Abstract
Background:
Patients with anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) are frequently accompanied by rapidly progressive-interstitial lung disease (RP-ILD). They are often refractory to intensive immunosuppressive therapy and have poor prognosis.
Case presentation:
A 73-year-old woman presented with fever, cold symptoms, and skin eruption for a month. She also exhibited muscle weakness on upper extremities slightly. The titer of anti-MDA5 antibody was extremely high, and computed tomography showed ground glass opacity and reticular shadows in the lungs. She was diagnosed as anti-MDA5 antibody-positive classical DM-associated RP-ILD and treated with intensive immunosuppressive therapy. However, the titer of anti-MDA5 antibody did not decrease satisfactorily, and plasma exchange was alternatively initiated. The titer decreased remarkably, and she obtained disease remission. Similarly, a 63-year-old woman presented with stiffness of the neck and hands, fever and cough. She was also diagnosed as anti-MDA5 antibody-positive classical DM-associated RP-ILD, because she had skin eruptions, slight muscle weakness, an elevation in anti-MDA5 antibody, and RP-ILD. She was unresponsive to intensive immunosuppressive therapy, but plasma exchange successfully improved the titer of anti-MDA5 antibody, the symptoms, and the findings of computed tomography.
Conclusions:
Although anti-MDA5 antibody-positive DM-associated RP-ILD has a high mortality rate, this report suggests the usefulness of plasma exchange to improve the prognosis.
Keywords: Anti-MDA5 antibody, dermatomyositis, plasma exchange, case report
Introduction
Anti-melanoma differentiation-associated gene 5 (MDA5) antibody is associated with dermatomyositis (DM). It was first reported in 2005 in cases of clinically amyopathic DM, but it has also been detectable in classical DM.1,2 Patients with anti-MDA5 antibody-positive DM are frequently accompanied by rapidly progressive-interstitial lung disease (RP-ILD), and they are often refractory to intensive immunosuppressive therapy. 2 The prognosis of the patients is extremely poor, and the results of previous studies have shown that 20% to 30% of patients with anti-MDA5 antibody-positive DM-associated RP-ILD do not survive even after an early diagnosis or intensive immunosuppressive therapy.2,3
Plasma exchange (PE) can remove pathological antibodies, immune complexes, and elevated inflammatory cytokines from the blood and has been shown to be effective for some autoimmune diseases.4,5 Several case reports have described successful PE treatment for the patients with anti-MDA5 antibody-positive DM-associated RP-ILD in recent years.3,6-11 However, as yet, standard protocols for the initiation/termination timing and the frequency of PE have not been established for these patients. Here, we present 2 cases of anti-MDA5 antibody-positive DM-associated RP-ILD for which PE was successfully executed. We also discuss the following points: (1) which patients should receive PE; (2) when PE should be started; and (3) how long PE should be continued for the patients with anti-MDA5 antibody-positive DM-associated RP-ILD.
Case Presentation
Case 1
A 73-year-old woman was admitted to our hospital in September 2019, because she had fever, cold symptoms, and skin eruption on hands, arms, and thighs for a month. Her medical history was unremarkable except for an operation for an ovarian cyst at the age of 48. She did not take any medications. Physical examination revealed a high fever of 39.1°C and a low SpO2 of 94% at room air. She had Gottron’s sign, inverse Gottron’s sign, Mechanic’s hands, and erythema around the fingernails and on the anterior chest. Fine crackles were audible in the lung fields. Muscle weakness on upper extremities was slightly detectable: the scores of manual muscle testing were 4 for deltoid, biceps, and triceps bilaterally. Laboratory findings on admission are shown in Table 1. Of these, the following data were notable: anti-MDA5 antibody 2610 index (normal range: <32 index); C-reactive protein (CRP) 2.48 mg/dL; and ferritin 208 ng/mL. Chest computed tomography (CT) exhibited ground glass opacity and reticular shadows on the peripheral and basilar regions of both lungs (Figure 1A). According to the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for idiopathic inflammatory myopathies (IIM), 12 she was classified as definite IIM (11.2 point without muscle biopsy), and she was diagnosed as anti-MDA5 antibody-positive classical DM-associated RP-ILD. Her respiratory status was poor, and alveolar-arterial oxygen difference (AaDO2) was 39.35 mmHg. Oxygen administration was started, and she was treated with methylprednisolone pulse therapy (1000 mg/day × 3 days, 3 times, and 500 mg/day × 3 days, once), prednisolone (starting from 50 mg/day), tacrolimus (1.5-4 mg/day), cyclophosphamide (1000 mg/day, 3 times), and rituximab (480 mg/day, 2 times). However, the titer of anti-MDA5 antibody did not decrease satisfactorily. Her symptoms did not improve, and AaDO2 level elevated to 75.54 mmHg. In addition, she developed pseudomonas aeruginosa pneumonia which might be due to immunosuppression (Figure 1B), and thus immunosuppressive drugs other than prednisolone were discontinued (Figure 2). Alternatively, PE 3 times a week was initiated in the middle of October by using 1920 mL of fresh frozen plasma (FFP) as the replacement fluid. The titer of anti-MDA5 antibody decreased remarkably from 1780 index to 560 index by 2 PE sessions and to 87 index by 5 PE sessions. KL-6 and AaDO2 also decreased, and RP-ILD, as assessed by chest CT, improved (Figure 1C). Oxygen administration was terminated at the end of November. The frequency of PE was tapered gradually, as shown in Figure 2. There was no increase in the titer of anti-MDA5 antibody, and the patient was finally discharged in April 2020.
Table 1.
Laboratory findings on admission.
| Unit | Case 1 | Case 2 | |
|---|---|---|---|
| White blood cells | /µL | 7200 | 5000 |
| Red blood cells | ×104/µL | 356 | 396 |
| Hemoglobin | g/dL | 9.4 | 11.4 |
| Platelets | ×104/µL | 40.4 | 29.6 |
| Total protein | g/dL | 6.7 | 6.9 |
| Albumin | g/dL | 2.8 | 2.9 |
| Urea nitrogen | mg/dL | 9.2 | 10.0 |
| Creatinine | mg/dL | 0.53 | 0.63 |
| Sodium | mEq/L | 139 | 138 |
| Potassium | mEq/L | 3.5 | 3.9 |
| Chloride | mEq/L | 100 | 100 |
| Calcium | mg/dL | 8.1 | 8.5 |
| Phosphorus | mg/dL | 2.8 | 4.1 |
| Aspartate aminotransferase | IU/L | 52 | 149 |
| Alanine aminotransferase | IU/L | 13 | 81 |
| Lactate dehydrogenase | IU/L | 429 | 429 |
| Creatine kinase | IU/L | 113 | 720 |
| Antinuclear antibody | <40 | <40 | |
| IgG | mg/dL | 1551 | 1869 |
| IgA | mg/dL | 453 | 228 |
| IgM | mg/dL | 53 | 193 |
| C-reactive protein | mg/dL | 2.48 | 0.37 |
| Ferritin | ng/mL | 208 | 549 |
| KL-6 | U/mL | 472 | 418 |
| SP-D | ng/mL | 32 | <17 |
| Anti-MDA5 antibody | Index | 2610 | 5530 |
The titers of anti-MDA5 antibody were determined by MESACUP enzyme-linked immunosorbent assay kits (MBL, Nagoya, Japan).
Figure 1.
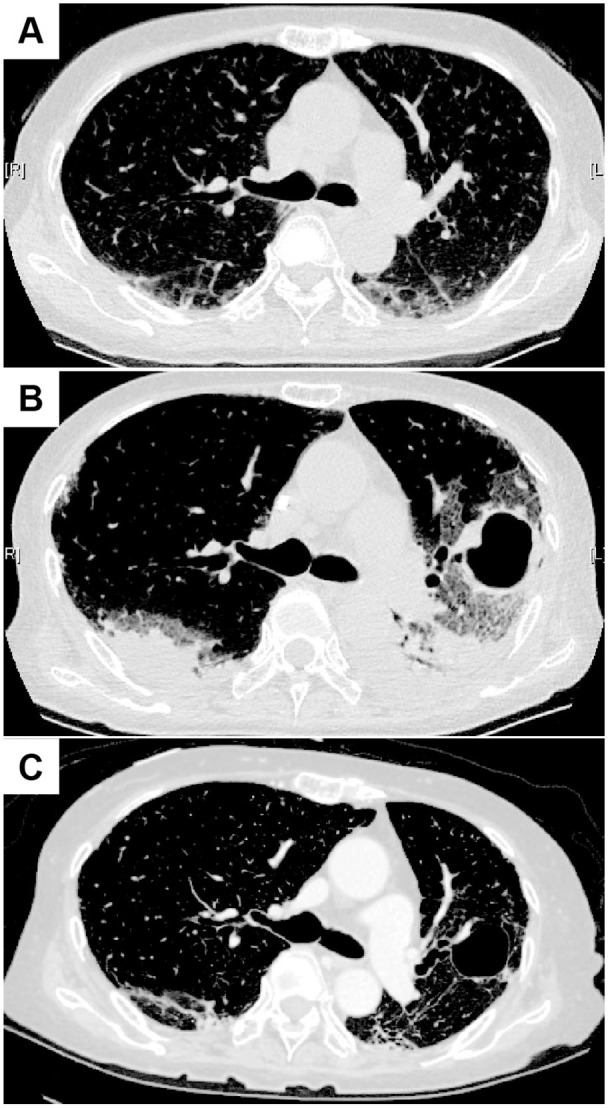
Chest CT images in Case 1. (A) At the admission. Ground glass opacity and reticular shadows on the peripheral and basilar regions of both lungs were seen. (B) Immediately after the start of the first PE session. Invasive shadows on the dorsal side of the both lungs were seen. In addition, a large cavity with wall thickening was seen in the left lung. (C) After the termination of PE sessions. The ground glass opacity on both lungs still remained, but improved.
Figure 2.
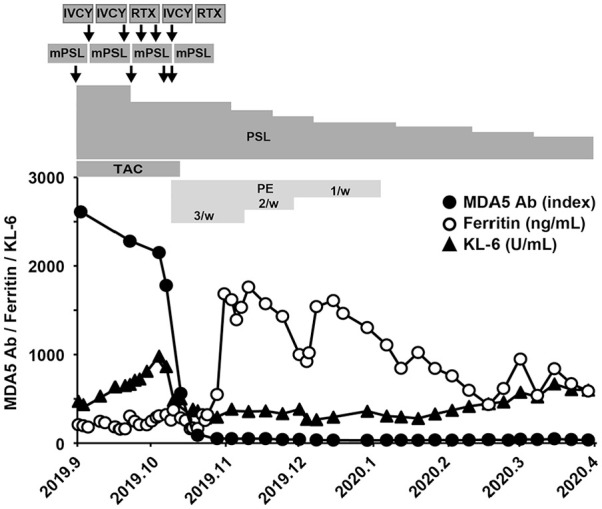
Clinical course of the patient with anti-MDA5 antibody-positive DM-associated RP-ILD (Case 1). The titer of anti-MDA5 antibody (black circle), serum ferritin level (white circle), and serum KL-6 level (black triangle) are shown. The patient was treated with methylprednisolone pulse therapy (mPSL), prednisolone (PSL), tacrolimus (TAC), intravenous administration of cyclophosphamide (IVCY), and rituximab (RTX). Plasma exchange (PE) 3 times a week was initiated in the middle of October, and the frequency of PE was tapered gradually.
Case 2
A 63-year-old woman presented with stiffness of the neck and hands, fever and cough in early October 2019. She didn’t have any remarkable medical history and did not take any medication. In November, she visited a hospital and was found to have Gottron’s sign, inverse Gottron’s sign, heliotrope rash, and shawl sign. Muscle weakness in upper and lower extremities were detectable bilaterally by manual muscle testing. The blood test showed high creatine kinase levels, and chest CT exhibited a slight interstitial shadow. Classical DM was suspected, and she was transferred to our hospital. Physical examination on admission revealed body temperature at 37.2°C and SpO2 of 95% at room air. Respiratory sounds were clear. Laboratory findings on admission are shown in Table 1. Anti-MDA5 antibody was 5530 index, and ferritin was high at 540 ng/mL. AaDO2 was 31.33 mmHg. Chest CT on admission showed the deterioration of ILD (Figure 3A) compared to the findings a week earlier. She was classified as definite IIM (7.6 point without muscle biopsy) according to the 2017 EULAR/ACR classification criteria for IIM, 12 and was diagnosed as anti-MDA5 antibody-positive classical DM-associated RP-ILD. Immediately, she was treated with methylprednisolone pulse therapy (1000 mg/day × 3 days, 3 times), prednisolone (starting from 50 mg/day), tacrolimus (4-7 mg/day), cyclophosphamide (1200 mg/day, 3 times), and rituximab (500 mg/day, 3 times). Serum ferritin levels decreased from 2533 to 922 ng/mL, and the titer of anti-MDA5 antibody decreased to 2140 index. However, chest CT showed the exacerbation of RP-ILD (Figure 3B). Therefore, PE 3 times a week was initiated in early February 2020 by using 1920 mL of FFP as the replacement fluid (Figure 4). PE was temporarily performed 4 times a week, because RP-ILD worsened just after the start of PE. The titer of anti-MDA5 antibody rapidly decreased to 19 index after 9 sessions. The CT findings improved (Figure 3C), and the frequency of PE was gradually tapered. Her AaDO2 level remarkably decreased to 3.98 mmHg, and PE sessions were terminated. Subsequently, she developed pulmonary aspergillosis in April, but it was improved by the treatment with voriconazole. After the completion of PE, the titer of anti-MDA5 antibody, the symptoms, and the CT findings remained stable. She was discharged in June 2020.
Figure 3.
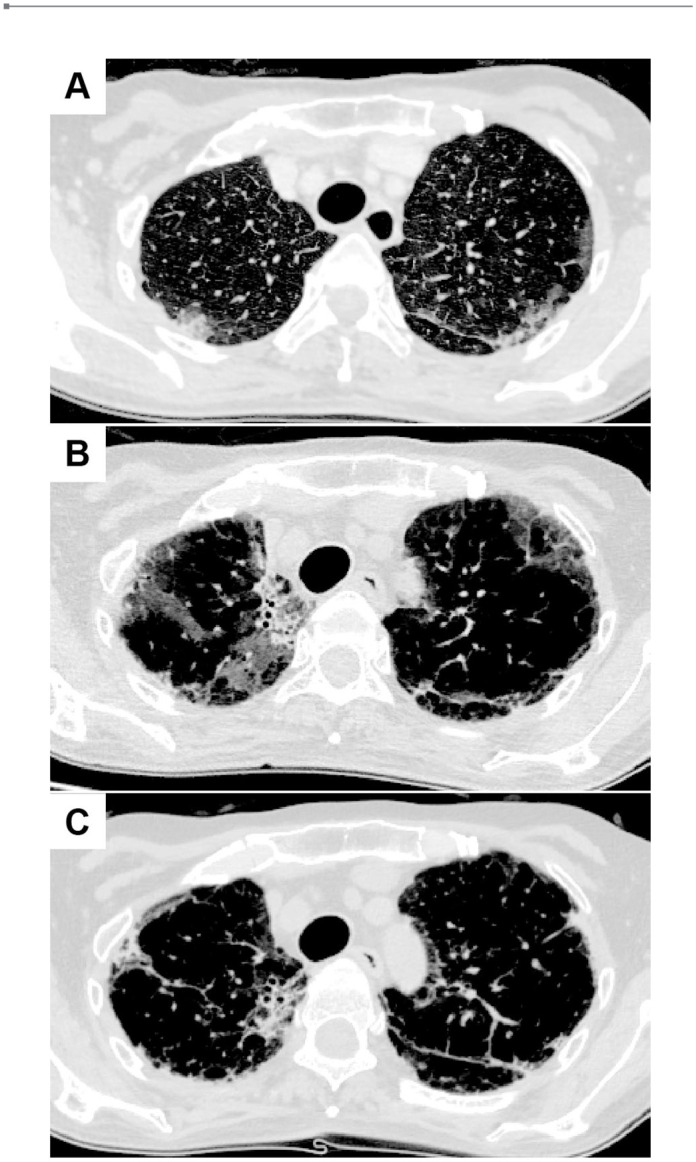
Chest CT images in Case 2. (A) At the admission. Ground glass opacity and reticular shadows on both lungs were seen. (B) Before starting the first PE session. The abnormal shadows were deteriorated. (C) After the termination of PE sessions. The ground glass opacity and reticular shadows still remained, but improved.
Figure 4.
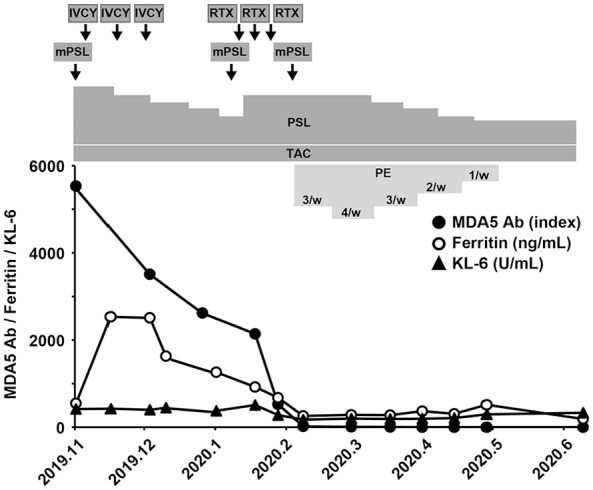
Clinical course of the patient with anti-MDA5 antibody-positive DM-associated RP-ILD (Case 2). The titer of anti-MDA5 antibody (black circle), serum ferritin level (white circle), and serum KL-6 level (black triangle) are shown. The patient was treated with methylprednisolone pulse therapy (mPSL), prednisolone (PSL), tacrolimus (TAC), intravenous administration of cyclophosphamide (IVCY), and rituximab (RTX). Plasma exchange (PE) 3 times a week was initiated in early February, and a total of 31 PE sessions were performed.
Discussion
We experienced 2 cases of anti-MDA5 antibody-positive DM-associated RP-ILD, for which PE was successfully performed. Both patients obtained disease remission following PE.
Treatment of anti-MDA5 antibody-positive DM-associated RP-ILD begins with 2-drug combination therapy with glucocorticoids and calcineurin inhibitors (cyclosporine or tacrolimus), or 3-drug combination therapy with glucocorticoids, calcineurin inhibitors, and cyclophosphamide. 2 If the patient is refractory to these drugs, additional immunosuppressive agents, such as rituximab, are used. 2 Both patients in the present report were initially treated with the triple therapy of glucocorticoids, tacrolimus, and cyclophosphamide, but since they were refractory to the triple therapy, rituximab was also used. In Case 1, 3-drug combination therapy as well as rituximab therapy did not decrease the titer of anti-MDA5 antibody sufficiently, and the patient continued to require oxygen administration. The strong immunosuppressive agents caused pseudomonas aeruginosa pneumonia, and further immunosuppression was thought to be difficult. Therefore, PE was initiated after 5 weeks of treatment with 3-drug combination therapy, and a total of 24 PE sessions were performed during 12 weeks. In Case 2, 3-drug combination therapy followed by rituximab treatment reduced the titer of anti-MDA5 antibody from 5530 to 2140 index, but the CT findings worsened. PE was introduced 12 weeks after the start of the treatment and was performed 31 times during 11 weeks.
PE has been recommended for the patients who are refractory to the immunosuppressive therapy, and several cases have been reported to respond to PE successfully.3,6-11 Moreover, the results of previous studies showed that PE significantly provided the higher survival rate for the patients who were refractory to immunosuppressive therapy.3,10 Abe et al 3 showed that in patients refractory to immunosuppressive drugs, the 1-year survival rate of the PE group was significantly higher than that of the non-PE group (100% and 25%, respectively, P = .033). Shirakashi et al 10 showed that in patients refractory to combined immunosuppressive treatment, the 3-year survival rate in the PE group was significantly higher than that in the non-PE group (P = .001). PE appears to be effective for the patients refractory to immunosuppressive therapy. However, there is a report showing that a patient with anti-MDA5 antibody-positive RP-ILD underwent PE using FFP as a replacement fluid, developed transfusion-related acute lung injury (TRALI), and died within 4 hours. 13 At present, although it is increasingly suggested that PE is effective, a consensus has not yet been established as to: (1) which patients should receive PE; (2) when PE should be started; and (3) how long PE should be continued for the patients with anti-MDA5 antibody-positive DM-associated RP-ILD. We would like to discuss these points as follows.
The first question is which patients should be treated with PE. Regarding this question, we need to consider the prognostic factors of anti-MDA5 antibody-positive DM-associated RP-ILD. High ferritin levels and severe respiratory impairment have been reported as prognostic factors for the patients with anti-MDA5 antibody-positive DM-associated RP-ILD.14,15 Gono et al 14 reported a significant decrease in the 5-year survival rate when ferritin was greater than 500 ng/mL compared to less than 500 ng/mL in patients with anti-MDA5 antibody-positive DM-associated RP-ILD. Fujiki et al 15 showed that an initial serum ferritin level of ⩾450 ng/mL and AaDO2 of ⩾30 mmHg were poor prognostic factors for these patients. In Case 1 in the present report, serum ferritin level was less than 450 ng/mL (208 ng/mL), but the patient had oxygen demand. On the contrary, there was no oxygen demand, but serum ferritin level at the peak was extremely high (2533 ng/mL) in Case 2. PE was required for both cases. It is possible that high ferritin levels and oxygen demand can be factors to determine whether PE is needed for patients with anti-MDA5 antibody-positive DM-associated RP-ILD. In addition to these factors, the age, titer of anti-MDA5 antibody, C-reactive protein levels, and KL-6 levels have also been reported to be possible factors to predict the prognosis in patients with anti-MDA5 antibody-positive DM-associated RP-ILD.16,17 By using these variables, the starting criteria of PE may be defined.
The next question is when to start PE. According to the previous report, 11 the duration from the diagnosis to the start of PE was 11.5 days, which was much shorter than the present cases (5 weeks for Case 1 and 12 weeks for Case 2). In addition, in patients with anti-MDA5 antibody-positive DM-associated RP-ILD, early initiation of treatment has been reported to be one of the prognostic factors. 11 Moreover, it takes about 2 weeks for cyclophosphamide, the key drug in the 3-drug combination therapy, to exert its effect. Since PE reduces the titer of autoantibodies immediately, the early initiation of PE may be a useful option until the effect of cyclophosphamide is achieved. We may consider starting PE earlier in some cases.
The third question is how long PE should be continued for the patients with anti-MDA5 antibody-positive DM-associated RP-ILD. Regarding the total number of PE sessions, the average number of PE was 9.5 to 11.4 sessions in the previous reports,10,11 which was much less than the present cases (24 sessions for Case 1 and 31 sessions for Case 2). In clinical practice, once PE is started, it is often difficult to decide the timing of the termination of PE treatment due to the high mortality rate of the disease. In the present cases, although a lot of PE sessions were performed, the patients achieved disease remission without reactivation of the disease. The number and frequency of PE require further data accumulation.
Although we described that PE successfully improved the prognosis of the 2 patients with anti-MDA5 antibody-positive DM-associated RP-ILD, we should be aware that both patients received rituximab treatment. It is known that rituximab targets CD20-positive cells (ie, B-cell precursors), leading to the depletion of B cells in the blood within several weeks of administration in most patients. 18 It is possible that the decrease in the titer of anti-MDA5 antibody in the present cases was caused in part by the delayed effect of rituximab. However, infections are common adverse events of rituximab and results of previous studies showed that only 50% of patients responded to rituximab even 20 weeks after use. 18 Therefore, even with the use of rituximab, PE should be considered for live-saving treatment in cases, such as Case 1, where continuous use of rituximab was difficult due to the infection and Case 2, where the use of rituximab did not improve the lung lesions.
Conclusions
In conclusion, we experienced 2 cases of anti-MDA5 antibody-positive DM-associated RP-ILD for which PE was successfully performed. Because the disease still has a high mortality rate, it is hoped that the data of the patients are accumulated, the mechanisms of the disease are clarified, and the standard therapy, including the timing and the frequency of PE, is established.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RT and TY wrote the manuscript. KM, YKo, JK, SS, SI, YKa, TT, HI, and MO contributed to the interpretation of patients’ data and critical revision of the manuscript. All authors read and approved the final manuscript.
Consent for Publication: Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
ORCID iD: Tadashi Yoshida  https://orcid.org/0000-0003-1626-7773
https://orcid.org/0000-0003-1626-7773
References
- 1. Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576. [DOI] [PubMed] [Google Scholar]
- 2. Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, et al. Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum. 2020;50:776-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abe Y, Kusaoi M, Tada K, Yamaji K, Tamura N. Successful treatment of anti-MDA5 antibody-positive refractory interstitial lung disease with plasma exchange therapy. Rheumatology (Oxford). 2020;59:767-771. [DOI] [PubMed] [Google Scholar]
- 4. Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164:342-351. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida T, Minakuchi H, Takahashi R, Morita S, Oya M. Safety and efficacy of plasma exchange via direct femoral vein puncture in autoimmune blistering diseases. J Clin Apher. 2020;35:172-177. [DOI] [PubMed] [Google Scholar]
- 6. Endo Y, Koga T, Suzuki T, et al. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis: a case report. Medicine (Baltimore). 2018;97:e0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silveira MG, Selva-O’Callaghan A, Ramos-Terrades N, Arredondo-Agudelo KV, Labrador-Horrillo M, Bravo-Masgoret C. Anti-MDA5 dermatomyositis and progressive interstitial pneumonia. QJM. 2016;109:49-50. [DOI] [PubMed] [Google Scholar]
- 8. Sasaki N, Ishii A, Kurabayashi T, et al. Early initiation of plasma exchange therapy for a patient with anti-MDA5 autoantibody-positive dermatomyositis developing rapidly progressive interstitial lung disease. Mod Rheumatol Case Rep. 2021;5:87-94. [DOI] [PubMed] [Google Scholar]
- 9. Yamagata A, Arita M, Tanaka A, et al. Therapeutic plasma exchange for clinically amyopathic dermatomyositis (CADM) associated with rapidly progressive interstitial pneumonia. J Clin Apher. 2020;35:435-443. [DOI] [PubMed] [Google Scholar]
- 10. Shirakashi M, Nakashima R, Tsuji H, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology (Oxford). 2020;59:3284-3292. [DOI] [PubMed] [Google Scholar]
- 11. Saito T, Mizobuchi M, Miwa Y, et al. Anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease treated with therapeutic plasma exchange: a case series. J Clin Apher. 2021;36:196-205. [DOI] [PubMed] [Google Scholar]
- 12. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76:1955-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kagawa H, Tsujino K, Yamamoto Y, et al. Acute lung injury after plasma exchange in a patient with anti-MDA5 antibody-positive, rapidly progressive, interstitial lung disease: a case report. Respir Med Case Rep. 2020;29:101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49:1713-1719. [DOI] [PubMed] [Google Scholar]
- 15. Fujiki Y, Kotani T, Isoda K, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. 2018;28:133-140. [DOI] [PubMed] [Google Scholar]
- 16. Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford). 2018;57:1212-1221. [DOI] [PubMed] [Google Scholar]
- 17. Gono T, Masui K, Nishina N, et al. Risk prediction modeling based on a combination of initial serum biomarker levels in polymyositis/dermatomyositis-associated interstitial lung disease. Arthritis Rheumatol. 2021;73:677-686. [DOI] [PubMed] [Google Scholar]
- 18. Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]


