Abstract
Objective
This meta-analysis evaluated the effect of probiotics and synbiotics on insulin resistance in patients with polycystic ovary syndrome (PCOS).
Methods
A systematic search was performed to identify all relevant publications listed on the electronic databases (PubMed®, Web of Science, Embase® and China National Knowledge Infrastructure) between inception and 30 October 2020. All statistical analyses were performed on randomized controlled trials (RCTs) using RevMan version 5.3 software provided by the Cochrane Collaboration.
Results
A total of 486 patients from seven RCTs were included in the meta-analysis. Probiotic and synbiotic supplementation appeared to improve levels of homeostatic model assessment of insulin resistance (mean difference = –0.37; 95% confidence interval –0.69, –0.05) and serum insulin (standardized mean difference = –0.66; 95% confidence interval –1.19, –0.12). The results failed to show any influence of probiotic and synbiotic supplementation on body mass index, waist circumference, hip circumference and fasting blood sugar.
Conclusions
Probiotics and synbiotics appear to have a partially beneficial effect on indices of insulin resistance in patients with PCOS.
Keywords: Insulin resistance, meta-analysis, obesity, polycystic ovary syndrome, probiotics, synbiotics
Introduction
Polycystic ovary syndrome (PCOS) is among the most common endocrine and metabolic disorders, affecting 6–21% of reproductive-aged women worldwide. 1 The clinical manifestations of PCOS are heterogeneous and complex. For example, it is characterized by ovulatory dysfunction, hyperandrogenism and polycystic ovaries on ultrasonography. 2 PCOS increases the risk of long-term complications such as infertility, endometrial cancer, obesity, metabolic disease, dyslipidaemia and cardiovascular disease. 3 These symptoms and complications seriously affect patients’ lives. 4 Despite the high prevalence and marked impact of PCOS, its pathogenesis and treatment vary and remain poorly defined.
In recent years, insulin resistance (IR) has been shown to be a key aetiological component of PCOS. 5 Metformin, an insulin sensitizer, is used as an important routine treatment for PCOS. 6 However, its gastrointestinal side-effects reduce treatment adherence rates and this has motivated the search for a novel treatment for IR. 7 IR is closely correlated with changes in the intestinal microbiota, with dynamic changes in the intestinal microbiota structure affecting the occurrence and development of a variety of endocrine metabolic diseases. 8 Hence, increasing attention is being paid to probiotics and synbiotics, which can shape the intestinal microbiota, leading to the cure of some diseases and the promotion of body health. 9 Recent studies demonstrated that probiotic and synbiotic supplementation affects the metabolic status of IR.10,11 However, the effects of probiotics and synbiotics on IR in women with PCOS remain controversial. For example, a previous study showed that synbiotic supplementation had no beneficial effects on insulin metabolism in patients with PCOS. 12 A meta-analysis reported that the effects on some metabolic indices (homeostatic model assessment of insulin resistance [HOMA-IR], quantitative insulin sensitivity check index [QUICKI] and fasting plasma glucose [FPG]) were negligible and not clinically significant. 13 In contrast, a randomized double-blind study reported that 12-week synbiotic supplementation significantly improved HOMA-IR and QUICKI in patients with PCOS. 14
The current meta-analysis summarized the available evidence and comprehensively evaluated the effects of probiotic supplementation on the markers of IR in women with PCOS in order to clarify the curative effects of probiotic supplementation on IR in PCOS and provide accurate nutritional advice for patients with PCOS.
Materials and methods
Search strategy
A systematic search of publications listed on the electronic databases (PubMed®, Web of Science, Embase® and China National Knowledge Infrastructure) between inception and 30 October 2020 was conducted using the following keywords: (i) polycystic ovary syndrome or polycystic ovary disease or PCOS; and (ii) probiotics, prebiotics or synbiotics. In addition, a manual review of the reference list of each identified article was undertaken to identify additional possible studies. The protocol of this meta-analysis was registered at INPLASY (no. INPLASY202150112).
Inclusion and exclusion criteria
Studies were eligible for inclusion if they met the following criteria: (i) randomized controlled trials (RCTs) evaluating the effects of probiotic and/or synbiotic supplementation in the treatment of patients with PCOS; (ii) studies that enrolled women with a standard diagnosis of PCOS according to the European Society of Human Reproduction and Embryology (ESHRE), the American Society of Reproductive Medicine (ASRM) or the National Institutes of Health (NIH); (iii) administration of probiotics and/or synbiotics as an intervention or the administration of metformin as a co-intervention in the intervention and control groups; (iv) indicators of insulin resistance including HOMA-IR, serum insulin and fasting blood sugar (FBS) were considered as the primary outcomes. Especially, HOMA-IR is a reliable homeostasis model of insulin resistance. The secondary outcomes that were used as indices to evaluate central obesity included body mass index (BMI), waist circumference (WC) and hip circumference (HC). The language was restricted to English and Chinese. The exclusion criteria were as follows: (i) studies that included patients with other diseases such as Cushing’s syndrome, type 1 or 2 diabetes mellitus, hyperthyroidism or other hormone-related disorders; (ii) studies with unavailable data or unreported target outcomes.
Study selection was based on an initial screening of identified abstracts or titles and a second screening of full-text articles. Two authors (X.J.F. and Y.C.) independently reviewed the articles for eligibility. Differences between the two reviewers were resolved by consensus. Any unresolved issues were referred to a third author (Q.G.G.).
Data extraction
Two authors (C.Y.M. and X.J.F.) read the selected studies for the meta-analysis and extracted the following information: study design, first author’s name, publication year, country, study size, detailed medications, control interventions, duration and main outcomes. If necessary, missing information was requested from the original authors.
Quality assessment
Two authors (Y.C. and Y.Z.) independently assessed the quality of the included studies. The evaluation was based on the following criteria: study design and case characteristic matching, patient inclusion and exclusion criteria, and the Jadad scale for RCTs (randomization, hidden allocation, blinding and follow-up). 15
Statistical analyses
RevMan software (version 5.3: Cochrane Collaboration, Oxford, UK) was used to conduct the statistical analyses according to the guidelines described in the Cochrane Handbook for Systematic Reviews of Interventions. If all the studies had the same scale, the results were combined for the meta-analysis as the mean difference (MD) with 95% confidence intervals (CIs). When the data were reported using different methods or scales, the standardized mean difference (SMD) was calculated. Two authors (C.Y.M. and X.J.F.) independently assessed the methodological quality of the included studies according to the Jadad scale and the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions in order to determine the risk of bias. The I2 statistic was calculated to measure the heterogeneity. If significant heterogeneity was observed between the studies (Cochran’s Q χ2-test), a random-effects model was utilized. If there was no significant heterogeneity between the studies, a fixed-effects model was utilized. Funnel plot were used to assess publication bias. A P-value < 0.05 was considered statistically significant.
Results
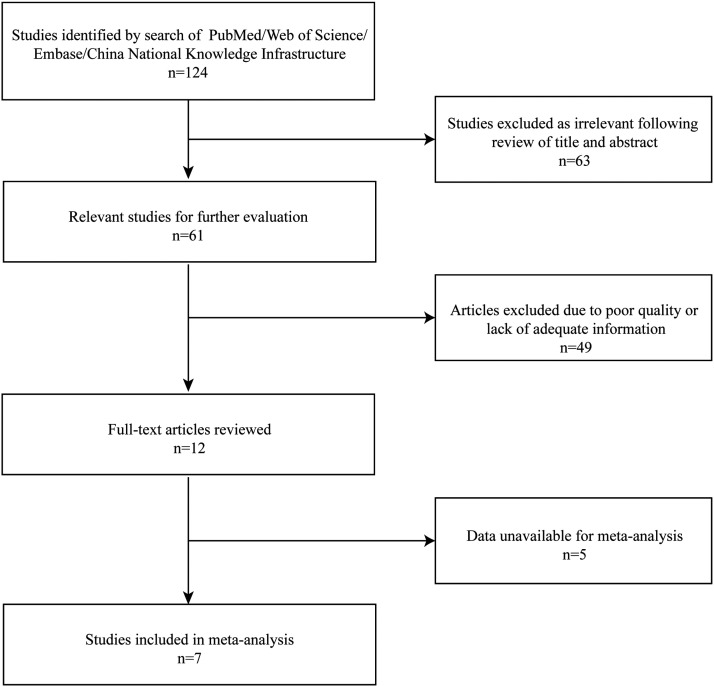
The preliminary search identified 124 studies (Figure 1). Of these, 63 were considered irrelevant or duplicates and were excluded. After the review of the article abstracts, 49 articles were excluded due to poor quality or lack of adequate information. After full-text review of the remaining 12 articles, five articles were excluded due to data unavailable. Seven studies were included in this meta-analysis.12,14,16–20
Figure 1.
Flow chart of eligible studies showing the number of citations identified, retrieved and included in the final meta-analysis.
The seven included RCTs included a total of 486 participants. The general characteristics of the included studies are shown in Table 1.12,14,16–20The diagnostic criteria for PCOS in all studies were based on the ESHRE and ASRM guidelines.
Table 1.
Studies included in a meta-analysis to evaluate the relationship between probiotic and synbiotic supplementation and insulin resistance in patients with polycystic ovary syndrome.12,14,16–20
| Author, year | Country | Diagnostic criteria | Sample size, n | Duration | Intervention arm | Control arm | Outcomes |
|---|---|---|---|---|---|---|---|
| Esmaeilinezhad et al., 2019 16 | Iran | Rotterdam | 46 | 8 weeks | Lactobacillus rhamnosus GG, Bacillus coagulans, Bacillus indicus | Unknown | HOMA-IR, weight, BMI, waist, hip, FBS, insulin, QUICKI, testosterone, LH, FSH, LH/FSH |
| Karimi et al., 2018 12 | Iran | Rotterdam | 99 | 12 weeks | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus rhamnosus, Bifidobacterium longus, Bifidobacterium breve, Streptococcus thermophilus | Placebo capsules containing starch and maltodextrin, but no bacteria | FBS, FPG, glycohemoglobin, insulin, CRP/HOMA-IR, QUICKI, apelin36 |
| Samimi et al., 2019 14 | Iran | Rotterdam | 60 | 12 weeks | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum | Placebo | FPG, insulin, HOMA-IR, QUICKI, TC, TG, LDL-C, HDL-C |
| Shoaei et al., 2015 17 | Iran | Rotterdam | 65 | 8 weeks | Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus Lactobacillus, Bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophiles | Placebo containing starch and maltodextrins but no bacteria | FBS, insulin, HOMA-IR, QUICKI, CRP |
| Gholizadeh Shamasbi et al., 2019 18 | Iran | Rotterdam | 62 | 3 months | 20 g of prebiotic | Placebo (maltodextrin) | BMI, weight, hip, waist |
| Karimi et al., 2020 19 | Iran | Rotterdam | 99 | 12 weeks | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus rhamnosus, Bifidobacterium longum, Bifidobacterium breve, Streptococcus thermophilus | Placebo capsules containing starch and maltodextrin but no bacteria | Weight, BMI, waist, hip, blood pressure systolic, blood pressure diastolic, TC, TG, LDL-C, HDL-C |
| Chen et al., 2018 20 | China | Rotterdam | 55 | 12 weeks | Bifid triple viable combined with metformin | Metformin | Weight, BMI, FBS, FSI, HOMA-IR, TC, TG, HDL-C, LDL-C |
HOMA-IR, homeostatic model assessment of insulin resistance; BMI, body mass index; CRP, C-reactive protein; FBS, fasting blood sugar; QUICKI, quantitative insulin sensitivity check index; LH, luteinizing hormone; FSH, follicle-stimulating hormone; FPG, fasting plasma glucose; CRP, C-reactive protein; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
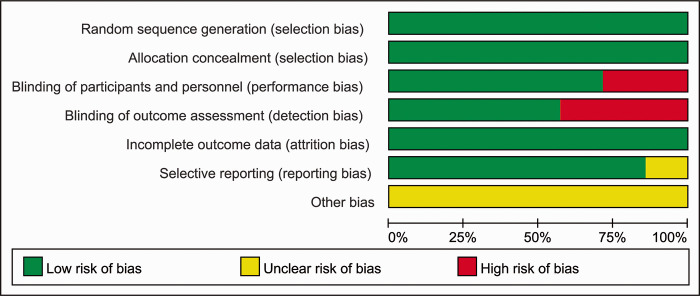
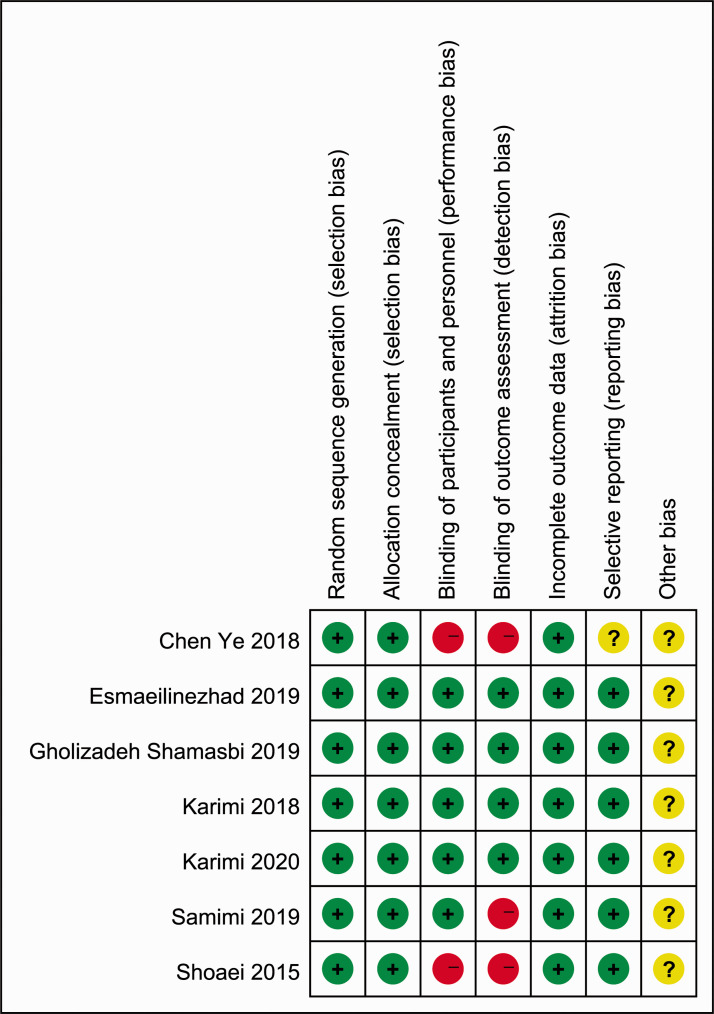
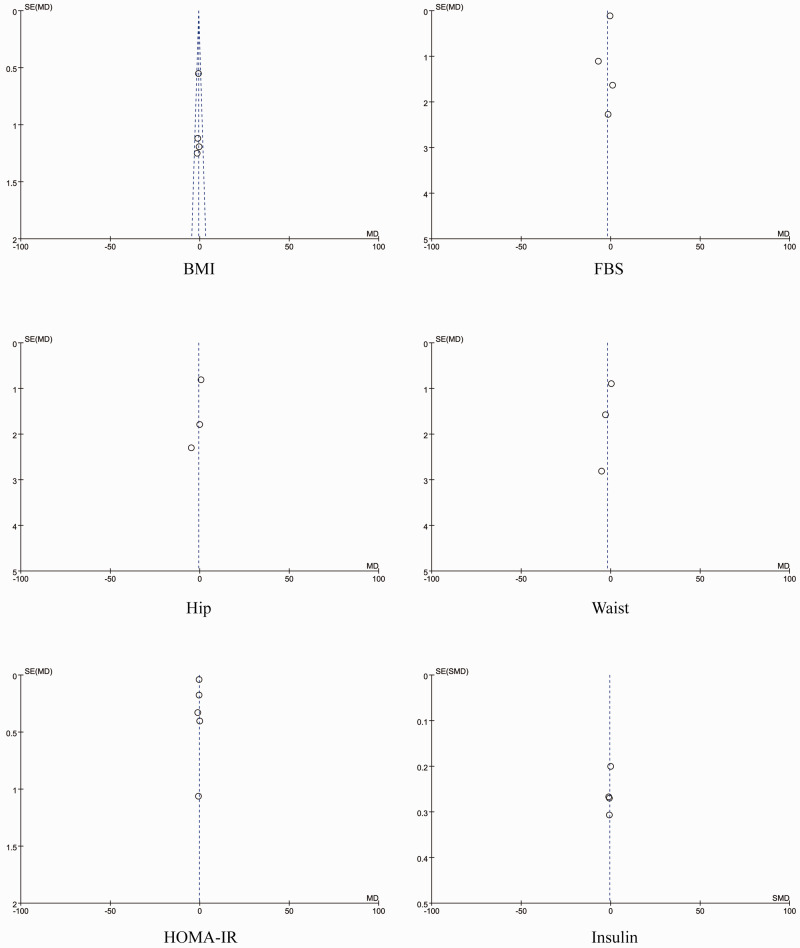
The results of the assessment of quality and bias risk of the included studies according to the Jadad scale and criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions are shown in Figures 2 and 3. The funnel plots were roughly symmetrical (Figure 4), which indicated that publication bias was not significant.
Figure 2.
The quality assessment used to evaluate the seven studies included in meta-analysis to evaluate the relationship between probiotic and synbiotic supplementation and insulin resistance in patients with polycystic ovary syndrome.12,14,16–20
Figure 3.
Quality assessment of the seven studies included in meta-analysis to evaluate the relationship between probiotic and synbiotic supplementation and insulin resistance in patients with polycystic ovary syndrome.12,14,16–20
Figure 4.
Funnel plots for publication bias based on the physical characteristic that was examined by the studies included in the meta-analysis. BMI, body mass index; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment of insulin resistance.12,14,16–20
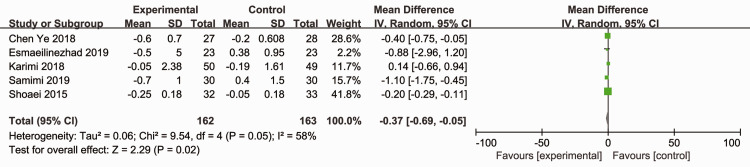
Five RCTs (325 participants) reported the effects of probiotics and synbiotics on HOMA-IR.12,14,16,17,20 As significant heterogeneity was observed (Cochran’s Q χ2-test: P = 0.05, I2 = 58%), a random-effects model was utilized. The overall analysis revealed that probiotic and synbiotic supplementation reduced HOMA-IR compared with placebo (MD = –0.37; 95% CI –0.69, –0.05; P = 0.02) (Figure 5).
Figure 5.
Effect of probiotic and synbiotic supplementation on homeostatic model assessment of insulin resistance in patients with polycystic ovary syndrome.12,14,16,17,20
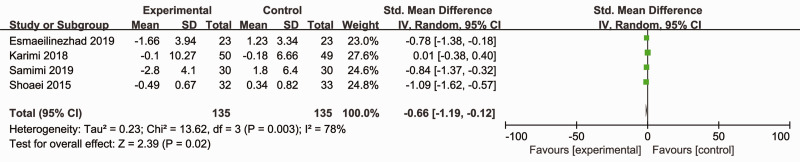
Four RCTs (270 participants) reported the effects of synbiotics on serum insulin.12,14,16,17 As significant heterogeneity was observed (Cochran’s Q χ2-test: P = 0.003, I2 = 78%), a random-effects model was utilized. The overall analysis revealed that synbiotic supplements reduced serum insulin levels compared with placebo (SMD = –0.66; 95% CI –1.19, –0.12; P = 0.02) (Figure 6). The exclusion of one study and recalculation of the pooled effect did not significantly affect the results (data not shown).
Figure 6.
Effect of synbiotic supplementation on serum insulin levels in patients with polycystic ovary syndrome.12,14,16,17
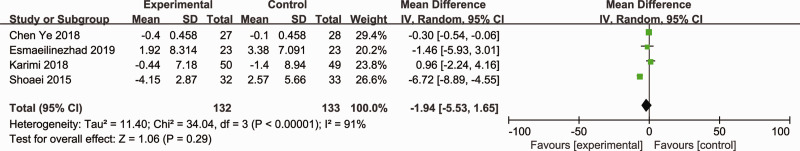
Four RCTs (265 participants) reported on the effects of probiotics and synbiotics on FBS.12,16,17,20 As significant heterogeneity was observed among the studies (Cochran’s Q χ2-test: P < 0.00001, I2 = 91%), a random-effects model was utilized. The overall analysis revealed that probiotic and synbiotic supplements did not reduce FBS compared with other treatments (MD = –1.94; 95% CI –5.53, 1.65; P = 0.29) (Figure 7).
Figure 7.
Effect of probiotic and synbiotic supplementation on fasting blood sugar levels in patients with polycystic ovary syndrome.12,16,17,20
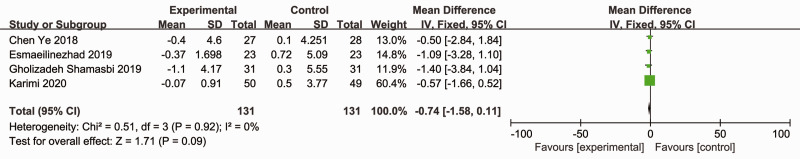
Four RCTs (262 participants) reported the effects of probiotics and synbiotics on BMI.16,18–20 There was no heterogeneity between the studies (Cochran’s Q χ2-test: P = 0.92, I2 = 0%), so a fixed-effects model was utilized. However, the data were pooled, and probiotic and synbiotic treatment showed no advantage for improving BMI compared with placebo (MD = –0.74; 95% CI –1.58, 0.11; P = 0.09) (Figure 8).
Figure 8.
Effect of probiotic and synbiotic supplementation on body mass index in patients with polycystic ovary syndrome.16,18–20
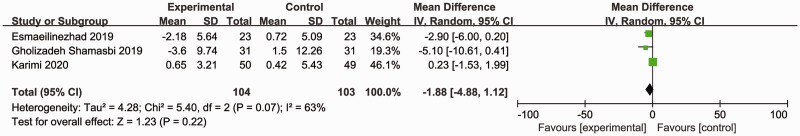
Three RCTs (207 participants) reported the effects of synbiotics on WC.16,18,19 As significant heterogeneity was observed (Cochran’s Q χ2-test: P = 0.07, I2 = 63%), a random-effects model was utilized. The overall analysis revealed that synbiotic supplementation did not reduce WC compared with placebo (MD = –1.88, 95% CI –4.88, 1.12; P = 0.22) (Figure 9).
Figure 9.
Effect of synbiotic supplementation on waist circumference in patients with polycystic ovary syndrome.16,18,19
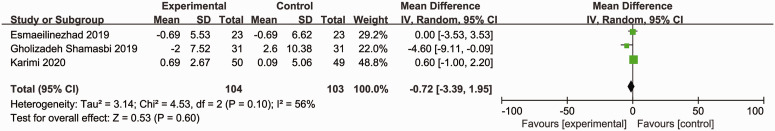
Three RCTs (207 participants) reported the effects of synbiotics on HC.16,18,19 As significant heterogeneity was observed (Cochran’s Q χ2-test: P = 0.10, I2 = 56%), a random-effects model was utilized. However, the data were pooled, and no advantages of synbiotics were observed for reducing HC compared with placebo (MD = –0.72; 95% CI –3.39, 1.95; P = 0.60) (Figure 10).
Figure 10.
Effect of synbiotic supplementation on hip circumference in patients with polycystic ovary syndrome.16,18,19
Discussion
Overall, the results of the current meta-analysis of seven RCTs on probiotic and synbiotic supplementation showed that this intervention significantly reduced HOMA-IR and serum insulin levels but did not affect the BMI, WC, HC or FBS in patients with PCOS.
It is well recognized that IR plays an important role in the pathogenesis of PCOS. 21 Approximately 40–70% of women with PCOS have IR. 22 PCOS can lead to increased steroidogenesis, deranged granulosa cell differentiation and arrested follicle growth. 3 Therefore, this current meta-analysis used the indices of IR, including HOMA-IR, serum insulin and FBS levels, as the main outcomes. HOMA-IR is widely accepted as the most sensitive index of IR. 23 However, in the current meta-analysis, FBS remained unchanged, which did not support the initial assumptions. Based on the changes in HOMA-IR and serum insulin levels, these current findings suggested that this intervention may improve IR in women with PCOS. The results were similar to those of several previous studies. For example, a previous meta-analysis suggested the beneficial effects of probiotic supplementation on FPG, insulin, HOMA-IR, QUICKI scores and markers of oxidative stress in patients with PCOS. 24 Probiotics and synbiotics can alleviate IR in other diseases. For example, a previous meta-analysis of 1119 participants from 15 studies reported that probiotics and synbiotics could decrease HOMA-IR and serum insulin levels and improve glucose metabolism in patients with gestational diabetes mellitus. 25 A randomized placebo-controlled clinical trial in patients with diabetic nephropathy indicated that 8 × 10 9 CFU per day probiotic supplementation for 12 weeks helped control glycaemic metabolism. 26 Compared with placebo, probiotic supplementation resulted in a significant reduction in FPG, serum insulin and HOMA-IR. 26
Previous research demonstrated that probiotics induced malondialdehyde levels and increased total glutathione and total antioxidant capacity in PCOS patients. 27 As a result, the antioxidant properties of probiotics play a crucial role in treating PCOS. 28 Additionally, probiotics and synbiotics affect IR by maintaining the homeostasis of the internal microbiota. 8 The gut microbiota are strongly associated with metabolism. 29 The mechanisms by which the gut microbiota improve IR include reducing intestinal permeability by maintaining the epithelial barrier function and reducing inflammation via lipopolysaccharide or short-chain fatty acids. 30
Central obesity is a pivotal risk factor for IR.31,32 Therefore, the evaluation of indicators of central obesity (BMI, WC, HC) were also used as secondary outcomes in the current meta-analysis. These results showed that probiotic and synbiotic supplementation did not affect these indices. The short duration of the intervention might be one possible explanation for this negative result. The optimal treatment duration for central obesity may be more than 12 weeks.33–35 The duration of the included studies was 8–12 weeks, which was too short to observe changes in anthropometric parameters and likely affected the accuracy of these current results.
The current meta-analysis had several limitations. First, due to the variety in the regimens, doses, durations, clinical settings and enrolled populations, most of the results reported in this study were influenced by significant heterogeneity, which may undermine the validity of the results. Among them, the various regimens may be an important factor of heterogeneity. A subgroup analysis based on different probiotic strain types was attempted to decrease the heterogeneity, but the number and sample sizes of the RCTs were too small to enable this analysis. Secondly, the geographic and ethnic groups included in the study were limited. Although there were no national restrictions, six studies were conducted in Iran and only one was conducted in China. This fact may limit the generalizability of the current findings. Thirdly, the effect was assessed by very few studies and grey literature was not included. Moreover, because several studies also did not apply appropriate randomization and allocation concealment, there was a risk of selection bias. Thus, the results should be interpreted with caution. Further studies including large sample sizes, different ethnic groups and long-term RCTs are needed to determine whether probiotic and synbiotic supplementation could be effective for the treatment of PCOS. As diet is associated with intestinal microbiota, 36 this factor should be strictly controlled in future studies.
In conclusion, despite its limitations, this current meta-analysis illustrated that probiotic and synbiotic supplementation was somewhat effective for IR in patients with PCOS. Although the effect of probiotics on IR in patients with PCOS appears to be supported by evidence, more information about the mechanisms by which this occurs in the treatment of PCOS will help increase the understanding of this topic.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211031758 for Effects of probiotic and synbiotic supplementation on insulin resistance in women with polycystic ovary syndrome: a meta-analysis by Chenyun Miao, Qingge Guo, Xiaojie Fang, Yun Chen, Ying Zhao and Qin Zhang in Journal of International Medical Research
Footnotes
Authors’ contributions: C.Y.M. and Q.G.G. were involved in the data management and statistics and drafted the manuscript. X.J.F. and Y.C. verified the extracted data after the literature search, monitored the study and drafted the manuscript. Q.Z. designed the study. X.J.F. and Y.C. conducted the searches and performed the statistical analyses. Q.G.G. and C.Y.M. extracted the data and contributed to the quality assessment. All authors contributed to drafting and revising the manuscript; and read and approved the final manuscript.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported through grants from the Programme of Zhejiang Provincial TCM Sci-tech Plan (no. 2020ZA078), the Medical and Health Science and Technology Plan of Zhejiang Province (no. 2021KY920) and the Zhejiang Zhangqin Famous Traditional Chinese Medicine Expert Inheritance Studio Project (no. GZS2012014).
ORCID iD: Qin Zhang https://orcid.org/0000-0002-9845-4099
References
- 1.Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 2016; 106: 6.– . DOI:10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19–25. DOI: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Dumesic DA, Oberfield SE, Stener-Victorin E, et al. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev 2015; 36: 487–525. DOI: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidra S, Tariq MH, Farrukh MJ, et al. Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. PLoS One 2019; 14: e0223329. DOI: 10.1371/journal.pone.0223329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018; 14: 270–284. DOI: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 6.Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update 2015; 21: 560–574. DOI: 10.1093/humupd/dmv025. [DOI] [PubMed] [Google Scholar]
- 7.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002; 137: 25–33. DOI: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev 2018; 31: 35–51. DOI: 10.1017/s095442241700018x. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Guevarra RB, Kim YT, et al. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J Microbiol Biotechnol 2019; 29: 1335–1340. DOI: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 10.Kassaian N, Feizi A, Aminorroaya A, et al. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double-blind randomized clinical trial. Acta Diabetol 2018; 55: 1019–1028. DOI: 10.1007/s00592-018-1175-2. [DOI] [PubMed] [Google Scholar]
- 11.Kijmanawat A, Panburana P, Reutrakul S, et al. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J Diabetes Investig 2019; 10: 163–170. DOI: 10.1111/jdi.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi E, Moini A, Yaseri M, et al. Effects of synbiotic supplementation on metabolic parameters and apelin in women with polycystic ovary syndrome: a randomised double-blind placebo-controlled trial. Br J Nutr 2018; 119: 398–406. DOI: 10.1017/s0007114517003920. [DOI] [PubMed] [Google Scholar]
- 13.Heshmati J, Farsi F, Yosaee S, et al. The Effects of Probiotics or Synbiotics Supplementation in Women with Polycystic Ovarian Syndrome: a Systematic Review and Meta-Analysis of Randomized Clinical Trials. Probiotics Antimicrob Proteins 2019; 11: 1236–1247. DOI: 10.1007/s12602-018-9493-9. [DOI] [PubMed] [Google Scholar]
- 14.Samimi M, Dadkhah A, Haddad Kashani H, et al. The Effects of Synbiotic Supplementation on Metabolic Status in Women With Polycystic Ovary Syndrome: a Randomized Double-Blind Clinical Trial. Probiotics Antimicrob Proteins 2019; 11: 1355–1361. DOI: 10.1007/s12602-018-9405-z. [DOI] [PubMed] [Google Scholar]
- 15.Clark HD, Wells GA, Huët C, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials 1999; 20: 448.–. DOI: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 16.Esmaeilinezhad Z, Babajafari S, Sohrabi Z, et al. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutr Metab Cardiovasc Dis 2019; 29: 201–208. DOI: 10.1016/j.numecd.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Shoaei T, Heidari-Beni M, Tehrani HG, et al. Effects of Probiotic Supplementation on Pancreatic β-cell Function and C-reactive Protein in Women with Polycystic Ovary Syndrome: A Randomized Double-blind Placebo-controlled Clinical Trial. Int J Prev Med 2015; 6: 27. DOI: 10.4103/2008-7802.153866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholizadeh Shamasbi S, Dehgan P, Mohammad-Alizadeh Charandabi S, et al. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: a randomized, triple-blind, controlled, clinical trial. Eur J Nutr 2019; 58: 629–640. DOI: 10.1007/s00394-018-1648-7. [DOI] [PubMed] [Google Scholar]
- 19.Karimi E, Heshmati J, Shirzad N, et al. The effect of synbiotics supplementation on anthropometric indicators and lipid profiles in women with polycystic ovary syndrome: a randomized controlled trial. Lipids Health Dis 2020; 19: 60. DOI: 10.1186/s12944-020-01244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Zhu M, Wang C. Effect of Probiotic Supplementation on Blood Sugar and Lipids in Patients with Polycystic Ovary Syndrome. J Int Obstet Gynecol 2018; 45: 4 [in Chinese]. [Google Scholar]
- 21.Cetkovic N, Pellicano R, Bjelica A, et al. Polycystic ovary syndrome and vitamin D serum levels. Minerva Endocrinol 2019; 44: 82–84. DOI: 10.23736/s0391-1977.18.02887-0. [DOI] [PubMed] [Google Scholar]
- 22.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril 2002; 77: 1095–1105. DOI: 10.1016/s0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 23.Rössner SM, Neovius M, Mattsson A, et al. HOMA-IR and QUICKI: decide on a general standard instead of making further comparisons. Acta Paediatr 2010; 99: 1735–1740. DOI: 10.1111/j.1651-2227.2010.01911.x. [DOI] [PubMed] [Google Scholar]
- 24.Tabrizi R, Ostadmohammadi V, Akbari M, et al. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Probiotics Antimicrob Proteins 2019. DOI: 10.1007/s12602-019-09559-0. [DOI] [PubMed]
- 25.Łagowska K, Malinowska AM, Zawieja B, et al. Improvement of glucose metabolism in pregnant women through probiotic supplementation depends on gestational diabetes status: meta-analysis. Sci Rep 2020; 10: 17796. DOI: 10.1038/s41598-020-74773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mafi A, Namazi G, Soleimani A, et al. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct 2018; 9: 4763–4770. DOI: 10.1039/c8fo00888d. [DOI] [PubMed] [Google Scholar]
- 27.Jamilian M, Mansury S, Bahmani F, et al. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res 2018; 11: 80. DOI: 10.1186/s13048-018-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Wu Y, Wang Y, et al. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017; 9: 521. DOI: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 2013; 27: 73–83. DOI: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda) 2016; 31: 283–293. DOI: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Hedderson MM, Quesenberry CP, et al. Central Obesity Increases the Risk of Gestational Diabetes Partially Through Increasing Insulin Resistance. Obesity (Silver Spring) 2019; 27: 152–160. DOI: 10.1002/oby.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoczen S, Wojcik M, Fijorek K, et al. Expression of the central obesity and Type 2 Diabetes mellitus genes is associated with insulin resistance in young obese children. Exp Clin Endocrinol Diabetes 2015; 123: 252–259. DOI: 10.1055/s-0034-1398503. [DOI] [PubMed] [Google Scholar]
- 33.Niklowitz P, Rothermel J, Lass N, et al. Link between chemerin, central obesity, and parameters of the Metabolic Syndrome: findings from a longitudinal study in obese children participating in a lifestyle intervention. Int J Obes (Lond) 2018; 42: 1743–1752. DOI: 10.1038/s41366-018-0157-3. [DOI] [PubMed] [Google Scholar]
- 34.Hansel B, Giral P, Gambotti L, et al. A Fully Automated Web-Based Program Improves Lifestyle Habits and HbA1c in Patients With Type 2 Diabetes and Abdominal Obesity: Randomized Trial of Patient E-Coaching Nutritional Support (The ANODE Study). J Med Internet Res 2017; 19: e360. DOI: 10.2196/jmir.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerchbaum E, Trummer C, Theiler-Schwetz V, et al. Effects of Vitamin D Supplementation on Body Composition and Metabolic Risk Factors in Men: A Randomized Controlled Trial. Nutrients 2019; 11: 1894. DOI: 10.3390/nu11081894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. DOI: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211031758 for Effects of probiotic and synbiotic supplementation on insulin resistance in women with polycystic ovary syndrome: a meta-analysis by Chenyun Miao, Qingge Guo, Xiaojie Fang, Yun Chen, Ying Zhao and Qin Zhang in Journal of International Medical Research