Abstract
Study Objectives:
To assess sex-related differences in the relationship between hypertension (HT), blood pressure (BP), and sleep apnea in the general population.
Methods:
We performed home polygraphy in a cohort of 1809 men and women in the general population. Office BP was measured. Presence of HT (drug-treated, physician-diagnosed, or high BP during study visit) was also recorded. HT rate and BP were assessed over a range of 7 sleep apnea severity categories based on the respiratory event index (REI).
Results:
The age-adjusted HT prevalence rate increased with higher REI in both sexes. After additional adjustment for obesity the association remained significant in women but not in men. In participants not treated with antihypertensive medications, age-adjusted BP increased with REI. Remarkably, the association was already significant within the normal range (REI < 5 events/h). The REI threshold for higher BP was situated at a distinctly lower cutoff point in women compared to men. After additional adjustment for obesity, the associations remained significant for diastolic but not systolic BP.
Conclusions:
Significant increases in the age-adjusted BP and HT rate in the general population were present at lower REI cutoffs in women compared to men. Even a very low number of respiratory events was associated with higher BP and HT prevalence. Adjustment for obesity attenuated these associations, especially in men. Sex differences in BP susceptibility across the sleep apnea spectrum may be present.
Citation:
Bauters FA, Hertegonne KB, Pevernagie D, De Buyzere ML, Chirinos JA, Rietzschel ER. Sex differences in the association between arterial hypertension, blood pressure, and sleep apnea in the general population. J Clin Sleep Med. 2021;17(5):1057–1066.
Keywords: sleep apnea, hypertension, blood pressure, sleep-disordered breathing, cohort study
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although sleep apnea and hypertension have been linked, little is known about sex differences. It is unknown how much sleep apnea is needed to develop high(er) blood pressure. We aimed to assess the relationships between sleep apnea, hypertension, and blood pressure in the general population.
Study Impact: There was an age-adjusted graded association of higher blood pressure and hypertension prevalence with increasing respiratory event index in both sexes, even within the normal range of respiratory event index. Additional adjustment for obesity attenuated these associations, especially in men. The graded associations were dissimilar between men and women, suggesting different susceptibility.
INTRODUCTION
Sleep apnea (SA) and arterial hypertension (HT), both prevalent conditions, are closely linked. Potential pathophysiological mechanisms are related to repetitive episodes of intermittent hypoxemia in SA, provoking sympathetic nervous system activation, negative intrathoracic pressure surges, oxidative stress, and inflammatory and metabolic responses, eventually resulting in endothelial dysfunction, increased arterial stiffness, and high blood pressure (BP).1 Despite accumulating evidence for causality in the relationship between SA and HT, the role of confounding factors such as obesity, age, and sex remains unclear. Cross-sectional data from community-based and clinical populations have indicated that SA is a risk factor for HT.2–8 Most reports focused on moderate to severe SA, with an apnea-hypopnea index (AHI; in case of polysomnography registration), or a respiratory event index [REI; in case of home polygraphy (PG) recording] exceeding 15, 20, or 30 respiratory events per hour of sleep or per hour of recording time, respectively. Until now, it remained unclear whether mild SA is independently associated with high BP. A research statement report of the American Thoracic Society stated that there is insufficient evidence for an independent correlation of mild SA with HT after adjustment for confounding variables.9 The same conclusion was made in a review paper on mild SA and its associated cardiovascular risks.10 In contrast, recent data from the European Sleep Apnea Database (ESADA) demonstrated a graded relationship of SA and HT in a sleep clinic population that was already significant below the threshold of 15 events per hour.6 Surprisingly, most data were not analyzed in a sex-stratified manner and, if so, the results from female study participants have often been underreported.11 In the last few years it became clear that the clinical profile of SA is different in women compared to men.12–15 It has been stated that women may be more vulnerable to SA-related cardiovascular complications than men.16–18 Little is known about sex differences in the association of mild SA and BP in the general population.
In the present community-based cohort study, we specifically aimed to investigate the relationship of an increasing exposure of respiratory events during sleep and systolic and diastolic BP and the prevalence of HT, unbiased by—and exploring beyond—the conventional AHI cutoff points. We assessed the “dose-response” relationships between REI and HT rate, and between REI and BP, differentiated for sex and adjusted for age and obesity.
METHODS
Study design and population
The study was conducted in the second measurement round (2011–2016) of the Asklepios cohort, an ongoing community-based cohort study with the focus on cardiovascular disease.19,20 In brief, the study focused on arterial ageing (arterial stiffness and atherosclerosis), early cardiac changes in systolic and diastolic function, and ventriculo-arterial function with a goal of better understanding the development of cardiovascular disease and ultimately help underpin better prevention strategies. The Asklepios population consisted of 2205 eligible study participants aged 44–75 years (52.3% women) from 2 Belgian communities, who were not diagnosed with or treated for SA. During the initial study visit, each volunteer underwent clinical assessment and was offered home PG. The study was approved by the Ethics Committee of the Ghent University Hospital. All participants gave written informed consent. Additional details on the study protocol were published earlier.20,21
Clinical assessments
All study participants underwent a standardized examination at the study center.20 We collected the personal and primary care physician–validated medical and treatment history, demographic data and lifestyle habits, and study questionnaires. Medication was classified according to the Anatomical Therapeutic Chemical Classification System of drugs.22 We measured height, weight, and circumference of waist, hip, and neck. The body mass index (BMI, kg/m2) was calculated from weight and height. Obesity was defined as a BMI ≥ 30 kg/m2.
Blood pressure and hypertension
BP was measured in the upright sitting position using a suitable cuff, after 10–15 minutes rest in a temperature-controlled environment (Omron HEM-907; Kyoto, Japan). The mean value both arms, each consisting of 3 measurements with 1-minute intervals was used.19 Systolic and diastolic BP were studied as continuous variables in our data analysis. Arterial HT was defined as ongoing treatment with antihypertensive drugs, currently having physician-diagnosed HT in medical record, having an average systolic BP (SBP) ≥ 140 mmHg or a diastolic BP (DBP) ≥ 90 mmHg, or a combination thereof. HT was used as a dichotomous variable in our data analysis.
Home polygraphy
Each volunteer was invited for home sleep testing during one night with the ApneaLink PlusTM (ResMed, San Diego, CA), a level 3 cardiorespiratory recording device. Respiratory events were manually scored according to the American Academy of Sleep Medicine criteria. An apnea was defined as a 90% airflow reduction for at least 10 seconds. Apneas with preserved respiratory effort were scored as obstructive apneas, whereas central apneas were scored if respiratory effort was absent. A hypopnea was defined as an airflow reduction of at least 30% with an associated ≥ 4% oxygen desaturation.23 Because we used home PG without electroencephalogram recordings, we denominated the number of apneas and hypopneas per hour evaluation time as “respiratory event index” (REI), rather than apnea-hypopnea index (AHI). Additional details on the procedures were published before.20
Other assessments
We collected venous blood samples after 8 hours of fasting and at least 6 hours of smoking abstinence. We defined type 2 diabetes as fasting blood glucose ≥ 126 mg/dL or the use of blood glucoselowering– medication. The Epworth Sleepiness Scale (ESS), providing information on self-reported sleepiness, was administered.24 A total ESS score greater than 10 was considered to be consistent with daytime sleepiness.
Statistical analysis
We examined the association between the REI and HT rate on the one hand, and systolic and diastolic BP values on the other. We created REI categories based on the observed distribution in the overall study population, resulting in 7 groups of approximately the same size (septiles). The conventional clinical REI cutoff points (5 events/h for mild and 15 events/h for moderate SA) were considered in the categorization: REI = 0 events/h, REI = 1 event/h, REI = 2 events/h, REI = 3 or 4 events/h, REI 5–7 events/h, REI 8–14 events/h, and REI ≥ 15 events/h. All subsequent analyses were sex-stratified. Significance was set at the .05 level. Chi-square tests were used to compare proportions. Analysis of variance or Kruskal-Wallis test were used to compare continuous variables. Logistic regression analysis was performed to calculate the age-adjusted and age- and obesity-adjusted odds ratios with 95% confidence interval of having HT by REI category (reference category REI = 0 events/h). In participants who were not treated with antihypertensive drugs, we used linear regression models to assess the estimated marginals means of the systolic and diastolic BP in each REI category. As for the logistic regression analyses, the linear models were adjusted for age alone, and for age and obesity. We used SPSS Statistics version 26 (IBM Corp, Armonk, NY).
RESULTS
Study participants
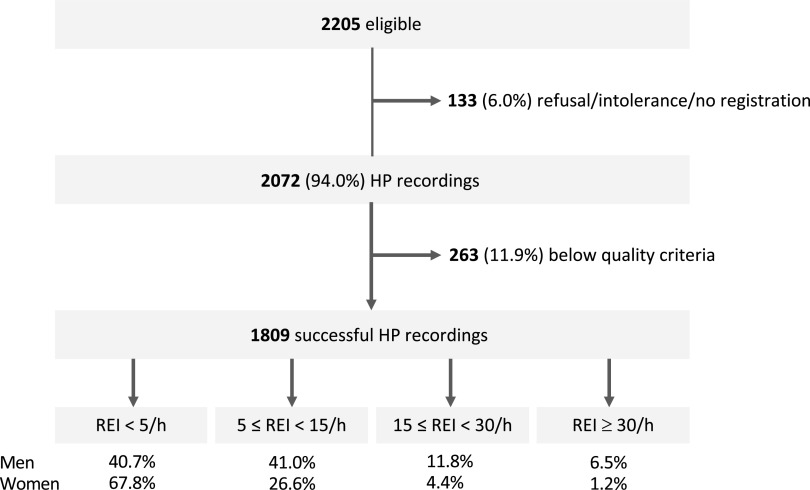
Of 2205 eligible participants, 133 had no available recordings (declined, intolerance, or other reasons) and in 263 the recordings were below strict predefined quality criteria.20 Included in the analysis were 1809 participants (82% of eligible) (Figure 1). The study population was almost exclusively Caucasian. The mean age was 56.0 ± 5.9 years (Table 1). Both sexes had similar age. Women had a significantly lower BMI compared to men. An REI below 15 events/h was seen in more than 80% men and more than 90% of women. The men/women ratio for the prevalence of moderate to severe SA (defined as a REI ≥ 15 events/h) was approximately 3:1. Systolic and diastolic BP were higher in men, as was HT prevalence.
Figure 1. Flow diagram of the study cohort.
HP = home polygraphy, REI = respiratory event index.
Table 1.
Baseline descriptive characteristics of the study participants, comparison between men and women.
| Variable | Total Group (n = 1,809) | Men (n = 862, 47.7%) | Women (n = 947, 52.3%) | P Value* |
|---|---|---|---|---|
| Age and body size | ||||
| Age (years) | 56.0 ± 5.9 | 56.1 ± 5.9 | 55.9 ± 5 .9 | .505 |
| BMI (kg/m2) | 26.8 ± 4.6 | 27.5 ± 4.1 | 26.1 ± 4.8 | < .001 |
| Obesity (%)† | 20.6 | 23.2 | 18.3 | < .001 |
| Blood pressure | ||||
| Systolic blood pressure (mm Hg) | 130 ± 15 | 134 ± 14 | 126 ± 15 | < .001 |
| Diastolic blood pressure (mm Hg) | 81 ± 10 | 84 ± 10 | 79 ± 9 | < .001 |
| Hypertension‡ (physician-diagnosed, suspected, or drug-treated; %) | 43.7 | 50.1 | 37.8 | < .001 |
| Apnea-hypopnea index (AHI) | ||||
| AHI | 4 (1–9) | 6 (2–12) | 2 (1–6) | < .001 |
| No sleep apnea (REI < 5) (%) | 54.9 | 40.7 | 67.8 | < .001 |
| Mild sleep apnea (5 ≤ REI < 15) (%) | 33.4 | 41.0 | 26.6 | < .001 |
| Moderate sleep apnea (15 ≤ REI < 30) (%) | 8.0 | 11.8 | 4.4 | < .001 |
| Severe sleep apnea (REI ≥ 30) (%) | 3.7 | 6.5 | 1.2 | < .001 |
Data are proportions, mean ± standard deviation, or median (interquartile range). *P value: comparison between men and women. †Obesity: BMI ≥ 30 kg/m2. ‡Hypertension: physician-diagnosed, drug-treated hypertension, or systolic blood pressure ≥ 140 mmH g or diastolic blood pressure ≥ 90 mm Hg during study visit. BMI = body mass index, REI = respiratory event index.
Clinical associations of increasing REI
Increasing REI was associated with older age, higher BMI, and more obesity (Table 2 and Table 3). The prevalence of type 2 diabetes mellitus also increased with increasing REI. As previously published, we found no difference in self-reported sleepiness between SA severity categories in our general population cohort.20 Considering lifestyle, there was no difference in smoking habits. Weekly alcohol intake was the same among REI groups in women, whereas in men it was higher with increasing REI category.
Table 2.
Cardiovascular risk factors, hypertension, and blood pressure in men, comparison between REI categories.
| Men | All Groups (n = 862) | REI 0 (n = 46) | REI 1 (n = 89) | REI 2 (n = 82) | REI 3–4 (n = 134) | REI 5–7 (n = 154) | REI 8–14 (n = 199) | REI ≥ 15 (n = 158) | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular risk factors | |||||||||
| Age (years) | 56 ± 6 | 54 ± 7 | 54 ± 6 | 55 ± 6 | 55 ± 6 | 56 ± 6 | 57 ± 6 | 58 ± 6 | < .001 |
| BMI (kg/m2) | 27.5 ± 4.1 | 24.8 ± 3.2 | 26.0 ± 3.5 | 26.2 ± 3.7 | 26.4 ± 3.2 | 27.1 ± 3.3 | 28.0 ± 3.9 | 30.4 ± 4.7 | < .001 |
| Obesity† (%) | 23.2 | 3.4 | 6.6 | 15.1 | 14.4 | 17.2 | 27.7 | 52.8 | < .001 |
| Type 2 diabetes mellitus‡ (%) | 9.4 | 4.3 | 2.2 | 3.7 | 5.2 | 11.0 | 14.1 | 13.9 | .001 |
| Lipid-lowering drugs (%) | 31.0 | 15.2 | 25.8 | 22.0 | 28.4 | 34.4 | 36.2 | 35.4 | .022 |
| Sleepiness§ (%) | 20.5 | 13.0 | 19.5 | 14.8 | 22.1 | 16.4 | 23.0 | 25.8 | .212 |
| Active smoking (%) | 9.6 | 8.7 | 9.0 | 11.0 | 11.2 | 9.1 | 7.0 | 12.0 | .886 |
| Alcohol (units/week) | 15 (8–27) | 8 (3–12) | 11 (3–19) | 8 (4–21) | 12 (4–25) | 12 (4–27) | 13 (4–25) | 16 (5–25) | .004 |
| Hypertension and blood pressure | |||||||||
| Hypertension¶ (%) | 50.1 | 34.8 | 38.2 | 36.6 | 46.3 | 51.9 | 55.8 | 62.7 | < .001 |
| Systolic BP (mm Hg) | 134 ± 14 | 130 ± 14 | 131 ± 13 | 130 ± 11 | 134 ± 16 | 134 ± 14 | 135 ± 14 | 136 ± 14 | .004 |
| Diastolic BP (mm Hg) | 84 ± 10 | 80 ± 11 | 81 ± 9 | 81 ± 10 | 83 ± 11 | 84 ± 10 | 84 ± 10 | 87 ± 9 | < .001 |
| Not drug-treated (number) | 647 | 38 | 74 | 70 | 105 | 115 | 141 | 104 | |
| Systolic BP (mm Hg) | 132 ± 14 | 127 ± 12 | 130 ± 12 | 129 ± 11 | 133 ± 15 | 133 ± 14 | 133 ± 14 | 135 ± 15 | .025 |
| Diastolic BP (mm Hg) | 83 ± 10 | 78 ± 10 | 82 ± 8 | 81 ± 10 | 83 ± 11 | 84 ± 10 | 84 ± 10 | 87 ± 10 | < .001 |
Data are number, percentage (%), mean ± standard deviation, or median (interquartile range). *P value: comparison between REI groups. †Obesity: BMI ≥ 30 kg/m2. ‡Type 2 diabetes mellitus: fasting blood glucose ≥ 126 mg/dL or the use of blood glucose lowering medication. §Sleepiness: Epworth Sleepiness Scale > 10. ¶Hypertension: drug-treated, physician-diagnosed, or systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg during study visit. BMI = body mass index, BP = blood pressure, REI = respiratory event index.
Table 3.
Cardiovascular risk factors, hypertension, and blood pressure in women, comparison between REI categories.
| Women | All Groups (n = 947) | REI 0 (n = 197) | REI 1 (n = 162) | REI 2 (n = 122) | REI 3–4 (n = 161) | REI 5–7 (n = 140) | REI 8–14 (n = 112) | REI ≥ 15 (n = 53) | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular risk factors | |||||||||
| Age (years) | 56 ± 6 | 52 ± 5 | 55 ± 6 | 56 ± 6 | 57 ± 6 | 58 ± 5 | 58 ± 5 | 58 ± 5 | < .001 |
| BMI (kg/m2) | 26.1 ± 4.8 | 23.5 ± 3.5 | 24.8 ± 3.5 | 25.5 ± 4.5 | 25.7 ± 3.7 | 27.8 ± 4.4 | 29.1 ± 6.3 | 31.8 ± 5.2 | < .001 |
| Obesity† (%) | 18.3 | 1.4 | 7.3 | 7.9 | 14.5 | 29.0 | 38.3 | 72.0 | < .001 |
| Type 2 diabetes mellitus‡ (%) | 4.3 | 1.0 | 1.2 | 2.5 | 6.8 | 4.3 | 8.0 | 15.1 | < .001 |
| Lipid-lowering drugs (%) | 26.7 | 17.3 | 19.1 | 31.1 | 26.1 | 39.3 | 33.9 | 28.3 | < .001 |
| Sleepiness§ (%) | 19.3 | 19.9 | 21.3 | 16.5 | 15.8 | 19.1 | 20.5 | 25.0 | .749 |
| Active smoking (%) | 9.4 | 7.6 | 9.9 | 13.9 | 11.2 | 5.7 | 8.0 | 11.3 | .318 |
| Alcohol (units/week) | 4 (0–8) | 4 (0–8) | 4 (1–10) | 2 (0–7) | 4 (0–8) | 5 (0–1) | 5 (0–11) | 4 (1–14) | .136 |
| Hypertension and blood pressure | |||||||||
| Hypertension¶ (%) | 37.8 | 20.8 | 30.9 | 37.7 | 37.3 | 47.1 | 54.5 | 64.2 | < .001 |
| Systolic BP (mm Hg) | 126 ± 15 | 121 ± 13 | 126 ± 15 | 128 ± 15 | 127 ± 16 | 128 ± 15 | 128 ± 15 | 134 ± 15 | < .001 |
| Diastolic BP (mm Hg) | 79 ± 9 | 75 ± 9 | 78 ± 9 | 81 ± 8 | 79 ± 9 | 80 ± 9 | 80 ± 10 | 85 ± 10 | < .001 |
| Not drug-treated (number) | 724 | 172 | 133 | 99 | 128 | 92 | 68 | 32 | |
| Systolic BP (mm Hg) | 124 ± 15 | 120 ± 13 | 125 ± 15 | 127 ± 14 | 126 ± 17 | 125 ± 15 | 124 ± 14 | 131 ± 13 | < .001 |
| Diastolic BP (mm Hg) | 78 ± 9 | 74 ± 8 | 78 ± 9 | 80 ± 8 | 79 ± 9 | 80 ± 10 | 80 ± 9 | 85 ± 9 | .002 |
Data are number, percentage (%), mean ± standard deviation, or median (interquartile range). *P value: comparison between REI groups. †Obesity: BMI ≥ 30 kg/m2. ‡Type 2 diabetes mellitus: fasting blood glucose ≥ 126 mg/dL or the use of blood glucose lowering medication. §Sleepiness: Epworth Sleepiness Scale > 10. ¶Hypertension: drug-treated, physician-diagnosed, or systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mmHg during study visit. BMI = body mass index, BP = blood pressure, REI = respiratory event index.
Blood pressure in mild sleep apnea
In both sexes, the prevalence of HT increased among the 7 REI-based SA categories (Table 2 and Table 3). The same graded effect was seen for SBP and DBP in a subgroup excluding study participants who were currently treated with antihypertensive drugs. We repeated the analyses for SBP and DBP in the whole group (including those with drug-treated HT), with very similar results demonstrating a graded increase of SBP and DBP among REI groups.
Adjusted associations between hypertension and sleep apnea
Age-adjusted logistic regression analysis demonstrated a stepwise increase in the odds ratios of having HT at different REI cutoff points in both sexes (reference category REI 0 events/h) (Figure 2). In women, an REI of 2 events/h was already associated with an increased prevalence of HT (reference category REI 0 events/h), whereas in men the increased odds for HT were situated at a higher cutoff point (REI ≥ 8 events/h, reference category REI 0 events/h). In all REI categories, the point estimates for HT in women exceeded those in men. Additional adjustment for obesity attenuated the odds, which remained significant in women at the same REI cutoff. In men, the association between REI categories and HT lost significance after obesity adjustment. In both models and in both sexes, age and obesity were associated with HT.
Figure 2. Adjusted odds ratios with 95% confidence intervals for hypertension by REI category.
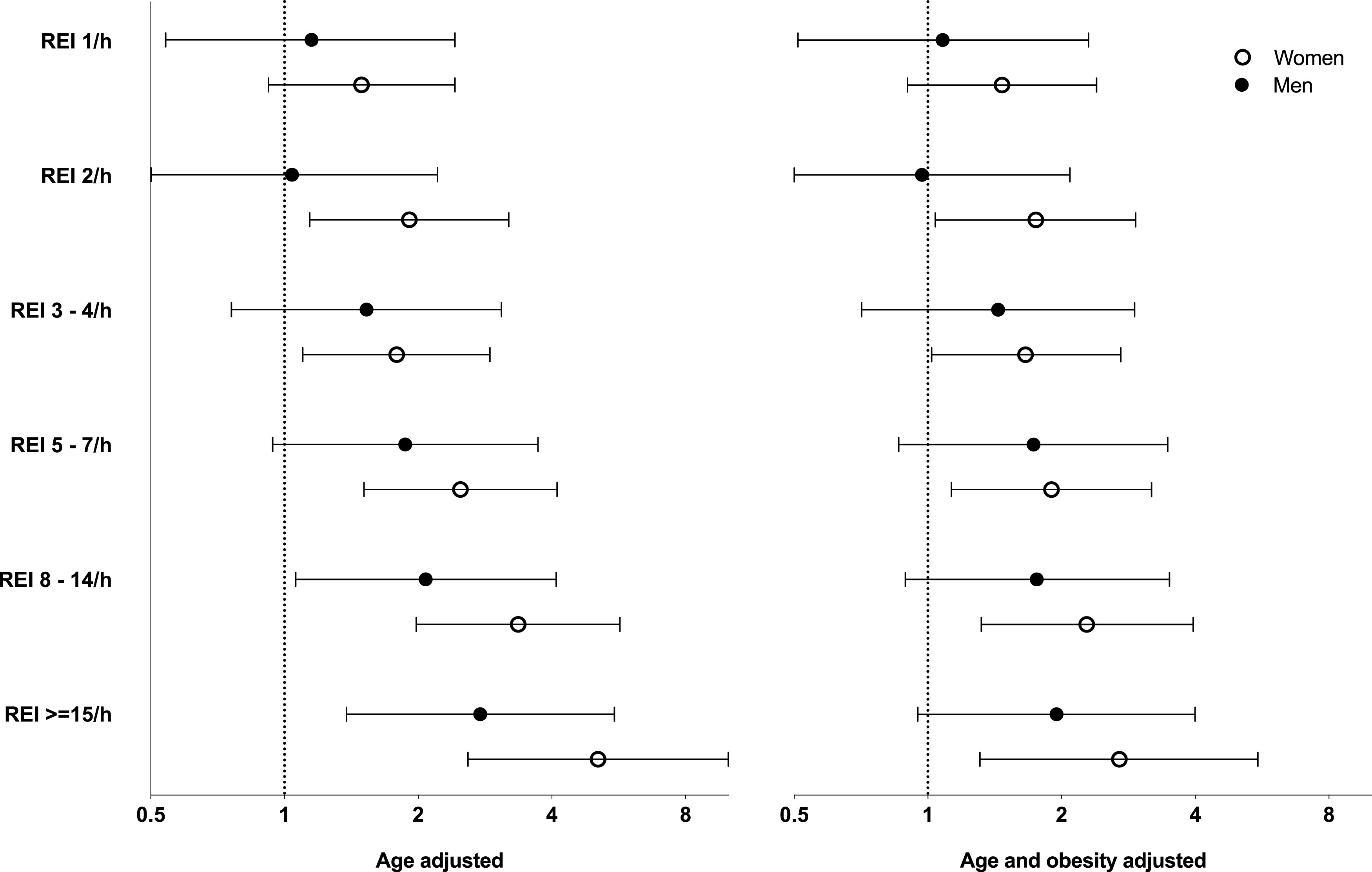
The x-axis has a logarithmic scale. The reference category is REI 0 events/h. Age-adjusted was significant in both sexes. Age- and obesity-adjusted was significant in women but no longer significant in men. REI = respiratory event index.
Adjusted associations between blood pressure and sleep apnea
Table 4 and Table 5 list the age-adjusted and the age- and obesity-adjusted estimated marginal means of BP across REI categories. The age-adjusted linear regression analysis showed a significant increase in BP with increasing REI in both sexes, except for SBP in men (P-value = .050). Remarkably, the BP increase was already significant at REI thresholds far below the normal cutoff of 5 events/h. After additional adjustment for obesity, DBP was independently associated with REI in both sexes. SBP was no longer significant in men, and borderline significant in women. The age- and obesity-adjusted estimates of SBP were 6 and 5 mm Hg higher in the highest SA category (reference category lowest REI group) in women and men, respectively, and the difference in DBP between the lowest and highest REI group was 8 mm Hg in both sexes. Visual inspection suggested a linear trend in men, but a cubic trend in women. Curve-fitting of age- and obesity-adjusted residuals of SBP and DBP confirmed a linear trend in men (P = .027 and < .001, respectively). In contrast, for SBP in women the data did not fit a linear model (P = .547) and a cubic model provided the best curve fitting (P = .012). For DBP in women, a linear model was possible (P < .001), but it was inferior to a cubic curve fitting (P < .001) (Figure S1 (663.8KB, pdf) in the supplemental material).
Table 4.
Adjusted estimated marginal means of systolic blood pressure per REI category in men and women not treated with antihypertensive drugs.
| Age-Adjusted SBP (mm Hg) | Age- and Obesity-Adjusted SBP (mm Hg) | |||
|---|---|---|---|---|
| EM Mean | P Value* | EM Mean | P Value* | |
| Men | ||||
| REI 0 events/h | 128 (123–132) | 130 (125–134) | ||
| REI 1 events/h | 131 (127–134) | .284 | 132 (129–136) | .361 |
| REI 2 events/h | 130 (126–133) | .449 | 132 (128–135) | .543 |
| REI 3–4 events/h | 133 (131–136) | .027 | 135 (132–138) | .044 |
| REI 5–7 events/h | 133 (131–136) | .029 | 135 (132–138) | .044 |
| REI 8–14 events/h | 133 (131–135) | .033 | 135 (132–137) | .065 |
| REI ≥ 15 events/h | 135 (132–137) | .007 | 135 (132–138) | .050 |
| Women | ||||
| REI 0 events/h | 121 (119–124) | 124 (122–127) | ||
| REI 1 events/h | 125 (123–128) | .032 | 128 (125–131) | .041 |
| REI 2 events/h | 127 (124–130) | .004 | 129 (126–132) | .008 |
| REI 3–4 events/h | 125 (123–128) | .506 | 128 (125–131) | .038 |
| REI 5–7 events/h | 124 (121–127) | .159 | 126 (123–129) | .469 |
| REI 8–14 events/h | 123 (120–127) | .402 | 125 (121–128) | .918 |
| REI ≥ 15 events/h | 130 (125–135) | .004 | 130 (125–135) | .080 |
Linear regression analysis showing age-adjusted and age- and obesity-adjusted estimated marginal means with 95% confidence interval for systolic blood pressure per REI category. Reference group: REI 0 events/h. *The overall P-values for the association with adjusted SBP for the categorical variable REI (combining all 7 REI groups) in the multivariate model were .050 for age-adjusted and .194 for age- and obesity-adjusted SBP in men, and .024 for age adjusted and .058 for age and obesity adjusted SBP in women. CI = confidence interval, EM mean = estimated marginal mean, REI = respiratory event index, SBP = systolic blood pressure.
Table 5.
Adjusted estimated marginal means of diastolic blood pressure per REI category in men and women not treated with antihypertensive drugs.
| Age-Adjusted DBP (mm Hg) | Age- and Obesity-Adjusted DBP (mm Hg) | |||
|---|---|---|---|---|
| EM Mean | P Value* | EM Mean | P Value* | |
| Men | ||||
| REI 0 events/h | 78 (75–81) | 80 (76–83) | ||
| REI 1 events/h | 81 (79–83) | .096 | 83 (80–85) | .141 |
| REI 2 events/h | 81 (79–83) | .116 | 82 (80–85) | .163 |
| REI 3–4 events/h | 83 (81–85) | .004 | 85 (83–87) | .009 |
| REI 5–7 events/h | 84 (83–86) | < .001 | 86 (84–88) | .001 |
| REI 8–14 events/h | 84 (83–86) | < .001 | 86 (84–87) | .001 |
| REI ≥ 15 events/h | 88 (86–89) | < .001 | 88 (86–90) | < .001 |
| Women | ||||
| REI 0 events/h | 74 (73–75) | 77 (75–78) | ||
| REI 1 events/h | 78 (76–80) | < .001 | 81 (79–82) | < .001 |
| REI 2 events/h | 80 (78–82) | < .001 | 82 (81–84) | < .001 |
| REI 3–4 events/h | 79 (77–80) | < .001 | 81 (79–83) | < .001 |
| REI 5–7 events/h | 80 (78–82) | < .001 | 82 (80–84) | < .001 |
| REI 8–14 events/h | 80 (78–82) | < .001 | 81 (79–83) | < .001 |
| REI ≥ 15 events/h | 85 (82–88) | < .001 | 85 (82–88) | < .001 |
Linear regression analysis showing age-adjusted and age- and obesity-adjusted estimated marginal means with 95% confidence interval for diastolic blood pressure per REI category. Reference group: REI 0 events/h. *The overall P values for the association with adjusted DBP for the categorical variable REI (combining all 7 REI groups) in the multivariate model were < .001 for age adjusted and for age- and obesity-adjusted DBP in men and women. CI = confidence interval, DBP = diastolic blood pressure, EM mean = estimated marginal mean, REI = respiratory event index.
Sensitivity analyses
Adjustments for BMI instead of obesity did not substantially change the results. Logistic and linear models with more anthropometric variables instead of obesity or BMI, such as neck circumference, waist circumference, waist-to-hip ratio, or body fat distribution were run. These adjustments provided no additional information and had no substantial impact on the results compared to obesity adjustment, as was seen in previous reports.3 Moreover, “full” models in which supplementary variables related to body composition are combined in the same model are over-adjustments and were therefore not reported.
DISCUSSION
We investigated the relationship of sleep-related respiratory events with HT and BP in 1809 men and women from a community-based cohort. Half of men and more than 1 out of 3 women had HT. We found an age-adjusted graded association of HT prevalence with increasing REI in both sexes. The magnitude of the association between SA and HT prevalence was small, as in previous reports.11 In participants not treated with antihypertensive medications, office systolic and diastolic BP were associated with a higher REI. Remarkably, the relationship with HT and BP was already apparent within the normal range (REI < 5 events/h) and in mild SA (5 ≤ REI < 15 events/h). Sex differences in the association of HT and BP with SA were seen in the adjusted analyses. First, the REI threshold for high(er) BP was situated at a lower cutoff point in women compared to men. Second, in the age- and obesity-adjusted analyses HT and REI were independently associated in women but not in men. Third, the association of BP and REI showed a linear trend in men, whereas a cubic model proved the best curve fitting in women.
Cross-sectional data from population-based studies have demonstrated a dose-response association between SA and HT prevalence after adjustment for confounders.2–4,25 The relationship between both conditions is partially or completely explained by BMI, and the effect size of SA on HT in the adjusted models was usually small.11 The Wisconsin Sleep Cohort study found a linear increase in BP with increasing AHI.2 In the Vitoria Sleep Cohort study, SA and HT were independently associated.4 Others, among which is the Sleep Heart Health Study, found an independent association in men but not in women.3,25 Data from longitudinal follow-up studies in the general population are conflicting. In the Wisconsin Sleep Cohort study, SA was associated with increased HT incidence,26 whereas in the Sleep Heart Health Study there was no independent association in the overall population and in men; however, it was significant in women.27 In the Vitoria Sleep Cohort, HT incidence and SA were not associated, and the sex-stratified analyses revealed no differences between men and women.28 However, post-hoc analyses in the same cohort showed that moderate SA was associated with incident stage 2 HT in men but not in women.29 Although the results from community-based studies are apparently conflicting in that the confidence intervals exceed 1 and are therefore not statistically significant, the trend in the point estimates of these analyses is consistent in direction and in general magnitude.3,25,27
More consistent results are seen in sleep clinic samples, in which HT rate seems to be associated with SA severity.5,7,8,12 In ESADA, a large European sleep-clinic database, oxygen desaturation index but not AHI was independently associated with HT.7 Although there is evidence for an independent dose-response correlation of moderate-to-severe SA with HT,11 the impact of mild SA on BP remains uncertain. This was addressed in a recent publication from ESADA, in which mild SA, compared to nonapneic snorers, was associated with a significantly higher HT rate after adjustment for confounding variables.6 The question remains: How much SA is needed to increase the risk for high(er) BP? Discrepancies in published reports may be related to differences in study population (age, BMI, men/women ratio, life-style habits, associated cardiovascular risk profile), small sample size, underrepresentation of women, variability of diagnostic methods (eg, 3% or 4% desaturation criterion for hypopneas), and differences in AHI categorization or cutoff points.
Although most reports were not stratified by sex, and if so, results from female participants were often underreported, several investigators explored sex-related differences. Some found no differences between men and women, whereas others report an increased propensity for HT either in men or in women. Several authors have stated that women with SA may have greater risk for HT and comorbid SA-related conditions than men.16–18
Several biological, clinical, and pathophysiological aspects of SA are influenced by sex. First, female sex hormones may have favorable impact. It has been shown that SA prevalence is much lower in premenopausal women, whereas it rises considerably after menopause, irrespective of BMI.30,31 Second, sex-related differences in sleep-related breathing can be explained by differences in anatomical and functional features, such as fat distribution and body composition, upper airway structure and collapsibility, and hypoxic ventilatory response.31,32 Third, an equal AHI does not necessarily implicate the same exposure to intermittent hypoxia in men and women. Indeed, sex differences in the characteristics of respiratory events have been described. REM-related SA is more common in women, and there are differences in event duration and severity of desaturation.13,14 Fourth, in clinic-based samples women tend to report different symptoms than men (with more insomnia, mood symptoms, and fatigue).32 Women are less likely to seek medical attention for concerns about SA, as SA is generally regarded as a condition affecting men. As a consequence, women are less likely to be diagnosed with and treated for SA.33 This diagnostic delay may result in a longer exposure before treatment is initiated. Finally, sex has differential effects on the interaction of SA with its comorbidities, as demonstrated by a large database in which more than 1,700,000 obstructive sleep apnea patients were compared to matched controls.12 Ischemic heart disease was more strongly associated with SA in men, whereas HT and depression were more prominent SA associations in women.
No unequivocal explanation for the cubic-shaped relationship between REI and BP in women was found. More extensive adjustments (including BMI, rather than obesity, estimated glomerular filtration rate, smoking, educational achievement, physical activity, smoking, alcohol intake, diabetes) did not alter the cubic correlation. Several hypotheses can be put forward for crossed effects resulting in a cubic model in women, such as nonlinear BMI or age gradients between REI groups, differences in compliance or susceptibility for therapeutic interventions with higher BMI or REI, selection bias, or even hormonal effects correlated with menopause.
Our data suggest that the REI threshold associated with increasing BP and prevalence of hypertension seems to be situated at a lower cutoff point in women compared to men. These results need to be interpreted with regard to the substantially lower baseline systolic and diastolic BP in women in the population. This might explain why, in previous reports on the relationship of SA and HT in the general population, there was often an association in men but not in women. Indeed, in women the absolute SBP and DBP are more likely to be situated below the threshold for “hypertension.” This again highlights the importance of a sex-specific approach in the analysis and the interpretation of clinical studies, especially in the field of SA.
Certain limitations need to be considered. First, the cross-sectional nature of our data does not allow evaluation of causality between the associations we found. Second, although a careful, bilateral triplicate automated office BP measurement was used (6 measurements each with 1-minute interval) during the study visit, a 24-hour or ambulatory home BP registration would have been preferable. However, office BP measurements have been used and validated in many studies. Data on the BP dipping pattern during nighttime would have been interesting though, since nocturnal nondipping BP can be an early marker for HT in obstructive sleep apnea.34,35 The HT definition we applied included drug-treated or physician-diagnosed HT, or increased office BP at study visit. The latter could potentially result in diagnostic inaccuracies in the classification of HT. We approached this by repeating the analyses with a more limited definition of HT without the measured office BP values. Since these results and estimates were very similar compared to the analyses of the more inclusive HT classification, we believe that imprecision was limited. Third, we used a 4-channel level 3 ambulatory PG without electroencephalogram. The REI obtained from PG is usually underestimated compared to the AHI obtained by polysomnography.36 However, the AHI, including arousals in its definition, is a more complex and variable metric, whereas the REI is more strongly related to the oxygen desaturation index, a better predictor for HT.5,7,37 In our study, the REI was highly correlated with the 4% oxygen desaturation index (Spearman correlation coefficient 0.939). Data from ESADA have shown that the type of sleep study did not affect the predictive value of the AHI on HT prevalence.7 Fourth, although our cohort is a community-based cohort study, a selection bias excluding already treated SA patients is possible. However, our study data do not indicate such a bias. Of all volunteers with newly diagnosed moderate-to-severe SA (REI ≥ 15 events/h), the majority (73%) and an equal proportion of men and women, had “clinically significant” SA (defined as having either sleepiness or HT). Fifth, no data on menopausal status of the women were available. Furthermore, as in other general population cohorts, we did not exclude participants with central apneas. The occurrence of central apneas could potentially cause limited imprecision. However, central apneas were very rare (99.5% had a central apnea index below 15 events/h). Moreover, in the absence of electroencephalogram registration central apneas are more likely to be “over-scored” since they often occur during wakefulness. Although the vast majority of apneas were obstructive, we felt it was more accurate to use the term “sleep apnea” instead of “obstructive sleep apnea.” Finally, the lack of racial diversity in our cohort limits the generalizability of the findings. The major strengths of our study were the large sample size, the random sample of men and women from the general population minimizing selection and referral bias, the equal sex distribution, and the well-powered assessment of sex differences. Data from a community-based population with a high proportion of participants having low REIs allow us to understand the impact of mild SA, in contrast to clinical populations in which the distribution of SA is situated in a higher degree of disease severity. Even though our sample was relatively large, we do not exclude that the age- and obesity-adjusted associations might become statistically significant for SBP in very large study populations.
CONCLUSIONS
Significant increases in BP and hypertension prevalence in the general population were present at distinctly lower REI values in women compared to men. Even a very low exposure to respiratory events during sleep (REI values within the normal range of < 5 events/h) was associated with a higher BP. Adjusting for obesity attenuated the associations, especially in men. There are major challenges to a better understanding of the sex differences in the clinical picture of SA and their health consequences. Upcoming research should ideally be performed in a sex-stratified manner. Addressing this research gap and achieving a better understanding of sex differences could allow a move toward more precise and personalized management of women and men with SA, and potentially improve decision-making from a public health perspective.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Work was performed at Ghent University Hospital and Ghent University, Belgium. F.A.B. received grant support from the Ghent University Hospital Clinical Research Fund (KOF). The Asklepios Study is supported by the Fund for Scientific Research Flanders (FWO research grants G042703 and G083810N). The funding sources did not have any involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. E.R.R. and J.A.C. have received research support (equipment loans) from ResMed (to Ghent University, Asklepios study). F.A.B reports travel grants from Vivisol and Vitalaire/AirLiquide, outside the submitted work. K.B.H. reports travel grants from Vivisol and Vitalaire/AirLiquide, outside the submitted work. J.A.C. has recently consulted for Bayer, Sanifit, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, and the Galway-Mayo Institute of Technology. He is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. He has received payments for editorial roles from the American Heart Association and the American College of Cardiology. J.A.C. is supported by National Institutes of Health grants R01-HL 121510, R33-HL-146390, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874, and R56-HL136730. E.R.R. has received unrestricted educational grants from Amgen, MSD, AstraZeneca, Sanofi, and Unilever and speakers’ fees from Novo Nordisk, Boehringer Ingelheim, Amgen, Sanofi-Aventis, Novartis, and Teva. These grants were paid to Ghent University and are outside the submitted work. D.P. and M.D.B. report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank the study participants who voluntarily participated in the Asklepios study, the general practitioners of Erpe-Mere and Nieuwerkerken, and Femke Van Hoeke for general secretarial assistance.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- DBP
diastolic blood pressure
- ESADA
European Sleep Apnea Database
- HP
home polygraphy
- HT
hypertension
- PG
polygraphy
- REI
respiratory event index
- SA
sleep apnea
- SBP
systolic blood pressure
REFERENCES
- 1.Bauters F, Rietzschel ER, Hertegonne KBC, Chirinos JA. The link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep. 2016;18(1):1–11. 10.1007/s11883-015-0556-z [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. 10.1001/archinte.1997.00440360178019 [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 4.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3):685–689. 10.1164/ajrccm.163.3.2005065 [DOI] [PubMed] [Google Scholar]
- 5.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. 10.1136/bmj.320.7233.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouloukaki I, Grote L, McNicholas WT, et al. European Sleep Apnoea Database Network . Mild obstructive sleep apnea increases hypertension risk, challenging traditional severity classification. J Clin Sleep Med. 2020;16(6):889–898. 10.5664/jcsm.8354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tkacova R, McNicholas WT, Javorsky M, et al. European Sleep Apnoea Database Study Collaborators . Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J. 2014;44(4):931–941. 10.1183/09031936.00225113 [DOI] [PubMed] [Google Scholar]
- 8.Mohsenin V, Yaggi HK, Shah N, Dziura J. The effect of gender on the prevalence of hypertension in obstructive sleep apnea. Sleep Med. 2009;10(7):759–762. 10.1016/j.sleep.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 9.Chowdhuri S, Quan SF, Almeida F, et al. ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea . An official American Thoracic Society Research Statement: impact of mild obstructive sleep apnea in adults. Am J Respir Crit Care Med. 2016;193(9):e37–e54. 10.1164/rccm.201602-0361ST [DOI] [PubMed] [Google Scholar]
- 10.McNicholas WT, Bonsignore MR, Lévy P, Ryan S. Mild obstructive sleep apnoea: clinical relevance and approaches to management. Lancet Respir Med. 2016;4(10):826–834. 10.1016/S2213-2600(16)30146-1 [DOI] [PubMed] [Google Scholar]
- 11.Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010405. 10.7189/jogh.08.010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47(4):1162–1169. 10.1183/13993003.01618-2015 [DOI] [PubMed] [Google Scholar]
- 13.Kulkas A, Duce B, Leppänen T, Hukins C, Töyräs J. Gender differences in severity of desaturation events following hypopnea and obstructive apnea events in adults during sleep. Physiol Meas. 2017;38(8):1490–1502. 10.1088/1361-6579/aa7b6f [DOI] [PubMed] [Google Scholar]
- 14.Mano M, Hoshino T, Sasanabe R, et al. Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health. 2019;16(6):1068. 10.3390/ijerph16061068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appleton S, Gill T, Taylor A, et al. Influence of gender on associations of obstructive sleep apnea symptoms with chronic conditions and quality of life. Int J Environ Res Public Health. 2018;15(5):93. 10.3390/ijerph15050930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27(6):1113–112. [DOI] [PubMed] [Google Scholar]
- 17.Won C, Guilleminault C. Gender differences in sleep disordered breathing: implications for therapy. Expert Rev Respir Med. 2015;9(2):221–231. 10.1586/17476348.2015.1019478 [DOI] [PubMed] [Google Scholar]
- 18.Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the atherosclerosis risk in communities-sleep heart health study. Circulation. 2015;132(14):1329–1337. 10.1161/CIRCULATIONAHA.115.016985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rietzschel E-R, De Buyzere ML, Bekaert S, et al. Asklepios Investigators . Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil. 2007;14(2):179–191. 10.1097/HJR.0b013e328012c380 [DOI] [PubMed] [Google Scholar]
- 20.Bauters FA, Hertegonne KB, De Buyzere ML, Joos GF, Chirinos JA, Rietzschel ER. Phenotype and risk burden of sleep apnea: a population-based cohort study. Hypertension. 2019;74(4):1052–1062. 10.1161/HYPERTENSIONAHA.119.13452 [DOI] [PubMed] [Google Scholar]
- 21.Bauters FA, Loof S, Hertegonne KB, Chirinos JA, De Buyzere ML, Rietzschel ER. Sex-specific sleep apnea screening questionnaires: closing the performance gap in women. Sleep Med. 2020;67:91–98. 10.1016/j.sleep.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 22.WHO Collaborating Center for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment. https://www.whocc.no/atc/structure_and_principles/. Accessed February 26, 2018. [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 25.Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J. 2006;27(3):564–57. 10.1183/09031936.06.00042105 [DOI] [PubMed] [Google Scholar]
- 26.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 27.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–1164. 10.1164/rccm.200712-1809OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cano-Pumarega I, Durán-Cantolla J, Aizpuru F, et al. Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med. 2011;184(11):1299–1304. 10.1164/rccm.201101-0130OC [DOI] [PubMed] [Google Scholar]
- 29.Cano-Pumarega I, Barbé F, Esteban A, Martínez-Alonso M, Egea C, Durán-Cantolla J; Spanish Sleep Network(∗) . Sleep apnea and hypertension: are there sex differences? The Vitoria Sleep Cohort. Chest. 2017;152(4):742–75. 10.1016/j.chest.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 30.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. 10.1164/rccm.200209-1055OC [DOI] [PubMed] [Google Scholar]
- 31.Huang T, Lin BM, Markt SC, et al. Sex differences in the associations of obstructive sleep apnoea with epidemiological factors. Eur Respir J. 2018;51(3):1702421. 10.1183/13993003.02421-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han MK, Arteaga-Solis E, Blenis J, et al. Female sex and gender in lung/sleep health and disease. Increased understanding of basic biological, pathophysiological, and behavioral mechanisms leading to better health for female patients with lung disease. Am J Respir Crit Care Med. 2018;198(7):850–858. 10.1164/rccm.201801-0168WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindberg E, Benediktsdottir B, Franklin KA, et al. Women with symptoms of sleep-disordered breathing are less likely to be diagnosed and treated for sleep apnea than men. Sleep Med. 2017;35:17–22. 10.1016/j.sleep.2017.02.032 [DOI] [PubMed] [Google Scholar]
- 34.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–80. 10.1093/sleep/31.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenner R, Fatureto-Borges F, Costa-Hong V, et al. Association of obstructive sleep apnea with arterial stiffness and nondipping blood pressure in patients with hypertension. J Clin Hypertens (Greenwich). 2017;19(9):910–918. 10.1111/jch.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escourrou P, Grote L, Penzel T, et al. ESADA Study Group . The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res. 2015;24(6):730–738. 10.1111/jsr.12318 [DOI] [PubMed] [Google Scholar]
- 37.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177(10):1150–1155. 10.1164/rccm.200712-1884OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.