Abstract
Background:
Cardiovascular (CVS) diseases are the leading cause of death worldwide and patients with rheumatic diseases have an increased CVS. CVS risk factors and CVS events are common in spondyloarthritis (SpA). Delineating the CVS risk in patients with SpA and identifying modifiable risk factors would be useful.
Methods:
Patients with SpA and patients with non-specific back pain (NSBP) were identified in rheumatology and orthopedics clinics, respectively. Clinical information and CVS events were retrieved. Baseline characteristics and incidence rates of CVS events were compared between two groups of patients using an age- and sex-matched cohort. Propensity score adjustment and Cox regression analysis were performed to determine the CVS risk associated with SpA.
Results:
A total of 5046 patients (SpA 2616 and NSBP 2430) were included from eight centers. Over 56,484 person-years of follow up, 160 strokes, 84 myocardial infarction (MI) and 262 major adverse cardiovascular events (MACE) were identified. Hypercholesterolemia was more prevalent in SpA (SpA 34.2%, NSBP 28.7%, p < 0.01). Crude incidence rates of MACE and stroke were higher in SpA patients. SpA was associated with a higher risk of MACE [hazard ratio (HR) 1.70; 95% confidence interval (CI) 1.29–2.26; p < 0.01] and cerebrovascular events (HR 1.50; 95% CI 1.08–2.07; p = 0.02). SpA patients with anti-TNF use had a reduced risk of MACE (HR 0.37, 95%CI 0.17–0.80, p = 0.01) and cerebrovascular events (HR 0.21, 95%CI 0.06–0.78, p = 0.02) compared with SpA patients without anti-TNF use.
Conclusion:
SpA is an independent CVS risk factor. Anti-tumor necrosis factor (TNF) drugs were associated with a reduced CVS risk in these patients.
Keywords: cardiovascular event, cerebrovascular event, myocardial infarction, spondyloarthritis, stroke
Introduction
Cardiovascular (CVS) risk is increased in patients with inflammatory arthritis compared with the general population.1–3 In addition to traditional risk factors, inflammation is believed to play a role in contributing to increased CVS risk. Over recent decades, biologics have become an important treatment option for patients with inflammatory arthritis, driven by their effectiveness in controlling inflammation. 4 The association between biologics use and CVS risk among patients with inflammatory arthritis is an important area for research. Delineating the CVS risk and the effects of anti-rheumatics drugs in patients with inflammatory arthritis were proposed as part of the research agenda in the European League Against Rheumatism (EULAR) recommendations for CVS risk management in patients with inflammatory joint disorders. 5
Spondyloarthritis (SpA) is a spectrum of inflammatory conditions with ankylosing spondylitis (AS) being the prototype disease. As shown in the ASAS-COMOSPA study, 6 traditional CVS risk factors are prevalent among patients with SpA, as well as CVS events such as stroke (1.3%) and myocardial infarction (MI) (2.7%). Studies in AS have shown that CVS risk is increased among these patients compared with the general population even after adjustment for traditional risk factors. 7 Quantifying the excess CVS risk and identifying potential modifiable factors are important in guiding the management of patients with SpA.
The effect of medications, including disease-modifying anti-rheumatic drugs (DMARDs), on CVS risk has been reported in patients with inflammatory arthritis. 8 Using subclinical atherosclerosis as a surrogate CVS endpoint, anti-tumor necrosis factor alpha (TNF-α) therapy was shown to reduce endothelial dysfunction in patients AS.9,10 Study dedicated to patients with SpA using CVS events as outcome will be useful to add to the current understanding of the potential role of biologics in reducing CVS risk in these patients.
This retrospective study was performed to determine CVS burden and risk in patients with SpA.
Methods
This is a retrospective analysis of data retrieved from a territory-wide centralized electronic database, the Hospital Authority Clinical Management System (CMS). The CMS has been in use since 1995 and contains complete medical records of all patients seen at the public hospitals in Hong Kong. The prevalence of traditional CVS risk factors was evaluated. Incidence rates of major adverse cardiovascular events (MACE) and cerebrovascular events were calculated. The CVS risk contributed by SpA was estimated after adjustment for traditional risk factors. The potential factors influencing the CVS risk in SpA patients, including the use of medications, were explored.
Study subjects
The author (HYC) identified patients with an expert diagnosis of SpA by manually searching all electronic medical records of patient in eight rheumatology centers (Queen Mary Hospital, Grantham Hospital, Tung Wah Hospital, Caritas Medical Center, Pamela Youde Nethersole Eastern Hospital, Tseung Kwan O Hospital, Queen Elizabeth Hospital, Kwong Wah Hospital). The diagnosis of SpA was checked randomly by another author (SCWC). A control group was selected from the orthopedics unit of a public hospital (Queen Mary Hospital) by including all patients with non-specific back pain (NSBP). NSBP was defined as back pain without a specific and identified pathology such as infection, tumor, trauma, deformity, and neurological cause. All patients with a history of stroke, transient ischemic attack (TIA), or MI prior to the date of first follow up were excluded.
Clinical data were retrieved from the CMS. This included age, gender, dates of first and last follow up, smoking and drinking status, history of psoriasis, and inflammatory bowel disease (IBD). Presence of traditional CVS risk factors such as diabetes mellitus (DM), hypertension (HT), and hypercholesterolemia was assessed. Other comorbidities including atrial fibrillation (AF), congestive heart failure (CHF), ischemic heart disease (IHD), peripheral vascular disease (PVD), and chronic kidney disease (CKD) stage 3 or above were recorded. DM was defined based on physician diagnosis and/or use of anti-diabetic drugs and/or by blood tests (fasting glucose > 7 mml/l or HbA1c > 6.5%). Hypertension was defined based on physician diagnosis and/or use of anti-hypertensive medications. Hypercholesterolemia was defined based on physician diagnosis or the use of lipid-lowering medication and/or by blood tests (total cholesterol > 5.2 mmol/l or low-density lipoprotein cholesterol > 3.4 mmol/l).
Use of medications including aspirin, anticoagulant, statin, non-steroidal anti-inflammatory drugs (NSAIDs), conventional and biological DMARDs were documented. Use of medications was defined as the prescription of drugs for any period of time over the duration of follow up.
Definition of events
The outcomes of interest included MACE and cerebrovascular events (stroke or TIA). MACE was defined as cerebrovascular events, MI, and CVS death. All identified cerebrovascular events were reviewed by a neurologist to confirm the diagnoses. MI was defined using the fourth universal definition and all MI events were verified by review of clinical records including electrocardiogram, cardiac enzymes, and imaging findings. 11
Duration of follow up
Duration of follow up to first MACE was defined as the time between first assessment at the rheumatology or orthopedic clinic and one of the following endpoints: first MACE (cerebrovascular event, MI, or CVS death), non-CVS death, or end of study.
Duration of follow up to first cerebrovascular event was defined as the time between first assessment at the rheumatology or orthopedic clinic and one of the following endpoints: first cerebrovascular event, death, or end of study.
Statistical analysis
All statistics were performed using the International Business Machines Corporation Statistical Package for the Social Sciences (IBM SPSS) package 26.0.
Baseline characteristics of the two groups were compared. Age- and sex-matching was performed in one-to-one ratio on SpA and NSBP groups according to sex and 10-year age categories. Non-overlapping parts were trimmed. Continuous variables were expressed as mean with standard deviation (SD) and compared using Student t test. Categorical variables were expressed as percentage and compared using Pearson’s chi-square test. List-wise deletion was performed for missing data.
Crude incidence rates (CIR) were calculated based on the age- and sex-matched cohort. CIR were reported as number of CVS events per 100,000 person-years in SpA and NSBP groups.
To delineate the association between SpA and CVS events, covariate adjustment using propensity score was performed. A propensity score is generated for each patient. PS was generated using logistic regression taking various covariates (age, sex, smoking, drinking DM, HT, hypercholesterolemia, AF, CHF, IHD, CKD, PVD, aspirin, anticoagulant, statin, and NSAIDs) into consideration. A Cox regression model was used to determine the risk of MACE and cerebrovascular event (stroke and TIA) based on propensity score and the two patient groups (SpA and NSBP). Duration of follow-up to MACE and cerebrovascular events was used as the time variable. Results were expressed as HR and 95% CI.
Potential factors affecting the risk of MACE and cerebrovascular event in patients with SpA were screened using univariate Cox regression analysis. This includes the above-mentioned risk factors as well as disease-related medications (NSAIDs, conventional and biological DMARDs). Variables with p value < 0.10 were included in the multivariate Cox regression model using enter mode. Results were expressed as HR and a p value < 0.05 was defined as statistically significant.
Results
Baseline characteristics of patients with SpA
A total of 5046 patients including 2616 patients with SpA and 2430 patients with NSBP were included in the analyses; 3070 patients (1535 patients from each group) were selected after age- and sex-matching. After matching, the two groups had similar baseline characteristics. SpA had shorter duration of follow up, more hypercholesterolemia, and more use of NSAIDs.
Amongst patients with SpA, 19.4% had psoriatic arthritis and 1.5% had IBD-related arthritis; 67.9% of patients met the modified New York Criteria for AS. 12 Almost all patients had NSAIDs use, more than half had conventional DMARDs and a quarter had biological DMARDs. Anti-TNF-α was the most commonly used biologic in our cohort.
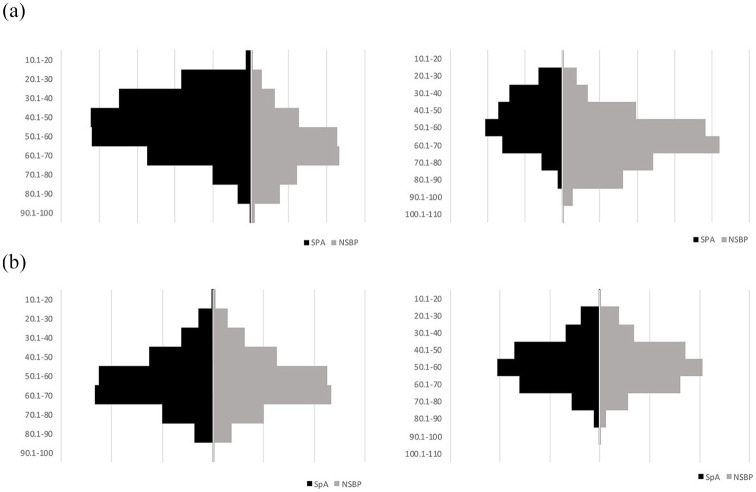
Details of baseline characteristics and age-and-sex distribution are described in Table 1 and Figure 1.
Table 1.
Baseline characteristics of patients with SpA and NSBP before and after age-and sex-matching.
| Baseline demographics | Before matching | Age- and sex-matched | ||||
|---|---|---|---|---|---|---|
| SpA | NSBP | p value | SpA | NSBP | p value | |
| Number of patients | 2616 | 2430 | NA | 1535 | 1535 | NA |
| Age (years) | 50.0 ± 14.7 | 61.6 ± 14.8 | <0.01 | 54.9 | 55.4 | NA |
| Male (%) | 68.7 | 36.4 | <0.01 | 53.3 | 53.3 | NA |
| Duration of FU (years) | 8.8 ± 5.8 | 13.7 ± 5.9 | <0.01 | 8.9 ± 5.8 | 12.5 ± 6.2 | <0.01 |
| Smoking (%) | 30.3 | 18.6 | <0.01 | 26.1 | 25.5 | 0.74 |
| Drinking (%) | 7.8 | 6.3 | 0.04 | 7.6 | 8.7 | 0.26 |
| DM (%) | 11.2 | 17.5 | <0.01 | 13.4 | 14.3 | 0.47 |
| HT (%) | 35.7 | 44.2 | <0.01 | 39.7 | 38.4 | 0.48 |
| Hypercholesterolemia (%) | 30.3 | 32.8 | 0.06 | 34.2 | 28.7 | <0.01 |
| AF (%) | 1.4 | 3.0 | <0.01 | 2.2 | 1.7 | 0.36 |
| CHF (%) | 0.7 | 1.0 | 0.25 | 0.9 | 0.5 | 0.27 |
| IHD (%) | 5.6 | 8.7 | <0.01 | 7.0 | 7.6 | 0.59 |
| CKD (%) | 6.2 | 12.6 | <0.01 | 8.4 | 8.3 | 0.90 |
| PVD (%) | 0.1 | 0.4 | 0.07 | 0.2 | 0.3 | 0.47 |
| Aspirin (%) | 6.6 | 11.6 | <0.01 | 8.3 | 9.6 | 0.19 |
| Anticoagulant (%) | 1.0 | 2.6 | <0.01 | 1.5 | 1.6 | 0.77 |
| Statin (%) | 19.3 | 24.5 | <0.01 | 22.4 | 21.0 | 0.33 |
| NSAIDs (%) | 95.1 | 84.1 | <0.01 | 94.3 | 84.5 | <0.01 |
| Psoriasis (%) | 19.4 | |||||
| IBD (%) | 1.5 | |||||
| Ankylosing spondylitis (%) | 67.9 | |||||
| cDMARDs | 57.2 | |||||
| Sulfasalazine (%) | 46.2 | |||||
| Methotrexate (%) | 26.3 | |||||
| Leflunomide (%) | 5.3 | |||||
| bDMARDs | 25.2 | |||||
| Anti-TNF (%) | 24.1 | |||||
| Secukinumab (%) | 2.3 | |||||
| Ustekinumab (%) | 0.5 | |||||
AF, atrial fibrillation; bDMARDS, biological disease-modifying anti-rheumatic drugs; cDMARDS, conventional disease-modifying anti-rheumatic drugs; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; FU, follow-up; HT, hypertension; IBD, inflammatory bowel disease; IHD, ischemic heart disease; NSAIDs, non-steroidal anti-inflammatory drugs; NSBP, non-specific back pain; PVD, peripheral vascular disease; SpA, spondyloarthritis; TNF, tumor necrosis factor.
Figure 1.
Side-by-side histogram showing age and sex distribution of SpA (black bars) and NSBP (grey bars) groups before and after age- and sex-matching: x-axis, number of patients; y-axis, 10-year age categories.
F, female; M, male; NSBP, non-specific back pain; SpA, spondyloarthritis.
Crude incidence rates of MACE and cerebrovascular event were higher in patients with SpA. Based on the age- and sex-matched cohort, there were 61 stroke, 10 TIA, and 98 MACE in patients with SpA over 13 703 person-years of follow up. In patients with NSBP, there were 58 stroke, 12 TIA, and 79 MACE over 19 277 person-years of follow up.
The CIR of MACE was higher in SpA group (male 922.4 per 100,000 patient-years, female 497.9 per 100,000 patient-years) compared with NSBP group (male 558.8 per 100,000 patient-years, female 249.5 per 100,000 patient-years).
The CIR of cerebrovascular event was higher in the SpA group (male 660.0 per 100,000 patient-years, female 428.3 per 100,000 patient-years) compared with NSBP group (male 539.2 per 100,000 patient-years, female 232.4 per 100,000 patient-years).
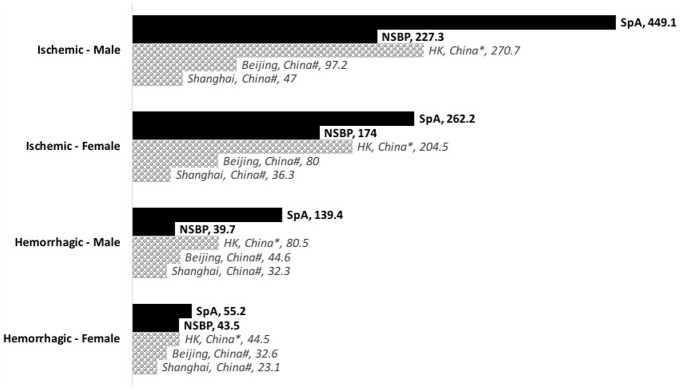
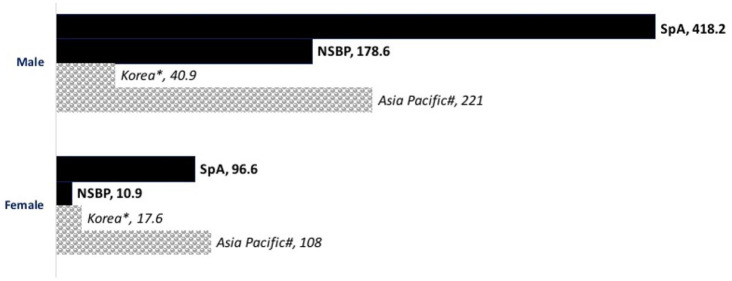
The CIR of stroke and MI in NSBP group were similar to those reported by previous studies in the general population (Figures 2 and 3).13–16
Figure 2.
Bar chart showing the incidence rate of first-ever stroke in SpA and NSBP (per 100,000 person-years). Age-standardized rates of the general population reported by previous studies for Chinese (shaded bars) were included for comparison.
*Age-standardized rate for general population in HK, study year 1999–2007. 13
#Age-standardized rate for general population in China, study year 1991–2000. 14
F, female; HK, Hong Kong; M, male; NSBP, non-specific back pain; SpA, spondyloarthritis.
Figure 3.
Bar chart showing the incidence rate of first-ever MI in SpA and NSBP (per 100 000 person-year). Age-standardized rates of the general population reported by previous studies for Asians (shaded bars) were included for comparison.
*Age-standardized rate for general population in Korea, study year 2006–2010. 15
#Age-standardized rate for general population in Asia Pacific, study year 2010. 16
F, female; HK, Hong Kong; M, male; MI, myocardial infarction; NSBP, non-specific back pain; SpA, spondyloarthritis.
Patients with SpA were associated with a higher risk of MACE and cerebrovascular events, even after adjustment for traditional risk factors.
Using adjustment by propensity score derived from age, sex, smoking and drinking status, CVS comorbidities, and use of medications (aspirin, anti-coagulation, statin, and NSAIDs), SpA was associated with a higher risk for MACE (HR 1.70; 95% CI 1.29–2.26; p < 0.01).
Using adjustment by propensity score derived from age, sex, smoking and drinking status, CVS comorbidities, and use of medications (aspirin, anti-coagulation, statin, and NSAIDs), SpA was associated with a higher risk for cerebrovascular events (HR 1.50; 95% CI 1.08–2.07; p = 0.02).
Use of anti-TNF drugs was associated with a reduced risk of MACE and cerebrovascular events among patients with SpA.
Among 2616 patients with SpA, statistically significant (p < 0.1) associations with MACE in the univariate Cox regression models were age, male sex, smoking, DM, HT, hypercholesterolemia, AF, CHF, IHD, CKD, aspirin, statin, and anti-TNF-α use. Multivariate cox regression analysis showed that HT and IHD were independent risk factors for MACE in SpA patients, whereas anti-TNF drugs were associated with a reduced risk of MACE (Table 2).
Table 2.
Factors associated with major adverse cardiovascular events in SpA patients.
| SpA patients (N = 2616) | Major adverse cardiovascular events | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Covariates | No. of patients | p-Value | HR (95% CI) | p-Value |
| Age | 2616 | <0.01 | 0.99 (0.98–1.01) | 0.51 |
| Male | 1798 | <0.01 | 1.12 (0.68–1.84) | 0.65 |
| Smoking | 777 | <0.01 | 1.17 (0.73–1.86) | 0.52 |
| Drinking | 199 | 0.64 | NA | NA |
| DM | 293 | <0.01 | 1.20 (0.75–1.94) | 0.45 |
| HT | 934 | <0.01 | 2.17 (1.26–3.73) | <0.01 |
| Dyslipidemia | 792 | <0.01 | 1.05 (0.51–2.13) | 0.90 |
| AF | 36 | <0.01 | 0.95 (0.35–2.56) | 0.92 |
| CHF | 18 | <0.01 | 1.90 (0.65–5.60) | 0.24 |
| IHD | 147 | <0.01 | 4.45 (2.14–9.28) | <0.01 |
| CKD | 161 | <0.01 | 1.35 (0.79–2.30) | 0.28 |
| PVD | 3 | 0.30 | NA | NA |
| Aspirin | 172 | <0.01 | 1.51 (0.71–3.23) | 0.28 |
| Anticoagulant | 26 | 0.12 | NA | NA |
| Statin | 504 | <0.01 | 0.86 (0.40–1.87) | 0.71 |
| NSAIDs | 2487 | 0.12 | NA | NA |
| Sulfasalazine | 1206 | <0.01 | 1.08 (0.73–1.61) | 0.71 |
| Methotrexate | 687 | 0.55 | NA | NA |
| Leflunomide | 139 | 0.41 | NA | NA |
| Anti-TNF | 649 | <0.01 | 0.37 (0.17–0.80) | 0.01 |
| Secukinumab | 60 | 0.91 | NA | NA |
| Ustekinumab | 14 | 0.59 | NA | NA |
AF, atrial fibrillation; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HR, hazard ratio; HT, hypertension; IHD, ischemic heart disease; NA, not applicable; NSAIDs, non-steroidal anti-inflammatory drugs; PVD, peripheral vascular disease; SpA, spondyloarthritis; TNF, tumor necrosis factor.
Statistically significant (p < 0.1) associations with cerebrovascular events in the univariate Cox regression models were age, male sex, smoking, drinking, DM, HT, hypercholesterolemia, AF, IHD, CKD, and anti-TNF-α use. Multivariate Cox regression analysis showed that smoking, HT and CKD were independent risk factors for cerebrovascular events in SpA patients, anti-TNF drugs were associated with a reduced risk of cerebrovascular events (Table 3).
Table 3.
Factors associated with cerebrovascular events in SpA patients.
| SpA patients (N = 2616) | Cerebrovascular events | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Covariates | No. of patients | p value | HR (95% CI) | p value |
| Age | 2616 | <0.01 | 1.01 (0.10–1.03) | 0.21 |
| Male | 1798 | <0.01 | 0.97 (0.65–1.45) | 0.89 |
| Smoking | 777 | <0.01 | 1.69 (1.14–2.52) | <0.01 |
| Drinking | 199 | 0.04 | 1.03 (0.60–1.78) | 0.91 |
| DM | 293 | <0.01 | 1.07 (0.71–1.60) | 0.75 |
| HT | 934 | <0.01 | 2.39 (1.57–3.64) | <0.01 |
| Dyslipidemia | 792 | <0.01 | 1.29 (0.88–1.91) | 0.20 |
| AF | 36 | <0.01 | 1.37 (0.71–2.62) | 0.35 |
| CHF | 18 | 0.62 | NA | NA |
| IHD | 147 | <0.01 | 1.04 (0.65–1.68) | 0.86 |
| CKD | 161 | <0.01 | 2.33 (1.53–3.53) | <0.01 |
| PVD | 3 | 0.48 | NA | NA |
| Aspirin | 172 | 0.10 | NA | NA |
| Anticoagulant | 26 | 0.28 | NA | NA |
| Statin | 504 | 0.35 | NA | NA |
| NSAIDs | 2487 | 0.23 | NA | NA |
| Sulfasalazine | 1206 | 0.14 | NA | NA |
| Methotrexate | 687 | 0.59 | NA | NA |
| Leflunomide | 139 | 0.84 | NA | NA |
| Anti-TNF | 649 | <0.01 | 0.21 (0.06–0.78) | 0.02 |
| Secukinumab | 60 | 0.83 | NA | NA |
| Ustekinumab | 14 | 0.68 | NA | NA |
AF, atrial fibrillation; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HR, hazard ratio; HT, hypertension; IHD, ischemic heart disease; NA, not applicable; NSAIDs, non-steroidal anti-inflammatory drugs; PVD, peripheral vascular disease; SpA, spondyloarthritis; TNF, tumor necrosis factor.
Anti-TNF drugs were associated with reduced risk of MACE (HR 0.37, 95% CI 0.17–0.80, p = 0.01) and cerebrovascular events (HR 0.21, 95% CI 0.06–0.78, p = 0.02) in patients with SpA.
Discussion
This study demonstrates that SpA is an independent CVS risk factor. SpA patients have a higher incidence of CVS events. The use of anti-TNF drugs is associated with lower risk for MACE and cerebrovascular events.
CVS comorbidities are common among patients with SpA. Hypercholesterolemia is significantly more prevalent in SpA compared with NSBP. Inflammation has been thought to account for the altered lipid profile commonly seen in patients with rheumatic diseases.17,18 In patients with AS, disease activity is shown to be associated with a deterioration of lipid profile. 19 In addition to its importance as a cytokine in the pathogenesis of SpA, 20 TNF-α has also been suggested as a link between inflammation, dyslipidemia, and metabolic syndrome.21,22 Disease activity and inflammation in SpA are potential contributors to the higher prevalence of hypercholesterolemia in patients with SpA.
SpA patients have a higher incidence of MACE and cerebrovascular events compared with patients with NSBP after age- and sex-matching, whereas male patients have more CVS events compared with female patients in both groups. The NSBP group serves as a good control group for comparison as the incidence of CVS events in NSBP reported in this study falls within the range reported for the general population among Chinese and Asians.13–16 The NSBP group therefore allows a good indirect comparison between patients with SpA and the general population.
This study identifies SpA as an independent CVS risk factor after adjustment for traditional risk factors. A potential explanation for the excess CVS risk contributed by SpA is the effect of inflammation on accelerated atherosclerosis. It is increasingly recognized that rheumatic diseases are associated with a higher CVS risk. In parallel, there is the growing understanding of atherosclerosis as an immune-mediated process.23–25 Evidence on the role of inflammation in contributing to accelerated atherosclerosis in rheumatic diseases are more robust in conditions like SLE and RA compared with SpA.26,27 This study also attempted to address this link by examining the association between medications and CVS risk.
Non-steroidal anti-inflammatory drugs are commonly used in patients with SpA and previous findings of their CVS safety in these patients have been inconclusive.28,29 NSAIDs inhibit cyclooxygenase (COX)-2, leading to an imbalance between prostaglandin I2 production by the vascular endothelium and prothrombotic thromboxane A2 production by platelets,30,31 resulting in an increased CVS events (stroke and MI) in the general population.32–34 On the contrary, there is no association between use of NSAIDs and CVS risk in patients with RA. 35 One explanation is that the beneficial impact of NSAIDs in lowering systemic inflammatory burden offsets the deleterious vascular effect of COX-2 inhibition. 36 Furthermore, NSAIDs can improve mobility and function in patient with SpA. 37 Another alternative explanation is a possible selection bias in clinicians avoiding the use of NSAIDs in patients with high CVS risk. The finding in this study echoes the CVS safety of NSAIDs reported in patients with RA. Future dedicated studies will be useful to delineate the relationship between NSAIDs and CVS safety more accurately by including more detailed information on NSAIDs use (class, dosage, duration, and frequency) and disease activity.
Conventional DMARDs are used in SpA for the management of peripheral arthritis and psoriasis. 38 The CVS protective effect of sulfasalazine in AS has been suggested in a Taiwanese nationwide cohort study. 39 Similarly, methotrexate is found to reduce CVS risk patients with rheumatoid arthritis. 40 In contrast to these findings, conventional DMARDs were not found to have a role in CVS protection in this study. This might be due to the difference in clinical efficacy of conventional DMARDs between RA and SpA. Also, there are major differences between this study and the Taiwanese study. A wider spectrum of SpA patients not limited to AS are included and more variables have been adjusted for analysis, especially medications such as aspirin, statins, and anticoagulants.
An association between anti-TNF therapies and reduced CVS risk in patients with SpA was shown in this study. It is increasingly recognized that inflammation plays a major role in atherosclerosis and studies attempting to alter CVS risk in using medications such as methotrexate and Canakinumab have been carried out.41,42 The effect of anti-TNF drugs on CVS risk has been studied in patients with RA and a protective role has been suggested. 43 In a prospective study with patients with inflammatory arthritis, Lee et al. showed that anti-TNF drugs were associated with a reduction of CVS events. 44 In SpA, there is indirect evidence that anti-TNF drugs may have a role in preventing arterial dysfunction in these patients.9,10,45,46 In this study, it is shown that anti-TNF drugs are associated with reduced CVS risk. Since TNF-α is the cytokine involved in the pathogenesis of both SpA and atherosclerosis, the observed CVS risk reduction might be partly explained by its effect on the inflammatory pathway.
Limitations
Several limitations exist in this study, including the lack of gold standard for SpA diagnosis, the control group selection and the retrospective study design. Although different classification criteria exist, SpA remains a clinical diagnosis by rheumatologists in daily practice. NSBP has been selected as the control group due to inaccessibility to data of healthy general population using our electronic database. It has been shown previously that low back pain is not associated with increased CVS risk, 47 supporting the validity of the control group selection.
Furthermore, detailed information of patients’ disease activity, inflammatory markers, and functional status were not available for analysis. This limited the evaluation regarding the possible mechanisms and key drivers for the increased cardiovascular risk observed in patients with spondyloarthritis. Future studies with detailed analysis in these aspects to ascertain the role of inflammation on CVS risk would be helpful.
Conclusion
CVS comorbidities, especially hypercholesterolemia, are common in patients with SpA and should be actively screened for and managed. Patients with SpA are associated with a higher CVS risk. The use of anti-TNF drugs is associated with a reduced CVS risk in these patients.
Footnotes
Author contributions: Study conception and design: SCWC, HYC, CSL. Acquisition of data: SCWC, HYC, PHL, GKKL. Analysis and interpretation of data: SCWC, KCT, HYC, CSL. Drafting the article: SCWC, HYC. Revising the article: SCWC, HYC, CSL.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval information: The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (reference number UW 18-263) and local ethics committees. It was conducted in accordance with the Declaration of Helsinki and the guidance of Good Clinical Practice, 30 November 2006. All participants gave written informed consent before recruitment.
Data sharing: data is available from Ho Yin Chung upon reasonable request.
ORCID iDs: Shirley Chiu Wai Chan  https://orcid.org/0000-0002-0640-0676
https://orcid.org/0000-0002-0640-0676
Ho Yin Chung  https://orcid.org/0000-0002-0175-1346
https://orcid.org/0000-0002-0175-1346
Contributor Information
Shirley Chiu Wai Chan, Division of Rheumatology and Clinical Immunology, The University of Hong Kong, Hong Kong.
Cheong Kay Teo, Division of Neurology, The University of Hong Kong, Hong Kong.
Philip Hei Li, Division of Rheumatology and Clinical Immunology, The University of Hong Kong, Hong Kong.
Kui Kai Lau, Division of Neurology, The University of Hong Kong, Hong Kong.
Chak Sing Lau, Division of Rheumatology and Clinical Immunology, The University of Hong Kong, Hong Kong.
Ho Yin Chung, Division of Rheumatology and Clinical Immunology, The University of Hong Kong, 102, Pokfulam Road, Hong Kong, China.
References
- 1. Crowson CS, Liao KP, Davis JM, III, et al. Rheumatoid arthritis and cardiovascular disease. Am Heart J 2013; 166: 622–628.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verhoeven F, Prati C, Demougeot C, et al. Cardiovascular risk in psoriatic arthritis, a narrative review. Joint Bone Spine 2020; 87: 413–418. [DOI] [PubMed] [Google Scholar]
- 3. Liew JW, Ramiro S, Gensler LS. Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol 2018; 32: 369–389. [DOI] [PubMed] [Google Scholar]
- 4. Luchetti MM, Benfaremo D, Gabrielli A. Biologics in inflammatory and immunomediated arthritis. Curr Pharm Biotechnol 2017; 18: 989–1007. [DOI] [PubMed] [Google Scholar]
- 5. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010; 69: 325–331. [DOI] [PubMed] [Google Scholar]
- 6. Molto A, Etcheto A, van der Heijde D, et al. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: results of the international cross-sectional ASAS-COMOSPA study. Ann Rheum Dis 2016; 75: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 7. Mathieu S, Soubrier M. Cardiovascular events in ankylosing spondylitis: a 2018 meta-analysis. Ann Rheum Dis 2019; 78: e57. [DOI] [PubMed] [Google Scholar]
- 8. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tam LS, Shang Q, Kun EW, et al. The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis-a randomized, placebo-controlled pilot trial. Rheumatology (Oxford) 2014; 53: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 10. van Sijl AM, van Eijk IC, Peters MJ, et al. Tumour necrosis factor blocking agents and progression of subclinical atherosclerosis in patients with ankylosing spondylitis. Ann Rheum Dis 2015; 74: 119–123. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264. [DOI] [PubMed] [Google Scholar]
- 12. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 13. Chau PH, Woo J, Goggins WB, et al. Trends in stroke incidence in Hong Kong differ by stroke subtype. Cerebrovasc Dis 2011; 31: 138–146. [DOI] [PubMed] [Google Scholar]
- 14. Jiang B, Wang WZ, Chen H, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke 2006; 37: 63–68. [DOI] [PubMed] [Google Scholar]
- 15. Kim RB, Kim BG, Kim YM, et al. Trends in the incidence of hospitalized acute myocardial infarction and stroke in Korea, 2006-2010. J Korean Med Sci 2013; 28: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forouzanfar MH, Moran AE, Flaxman AD, et al. Assessing the global burden of ischemic heart disease, part 2: analytic methods and estimates of the global epidemiology of ischemic heart disease in 2010. Glob Heart 2012; 7: 331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung CP, Oeser A, Solus J, et al. Inflammatory mechanisms affecting the lipid profile in patients with systemic lupus erythematosus. J Rheumatol 2007; 34: 1849–1854. [PubMed] [Google Scholar]
- 18. Gonzalez-Gay MA, Gonzalez-Juanatey C. Inflammation and lipid profile in rheumatoid arthritis: bridging an apparent paradox. Ann Rheum Dis 2014; 73: 1281–1283. [DOI] [PubMed] [Google Scholar]
- 19. van Halm VP, van Denderen JC, Peters MJ, et al. Increased disease activity is associated with a deteriorated lipid profile in patients with ankylosing spondylitis. Ann Rheum Dis 2006; 65: 1473–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tam LS, Gu J, Yu D. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol 2010; 6: 399–405. [DOI] [PubMed] [Google Scholar]
- 21. Borst SE. The role of TNF-alpha in insulin resistance. Endocrine 2004; 23: 177–182. [DOI] [PubMed] [Google Scholar]
- 22. Hardardottir I, Moser AH, Memon R, et al. Effects of TNF, IL-1, and the combination of both cytokines on cholesterol metabolism in Syrian hamsters. Lymphokine Cytokine Res 1994; 13: 161–166. [PubMed] [Google Scholar]
- 23. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12: 204–212. [DOI] [PubMed] [Google Scholar]
- 24. IL6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012; 379: 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerli R, Bartoloni Bocci E, Sherer Y, et al. Association of anti-cyclic citrullinated peptide antibodies with subclinical atherosclerosis in patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67: 724–725. [DOI] [PubMed] [Google Scholar]
- 27. Kerekes G, Szekanecz Z, Der H, et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J Rheumatol 2008; 35: 398–406. [PubMed] [Google Scholar]
- 28. Tsai WC, Ou TT, Yen JH, et al. Long-term frequent use of non-steroidal anti-inflammatory drugs might protect patients with ankylosing spondylitis from cardiovascular diseases: a nationwide case-control study. PLoS One 2015; 10: e0126347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubreuil M, Louie-Gao Q, Peloquin CE, et al. Risk of myocardial infarction with use of selected non-steroidal anti-inflammatory drugs in patients with spondyloarthritis and osteoarthritis. Ann Rheum Dis 2018; 77: 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coxib and traditional NSAID Trialists’ (CNT) Collaboration, Bhala N, Emberson J, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caughey GE, Cleland LG, Penglis PS, et al. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol 2001; 167: 2831–2838. [DOI] [PubMed] [Google Scholar]
- 32. Varas-Lorenzo C, Riera-Guardia N, Calingaert B, et al. Stroke risk and NSAIDs: a systematic review of observational studies. Pharmacoepidemiol Drug Saf 2011; 20: 1225–1236. [DOI] [PubMed] [Google Scholar]
- 33. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011; 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ 2017; 357: j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grigoriou A, Ibrahim F, Chaabo K, et al. Cardiovascular risk with NSAIDs in rheumatoid arthritis: an analysis using routinely collected data. Rheumatology (Oxford) 2016; 55: 763–764. [DOI] [PubMed] [Google Scholar]
- 36. Tarp S, Bartels EM, Bliddal H, et al. Effect of nonsteroidal antiinflammatory drugs on the C-reactive protein level in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2012; 64: 3511–3521. [DOI] [PubMed] [Google Scholar]
- 37. Kroon FP, van der Burg LR, Ramiro S, et al. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev 2015; 7: CD010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 39. Tam HW, Yeo KJ, Leong PY, et al. Sulfasalazine might reduce risk of cardiovascular diseases in patients with ankylosing spondylitis: a nationwide population-based retrospective cohort study. Int J Rheum Dis 2017; 20: 363–370. [DOI] [PubMed] [Google Scholar]
- 40. Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010; 49: 295–307. [DOI] [PubMed] [Google Scholar]
- 41. Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019; 380: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 43. Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011; 63: 522–529. [DOI] [PubMed] [Google Scholar]
- 44. Lee JL, Sinnathurai P, Buchbinder R, et al. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther 2018; 20: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zardi EM, Pipita ME, Giorgi C, et al. Differences in carotid atherosclerosis between patients with ankylosing spondylitis treated with tumor necrosis factor-alpha antagonists and healthy matched controls. Medicine (Baltimore) 2018; 97: e11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Angel K, Provan SA, Gulseth HL, et al. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 2010; 55: 333–338. [DOI] [PubMed] [Google Scholar]
- 47. Heliovaara M, Makela M, Aromaa A, et al. Low back pain and subsequent cardiovascular mortality. Spine (Phila Pa 1976) 1995; 20: 2109–2111. [DOI] [PubMed] [Google Scholar]