Abstract
Study Objectives:
The sleep patterns of humans are greatly influenced by age and sex and have various effects on overall health as they change continuously during the lifespan. We investigated age-dependent changes in sleep properties and their relation to sex in middle-aged individuals.
Methods:
We analyzed data from 2,640 participants (mean age of 49.8 ± 6.8 years at baseline, 50.6% women) in the Korean Genome and Epidemiology Study, which assessed sleep habits using the Pittsburgh Sleep Quality Index and other clinical characteristics. We analyzed the sleep habit changes that occurred between baseline and a follow-up point (mean interval: 12.00 ± 0.16 years). Associations of age and sex with 9 sleep characteristics were evaluated.
Results:
Age was associated with most of the sleep characteristics cross-sectionally and longitudinally (P < .05), except for the time in bed at baseline (P = .455) and change in sleep duration (P = .561). Compared with men, women had higher Pittsburgh Sleep Quality Index scores, shorter time in bed, shorter sleep duration, and longer latency at baseline (P ≤ .001). Longitudinal deterioration in Pittsburgh Sleep Quality Index score, habitual sleep efficiency, duration, and latency was more prominent in women (P < .001). The sex differences in these longitudinal sleep changes were mainly noticeable before age 60 years (P < .05). Worsening of Pittsburgh Sleep Quality Index scores, habitual sleep efficiency, and latency was most evident in perimenopausal women. Men presented with greater advancement of chronotype (P = .006), with the peak sex-related difference occurring when they were in their late 40s (P = .048).
Conclusions:
Aging is associated with substantial deterioration in sleep quantity and quality as well as chronotype advancement, with the degree and timing of these changes differing by sex.
Citation:
Kim HJ, Kim REY, Kim S, et al. Sex differences in deterioration of sleep properties associated with aging: a 12-year longitudinal cohort study. J Clin Sleep Med. 2021;17(5):964–972.
Keywords: sleep, age, sex, menopause, chronotype, cohort
BRIEF SUMMARY
Current Knowledge/Study Rationale: To date, few studies have presented longitudinal follow-up data of individual sleep measures, and even fewer have measured phase advance in relation to biological sex during the aging process. The identification of sleep profile changes in the pre-aging population is fundamental for understanding the role of sleep in healthy aging.
Study Impact: Our study presents the interactive effects of age and sex on sleep behavior changes stratified by age, which could be used to guide future research on the extent to which sleep behavior changes should be addressed in men and women in late adulthood. Studies on the association between aging and sleep properties should further consider the multifactorial effects of sex and hormonal status.
INTRODUCTION
Age-related changes in sleep profiles usually start in middle age around the 50s or even earlier, accompanied by a gradual increase in sleep complaints.1 In addition to the aging process, medical and psychiatric comorbidities in middle-aged adults, as well as primary sleep disorders, contribute to further deterioration in sleep properties.2,3 Consequently, it is quite challenging to distinguish general aging-associated sleep changes from pathological sleep conditions in this age group. Since evidence for the effect of sleep on the overall physical and mental health of the adults aged 40s and older population has increased in recent years, establishing a norm for how sleep changes with age will provide a valuable foundation for clinicians and researchers.
The circadian system is a core determinant of sleep timing and structure, interacting with the homeostatic sleep-wake cycle to achieve consolidated sleep.4 The endogenous oscillation of biological timing in humans is synchronized to the 24-hour cycle by external inputs through entrainment. Age-related degeneration in any of the coordination systems involved in generating the circadian rhythms may, therefore, contribute to altered sleep-wake propensity.5,6 Age-related neural dysfunctions leading to decreased homeostatic sleep pressure buildup during wakefulness or decreased sleep need in itself are other suggested main factors leading to changes in sleep behavior.7,8
Chronological regression of sleep patterns after middle age differs between individuals and, therefore, interindividual sleep profile variability is much more significant in adults aged 40s and older compared with the younger population.9 In addition to aging, sex is well known to influence intrinsic sleep structure.10 There is substantial evidence that women generally have more sleep-related self-reported complaints than men, but these are not always consistent with objective measures.11 Investigations on sex-related differences in sleep at different levels, from genetic regulation to regulation by the hypothalamic-pituitary-gonadal axis and reproductive hormones, have mostly been confined to the laboratory setting.12,13 Therefore, the interplay between sex-associated and aging effects on sleep patterns in real life remains unclear. Moreover, most of the research so far has focused on the amount of sleep without considering sleep timing preference (the chronotype) and has not included many middle-aged people,14 many of whom are also undergoing radical sex hormone changes.
A thorough investigation of sleep behavior evolution in a middle-aged population stratified by age group in each sex is needed to provide a coherent interpretation of the research data on the consequences of inadequate sleep. Considering the substantial interindividual variability, the effects of the aging process on sleep physiology in individuals should be compared with the norm in their age group to assess the degree of pathological deviation.15,16 For this, reference data on sleep profiles derived from a representative cohort are crucial for selecting a target group that needs to be closely monitored. To date, however, there have been few large-scale longitudinal studies investigating a comprehensive panel of sleep measures including chronotype and their influencing factors, and even fewer in the Asian population.17–20 Our study aimed to fill this knowledge gap by delineating the sex-related differences in age-related effects on longitudinal sleep behavior changes in middle to late adulthood in a Korean population.
METHODS
Study design and participants
We studied a subset of participants in the Korean Genome and Epidemiology (KoGES) Ansan Study.21 This prospective study recruited randomly selected community-dwelling individuals aged 40–69 years in 2001 to investigate the genetic and environmental etiology of common complex diseases in Koreans and causes of death with long-term follow-up. A physical/medical examination and a questionnaire-based interview were administered every 2 years to compile longitudinal data. Each participant provided an informed-consent form, and the Institutional Review Board of the Korea University Ansan Hospital approved the study procedure.
Due to multiple revisions in the sleep behavior survey format during the KoGES study, we only compared data from the second and eighth examinations, which we used as the baseline and follow-up time points, respectively, for our study. Among 4,023 participants for whom baseline sleep behavior data were available (collected during the second KoGES examination, 2003–2004), 3,083 participants completed the follow-up examination (eighth KoGES examination, 2015–2016), with a mean interval of 12.00 ± 0.16 years. The main reasons for attrition were contact loss (n = 666), refusal (n = 153), moving away (n = 59), and hospitalization (n = 56). We observed no distinct differences in the baseline characteristics between responders and nonresponders. We excluded participants who reported psychiatric illness (n = 3) or shift-working history (n = 206) as well as those taking any hypnotic medication (n = 69). After further elimination of those who reported extremes of any sleep behavior (n = 24; those who reported a midsleep time between 9 am and 6 pm on workdays), our final cohort had a total of 2,640 participants.
Sleep questionnaires
We investigated sleep behavior profiles on workdays and free days separately. The weekly average was then calculated as follows:
The Pittsburgh Sleep Quality Index (PSQI) components related to sleep quantity include sleep latency and self-aware actual sleep duration in addition to bedtime and wake time during the past month.22 Time in bed (TIB) was calculated as the time difference between bedtime and wake time and then used to calculate habitual sleep efficiency (HSE; = % sleep duration/TIB). Social jetlag was defined as the discrepancy between the midsleep time on free days vs workdays, which represents the discrepancy between social and biological time.23 Mid-sleep time on free days corrected for sleep debt on weekdays (MSFsc) was also calculated as the midpoint between bedtime and wake time to estimate each individual’s chronotype, as follows:24
Statistical analysis
Statistical analysis was performed using the statistical software R (R version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/). In the demographic characteristics analysis, age at baseline (second KoGES examination) was used and sex differences were evaluated using the t test or chi-square tests. In the cross-sectional sleep profiles analysis, the effects of age and sex were reported from 1 linear regression model after adjusting for body mass index (kg/m2), smoking intensity (pack-years), alcohol consumption (g/d), hypertension, diabetes mellitus, and occupation.21,25
In the longitudinal change analysis, values at baseline were subtracted from the corresponding values at the follow-up point and adjustments were made for each individual’s baseline measurements and precise follow-up interval.
Correlation matrices for the paired association between sleep properties were computed and, after adjusting for the covariates mentioned above, corresponding P values for each longitudinal change in sleep behavior were reported.
To investigate associations between sleep behaviors and menstruation status, we categorized the female study participants into 3 groups based on their menopausal status during our study period26: (1) the premenopause group with persistent menstruation up to the end of the data-collection period, (2) the perimenopause group of women who had menstruation at baseline but reported menopause at the final visit, and (3) the postmenopause group with menopause reported at baseline. A linear regression model was used to analyze changes in sleep properties across menopausal groups after adjusting for the covariates mentioned above. Strata of menopausal status were used to show patterns of association, and tests for trend (P for trend) were conducted based on a general linear regression model with status strata ordered from pre- to peri- to postmenopausal.27
RESULTS
Demographic characteristics
We analyzed data from 2,640 individuals (50.6% female) whose average age was 49.8 years at baseline. Detailed sex-segregated demographic characteristics at baseline are presented in Table 1. The mean follow-up interval was slightly longer in women (P = .040). Most of the men were economically active (88.8%) at baseline. In contrast, a majority of female participants reported being homemakers (63.6%). Hypertension (22.9% vs 32.3%; P < .001) and diabetes (13.2% vs 18.1%; P < .001) were reported more frequently in men than in women. Body mass index was not different between the sexes (P = .181). Men reported considerably more unhealthy lifestyles, including smoking experience and alcohol consumption (P < .001, respectively).
Table 1.
Demographic characteristics at baseline.
| Women (n = 1,335) (50.6%) | Men (n = 1,305) (49.4%) | P* | |
|---|---|---|---|
| Age, y | 49.82 ± 6.93 | 49.71 ± 6.70 | .685 |
| Follow-up duration, y | 12.00 ± 0.15 | 11.99 ± 0.17 | .040 |
| Occupation,† n (%) | <.001 | ||
| Intellectual | 185 (13.9) | 595 (45.6) | |
| Mixed | 232 (17.4) | 523 (40.1) | |
| Elementary | 52 (3.9) | 40 (3.1) | |
| Homemaker | 849 (63.6) | 1 (0.1) | |
| Hypertension, n (%) | 305 (22.9) | 421 (32.3) | <.001 |
| Systolic blood pressure, mm Hg | 109.64 ± 14.68 | 115.38 ± 14.03 | |
| Diastolic blood pressure, mm Hg | 72.63 ± 10.22 | 78.57 ± 10.43 | |
| Diabetes, n (%) | 176 (13.2) | 236 (18.1) | <.001 |
| Body mass index, kg/m2 | 24.46 ± 2.92 | 24.61 ± 2.70 | .181 |
| Smoking status, n (%) | <.001 | ||
| Never | 1,289 (96.6) | 294 (22.5) | |
| Ex-smoker | 15 (1.1) | 545 (41.8) | |
| Current | 31 (2.3) | 466 (35.7) | |
| Alcohol, g/d | 1.89 ± 6.19 | 19.87 ± 30.82 | <.001 |
| Heavy (≥15 g/d), n (%) | 38 (2.9) | 527 (40.4) | <.001 |
Data are means ± SDs or the number of observations, n (%). n = 2,640. *P = overall group differences between the sexes by t test or chi-square test. †Occupation was investigated and classified into 4 categories: intellectual (including senior officials, managers, and professionals), mixed (including armed forces, technicians and associate professionals, service workers and shop and market sales workers, skilled agricultural and fishery workers, craft and related trades workers, plant and machine operators and assemblers), elementary (elementary occupations consist of simple and routine tasks which mainly require the use of hand-held tools and often some physical effort), and homemakers. Students, retirees, and unemployed people were classified as economically inactive. SD = standard deviation.
Sleep properties with aging
The mean values of 9 sleep properties and their associations with age at baseline are summarized in the upper left portion of Table 2. Cross-sectionally, older age was substantially associated with deterioration in all sleep characteristics except for TIB (P = .455). Older age was associated with higher PSQI score (P < .001); longer sleep latency (P = .001); lower HSE (P < .001), which was mostly driven by shorter sleep duration (P < .001); smaller social jetlag (P < 0.001); and earlier chronotype (MSFsc, P < .001).
Table 2.
Sleep properties at baseline and their change at follow-up.
| Total | βAge† | P† | Women | Men | βW/M‡ | P‡ | |
|---|---|---|---|---|---|---|---|
| Baseline measure | |||||||
| PSQI, score | 4.2 (2.3) | 0.03 | <.001 | 4.4 (2.4) | 4.0 (2.2) | 0.67 | <.001 |
| Sleep latency, min | 16.0 (16.9) | 0.16 | .001 | 17.0 (18.1) | 15.0 (15.6) | 4.22 | <.001 |
| HSE, % | 94.2 (10.6) | −0.24 | <.001 | 94.3 (11.2) | 94.1 (10.0) | −0.34 | .508 |
| Time in bed, h | 6.7 (1.2) | 0.00 | .455 | 6.6 (1.3) | 6.9 (1.1) | −0.18 | .001 |
| Sleep duration, h | 6.3 (1.2) | −0.01 | <.001 | 6.2 (1.2) | 6.4 (1.2) | −0.21 | <.001 |
| MSFsc, 24-h | 3:05 (1:11) | −0:03 | <.001 | 2:59 (1:07) | 3:12 (1:14) | −0:03 | .307 |
| Midsleep time (F), 24-h | 3:15 (1:15) | −0:04 | <.001 | 3:11 (1:12) | 3:18 (1:18) | 0:03 | .289 |
| Midsleep time (W), 24-h | 2:54 (1:05) | −0.02 | <.001 | 2:48 (0:59) | 2:59 (1:10) | −0:02 | .346 |
| Social jetlag, 24-h | 0:24 (0:40) | −0:01 | <.001 | 0:26 (0:37) | 0:23 (0:42) | 0:05 | .004 |
| Longitudinal change | |||||||
| ΔPSQI, score | 0.7 (3.0) | 0.02 | .011 | 0.9 (3.1) | 0.5 (2.8) | 0.80 | <.001 |
| ΔSleep latency, min | 1.2 (22.7) | 0.22 | <.001 | 3.5 (26.7) | −1.1 (17.6) | 6.86 | <.001 |
| ΔHSE, % | −4.0 (14.4) | −0.26 | <.001 | −5.6 (14.9) | −2.3 (13.7) | −3.71 | <.001 |
| ΔTime in bed, h | 0.3 (1.4) | 0.02 | <.001 | 0.4 (1.4) | 0.2 (1.4) | 0.02 | .769 |
| ΔSleep duration, h | 0.0 (1.4) | 0.00 | .561 | −0.1 (1.4) | 0.0 (1.3) | −0.23 | <.001 |
| ΔMSFsc, 24-h | −0:29 (1:22) | −0:01 | <.001 | −0:19 (1:16) | −0:38 (1:26) | 0:09 | .006 |
| ΔMidsleep time (F), 24-h | −0:35 (1:19) | −0:01 | <.001 | −0:29 (1:12) | −0:42 (1:25) | 0:08 | .013 |
| ΔMidsleep time (W), 24-h | −0:23 (1:12) | −0:01 | <.001 | −0:16 (1:07) | −0:31 (1:16) | 0:07 | .023 |
| ΔSocial jetlag, 24-h | −0:11 (0:54) | −0:01 | <.001 | −0:11 (0:55) | −0:11 (0:53) | 0:03 | .126 |
The adjusted mean (SD) values at baseline (upper portion) and change at follow-up (lower portion) are shown. βAge† and βW/M‡ were computed in the same model. βAge† (presented with its corresponding P†) is the estimated association of age to the corresponding sleep characteristic after adjusting for sex, body mass index (kg/m2), smoking intensity (pack-years), alcohol consumption (g/d), hypertension, diabetes mellitus, and occupation. For longitudinal comparisons, additional adjustments for the variable at baseline and the visit interval (years) were made. βW/M‡ (presented with its corresponding P‡) reflects the association of the measures in women relative to men. F = free days, HSE = habitual sleep efficiency, MSFsc = mid-sleep time on free days corrected for sleep debt on weekdays, PSQI = Pittsburgh Sleep Quality Index, SD = standard deviation, W = workdays, Δ = change.
The longitudinal observations were consistent with our baseline findings in that advancing age was associated with accelerated deterioration in sleep properties (Table 2; lower left, βAge†). We observed accelerated changes in PSQI scores (+0.02/year, P = .011), sleep latency (+0.22 minutes/year, P < .001), and HSE (−0.26%/year, P < .001) driven by an accelerated increase in TIB (+0.02 hours/year, P < .001). Also, the 1-year increase in age was associated with accelerated advance in chronotype (MSFsc, −1 minute/year, P < .001) and decrease in social jetlag (−1 minute/year, P < .001).
Sleep properties by sex
The right side of Table 2 summarizes the sleep characteristics at baseline and after 12 years for each sex. At baseline, women reported higher PSQI scores (P < .001), longer sleep latency (P < .001), shorter TIB (P = .001), shorter sleep duration (P < .001), and larger social jetlag (P = .004) than men. There were no observed sex-associated differences in HSE, MSFsc, or midsleep time (either on free days or workdays) at baseline.
The longitudinal change in many sleep properties also differed between the sexes. Women reported a greater reduction in sleep quality (represented by a larger increase in PSQI, 0.9 vs 0.5; P < .001). Sleep latency increased in women but decreased slightly in men (+3.5 vs −1.1 minutes, P < .001). Women exhibited a greater decrease in HSE (−5.6% vs −2.3%, P < .001) resulting from a larger decrease in sleep duration (−0.1 vs 0.0 hours, P < .001) in addition to increased TIB (P = .769). No definite sex-related difference was observed in longitudinal social jetlag change (P = .126). The overall degree of chronotype advancement at the 12-year follow-up point was more remarkable in men than in women as revealed by changes in MSFsc (−38 vs −19 minutes, P = .006) and midsleep time (P = .013 for free days and P = .023 for workdays).
Longitudinal sleep deteriorations across age groups in each sex
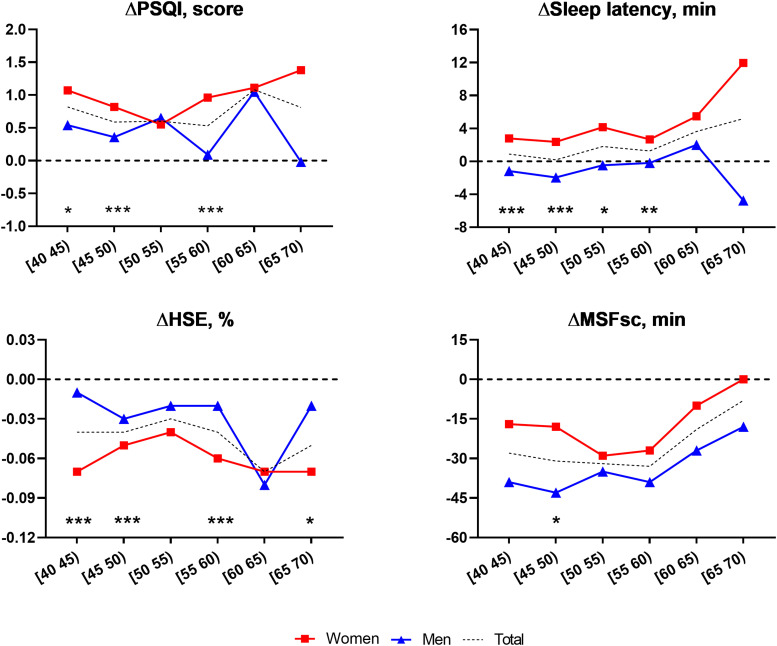
Further investigation by age and sex is summarized in Figure 1 and Table S1 (82KB, pdf) in the supplemental material. The results suggest that sex differences in longitudinal sleep changes could differ by age subgroup. The degree of longitudinal PSQI increase was generally higher for women in most age groups, showing a tendency for accelerated deterioration after the mid-50s. The increment of sleep latency was more remarkable for women than for men in all age groups, and the sex-related difference in this variable was mainly observed before age 60 years. The degree of HSE decrease was generally greater for women participants in most age groups, and the sex differences in HSE change were observed prominently in participants in their 40s and late 50s.
Figure 1. Longitudinal changes in sleep properties between the sexes across age groups.
Dotted lines represent the mean change in sleep properties over the follow-up period in men (blue triangles) and women (red squares) participants by age group. Significant differences between the sexes after adjusting for all covariates are marked by asterisks: *P < .05, **P < .005, and ***P < .001. The black dotted horizontal lines (zero on the y axis) indicate the level that would represent no change. HSE = habitual sleep efficiency, MSFsc = mid-sleep time on free days corrected for sleep debt on weekdays, PSQI = Pittsburgh Sleep Quality Index.
The most substantial chronotype advance was observed in men in their 40s and women in their 50s. The degree of chronotype advancement was generally greater in men than in women in all age groups, and a sex-related difference was mainly noticeable in participants in their 40s (45–49 years old for change [Δ] in MSFsc, −43 minutes in men and −18 minutes in women; P < 0.05). In participants older than their mid-50s, the degree of chronotype advancement tended to be gradually attenuated in both sexes.
Pairwise correlation of longitudinal sleep change properties
We next investigated paired correlations between longitudinal sleep change properties in each sex (Table 3). In both sexes, sleep quality deterioration reflected in increased PSQI during the follow-up period was associated with the following measures (in order of strongest to weakest correlation): decreased sleep duration (r = −.60 in women, r = −.61 in men; both P < .001), decreased HSE (r = −.57 in women, r = −.56 in men; both P < .001), increased sleep latency (r = .45 in women, r = .44 in men; both P < .001), and decreased TIB (r = −.15 in women, r = −.20 in men; both P < .001).
Table 3.
Adjusted correlation matrices of longitudinal changes in sleep properties in each sex.
| ΔPSQI | ΔLatency | ΔHSE | ΔTIB | ΔDuration | ΔMSFsc | ΔMST (F) | ΔMST (W) | |
|---|---|---|---|---|---|---|---|---|
| Women | ||||||||
| ΔLatency | 0.45*** | 1.00 | ||||||
| ΔHSE | −0.57*** | −0.32*** | 1.00 | |||||
| ΔTIB | −0.15*** | 0.13*** | −0.31*** | 1.00 | ||||
| ΔDuration | −0.60*** | −0.15*** | 0.52*** | 0.62*** | 1.00 | |||
| ΔMSFsc | 0.01 | 0.03 | 0.03 | −0.02 | 0.03 | 1.00 | ||
| ΔMST (F) | 0.02 | 0.02 | 0.05 | −0.03 | 0.03 | 0.70*** | 1.00 | |
| ΔMST (W) | 0.02 | 0.04 | 0.02 | −0.07 | −0.01 | 0.76*** | 0.66*** | 1.00 |
| ΔSJL | 0.04 | −0.03 | 0.01 | 0.02 | 0.02 | 0.33*** | 0.26*** | −0.03 |
| Men | ||||||||
| ΔLatency | 0.44*** | 1.00 | ||||||
| ΔHSE | −0.56*** | −0.33*** | 1.00 | |||||
| ΔTIB | −0.20*** | 0.07* | −0.27*** | 1.00 | ||||
| ΔDuration | −0.61*** | −0.16*** | 0.51*** | 0.66*** | 1.00 | |||
| ΔMSFsc | 0.00 | −0.01 | 0.02 | 0.04 | 0.07* | 1.00 | ||
| ΔMST (F) | −0.01 | −0.01 | 0.07** | 0.00 | 0.09* | 0.82*** | 1.00 | |
| ΔMST (W) | −0.02 | −0.02 | 0.06* | −0.03 | 0.04 | 0.77*** | 0.76*** | 1.00 |
| ΔSJL | 0.05 | 0.01 | −0.01 | 0.09** | 0.07* | 0.26*** | 0.20*** | 0.06*** |
Correlation significances are noted after adjusting for body mass index (kg/m2), smoking intensity (pack-years), alcohol consumption (g/d), hypertension, diabetes mellitus, occupation, and visit interval (years). *P < .05, **P < .005, ***P < .001. F = free days, HSE = habitual sleep efficiency, MSFsc = mid-sleep time on free days corrected for sleep debt on weekdays, MST = midsleep time, PSQI = Pittsburgh Sleep Quality Index, SJL = social jetlag, TIB = time in bed, W = workdays.
Longitudinal changes in chronotype measures (ΔMSFsc, Δmidsleep time) were highly correlated with each other in both sexes (r > .6, P < .001) and also correlated with decreased social jetlag (for ΔMSFsc: r = .33 in women, r = .26 in men; both P < .001). However, we did not find any significant linear association of longitudinal chronotype advance and sleep quality/quantity deteriorations. Only in men did advanced chronotype measured by midsleep time (free days and workdays) exhibit a slight tendency to correspond with decreased HSE and duration, but this finding was marginal (r = .07 and r = .06, respectively; both P < .05).
Effects of menstruation status on sleep characteristics
Baseline characteristics of the women across menopausal statuses are shown in Table S2 (82KB, pdf) . At baseline, similar numbers of participants were in the premenopause and postmenopause groups (n = 728 [54.5%] vs n = 607 [45.5%]). The postmenopause group’s average age was higher, as expected (55.07 ± 6.62 years vs 45.44 ± 3.03 years, respectively; P < .001). Sleep profiles differed widely between the pre- and postmenopause groups cross-sectionally at baseline. However, after adjusting for general health, lifestyle, and age characteristics, only sleep latency (15.2 vs 19.1 minutes, P = .018) and social jetlag (35 vs 15 minutes, P = .005) remained significantly different.
The effects of menstruation status transition in women on the longitudinal sleep profile changes are shown in Table 4. At follow-up, only 29 women remained in the premenopause group. When comparing the 3 groups classified by menopausal status, longitudinal PSQI scores, sleep latency, and HSE seemed to deteriorate the most in the group of women who underwent menopause during the course of the study (perimenopause), but there was no significant association after covariate adjustment. Although chronotype advancement was most prominent in the group of postmenopausal women from the baseline, workday midsleep time deterioration was affected significantly by menopausal status transition even after covariate adjustment (P = .011).
Table 4.
Adjusted mean (95% confidence interval) changes in the sleep characteristics stratified by menopausal status in women.
| Menopausal Status | P for Trend | |||
|---|---|---|---|---|
| Pre (n = 29) | Peri (n = 697) | Post (n = 606) | ||
| ΔPSQI, score | 0.7 (−0.5 to 1.8) | 1.1 (0.8 to 1.4) | 0.7 (0.4 to 1.0) | .225 |
| ΔSleep latency, min | 4.7 (−5.2 to 14.5) | 5.2 (2.9 to 7.5) | 1.5 (−1.1 to 4.1) | .098 |
| ΔHSE, % | −4.1 (−9.7 to 1.5) | −6.0 (−7.3 to −4.7) | −5.2 (−6.7 to −3.7) | .697 |
| ΔTime in bed, min | 9.9 (−21.2 to 41.0) | 19.6 (12.3 to 26.9) | 25.3 (17.0 to 33.5) | .283 |
| ΔSleep duration, min | −7.6 (−38.5 to 23.2) | −6.9 (−14.1 to 0.4) | 1.4 (−6.8 to 9.5) | .220 |
| ΔMSFsc, 24-h | −0:05 (−0:34 to 0:22) | −0:15 (−0:22 to −0:08) | −0:25 (−0:33 to −0:18) | .061 |
| ΔMidsleep time (F), 24-h | −0:21 (−0:48 to 0:05) | −0:27 (−0:33 to −0:20) | −0:32 (−0:39 to −0:24) | .322 |
| ΔMidsleep time (W), 24-h | −0:06 (−0:31 to 0:18) | −0:10 (−0:16 to −0:04) | −0:24 (−0:30 to −0:17) | .011 |
| ΔSocial jetlag, 24-h | −0:18 (−0:39 to 0:02) | −0:15 (−0:19 to −0:10) | −0:06 (−0:12 to −0:01) | .059 |
P for trend is reported for menopausal status after adjusting for body mass index (kg/m2), smoking intensity (pack-years), alcohol consumption (g/d), hypertension, diabetes mellitus, occupation, and visit interval (years) in addition to baseline age. F = free days, HSE = habitual sleep efficiency, MSFsc = mid-sleep time on free days corrected for sleep debt on weekdays, n = number of observations, PSQI = Pittsburgh Sleep Quality Index, W = workdays.
DISCUSSION
This study described the effects of sex and age on sleep patterns cross-sectionally and longitudinally in a community-dwelling Korean cohort. We further investigated the degree and timing of sleep changes in each sex on age-stratified groups. To our knowledge, this is the first study to investigate the long-term association between sex and sleep profile changes in the normal aging process. Although many studies have demonstrated differences in sleep behavior across the lifespan cross-sectionally, not many have investigated longitudinal follow-up data to consider the independent and interactive effects of sex in a late-adulthood population experiencing significant hormonal changes in addition to aging.9,14,28 The findings of this study can be summarized as follows:
Sleep profiles undergo substantial deterioration from late adulthood onward.
The degree of deterioration in the quality and quantity of sleep tends to be more accelerated with aging.
Sleep profiles and their aging-related deterioration vary with sex, with differences mainly noticeable before age 60.
Chronotype advance occurs mainly before age 50 with more pronounced changes in men.
Radical changes in hormonal status at the beginning of the aging process are also important factors that must be considered in studying the sleep profiles after the 40s.
Our results are well in line with known aging-related sleep deteriorations. Common sleep problems associated with the aging process include increased sleep fragmentation, reduced total sleep time, and advanced sleep timing.9,16,29 One of our study’s strengths is that we investigated longitudinal sleep quality deteriorations and associations between their subproperties in great detail. We observed an increase in the PSQI score, which was most affected by decreased HSE due to decreased sleep duration, in addition to increased sleep latency during aging. Also, by examining results across a spectrum of measures, our data suggested that a substantive understanding of each of the self-reported sleep measures should be considered for drawing inferences in future studies.30 For example, we should more accurately describe the effects of sleep quantity on the adults’ aged 40s and older health outcomes as the self-aware sleep duration and TIB change in opposite directions with aging. However, to investigate the impact of sleep behavior on various health outcomes more precisely, it is necessary to plot more detailed individual trajectories through a continuous follow-up assessment of these measures. Due to changes in the sleep behavior survey format during the early years of the study, we were unable to investigate the trajectories using additional assessment points; however, we expect this to be possible in the future as KoGES in an ongoing project now consistently using a more detailed format for the sleep survey.
When conducted with precisely defined operational measurements pertinent to the various aspects of sleep properties, sleep data obtained through questionnaires can often be a better reflection of regular sleep patterns than objective methods such as polysomnography over a small number of consecutive nights.31 In this study, we established a normative range of longitudinal chronotype advance by slightly modifying the existing PSQI to investigate the sleep patterns during the workdays and the free days separately, which allowed us to calculate MSFsc. MSFsc is practical to measure and highly relevant to conventional circadian process markers such as dim-light melatonin onset and core body temperature.32 Clearly, the way we calculated MSFsc has limitations because there was no investigation into the use of alarm clocks on free days as in the formal Munich Chronotype Questionnaire. However, the social jetlag parameter confirmed the difference in sleep patterns between workdays and free days. Moreover, in our study, MSFsc at baseline was skewed to earlier timing in women, which is consistent with previous cross-sectional studies using the conventional Munich Chronotype Questionnaire survey showing that maximum lateness is reached during adolescence at an earlier age but to a lesser degree in women than men.24 Also consistent with previous studies, the longitudinal advance of MSFsc during the follow-up period was more significant in men, resulting in a tendency to reduce differences between the sexes.14,18
Another point to note for our MSFsc metric is that it has relatively little dependence on other subcomponents of the PSQI. Circadian rhythm attenuation in the adults aged 40s and older population results in unconsolidated sleep, especially for the latter part of the night, which leads to further deterioration caused by sleep phase misalignment and advancement.33 However, current literature regarding inadequate sleep and its consequences in adults aged 40s and older has been mainly focused on the amount of sleep itself, with little regard for the relevance of sleep quality, efficiency, or phase.34 Moreover, few studies have investigated the effect of intensive advance in sleep timing in late adulthood compared with the more favorable health outcomes of the younger population’s morningness trait.35 It is essential to provide reference points of sleep behavior trajectory to which possible pathologic circadian phase changes can be compared because it remains unclear whether sleep disruption with age comes about as a result of degeneration in circadian or homeostatic facets of sleep regulation or both.7,33 Our results also suggest the need for further research into how cognitive behavior therapy, which focuses on putting back bedtime, works for groups of people in their 40s and older who are uncomfortable due to reduced sleep duration or increased sleep latency.36,37
Our results have confirmed that sex-related differences in sleep behavior changes are mainly noticeable before age 60. These differences in sleep regulation and profiles can arise from many factors, with the higher incidence of sleep complaints in women known to be closely related to psychological comorbidity and socioeconomic status in addition to biological factors.11,38 Our initial survey format did not include specific measures for depression or anxiety symptoms, although individuals with a history of psychiatric illness were excluded from the analysis. Moreover, as some previous studies have demonstrated that adjustments for socioeconomic status factors such as years of education or marital status can as much as halve sex-related differences in self-reported sleep problems, the lack of detailed investigation and correction of these factors is an unfortunate limitation of our study.39,40 Also, if we had been able to plot a more detailed trajectory through the collection of additional assessment points near the age of retirement, we might have identified how retirement relates to men in their 60s having the most severe deterioration in PSQI, sleep latency, and HSE. However, transitioning to retirement is generally associated with longer sleep duration and later bedtime/wake time,41 which is the opposite of our results. Also, the average age at which Koreans retire from their longest-serving occupation is 49.4 years,42 and a majority of our female participants reported being homemakers (63.6%). Moreover, as we have found a tendency for gradual acceleration in sleep quality/quantity deterioration and gradual attenuation in chronotype advance after ages in the mid-50s, we decided to focus more on the biological factors that affect the long-term change trend of sleep profiles in late adulthood rather than socioeconomic status factors with high interindividual variability.
To further investigate biological factors other than aging, we examined the effects of another factor that has a notable crucial association with sleep profiles but has frequently been overlooked in previous population-based studies of the middle-aged radical changes in sex hormones.12,43 The average age of menopause for Korean women is in their 50s,44 and we previously reported that insomnia is highly associated with the menopausal transition in our cohort and that sleep initiation difficulty is the most relevant symptom.26 Our current study confirmed significant differences in sleep latency dependent on the menopausal stage at baseline, and longitudinal deterioration in overall sleep quality was also most severe in the group of participants who underwent menopause during the study. However, unequal group sizes have limited the statistical power of our examination of longitudinal sleep profile changes, suggesting the need for a study design that includes more middle-aged women with continued menorrhea. Also, the sleep quality of women undergoing transition to menopause is known to be influenced by a variety of factors, such as the cause of menopause, whether they take hormone replacement therapy, and whether they have symptoms such as hot flashes.45 To better understand the effects and etiology of radical sex hormone changes on sleep physiology, further investigations are required to carefully consider the confounding factors mentioned above in the general middle-aged female population. Estrogen hormone replacement therapy generally enhances sleep amount and continuity,46 whereas androgens appear to have a beneficial impact on rapid eye movement sleep but disrupt sleep consolidation.13,47,48 Therefore, we also need to find practical ways to study the andropause process and its impact on sleep profiles in middle-aged men.
Chronological regression of sleep quality and quantity in the adults aged 40s and older population has received much attention in recent years with the emerging evidence of its short- and long-term effects on physical and mental health. However, we still do not have definite answers as to the fundamental questions regarding the underlying mechanisms or critical biomarkers related to the effects of inadequate sleep, particularly concerning the normal aging process. An in-depth understanding of associations between sex/hormone status with sleep properties in the general population from middle age is fundamental to answering these questions. Our results provide a more comprehensive set of norm references than previously available, which will increase the ability of physicians and researchers to more precisely determine the degree to which an individual’s sleep profile is outside of the norm for their age and sex.
DISCLOSURE STATEMENT
This study was funded by grants from the Korean Centers for Disease Control and Prevention, Korean Ministry for Health and Welfare (grant numbers 2003-347-6111-221, 2004-E71001-00, 2015-P71001-00, and 2016-E71003-00; to C.S.), grants from the Basic Science Research Program, Convergent Technology R&D Program for Human Augmentation and the BK21 Plus Program through the National Research Foundation of Korea funded by the Ministry of Science, Information, and Communication Technologies & Future Planning (NRF-2017R1A2A2A05069647, 2017R1B5A2086553, 2018M3C1B8016147, 2019M3C1B8090803, and 2020R1A2C2013216; to H.W.L.), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI19C1065; to H.J.K.). The authors confirm that they have read the journal’s position on issues involved in ethical publication and affirm that this article is consistent with those guidelines. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors acknowledge all members of KoGES for their assistance in completing this analysis. The authors thank the KoGES Ansan sites and the study participants.
ABBREVIATIONS
- HSE
habitual sleep efficiency
- KoGES
Korean Genome and Epidemiology Study
- MSFsc
mid-sleep time on free days corrected for sleep debt on weekdays
- PSQI
Pittsburgh Sleep Quality Index
- TIB
time in bed
REFERENCES
- 1.Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the Sleep Heart Health Study. J Am Geriatr Soc. 2008;56(7):1218–1227. 10.1111/j.1532-5415.2008.01755.x [DOI] [PubMed] [Google Scholar]
- 2.Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med Rev. 2016;25:21–30. 10.1016/j.smrv.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. 10.1016/j.jpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 5.Duffy JF, Zitting K-M, Chinoy ED. Aging and circadian rhythms. Sleep Med Clin. 2015;10(4):423–434. 10.1016/j.jsmc.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3(4):299–309. 10.1016/0197-4580(82)90018-5 [DOI] [PubMed] [Google Scholar]
- 7.Skeldon AC, Derks G, Dijk D-J. Modelling changes in sleep timing and duration across the lifespan: changes in circadian rhythmicity or sleep homeostasis? Sleep Med Rev. 2016;28:96–107. 10.1016/j.smrv.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 8.Dijk D-J, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33(2):211–223. 10.1093/sleep/33.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moraes W, Piovezan R, Poyares D, Bittencourt LR, Santos-Silva R, Tufik S. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15(4):401–409. 10.1016/j.sleep.2013.11.791 [DOI] [PubMed] [Google Scholar]
- 10.Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. 10.1016/j.yfrne.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32(10):1367–1375. 10.1093/sleep/32.10.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrier J, Semba K, Deurveilher S, et al. Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;47:66–85. 10.1016/j.yfrne.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Paul KN, Turek FW, Kryger MH. Influence of sex on sleep regulatory mechanisms. J Womens Health (Larchmt). 2008;17(7):1201–1208. 10.1089/jwh.2008.0841 [DOI] [PubMed] [Google Scholar]
- 14.Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US—influence of age and sex. PLoS One. 2017;12(6):e0178782. 10.1371/journal.pone.0178782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16(2):170–180. 10.1111/j.1365-2869.2007.00594.x [DOI] [PubMed] [Google Scholar]
- 16.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 17.Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. Circadian preference links to depression in general adult population. J Affect Disord. 2015;188:143–148. 10.1016/j.jad.2015.08.061 [DOI] [PubMed] [Google Scholar]
- 18.Duarte LL, Menna-Barreto L, Miguel MA, et al. Chronotype ontogeny related to gender. Braz J Med Biol Res. 2014;47(4):316–320. 10.1590/1414-431X20143001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randler C. Age and gender differences in morningness-eveningness during adolescence. J Genet Psychol. 2011;172(3):302–308. 10.1080/00221325.2010.535225 [DOI] [PubMed] [Google Scholar]
- 20.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30-49 years). J Biol Rhythms. 2006;21(1):68–76. 10.1177/0748730405283154 [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Han B-G; KoGES group . Cohort profile: the Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol. 2017;46(2):e20. 10.1093/ije/dyv316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 23.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 24.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. 10.1016/j.smrv.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 25.Foubert-Samier A, Catheline G, Amieva H, et al. Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging. 2012;33(2):423, e15–25. 10.1016/j.neurobiolaging.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 26.Shin C, Lee S, Lee T, et al. Prevalence of insomnia and its relationship to menopausal status in middle-aged Korean women. Psychiatry Clin Neurosci. 2005;59(4):395–402. 10.1111/j.1440-1819.2005.01391.x [DOI] [PubMed] [Google Scholar]
- 27.Patino CM, Ferreira JC. Test for trend: evaluating dose-response effects in association studies. J Bras Pneumol. 2016;42(4):240. 10.1590/s1806-37562016000000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broms U, Pitkäniemi J, Bäckmand H, et al. Long-term consistency of diurnal-type preferences among men. Chronobiol Int. 2014;31(2):182–188. 10.3109/07420528.2013.836534 [DOI] [PubMed] [Google Scholar]
- 29.Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. 10.1016/j.jsmc.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savitz DA. Interpreting epidemiologic evidence: strategy for study design and analysis.Oxford, UK: Oxford University Press; 2003. 10.1093/acprof:oso/9780195108408.001.0001 [DOI] [Google Scholar]
- 31.Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:166. 10.3389/fnagi.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantermann T, Sung H, Burgess HJ. Comparing the morningness-eveningness questionnaire and Munich Chronotype Questionnaire to the dim light melatonin onset. J Biol Rhythms. 2015;30(5):449–453. 10.1177/0748730415597520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23(1–2):461–474. 10.1080/07420520500545813 [DOI] [PubMed] [Google Scholar]
- 34.Devore EE, Grodstein F, Schernhammer ES. Sleep duration in relation to cognitive function among older adults: a systematic review of observational studies. Neuroepidemiology. 2016;46(1):57–78. 10.1159/000442418 [DOI] [PubMed] [Google Scholar]
- 35.Merikanto I, Lahti T, Puolijoki H, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30(4):470–477. 10.3109/07420528.2012.741171 [DOI] [PubMed] [Google Scholar]
- 36.Joshi S. Nonpharmacologic therapy for insomnia in the elderly. Clin Geriatr Med. 2008;24(1):107–119. 10.1016/j.cger.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 37.Lovato N, Lack L, Wright H, Kennaway DJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep. 2014;37(1):117–126. 10.5665/sleep.3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Wing Y-K. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. 10.1093/sleep/29.1.85 [DOI] [PubMed] [Google Scholar]
- 39.Arber S, Bote M, Meadows R. Gender and socio-economic patterning of self-reported sleep problems in Britain. Soc Sci Med. 2009;68(2):281–289. 10.1016/j.socscimed.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 40.Arber S, Hislop J, Bote M, Meadows R. Gender roles and women’s sleep in mid and later life: a quantitative approach. Sociol Res Online. 2007;12(5):182–199. 10.5153/sro.1609 [DOI] [Google Scholar]
- 41.Hagen EW, Barnet JH, Hale L, Peppard PE. Changes in sleep duration and sleep timing associated with retirement transitions. Sleep. 2016;39(3):665–673. 10.5665/sleep.5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korean Statistical Information Service . Supplementary Results of the Economically Active Population Survey for the Old Population in May 2019 http://kostat.go.kr/portal/eng/pressReleases/5/5/index.board. Accessed September 24, 2020.
- 43.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 44.Hee-Ran C. Korean women’s reproductive health indicators. Health Welfare Policy Forum. 2016;235:34–46. [Google Scholar]
- 45.Xu Q, Lang CP. Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause. 2014;21(12):1301–1318. 10.1097/GME.0000000000000240 [DOI] [PubMed] [Google Scholar]
- 46.Cintron D, Lipford M, Larrea-Mantilla L, et al. Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine. 2017;55(3):702–711. 10.1007/s12020-016-1072-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanisch LJ, Gooneratne NS, Soin K, Gehrman PR, Vaughn DJ, Coyne JC. Sleep and daily functioning during androgen deprivation therapy for prostate cancer. Eur J Cancer Care (Engl). 2011;20(4):549–554. 10.1111/j.1365-2354.2010.01226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett-Connor E, Dam T-T, Stone K, Harrison SL, Redline S, Orwoll E; Osteoporotic Fractures in Men Study Group . The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93(7):2602–2609. 10.1210/jc.2007-2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.