheung DST, Chau PH, Yeung W-F, Deng W, Hong AWL, Tiwari AFY. Assessing the effect of a mind-body exercise, qigong Baduanjin, on sleep disturbance among women experiencing intimate partner violence and possible mediating factors: a randomized-controlled trial. J Clin Sleep Med. 2021;17(5):993–1003.
Keywords: intimate partner violence, sleep, randomized control trial, qigong, Baduanjin, mind-body, complementary and alternative medicine, non-pharmacological intervention
BRIEF SUMMARY
Current Knowledge/Study Rationale: Women experiencing intimate partner violence often report sleep disturbances. Although qigong Baduanjin has demonstrated benefits in improving sleep among patients with physical illnesses, its effects in abused women, a population whose sleep problems may be associated with chronic stress, have not been examined in the literature.
Study Impact: Our research shows that qigong Baduanjin, compared to wait-list control, resulted in improvement in self-reported sleep disturbances. More research is needed to understand the clinical importance of the observed improvement in sleep.
INTRODUCTION
Intimate partner violence (IPV), encompassing physical, psychological, and sexual abuse, is a serious public health problem that affects women worldwide. Globally, nearly one-third (30%) of women who have been in an intimate relationship have experienced physical or sexual abuse inflicted by their intimate partners.1 Psychological violence is more prevalent than are other forms of IPV, affecting up to 43% of the women.2 IPV victimization has been associated with numerous adverse mental and physical health outcomes among women.1 However, sleep disturbance is an area that has received limited attention in the IPV literature.
A systematic review has shown that sexually abused women were more likely to experience sleep disturbances than were individuals who had not been abused.3 Approximately half of IPV victims had clinically significant insomnia.4 Specifically, a majority of women reported moderate to severe sleep initiation insomnia, middle of the night insomnia, nightmares that replayed elements of their abusive events, and early morning awakening.4 IPV may be more salient in causing enduring sleep disturbances than are other stressors and life events because the abused victim’s sleep environment may be the venue where the violence took/takes place and it thus represents past/present danger. Also, the sleep environment may be accessible to the perpetrator, causing the woman to experience heightened vigilance during sleep. The feelings of threats and chronic stress inhibit sleep and may contribute to the persistence of sleep disturbances, even if abuse has terminated.5
Sleep disturbance in IPV victims has been associated with other mental health issues, including depression, anxiety, suicidality, social dysfunction, and posttraumatic stress disorder.4,6 Another study found that sleep disturbance partially mediates the relationship between IPV and mental/physical health.5 Therefore, sleep interventions may potentially mitigate the negative effects of IPV. Despite the well-documented prevalence and impacts of sleep disturbance resulting from IPV, interventions addressing this problem are scarce. Long-term use of conventional pharmacotherapies has uncertain efficacy and is associated with drug abuse, dependence, and increased mortality.7 Psychological and behavioral therapies are effective, but they have remained largely underused in primary care, probably due to the high costs and extensive behavioral changes required.8 Because sleep disturbance is often chronic in abused women, long-term and safe nonpharmacological interventions are warranted. In this connection, qigong may hold promise as such an intervention.
Qigong is a mind-body intervention rooted in a traditional Chinese medicine concept and is a preferable complementary and alternative medicine for insomnia in the Chinese population.9 Qi refers to the vital energy flowing within the body along the meridian. Qigong exercises are practiced with a combination of body posture and movement, breath control, and mindful meditation, all designed to promote qi flow for healing, preventing disease, and promoting psychological and physical health.10 The most commonly studied form of qigong is Baduanjin. This consists of 8 easy-to-follow movements, and thus is suitable for a wide range of participants. A meta-analysis has demonstrated an overall significant result of qigong Baduanjin in improving sleep among patients with physical illnesses.11 However, the results were not conclusive because the heterogeneity of the included studies was high (83%). Among the 3 studies reporting significant findings, the 1 presenting the greatest effect size also included educational lessons for reducing hypertension in the intervention.12 It is speculated that if Chen et al’s study12 were not included, the overall effect estimate might not be significant. More research is therefore needed to examine the specific effects of Baduanjin for improving sleep in different populations including those without chronic physical illnesses.
In addition to studying the effects of qigong on sleep, it is crucial to explain the mechanisms by which the intervention is successful. Cytokines in the inflammatory system have long been suggested to participate in the central nervous system regulation of physiological sleep.13 A meta-analysis has revealed that sleep problems were associated with increases in markers of inflammation.14 In addition, mental health comorbidities such as depression may have a negative impact on sleep.15 Given that inflammation, perceived stress, and depression have been found to be amenable to modification through qigong,16,17 we hypothesize that they are potential mediators able to produce the intervention effects. Analyses that elucidate mediators of the qigong impact on sleep will provide an important next step for guiding clinical interpretations of trials and informing the design and targeting of future interventions.
The effects of qigong for improving sleep disturbance in abused women, a population whose sleep problems may be associated with chronic stress related to IPV, have not been examined in the literature. The aim of the study is to evaluate the effect of qigong Baduanjin intervention on sleep disturbance in abused women and explore the mediating role of depressive symptoms, perceived stress, and inflammation in producing the effects.
METHODS
Design
The data set was a subset from a 2-group, parallel randomized controlled trial comparing the effects of a qigong Baduanjin intervention on telomerase activity among abused women with a wait-list control. From a qualitative evaluation of the first phase of the study (n = 85), a substantial number of participants highlighted sleep improvements after the intervention as shown in the following narrative comments, for example: “It feels like I can go into deeper sleep at night. During daytime, I feel less fatigue and more energetic.” “My sleep quality at night has become better, so I wake up feeling more rested and cheerful. My husband sleeps better as well and we fight less.” “In the past, my mind was active and worked continuously from day to night. During qigong practice, the master taught us to focus our mind on the present moment. My mind has become calmer and I can fall asleep more easily.” “I have had trouble remaining asleep during the night. Whenever I was awake in the middle of night, I became very anxious that I could not sleep again. After practicing qigong, I have learnt how to relax and breathe slowly. I am now less worried and often able to return to sleep.” Hence, sleep measure was added in the quantitative evaluation in subsequent phases (n = 186). A complete description of the design and methods of the parent study, without the sleep measurement described herein, was published previously.16,18 The parent study was conducted between March 12, 2014 and May 26, 2016.
Participants
The inclusion criteria were as follows: participants were Chinese women, 18 years of age or older, willing to undertake the qigong intervention, available for all of the data collection points, receptive to random allocation, and determined to be positive for IPV, using the Abuse Assessment Screen,19 in the preceding 1–2 years. Individuals were excluded if they had participated in qigong training within the previous 6 months, were pregnant, or had serious medical conditions that might limit their participation in qigong.
Intervention and control conditions
The intervention lasted 22 weeks and was comprised of 1) group training—qigong sessions provided by a qigong master twice per week for 6 weeks (24 hours), 2) weekly group follow-up—qigong follow-up sessions with reinforcement of practice by the same qigong master once per week for 16 weeks (16 hours), and 3) self-practice—participants were encouraged to practice qigong 30 minutes per day for the entire intervention period. To monitor the self-practice of qigong, each participant entered on a record card the duration and frequency of qigong undertaken every day, and this card was submitted to a research assistant at the weekly group follow-up sessions to verify compliance.
Participants in the wait-list control group received monthly health education sessions unrelated to qigong and could choose to receive qigong training after data collection had been completed.
Procedure
Ethical approval for the study was obtained from the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB: UW 12–555). Participants were recruited from the catchment area of a large community center that provides diversified health and social services for users of all ages in Hong Kong. Fliers promoting the study were posted on the notice boards of the host community center and its outreach sites. Interested clients contacted center staff members to make an appointment for further screening. To confirm a woman’s eligibility, a face-to-face interview was conducted by a trained research assistant in a private area without the presence of the participant’s partner. All participants provided written informed consent.
Eligible participants were randomly assigned to the intervention or the wait-list control group (1:1) based on a list of random permutations prepared by block randomization. The list was computer generated, recorded by an investigator not involved in participant recruitment, and placed in numbered sequential sealed envelopes. The list was also centrally controlled to avoid any bias in selection.
Data collection
Data were collected at the following time points: baseline, posttraining (week 6, ie, upon completion of the 6-week group training), and postintervention (week 22, ie, upon completion of the entire qigong intervention). At each time point, questionnaires including the General Sleep Disturbance Scale (GSDS), Perceived Stress Scale, and Beck Depression Inventory version II (BDI-II) were administered to participants in both groups. The Abuse Assessment Screen and Demographic Questionnaire were administered only at the baseline point. For measurements of the plasma levels of proinflammatory cytokines, tumor necrosis factor (TNF) and interleukin 6 (IL-6), 10 mL of peripheral blood was collected from each participant at baseline and after 22 weeks. Research assistants who administered the questionnaires and entered data were blinded. The nurse who collected the blood and laboratory technicians who processed the blood samples and measurements were also blinded to the group assignment.
Study measures
The Abuse Assessment Screen was used to screen potential participants for IPV. Participants were required to respond “yes” or “no” to 5 items addressing physical, psychological, and sexual abuse and fear toward the partner. The Chinese version demonstrated satisfactory screening accuracy.19
The GSDS was used to assess symptoms of sleep disturbance exhibited in the past week (ranging from 0 [“not at all”] to 7 [“everyday”]). The instrument includes 21 items related to a number of general sleep issues, which are summed to form 7 subscales: 1) difficulty getting to sleep, 2) waking up during sleep, 3) early waking, 4) quality of sleep, 5) quantity of sleep, 6) daytime sleepiness, and 7) self-medicating to assist sleeping. Each subscale has a range from 0 to 7, with scores 3 or higher indicating significant sleep problems on 3 or more nights per week. The 21 items were also summed to yield an overall sleep disturbance score ranging from 0 (“no disturbance”) to 147 (“extreme disturbance”); scores ≥ 43 represented poor sleep. Among various sleep measures, the GSDS covers a comprehensive assessment of both daytime impairment (7 items) and nighttime sleep experience (5 items), while other commonly used instruments such as the Pittsburgh Sleep Quality Index (2 items on daytime functioning) and Insomnia Severity Index (1 item on daytime functioning) focus more on nighttime sleep disturbances. Indeed, daytime assessment is equally important because people experiencing insomnia symptoms generally report daytime consequences.20 Therefore, the GSDS was selected as the outcome measure in the present study. The GSDS has been used with a variety of clinical groups, including postpartum women21 and cancer patients.22 The internal consistency and construct validity of the Chinese GSDS has been proven satisfactory.23
The Perceived Stress Scale was used to measure perception of stress during the past month. It consists of 10 items, for which respondents rate the degree to which life situations are perceived as stressful on a 4-point Likert-type scale ranging from 0 (never) to 4 (very often). The total scores range from 0 to 40, with higher scores representing higher stress. The Chinese Perceived Stress Scale has shown satisfactory internal consistency in Chinese adults.21
The BDI-II was used to assess depressive symptoms exhibited in the past 2 weeks. It assesses cognitive, affective, and somatic components of depression, including, for example, concentration problems, suicide ideation, and sleep disturbances. It consists of 21 items rated on a 4-point Likert-type scale. The total score ranges from 0 to 63, with higher scores indicating more severe depressive symptoms. The Chinese BDI-II has exhibited construct validity and reliability for Chinese populations.24
For measuring levels of proinflammatory cytokines (TNF and IL-6), plasma was first separated from the blood sample. Levels of the cytokines in plasma were then measured with enzyme-linked immunosorbent assay kits (Bio-Station Limited) in the laboratory.
The Demographic Questionnaire was designed by the research team to elicit background, lifestyle, and health-related information.
Selected mediators
We chose mediators based on the literature report that qigong practice reduces depressive symptoms, perceived stress,16 and inflammation,17 which in turn reduces sleep disturbance in abused women. Therefore, changes in levels of perceived stress (measured by the Perceived Stress Scale), depressive symptoms (measured by the BDI-II), and inflammation (measured by TNF and IL-6) occurring between baseline and postintervention periods were hypothesized to be the mediators of the relationship between the qigong intervention and changes in sleep (measured by the GSDS).
Sample size
A meta-analysis has reported that the effect size of Baduanjin for improving sleep quality among healthy individuals and patients with physical health problems compared to usual care was 0.55.11 Taking into account the chronicity of sleep disturbance experienced by abused women relative to the general population,3 a conservative estimate of effect size of 0.45 was used. With the statistical program G*Power and assuming a type I error rate of 5%, n = 158 is needed to achieve power greater than 80%. Allowing for an attrition rate of 10%, a sample size of at least 176 is needed.
Data analysis
Descriptive statistics included participants’ background characteristics, adherence, and baseline sleep profile. A t test and chi-square test were performed to identify the heterogeneity of the between-group baseline data.
Intervention effects on sleep were tested on an intention-to-treat basis using mixed-effects models, which can accommodate missing observations in the follow-up data and does not require imputation, providing a natural way to deal with missing values or dropouts. All tests involved a 2-sided significance level of ⍺ = 0.05. A linear mixed-effects model was conducted using SPSS to compare the mean changes in the GSDS total and subscale scores between the intervention and control groups at different time points (as compared with baseline). The analyses were repeated by including poor sleepers at baseline only (baseline GSDS scores ≥ 43) and using the per-protocol approach (using data of adherents only). The adherents in this study were defined as follows: 1) in the intervention group, those who attended over 80% of the training sessions and completed all measurements, 2) in the control group, those who completed all measurements. All analyses were repeated while adjusting for potential covariates including age25 and type of IPV26 (psychological abuse only, physical/sexual abuse combined with psychological abuse, and physical abuse plus sexual abuse plus psychological abuse).
The mediation model of the intervention effect on sleep was tested using the PROCESS function V3.4.1 in SPSS (model 4).27 Only participants without missing data in the respective variables were included in the analysis. Changes in perceived stress, depressive symptoms, and inflammation were entered as mediators; the qigong intervention was the independent variable and change in sleep was the outcome variable. To test the significance of the indirect effects (IEs), the macro generated bias-corrected bootstrapped 95% confidence intervals (CIs). Mediation was significant if the upper and lower bound of the bias corrected CI for the IE did not include zero.27 The proportion of the total effect that was mediated was also calculated.
RESULTS
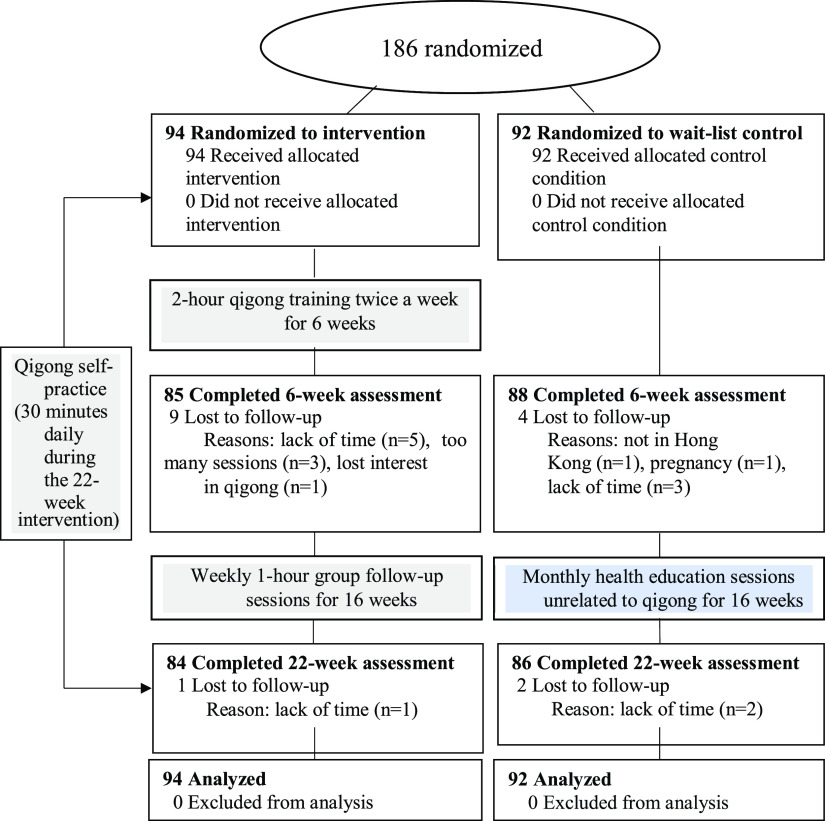
The sample available for the current analysis was comprised of 186 participants (94 in the intervention group and 92 in the wait-list control group). Figure 1 shows the study flow. The study was completed by 89% (84 of 94) of the participants in the intervention group and 94% (86 of 92) of those in the wait-list control group. Overall, 16 (9%) participants withdrew from the study, frequently because of a lack of time. Of the participants in the intervention group, 71% attended ≥ 80% of training sessions (n = 60). No adverse effects resulted.
Figure 1. Study flow.
At baseline, sociodemographic characteristics (Table 1) for the intervention and wait-list control groups did not differ significantly. All participants’ data were analyzed by the original assigned groups.
Table 1.
Baseline characteristics.
| Characteristics | Intervention (n = 94) | Control (n = 92) | P* |
|---|---|---|---|
| Age, years, mean ± SD | .972 | ||
| Self | 41.63 ± 8.89 | 41.67 ± 9.00 | |
| Partner | 47.29 ± 9.39 | 47.47 ± 10.16 | |
| Education, n (%) | .488 | ||
| ≤ 6 years | 15 (16) | 10 (11) | |
| 7–13 years | 71 (76) | 76 (83) | |
| Tertiary | 8 (9) | 6 (7) | |
| Place of birth, n (%) | .796 | ||
| Hong Kong | 17 (18) | 18 (20) | |
| Mainland China | 77 (82) | 74 (90) | |
| Years of marriage, mean ± SD | 20.19 ± 23.64 | 19.04 ± 20.45 | .725 |
| Marital status, n (%) | .250 | ||
| Married/cohabiting | 85 (90) | 88 (96) | |
| Single/divorced | 9 (10) | 4 (4) | |
| ≤ 1 Child, n (%) | 36 (38) | 39 (42) | .654 |
| Chronic illness, n (%) | |||
| Self | 15 (16) | 14 (15) | 1.000 |
| Partner | 10 (11) | 12 (13) | .655 |
| Employed, n (%) | .142 | ||
| Self | 23 (25) | 14 (15) | |
| Partner | 77 (83) | 79 (87) | |
| Experiencing financial hardship, n (%) | 62 (67) | 61 (66) | |
| Regular exercise, n (%) | 32 (34) | 25 (27) | .342 |
| Daily smoking, n (%) | 4 (4) | 3 (3) | 1.000 |
| Alcohol drinking, n (%) | 4 (4) | 4 (4) | 1.000 |
| Postmenopausal, n (%) | 17 (18) | 15 (16) | .749 |
| Illness, n (%) | |||
| Diabetes | 2 (2) | 1 (1) | .576 |
| Cardiovascular disease | 1 (1.1) | 0 (0) | .324 |
| Cancer | 0 (0) | 1 (1) | .313 |
| Hypertension | 3 (3) | 7 (7) | .184 |
| Body mass index, n (%) | .410 | ||
| < 18.5 kg/m2 (underweight) | 10 (11) | 8 (9) | |
| 18.5–22.9 kg/m2 (normal) | 55 (59) | 47 (51) | |
| > 22.9 kg/m2 (overweight/obese) | 29 (31) | 37 (40) | |
| Type of abuse experienced, n (%) | .506 | ||
| Psychological abuse only | 54 (57) | 45 (49) | |
| Physical/sexual abuse combined with psychological abuse | 28 (29) | 33 (36) | |
| Physical abuse plus sexual abuse plus psychological abuse | 12 (13) | 14 (15) | |
| Baseline PSS scores, mean ± SD | 20.93 ± 6.44 | 20.47 ± 5.23 | .596 |
| Baseline BDI-II scores, mean ± SD | 19.33 ± 12.16 | 17.32 ± 10.43 | .227 |
| Baseline TNF levels, mean ± SD | 1.68 ± 1.84 | 1.63 ± 1.73 | .834 |
| Baseline IL-6 levels, mean ± SD | 0.33 ± 1.06 | 0.16 ± 1.02 | .246 |
*P values obtained by chi-square test or t test. BDI-II = Beck Depression Inventory, version II, GSDS = General Sleep Disturbance Scale, IL-6 = interleukin 6, PSS = Perceived Stress Scale, SD = standard deviation, TNF = tumor necrosis factor.
Table 2 shows the intervention effect on sleep. The decrease in GSDS total scores in the intervention group from baseline was significant at 6 weeks (−7.06; 95% CI, −11.20 to −2.93; P < .001) and 22 weeks (−8.57; 95% CI, −12.56 to −4.58; P < .001). In the wait-list control group, the decrease in GSDS total scores from baseline was not statistically significant at 6 weeks (−1.56; 95% CI, −5.66 to 2.54; P = 1.000) or 22 weeks (−3.86; 95% CI, −7.80 to 0.09; P = .06). There were differences between the groups, and the GSDS total scores of the intervention group were significantly lower than were those of the wait-list control group at week 6 (difference = −7.96; 95% CI, −13.63 to −2.30; P = .006; effect size = −0.40) and week 22 (difference = −7.17; 95% CI, −12.58 to −1.76; P = .01; effect size = −0.38). There was a significant change in the between-group difference in the GSDS total scores over time, as indicated by the group × time interaction effect (P = .045).
Table 2.
Intention-to-treat analysis of outcomes at baseline and at the end of weeks 6 and 22.
| Intervention (n = 94) | Control (n = 92) | Between-Group Difference at Each Time Point | P (Group × Time) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Within-Group Change from Baseline (95% CI) | P | Mean (95% CI) | Within-Group Change from Baseline (95% CI) | P | Mean (95% CI) | P | Effect Size* | ||
| GSDS total score | ||||||||||
| 0.045 | ||||||||||
| Baseline | 49.66 (45.72, −53.60) | / | / | 52.12 (48.13, 56.11) | / | / | −2.46 (−8.07, 3.15) | .39 | ||
| Week 6 | 42.60 (38.58, 46.61) | −7.06 (−11.2, −2.93) | < .001 | 50.56 (46.56, 54.56) | −1.56 (−5.66, 2.54) | 1.000 | −7.96 (−13.63, −2.30) | .006 | −0.40 (−0.69, −0.11) | |
| Week 22 | 41.09 (37.26, 44.93) | −8.57 (−12.56, −4.58) | < .001 | 48.26 (44.45, 52.07) | −3.86 (−7.80, 0.09) | .06 | −7.17 (−12.58, −1.76) | .01 | −0.38 (−0.67, −0.09) | |
| GSDS subscale scores | ||||||||||
| Difficulty getting to sleep | .03 | |||||||||
| Baseline | 2.47 (2.04, 2.90) | 2.60 (2.16, 3.03) | −0.13 (−0.74, 0.48) | .67 | ||||||
| Week 6 | 1.74 (1.31, 2.18) | −0.72 (−1.24, −0.21) | .002 | 2.68 (2.25, 3.11) | 0.08 (−0.43, 0.59) | 1.000 | −0.93 (−1.55, −0.32) | .003 | −0.44 (−0.73, −0.15) | |
| Week 22 | 1.76 (1.36, 2.16) | −0.71 (−1.25, −0.17) | .005 | 2.31 (1.91, 2.70) | −0.29 (−0.83, 0.26) | .57 | −0.55 (−1.11, 0.01) | .05 | −0.28 (−0.57, 0.01) | |
| Waking up during sleep | .47 | |||||||||
| Baseline | 3.73 (3.28, 4.19) | 4.02 (3.56, 4.48) | −0.29 (−0.93, 0.36) | .38 | ||||||
| Week 6 | 3.34 (2.85, 3.83) | −0.40 (−0.98, 0.19) | .31 | 3.84 (3.36, 4.33) | −0.18 (−0.75, 0.40) | 1.000 | −0.50 (−1.19, 0.18) | .15 | −0.21 (−0.50, 0.080) | |
| Week 22 | 2.89 (2.40, 3.37) | −0.85 (−1.46, −0.24) | .003 | 3.61 (3.13, 4.09) | −0.41 (−1.02, 0.19) | .30 | −0.72 (−1.40, −0.04) | .04 | −0.30 (−0.59, −0.01) | |
| Early waking | .83 | |||||||||
| Baseline | 2.97 (2.49, 3.45) | 3.54 (3.06, 4.03) | −0.58 (−1.26, 0.11) | .10 | ||||||
| Week 6 | 2.36 (1.87, 2.85) | −0.61 (−1.29, 0.07) | .09 | 2.76 (2.28, 3.24) | −0.78 (−1.45, −0.11) | .02 | −0.41 (−1.09, 0.28) | .24 | −0.17 (−0.46, 0.12) | |
| Week 22 | 2.36 (1.89, 2.83) | −0.61 (−1.29, 0.07) | .10 | 3.01 (2.54, 3.48) | −0.53 (−1.21, 0.14) | .17 | −0.65 (−1.31, 0.02) | .06 | −0.28 (−0.57, 0.01) | |
| Quality of sleep | .001 | |||||||||
| Baseline | 4.57 (4.23, 4.91) | 4.28 (3.93, 4.62) | 0.29 (−0.19, 0.78) | .24 | ||||||
| Week 6 | 3.83 (3.48, 4.17) | −0.74 (−1.18, −0.31) | < .001 | 4.52 (4.18, 4.86) | 0.24 (−0.19, 0.67) | .55 | −0.69 (−1.17, −0.21) | .005 | −0.47 (−0.76, −0.18) | |
| Week 22 | 3.88 (3.56, 4.21) | −0.69 (−1.13, −0.25) | .001 | 4.03 (3.71, 4.36) | −0.25 (−0.68, 0.19) | .52 | −0.15 (−0.61, 0.31) | .52 | −0.10 (−0.39, 0.19) | |
| Quantity of sleep | .42 | |||||||||
| Baseline | 2.28 (2.00, 2.56) | 2.39 (2.11, 2.67) | −0.11 (−0.51, 0.28) | .57 | ||||||
| Week 6 | 1.91 (1.60, 2.21) | −0.37 (−0.76, 0.01) | .06 | 2.32 (2.02, 2.61) | −0.08 (−0.46, 0.31) | 1.000 | −0.41 (−0.83, 0.01) | .06 | −0.27 (−0.56, 0.02) | |
| Week 22 | 2.05 (1.75, 2.35) | −0.23 (−0.64, 0.19) | .57 | 2.35 (2.06, 2.65) | −0.04 (−0.45, 0.37) | 1.000 | −0.30 (−0.72, 0.12) | .16 | −0.21 (−0.50, 0.08) | |
| Daytime sleepiness | .32 | |||||||||
| Baseline | 2.96 (2.68, 3.24) | 3.21 (2.93, 3.50) | −0.25 (−0.65, 0.15) | .22 | ||||||
| Week 6 | 2.64 (2.35, 2.92) | −0.33 (−0.63, −0.02) | .03 | 3.07 (2.78, 3.35) | −0.15 (−0.45, 0.16) | .74 | −0.43 (−0.83, −0.03) | .04 | −0.30 (−0.59, −0.01) | |
| Week 22 | 2.49 (2.23, 2.75) | −0.47 (−0.77, −0.18) | < .001 | 3.00 (2.74, 3.26) | −0.22 (−0.51, 0.08) | .22 | −0.51 (−0.88, −0.14) | .008 | −0.40 (−0.69, −0.11) | |
| Self-medication to assist sleeping | .96 | |||||||||
| Baseline | 0.25 (0.12, 0.37) | 0.31 (0.18, 0.43) | −0.06 (−0.24, 0.12) | .51 | ||||||
| Week 6 | 0.20 (0.07, 0.33) | −0.05 (−0.16, 0.07) | 1.000 | 0.28 (0.15, 0.41) | −0.03 (−0.14, 0.08) | 1.000 | −0.08 (−0.26, 0.11) | .41 | −0.13 (−0.41, 0.16) | |
| Week 22 | 0.21 (0.07, 0.35) | −0.03 (−0.15, 0.08) | 1.000 | 0.26 (0.12, 0.41) | −0.04 (−0.16, 0.07) | 1.000 | −0.05 (−0.25, 0.15) | .62 | −0.07 (−0.36, 0.22) | |
*Effect size computed by dividing the difference between group means by the pooled standard deviation. CI = confidence interval, GSDS = General Sleep Disturbance Scale.
Regarding subscales, the scores of intervention group were significantly lower than those in the wait-list control group at week 6 in terms of difficulty in getting to sleep (−0.93; 95% CI, −1.55 to −0.32; P = .003; effect size = −0.44), quality of sleep (−0.69; 95% CI, −1.17 to −0.21; P = .005; effect size = −0.47), and daytime sleepiness (−0.43; 95% CI, −0.83 to −0.03; P = .04; effect size = −0.30). At week 22, only the daytime sleepiness data for the 2 groups still exhibited a significant difference (−0.51; 95% CI, −0.88 to −0.14; P = .008; effect size = −0.40), and waking during sleep was found to be significantly lower in the intervention group (−0.72; 95% CI, −1.40 to −0.04; P = .04; effect size = −0.30). In the analysis including poor sleepers at baseline only (n = 124; see Table S1 (43.4KB, pdf) in the supplemental material), the between-group differences that were insignificant at week 6 and week 22 in the total sample analysis remained insignificant, suggesting that the null findings for those subscales were not due to “ceiling effect” at baseline.
The results of analyses controlling for potential covariates (age and type of IPV) remained similar. In addition, per-protocol analysis was performed on adherents (intervention group: n = 60; wait-list control group: n = 86). The baseline characteristics were comparable between the 2 study groups. The results of the per-protocol analysis (Table S2 (43.4KB, pdf) ) were similar to those of intention-to-treat, with 2 exceptions: 1) the reduction from baseline to week 6 in early waking in the intervention group became significant (P = .04; vs 0.09 in intention-to-treat) and 2) the between-group difference in quantity of sleep at week 6 changed from marginally nonsignificant to significant (P = .04; vs 0.06 in intention-to-treat).
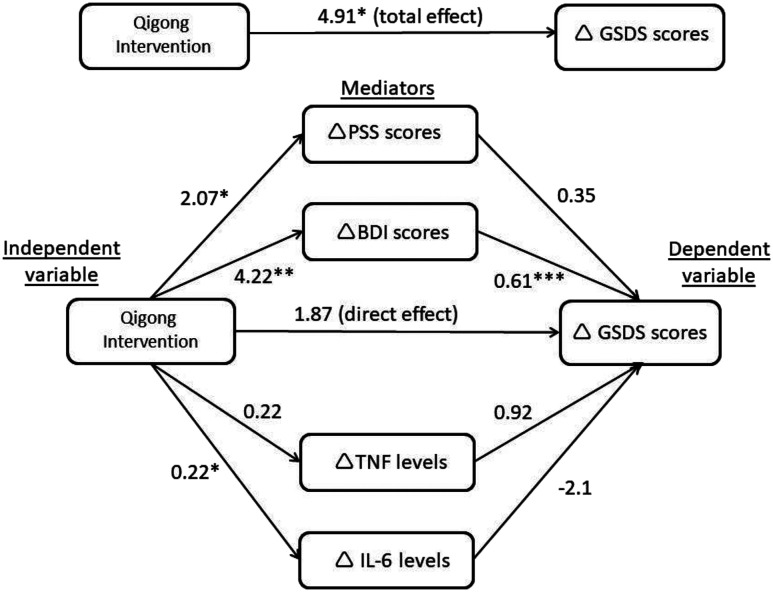
Results of the mediation analyses for improved sleep (n = 169 after removing missing data) are presented in Figure 2 and Table 3. The total effect of qigong intervention on sleep was significant (βtotal = 4.91; 95% CI, 0.24 to 9.57; P = .04). Significant overall IEs (βoverallIE = 3.03, 95% CI, 0.59 to 5.79) were exhibited, while the direct effect was not significant (βdirect = 1.87; 95% CI: −2.55 to 6.29; P = .40). Specifically, there is a statistically significant IE, in that the intervention improved sleep through depressive symptoms (IEdepression = 2.58, 95% CI: 0.69 to 5.09), such that participants who received the qigong intervention were more likely, relative to control group participants, to exhibit improvements in the effects of depressive symptoms, and were, therefore, more likely to report improvements in sleep disturbance. However, reductions in perceived stress, IL-6, and TNF did not mediate the relationship between the intervention and improved sleep (IEstress = 0.72, 95% CI: −0.19 to 2.06; IEIL-6 = −0.47, 95% CI: −1.51 to 0.34; IETNF = 0.21, 95% CI: −0.32 to 0.79). Overall, the indirect paths explained 62% of the total effect observed (3.03/4.91) and the mediating effect of depressive symptoms was found to account for 53% of the total intervention effect (2.58/4.91). The significance of the mediation pathways remained unchanged after controlling for potential covariates (age, duration of relationship, and type of IPV).
Figure 2. Diagram showing the mediation pathways (n = 169).
*P < .05, **P < .01, ***P < .001. Values on pathways represent unstandardized regression coefficients. Δ Denotes the change from T0 to T2, such that positive numbers indicate decrease in sleep disturbance/stress/depressive symptoms/TNF/IL-6 levels from T0 to T2 (ie, improvement). BDI-II = Beck Depression Inventory, version II, GSDS = General Sleep Disturbance Scale, IL-6 = interleukin 6, PSS = Perceived Stress Scale, TNF = tumor necrosis factor.
Table 3.
Results of mediation analyses.
| Potential mediators | Intervention Effect on Change in Potential Mediators* (A) | Association between Change in Potential Mediators and Change in GSDS Scores* (B) | Indirect Effect (AB), β Coefficient (95% CI) | ||
|---|---|---|---|---|---|
| β Coefficient (95% CI) | P | β Coefficient (95% CI) | P | ||
| PSS | 2.07 (0.37, 3.76) | .02 | 0.35 (−0.05, 0.74) | .09 | 0.72 (−0.19, 2.06) |
| BDI-II | 4.22 (1.31, 7.12) | .005 | 0.61 (0.38, 0.84) | < .001 | 2.58 (0.69, 5.09) |
| TNF | 0.22 (−0.11, 0.55) | .18 | 0.92 (−1.04, 2.88) | .36 | 0.21 (−0.32, 0.79) |
| IL-6 | 0.22 (0.01, 0.44) | .04 | −2.10 (−5.15, 0.95) | .18 | −0.47 (−1.51, 0.34) |
Total effect (C): β Coefficient = 4.91; 95% CI = 0.24, 9.57; P = .04. Direct effect (C′): β Coefficient = 1.87; 95% CI = −2.55, 6.29; P = .40. Overall indirect effect: β Coefficient = 3.03; 95% CI = 0.59, 5.79. *Denotes the change from T0 to T2, such that positive numbers indicate decrease in sleep disturbance/stress/depressive symptoms/TNF/IL-6 levels from T0 to T2 (ie, improvement). BDI-II = Beck Depression Inventory, version II; CI = confidence interval; GSDS = General Sleep Disturbance Scale; IL-6 = interleukin 6; PSS = Perceived Stress Scale; TNF = tumor necrosis factor.
DISCUSSION
Our findings indicate that Baduanjin qigong resulted in a statistically significant improvement in sleep among abused women, relative to the wait-list control group. Specifically, significant benefits were observed from relieving difficulty in sleep initiation, improving quality of sleep, and reducing daytime sleepiness. Mediation analysis reveals that women who received the qigong intervention were more likely to exhibit reductions in depressive symptoms, and, through this, participants were more likely to experience improved sleep.
To the best of our knowledge, this is the first randomized controlled trial to examine the effect of qigong on reducing sleep disturbances among women experiencing IPV. Although relocating to a shelter may be efficacious in reducing reabuse and psychological distress of abused women, sheltered women may still experience disturbed sleep and daytime fatigue probably due to environmental factors.28 Also, many women may not leave their partner due to such barriers as fear, cultural values for maintaining a 2-parent family, financial dependence, religious beliefs, and increased vulnerability to stalking and homicide.29 Interventions directed toward improving sleep for abused women are crucial. However, research on interventions for sleep disturbance experienced by abused women is scarce. The existing literature only includes: 1) a study reporting significant benefits from music therapy for improving insomnia in 28 abused women using a pre-post design,30 and 2) 1 study protocol of an randomized controlled trial targeting survivors of interpersonal violence using cognitive-behavioral therapy, with results not yet published.31 Compared to these 2 interventions, qigong is more culturally appropriate for Chinese people, because Chinese abused women were receptive to engaging in health-promoting behaviors based on the concepts of Chinese medicine.32 Also, qigong is inexpensive, safe, and can be practiced at home without equipment, making treatments accessible to IPV survivors from a wide range of sociodemographic backgrounds.33 Most importantly, the current study uses a rigorous design to confirm the sleep benefits experienced by participants in an abused population.
Though not within the context of IPV, the effects of qigong Baduanjin for improving sleep have been investigated with patients having chronic physical illnesses such as hypertension,12 diabetes,34 and Parkinson’s disease.35 Unlike chronic physical illnesses in which insomnia may be caused by physical symptoms arising from the illness or the side effects of treatment (eg, nocturia in diabetes)36 or nocturnal cramping in Parkinson’s disease,37 insomnia in abused women is more likely attributable to psychological issues such as feelings of threats and chronic stress.5 In our study, the effect size of sleep improvement postintervention in abused women was −0.40 (Cohen’s d), while that of a meta-analysis which covered 6 randomized controlled trials conducted among patients with physical illnesses was −0.55 (Hedge’s g).11 Although our effect size is somewhat smaller, our study provides new information indicating that the benefits of qigong for sleep are not limited to populations with physical illnesses but are also effective for populations experiencing psychological stress (such as abused women).
To explore the mechanism by which qigong benefits sleep, we performed additional analyses to identify mediators. To date, diverse nonpharmacological interventions have been proposed to be effective in altering sleep in various populations8; however, whether the benefits are mediated in part by psychological, physiological, or neurobiological changes remains largely unknown. Contrary to speculation that the alterations in sleep promoted by exercise are mediated by cytokines,38 IL-6 and TNF did not exhibit mediating effects in our study. However, this negative finding does not exclude other possible biological mechanisms such as C-reactive protein modulation,39,40 which can be explored in future studies. Data from this study showed that the effect of qigong in improving sleep was mediated by reductions in depressive symptoms. A multicountry population-based study showed that depression is a strong predictor of sleep problems.15 A plausible explanation to the mediating effect of depression on sleep outcomes is the ability of qigong, as a mind-body exercise, to counteract intrusive thoughts, foster relaxation states, and reduce body tension.33 In addition, the neurobiological pathways (monoamine neurotransmitters in the brain, the hypothalamic-pituitary-adrenal axis, and brain-derived neurotropic factors) that have been proposed to explain the antidepressive effects of qigong practice41 may constitute a partial explanation for the observed improvement in sleep. Further investigation is needed to understand fully the psychobiological mechanisms underlying the mediating effects of depression on sleep in qigong interventions. On the other hand, researchers have sought to conceptualize the sleep-depression relationship as bidirectional42; thus, caution must be taken when interpreting the results. Additionally, other plausible mediators, such as self-efficacy, cortisol, and C-reactive protein could be assessed in future studies.
Limitations
A limitation of the present study is the absence of objective forms of outcome measurement on sleep and psychological well-being. The reliance on self-reported measures of sleep and psychological well-being risks responses of participants being subject to retrospective reporting biases. Second, other conditions that may contribute to participants’ sleep problems such as shift work were not excluded. This may have increased heterogeneity of the sample. Third, we focused on outcomes immediately postintervention and are unable to assess the sustainability of the effects on sleep. Fourth, our mediation modeling was not derived from an experimental design, and the data were not specifically powered for mediation analysis. Although we have emphasized the indirect effects of qigong on sleep mediated by depression, there might be a bidirectional component to the relationship between depression and sleep that was not evaluated in the present study. Fifth, given that qigong was practiced in a group on a regular basis, social interactions with other participants and attention from research personnel may have contributed partly to the intervention effect and cannot be isolated. Finally, our measure of depression (ie, BDI-II) incorporates 1 item assessing sleep symptoms. However, this item accounts for only a small proportion of the total scale score, so the chances that changes in this item alone could account for the observed mediating effect are minimal.
Implications
In practice settings, service providers may recommend qigong Baduanjin for abused women who report sleep disturbances. It is safe, free, and can be practiced at any time. Specifically, the observed effects included 1) reduced difficulty in sleep initiation, 2) reduced daytime sleepiness, and 3) improved sleep quality. To enable the wide adoption of qigong as a complementary intervention in service settings, more efforts are needed to increase programming availability and to enhance the awareness of the benefits of qigong among the public and service communities.
In terms of research, our findings add to the information on underlying mechanisms that can explain the effects of nonpharmacological interventions on sleep by demonstrating the possible mediating effect of depressive symptoms. In the context of IPV, a number of psychological interventions have proved to be effective for improving depressive symptoms43 and may also improve sleep outcomes. It is recommended that future interventional trials should include both depression and sleep in the outcome evaluations. Particularly, trials should report item-level data or use depressive measures that do not include items assessing sleep symptoms, so as to enable investigations of the association among the intervention, non-sleep depressive symptoms, and sleep. Also, further research is needed to understand the clinical importance of the observed sleep improvements. Findings complemented by standard sleep outcome measures such as sleep diary can provide a more complete picture of the intervention effect. Particularly, actigraphy, a noninvasive device that allows for continuous sleep monitoring for days to weeks, could be used as an objective sleep measurement in future studies. Of note, IPV researchers should consider the safety of participants when designing the measurement, as wearing actigraphy devices may lead to heightened suspicion of perpetrators. In addition, discrepancies between self-reported and objective sleep assessment have been reported in previous studies.44,45 Given the subjective nature of sleep complaints and rising importance of evaluating treatment effectiveness from the patient's perspective,46 self-reported sleep measures could be prioritized in the context of IPV. An active control group such as simple stretching can be used to control for the nonspecific effects of social gathering and light physical activity.
CONCLUSIONS
In this study involving abused women, as compared with results for a wait-list control group, qigong Baduanjin resulted in improvement in self-reported sleep disturbances based on its effect on reducing depressive symptoms. More research is needed to understand the clinical importance of the observed improvement in sleep.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at The University of Hong Kong. This study was funded by the Health and Medical Research Fund, Food and Health Bureau of the Hong Kong SAR Government (Project Number 11121361). The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank all the participants for taking part in the study and the Hong Kong Sheng Kung Hui Lady MacLehose Centre for providing space for study sites.
Author contributions: Study concept and design: Cheung, Deng, Hong, Tiwari; Acquisition of data: Cheung, Deng, Hong; Analysis and interpretation of data: Cheung, Chau, Yeung, Deng, Tiwari; Drafting of the manuscript: Cheung, Chau, Yeung, Tiwari; Critical revision of the manuscript for important intellectual content: Cheung, Chau, Yeung, Deng, Hong, Tiwari; Statistical analysis: Cheung, Chau; Obtained funding: Deng, Hong, Tiwari; Study supervision: Cheung, Deng, Tiwari.
ABBREVIATIONS
- BDI-II
Beck Depression Inventory, version II
- CI
confidence interval
- GSDS
General Sleep Disturbance Scale
- IE
indirect effect
- IL-6
interleukin 6
- IPV
intimate partner violence
- TNF
tumor necrosis factor
REFERENCES
- 1.World Health Organization . Global and Regional Estimates of Violence Against Women: Prevalence and Health Effects of Intimate Partner Violence and Non-Partner Sexual Violence. Geneva: World Health Organization; 2013. [Google Scholar]
- 2.European Union Agency for Fundamental Rights . Violence against women, an EU-wide survey: Main results; 2014. https://fra.europa.eu/en/publication/2014/violence-against-women-eu-wide-survey. Accessed June 30, 2020.
- 3.Steine IM, Harvey AG, Krystal JH, et al. Sleep disturbances in sexual abuse victims: a systematic review. Sleep Med Rev. 2012;16(1):15–25. 10.1016/j.smrv.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Pigeon WR, Cerulli C, Richards H, He H, Perlis M, Caine E. Sleep disturbances and their association with mental health among women exposed to intimate partner violence. J Womens Health (Larchmt). 2011;20(12):1923–1929. 10.1089/jwh.2011.2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalley-Chareczko L, Segal A, Perlis ML, Nowakowski S, Tal JZ, Grandner MA. Sleep disturbance partially mediates the relationship between intimate partner violence and physical/mental health in women and men. J Interpers Violence. 2017;32(16):2471–2495. 10.1177/0886260515592651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matos M, Gonçalves M. Sleep and women intimate partner victimization: prevalence, effects and good practices in health care settings. Sleep Sci. 2019;12(1):35–42. 10.5935/1984-0063.20190057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovato N, Lack L. Insomnia and mortality: a meta-analysis. Sleep Med Rev. 2019;43:71–83. 10.1016/j.smrv.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 8.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. 10.1016/S0140-6736(11)60750-2 [DOI] [PubMed] [Google Scholar]
- 9.Yeung WF, Chung KF, Yung KP, et al. The use of conventional and complementary therapies for insomnia among Hong Kong Chinese: a telephone survey. Complement Ther Med. 2014;22(5):894–902. 10.1016/j.ctim.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 10.Jahnke R, Larkey L, Rogers C, Etnier J, Lin F. A comprehensive review of health benefits of qigong and tai chi. Am J Health Promot. 2010;24(6):e1–e25. 10.4278/ajhp.081013-LIT-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou L, SasaKi JE, Wang H, Xiao Z, Fang Q, Zhang M. A systematic review and meta-analysis Baduanjin Qigong for health benefits: randomized controlled trials. Evid Based Complement Alternat Med. 2017;2017:4548706. 10.1155/2017/4548706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YQ, Liu RZ, He R. Effect of Baduanjin on sleep quality in people with hypertension. Hunan J Tradit Chin Med. 2015;31(4):52–54. [Google Scholar]
- 13.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210. 10.1038/nrn2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin MR, Olmstead R, Carroll JE. sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyanagi A, Garin N, Olaya B, et al. Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multi-country study. PLoS One. 2014;9(12):e114742. 10.1371/journal.pone.0114742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung DST, Deng W, Tsao SW, et al. Effect of a Qigong intervention on telomerase activity and mental health in Chinese women survivors of intimate partner violence: a randomized clinical trial. JAMA Netw Open. 2019;2(1):e186967. 10.1001/jamanetworkopen.2018.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh B, Butow P, Mullan B, et al. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann Oncol. 2010;21(3):608–614. 10.1093/annonc/mdp479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari A, Chan CLW, Ho RTH, et al. Effect of a qigong intervention program on telomerase activity and psychological stress in abused Chinese women: a randomized, wait-list controlled trial. BMC Complement Altern Med. 2014;14:300. 10.1186/1472-6882-14-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari A, Fong DYT, Chan KL, Leung WC, Parker B, Ho PC. Identifying intimate partner violence: comparing the Chinese abuse assessment screen with the Chinese revised conflict tactics scales. BJOG. 2007;114(9):1065–1071. 10.1111/j.1471-0528.2007.01441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RA, Lack LC, Lovato N, Wright H. The relationship between a night’s sleep and subsequent daytime functioning in older poor and good sleepers. J Sleep Res. 2015;24(1):40–46. 10.1111/jsr.12237 [DOI] [PubMed] [Google Scholar]
- 21.Lee S-Y, Hsu H-C. Stress and health-related well-being among mothers with a low birth weight infant: the role of sleep. Soc Sci Med. 2012;74(7):958–965. 10.1016/j.socscimed.2011.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halle IH, Westgaard TK, Wahba A, Oksholm T, Rustøen T, Gjeilo KH. Trajectory of sleep disturbances in patients undergoing lung cancer surgery: a prospective study. Interact Cardiovasc Thorac Surg. 2017;25(2):285–291. 10.1093/icvts/ivx076 [DOI] [PubMed] [Google Scholar]
- 23.Lee SY. Validating the General Sleep Disturbance Scale among Chinese American parents with hospitalized infants. J Transcult Nurs. 2007;18(2):111–117. 10.1177/1043659606298502 [DOI] [PubMed] [Google Scholar]
- 24.Byrne BM, Stewart SM, Lee PWH. Validating the Beck Depression Inventory-II for Hong Kong community adolescents. Int J Test. 2004;4(3):199–216. 10.1207/s15327574ijt0403_1 [DOI] [Google Scholar]
- 25.Madrid-Valero JJ, Martínez-Selva JM, Ribeiro do Couto B, Sánchez-Romera JF, Ordoñana JR. Age and gender effects on the prevalence of poor sleep quality in the adult population. Gac Sanit. 2017;31(1):18–22. 10.1016/j.gaceta.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 26.Sanchez SE, Islam S, Zhong Q-Y, Gelaye B, Williams MA. Intimate partner violence is associated with stress-related sleep disturbance and poor sleep quality during early pregnancy. PLoS One. 2016;11(3):e0152199. 10.1371/journal.pone.0152199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- 28.Humphreys JC, Lee KA, Neylan TC, Marmar CR. Sleep patterns of sheltered battered women. Image J Nurs Sch. 1999;31(2):139–143. 10.1111/j.1547-5069.1999.tb00452.x [DOI] [PubMed] [Google Scholar]
- 29.Bell ME, Goodman LA, Dutton MA. The dynamics of staying and leaving: implications for battered women’s emotional well-being and experiences of violence at the end of a year. J Fam Violence. 2007;22(6):413–428. 10.1007/s10896-007-9096-9 [DOI] [Google Scholar]
- 30.Hernández-Ruiz E. Effect of music therapy on the anxiety levels and sleep patterns of abused women in shelters. J Music Ther. 2005;42(2):140–158. 10.1093/jmt/42.2.140 [DOI] [PubMed] [Google Scholar]
- 31.Pigeon WR, Heffner KL, Crean H, et al. Responding to the need for sleep among survivors of interpersonal violence: A randomized controlled trial of a cognitive-behavioral insomnia intervention followed by PTSD treatment. Contemp Clin Trials. 2015;45(Pt B):252–260. 10.1016/j.cct.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari AF, Salili F, Chan RYP, Chan EK, Tang D. Effectiveness of an empowerment intervention in abused Chinese women. Hong Kong Med J. 2010;16(Suppl 3):25–28. [PubMed] [Google Scholar]
- 33.Neuendorf R, Wahbeh H, Chamine I, Yu J, Hutchison K, Oken BS. The Effects of Mind-Body Interventions on Sleep Quality: A Systematic Review. Evid Based Complement Alternat Med. 2015;2015:902708. 10.1155/2015/902708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Wang WD, Zhang RR. Effects of Qigoing on sleep quality in patients with type 2 diabetes. J Beijing Univ Tradit Chin Med. 2009;32(9):636–640. [Google Scholar]
- 35.Xiao CM, Zhuang YC. Effect of health Baduanjin Qigong for mild to moderate Parkinson’s disease. Geriatr Gerontol Int. 2016;16(8):911–919. 10.1111/ggi.12571 [DOI] [PubMed] [Google Scholar]
- 36.Chang WP, Lin CC. Relationships of salivary cortisol and melatonin rhythms to sleep quality, emotion, and fatigue levels in patients with newly diagnosed lung cancer. Eur J Oncol Nurs. 2017;29:79–84. 10.1016/j.ejon.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 37.Smith MC, Ellgring H, Oertel WH. Sleep disturbances in Parkinson’s disease patients and spouses. J Am Geriatr Soc. 1997;45(2):194–199. 10.1111/j.1532-5415.1997.tb04506.x [DOI] [PubMed] [Google Scholar]
- 38.Santos RVT, Tufik S, De Mello MT. Exercise, sleep and cytokines: is there a relation? Sleep Med Rev. 2007;11(3):231–239. 10.1016/j.smrv.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 39.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37(9):1543–1552. 10.5665/sleep.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liukkonen T, Räsänen P, Ruokonen A, et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69(8):756–761. 10.1097/PSY.0b013e318157cb96 [DOI] [PubMed] [Google Scholar]
- 41.Tsang HW, Fung KMT. A review on neurobiological and psychological mechanisms underlying the anti-depressive effect of qigong exercise. J Health Psychol. 2008;13(7):857–863. 10.1177/1359105308095057 [DOI] [PubMed] [Google Scholar]
- 42.Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020;45(1):74–89. 10.1038/s41386-019-0411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan M, O'Doherty L, Gilchrist G, et al. Psychological therapies for women who experience intimate partner violence. Cochrane Database Syst Rev. 2018(5):CD013017. 10.1002/14651858.CD013017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosom Med. 2011;73(9):810–816. 10.1097/PSY.0b013e3182359e77 [DOI] [PubMed] [Google Scholar]
- 45.Aili K, Åström-Paulsson S, Stoetzer U, Svartengren M, Hillert L. Reliability of actigraphy and subjective sleep measurements in adults: the design of sleep assessments. J Clin Sleep Med. 2017;13(1):39–47. 10.5664/jcsm.6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. 10.2147/PROM.S156279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.