Abstract
Objective
To retrospectively analyze the biological compatibility and oncologic outcomes of autogenous, allogeneic, or combined bone grafting.
Methods
From April 2000 to December 2016, 37 patients with histologically confirmed low-grade intramedullary chondrosarcoma of the long bones at Kyungpook National University Hospital were enrolled in this retrospective study. All 37 patients underwent intralesional curettage (with or without cryotherapy) followed by bone grafting. Among the 24 patients who underwent cryotherapy, 13 were treated by prophylactic internal fixation (10 in the femur, 1 in the tibia, and 2 in the humerus). Thirteen patients underwent the same treatment without cryotherapy, whereas 12 did not undergo preventive internal fixation.
Results
A single intraoperative fracture was managed by plate fixation. One patient who underwent cryotherapy and internal fixation developed a fracture distal to the operation site 25 days after surgery, and this fracture was repaired with a long plate. None of the 37 patients showed any recurrence or metastasis.
Conclusions
Adequate intralesional curettage (with or without cryosurgery) combined with bone grafting using autogenous and allogeneic bone chips was effective for the treatment of low-grade intramedullary chondrosarcoma. Therefore, prophylactic internal fixation using a plate is recommended in the cryotherapy of definite cortical invasion in weight-bearing bones.
Keywords: Low-grade chondrosarcoma, long bone, curettage, bone graft, cryosurgery, internal fixation
Introduction
Chondrosarcoma is the third most common primary bone malignancy after osteosarcoma and myeloma.1,2 Based on its mitotic rate, cellularity, and nuclear size, chondrosarcoma can be classified into three grades: I, II, and III. 3 Grade I, also known as low-grade chondrosarcoma, is the most common type of chondrosarcoma.4,5 However, the prevalence of grade I chondrosarcoma remains controversial because of variation in its histologic grading.4,6 Local recurrence rates range from 0% to 13%,7–15 and distant metastasis of low-grade chondrosarcoma is very rare (0%–5% of cases).3,10,16 An obvious prognostic difference is present between chondrosarcoma of the axial skeleton and limb bones. 4 Although some reports have described wide excision of grade I chondrosarcoma of the long bones, intralesional curettage is currently the first-choice treatment.12,13,15,17,18 Adjuvant therapy includes cryotherapy and phenol, alcohol, and thermal cauterization.4,8,11,13,15,19–21
Bone cement filling is the most commonly used postoperative method to enhance mechanical stability and increase tumor cytotoxicity.21,22 However, this method is associated with adverse effects in some patients, such as the risk of postoperative fracture and infections.11,15,18 In addition, bone cement cannot be absorbed, and the original physiological state of the bone can never be restored. Therefore, in the present study, we evaluated the biologic compatibility and oncologic outcomes of autogenous and allogeneic bone grafting.
Materials and methods
Study design and setting
We retrospectively evaluated 53 consecutive patients who were treated for low-grade intramedullary chondrosarcoma of the long bones at Kyungpook National University Hospital from April 2000 to December 2016. This study was approved by the Ethics Committee of Kyungpook National University Hospital (approval no. KNUH 2017-07-030). All patients and their families provided written informed consent.
Study participants
The inclusion criteria were histologically confirmed grade I chondrosarcoma of the long bone, curettage with or without cryotherapy followed by bone grafting, and more than 2 years of follow-up. After excluding 4 patients with bone cement infilling and 12 patients with <2 years of follow-up, we included 37 patients in the study.
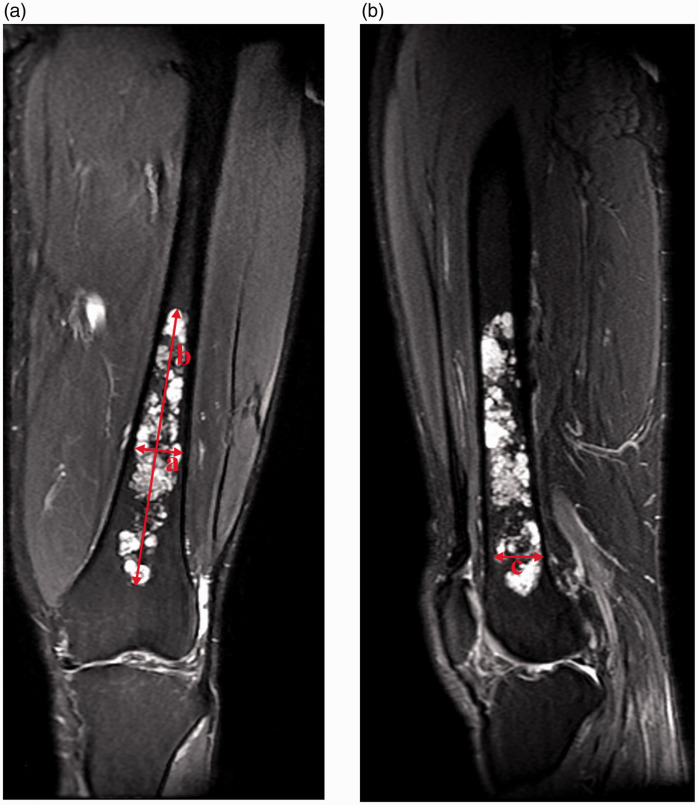
Preoperative plain radiography, magnetic resonance imaging (MRI), computed tomography (CT), and technetium-99m (Tc99m) bone scintigraphy were routinely performed. A clinico-radiologic-pathologic meeting was conducted to diagnose all cases. General characteristics such as patient age and sex; tumor location; and clinical outcomes of recurrence, metastasis, and complications were analyzed. We used the ellipsoid volume formula 4/3 π × × × to measure the tumor volume based on the maximum transverse and longitudinal diameters in the coronal section and the maximum transverse diameter in the sagittal section as obtained by MRI 23 (Figure 1). Evans histological grading was used. 3
Figure 1.
(a, b) Calculation of tumor volume using the ellipsoid volume formula 4/3 π × × × , where a is the maximum transverse diameter in the coronal section, b is the longitudinal diameter in the coronal section, and c is the maximum transverse diameter in the sagittal section obtained by T2-weighted magnetic resonance imaging.
Description of experiment, treatment, and surgery
The surgical indications were the presence of calcified intramedullary shadows upon simple X-ray examination, cartilage signals of either >5 cm3 (roughly jujube-sized) or <5 cm3 with invasion of the cortex upon MRI or CT examination, and moderate to high uptake on Tc99m bone scintigraphy.
Six patients underwent trephine biopsy before surgery. Intramedullary cartilage tissue was confirmed by gross examination during the operation, and intralesional curettage was then performed. C-arm fluoroscopy was used to inspect the curettage range. A minimal skin incision of approximately 1 inch in length was made over the iliac crest. A bone graft harvesting system (Acumed, Hillsboro, OR, USA) was then used to obtain pure autogenous cancellous bone. Approximately 10 mL of bone marrow was aspirated through the milling holes before skin closure. A routine indwelling drainage tube was used for iliac bone procurement; the drainage tube was always removed when the drainage fluid volume was <5 mL in 24 hours. Cryotherapy was performed using a liquid nitrogen spraying device (Cry-Ac® B-700; Brymill Cryogenic Systems, Ellington, CT, USA) followed by three rounds of saline irrigation.
The criteria for using cryotherapy were based on preoperative imaging and intraoperative observation of the extent of tumor invasion, obvious cortical bone invasion (scallop-like appearance), the presence or absence of internal cortical septal formation, and difficulty in performing complete curettage of the tumor.
The aspirated marrow was sprayed into the allogeneic cancellous chips, which were then placed in the tumor cavity. Although the autogenous bone was mixed with a small amount of allogeneic cancellous chips, most were set below and over the bone window to accelerate window healing. Additive internal fixation was performed depending on the local conditions of each case.
We performed preventive internal fixation in patients who had undergone cryotherapy of weight-bearing bones, such as the femur and tibia, that showed obvious cortical bone invasion (scallop-like appearance) (Figure 2). If cryosurgery was not performed, no additional internal plate fixation was administered.
Figure 2.
(a, b) A large amount of lobulated cartilage-like stroma was observed in the intramedullary femoral shaft with low signal intensity on T1-weighted magnetic resonance imaging and high signal intensity on T2-weighted magnetic resonance imaging. (c, d) Computed tomography and preoperative radiography showing massive chondrogenic punctate calcification in the intramedullary femoral shaft. (e) Radiography immediately after intralesional curettage with cryotherapy followed by bone grafting and internal fixation. (f) Radiography 2.3 years after surgery showing that the bone had healed well after removal of the plate and screws because of discomfort from the heaviness of the plate.
Variables, outcome measures, data sources, and bias
We investigated postoperative complications, including fractures, infections, and nerve injuries, as well as oncologic outcomes such as recurrence, metastasis, and comorbidities. Our routine postoperative monitoring procedure included the following steps. After the histologic diagnosis of low-grade chondrosarcoma, positron emission tomography–CT was performed once from the neck to the knee joint to detect any other tumor tissue or pulmonary metastases. Simple radiography was performed monthly until radiographic confirmation of window site union, which usually occurred 3 to 6 months after surgery. Enhanced chest CT was then typically performed annually until 5 years postoperatively. Other monitoring examinations, such as plain radiography, Tc99m bone scintigraphy, and rarely MRI, were typically performed every 6 to 12 months.
Results
Biological compatibility
In total, 32 patients underwent autogenous and allogeneic combined bone grafting, 4 underwent allogeneic bone grafting only, and 1 underwent autogenous bone grafting only. Fourteen patients underwent internal fixation. Among the 24 patients who received cryotherapy, 13 underwent prophylactic internal fixation (10 in the femur, 1 in the tibia, and 2 in the humerus). In one patient who received cryotherapy and internal fixation, a fracture distal to the operation site occurred 25 days after surgery and was repaired via internal fixation using a long plate. Among the 13 patients who did not receive cryotherapy, 12 did not undergo preventive internal fixation and 1 developed a pathologic fracture during the operation and underwent internal fixation (Table 1). The remaining patients had no fractures, and the bones healed well.
Table 1.
Surgical options.
| Cryotherapy | Non-cryotherapy | ||
|---|---|---|---|
| Total | 24 | 13 | |
| Internal fixation | 14 | 13 | 1 |
| Non-internal fixation | 23 | 11 | 12 |
Oncologic outcomes
During the follow-up period, all patients showed good functional recovery with no recurrence or metastasis (Figures 3 and 4). Residual tumor tissue was suspected in one patient. Follow-up observation of this patient revealed no disease progression at 6.2 years after surgery (Table 2).
Figure 3.
(a) Preoperative radiography showing a lytic lesion with slight chondrogenic calcification in the proximal humerus. (b, c) The intramedullary low signal intensity on T1-weighted magnetic resonance imaging and high signal intensity on T2-weighted magnetic resonance imaging indicates the presence of a chondromatous matrix in the lesion. (d) Technetium-99m bone scintigraphy showing characteristic hot uptake. (e) Computed tomography showing the presence of dense intramedullary calcifications in the proximal humerus. (f) Postoperative pathology showing mild atypical cells and extensive mucinous degeneration of cartilage-like matrix (×100, hematoxylin–eosin).
Figure 4.
(a, b) T1- and T2-weighted magnetic resonance imaging 2.5 years after the surgery showing that the incorporated bone graft appeared healthy. A slightly increased signal of bone marrow edema was present. (c) Radiography 4.5 years after the surgery showing good healing of the bone graft. (d) Technetium-99m bone scintigraphy showing only slightly increased uptake at the operation site.
Table 2.
Patient demographics.
| Characteristic | No. | Percentage (%) |
|---|---|---|
| Total | 37 | |
| Sex | ||
| Male | 6 | 16.2 |
| Female | 31 | 83.8 |
| Mean (range) age at surgery, years | 48.8 (20–65) | |
| Location of tumor | ||
| Humerus | 19 | 51.4 |
| Femur | 14 | 37.8 |
| Tibia | 2 | 5.4 |
| Fibula | 2 | 5.4 |
| Average (range) tumor volume, cm³ | 10.9 (1.2–49.6) |
Other relevant findings
No infections or nerve injuries were observed at the lesion sites. Apart from mild pain 2 to 3 days after surgery, there was no obvious pain at the tumor or iliac bone procurement site. During the follow-up period, we observed three cases of thyroid cancer, one case of a benign thyroid nodule, and one case of lung cancer. The average tumor volume was 10.9 cm3 (range, 1.2–49.6 cm3) (Table 2). In nine patients, a lesion of <5 cm3 showed invasion of the cortex on MRI or CT. The average follow-up period was 5.3 years (range, 2.1–12.8 years); 37 patients had a follow-up period of >2 years, 29 had a follow-up period of >3 years, and 18 (48.6%) had a follow-up period of >5 years. No patient was lost to follow-up (Table 3).
Table 3.
Overview of patient follow-up outcomes, oncologic outcomes, and complications.
| Patients | No. | Percentage (%) |
|---|---|---|
| Total | 37 | 100.0 |
| Average (range) follow-up time, years | 5.3 (2.1–12.8) | |
| Oncologic outcomes | ||
| Recurrence | 0 | 0.0 |
| Metastasis | 0 | 0.0 |
| Residual tumor* | 1 | 2.7 |
| Complications | ||
| Fracture | 2 | 5.4 |
*Proximal humerus lesion still undergoing follow-up 6.2 years postoperatively.
Discussion
We conducted a study on intralesional curettage (with or without cryosurgery) using bone grafting rather than bone cement filling for the treatment of low-grade intramedullary chondrosarcoma of the long bones. The use of bone cement filling or bone transplantation after curettage largely depends on the surgeon’s preference (Table 4). To the best of our knowledge, no retrospective case–control studies or meta-analyses have compared these approaches, and prospective controlled studies are difficult to undertake. Although bone grafting has been used to fill bone defects in some studies, no consensus exists in the field.
Table 4.
Oncologic result and complications of intralesional treatment for low-grade chondrosarcoma.
| Authors (year) | n | Age in years, mean (range) | Sex, M/F | Site (n) | Stage (n) | Biopsy (n) | Treatment | Adjunct | Filling | IF | Follow-up in years, mean (range) | LR, n (%) | Metastases, n (%) | DFD, n (%) | Complications (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bauer et al. (1995) 31 | 17 | 53.2 (30–69) | 2/4 | Long bone (15) | IA (14) | 2 | Curettage | NA | Bone graft | NA | 7.0 (3–11) | 1 (5.9%)a | 0 (0.0%) | 0 (0.0%) | Reoperation (3) |
| Calcaneus (1) | IB (3) | ||||||||||||||
| Patella (1) | |||||||||||||||
| 6 | 37.8 (14–65) | 11/6 | Long bone | IA | 1 | Curettage | NA | Bone cement | NA | 5.2 (4–8) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | Reoperation (2) | |
| Schreuder et al. (1998) 30 | 9 | 42.9 | 3/6 | Extremities | IA | All | Curettage | LN | Bone graft | 3 | 2.3 (1.3–3.1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | Post. Fx. (1) |
| Wound infection (1) | |||||||||||||||
| Ahlmann et al. (2006) 19 | 10 | 54.4 (29–83) | 8/2 | Long bone (7) | IA | All | Curettage + burring | LN | Bone cement | 8b | 3.2 (2–5) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | Loosening of intramedullary nail (1) |
| Axial bone (3) | Reoperation (1) | ||||||||||||||
| Leerapun et al. (2007) 9 | 12 | 36.8 ± 19.3 | 7/6 | Long bone | IA | All | Curettage | P | Bone graft | NA | 10.7 (0.2–22.8) | 1 (8.3%) | 1 (8.3%) | 1 (8.3%)c | None |
| 1 | Bone cement | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | None | ||||||||||
| Hanna et al. (2009) 32 | 39 | 55.5 (32–82) | 10/29 | Long bone | IA | All | Curettage | NA | Bone cement | NA | 5.1 (3–8.7) | 2 (5.1%) | 0 (0.0%) | 0 (0.0%) | Reoperation (3) |
| Mild pain (4) | |||||||||||||||
| Mohler et al. (2010) 11 | 17 | 45.2 (18–70) | 7/10 | Long bone (14) | NA | NA | Curettage | LN + HP | Bone cement | NA | 3.5 (1.5–11.2) | 2 (11.8%) | 0 (0.0%) | 0 (0.0%) | Post. Fx. (1) |
| Other bone (3) | Reoperation (2) | ||||||||||||||
| Verdegaal et al. (2012) 13 | 85d | 47.5 (15.6–72.3) | NA | Long bone | NA | 60 | Curettage | P+E | Bone graft | None | 6.8 (0.2–14.1) | 5 (5.9%) | 0 (0.0%) | 0 (0.0%) | Post. Fx. (2) |
| Superficial infection (1) | |||||||||||||||
| Reoperation (13) | |||||||||||||||
| Meftah et al. (2013) 15 | 43e | 44.9 ± 11.3 (21.8–66.4) | 13/29 | All | IA (37) | NA | Curettage + burring | LN | Bone cement | NA | 10.2 ± 4.6 (5–22.5) | 4 (9.3%)f | 0 (0.0%) | 0 (0.0%) | Post. Fx. (post-traumatic) (2) |
| IB (6) | Infection (1) | ||||||||||||||
| Reoperation (7) | |||||||||||||||
| Mermerkaya et al. (2014) 33 | 21 | 48.7 (18–71) | 7/14 | Long bone | IA | All | Curettage + burring | TC | Bone cement | None | 4.9 (2.2–7.1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | Wound infection (1) |
| Kim et al. (2015) 18 | 11 | 46 (18–67) | 13/23 | Long bone | IA | NA | Curettage + burring | E | Bone graft | None | 5.2 (2–14.0) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | Post. Fx. (1) |
| Reoperation (1) | |||||||||||||||
| 25 | Bone cement | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | Post. Fx. (3) | ||||||||||
| Intraarticular loose body (1) | |||||||||||||||
| Joint stiffness (1) | |||||||||||||||
| Reoperation (4) | |||||||||||||||
| Chen et al. (2017) 34 | 7 | 34 (20–55) | 3/4 | Long bone (5) | IA | 7 | Curettage | LN (2) | Bone graft | NA | 8.0 (4–16.2) | 1 (14.3%)g | 0 (0.0%) | 0 (0.0%) | None |
| Axial bone (2) | |||||||||||||||
| Kim et al. (2018) 10 | 8 | 45 (18-62) | 9/15 | Long bone | IA | 21 | Curettage + burring | HP | Bone graft | 16 | 5.5 (4–11.1)h | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | None |
| 16 | Bone cement |
M = male, F = female, IF = internal fixation, LR = local recurrence, DFD = death from disease, CS = chondrosarcoma, LG-CS = low-grade chondrosarcoma, ACTs = atypical cartilaginous tumors, LN = liquid nitrogen, P = phenol, TC = thermal cauterization, HP = hydrogen peroxide, E = ethanol, Post. Fx. = postoperative fracture, NA = not available.
aCalcaneus, IA. bLong bone (n = 7), axial bone (n = 1). cRecurrence, metastasis, and death occurred in the same patient. dThree patients were lost to follow-up. e42 patients. fIB. gLong bone. hMedian follow-up duration, months (range).
Bone cement filling was originally used after intralesional curettage because of the fear of tumor recurrence as well as to ensure mechanical stability. We have performed bone cement filling in four patients at our hospital. However, this number of patients is too small to constitute a control group; therefore, these patients were not included in the present study. Regardless of whether doctors or their patients fear tumor recurrence, excessive local treatment is unacceptable. The ability to continuously evaluate disease progression and improvements in treatment modalities has changed our approach of using bone cement filling to the more physiologic method of bone grafting. In this study, we primarily evaluated the effectiveness of bone grafting to demonstrate its potential as a treatment option for low-grade chondrosarcoma.
The limitations of our study include those inherent to all retrospective analyses as well as the lack of a control group. Our previous case series included two patients who had follow-up periods of 2.1 and 2.9 years, respectively, with no recurrence or metastasis. There was no follow-up in the subsequent 15 to 16 years, which is also a limitation of this study.
Ahlmann et al. 19 described 10 patients with low-grade chondrosarcoma who were treated with intralesional curettage, cryosurgery, and bone cement packing. They reported that no patient developed a pathologic fracture, recurrence, or metastasis; furthermore, there were no cases of neurovascular injury, cutaneous necrosis, or infection during a mean follow-up period of 38.5 months (range, 24–60 months). Loosening of the intramedullary nail was observed in one case, which required surgical treatment. 19 Meftah et al. 15 reported the long-term results of low-grade chondrosarcoma after intralesional curettage, cryotherapy, and bone cement filling. In this cohort, 2 patients developed postoperative fractures, 1 developed infection, and 7 underwent reoperations; additionally, 4 patients developed local recurrence among 43 lesions in 42 patients, with a mean follow-up period of 10.2 ± 4.6 years. The mean time to recurrence was 2.4 ± 2.3 years (range, 0.6–5.6 years), and no patients developed metastatic disease during the follow-up period. 15 Kim et al. 18 described 36 patients with low-grade chondrosarcoma of the long bones who were treated with intralesional curettage and alcohol adjuvant therapy; no local recurrence or distant metastasis was detected during a mean follow-up period of 62 months. In the bone cement group, postoperative fractures were observed in three patients, an intra-articular loose body in one patient, postoperative joint stiffness in one patient, and reoperation in four patients. In the bone graft group, a postoperative fracture was observed in one patient, and another patient required reoperation. 18 Verdegaal et al. 13 reported the results of intralesional curettage treatment of 85 patients with low-grade chondrosarcoma with additional phenol, alcohol, and bone grafting. Five (5.8%) patients showed recurrence, 2 developed postoperative fractures, 1 developed a superficial infection, and 13 required reoperation during a mean follow-up period of 6.8 years (range, 0.2–14.1 years). 13
The stiffness of cement differs from that of adjacent bone. 24 In addition, bone cement cannot be absorbed; its Young’s modulus lies between that of cancellous and cortical bones, and there is a long-term risk of fracture. 25 However, bone grafting facilitates restoration of the original physiological characteristics of the bone after healing, which is the most significant advantage of this method (Figure 4).
Cryosurgery is a powerful adjuvant therapy, but it can induce deep osteonecrosis at 7 to 12 mm 26 and increase the risk of fracture. Weight-bearing bones, such as the femur and tibia, carry a large amount of force while walking, and obvious cortical bone invasion increases the risk of fracture. In our study, cryotherapy, involvement of weight-bearing bones, and obvious cortical bone invasion were important considerations for the administration of preventive internal fixation. Thirteen of the 14 patients who underwent preventive internal fixation were treated with cryotherapy, and most of these fixation procedures involved weight-bearing bones such as the femur and tibia. In the two cases occurring in the humerus, the cortical bone was at a significant risk of invading the fractures; therefore, prophylactic internal fixation was also performed in these cases. In one case of distal femoral lesions, which were mostly confined to cancellous bone, only a small part of the invasion reached the cortex; therefore, cryotherapy was performed instead of preventive internal fixation. Thirteen patients did not receive cryotherapy, and 12 of these were not treated with internal fixation.
Two fractures were observed in our series. The first occurred in a 46-year-old man who developed a pathologic fracture crossing the tumor lesion of the humerus during surgery, and the fracture was repaired with a plate. The other fracture occurred in a 20-year-old man who had low-grade chondrosarcoma in the humerus. He was discharged after satisfactory intralesional curettage, cryotherapy, bone grafting, and internal fixation with a plate. However, 25 days after surgery, he developed a fracture at the distal end of the initial plate fixation, which was changed to a long plate. We recommend prophylactic internal fixation in patients undergoing cryotherapy of weight-bearing bones, such as the femur and tibia, or in patients showing obvious cortical bone invasion (scallop-like appearance) with a high risk of fracture.
Intralesional treatment of low-grade intramedullary chondrosarcoma in the long bones results in average postoperative Musculoskeletal Tumor Society scores of 92% to 94%.4,10,22 No patient in our study had any apparent discomfort or dysfunction. Therefore, the Musculoskeletal Tumor Society score was not recorded for any patient.
For histologically confirmed enchondroma with postoperative recurrence and when the reoperation specimens show low-grade chondrosarcoma, it is unclear whether the tumor was initially chondrosarcoma or secondary chondrosarcoma. The entire process from diagnosis to treatment of low-grade chondrosarcoma remains controversial. 27 It is impossible to distinguish low-grade chondrosarcoma from enchondroma by histology alone.28,29 Other reports of low-grade chondrosarcoma may include some cases of enchondroma, resulting in the favorable clinical effects shown in other studies. 29 For this reason, the histologic diagnosis of all cases in the present study was based on a weekly clinical-radiologic-pathologic team-based approach.
Although wide excision may reduce the recurrence rate, it dramatically impairs patient functions, indicating that this therapy may be excessive. An 85% recurrence rate has been reported upon simple intralesional curettage of low-grade chondrosarcoma. 16 In a meta-analysis, Hickey et al. 17 reported no statistically significant difference in local recurrence or metastasis between intralesional resection (78 patients) and wide resection (112 patients). Adjuvant therapy includes cryotherapy and phenol, alcohol, and thermal cauterization; however, a uniform standard is currently absent. Therefore, the appropriate surgical modality also remains unclear. Considering this, adjuvant therapy may not be essential, particularly if curettage provides an adequate surgical margin. Schreuder et al. 30 reported that 8 of 11 cases of uncertain tumors (enchondroma or low-grade chondrosarcoma) were finally diagnosed as low-grade chondrosarcoma by preoperative needle biopsy. However, Bauer et al. 31 suggested that tissue biopsy is not required in cases without pain and in those showing radiological inactivity. We also agree that most cases do not require biopsy. According to Tsuda et al., 3 the local recurrence rate primarily depends on the sufficiency of the operative treatment rather than the histologic grading. However, some experts believe histologic tumor grading to be an important index of local recurrence and metastasis.3,4
Although local recurrence is common 2 years after surgery, our study only had a minimum follow-up of 2 years, which could be another limitation. Nonetheless, our 3- and 5-year follow-up patients showed no local recurrence or metastasis. Moreover, all of our patients had stage IA low-grade chondrosarcoma of the long bones; patients with highly aggressive axial bone tumors were excluded. One patient was suspected to have a residual tumor by gadolinium-enhanced MRI, but no surgery was performed. No significant progression in the lesions was found after 6.2 years of follow-up. Residual tumors were mainly incompletely scraped. In the case of bone grafting, it is difficult to differentiate between tumor recurrence and residual tumor tissue. We used a combined approach of gadolinium-enhanced MRI and Tc99m bone scan in the follow-up cases. We believe that any residual lesions were small, and no significant progression was observed.
In conclusion, after adequate intralesional curettage with or without cryosurgery, combined bone grafting of autogenous and allogeneic bone chips was effective for curing low-grade intramedullary chondrosarcoma, thereby establishing physiologic and biologic reconstruction and achieving good oncologic outcomes. Furthermore, prophylactic internal fixation with a plate is recommended in patients undergoing cryotherapy of definite cortical invasion and weight-bearing bones.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211025403 for Evaluation of bone grafting for treatment of low-grade chondrosarcoma of long bones by Guofeng Zhang, Sangho Cheon and Ilhyung Park in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211025403 for Evaluation of bone grafting for treatment of low-grade chondrosarcoma of long bones by Guofeng Zhang, Sangho Cheon and Ilhyung Park in Journal of International Medical Research
Acknowledgment
We would like to thank Ms. Hyunhee Bang, Master of Nursing (Medical Device and Robot Institute of Park, Kyungpook National University) for her role in the review process of the Ethics Review Committee.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ilhyung Park https://orcid.org/0000-0003-3865-183X
References
- 1.Morales AG, Sabrido JLG, Calvo JA, et al. Total sacrectomy for the treatment of advanced pelvic chondrosarcoma. Indian J Surg Oncol 2020; 11: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramaniam S, Pawar V. Cytologic diagnosis of chondrosarcoma on fine needle aspiration cytology: a challenge! Journal of Krishna Institute of Medical Sciences University 2019; 8: 101–104. https://www.jkimsu.com/jkimsu-vol8no4/JKIMSU,%20Vol.%208,%20No.%204,%20October-December%202019%20Page%20101-104.pdf [Google Scholar]
- 3.Tsuda Y, Tsoi K, Stevenson JD, et al. Development and external validation of nomograms to predict sarcoma-specific death and disease progression after surgical resection of localized high-grade conventional primary central chondrosarcoma and dedifferentiated chondrosarcoma. Bone Joint J 2020; 102-b: 1752–1759. [DOI] [PubMed] [Google Scholar]
- 4.Dierselhuis EF, Goulding KA, Stevens M, et al . Intralesional treatment versus wide resection for central low-grade chondrosarcoma of the long bones. Cochrane Database Syst Rev 2019; 3: Cd010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumstein G, Kelley B, Nelson S, et al. Low-grade spinal malignancies: chordoma and chondrosarcoma. In: Singh K, Colman M. (eds) Surgical spinal oncology. Cham: Springer, 2020, pp.89–113. 10.1007/978-3-030-50722-0_7 [DOI] [Google Scholar]
- 6.Weber KL, Pring ME, Sim FH. Treatment and outcome of recurrent pelvic chondrosarcoma. Clin Orthop Relat Res 2002: 19–28. [DOI] [PubMed] [Google Scholar]
- 7.Schwab JH, Wenger D, Unni K, et al. Does local recurrence impact survival in low-grade chondrosarcoma of the long bones? Clin Orthop Relat Res 2007; 462: 175–180. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Geest IC, De Valk MH, De Rooy JW, et al. Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol 2008; 98: 421–426. [DOI] [PubMed] [Google Scholar]
- 9.Leerapun T, Hugate RR, Inwards CY, et al. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res 2007; 463: 166–172. [DOI] [PubMed] [Google Scholar]
- 10.Kim W, Lee JS, Chung HW. Outcomes after extensive manual curettage and limited burring for atypical cartilaginous tumour of long bone. Bone Joint J 2018; 100-b: 256–261. [DOI] [PubMed] [Google Scholar]
- 11.Mohler DG, Chiu R, McCall DA, et al. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res 2010; 468: 2765–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Giorgio L, Touloupakis G, Vitullo F, et al. Intralesional curettage, with phenol and cement as adjuvants, for low-grade intramedullary chondrosarcoma of the long bones. Acta Orthop Belg 2011; 77: 666–669. [PubMed] [Google Scholar]
- 13.Verdegaal SH, Brouwers HF, Van Zwet EW, et al. Low-grade chondrosarcoma of long bones treated with intralesional curettage followed by application of phenol, ethanol, and bone-grafting. J Bone Joint Surg Am 2012; 94: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 14.Campanacci DA, Scoccianti G, Franchi A, et al. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol 2013; 14: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meftah M, Schult P, Henshaw RM. Long-term results of intralesional curettage and cryosurgery for treatment of low-grade chondrosarcoma. J Bone Joint Surg Am 2013; 95: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 16.Sanerkin NG, Gallagher P. A review of the behaviour of chondrosarcoma of bone. J Bone Joint Surg Br 1979; 61-b: 395–400. [DOI] [PubMed] [Google Scholar]
- 17.Hickey M, Farrokhyar F, Deheshi B, et al. A systematic review and meta-analysis of intralesional versus wide resection for intramedullary grade I chondrosarcoma of the extremities. Ann Surg Oncol 2011; 18: 1705–1709. [DOI] [PubMed] [Google Scholar]
- 18.Kim W, Han I, Kim EJ, et al. Outcomes of curettage and anhydrous alcohol adjuvant for low-grade chondrosarcoma of long bone. Surg Oncol 2015; 24: 89–94. [DOI] [PubMed] [Google Scholar]
- 19.Ahlmann ER, Menendez LR, Fedenko AN, et al. Influence of cryosurgery on treatment outcome of low-grade chondrosarcoma. Clin Orthop Relat Res 2006; 451: 201–207. [DOI] [PubMed] [Google Scholar]
- 20.Yasko AW, Ravi V, Guadagnolo A. Chondrosarcoma. In: Lin P, Patel S. (eds) Bone sarcoma. MD Anderson Cancer Care Series. Boston: Springer, 2013, pp.117–130. 10.1007/978-1-4614-5194-5_7 [DOI] [Google Scholar]
- 21.Pountos I, Giannoudis PV. Drug-eluting implants for the suppression of metastatic bone disease: current insights. Expert Rev Med Devices 2018; 15: 301–311. [DOI] [PubMed] [Google Scholar]
- 22.Phull SS, Yazdi AR, Ghert M, et al. Bone cement as a local chemotherapeutic drug delivery carrier in orthopedic oncology: a review. J Bone Oncol 2021; 26: 100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Göbel V, Jürgens H, Etspüler G, et al . Prognostic significance of tumor volume in localized Ewing's sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol 1987; 113: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res 1997; 38: 155–182. [DOI] [PubMed] [Google Scholar]
- 25.Miller M, Thompson SR. Miller's Review of Orthopaedics. Elsevier Health Sciences, 2016. [Google Scholar]
- 26.Marcove RC, Stovell PB, Huvos AG, et al. The use of cryosurgery in the treatment of low and medium grade chondrosarcoma. A preliminary report. Clin Orthop Relat Res 1977; 147–156. [PubMed] [Google Scholar]
- 27.Abbas K, Tahir A. Evaluation of different treatment and management options for chondrosarcoma; the prognostic factors determining the outcome of the disease. Int J Surg Oncol 2018; 3: e58. 10.1097/IJ9.0000000000000058. [DOI] [Google Scholar]
- 28.Wang XL, De Beuckeleer LH, De Schepper AM, et al. Low-grade chondrosarcoma vs enchondroma: challenges in diagnosis and management. Eur Radiol 2001; 11: 1054–1057. [DOI] [PubMed] [Google Scholar]
- 29.Mirra JM, Gold R, Downs J, et al. A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones. A clinicopathologic analysis of 51 cases. Clin Orthop Relat Res 1985: 214–237. [PubMed] [Google Scholar]
- 30.Schreuder HW, Pruszczynski M, Veth RP, et al. Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur J Surg Oncol 1998; 24: 120–126. [DOI] [PubMed] [Google Scholar]
- 31.Bauer HC, Brosjö O, Kreicbergs A, et al. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities. 80 patients followed for 2-25 years. Acta Orthop Scand 1995; 66: 283–288. [DOI] [PubMed] [Google Scholar]
- 32.Hanna SA, Whittingham-Jones P, Sewell MD, et al. Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg Oncol 2009, 35: 1343–1347. [DOI] [PubMed] [Google Scholar]
- 33.Mermerkaya MU, Bekmez S, Karaaslan F, et al. Intralesional curettage and cementation for low-grade chondrosarcoma of long bones: retrospective study and literature review. World J Surg Oncol 2014; 12: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YC, Wu PK, Chen CF, et al. Intralesional curettage of central low-grade chondrosarcoma: a midterm follow-up study. J Chin Med Assoc 2017; 80: 178–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211025403 for Evaluation of bone grafting for treatment of low-grade chondrosarcoma of long bones by Guofeng Zhang, Sangho Cheon and Ilhyung Park in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211025403 for Evaluation of bone grafting for treatment of low-grade chondrosarcoma of long bones by Guofeng Zhang, Sangho Cheon and Ilhyung Park in Journal of International Medical Research