Abstract
Objective
Synovitis is a joint disease that seriously affects patient quality of life, but there are currently no diagnostic markers. The albumin to fibrinogen ratio (AFR) and monocyte to lymphocyte ratio (MLR) are non-invasive and cost-effective markers for various systemic inflammatory diseases. However, these markers have not yet been investigated for synovitis. This cross-sectional study evaluated the predictive ability of AFR and MLR in patients with non-specific knee synovitis.
Methods
One hundred fifty-five patients with knee synovitis and 108 healthy control patients were enrolled. Patient characteristics, blood parameters, AFRs, and MLRs were assessed, and the diagnostic value of these factors was determined.
Results
Among 125 patients included, patients with synovitis had a lower AFR and higher MLR than control subjects. The diagnostic values of AFR and MLR were 0.86 and 0.84, respectively, and higher compared with other parameters by receiver operating characteristic curve assessments. Additionally, MLR was negatively correlated with AFR. Late-stage patients showed significantly lower AFRs and significantly higher MLRs than early-stage patients. Binary logistic regression analyses indicated that AFR was an independent predictor for synovitis severity.
Conclusions
The AFR and MLR had high diagnostic value for knee synovitis. The AFR was an independent predictor for synovitis severity.
Keywords: Knee synovitis, albumin to fibrinogen ratio, monocyte to lymphocyte ratio, predictor, diagnostic, marker
Introduction
The synovium is an important component of the joint system. It is a thin and soft layer of loose connective tissue that lines the inner side of the joint capsule. The edges of the synovium are attached to the periphery of articular cartilage and form a closed capsule around the joint cavity.1,2 Synovitis is a joint disease in which inflammation of the synovium is stimulated by various factors, such as trauma, infection, hyperosteogeny, tuberculosis, joint degeneration, rheumatism, pigmented villonodular, or surgery, resulting in the impaired secretion of synovial cells.3,4 The onset and progression of synovitis lead to the development of symptoms characteristic of joint disease, which negatively affect patient quality of life. 5 Currently, there are no specific diagnostic indicators for synovitis. Early screening, diagnosis, and treatment for this disease are important to shorten the course of therapy and reduce patient pain. Therefore, identifying a rapid, simple, and effective method to evaluate the degree of inflammatory responses in synovitis is of great clinical significance.
It has been reported that immune and inflammatory systems are activated in cases with knee synovitis.6,7 Several studies examined inflammation and concluded that albumin (ALB), fibrinogen (Fib), C-reactive protein (CRP), and their ratio are useful biomarkers.8,9 In patients with rheumatoid arthritis, the albumin to fibrinogen ratio (AFR) and albumin to CRP ratio (ACR) were highly correlated with disease activity and thus proposed as novel inflammatory markers. 10 In several types of inflammatory and immune diseases, the monocyte to lymphocyte ratio (MLR), neutrophil to lymphocyte ratio (NLR), and platelet to lymphocyte ratio (PLR) have been reported to be inexpensive and independent disease predictors.11–14 Previous research, which included the authors of this article, examined severe cases of knee osteoarthritis by receiver operating characteristic (ROC) curve analysis and found that the MLR showed a high diagnostic value of 0.81, high sensitivity of 84%, and a relatively high specificity of 66.7%, indicating that it is a good predictor for knee osteoarthritis. 15
Similar assessments regarding the diagnostic value of AFR, ACR, MLR, NLR, and PLR in patients with synovitis have not been reported. In this study, we aimed to evaluate the predictive ability of these relatively novel inflammatory blood markers in patients with knee synovitis. Our findings provide valuable information for the early diagnosis of synovitis.
Patients and methods
Patient characteristics
Patients with synovitis hospitalized in the Department of Orthopedics, Shenzhen Traditional Chinese Medicine Hospital between 1 June 2017 and 31 June 2019 were recruited for this study. Healthy control subjects without evidence of illness who visited the hospital for routine physical examinations during the same period were enrolled. An orthopedic doctor with more than 10 years of work and research experienced selected the subjects. The study was approved by the Ethics Committee of Shenzhen Traditional Chinese Medicine Hospital (No. 2018-67). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study. The reporting of this study conforms to the STROBE statement. 16
Inclusion and exclusion criteria
Patients who met the following inclusion criteria were included in this study. (1) All patients fulfilled the 2016 Expert Consensus on Diagnosis and Clinical Efficacy Evaluation of Knee Synovitis in Adults published by the Chinese Society of Traditional Chinese Medicine, 17 including a) young adults (18–29 years) with a history of trauma (e.g., strain, ligament injury, or soft tissue injury); b) middle-aged (30–59 years) and older individuals (60+ years), especially those who are obese and overweight; c) swelling of a knee joint; d) knee joint distended and painful; e) distended causing discomfort or pain, obvious aggravation when knee joint extended or fully flexed; f) increased skin temperature by palpation; g) tenderness point is uncertain and may be tender at the primary injury; h) positive floating patella test or excessive effusion of joint via B-ultrasound and magnetic resonance imaging; i) atrophy of quadriceps femoris; and j) yellow or light-yellow joint puncture fluid and lacking fat on the surface drop. To be diagnosed with non-specific synovitis of the knee joint, patients met both c) and d) and had at least one other symptom (or multiple other symptoms). (2) All subjects were provided with and signed informed consent forms.
Patients were excluded if they met the any of following criteria: (1) patients who had synovitis caused by rheumatoid arthritis, infectious arthritis, gouty arthritis, tuberculosis, pigmented villonodular arthritis, hemophilic arthritis, synovial chondrosarcoma, or knee surgery; (2) patients with hypertension, diabetes mellitus, malignancy, renal and liver failure, or active infection; or (3) patients who used prescribed medications up to one month prior to the assessments for inclusion.
Laboratory and clinical assessments
Age, sex, weight, body mass index, and other basic data were collected for all participants. Baseline data were recorded for the two groups. For all patients, ALB, Fib, CRP, red blood cell distribution widths, white blood cells (WBCs), erythrocyte sedimentation rate (ESR), red blood cells (RBCs), and monocyte, neutrophil, lymphocyte, and platelet counts were measured. The AFR, MLR, NLR, and PLR were also calculated. Single-factor and multi-factor logistic regression analyses were performed to identify the most important independent risk factors for patients with synovitis. ROC curves were generated to facilitate the analysis of predictive values for AFR, MLR, NLR, and PLR in patients with synovitis.
Clinical stage of synovitis
The clinical stage of synovitis was determined according to the 2016 Expert Consensus on Diagnosis and Clinical Efficacy Evaluation of Knee Synovitis in Adults published by the Chinese Society of Traditional Chinese Medicine. 17
Statistical analysis
Data analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). Data for measurements were expressed as the mean ± standard deviation and compared by t-tests. Count data were expressed by frequency or percentage and compared by the χ2 test, and risk factors were analyzed by logistic regression. Diagnostic values for AFR, MLR, NLR, and PLR were analyzed by ROC curves. Pearson correlation tests were conducted to assess the relationships between variables of interest. A P value of < 0.050 was used as the level of statistical significance, at which the null hypothesis of no differences between the groups being compared was rejected.
Results
Basic patient characteristics
One hundred fifty-five patients with synovitis were enrolled in this study. Based on the inclusion and exclusion criteria, 125 patients with synovitis and 108 healthy controls were analyzed. The clinical stages of patients with synovitis were based on the Visual Analog Scale score, degree of swelling, and findings on B-ultrasound 17 and are shown in Table 1. Hematologic parameters and clinical characteristics of the two groups are shown in Table 2. In patients with synovitis, the AFR was 10.69 ± 4.02, which was significantly lower compared with the healthy group (16.07 ± 3.42; P < 0.050). The MLR (0.16 ± 0.07), NLR (2.15 ± 0.86), and PLR (111.78 ± 45.27) were significantly higher in patients with synovitis compared with healthy controls (P < 0.050).
Table 1.
Clinical stage of synovitis.
| Stage of synovitis | I | II | III | IV |
|---|---|---|---|---|
| Pain | VAS ≤ 3 | 3 < VAS ≤ 5 | 6 < VAS ≤ 8 | 8 < VAS ≤ 10 |
| Swelling | None | Mild | Moderate | Severe |
| B-ultrasound | Normal | Mild synovium thickening without effusion | Moderate synovium thickening with minimal effusion | Severe synovium thickening with extensive effusion |
VAS, visual analog scale.
Table 2.
Basic characteristics of patients with synovitis and controls.
| Control (n = 108) | Synovitis (n = 125) | P | |
|---|---|---|---|
| Age (years) | 44.08 ± 9.75 | 45.36 ± 10.38 | 0.335 |
| Sex (male/female) (%) | 51/57 (47.22%) | 69/56 (55.20%) | 0.760 |
| WBC (10^9/L) | 5.25 ± 1.02 | 6.05 ± 2.02 | <0.001* |
| Lymphocyte (10^9/L) | 2.42 ± 0.74 | 2.01 ± 0.62 | <0.001* |
| ALB (g/L) | 45.88 ± 4.40 | 39.01 ± 5.68 | <0.001* |
| Fib (g/L) | 2.96 ± 0.60 | 3.95 ± 0.94 | <0.001* |
| Neutrophil (10^9/L) | 3.23 ± 0.89 | 4.32 ± 1.75 | <0.001* |
| Monocyte (10^9/L) | 0.25 ± 0.08 | 0.32 ± 0.06 | <0.001* |
| Platelet (10^9/L) | 214.36 ± 59.35 | 224.68 ± 70.25 | 0.231 |
| Hemoglobin (g/L) | 136.65 ± 18.32 | 131.54 ± 20.38 | 0.046* |
| RBC (10^12/L) | 4.67 ± 0.52 | 4.53 ± 0.68 | 0.083 |
| RDW CV (%) | 13.32 ± 1.92 | 13.23 ± 1.85 | 0.716 |
| CRP (mg/L) | undetected | 60.48 ± 30.24 | N/A |
| AFR (%) | 16.07 ± 3.42 | 10.69 ± 4.02 | <0.001* |
| ACR (%) | – | 0.65 ± 0.16 | N/A |
| MLR (%) | 0.10 ± 0.04 | 0.16 ± 0.07 | <0.001* |
| NLR (%) | 1.33 ± 0.52 | 2.15 ± 0.86 | <0.001* |
| PLR (%) | 88.58 ± 25.96 | 111.78 ± 45.27 | <0.001* |
| ESR (mm/hour) | undetected | 26.57 ± 14.38 | N/A |
| ALT (U/L) | undetected | 26.32 ± 4.48 | N/A |
| AST (U/L) | undetected | 18.35 ± 4.24 | N/A |
| CREA (µmol/L) | undetected | 75.65 ± 28.35 | N/A |
WBC, white blood cells; ALB, albumin; Fib, fibrinogen; RDW CV, red blood cell distribution width coefficient of variation; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RBC, red blood cells; AFR, albumin to fibrinogen ratio; ACR, albumin to CRP ratio; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; ESR, erythrocyte sedimentation rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CREA, creatinine; N/A, not applicable.
Diagnostic value of AFR, NLR, MLR, and PLR for synovitis
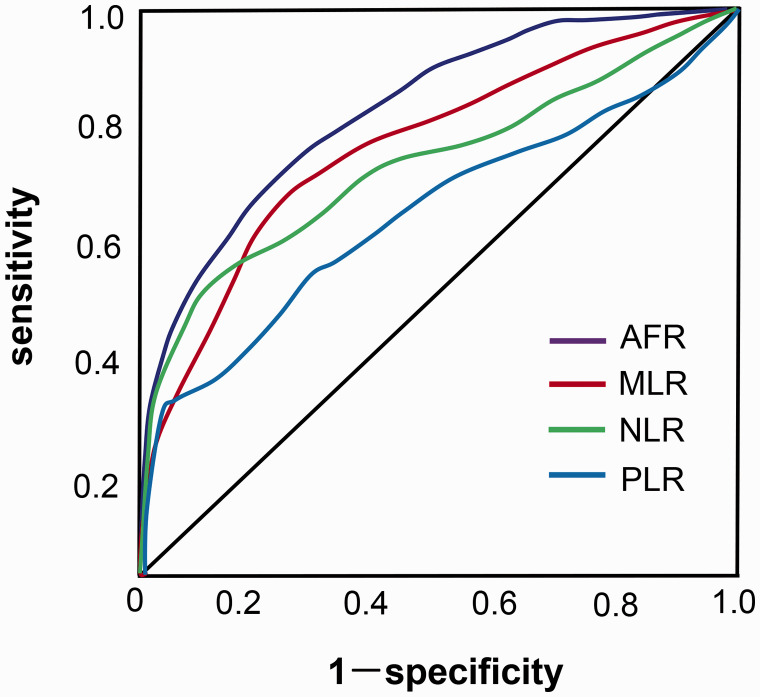
We assessed the diagnostic values of the AFR, NLR, MLR, and PLR for synovitis by ROC curve analysis. Using the optimal cut-off value of 12.37, the area under the curve (AUC) of AFR was 0.86 [95% confidence interval (CI): 0.818–0.917], which was higher than the AUCs of ALB and Fib, resulting in a sensitivity of 78.2% and specificity of 88.3%. The AUC of MLR was 0.84 (95% CI: 0.745–0.872), which was higher than the AUCs of NLR (0.76) and PLR (0.62) (Figure 1).
Figure 1.
ROC curve analysis of various markers for diagnosing synovitis. One hundred twenty-five patients with knee synovitis and 108 healthy control patients were analyzed. The diagnostic values of AFR, MLR, NLR, and PLR for synovitis were assessed by ROC curve analysis.
ROC, receiver operating characteristic; AFR, albumin to fibrinogen ratio; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Correlations between AFR, MLR, NLR, PLR, and variables
Correlations between AFR, MLR, NLR, PLR, and variables are shown in Table 3. AFR was negatively correlated with monocytes (r = −0.186, P < 0.050) and MLR (r = −0.235, P < 0.050). MLR was positively correlated with ESR (r = 0.225, P < 0.050), CRP (r = 0.336, P < 0.050), WBCs (r = 0.256, P < 0.050), Fib (r = 0.277, P < 0.050), NLR (r = 0.625, P < 0.05), and PLR (r = 0.459, P < 0.050) and negatively correlated with lymphocytes (r = −0.432, P < 0.050) and AFR (r = −0.235, P < 0.050).
Table 3.
Analysis of the correlation between variables.
|
MLR |
NLR |
PLR |
AFR |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Age | −0.152 | 0.021 | −0.154 | 0.336 | −0.121 | 0.350 | −0.002 | 0.979 |
| Sex | −0.122 | 0.282 | −0.125 | 0.235 | −0.245 | 0.424 | −0.073 | 0.433 |
| ESR | 0.225 | 0.012* | 0.384 | 0.015* | 0.211 | 0.032* | −0.008 | 0.616 |
| CRP | 0.336 | 0.024* | 0.475 | <0.001* | 0.230 | 0.024* | −1.00 | 0.545 |
| WBC | 0.256 | 0.004* | 0.518 | <0.001* | 0.025 | 0.785 | −0.034 | 0.715 |
| Lymphocyte | −0.432 | <0.001* | −0.480 | <0.001* | −0.559 | <0.001* | 0.101 | 0.275 |
| ALB | −0.082 | 0.374 | −0.079 | 0.392 | −0.101 | 0.274 | 0.535 | <0.001* |
| Fib | 0.277 | 0.002* | 0.109 | 0.238 | 0.109 | 0.236 | −0.808 | <0.001* |
| Neutrophil | 0.373 | <0.001* | 0.720 | <0.001* | 0.201 | 0.028* | −0.049 | 0.599 |
| Monocyte | 0.644 | <0.001* | 0.293 | <0.001* | 0.051 | 0.580 | −0.186 | 0.043* |
| AFR | −0.235 | 0.010* | −0.081 | 0.379 | −0.128 | 0.164 | 1 | – |
| MLR | 1 | – | 0.625 | <0.001* | 0.459 | <0.001* | −0.235 | 0.010* |
WBC, white blood cells; ALB, albumin; Fib, fibrinogen; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RBC, red blood cells; AFR, albumin to fibrinogen ratio; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
AFR, MLR, NLR, and PLR values in patients with early-stage and late-stage synovitis
We divided all patients with synovitis into early-stage and late-stage groups according to the 2016 Expert Consensus Guidelines for Diagnosis and Clinical Evaluation of Knee Synovitis in Adults published by the Chinese Society of Traditional Chinese Medicine. Late-stage patients had a significantly lower AFR (8.59 ± 2.26) and higher MLR (0.21 ± 0.06) than early-stage patients (P < 0.001 for each comparison; Table 4).
Table 4.
Characteristics of early-stage and late-stage patients.
| Late stage (n = 47) | Early stage (n = 78) | P | |
|---|---|---|---|
| Age (years) | 43.25 ± 6.72 | 44.35 ± 9.68 | 0.494 |
| Sex (male/female) (%) | 24/23 (51.06%) | 35/43 (44.87%) | 0.320 |
| WBC (10^9/L) | 8.28 ± 2.86 | 7.25 ± 1.05 | 0.005* |
| Lymphocyte (10^9/L) | 1.65 ± 0.32 | 2.04 ± 0.65 | <0.001* |
| ALB (g/L) | 36.76 ± 4.42 | 39.86 ± 5.88 | 0.002* |
| Fib (g/L) | 4.42 ± 0.95 | 3.77 ± 0.91 | <0.001* |
| Neutrophil (10^9/L) | 4.84 ± 2.02 | 4.23 ± 1.86 | 0.088 |
| Monocyte (10^9/L) | 0.45 ± 0.35 | 0.36 ± 0.15 | 0.048* |
| Platelets (10^9/L) | 215.38 ± 50.25 | 224.58 ± 62.59 | 0.394 |
| Hemoglobin (g/L) | 132.21 ± 22.35 | 139.26 ± 33.78 | 0.206 |
| RBC (10^12/L) | 4.32 ± 0.85 | 5.08 ± 3.45 | 0.141 |
| RDW CV (%) | 14.03 ± 2.38 | 13.27 ± 2.15 | 0.068 |
| AFR | 8.59 ± 2.26 | 11.50 ± 4.23 | <0.001* |
| MLR | 0.21 ± 0.06 | 0.15 ± 0.07 | <0.001* |
| NLR | 2.93 ± 1.85 | 2.07 ± 1.52 | 0.005* |
| PLR | 130.55 ± 40.51 | 112.18 ± 38.24 | 0.012 |
| ESR (mm/hour) | 27.28 ± 28.32 | 24.33 ± 20.12 | 0.498 |
| CRP (mg/hour) | 22.26 ± 28.25 | 25.25 ± 33.75 | 0.398 |
| ALT (U/L) | 18.53 ± 9.25 | 20.25 ± 10.32 | 0.350 |
| AST (U/L) | 22.24 ± 8.72 | 24.32 ± 9.25 | 0.216 |
| CREA (µmol/hour) | 74.12 ± 13.48 | 75.32 ± 32.18 | 0.809 |
WBC, white blood cells; ALB, albumin; Fib, fibrinogen; RDW CV, red blood cell distribution width coefficient of variation; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RBC, red blood cells; AFR, albumin to fibrinogen ratio; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CREA, creatinine.
Independent association of AFR with the severity of synovitis
Using binary logistic regression, we explored the associations of AFR, NLR, MLR, and PLR with the severity of synovitis. Our findings indicated that AFR (odds ratio = 0.71, P < 0.050) was a significant independent predictor for synovitis severity (Table 5).
Table 5.
Factors independently associated with disease activity by binary logistic regression.
| Risk factors | OR (95%CI) | P |
|---|---|---|
| AFR | 0.71 (0.564–0.887) | 0.003* |
| MLR | 48.60 (0.108–219.01) | 0.213 |
| NLR | 0.97 (0.625–1.507) | 0.894 |
| PLR | 1.00 (0.989–1.015) | 0.757 |
OR, odds ratio; CI, confidence interval; AFR, albumin to fibrinogen ratio; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Discussion
In recent years, the incidence of synovitis has increased. The identified causes are diverse and complex, consisting of both non-infectious and infectious mechanisms. Non-infectious causes include trauma, gout, pseudogout, autoimmune disorders, tumors, and other factors. Infectious synovitis results from both common and rare factors related to tuberculosis, bacterial infection, and other types of infection. 5 Thus, using the cross-sectional approach in our study, we evaluated the potential diagnostic values of AFR, MLR, NLR, and PLR in knee synovitis without a specific etiology. Our findings indicated that patients in the synovitis group had a significantly lower AFR and significantly higher MLR, NLR, and PLR compared with patients in the healthy control group. The diagnostic value of AFR and MLR for use as markers was higher than that observed for NLR, PLR, and other parameters. Correlation assessments revealed that AFR was negatively associated with monocytes and MLR. In addition, MLR was positively correlated with ESR, CRP, WBCs, and Fib. We further sub-divided all patients with synovitis into two groups based on the disease stage and found that late-stage patients had a lower AFR and higher MLR compared with patients in the early-stage group. Binary logistic regression analyses indicated that AFR was an important independent predictor for synovitis severity. Therefore, we conclude that AFR and MLR had the highest potential for use as diagnostic markers in synovitis, and AFR was an important independent predictor for synovitis severity.
Besides secretion, phagocytosis, and lubrication, the synovium functions to nourish the associated joint. Once the synovium is negatively impacted by trauma or other factors, associated changes may lead to articular cartilage destruction and directly reduce the range and dynamics of movement in the affected joint. These and other related impacts ultimately result in patients losing the ability to perform labor-intensive tasks.1,18 Thus, potential approaches that facilitate early detection, diagnosis, and treatment for synovitis have received great interest. ALB and globulin have been demonstrated to play important roles in systemic inflammatory responses, and decreases in serum ALB indicate a poor nutritional status and chronic inflammatory response in corresponding patients. 19 Research has indicated that an increased serum Fib level is an independent prognostic marker for both non-small cell lung cancer and colon cancer.20,21 In addition, low ALB has been identified as an independent prognostic factor for multiple types of malignant tumors. 22 Blood Fib is an important facilitator of coagulation and platelet aggregation and promotes the synthesis of inflammatory cytokines in inflamed microenvironments as an acute phase molecule of the inflammatory response. Blood levels of Fib also reflect the state of the inflammatory response.23,24 However, the expression levels of these factors are influenced by metabolic processes, associated physiological states, and various types of diseases, including dehydration, cachexia, and sodium retention. 25 Therefore, the accuracy of prognosis prediction is higher when using both ALB and Fib compared with only a single inflammatory factor. 25
The AFR has been proposed as a new predictive marker in cancer and systemic inflammatory disease. Yang et al. reported that the AFR was a novel inflammatory marker for monitoring disease activity in patients with rheumatoid arthritis, with a lower ratio detected compared with the control group. 10 We found that AFR values were significantly lower in patients with synovitis than healthy controls, indicating the high diagnostic value of this factor. These findings were similar to those from Yang et al. Furthermore, several studies have reported that lymphocyte, neutrophil, monocyte, and platelet levels changed significantly in cases of systemic inflammation compared with unaffected cases.11,12,26 Our findings indicated that the MLR, NLR, and PLR were all significantly higher in patients with synovitis compared with healthy controls. These findings are similar to previous research that examined the dynamics of these factors in patients with arthritis, which was carried out by the authors of this article. 15
Based on ROC curve analyses, AFR and MLR had higher diagnostic values than NLR and PLR. In addition, correlation analyses indicated that AFR was negatively correlated with monocytes and MLR. Furthermore, MLR, NLR, and PLR were highly correlated with ESR and CRP but not patient age or sex. According to the guidelines from the 2016 Expert Consensus on Diagnosis and Clinical Efficacy Evaluation of Knee Synovitis in Adults published by the Chinese Society of Traditional Chinese Medicine, 17 we divided all patients with synovitis into two groups based on the stage of disease as follows: 1) early stage (I and II) and 2) later stage (III and IV). The results indicated that AFR was significantly lower in the late-stage group compared with the early-stage group, whereas MLR and NLR were significantly higher in the late-stage group. Furthermore, binary logistic regression analyses indicated that only AFR was a significant independent predictor for synovitis severity. Therefore, our findings support the use of AFR as a potential marker for disease severity predictions in patients with synovitis.
Despite our findings, the mechanisms and dynamics underlying the decreased AFR and elevated MLR remain unclear. Previously, it has been reported that pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor alpha, inhibit hepatocyte synthesis, leading to decreased ALB levels. In addition, the inflammation-mediated overproduction of inflammatory cytokines, such as IL-6, has been shown to increase Fib levels.27,28 Therefore, these factors may also contribute to the reduced AFR in patients with synovitis.
There were some limitations in our study. First, our sampling was carried out at a single center. Furthermore, we only sampled a relatively small number of patients. Thus, the findings from our study need to be expanded upon and further verified by a multi-center study with a larger number of samples. Second, we did not examine the mechanisms or perform a comprehensive analysis of potential factors that may have affected our results, particularly with respect to AFR and MLR. Therefore, further study of the underlying mechanisms of these factors is needed.
In conclusion, we found a lower AFR and higher MLR, NLR, and PLR in patients with synovitis compared with the healthy group. AFR and MLR had higher diagnostic values for synovitis than the other factors we assessed. AFR was the most important and was identified as an independent predictor for the severity of synovitis. To our knowledge, our study is the first to show the potential of AFR and MLR as novel and accurate predictors for assessing the state of synovitis.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was funded by the Shenzhen Scientific Research Fund (No. JCYJ20180302173509878) and the Sanming Project of Medicine in Shenzhen (No. SZSM201812066).
ORCID iDs: Kun Gao https://orcid.org/0000-0003-0323-4219
Yafei Cao https://orcid.org/0000-0001-9193-376X
References
- 1.Bhattaram P, Chandrasekharan U. The joint synovium: A critical determinant of articular cartilage fate in inflammatory joint diseases. Semin Cell Dev Biol 2017; 62: 86–93. 10.1016/j.semcdb.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 2.Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep 2013; 15: 323. 10.1007/s11926-013-0323-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017; 19: 18. 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asif Amin M, Fox DA, Ruth JH. Synovial cellular and molecular markers in rheumatoid arthritis. Semin Immunopathol 2017; 39: 385–393. 10.1007/s00281-017-0631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012; 51: 249–257. 10.1016/j.bone.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 2015; 11: 35–44. doi: 10.1038/nrrheum.2014.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlowsky EW, Kraus VB. The Role of Innate Immunity in Osteoarthritis: When Our First Line of Defense Goes On the Offensive. J Rheumatol 2015; 42: 363–371. doi: 10.3899/jrheum.140382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizuka M, Nagata H, Takagi K, et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol 2016; 23: 900–907. [DOI] [PubMed] [Google Scholar]
- 9.Sahebari M, Ayati R, Mirzaei H, et al. Serum trace element concentrations in rheumatoid arthritis. Biol Trace Elem Res 2016; 171: 237–245. [DOI] [PubMed] [Google Scholar]
- 10.Yang WM, Zhang WH, Ying HQ, et al. Two new inflammatory markers associated with disease activity score-28 inpatients with rheumatoid arthritis: Albumin to fibrinogen ratio and C-reactive protein to albumin ratio. Int Immunopharmacol 2018; 62: 293–298. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Liu A, Liang L, et al. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol 2018; 64: 10–15. doi: 10.1016/j.intimp.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 12.Taşoğlu Ö, Bölük H, Şahin Onat Ş, et al. Is blood neutrophil-lymphocyte ratio an independent predictor of knee osteoarthritis severity? Clin Rheumatol 2016; 35: 1579–1583. doi: 10.1007/s10067-016-3170-8 [DOI] [PubMed] [Google Scholar]
- 13.Xiang J, Zhou L, Li X, et al. Preoperative Monocyte-to-Lymphocyte Ratio in Peripheral Blood Predicts Stages, Metastasis, and Histological Grades in Patients with Ovarian Cancer. Transl Oncol 2017; 10: 33–39. doi: 10.1016/j.tranon.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Deng W, Zheng S, et al. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int Immunopharmacol 2018; 57: 43–46. doi: 10.1016/j.intimp.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 15.Gao K, Zhu W, Liu W, et al. Diagnostic value of the blood monocyte-lymphocyte ratio in knee osteoarthritis. J Int Med Res 2019; 47: 4413–4421. 10.1177/0300060519860686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 17.Zhan H, Zheng Y. Expert consensus on diagnosis and clinical efficacy evaluation of knee synovitis in adults. Chinese Journal of Orthopedics 2016; 24: 1–3. [In Chinese] [Google Scholar]
- 18.Schett G. Synovitis–an inflammation of joints destroying the bone. Swiss Med Wkly 2012; 142: w13692. 10.4414/smw.2012.13692 [DOI] [PubMed] [Google Scholar]
- 19.Chen WZ, Yu ST, Xie R, et al. Preoperative albumin/globulin ratio has predictive value for patients with laryngeal squamous cell carcinoma. Oncotarget 2017; 8: 48240–48247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng L, Luo M, Sun X, et al. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer 2013; 133: 2720–2725. [DOI] [PubMed] [Google Scholar]
- 21.Son HJ, Park JW, Chang HJ, et al. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol 2013; 20: 2908–2913. [DOI] [PubMed] [Google Scholar]
- 22.Sawada N, Iwasaki M, Inoue M, et al. Plasma testosterone and sex hormone-binding globulin concentrations and the risk of prostate cancer among Japanese men: a nested case-control study. Cancer Sci 2010; 101: 2652–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan Z, Zhang M, Han Q, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer 2017; 8: 1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen T, Kierulf P, Sandset PM, et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemos 2007; 97: 822–829. [DOI] [PubMed] [Google Scholar]
- 25.Azab BN, Bhatt VR, Vonfrolio S, et al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. Am J Surg 2013; 206: 764–770. [DOI] [PubMed] [Google Scholar]
- 26.Tasoglu I, Cicek OF, Lafci G, et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. Ann Vasc Surg 2014; 28: 606–613. doi: 10.1016/j.avsg.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 27.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005; 39: S143–S146. [DOI] [PubMed] [Google Scholar]
- 28.Ozkurt ZN, Yagci M, Sucak GT, et al. Thrombopoietic cytokines and platelet count in multiple myeloma. Platelets 2010; 21: 33–36. [DOI] [PubMed] [Google Scholar]