Abstract
Objective
Magnesium sulfate is considered to be an effective adjuvant to rocuronium in general anaesthesia. We conducted a meta-analysis to clarify its efficacy.
Methods
We searched the PubMed, Embase, Web of Science, Cochrane Library, WanFang, Chinese Biomedical Literature, and China National Knowledge Infrastructure databases for randomized controlled trials (RCTs) of magnesium sulfate as an adjuvant to rocuronium from the start of the database establishment until May 2020.
Results
Eleven RCTs were analysed. The pooled meta-analysis showed that using magnesium sulfate as an adjuvant significantly shortened the onset time and prolonged the clinical duration of neuromuscular blockade by rocuronium compared with the control group without magnesium sulfate. However, there was no significant difference in recovery index of neuromuscular block between the magnesium and control groups. Furthermore, magnesium sulfate significantly increased the rates of excellent and clinically acceptable intubation conditions.
Conclusion
Adding magnesium sulfate to rocuronium during general anaesthesia can alter the neuromuscular parameters, including shortening the anaesthesia-onset time and prolonging the clinical duration, without significantly increasing the recovery time. Pretreatment with magnesium sulfate may also improve intubation conditions during general anaesthesia.
Keywords: Magnesium sulfate, adjuvant, rocuronium, general anaesthesia, intubation, neuromuscular block
Introduction
Rocuronium induces rapid-onset profound neuromuscular blockade that can be completely reversed by sugammadex, making it a useful alternative to succinylcholine for facilitating endotracheal intubation during rapid sequence induction in certain situations.1–3 However, the onset time and time to achieve maximum muscle block with a 2ED95 dose of rocuronium (0.6 mg/kg) is still slower than for succinylcholine (1.0 mg/kg), 4 and a higher dose of rocuronium is needed to achieve the same onset time and intubation conditions. 5 However, rocuronium may have a ceiling effect for onset time, 6 and increasing the dose beyond a particular amount does not always guarantee a shortened onset time, 6 and can markedly prolong the duration of action.5,6 Moreover, the pharmacodynamics of muscle relaxants are influenced by numerous factors such as the potency of the drug, the dose administered, the cardiovascular status,7–9 and other drugs, particularly volatile anaesthetics, antibacterial drugs (procainamide, quinidine), calcium antagonists, phenytoin, lithium, and magnesium.10,11 Various alternative methods have thus been studied to accelerate the onset of neuromuscular blockade with a standard intubating dose of rocuronium.
Magnesium sulfate is becoming widely used as an adjuvant for anaesthesia because of its effects as an N-methyl-D-aspartate (NMDA) receptor antagonist and a sympathetic blocking agent.12–14 Magnesium exerts anaesthetic and analgesic effects by acting as an antagonist of NMDA receptors in the central nervous system, and modulates the hemodynamic response to stress via its vasodilatory and antiarrhythmic properties and inhibition of catecholamine release. Magnesium also inhibits the motor plate release of acetylcholine, 15 thus facilitating the actions of neuromuscular blocking agents. Although numerous clinical studies have investigated the effects of adding magnesium sulfate as an adjuvant to rocuronium in general anaesthesia, the results remain inconclusive: magnesium sulfate has been associated with a shorter onset time and prolonged total recovery time of neuromuscular block in some studies,16,17 while other studies18,19 have found contradictory results regarding the interaction between magnesium sulfate and rocuronium during general anaesthesia. We therefore conducted a meta-analysis of relevant randomized clinical trials (RCTs) to assess the efficacy of magnesium sulfate pretreatment on pharmacodynamic parameters and intubation conditions after anaesthesia induction using rocuronium.
Materials and methods
This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines. 20 The protocol was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) (registration no.: INPLASY202060070). All analyses were based on previously published studies, and no ethical approval or patient consent was therefore required.
Search strategy
Two researchers independently carried out a comprehensive literature search. Trials examining the outcomes of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia were retrieved from the PubMed, Embase, Web of Science, Cochrane Library, WanFang, Chinese Biomedical Literature, and China National Knowledge Infrastructure databases, from the start of the database until May 2020. The following Medical Subject Headings(MeSH) or non-MeSH terms and their combinations were searched in the title and abstract: “Rocuronium”, “Esmeron”, “Esmerone”, “Zemuron”, “Rocuronium Bromide”, “Magnesium Sulfate”, “magnesium sulphate”, “Sulfate, Magnesium”, “Magnesium Sulfate, Heptahydrate”, “Heptahydrate Magnesium Sulfate”, “randomized controlled trial”, “controlled clinical trial”, “randomized”, “placebo”, “drug therapy”, “randomly”, “trial” and “groups”. The study design was limited to RCTs.
Eligibility and exclusion criteria
We selected all studies that met the following eligibility criteria: randomized controlled trials; adult patients (≥18 years old) who underwent general anaesthesia; perioperative administration of intravenous magnesium sulfate as an adjuvant to rocuronium compared with rocuronium alone (control group), regardless of the dose administered; pharmacodynamic parameters and intubation conditions as a primary or secondary outcome; and availability of the full text in English or Chinese. The exclusion criteria were: studies that reported on patients with myasthenia gravis; abstract, comment, review/editorial review, guideline, meeting, or case report; and studies published without sufficient data or for which the relevant raw data could not be abstracted.
Data extraction and outcomes assessed
Selected studies that fulfilled the eligibility criteria were included. The data were extracted from each eligible study by two independent reviewers, and any discrepancies were reconciled after discussion with a third reviewer. The extracted data included primary author, year of publication, sample size, basic characteristics of the participants (age, sex, weight), surgery type, anaesthesia method, intervention study, comparators, neuromuscular monitoring place, and outcome measures.
The primary outcomes for the current study were the effects of adjuvant magnesium on the pharmacodynamics of rocuronium in terms of onset time, clinical duration, and recovery index. Based on previous studies,16–19,21–27 the time from injection of rocuronium to 80% to 100% depression of the single twitch, 95% to 100% depression of the first twitch, or when the train-of-four (TOF) count reached 0 as defined in the eligible studies were all accepted as the onset time. Clinical duration was defined as the time from injection of rocuronium to 25% recovery of first twitch of TOF stimulation or TOF count recovered to 2. Recovery index was the time from 25% recovery of first twitch of TOF stimulation to 75% recovery of first twitch of TOF stimulation. For published studies that reported pharmacodynamic parameter values without specifying the time period, the standard definition provided by the Stockholm Guide for Clinical Practice was followed. 28
Intubation conditions were assessed as a secondary outcome using the intubation scoring system of the good clinical research practice guidelines developed by Fuchs-Buder et al. 28 or the criteria of Cooper et al. 29 The grade “excellent” was considered as excellent intubation conditions, and a grade of either “excellent” or “good” was considered clinically acceptable. Data reported as a graph were extracted using the software WebPlotDigitizer 4.3 (https://automeris.io/WebPlotDigitizer/).
Risk-of-bias assessment
The quality of each included trial was independently estimated by two investigators using the Cochrane Collaboration’s tool for evaluating the risk of bias, with any discrepancies reconciled by a third reviewer. The tool includes seven quality items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. 30 Each item was classified as indicating a low, unclear, or high risk of bias. 30 Additionally, the main outcome of onset time was graded and evaluated according to the GRADE system.
Statistical analysis
All statistical analyses were performed using RevMan software version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark) and Stata version 16.0 (Stata Corp LP, College Station, TX, USA). Continuous outcomes were expressed as standard mean differences (SMD) with 95% confidence intervals (CIs) because different definitions were used to assess the same variable in the case of pharmacodynamic parameters. Intubation conditions were presented as dichotomous data, with risk ratios (RRs) and 95% CIs.
We assessed the heterogeneity among studies using Cochran’s Q test (P < 0.10 for statistical significance) and the I2 index (I2 > 50% for significant heterogeneity). Data with significant heterogeneity were analysed with a random-effect model; otherwise, a fixed-effect model was selected. Additionally, we performed a sensitivity analysis by excluding one trial at a time, to evaluate the effect of each individual study with a high risk of bias on the stability of the pooled data. Finally, publication bias was detected by funnel plot asymmetry with Begg’s and Egger’s regression tests.
A P-value of <0.05 was considered to represent statistical significance.
Results
Literature search
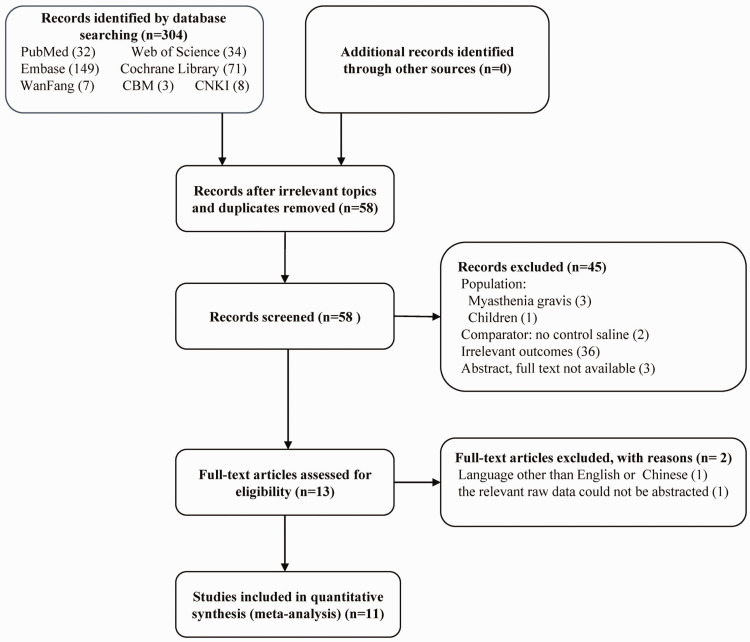
The initial literature search yielded 304 studies, of which 246 were removed as duplicate articles or irrelevant topics, and 45 were excluded after screening the titles and abstracts. The remaining 13 studies were reviewed for eligibility by scrutinizing the full-text articles. The relevant raw data could not be abstracted for one study, and one study was in a language other than English or Chinese. Eleven full-text RCTs16–19,21–27 were therefore included in the final analysis. Figure 1 shows the PRISMA 20 flow diagram, including the reasons for exclusion. The inter-rater reliability measures between the two reviewers showed high agreement for study selection (κ = 0.91).
Figure 1.
Flow diagram of included studies.
Characteristics of studies
Eleven studies16–19,21–27 involving 460 participants (≥18 years old) were included in the meta-analysis. The characteristics of the included trials are presented in Tables 1 and 2. There was complete agreement between the two reviewers regarding data extraction (κ = 1.0). The participants were all ASA status I–III, scheduled for elective surgery, with no neuromuscular disorders or anticipated difficult airway. In the case of missing data, the reviewers attempted to contact the authors to obtain more information, but no further information was obtained.
Table 1.
Characteristics of included trials.
| Author (year) | Sample size (magnesium/control groups) | Age (years, mean±SD) | Sex (n, male:female) | Weight (kg, mean±SD) | Surgery type | Anaesthetics (induction/maintenance) |
|---|---|---|---|---|---|---|
| Kussman et al. 18 | 14/14 | Similar | Similar | Similar | Surgery requiring tracheal intubation | Thiopentone+fentanyl /isoflurane+N2O |
| Gupta et al. 21 | 25/25 | 43.12±12.28/49.36 ±10.19 | 19:6/14:11 | 65.76 ±12.02/70.00 ±10.91 | Spinal surgery | Propofol+fentanyl/propofol |
| Czarnetzki et al. 16 | 35/37 | 35.3 ±11.8/38.6 ±12.8 | 28:12/27:13 | 70.0 ±10.3/70.6±12.2 | Elective surgery lasting at least 120 min | Propofol+sufentanil/propofol |
| Kim et al. 17 | 23/23 | 39 ±12/46±12 | 9:14/10:13 | 58±10/56±8 | Elective surgery under general anaesthesia | Propofol+remifentanil/propofol |
| Khafagy et al. 22 | 30/30 | 40.7±3.0/40.5±2.7 | 8:22/5:25 | 82.0±6.9/81.5±8.8 | Open cholecystectomy | Propofol+ fentanyl /propofol |
| Rotava et al. 19 | 30/30 | 68±8/69±8 | 22:8/20:10 | 63±13/69±15 | Elective head and neck cancer surgery | Propofol+ fentanyl /propofol |
| Park et al. 23 | 51/51 | 46.5±12.4/45.5±10.9 | 20:31/18:33 | 60.3±9.8/58.9±6.2 | Elective operations under general anaesthesia | Propofol+ alfentanil/sevoflurane |
| Wang et al. 24 | 20/20 | 45.5±10.9/44.78±11.81 | 17:3/15:5 | 72.33±9.37/66.92±12.93 | Elective surgery under general anaesthesia | Propofol+ alfentanil/sevoflurane |
| Liang et al. 25 | 28/28 | 35.6±10.9/35.8±10.0 | 13:15/15:13 | ND | Elective surgery under general anaesthesia | Etomidate+fentanyl/propofol+sevoflurane |
| Kim et al. 26 | 20/20 | 43±19/42±15 | 9:11/8:12 | 59±8/60±8 | Elective operation under general anaesthesia | Propofol+remifentanil/ND |
| Choi et al. 27 | 25/23 | 50.0±12.2/49.0±12.9 | ND | 64.3±9.6/64.7±8.3 | Laryngeal microsurgery | Propofol+remifentanil/sevoflurane |
SD, standard deviation; ND, not described; RCT, randomized controlled trial; Similar, both groups similar age, weight, and sex distribution in Kussman et al. 18
Table 2.
Additional characteristics of included trials.
| Author (year) | Intervention study (magnesium dose/administration mode) | Comparators | Neuromuscular monitoring site | Onset time | Clinical duration | Recovery index | Criteria for assessing intubation conditions |
|---|---|---|---|---|---|---|---|
| Kussman et al. 18 | Bolus MgSO4 (60 mg/kg, >1 minute) | Saline | Ulnar nerve | Yes | Yes | ND | ND |
| Gupta et al. 21 | Bolus MgSO4 (30 mg/kg, unclear) + continuous infusion (10 mg/kg/hour MgSO4) during surgery | Saline | Ulnar nerve | Yes | Yes | ND | ND |
| Czarnetzki et al. 16 | Bolus MgSO4 (60 mg/kg, 15 minutes) | Saline | Ulnar nerve | Yes | Yes | Yes | ND |
| Kim et al. 17 | Bolus MgSO4 (50 mg/kg, 10 minutes) | Saline | Ulnar nerve | Yes | Yes | ND | Fuchs-Buder |
| Khafagy et al. 22 | Bolus MgSO4 (50 mg/kg, 15 minutes) + continuous infusion (8 mg/kg/hour MgSO4) during surgery | Saline | Ulnar nerve | Yes | Yes | Yes | ND |
| Rotava et al. 19 | Bolus MgSO4 (30 mg/kg, 10 minutes) + continuous infusion (1 g/hour MgSO4 <3 hours) during surgery | Saline | Ulnar nerve | Yes | Yes | Yes | ND |
| Park et al. 23 | Bolus MgSO4 (50 mg/kg, 15 minutes) | Saline | ND | ND | ND | ND | Fuchs-Buder |
| Wang et al. 24 | Bolus MgSO4 (50 mg/kg, 15 minutes) | Saline | ND | ND | ND | ND | Fuchs-Buder |
| Liang et al. 25 | Bolus MgSO4 (50 mg/kg, 15 minutes) | Saline | Ulnar nerve | Yes | Yes | ND | Cooper |
| Kim et al. 26 | Bolus MgSO4 (50 mg/kg, 10 minutes) | Saline | Ulnar nerve | Yes | Yes | ND | Fuchs-Buder |
| Choi et al. 27 | Bolus MgSO4 (30 mg/kg, 10 minutes) | Saline | Ulnar nerve | Yes | Yes | Yes | Fuchs-Buder |
Risk-of-bias assessment
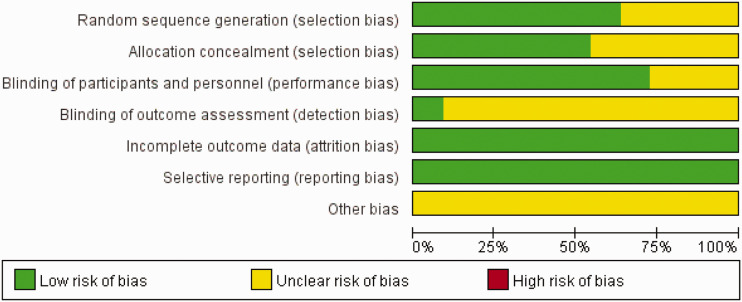
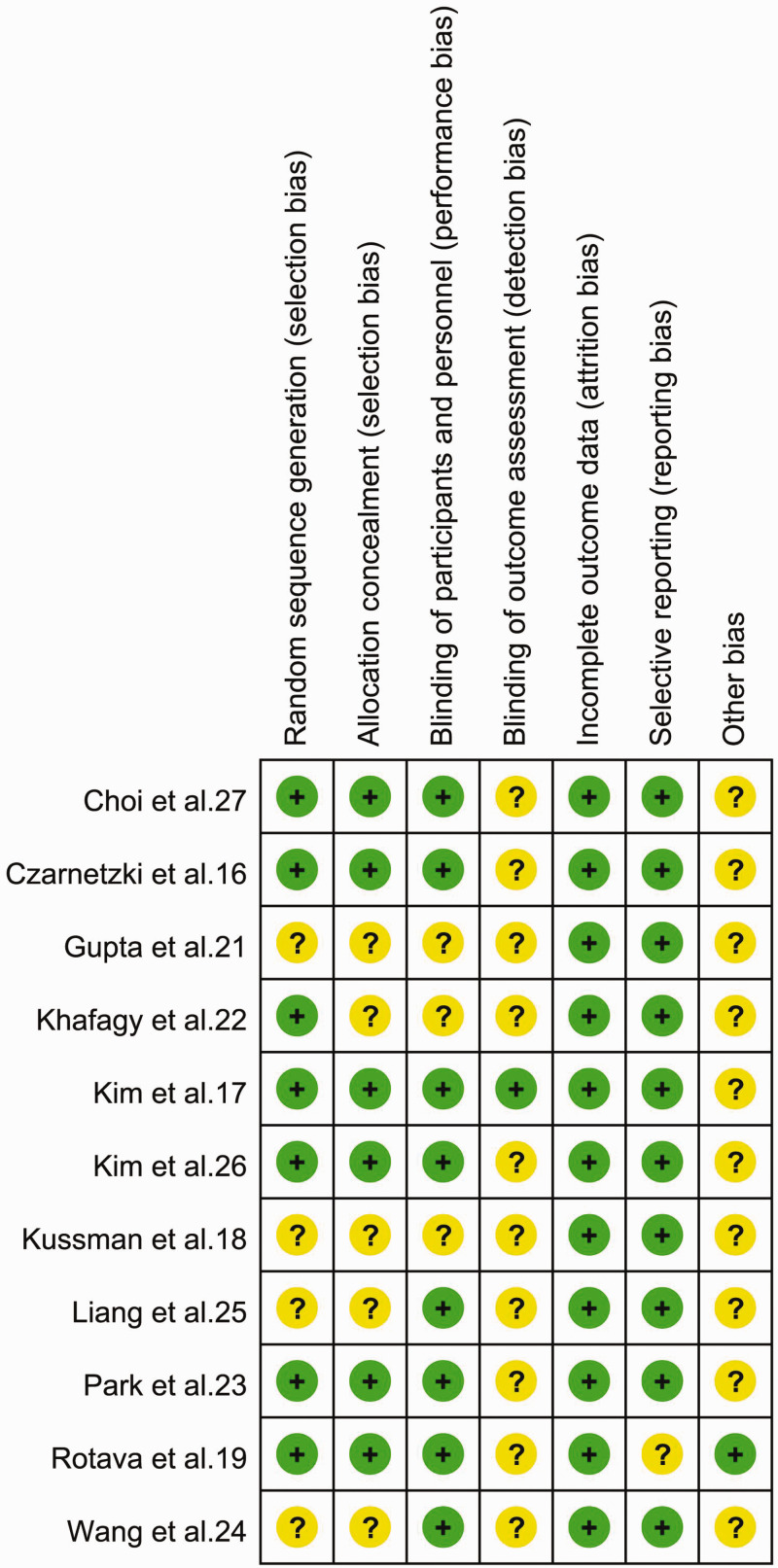
Some of the reviewed studies lacked sufficient details to permit full evaluation of the risk of bias; in such cases, we evaluated risk of bias conservatively by classifying trials lacking details allowing the exclusion of selection, performance, and detection biases as having an “unclear risk of bias”. Furthermore, we judged the risk of attrition bias to be low in 11 studies, and the number of participants with missing outcome data was balanced between groups. There were no concerns regarding selective reporting of results because all outcomes prespecified in the methods section were reported. However, very few studies had large effect sizes, and other potential sources of bias could therefore not be entirely ruled out. Authors’ judgments of each risk of bias item presented as percentages across all included studies are shown in Figure 2, and the authors’ judgements about each risk of bias item for each included study are shown in Figure 3.
Figure 2.
Risk of bias graph. Authors’ judgements of risk of bias items presented as percentages across all included studies.
Figure 3.
Risk of bias summary. Authors’ judgements of risk of bias items for each included study.
Summary of findings by the GRADE system
The main outcome of onset time was graded according to the GRADE system standard. The level of evidence for this finding was rated as low. The overall quality assessment was downgraded by quality and consistency limitations.
Primary outcomes
Onset time
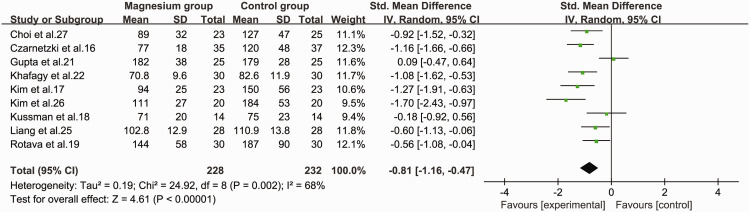
Nine studies16–19,21,22,25–27 assessed the onset time of rocuronium by monitoring neuromuscular responses, and provided sufficient information to allow the results to be pooled. The pooled data suggested that use of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia significantly shortened the onset time of neuromuscular blockade (SMD = −0.81, 95%CI −1.16 to −0.47, P < 0.001, I2 = 68%) compared with the control group (Figure 4).
Figure 4.
Forest plot of onset time of rocuronium in the magnesium and control groups.
CI, confidence interval; SD, standard deviation; IV, inverse variance.
We also performed subgroup analyses to reduce the possible bias of our conclusion caused by heterogeneity. Studies were grouped according to the dosage of magnesium: a dose of 50 mg/kg significantly reduced the onset time of neuromuscular block (SMD = −1.12, 95% CI −1.55 to −0.68, P < 0.01) and heterogeneity was observed (I2 = 52%, P = 0.1). However, there was no significant effect at doses of 30 mg/kg (SMD = −0.46, 95% CI −1.02 to 0.11) or 60 mg/kg (SMD = −0.71, 95% CI −1.67 to 0.25). In terms of statistical heterogeneity, the I2 values for 30 and 60 mg/kg were about 68% (P = 0.05) and 78% (P = 0.03), indicating heterogeneity among the datasets in these subgroups. We also stratified the data according to the time of administration of magnesium. We focused on magnesium administration over a 10- or 15-minute period before induction of anaesthesia because only one study administered magnesium for over 1 minute 18 or described the administration time as “unclear”, 21 respectively. Magnesium administration for 10 minutes (SMD = −1.07, 95% CI −1.54 to −0.6, P < 0.01) and 15 minutes (SMD = −0.95, 95% CI −1.3 to −0.61, P < 0.01] significantly reduced the onset time, with significant heterogeneity for the 10 minute group (I2 = 57%, P = 0.07) but not for the 15 minute group (I2 = 21%).
Clinical duration
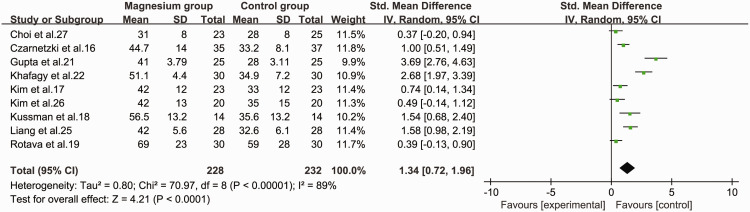
Nine studies16–19,21,22,25–27 were included in the pooled analysis comparing the clinical duration of rocuronium between the magnesium group and control group. The pooled data suggested that using magnesium sulfate as an adjuvant to rocuronium in general anaesthesia significantly prolonged the clinical duration of neuromuscular blockade (SMD = 1.34, 95%CI 0.72 to 1.96, P < 0.001, I2 = 89%) compared with the control group (Figure 5).
Figure 5.
Forest plot of the clinical duration time of rocuronium in the magnesium group and control groups.
CI, confidence interval; SD, standard deviation; IV, inverse variance.
Additionally, we performed subgroup analysis of these nine studies according to the dosage of magnesium (30, 50, 60 mg/kg). The SMDs for clinical duration were 1.43 (95%CI −0.25 to 3.12, P = 0.1) for 30 mg/kg, 1.36 (95%CI 0.44 to 2.28, P = 0.004) for 50 mg/kg, and 1.15 (95%CI 0.68 to 1.62, P < 0.01) for 60 mg/kg, with significant heterogeneity in the 30 and 50 mg/kg groups (I2 = 95%, P < 0.001 and I2 = 88%, P < 0.001, respectively) but not in the 60 mg/kg group. We also carried out subgroup analysis according to the time of administration of magnesium. The SMDs for magnesium administered for 10 minutes and 15 minutes were 0.48 (95% CI 0.20 to 0.77, P < 0.01) and 1.73, (95% CI 0.80 to 2.66, P < 0.01), with significant heterogeneity in the 15 minute group (I2 = 86%, P = 0.007) but not in the 10 minute group. Finally, the data were stratified based on the administration of magnesium as a continuous infusion. The clinical duration time was significantly prolonged following continuous (SMD=2.23, 95% CI 0.21 to 4.24, P = 0.03) and non-continuous infusion (SMD=0.92,[95% CI 0.53 to 1.32, P < 0.01); however, the heterogeneity between the groups did not disappear when the data were sorted according to continuous infusion or not (I2 = 96%, P < 0.001 and I2 = 60%, P = 0.03, respectively).
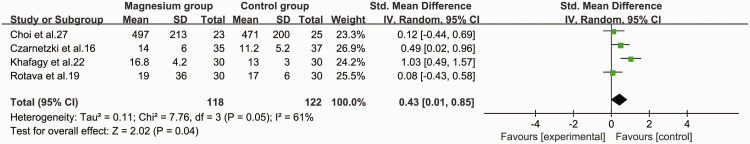
Recovery index
The recovery index of neuromuscular block was examined in four studies.16,17,19,27 We found that the recovery index of neuromuscular block was significantly longer in the magnesium group than in the control group (SMD = 0.43, 95%CI 0.01 to 0.85, P = 0.04, I2 = 61%) (Figure 6).
Figure 6.
Forest plot of the recovery index of rocuronium in the magnesium and control groups.
CI, confidence interval; SD, standard deviation; IV, inverse variance.
Sensitivity analysis
Sensitivity analyses of onset time and clinical duration of neuromuscular block revealed no significant changes in point estimates of SMD. However, sensitivity analysis of the recovery index of neuromuscular block identified one study 17 using various anaesthetic adjuvants with total intravenous anaesthesia guided by bispectral index that may have resulted in heterogeneity, and there was no heterogeneity among the remaining studies after removal of this study. A meta-analysis was performed using the fixed-effect model, and the results showed no significant difference between the two groups (SMD = 0.25, 95% CI −0.04 to 0.55).
Secondary outcomes
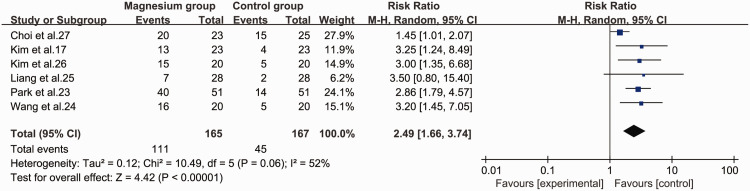
Excellent intubation conditions
Six studies17,23–27 assessed excellent and clinically acceptable intubation conditions as outcomes. The magnesium group was significantly superior to the control group in terms of creating excellent intubation conditions (RR = 2.49, 95% CI 1.66 to 3.74, P < 0.001, I2 = 52%) (Figure 7).
Figure 7.
Forest plot of excellent intubation conditions in the magnesium and control groups.
CI, confidence interval; M-H, Mantel–Haenszel.
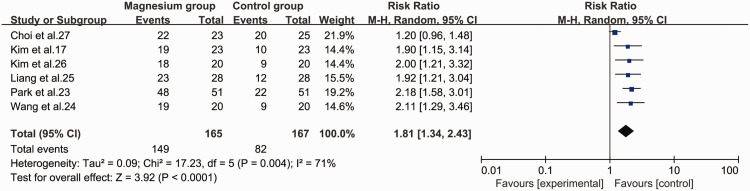
Clinically acceptable intubation conditions
The magnesium group was also significantly superior to the control group in creating clinically acceptable intubation conditions (RR = 1.81, 95% CI 1.34 to 2.43, P < 0.001, I2 = 71%) (Figure 8).
Figure 8.
Forest plot of clinically acceptable intubation conditions in the magnesium and control groups.
CI, confidence interval; M-H, Mantel–Haenszel.
Sensitivity analysis
We performed sensitivity analyses for excellent and clinically acceptable intubation conditions. One of the six included studies, 27 which used low-dose (30 mg/kg) magnesium as an adjuvant to low-dose (0.45 mg/kg) rocuronium in general anaesthesia, may have led to heterogeneity among the studies. Removing this study eliminated the heterogeneity between the remaining studies for both excellent and clinically acceptable intubation conditions. The results of a meta-analysis using a fixed-effect model showed that the difference remained significant for both excellent (RR = 3.03, 95% CI 2.18 to 4.22, P < 0.001) and clinically acceptable intubation conditions (RR = 2.05, 95% CI 1.69 to 2.49, P < 0.001).
Publication bias
Funnel plots require a minimum of 10 studies; however, the primary outcome of onset time was only measured in nine studies, and publication bias could therefore not be assessed by this method. We therefore performed Begg’s and Egger’s regression tests to evaluate the asymmetry and publication bias. The analysis did not identify any potential publication bias.
Discussion
This review and meta-analysis of 11 RCTs demonstrated that the addition of magnesium sulfate as an adjuvant in general anaesthesia altered the pharmacodynamic parameters of rocuronium. Pretreatment with magnesium sulfate before rocuronium general anaesthesia significantly shortened the onset time and prolonged the clinical duration and recovery time of neuromuscular blockade. However, the clinical significance of our results may be limited by the high degree of heterogeneity among the included studies, and a sensitivity analysis excluding a study that reported “recovery index” values without specifying the time period found no significant difference in recovery time of neuromuscular blockade in relation to magnesium. This indicated that although the definition was constant within a study, differences in definitions among studies may affect the results.
Our results were consistent with those of another recent meta-analysis that assessed pharmacological interventions to accelerate the onset time of rocuronium; however, this previous analysis only included four studies related to magnesium sulfate. 8 Furthermore, a systematic review and meta-analysis assessing the interaction between magnesium sulfate and neuromuscular blockers during the perioperative period 31 only included six RCTs referring to rocuronium. The current study thus included a broader search, assessed more RCTs concerning the use of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia, assessed its efficacy in terms of the pharmacodynamic parameters of rocuronium, and evaluated the effect of magnesium sulfate on intubation conditions after anaesthesia induction. Unfortunately, the results regarding the recovery index of neuromuscular block differed between the current and previous meta-analyses. 31 Moreover, the results lacked the power to determine equivalence owing to the small sample size. Further research is therefore needed to clarify the uncertainty regarding the efficacy of magnesium sulfate.
In addition, we found that the magnesium group was superior to the control group in terms of intubation conditions, and increased the rate of excellent intubation conditions by more than 3.03 times to 64.1%. Magnesium also increased the rate of clinically acceptable intubation conditions by more than 2.05 times to 89.4%. After excluding a study 27 using low-dose (30 mg/kg) magnesium as an adjuvant to low-dose (0.45 mg/kg) rocuronium in general anaesthesia, there was no significant heterogeneity with regard to excellent or clinically acceptable intubation conditions, indicating that an initial dose of magnesium sulfate may improve the effect of rocuronium in creating superior intubation conditions, and that the induction dose of rocuronium should also not be neglected.
This meta-analysis had several limitations. First, we disregarded data from studies published in any language other than English or Chinese. Although language-restricted meta-analyses have repeatedly been reported to cause no bias for estimating the effectiveness of different interventions, 32 the conclusions should still be interpreted with caution. Second, The definition of onset time was derived from the included literature, but the range was broad, and time to 80%, 90%, 95%, or 100% neuromuscular blockade as defined in the eligible studies were all accepted as the onset time, while the time from injection of rocuronium to 25% recovery of first twitch of TOF stimulation or TOF count recovered to 2 were counted as the clinical duration. Moreover, one study that reported “pharmacodynamic parameters” values without specifying the time period measured might have been a major cause of heterogeneity. Third, the models or devices used for neuromuscular stimulation and detection differed, which might also have caused heterogeneity. Fourth, the evidence in this study was graded as low quality, mainly because of the methodological shortcomings of the included studies and inconsistencies in the definitions and assessments of outcomes among the studies. Finally, although there was no statistical evidence of publication bias, the probability of bias still exists as a consequence of the low statistical power caused by the limited quantity of included studies and because the search strategy did not include unpublished research results. This omission could lead to either overestimation or underestimation of the net effects of neuromuscular blockade.
Conclusion
Overall, the results of this review and meta-analysis suggest that magnesium sulfate as an adjuvant to rocuronium in general anaesthesia has the potential to reduce the onset time of neuromuscular block and improve intubation conditions, without increasing the dose of rocuronium. However, the results must be interpreted with caution because of the clinical heterogeneity. Although there is no evidence to suggest that magnesium sulfate is harmful in the clinical doses used, there are also no long-term studies examining the safety of magnesium sulfate, 33 and more RCTs and high-quality studies are warranted in the future. We await with interest the completion and publication of ongoing trials that are currently investigating the efficacy of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211027736 for Efficacy of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia: a meta-analysis by Haiyan Sun, Tao Jin, Xiping Wu, Lei Yang, Yunxia Zuo and Ren Liao in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211027736 for Efficacy of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia: a meta-analysis by Haiyan Sun, Tao Jin, Xiping Wu, Lei Yang, Yunxia Zuo and Ren Liao in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Haiyan Sun https://orcid.org/0000-0002-1923-1250
Tao Jin https://orcid.org/0000-0003-4248-1466
Xiping Wu https://orcid.org/0000-0002-7312-5280
References
- 1.Tran DT, Newton EK, Mount VA, et al . Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev 2015; 2015: Cd002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soto R, Jahr JS, Pavlin J, et al. Safety and efficacy of rocuronium with sugammadex reversal versus succinylcholine in outpatient surgery-a multicenter, randomized, safety assessor-blinded trial. Am J Ther 2016; 23: e1654–e1662. [DOI] [PubMed] [Google Scholar]
- 3.Chambers D, Paulden M, Paton F, et al. Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment. Health Technol Assess 2010; 14: 1–211. [DOI] [PubMed] [Google Scholar]
- 4.Tran DTT, Newton EK, Mount VAH, et al. Rocuronium vs. succinylcholine for rapid sequence intubation: a Cochrane systematic review. Anaesthesia 2017; 72: 765–777. [DOI] [PubMed] [Google Scholar]
- 5.El-Orbany M, Connolly LA. Rapid sequence induction and intubation: current controversy. Anesth Analg 2010; 110: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 6.Han T, Kim H, Bae J, et al . Neuromuscular pharmacodynamics of rocuronium in patients with major burns. Anesth Analg 2004; 99: 386–392. [DOI] [PubMed] [Google Scholar]
- 7.Won YJ, Shin YS, Lee KY, et al. The effect of phenylephrine on the onset time of rocuronium. Korean J Anesthesiol 2010; 59: 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, Gao L, Lu W, et al. Pharmacological interventions for acceleration of the onset time of rocuronium: a meta-analysis. PLoS One 2014; 9: e114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YB, Sung TY, Yang HS. Factors that affect the onset of action of non-depolarizing neuromuscular blocking agents. Korean J Anesthesiol 2017; 70: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang DX, Rao YQ, Ji B, et al. Effects of sevoflurane and desflurane on pharmacodynamics of rocuronium in children. Zhonghua Yi Xue Za Zhi 2017; 97: 429–433. [DOI] [PubMed] [Google Scholar]
- 11.Queiroz Rangel Micuci AJ, Verçosa N, Filho PAG, et al. Effect of pretreatment with magnesium sulphate on the duration of intense and deep neuromuscular blockade with rocuronium: A randomised controlled trial. Eur J Anaesthesiol 2019; 36: 502–508. [DOI] [PubMed] [Google Scholar]
- 12.Srebro D, Vuckovic S, Milovanovic A, et al. Magnesium in pain research: state of the art. Curr Med Chem 2017; 24: 424–434. [DOI] [PubMed] [Google Scholar]
- 13.Soave PM, Conti G, Costa R, et al. Magnesium and anaesthesia. Curr Drug Targets 2009; 10: 734–743. [DOI] [PubMed] [Google Scholar]
- 14.Dubé L, Granry JC. The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: a review. Can J Anaesth 2003; 50: 732–746. [DOI] [PubMed] [Google Scholar]
- 15.Feldman S, Karalliedde L. Drug interactions with neuromuscular blockers. Drug Saf 1996; 15: 261–273. [DOI] [PubMed] [Google Scholar]
- 16.Czarnetzki C, Lysakowski C, Elia N, et al. Time course of rocuronium-induced neuromuscular block after pre-treatment with magnesium sulphate: a randomised study. Acta Anaesthesiol Scand 2010; 54: 299–306. [DOI] [PubMed] [Google Scholar]
- 17.Kim MH, Oh AY, Jeon YT, et al. A randomised controlled trial comparing rocuronium priming, magnesium pre-treatment and a combination of the two methods. Anaesthesia 2012; 67: 748–754. [DOI] [PubMed] [Google Scholar]
- 18.Kussman B, Shorten G, Uppington J, et al. Administration of magnesium sulphate before rocuronium: effects on speed of onset and duration of neuromuscular block. Br J Anaesth 1997; 79: 122–124. [DOI] [PubMed] [Google Scholar]
- 19.Rotava P, Cavalcanti IL, Barrucand L, et al. Effects of magnesium sulphate on the pharmacodynamics of rocuronium in patients aged 60 years and older: A randomised trial. Eur J Anaesthesiol 2013; 30: 599–604. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 21.Gupta K, Vohra V, Sood J. The role of magnesium as an adjuvant during general anaesthesia. Anaesthesia 2006; 61: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 22.Khafagy HF, Ebied RS, Osman ES, et al. Perioperative effects of various anesthetic adjuvants with TIVA guided by bispectral index. Korean J Anesthesiol 2012; 63: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SJ, Cho YJ, Oh JH, et al. Pretreatment of magnesium sulphate improves intubating conditions of rapid sequence tracheal intubation using alfentanil, propofol, and rocuronium - a randomized trial. Korean J Anesthesiol 2013; 65: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JP, Yang BH, Lu LC, et al. Effect of pretreatment with magnesium sulphate on hemodynamics in patients with rapid sequential induction of general anesthesia. Journal of Qiqihar University of Medicine 2014; 35: 1607–1609. Available at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2014&filename=QQHB201411030&v=1LhTlJSwGapgUcmq%25mmd2BU%25mmd2FT80jgBSbJNBS0KTYEpngziXxpCaXepPWBTOBPf%25mmd2BPsNBOg [Google Scholar]
- 25.Liang XL, Liu TJ, Zhang Y. Effect of magnesium sulphate and rocuronium priming on rapid sequence intubation. Journal of Medical Research 2015; 44: 110–112. DOI: 10.3969/j.issn.1673-548X.2015.01.032 [Google Scholar]
- 26.Kim MH, Oh AY, Han SH, et al. The effect of magnesium sulphate on intubating condition for rapid-sequence intubation: a randomized controlled trial. J Clin Anesth 2015; 27: 595–601. [DOI] [PubMed] [Google Scholar]
- 27.Choi ES, Jeong WJ, Ahn SH, et al. Magnesium sulfate accelerates the onset of low-dose rocuronium in patients undergoing laryngeal microsurgery. J Clin Anesth 2017; 36: 102–106. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs-Buder T, Claudius C, Skovgaard LT, et al. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand 2007; 51: 789–808. [DOI] [PubMed] [Google Scholar]
- 29.Cooper R, Mirakhur RK, Clarke RS, et al. Comparison of intubating conditions after administration of Org 9246 (rocuronium) and suxamethonium. Br J Anaesth 1992; 69: 269–273. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Rubio L, Solis Garcia Del Pozo J, Nava E, et al. Interaction between magnesium sulfate and neuromuscular blockers during the perioperative period. A systematic review and meta-analysis. J Clin Anesth 2016; 34: 524–534. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol 2000; 53: 964–972. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht E, Kirkham KR, Liu SS, et al. The analgesic efficacy and safety of neuraxial magnesium sulphate: a quantitative review. Anaesthesia 2013; 68: 190–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211027736 for Efficacy of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia: a meta-analysis by Haiyan Sun, Tao Jin, Xiping Wu, Lei Yang, Yunxia Zuo and Ren Liao in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211027736 for Efficacy of magnesium sulfate as an adjuvant to rocuronium in general anaesthesia: a meta-analysis by Haiyan Sun, Tao Jin, Xiping Wu, Lei Yang, Yunxia Zuo and Ren Liao in Journal of International Medical Research