Abstract
Objective
To explore the significance of the prostate central gland to total gland volume ratio (PVc/PV) in the diagnosis of prostate cancer (PCa) in patients with prostate specific antigen (PSA) levels in the grey zone (4–10 ng/ml).
Methods
This retrospective study enrolled patients that had undergone prostate biopsy. The volume of the prostate and the central prostate gland were measured. The differences in PSA, the ratio of free to total PSA (f/tPSA), PSA density (PSAD) and PVc/PV between the PCa and non-PCa groups were compared. Receiver operating characteristic curve analysis for PCa and clinically significant PCa (csPCa) diagnosis were calculated according to PSA (reference), f/tPSA, PSAD and PVc/PV.
Results
This study enrolled 136 patients. There was no significant difference in PSA and f/tPSA between the PCa and non-PCa groups, while there were significant differences in PSAD and PVc/PV. The area under the curve values of PVc/PV for PCa or csPCa diagnosis were 0.876 and 0.933, respectively; and for PSAD, they were 0.705 and 0.790, respectively. These were significantly different compared with the PSA curve, whereas f/tPSA showed no significant difference from the PSA curve.
Conclusion
PVc/PV could be a predictor of PCa when PSA is between 4–10 ng/ml.
Keywords: Prostate cancer, prostate hyperplasia, central gland, prostate specific antigen
Introduction
In recent years, the incidence and mortality of prostate cancer (PCa) in China has increased significantly. 1 PCa was estimated to account for 21% of all new cancer cases in the US in 2020. 2 The early diagnosis of PCa is very important. Prostate specific antigen (PSA) is the most important tumour marker of PCa, but when its value is between 4–10 ng/ml, namely the PSA grey zone, the incidence of PCa is only 25%. 3 As a consequence, it is important to study the related factors that contribute to a PCa positive biopsy when the PSA level is in the grey zone. Currently, the ratio of free to total PSA (f/tPSA) and PSA density (PSAD) are the most commonly-used related factors affecting the positive rate of prostate biopsies. 4 However, problems remain in terms of clinical prediction. It is commonly accepted that benign prostatic hyperplasia (BPH) lesions are mostly located in the central gland, while PCa usually occurs in the peripheral zone of the prostate. 5 Using multiparametric magnetic resonance imaging (mpMRI) alone is not sufficient in deciding when to perform a prostate biopsy.6–8 Using a combination of biomarkers such as the 4Kscore (a blood-based test that combines the four prostate-specific biomarkers of PSA, free PSA, intact PSA and human glandular kallikrein 2 [hK2]) in patients with positive mpMRI results can predict the presence of cancer outside the index lesion. 9 Patients with PCa and those with BPH have different values for the ratio of the central gland volume to total prostate volume (PVc/PV) as measured by mpMRI, so this is a potential new predictor of PCa and BPH. 10 This retrospective study analysed patients with PSA levels in the grey zone that underwent prostate biopsy in order to determine if PVc/PV could contribute to the diagnosis of PCa.
Patients and methods
Patient population
This retrospective study enrolled consecutive patients with PSA levels of 4–10 ng/ml that underwent a prostate biopsy between July 2015 and December 2020 in the Department of Urology, Minhang Hospital, Fudan University, Shanghai, China. The inclusion criteria were as follows: (i) PSA of 4–10 ng/ml; (ii) no 5α-reductase inhibitor or other endocrine therapy drugs were taken within 1 year; (iii) patients and their families both provided consent and signed the consent form. The exclusion criteria were as follows: (i) they had a clear history of PCa; (ii) they had acute urinary retention or indwelling catheterization within the last 2 weeks; (iii) they had lower urinary tract surgery or related treatment within the recent 3 months; (iv) they had urinary tract infection, acute prostatitis or coagulation dysfunction; (v) they had a local skin infection, severe infection, abnormal blood coagulation or other diseases that would not be suitable for an invasive examination; (vi) they had severe anal disease or anal diversion; (vii) those that were unable to cooperate with the investigators. PSA, f/tPSA, PSAD and PVc/PV values were collected from all patients.
Ethical approval was obtained from Ethics Committee of Minhang Hospital Affiliated to Fudan University (no. 2019-the certificate-006-01K). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Determination of f/tPSA and PSA
Whole peripheral blood samples (5 ml) were obtained prior to any treatment. These samples were then left at room temperature for 2 h to coagulate, centrifuged at 3000 g for 10 min using an Heraeus™ Labofuge™ 400 centrifuge (Thermo Fisher Scientific, Rockford, IL, USA). The serum was removed and stored at −80 °C until analysis. Immunofluorescence assay kits (Sorin, Milan, Italy) were used to measure PSA and f/tPSA in the blood samples.
All specimens with a diameter of 1 mm and a length of 10–15 mm taken from each patient were fixed with 4% neutral formaldehyde and then embedded in paraffin. Sections (3 μm) were cut and processed through xylene dewaxing and a graded series of ethanol. Sections were stained with haematoxylin and eosin. Sections also underwent immunohistochemical labelling to detect the protein levels of P504S, CK5/6, CK14 and P63 using mouse anti-human monoclonal antibodies and an S-P immunohistochemical detection kit (Maixin Biotech, Fuzhou, China) in accordance with the manufacturer’s instructions. The detection time was 48 h after digital rectal examination, cystoscopy and catheterization and 1 week after prostate massage.
Determination of PVc/PV
All patients underwent a routine clinical prostate mpMRI (GE 1.5T Signa Excite HD; GE Healthcare, Piscataway, NJ, USA). The anteroposterior diameter (cm), left and right diameter (cm) and upper and lower diameter (cm) of the prostate and central gland were measured. The prostate mpMRI acquisition protocol consisted of axial, sagittal and coronal T2-weighted imaging, diffusion-weighted imaging and dynamic contrast-enhanced imaging. The most commonly-used diffusion-weighted imaging was carried out using five b-values in the range of b50–b2000, while gadodiamide (Omniscan; GE Healthcare) was used as an intravenous contrast agent. Image interpretation was carried out by one of the three fellowship trained radiologists according to PI-RADS-V2.0 recommendations. 11 The volume of the anterior column gland and the central gland was calculated as π/6 × anteroposterior diameter × left and right diameter × upper and lower diameter (ml). PSAD was determined as follows: PSAD = PSA/PV.
Standard biopsy methods
Twelve standard biopsy cores + X biopsy (one extra biopsy from a suspicious part) were collected using an 18G TSK biopsy gun (TSK Laboratory China, Shanghai, China) guided by a B-ultrasound (MyLab™60 ultrasound system; Esaote, Genoa, Italy; transrectal biplane probe, frequency 3-9MHz) in the lithotomy position, including one needle at the apex, middle and bottom of the median sagittal section; and one needle at the tip, middle and bottom of the bilateral peripheral zone. For any suspicious parts, one more needle was inserted. Pathological specimens were fixed with 10% formaldehyde for biopsy and a senior pathologist reviewed all prostate biopsy specimens, reporting the International Society of Urological–Gleason grading according to the latest recommendations. 12
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for Windows® and Stata Statistical Software (Release 14; StataCorp LP, College Station, TX, USA). Categorical data and continuous data were compared between groups using χ2-test and Student’s t-test, respectively. Receiver operating characteristic (ROC) curve analysis was used to compare the accuracy of PSA, f/tPSA, PSAD and PVc/PV in predicting the prostate biopsy results. The area under the ROC curve (AUC) was compared using the Z-test. A P-value < 0.05 was considered statistically significant.
Results
This retrospective study enrolled 136 patients with PSA levels of 4–10 ng/ml that underwent prostate biopsies. All biopsies were completed successfully. The demographic and clinical characteristics of the study population are presented in in Table 1. Of these 136 patients, 39 were diagnosed with PCa. There were significant differences in PSAD and PVc/PV between the PCa group and the non-PCa group (P < 0.05 for both comparisons) (Table 2), but there were no significant differences in PSA and f/tPSA between the two groups.
Table 1.
Demographic and clinical characteristics of the study population (n = 136) of patients with prostate specific antigen (PSA) levels of 4–10 ng/ml that underwent prostate biopsies.
| Characteristic | Study cohort n = 136 |
|---|---|
| Age, years | 70.6 ± 8.7 |
| PSA, ng/ml | 6.6 ± 2.0 |
| DRE | |
| Negative | 105 (77.2%) |
| Suspicious | 31 (22.8%) |
| Biopsy history | |
| Biopsy naïve | 127 (93.4%) |
| Previous negative biopsy | 9 (6.6%) |
| PI-RADS | |
| 1–2 | 42 (30.9%) |
| 3 | 65 (47.8%) |
| 4–5 | 29 (21.3%) |
| Biopsy results | |
| Negative | 97 (71.3%) |
| GG 1 | 25 (18.4%) |
| csPCa | 14 (10.3%) |
Data presented as mean ± SD or n of patients (%).
DRE, digital rectal examination; PI-RADS, Prostate Imaging-Reporting and Data System; GG 1, Gleason group 1 (3 + 3); csPCa, clinically significant prostate cancer Gleason group ≥ 2 (≥ 3 + 4).
Table 2.
Prostate-related characteristics of patients with prostate specific antigen (PSA) levels of 4–10 ng/ml that underwent prostate biopsies stratified according to a diagnosis of prostate cancer (PCa).
| Characteristic | PCa group n = 39 | Non-PCa group n = 97 | Statistical analysisa |
|---|---|---|---|
| PSA, ng/ml | 6.64 ± 1.57 | 6.54 ± 2.01 | NS |
| f/tPSA | 0.15 ± 0.07 | 0.17 ± 0.06 | NS |
| PSAD, ng/ml/cm3 | 0.25 ± 0.12 | 0.16 ± 0.08 | P = 0.0004 |
| PVc/PV | 0.41 ± 0.14 | 0.63 ± 0.15 | P < 0.0001 |
Data presented as mean ± SD.
aStudent’s t-test; NS, no significant between-group difference (P ≥ 0.05).
f/tPSA, ratio of free to total PSA; PSAD, PSA density; PVc/PV, ratio of the central gland volume to total prostate volume.
The ROC curve analysis of PSA, f/tPSA, PSAD and PVc/PV in the diagnosis of all patients with PCa (Figure 1) and clinically significant PCa (csPCa) (Figure 2) are presented. The AUC values of the ROC curve for each parameter are shown in Table 3 and Table 4.
Figure 1.
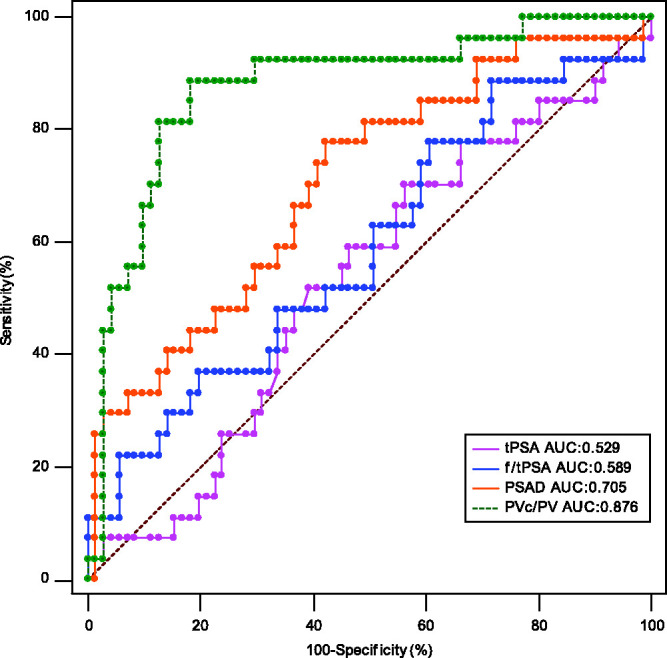
Receiver operating characteristic (ROC) curve analysis compared the predictive value of the ratio of the central gland volume to total prostate volume (PVc/PV) and other parameters in the diagnosis of all patients with prostate cancer. tPSA, total prostate specific antigen; AUC, area under the curve; f/tPSA, ratio of free to total prostate specific antigen; PSAD, prostate specific antigen density. The colour version of this figure is available at: http://imr.sagepub.com.
Figure 2.
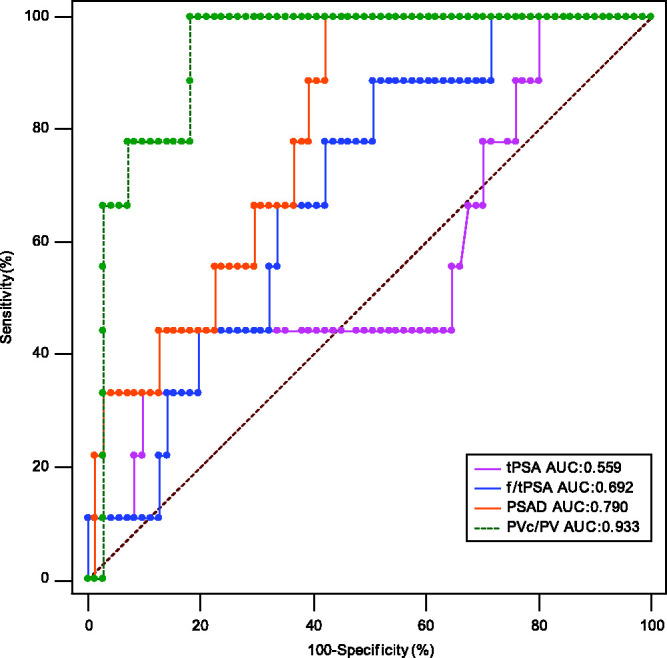
Receiver operating characteristic (ROC) curve analysis compared the predictive value of the ratio of the central gland volume to total prostate volume (PVc/PV) and other parameters in the diagnosis of all patients with clinically significant prostate cancer (Gleason group ≥ 2 [≥ 3 + 4]). tPSA, total prostate specific antigen; AUC, area under the curve; f/tPSA, ratio of free to total prostate specific antigen; PSAD, prostate specific antigen density. The colour version of this figure is available at: http://imr.sagepub.com.
Table 3.
Comparison of area under the curve (AUC) values of the receiver operating characteristic curve analysis of each characteristic for the diagnosis of all patients with prostate cancer.
| Characteristic | AUC | Σ | 95% CI | Statistical analysisa |
|---|---|---|---|---|
| PSA, ng/ml | 0.529 | 0.0645 | 0.426, 0.631 | – |
| f/tPSA | 0.589 | 0.0662 | 0.486, 0.688 | NS |
| PSAD, ng/ml/cm3 | 0.705 | 0.0595 | 0.605, 0.793 | P = 0.0138 |
| PVc/PV | 0.876 | 0.0416 | 0.794, 0.934 | P < 0.0001 |
aPSA was used as the reference control; Z-test; NS, no significant association (P ≥ 0.05).
CI, confidence interval; PSA, prostate specific antigen; f/tPSA, ratio of free to total PSA; PSAD, PSA density; PVc/PV, ratio of the central gland volume to total prostate volume.
Table 4.
Comparison of area under the curve (AUC) values of the receiver operating characteristic curve analysis of each characteristic for the diagnosis of all patients with clinically significant prostate cancer (Gleason group ≥ 2 [≥ 3 + 4]).
| Characteristic | AUC | Σ | 95% CI | Statistical analysisa |
|---|---|---|---|---|
| PSA, ng/ml | 0.559 | 0.111 | 0.444, 0.670 | – |
| f/tPSA | 0.692 | 0.0793 | 0.579, 0.790 | NS |
| PSAD, ng/ml/cm3 | 0.790 | 0.0632 | 0.685, 0.873 | P = 0.0259 |
| PVc/PV | 0.933 | 0.0310 | 0.854, 0.977 | P = 0.0004 |
aPSA was used as the reference control; Z-test; NS, no significant association (P ≥ 0.05).
CI, confidence interval; PSA, prostate specific antigen; f/tPSA, ratio of free to total PSA; PSAD, PSA density; PVc/PV, ratio of the central gland volume to total prostate volume.
Discussion
There were approximately 1.27 million new cases of PCa worldwide in 2018 and PCa accounted for the world’s second highest incidence of all cancers for men. 13 Many laboratory and imaging methods are widely used in the early screening of PCa, but the gold standard for diagnosis is prostate biopsy. 14 PSA is not a specific marker of PCa because prostatitis, BPH, prostate compression and related endoscopic operations via the prostate also increase levels of PSA. 15 PCa antigen 3 (PCA3), prostate health index (phi) and sarcosine are predictors of PCa characteristics during final pathology. 16 Published data show that phi has a good diagnostic performance in identifying csPCa. 17 Currently, there remains great controversy regarding the performance of a biopsy on patients with PSA levels in the grey zone. 18 Therefore, some other PSA-based indicators such as f/tPSA and PSAD can also be used as biomarkers for PCa diagnosis. 4 This current study demonstrated that there was no significant difference in the level of PSA between the PCa group and the non-PCa group, indicating that the specificity of PSA was low when its level is within the grey zone. Previous studies had confirmed the value of f/tPSA in the diagnosis of PCa in patients with PSA levels in the grey zone, 19 in which a low f/tPSA predicted a high risk of PCa. 20 However, the value of f/tPSA in the diagnosis of PCa in patients with a PSA level in the grey zone in East Asia remains controversial. For example, a previous study found that the predictive value of f/tPSA in grey zone PCa diagnosis in Chinese people was low. 21 Another study reported that f/tPSA did not improve the diagnostic accuracy of PSA grey zone PCa for Korean men aged 50–65 years in a prospective multicentre study. 22 A meta-analysis showed that the reasons for heterogeneity regarding the predictive value of f/tPSA included race, age, detection reagents and standards. 23 In this current study, there was no significant difference in f/tPSA between the PCa group and the non-PCa group. However, considering the small number of patients in this current study and the existence of age heterogeneity factors, it will be necessary to expand the sample and stratify the age to further verify the diagnostic value of f/tPSA in men with a PSA level in the grey zone.
Destruction of the barrier between the acinar epithelium, ductal epithelium and capillaries in the prostate can also lead to an increase in PSA and per unit volume of PSA. 24 PSAD has been used to distinguish PCa from BPH since 1992. 25 A previous study demonstrated that the accuracy of PSA in predicting PCa was inferior to that of PSAD. 26 The results of this current study also demonstrated that the value of PSAD in the diagnosis of PCa for patients with a PSA level in the grey zone was better than that of PSA.
The increase of PSA in patients with BPF is mainly caused by hyperplasia in the transitional zone of the central gland, 27 while PSA produced by the peripheral zone is relatively stable. 28 Enlargement of an aging prostate due to BPH is typically contributed to by the transition zone, while the peripheral zone is typically considered as age-irrelevant.29,30 Research shows that the PSAD in the prostate transitional zone of a PCa patient is significantly higher than that in a BPH patient, which means when presented with the same level of PSA, the ratio of transitional zone volume to total volume in patients with PCa was smaller than those with BPH.31,32 Since the transitional zone is the main component of the prostate’s central gland, this also means that the ratio of central gland volume to total volume is smaller in PCa patients. 33 The central gland volume is also related to bladder outlet obstruction parameters and prostatic inflammation; and is consistently used in predictive models and risk calculators for PCa.34,35 Although transrectal ultrasounds are widely used in the measurement of prostate volume, there are obvious limitations such as measurement error and incomplete image preservation. 36 There are obvious advantages in the measurement of prostate volume when using mpMRI, especially the T2 sequence, which can readily distinguish the different zones of the prostate.37,38 In order to ensure the stability and accuracy of data used in the study, prostate volume was measured by the same clinician. The PVc/PV value in the PCa group was significantly lower than that of the non-PCa group, which confirmed the correlation between PVc/PV value and PCa risk in the PSA grey zone. The AUC value of the ROC curve corresponding to PVc/PV in the PCa and csPCa patients was 0.876 and 0.933, respectively, which were the highest two values of all parameters tested. In comparison with the AUC values of PSA and f/tPSA, the AUC value of PVc/PV was statistically significant (P < 0.001). This finding suggests that PVc/PV could be used as a predictive parameter for the diagnosis of both PCa and csPCa in patients with a PSA level in the grey zone. Novel prostate MRI protocols such as biparametric MRI are promising because they could make the acquisition time much shorter. 39 In this context, PVc/PV could be used as a quantitative parameter added to the MRI report. Furthermore, mpMRI also demonstrated similar accuracies irrespective of race when undergoing radical prostatectomy.40,41
In conclusion, this current study suggests that PVc/PV could be a predictor of PCa when PSA is in the grey zone of 4–10 ng/ml. This could provide several clinical benefits such as improving the accuracy of PCa and csPCa diagnosis when PSA is in the grey zone and reducing unnecessary prostate biopsies. This diagnostic measure is worthy of further clinical investigation in larger, racially diverse populations in order to externally validate this preliminary finding.
Acknowledgement
The authors thank P.R. Cai from the key laboratory of whole-period monitoring and precise intervention of digestive cancer (SMHC) for their excellent technical assistance.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This work was supported by Minhang Hospital, Fudan University (no. 2020MHJC08).
ORCID iD: Zhui-Feng Guo https://orcid.org/0000-0003-0820-4748
References
- 1.Liu X, Yu C, Bi Y, et al. Trends and age-period-cohort effect on incidence and mortality of prostate cancer from 1990 to 2017 in China. Public Health 2019; 172:70.–. [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Brassell SA, Kao TC, Sun L, et al. Prostate-specific antigen versus prostate-specific antigen density as predictor of tumor volume, margin status, pathologic stage, and biochemical recurrence of prostate cancer. Urology 2005; 66: 1229–1233. [DOI] [PubMed] [Google Scholar]
- 4.Djavan B, Remzi M, Zlotta AR, et al. Complexed prostate-specific antigen, complexed prostate-specific antigen density of total and transition zone, complexed/total prostate-specific antigen ratio, free-to-total prostate-specific antigen ratio, density of total and transition zone prostate-specific antigen: results of the prospective multicenter European trial. Urology 2002; 60: 4–9. [DOI] [PubMed] [Google Scholar]
- 5.Pavelić J and Zeljko Z. Prostate gland-transition zone lesions. Etiology, growth regulation, growth factors, genetic changes. Lijec Vjesn 2002; 124: 211–219 [Article in Croatian, English abstract]. [PubMed] [Google Scholar]
- 6.Wajswol E, Winoker JS, Anastos H, et al. A cohort of transperineal electromagnetically tracked magnetic resonance imaging/ultrasonography fusion-guided biopsy: assessing the impact of inter-reader variability on cancer detection. BJU Int 2020; 125: 531–540. [DOI] [PubMed] [Google Scholar]
- 7.Falagario UG, Martini A, Wajswol E, et al. Avoiding Unnecessary Magnetic Resonance Imaging (MRI) and Biopsies: Negative and Positive Predictive Value of MRI According to Prostate-specific Antigen Density, 4Kscore and Risk Calculators. Eur Urol Oncol 2020; 3: 700–704. [DOI] [PubMed] [Google Scholar]
- 8.Falagario UG, Jambor I, Lantz A, et al. Combined Use of Prostate-specific Antigen Density and Magnetic Resonance Imaging for Prostate Biopsy Decision Planning: A Retrospective Multi-institutional Study Using the Prostate Magnetic Resonance Imaging Outcome Database (PROMOD). Eur Urol Oncol 2020: S2588-9311(20)30142-5. [DOI] [PubMed] [Google Scholar]
- 9.Falagario UG, Lantz A, Jambor I, et al. Using biomarkers in patients with positive multiparametric magnetic resonance imaging: 4Kscore predicts the presence of cancer outside the index lesion. Int J Urol 2021; 28: 47–52. [DOI] [PubMed] [Google Scholar]
- 10.Sellers J, Wagstaff RG, Helo N, et al. Quantitative measurements of prostatic zones by MRI and their dependence on prostate size: possible clinical implications in prostate cancer. Ther Adv Urol 2021; 13:17562872211000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver Reproducibility of the PI-RADS Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology 2016; 280: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016; 40: 244–252. [DOI] [PubMed] [Google Scholar]
- 13.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 14.Borghesi M, Ahmed H, Nam R, et al. Complications After Systematic, Random, and Image-guided Prostate Biopsy. Eur Urol 2017; 71: 353–365. [DOI] [PubMed] [Google Scholar]
- 15.Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery –what we have learned and where we are going. J Urol 1999; 162: 293–306. [DOI] [PubMed] [Google Scholar]
- 16.Ferro M, Lucarelli G, Bruzzese D, et al. Improving the prediction of pathologic outcomes in patients undergoing radical prostatectomy: the value of prostate cancer antigen 3 (PCA3), prostate health index (phi) and sarcosine. Anticancer Res 2015; 35: 1017–1023. [PubMed] [Google Scholar]
- 17.Ferro M, De Cobelli O, Lucarelli G, et al. Beyond PSA: The Role of Prostate Health Index (phi). Int J Mol Sci 2020; 21: 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinkler FB, Schmid DM, Hauri D, et al. Free/total prostate-specific antigen ratio can prevent unnecessary prostate biopsies. Urology 1998; 52: 479–486. [DOI] [PubMed] [Google Scholar]
- 19.Kalish LA, McKinlay JB. Serum prostate-specific antigen levels (PSA) in men without clinical evidence of prostate cancer: age-specific reference ranges for total PSA, free PSA, and percent free PSA. Urology 1999; 54: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 20.Chan DW, Kelley CA, Ratliff TL, et al. Analytical and clinical performance characteristics of Hybritech's Tandem-R free PSA assay during a large multicenter clinical trial to determine the clinical utility of percentage of free prostate-specific antigen. Clin Chem 1999; 45: 1863–1865. [PubMed] [Google Scholar]
- 21.Huang M, Lin Y, Xu A, et al. Percent free prostate-specific antigen does not improve the effectiveness of prostate cancer detection in Chinese men with a prostate-specific antigen of 2.5-20.0 ng/ml: a multicenter study. Med Oncol 2014; 31: 925. [DOI] [PubMed] [Google Scholar]
- 22.Jeong IG, Lee KH. . Percent free prostate specific antigen does not enhance the specificity of total prostate specific antigen for the detection of prostate cancer in Korean men 50 to 65 years old: a prospective multicenter study. J Urol 2008; 179: 111–116. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Sun G, Pan JG, et al. Performance of tPSA and f/tPSA for prostate cancer in Chinese. A systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2006; 9: 374–378. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran NM, Casey RG, Hong MK, et al. The ability of prostate-specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumour grade due to reduced PSA secretion per unit tumour volume. BJU Int 2012; 110: 36–42. [DOI] [PubMed] [Google Scholar]
- 25.Benson MC, Whang IS, Olsson CA, et al. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol 1992; 147: 817–821. [DOI] [PubMed] [Google Scholar]
- 26.Ghafoori M, Varedi P, Hosseini SJ, et al. Value of prostate-specific antigen and prostate-specific antigen density in detection of prostate cancer in an Iranian population of men. Urol J 2009; 6: 182–188. [PubMed] [Google Scholar]
- 27.Hammerer PG, McNeal JE, Stamey TA. Correlation between serum prostate specific antigen levels and the volume of the individual glandular zones of the human prostate. J Urol 1995; 153: 111–114. [DOI] [PubMed] [Google Scholar]
- 28.Lee JJ, Thomas IC, Nolley R, et al. Biologic differences between peripheral and transition zone prostate cancer. Prostate 2015; 75: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turkbey B, Huang R, Vourganti S, et al. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int 2012; 110: 1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karademir I, Shen D, Peng Y, et al. Prostate volumes derived from MRI and volume-adjusted serum prostate-specific antigen: correlation with Gleason score of prostate cancer. AJR Am J Roentgenol 2013; 201: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohigashi T, Kanao K, Kikuchi E, et al. Prostate specific antigen adjusted for transition zone epithelial volume: the powerful predictor for the detection of prostate cancer on repeat biopsy. J Urol 2005; 173: 1541–1545. [DOI] [PubMed] [Google Scholar]
- 32.Shen P, Zhao J, Sun G, et al. The roles of prostate-specific antigen (PSA) density, prostate volume, and their zone-adjusted derivatives in predicting prostate cancer in patients with PSA less than 20.0 ng/mL. Andrology 2017; 5: 548–555. [DOI] [PubMed] [Google Scholar]
- 33.Guo ZF, Lu XW, Yang F, et al. Significance of prostate central gland/total gland volume ratio combined with PSA in the diagnosis of prostate cancer patients. Zhonghua Yi Xue Za Zhi 2019; 99: 2836–2839 [Article in Chinese. English abstract]. [DOI] [PubMed] [Google Scholar]
- 34.Sanguedolce F, Falagario UG, Castellan P, et al. Bioptic intraprostatic chronic inflammation predicts adverse pathology at radical prostatectomy in patients with low-grade prostate cancer. Urol Oncol 2020; 38: 793.e19–793.e25. [DOI] [PubMed] [Google Scholar]
- 35.Cormio L, Cindolo L, Troiano F, et al. Development and Internal Validation of Novel Nomograms Based on Benign Prostatic Obstruction-Related Parameters to Predict the Risk of Prostate Cancer at First Prostate Biopsy. Front Oncol 2018; 8: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong S, Cardinal HN, McLoughlin RF, et al. Intra- and inter-observer variability and reliability of prostate volume measurement via two-dimensional and three-dimensional ultrasound imaging. Ultrasound Med Biol 1998; 24: 673–681. [DOI] [PubMed] [Google Scholar]
- 37.Rahmouni A, Yang A, Tempany CM, et al . Accuracy of in-vivo assessment of prostatic volume by MRI and transrectal ultrasonography. J Comput Assist Tomogr 1992; 16: 935–940. [DOI] [PubMed] [Google Scholar]
- 38.Swindle P, Ramadan S, Stanwell P, et al. Proton magnetic resonance spectroscopy of the central, transition and peripheral zones of the prostate: assignments and correlation with histopathology. MAGMA 2008; 21: 423–434. [DOI] [PubMed] [Google Scholar]
- 39.Falagario U, Jambor I, Taimen P, et al. Added value of systematic biopsy in men with a clinical suspicion of prostate cancer undergoing biparametric MRI-targeted biopsy: multi-institutional external validation study. World J Urol 2021; 39: 1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez IM, Jambor I, Kauko T, et al. Qualitative and Quantitative Reporting of a Unique Biparametric MRI: Towards Biparametric MRI-Based Nomograms for Prediction of Prostate Biopsy Outcome in Men With a Clinical Suspicion of Prostate Cancer (IMPROD and MULTI-IMPROD Trials). J Magn Reson Imaging 2020; 51: 1556–1567. [DOI] [PubMed] [Google Scholar]
- 41.Falagario UG, Ratnani P, Lantz A, et al. Staging Accuracy of Multiparametric Magnetic Resonance Imaging in Caucasian and African American Men Undergoing Radical Prostatectomy. J Urol 2020; 204: 82–90. [DOI] [PubMed] [Google Scholar]


