Abstract
Objective
Two critical processes in the coronavirus disease 2019 (COVID-19) pandemic involve assessing patients’ intensive care needs and predicting disease progression during patients’ intensive care unit (ICU) stay. We aimed to evaluate oxidative stress marker status at ICU admission and ICU discharge status in patients with COVID-19.
Methods
We included patients in a tertiary referral center ICU during June–December 2020. Scores of Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), and clinical severity, radiologic scores, and healthy discharge status were noted. We collected peripheral blood samples at ICU admission to evaluate total antioxidants, total oxidants, catalase, and myeloperoxidase levels.
Results
Thirty-one (24 male, 7 female) patients were included. At ICU admission, patients’ mean APACHE II score at ICU admission was 17.61 ± 8.9; the mean SOFA score was 6.29 ± 3.16. There was no significant relationship between clinical severity and oxidative stress (OS) markers nor between radiological imaging and COVID-19 data classification and OS levels. Differences in OS levels between patients with healthy and exitus discharge status were not significant.
Conclusions
We found no significant relationship between oxidative stress marker status in patients with COVID-19 at ICU admission and patients’ ICU discharge status.
Keywords: Catalase, coronavirus disease 2019, myeloperoxidase deficiency, critical care, oxidative stress, discharge status
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has undergone numerous mutations since its emergence, with different clinical manifestations such as asymptomatic infection, mild upper respiratory tract symptoms, or involvement of the cardiovascular, neurologic, gastrointestinal, and respiratory systems. Reported mortality rates of SARS-CoV-2 infection are between 1.7% and 5%. 1 The unusual clinical course and involvement of different systems has led clinicians to believe that the pathophysiology of coronavirus disease 2019 (COVID-19) might be related to acute inflammation, an infection-related cytokine storm, acute thromboembolic conditions, or oxidative stress (OS)-related disruption of the oxidant–antioxidant balance. Among these mechanisms, OS has been garnering attention because it is involved in inflammation, programmed cell death, the immune response, and natural defense against pathogens and viral diseases. 2
SARS-CoV-2 enters human cells via angiotensin-converting enzyme 2 (ACE2) receptors. The interaction between the virus and ACE2 receptors is also important for increasing OS markers in cells.3,4 Recent studies have emphasized the importance of OS in patients with COVID-19 in terms of disease severity leading to complications, a greater likelihood of admission to the intensive care unit (ICU), and an increased mortality rate.5–7
The most critical point in the clinical course of COVID-19 is patient deterioration and emergence of the need for ICU admission. Determining the priority of patient referral to the ICU during peaks in the pandemic is of great importance because the capacity of ICU services worldwide are limited. For this reason, objective parameters for use in in this evaluation process are needed. Although the criteria for ICU admission might vary according to the severity of an epidemic wave and available resources, the main criteria for ICU admission are hypoxia (oxygen saturation <90% even with oxygen support of 6 L/minute), hemodynamic instability necessitating vasoactive agents, the presence of acute respiratory distress syndrome (ARDS), and the need for mechanic ventilation. Apart from these, use of certain laboratory values has also been proposed, such as elevated inflammatory or coagulation markers (D-dimer level >1 µg/mL, elevated fibrin degradation products, prolonged activated partial thromboplastin time and prothrombin time, worsening lymphopenia, neutrophil count, elevated troponin, alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase levels. 8 However, the search for objective parameters is ongoing. Another critical factor is prediction of disease progression in the ICU using objective parameters.
Although it is widely speculated that OS is involved in the pathophysiology of COVID-19, to our knowledge, no other studies have evaluated OS as a marker to predict the need for ICU admission and disease progression in the ICU period. For this reason, we primarily aimed to investigate the relationship between OS markers (total antioxidant status [TAS], total oxidant status [TOS], myeloperoxidase [MPO], catalase [CAT], ferritin, D-dimer) in samples collected on the day of ICU admission from patients with COVID-19. We examined COVID-19 clinical severity classification, radiological scoring, and ferritin and D-dimer levels, which are accepted laboratory criteria according to previous reports. Furthermore, we secondarily aimed to investigate the relationship between initial OS markers at ICU admission and patients’ discharge status (exitus [EX] or healthy) from the ICU.
Methods
This study was conducted at a tertiary referral center between June and December 2020 and was approved by the Bezmialem Vakif University ethics committee for non-invasive studies (approval no. 54022451-050.05.04). Patients were admitted to the ICU according to the ICU triage criteria (Table 1), which is established as a routine work-up at our hospital. Informed consent was obtained from patients if possible, or from first-degree relatives for patients who were unable to provide their consent. We excluded patients who has bacterial sepsis, bacterial pneumonia, diagnosed immune suppression, chronic granulomatous diseases, and smoking, which might influence OS status.
Table 1.
ICU triage criteria.
| Recommendations for intensive care unit admission |
|---|
| • In case of dyspnea and respiratory distress • Respiratory rate ≥30/minute • Oxygen saturation <93% despite nasal oxygen support of 5 L/minute and above • Partial oxygen pressure <60 mmHg despite nasal oxygen support of 5 L/minute and above • PaO2/FiO2 <300 • Bilateral or multilobar infiltrations on chest radiography or computed tomography with clinical deterioration or increase in infiltrations compared with previous imaging • Hypotension (systolic blood pressure <90 mmHg, drop in usual systolic blood pressure >40 mmHg, mean arterial pressure <65 mmHg) or vasopressor requirement • Signs of hypoperfusion in the skin, lactate >2 mmol/L, increase in SOFA score (>2) • Elevation in cardiac enzymes (troponin) or arrhythmia • Kidney and liver abnormalities, thrombocytopenia • Development of MAS |
ICU, intensive care unit; PaO2/FiO2, partial pressure of arterial oxygen to fraction of inspired oxygen; SOFA, Sequential Organ Failure Assessment; MAS, macrophage activation syndrome.
For all patients, we collected demographic characteristics, comorbid diseases, need for mechanic ventilation, Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores at ICU admission, ICU process, and patient discharge status from the ICU (healthy or EX).
On the day of ICU admission, we collected blood samples from all patients. The samples were centrifuged at 3000 × g for 10 minutes. Serum samples were collected and stored at −80°C for subsequent testing. We examined serum D-dimer, ferritin, C-reactive protein (CRP), and procalcitonin levels and markers of OS including TAS, TOS, CAT, and MPO in the patient samples.
Measurements
TAS levels were measured using an auto-analyzer (Siemens ADVIA 1200; Siemens Healthcare Diagnostics, Deerfield, IL, USA), following the method developed by Erel. 9 The results are expressed as mmol Trolox equiv/L. TOS levels were also measured according to the method described by Erel, with an automated calorimetric process (Siemens ADVIA 1200). 10 Results are expressed as µmol H2O2 equiv/L.
Antioxidative status indicator CAT activity was measured using a spectrophotometric method that is based on H2O2 decrement with degradation, as described by Aebi et al. 11 (Siemens ADVIA 1200) and is expressed as IU/mL. MPO activity was measured with an auto-analyzer (Siemens ADVIA 1200) using the method described by Krawisz et al. 12 and results are expressed as IU/mL.
The clinical condition of patients was standardized according to the Chinese National Health Commission clinical classification scoring system (Table 2). 13 In all patients, chest computed tomography images were reviewed and evaluated according to the COVID-19 imaging reporting and data system (COVID-RADS) scoring system. This evaluation was conducted by a specialized radiologist who was blinded to patients’ clinical characteristics. 14 We recorded the discharge status of patients as either EX or healthy at the end of the period in the ICU.
Table 2.
Clinical classification of COVID-19, National Health Commission of China.
| Mild | Common | Severe | Critically severe |
|---|---|---|---|
| Mild clinical manifestations, No imaging performed |
Fever, respiratory symptoms, pneumonia on X-ray or CT |
Meeting any of the following: 1. Respiratory distress, RR ≥30 breaths/minute 2. Oxygen saturation ≤93% at rest 3. Arterial partial pressure of oxygen (PaO2)/fraction of inspired O2 (FiO2) ≤ 300 mmHg, 1 mmHg=0.133 kPa |
Meeting any of the following: 1. Respiratory failure, need for mechanical ventilation 2. Shock 3. Combined with other organ failure, need for ICU monitoring and treatment |
COVID-19, coronavirus disease 2019; CT, computed tomography; RR, respiration rate; ICU, intensive care unit.
Statistical analysis
The analysis was performed with IBM SPSS for Windows 20.0 (IBM Corp., Armonk, NY, USA). Descriptive data are expressed as median (minimum–maximum). Continuous variables were compared between radiologic classification groups and between clinical severity groups with the Mann–Whitney U test. The Spearman correlation test was used to evaluate the relationship of APACHE and SOFA scores with laboratory results. A value p<0.05 was considered to indicate statistical significance. To evaluate TAS, TOS, MPO, CAT, ferritin, and D-dimer in predicting the discharge status of patients at the end of the ICU stay, we used specificity, sensitivity, cut-off point, and area under the receiver operating characteristic (ROC) curve.
Results
Thirty-one (24 male, 7 female) patients who fulfilled the inclusion criteria were included in the study. Patients’ demographic characteristics are shown in Table 3. The results of patient blood sample testing on the day of ICU admission for CRP, procalcitonin, and ferritin, as well as the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (PaO2/FiO2) and serum MPO and CAT values are listed in Table 3.
Table 3.
Patients’ demographic and initial laboratory findings at ICU admission.
| n | Min. | Max. | Mean | SD | |
|---|---|---|---|---|---|
| Age (years) | 31 | 43 | 91 | 68.42 | 14.01 |
| Weight (kg) | 31 | 56 | 160 | 79.94 | 18.17 |
| APACHE | 31 | 5 | 41 | 17.61 | 8.98 |
| SOFA | 31 | 2 | 13 | 6.29 | 3.16 |
| ICU (days) | 31 | 5 | 46 | 17.10 | 11.51 |
| Lymphocytes (103/uL) | 31 | 0.36 | 26.3 | 4.14 | 6.42 |
| CRP (mg/mL) | 31 | 2 | 338 | 98.85 | 94.18 |
| Procalcitonin (ng/mL) | 31 | 0 | 37.5 | 4.88 | 9.47 |
| Ferritin (ng/mL) | 30 | 36 | 49000 | 3713.20 | 9386.89 |
| PaO2/FiO2 | 31 | 84.7 | 502.8 | 171.27 | 80.10 |
| MPO (IU/mL) | 31 | −3 | 884 | 167.06 | 259.89 |
| CAT (IU/mL) | 31 | 2 | 60 | 23.74 | 21.23 |
| TAS (mmol Trolox equiv/L) | 31 | 0.11 | 0.53 | 0.26 | 0.07 |
| TOS (μmol H2O2 equiv/L) | 31 | 0.06 | 0.92 | 0.23 | 0.20 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein; PaO2/FiO2, partial pressure of arterial oxygen/fraction of inspired oxygen; MPO, myeloperoxidase; CAT, catalase; ICU, intensive care unit; SD, standard deviation; TAS, total antioxidant status; TOS, total oxidant status.
No significant correlation was found between APACHE II and SOFA scores and TAS, TOS, CAT, and MPO levels (Table 4).
Table 4.
Correlation of patients’ APACHE II and SOFA scores with laboratory findings.
| APACHE | SOFA | |
|---|---|---|
| TAS (mmol Trolox equiv/L) | 0.680 | 0.104 |
| TOS (µmol H2O2 equiv/L) | 0.265 | 0.445 |
| TAS/TOS | 0.243 | 0.225 |
| CAT (IU/mL) | 0.541 | 0.422 |
| MPO (IU/mL) | 0.586 | 0.492 |
| Ferritin (ng/mL) | 0.052 | 0.248 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; TAS, total antioxidant status; TOS, total oxidant status; MPO, myeloperoxidase; CAT, catalase.
As for the clinical condition of patients, 13 patients were classified as severe and 18 patients as critically severe, according to the Chinese National Health Committee COVID-19 clinical classification scoring system. A comparison of the groups revealed no statistically significant difference in terms of TAS, TOS, TAS/TOS, CAT, MPO, and ferritin levels (Table 5).
Table 5.
Comparison of patients’ clinic classification and laboratory findings.
| Severe | Critically severe | p | |
|---|---|---|---|
| TAS (mmol Trolox equiv/L) | 0.28 (0.18–0.38) | 0.24 (0.11–0.53) | 0.125 |
| TOS (μmol H2O2 equiv/L) | 0.15 (0.09–0.92) | 0.16 (0.06–0.54) | 0.767 |
| TAS/TOS | 1.92 (0.38–3.56) | 1.64 (0.39–4.00) | 0.859 |
| CAT (IU/mL) | 11 (2–60) | 14 (2–58) | 0.953 |
| MPO (IU/mL) | 33 (−3–884) | 49 (0–667) | 0.890 |
| Ferritin (ng/mL) | 1053.62 (35.96–4556) | 765.80 (73.96–49000) | 0.680 |
Note: The values in parentheses in the table indicate the range.
TAS, total antioxidant status; TOS, total oxidant status; MPO, myeloperoxidase; CAT, catalase.
According to COVID-RADS classification, 6 patients were grade 2A, 13 patients were grade 2B, and 12 patients were grade 3. In patients with grades 2A, 2B, and 3 patients, serum levels of TAS, TOS, TAS/TOS, CAT and MPO showed no significant difference; however, ferritin levels were significantly higher in patients with grade 2A compared with those who were classified as grade 2B (p = 0.045) (Table 6).
Table 6.
Comparison of patients’ radiologic evaluation and laboratory findings.
| COVID-RADS grade | 2A | 2B | 3 | p |
|---|---|---|---|---|
| TAS (mmol Trolox equiv/L) | 0.26 (0.22–0.36) | 0.28 (0.11–0.38) | 0.24 (0.18–0.53) | 0.623 |
| TOS (μmol H2O2 equiv/L) | 0.13 (0.06–0.20) | 0.15 (0.11–0.92) | 0.19 (0.09–0.78) | 0.258 |
| TAS/TOS | 2.20 (1.30–4.00) | 1.57 (0.41–2.73) | 1.60 (0.28–3.56) | 0.195 |
| CAT (IU/mL) | 13 (2–41) | 8 (2–59) | 28.5 (2–60) | 0.176 |
| MPO (IU/mL) | 41 (7–56) | 29 (3–733) | 73 (0–884) | 0.487 |
| Ferritin (ng/mL) | 3443 (465–4521) | 321 (35.96–3445) | 1482 (95.62–49000) | 0.020* 0.045** |
*Comparison of all groups. **Comparison of groups 2A and 2B.
Note: The values in parentheses in the table indicate the range.
COVID-RADS, COVID-19 imaging reporting and data system; TAS, total antioxidant status; TOS, total oxidant status; MPO, myeloperoxidase; CAT, catalase.
A comparison of TAS, TOS, TAS/TOS, CAT, and MPO levels between patients with healthy ICU discharge status and EX discharge status revealed no significant differences. However, ferritin levels were significantly higher in patients with a healthy ICU discharge status compared with their counterparts who had an EX discharge status (p = 0.040) (Table 7).
Table 7.
Comparison of patients’ ICU discharge status and laboratory findings.
| Healthy discharge | Exitus discharge | p | |
|---|---|---|---|
| TAS (mmol Trolox equiv/L) | 0.23 (0.17–0.36) | 0.27 (0.11–0.53) | 0.187 |
| TOS (μmol H2O2 equiv/L) | 0.14 (0.10–0.78) | 0.16 (0.06–0.92) | 0.674 |
| TAS/TOS | 1.89 (0.32–2.40) | 1.72 (0.28–4.00) | 0.642 |
| CAT (IU/mL) | 12.5 (2–60) | 14 (2–59) | 0.947 |
| MPO (IU/mL) | 35 (0–884) | 49 (3–733) | 0.842 |
| Ferritin (ng/mL) | 2894 (219.20–49000) | 562.62 (35.96–21094.15) | 0.040 |
Note: The values in parentheses in the table indicate the range.
ICU, intensive care unit; TAS, total antioxidant status; TOS, total oxidant status; MPO, myeloperoxidase; CAT, catalase.
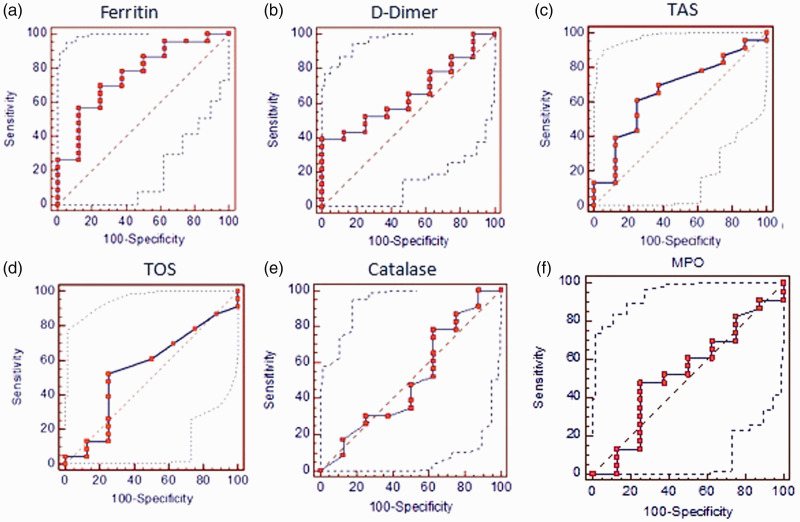
ROC curve analysis was used to evaluate ferritin, D-dimer, TAS, TOS, CAT, and MPO in predicting EX or healthy ICU discharge among patients (Figure 1). The results were as follows.
Figure 1.
Receiver operating characteristic curve analysis to evaluate ferritin (a) D-dimer (b) TAS (c) TOS (d) catalase (e) and MPO (f) levels in predicting discharge status from the intensive care unit
MPO: myeloperoxidase; TAS: total antioxidant status; TOS: total oxidant status.
Ferritin showed AUC = 0.750 (p = 0.039) and 95% confidence interval (CI) (0.549–0.951) (Figure 1a). The cut-off point of ferritin for healthy ICU discharge was ≥1482.86 with 75.0% sensitivity and 68.2% sensitivity. For D-dimer, we found an AUC = 0.652 and 95% CI (0.461–0813) (Figure 1b). D-dimer was not found to be significantly specific or sensitive for ICU discharge status: AUC = 0.663 and 95% CI (0.17–0.31) (Figure 1c). TAS was also not significantly specific or sensitive for ICU discharge status.
TOS showed an AUC = 0.552 and 95% CI (0.36–0.73) (Figure 1d) and was therefore not significantly specific and sensitive for ICU discharge. For CAT, AUC = 0.508 and 95% CI (0.32–0.69) (Figure 1e). CAT was also not significantly specific or sensitive for ICU discharge. Finally, an AUC = 0.524 and 95% CI (0.34–0.70) were found for MPO (Figure 1f), which was not significantly specific or sensitive for ICU discharge.
Discussion
The most critical periods during the COVID-19 pandemic are when ICU admissions are increased. Under conditions of limited critical care services, it is of critical importance to predict those patients that will benefit most from ICU care so as to optimize the selection of patients for admission to the ICU and ICU bed occupation intervals. 1 , 8 Laboratory, radiology, and clinical assessment are widely used for this purpose. TAS and TOS, which have key roles in the pathophysiology of COVID-19, have rarely been investigated to date.
OS and antioxidant capacity are balanced in healthy patients. 15 However, this balance is disrupted with aging, cancer, inflammation, allergy, and certain systemic or localized diseases. When antioxidant status is overwhelmed by OS, metabolic changes can occur, such as apoptosis, vascular endothelial changes, mitochondrial damage, expression of inflammatory cytokines, disruption of cellular repair mechanisms, and disease progression.4,16
SARS-CoV-2 uses the ACE2 receptor to enter cells and affects the production of nitric oxidase, increasing oxidation and producing oxidized phospholipids, which are increased in the lungs of patients with COVID-19. Infection with the virus also changes intracellular ferritin mechanisms and disrupts the endothelial barrier, causing vascular damage. 17 Studies have shown that deficiencies in antioxidant capacity result in ferroptosis-related cellular death, and viral infections cause cell death via this mechanism. 18 The findings of these previous studies and other smaller-sized studies18,19 suggest that it is important to investigate the role of OS in the pathophysiology of COVID-19.
Muhammad et al. 20 showed that patients with COVID-19 had increased OS and decreased antioxidant status, with deteriorated CAT and MPO activities. In contrast, Gaud et al. 21 revealed increased CAT and MPO levels in viral infections, which are correlated with clinical progression. Thus, it has been observed that OS levels change in COVID-19 and other viral infections; however, which parameters change and the pathophysiological mechanisms have remained unclear. In ARDS animal models of SARS-CoV-2 infection, deficiencies in antioxidant systems have been implicated in the pathophysiology of respiratory disease progression, accelerated clinical deterioration, and slowing of the healing process.22–24 In another study, peripheral blood samples of patients who recovered from SARS-Cov-2 infection showed upregulation of mitochondrial genes in mononuclear cells, which are responsible for reactions to OS; it was speculated that the antioxidant system might have a role in the recovery process. 25 Compatible with these findings, recent studies have revealed that antioxidant administration via nebulizer or systemically might have an impact on the healing process in patients with COVID-19. 23 , 26
Although research is ongoing regarding the importance of OS in diagnosis and treatment, no studies to date have evaluated OS status at the time of ICU admission, a critical point in the disease process that has a possible effect on clinical progress. Previous studies have mostly been hypothetical and included a limited number of patients, with no group homogeneity. Therefore, the relationship of OS with COVID-19 is still controversial.
Our study was based on the hypothesis that OS levels might change over time among patients admitted to the ICU. However, no clinical association was found between OS parameters and clinical or radiological findings. Furthermore, from the viewpoint of disease progression, OS also had no effect on patients’ ICU discharge status, i.e., whether patients had a healthy or an EX discharge status.
There are some limitations in our research. First, we used a retrospective design in this cross-sectional study to evaluate OS and antioxidant parameters. The dynamic changes in OS during the ICU period cannot be captured in a cross-sectional study. A prospective study design where disease progress can be dynamically monitored is more appropriate because the OS of each patient will differ according to age, sex, and concomitant diseases, among other factors. Another limitation of our study is the small number of patients. Owing to the study design, we could not calculate the sample size, which would improve the reliability of our results. Related to this, the hypothesis proposed in previous studies that OS is central to the pathophysiology of COVID-19 could be confirmed or ruled out in the present study, and it remains unclear whether oxidative markers are related to the disease process. Therefore, more homogenous, large-scale studies with longer follow-up and daily OS level measurement are needed to reach a more definitive conclusion related to this topic.
Conclusion
Although OS had been speculated in the literature to have an impact on COVID-19 pathogenesis, disease severity, prognosis, and treatment, we did not find any relationship of OS levels with widely used criteria for ICU admission nor between OS markers and patients’ ICU discharge status. According to the current evidence, OS does not seem to be an objective parameter for decision making regarding admission of patients with COVID-19 to the ICU or for predicting the ICU discharge status of this patient population.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Hayrettin Daskaya https://orcid.org/0000-0002-0155-1387
Harun Uysal https://orcid.org/0000-0003-0426-8525
References
- 1.WorldOMeter. Coronavirus Worldwide Graphs. https://www.worldometers.info/coronavirus/worldwide-graphs/#europe-usa-deaths (Accessed: 12.03.2021)
- 2.Ayar G, Atmaca YM, Alışık M, et al. Effects of paraoxonase, arylesterase, ceruloplasmin, catalase, and myeloperoxidase activities on prognosis in pediatric patients with sepsis. Clin Biochem 2017; 50: 414–417. [DOI] [PubMed] [Google Scholar]
- 3.Fratta Pasini AM, Stranieri C, Cominacini L, et al. Potential Role of Antioxidant and Anti-Inflammatory Therapies to Prevent Severe SARS-Cov-2 Complications. Antioxidants 2021; 10: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suhail S, Zajac J, Fossum C, et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J 2020; 39: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses 2020; 143: 110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat Rev Immunol 2020; 20: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernyak BV, Popova EN, Prikhodko AS, et al. COVID-19 and oxidative stress. Biochemistry (Mosc) 2020; 85: 1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004; 37: 112–119. [DOI] [PubMed] [Google Scholar]
- 10.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005; 38: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 11.Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105: 121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed]
- 12.Methods of enzymatic analysis: Third edition: Editor‐in‐Chief: Hans Ulrich Bergmeyer. Verlag Chemie, 1983, p.273. [Google Scholar]
- 13.Krawisz J, Sharon P, Stenson W. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984; 87: 1344–1350. [PubMed] [Google Scholar]
- 14.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. (Accessed: 12.03.2021)
- 15.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol 2020; 30: 4930–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams L, Franco MC, Estevez AG. Reactive nitrogen species in cellular signaling. Exp Biol Med (Maywood). 2015; 240: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busse LW, Chow JH, McCurdy MT, et al. COVID-19 and the RAAS-a potential role for angiotensin II? Crit Care 2020; 24: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis 2020; 97: 303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komaravelli N, Casola A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J Pharmacogenomics Pharmacoproteomics 2014; 5: 1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhammad Y, Kani YA, Iliya S, et al. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med 2021; 9: 2050312121991246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goud PT, Bai D, Abu-Soud HM. A Multiple-Hit Hypothesis Involving Reactive Oxygen Species and Myeloperoxidase Explains Clinical Deterioration and Fatality in COVID-19. Int J Biol Sci 2021; 17: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polonikov A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect Dis 2020; 6: 1558–1562. [DOI] [PubMed] [Google Scholar]
- 24.Silvagno F, Vernone A, Pescarmona GP. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020; 9: 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Den Brand JM, Haagmans BL, Van Riel D, et al. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. J Comp Pathol 2014; 151: 83–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao H, Lan D, Duan Z, et al. Upregulation of mitochondrial gene expression in PBMC from convalescent SARS patients. J Clin Immunol 2006; 26: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]