Graphical abstract
Abbreviations: AAALAC, Association for Assessment and Accreditation of Laboratory Animal Care; bw or BdW, body weight; CAS, Chemical Abstracts Service; CFR, Code of Federal Regulation; EFSA, European Food Safety Authority; EPA, Environmental Protection Agency; FEMA, Flavor and Extract Manufacturers Association; FDA, Food and Drug Administration; GRAS, Generally Recognized as Safe; GLP, Good Laboratory Practice; JECFA, Joint FAO/WHO Expert Committee on Food Additives; MW, molecular weight; NOAEL, no-observed-adverse-effect level; OECD, Organisation of Economic Cooperation and Development; ppm, parts per million; RSD, relative standard deviation; SD, standard deviation
Keywords: beta-Ionone epoxide, FEMA GRAS, flavoring ingredient, fragrance material, Rat, Toxicity
Highlights
-
•
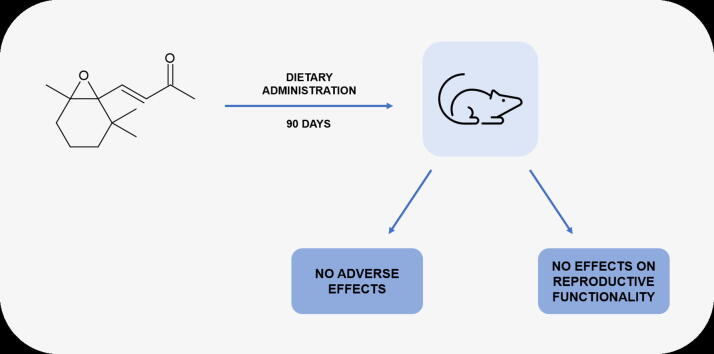
GLP-compliant 90-day study on β-ionone epoxide following OECD guideline.
-
•
β-Ionone epoxide produces no adverse effects at doses up to 80 mg/kg bw/day in rats.
-
•
β-Ionone epoxide had no effect on reproductive functionality parameters.
Abstract
In a 90-day GLP-compliant study groups of Sprague-Dawley rats (10/sex/group) were fed diets containing β-ionone epoxide, a fragrance material and a flavoring substance, at dietary concentrations providing target intakes of 0, 20, 40 and 80 mg/kg bw/day. There were no deaths and no adverse changes in clinical observations, ophthalmological examinations, body weight, body weight gain, food consumption, food efficiency; hematology, serum chemistry, urinalysis parameters; or in macroscopic findings attributable to β-ionone epoxide administration. Increased absolute and relative liver weights in high dose females without correlating hepatic histopathological findings were considered non-adverse. Cortical vacuolation of adrenal zona fasciculata was observed in high-dose males but was considered non-adverse due to the nondegenerative nature of this alteration. β-Ionone epoxide did not influence estrus cyclicity in females and did not affect sperm morphology or epididymal sperm count, homogenization-resistant spermatid count and motility measurements in male rats. The no-observed-adverse-effect level (NOAEL) for administration of β-ionone epoxide in the diet was determined to be the highest dose tested of 80 mg/kg bw/day.
Introduction
β-Ionone epoxide (Fig. 1) is a flavoring substance with a fruity and woody flavor profile. It occurs naturally in its trans (E-), cis (Z-) or non-specified isomeric configuration in many foods, with measured levels in apricots, dill, endive, raspberry, tea and tomato up to 8 ppm and in lemon balm up to 11,000 ppm (Nykanen and Nykanen, 1986, Schreier et al., 1981, Kawakami and Yamanishi, 1983, Buttery et al., 1993, Aprea et al., 2015, Takeoka et al., 1990). It is also used as a fragrance ingredient with an intensely sweet, fruity-woody odor with floral notes similar to raspberry (Arctander, 1969).
Fig. 1.
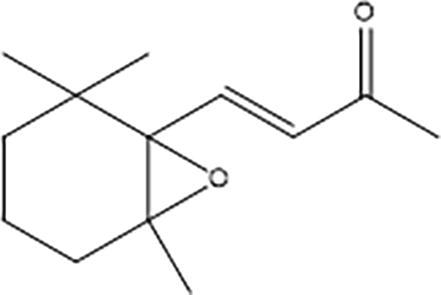
Chemical structure of β-ionone epoxide.
The use of β-ionone epoxide as a fragrance and flavoring substance was evaluated in the United States, Europe and internationally by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). In the United States, the Flavor and Extract Manufacturers Association (FEMA) Expert Panel evaluated β-ionone epoxide (FEMA No. 4144) as part of a group of similar epoxides in 2004 and reaffirmed it as a “Generally Recognized as Safe” (GRAS) flavoring substance (Smith et al., 2005). JECFA evaluated β-ionone epoxide (JECFA No. 1571) at its 65th meeting in 2006, as part of the group “Epoxides” and concluded that it was of no safety concern considering its natural occurrence and the low estimated daily intakes based on annual production volumes (FAO/WHO Joint Expert Committee on Food Additives, 2006, FAO/WHO Joint Expert Committee on Food Additives, 2009). In Europe, the European Food Safety Authority (EFSA) reviewed β-ionone epoxide [FL-No. 07.170] in 2009 and assigned it to further review for genotoxicity as part of a group of α,β-unsaturated ketones (European Food Safety Authority, 2009). Genotoxicity data were provided that ruled out concerns of genotoxic potential. In fact, β-ionone epoxide showed no mutagenic activity when tested in two different bacterial reverse mutation assays and in a mouse lymphoma thymidine kinase (tk+/−) gene mutation assay (Flanders, 2006, Jones, 1988, Kringstad, 2005). Once cleared of genotoxicity concerns (European Food Safety Authority, 2014), EFSA reviewed all available data for β-ionone epoxide in the same year (European Food Safety Authority, 2014). However, for this flavoring substance to be evaluated through EFSA’s safety evaluation procedure, a subchronic toxicity study was required to establish a no-observed-adverse-effect level (NOAEL). As a result, additional toxicological data were requested to complete the safety evaluation. In response to EFSA’s request, the OECD guideline-compliant subchronic toxicity study presented here was conducted and appropriately designed to derive a NOAEL. Additional endpoints of reproductive function were included for β-ionone epoxide in this study due to lack of relevant data in the broader database for this and similar epoxides. At the time of this publication, the EFSA safety evaluation for this material was ongoing, based on the newly submitted toxicity data. The purpose of this article is to present the results of the subchronic toxicity study on β-ionone epoxide.
Methods and materials
Study compliance
This study (Bauter, 2016) was conducted at the contract research organization (CRO) Product Safety Labs (PSL; Dayton, NJ) under the sponsorship of the International Organization of the Flavor Industry (IOFI) and the Research Institute for Fragrance Materials (RIFM). It was conducted according to the general principles outlined in OECD Guideline 408 (Organisation for Economic Cooperation and Development, 1998); the US FDA Toxicological Principles for the Safety Assessment of Food Ingredients (Redbook 2000) (US Food and Drug Administration, 2007); and principles of Good Laboratory Practice (GLP) (US Food and Drug Administration, 1987, Organisation for Economic Cooperation and Development, 1998). Clinical pathology evaluations were performed by Product Safety Labs.
Test substance
β-Ionone epoxide (IUPAC Name: 4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)but-3-en-2-one; MW 208.30; CAS No. 23267–57-4) was sourced from a flavor supplier as a light yellow liquid to crystalline product with a purity of > 95% (by area using GC–MS) of 5,6-epoxy ionone (a.k.a., β-ionone epoxide). The test material is representative of the flavoring substance in the market (referred to henceforth as “test substance”) and complied with EFSA and JECFA purity specifications (assay minimum > 95%). The test substance was stored at ambient conditions prior to initiation of the study. The stability, concentration verification and homogeneity in the dietary mixtures were confirmed as part of the study using gas chromatography (Product Safety Labs, Dayton, NJ).
Dietary preparation
Appropriate concentrations of β-ionone epoxide were added to the diet (excluding control diets) and thoroughly mixed. The stability of β-ionone epoxide in the diet was evaluated in samples taken from the lowest and highest dose concentrations on Days 0, 4, 7 and 10 following initial and final diet preparation using gas chromatography. Test substance homogeneity in the diet was assessed at the beginning of the study in samples taken from the top, middle and bottom strata of the diet preparations. Diet samples for concentration verification of β-ionone epoxide were taken at the beginning, middle and end of the study. All samples were kept frozen (-20 °C) until analysis.
Chemical analysis
Samples of neat substance and dietary mixtures at the lowest and highest dietary concentrations were analyzed using gas chromatography with flame ionization detector (GC-FID) (Hewlett Packard HP 6890 Series) for homogeneity, concentration verification and stability (Bellizio, 2015). The chemical analysis was conducted according to US EPA GLP Standards 40 CFR Part 160.113. The analytical method was developed and validated by the testing laboratory for the purpose of this study. Briefly, a Rtx-5MS capillary column (30 m × 0.25 mm, 0.25 µm film thickness) was used with carrier gas set to a constant flow rate of 1.8 mL/min. Split mode injections (ratio 10:1) were utilized with an inlet temperature of 230 °C. Oven operating conditions were as follows: initial temperature of 50 °C for 5 min, increasing to 240 °C at 25 °C/min and then held at 240 °C for 5 min. The detector temperature was set to 250 °C, with air and hydrogen flow rates of 400 and 40 mL/min, respectively. Injection volumes of 1 µL were used for all sample and standard solutions. All samples were extracted with methanol containing an internal standard (undecane) at a concentration of 100 µg/mL.
Experimental design
Target concentrations for the test diets in the main 90-day toxicity study were based on an unpublished non-GLP 14-day range finding toxicity study (Bauter, 2015), conducted in accordance with OECD Guideline 407 (Organisation for Economic Cooperation and Development, 2008) and Redbook 2000, IV.C.3.a. Short-Term Toxicity Studies with Rodents (US Food and Drug Administration, 2003). In the 14-day range-finding study, groups of CRL Sprague-Dawley CD® IGS rats (5/sex/group) were fed diets with nominal concentrations of 0 (control), 1200, 2400 or 4800 ppm β-ionone epoxide (equivalent to a mean overall dietary intake of 0, 106.1, 214.2 or 419.0 mg/kg bw/day for males and 0, 102.6, 210.8 or 421.1 mg/kg bw/day for females, respectively).
In the 90-day repeat dose toxicity study, test diets were prepared at concentrations calculated to provide target dose levels of 0, 20, 40 or 80 mg β-ionone epoxide/kg bw/day. The dietary intake of β-ionone epoxide (mg/kg bw/day) was calculated based on the animals’ most recent body weights and food consumption data each week and for the study overall. While doses up to 400 -mg/kg bw/day were expected to be welltolerated based on the results of the range finding study, the dose range selected for the 90-day study was limited by test substance availability as the annual production volume for this substance is low. Diets were prepared weekly in 2016C Envigo Teklad Global Certified Rodent Diet® (Envigo Teklad, Inc.) as a meal and were stored refrigerated between administrations. For test diets, the test substance was mixed with a small amount of acetone to facilitate incorporation into the basal diet (the final amount of acetone in the test diet was considered negligible as it was anticipated to fully evaporate during the mixing process). Acetone was also added at an equivalent quantity to the basal control diet. Once added to the basal diet, test diets were thoroughly mixed and administered to animals, while excess diet was stored refrigerated. Additional diet was provided, with or without test substance as needed throughout the study, to ensure ad libitum feeding.
Animals
In the range-finding study, the animals were housed under the same conditions and handled humanely, as described for the main study. CRL Sprague-Dawley CD® IGS rats were obtained from Charles River Laboratories, Inc. (Kingston, NY) at approximately 6–7 weeks of age. For the main study, following a 6-day acclimation period, healthy animals were randomly assigned to one of 4 groups (10/sex/group) stratified by body weight (±20% of the mean weight) and were approximately 7–8 weeks of age with body weights of 203–252 g for males and 164–201 g for females at study initiation. Animals were handled humanely in an AAALAC-accredited facility and were individually housed in suspended stainless steel caging with mesh floors conforming to the size recommendations in the most recent Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Litter paper was placed beneath the cages and was changed at least three times per week. During urine collection, animals were housed in metabolism cages. The animals were kept at a room temperature of 19–25 °C with a relative humidity of 46–71% and a 12-hour light/12-hour dark cycle and were provided with 2016C Envigo Teklad Global Certified Rodent Diet® as a meal and filtered tap water ad libitum.
Observations
Range-finding study
The animals were observed daily for viability, signs of gross toxicity and behavioral changes, and on Days 0, 7 and 14 for a battery of detailed observations. Body weights were recorded twice during the acclimation period including on Day 0, on Days 7 and 10 and on Day 14 prior to terminal sacrifice. Individual food consumption was also recorded at the same time as body weights. All animals were subject to gross necropsy and gross lesions were recorded. Tissues were collected and preserved when warranted by potential toxicity. Animals were not fasted prior to necropsy.
Main toxicity study
Ophthalmological examinations
Prior to treatment and just prior to study termination (Day 88), the eyes of all animals were examined by a veterinary ophthalmologist using mydriatic eye drops, focal illumination, indirect ophthalmoscopy and, when indicated, slit-lamp microscopy.
Clinical signs
The animals were observed at least twice daily for viability during the study and were also observed weekly for a battery of detailed clinical observations, including signs of gross toxicity and behavioral changes.
Body weight
Body weights were recorded twice during acclimation, including prior to test initiation (Day 0) and weekly thereafter until immediately prior to euthanasia. Body weight gain was also calculated.
Food consumption
Food consumption was measured and recorded to coincide with body weight measurements. Food efficiency and dietary intake of the test substance (mg/kg bw/day) was also calculated.
Hematology and clinical chemistry
Rats were fasted overnight prior to blood collection. Blood samples for hematology (500 µL blood collected in K3EDTA-containing tubes) and clinical chemistry (1000 µL blood collected without preservative) were drawn from overnight fasted rats via sublingual bleeding under isoflurane anesthesia during Weeks 8 and 13. Blood samples (1.8 mL) for coagulation parameters (prothrombin time and activated partial thromboplastin time) were collected (in tubes containing 3.2% sodium citrate) via the inferior vena cava under isoflurane anesthesia at termination.
The following hematology parameters were analyzed: erythrocyte count, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, red cell distribution width, absolute reticulocyte count, platelet count, total white blood cell and differential leukocyte count and mean corpuscular hemoglobin concentration. Blood smears stained with new methylene blue or Wright-Giemsa stain also were prepared. The following clinical chemistry parameters were analyzed: serum aspartate aminotransferase, serum alanine aminotransferase, sorbitol dehydrogenase, alkaline phosphatase, total bilirubin, urea nitrogen, blood creatinine, total cholesterol, triglycerides, fasting glucose, total serum protein, albumin, globulin, calcium, inorganic phosphorus, sodium, potassium and chloride.
Urinalysis
Rats were fasted at least 15 h prior to blood collection and placed in metabolism cages one day prior to blood collection. Urine samples for urinalysis were collected from all surviving study animals. The urinary parameters analyzed included pH, ketone, color, glucose, bilirubin, clarity, specific gravity, blood, volume, protein, urobilinogen and microscopic urine sediment.
Necropsy and organ weights
At termination, all rats were euthanized by carbon dioxide asphyxiation and underwent full necropsies. Wet organ weights were recorded for adrenals, kidneys, spleen, brain, liver, testes, epididymides, ovaries with oviducts, thymus, heart, pituitary and uterus. Ventral prostate, seminal vesicles and thyroid/parathyroid were weighed after preservation in 10% neutral buffered formalin. Selected organs and tissues from all animals were preserved in 10% neutral buffered formalin except for eyes with optic nerve, epididymides and testes, which were preserved in modified Davidson’s fixative and stored in ethanol for histological evaluation.
Histopathology
Histopathology evaluations were performed by a board-certified veterinary pathologist on the preserved organs and tissues of all animals from the control and high-dose groups, on any gross lesions and on tissues and organs from the low- and mid-dose groups to further investigate changes of potential toxicological interest, including the adrenal gland of one mid-dose male and the uteri of three low-dose and six mid-dose females. The fixed tissues were trimmed, processed, embedded in paraffin, sectioned with a microtome, placed on glass microscope slides, stained with hematoxylin and eosin and examined by light microscopy.
Estrus cyclicity
During Weeks 6–7 and again during Weeks 12–13 of the treatment period, vaginal smears and estrus cycle stages were taken and recorded daily for each 14-day interval for all females to evaluate reproductive functionality.
Sperm analysis
At the end of the study, all surviving male rats were evaluated for reproductive function by analysis of sperm samples collected at termination for motility, epididymal sperm count, homogenization-resistant spermatid (from the testis) count and morphology.
For motility evaluation, the left vas deferens was excised, placed into a petri dish containing 1% bovine serum albumin and left for 2 min to allow sperm to disperse from the tissue. Then a sample of sperm was collected from the petri dish into a 100 µm-deep glass chamber and immediately loaded into a pre-warmed stage of the Hamilton-Thorne IVOS automated sperm analyzer (Hamilton Thorne Biosciences, Beverly, MA). Motility was assessed for a minimum number of sperm (2 0 0) or until a maximum of 20 fields total were evaluated. Increments of five fields per animal were selected, stored and analyzed as digital images as percent motile sperm to total sperm analyzed per animal.
For total sperm count determination, the left epididymis, which was previously excised and frozen (−70 ± 10 °C), was thawed, weighed, trimmed and then minced in deionized water for preparation of sperm smear slides. For homogenization-resistant spermatid count, the left testis, which was previously removed and frozen (−70 ± 10 °C), was slightly thawed; and the tunica albuginea, along with any associated blood vessels, were removed and discarded. Each testis was weighed, placed into a vial containing deionized water and then homogenized in a Waring® blender. A 100 µL sample of the epididymis and testis suspensions were stained with a dye that uniquely stains sperm heads. These stained suspensions were placed onto 20 µm deep glass slides and loaded into the Hamilton-Thorne IVOS (Hamilton Thorne Biosciences, Beverly, MA) automated sperm analyzer for total sperm count and homogenization-resistant spermatid determination. Twenty fields/animal were selected, reported and adjusted for caudal epididymal weight.
For sperm morphological examination, two eosin-stained slides were prepared for each rat. The slides were evaluated and, where possible, a minimum of 200 sperm cells/rat were examined for morphological development using a light microscope.
Statistical analysis
In-life and organ weight data
Mean and standard deviations were calculated for all quantitative data. When warranted by sufficient group sizes, data within groups were evaluated for homogeneity of variances and normality by Bartlett’s test (Bartlett, 1937). Where Bartlett’s test indicated variance homogeneity, treated and control groups were compared using a one-way analysis of variance (ANOVA). When one-way analysis of variance was significant, a comparison of the treated groups to control by Dunnett’s test (Dunnett, 1964, Dunnett, 1980) for multiple comparisons was performed. Where variances were considered significantly different by Bartlett’s test, groups were compared using a non-parametric analysis of variance (Kruskal and Wallis, 1952). When non-parametric analysis of variance was significant, a comparison of treated groups to control was performed using Dunn’s test (Dunn, 1964).
Clinical pathology
Significance was evaluated at a probability value of p < 0.05. Males and females were analyzed separately. Initially, Levene’s test (Levene and Olkin, 1960) for homogeneity and Shapiro-Wilk test (Shapiro and Wilk, 1965) for normality were performed; if not significant, an ANOVA with Dunnett’s test (Dunnett, 1964, Dunnett, 1980) were conducted. If the preliminary tests were significant, transforms of the data to achieve normality and variance homogeneity were applied. The order of transforms attempted was log, square root and rank-order. If the log and square root transforms failed, the rank-order was used, and a non-parametric analysis of variance was performed for group comparison.
Sperm analysis
Means and standard deviations for sperm motility, epididymal sperm count, homogenization-resistant spermatid (HRS; from the testis) count and morphology data were calculated for each control and treatment group. Data were compared across groups using a non-parametric analysis of variance (Kruskal and Wallis, 1952). If a significant difference was found (p < 0.05), the Mann-Whitney U test (Mann and Whitney, 1947) was used for pair-wise comparisons of each treated group to the control group.
Results
The neat test substance was stable under storage conditions over the course of the study, with measured concentrations within 96.7%, 96% and 99.2% of target, respectively, at the beginning, middle and end of the study. The diets were prepared weekly and kept refrigerated following preparation, unless immediately administered to the test animals on the day of preparation. The dietary preparations at the low and high concentrations were homogeneously distributed within an acceptable margin of variation at the start [relative standard deviation (RSD) of 7.1% and 18%, respectively] and the end (0.6 and 0.8% RSD, respectively) of the study. Dietary preparations were stable for the duration of weekly intervals between preparations, with concentrations within 95.0–102.5% of target at the low dietary concentration and within 98.6–101.8% of target at the high dietary concentration. The concentration verification analysis, conducted at the beginning, middle and end of the study, confirmed that the weekly prepared test diets were consistent for test substance concentrations which ranged from 84.0 to 92.9% of target.
Observations
Range finding study
There were no deaths; adverse clinical observations; or changes in body weight, body weight gain, food consumption, food efficiency or gross findings that were considered a result of test substance administration. The results from the 14-day study indicated that male and female rats should tolerate dietary concentrations up to 4800 ppm β-ionone epoxide (approximately 420 mg/kg bw/day), the highest concentration tested, in a study of longer duration.
Main toxicity study
There were no deaths during the 90-day study. Clinical observations (e.g., slight to moderate alopecia, hair loss and broken incisor) reported were considered incidental and not relevant to the toxicological evaluation of the test material. All animals in the study were normal upon ophthalmic exam.
Mean weekly body weights, mean daily body weight gain, food consumption and food efficiency (Table 1) were comparable to controls in all animals administered β-ionone epoxide.
Table 1.
Mean (±SD) body weights, body weight gains, food consumption and food efficiency in Sprague-Dawley rats (n = 10) fed β-ionone epoxide in the diet for 90 days.
| Nominal Dose Level (mg/kg bw/day) |
||||
|---|---|---|---|---|
| 0 | 20 | 40 | 80 | |
| Males | ||||
| Body weighta (g) | 498 ± 42 | 513 ± 56 | 509 ± 42 | 489 ± 40 |
| Body weight gainb (g/d) | 3.0 ± 0.4 | 3.2 ± 0.6 | 3.1 ± 0.4 | 2.9 ± 0.3 |
| Food consumptionb (g/d) | 26.5 ± 2.3 | 27.3 ± 2.4 | 26.7 ± 2.5 | 25.9 ± 1.6 |
| Food efficiencyb | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 |
| Females | ||||
| Body weighta (g) | 281 ± 20 | 274 ± 26 | 280 ± 32 | 290 ± 30 |
| Body weight gainb (g/d) | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 |
| Food consumptionb (g/d) | 19.1 ± 0.9 | 18.9 ± 1.7 | 19.2 ± 2.2 | 19.3 ± 1.9 |
| Food efficiencyb | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 |
SD, standard deviation
values reported for Day 91
values reported for Day 0 to Day 91 (overall mean for study period)
Dietary intake
The rats were fed diets with β-ionone epoxide at target doses of 20, 40 or 80 mg/kg bw/day. Using body weight and food consumption data collected throughout the study, mean dietary β-ionone epoxide intake during the study (Days 0–91) was 19.3, 38.5 or 77.4 mg/kg bw/day for males and 19.5, 39.0 or 77.7 mg/kg bw/day for females.
Clinical pathology
There were no adverse and/or toxicologically relevant changes in hematology, clinical chemistry or coagulation parameters attributable to administration of β-ionone epoxide. The only statistically significant changes in hematological parameters reported (Table 2) included increased absolute large unstained cell count and decreased absolute reticulocyte count in high-dose males on Day 51 and increased absolute reticulocyte count in high-dose females on Day 51 (Table 2). Prothrombin time showed a small but statistically significant decrease in high-dose females on Day 95. Statistically significant changes also were reported for clinical chemistry parameters and included: on Days 51 and 86, increased triglycerides in high-dose females, decreased alkaline phosphatase in all treated females and increased calcium in mid-dose males; on Day 51, increased cholesterol in high-dose females, decreased aspartate aminotransferase in high-dose males, decreased alanine aminotransferase in high-dose females, decreased sorbitol dehydrogenase in low-dose females, decreased creatinine in mid-dose females, decreased bilirubin in low- and high-dose males and in mid- and high-dose females and decreased chloride in low-and mid-dose males; and on Day 86, decreased bilirubin in high-dose females, decreased creatinine in all treated females and decreased chloride in mid-dose females (Table 2). The only statistically significant differences in urinalysis parameters were increased volume in high-dose females on Days 51 and 86, decreased specific gravity in high-dose females on Day 86 and decreased urine protein in mid and high dose females on Day 86 (Table 2).
Table 2.
Selected mean (±SD) hematology, coagulation, clinical chemistry and urinalysis parameters of Sprague-Dawley rats (n = 10) fed β-ionone epoxide in the diet for 90 days.
| Nominal Dose Level (mg/kg bw/day) |
Historical Control Data |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 80 | Mean | Range | ||
| Males | |||||||
| Hematology | |||||||
| RBC (x106/µL) | Day 51 | 8.5±0.3 | 8.4±0.4 | 8.3±0.4 | 8.3±0.4 | 8.75 | 5.07-10.04 |
| Day 86 | 8.7±0.3 | 8.6±0.3 | 8.5±0.3 | 8.6±0.5 | |||
| HGB (g/dL) | Day 51 | 16.1±0.4 | 15.9±0.6 | 16.0±0.5 | 16.0±0.5 | 15.6 | 10.5-17.3 |
| Day 86 | 16.1±0.4 | 16.1±0.6 | 15.9±0.6 | 16.1±0.4 | |||
| HCT (%) | Day 51 | 46.3±1.2 | 45.9±1.6 | 46.1±1.4 | 45.9±1.3 | 46.27 | 34.8-50.6 |
| Day 86 | 46.0±1.1 | 45.9±1.5 | 45.6±1.5 | 46.2±1.3 | |||
| WBC (x103/µL) | Day 51 | 12.9±1.9 | 13.6±3 | 14.7±3.2 | 13.8±2.1 | 12.77 | 7.29-23.97 |
| Day 86 | 8.7±1.5 | 10±2.6 | 10.3±1.7 | 9.3±1.8 | |||
| NEU (x103/µL) | Day 51 | 2.1±0.8 | 2.5±1.1 | 2.1±1.3 | 1.8±0.6 | 2.23 | 0.53-9.39 |
| Day 86 | 1.3±0.4 | 2.0±0.9 | 1.6±0.8 | 1.3±0.4 | |||
| LYM (x103/µL) | Day 51 | 10.0±1.5 | 10.4±2.4 | 11.8±3 | 11.3±2.2 | 9.74 | 3.33-19.97 |
| Day 86 | 7.0±1.1 | 7.5±2.2 | 8.3±1.8 | 7.6±1.7 | |||
| MON (x103/µL) | Day 51 | 0.37±0.06 | 0.34±0.16 | 0.41±0.26 | 0.33±0.11 | 0.39 | 0.14-0.94 |
| Day 86 | 0.17±0.06 | 0.18±0.07 | 0.19±0.07 | 0.15±0.05 | |||
| LUC (x103/µL) | Day 51 | 0.06±0.02 | 0.07±0.03 | 0.09±0.05 | 0.08±0.01** | 0.13 | 0-0.47 |
| Day 86 | 0.03±0.01 | 0.04±0.01 | 0.04±0.02 | 0.04±0.02 | |||
| RET (x103/µL) | Day 51 | 277±34 | 252±65 | 237±54 | 203±32* | 231 | 121-1913 |
| Day 86 | 201±28 | 193±46 | 182±47 | 164±24 | |||
| Coagulation | |||||||
| PT (sec) | Day 94 | 10.5±0.3 | 10.4±0.3d | 10.3±0.2 | 10.5±0.4 | 10.5 | 9.5-11.5 |
| Clinical Chemistry | |||||||
| AST (U/L) | Day 51 | 86±9a | 86±9b | 83±8c | 75±5d* | 95 | 56-345 |
| Day 86 | 92±2e | 82±9c | 84±10f | 81±15e | |||
| ALT (U/L) | Day 51 | 37±2 | 37±5 | 37±4 | 36±4 | 40 | 18-221 |
| Day 86 | 32±4 | 34±6 | 33±5 | 35±6 | |||
| SDH (U/L) | Day 51 | 4.5±0.9a | 8.0±3.0b | 8.8±6.0c | 6.8±3.1d | 9.4 | 0-126.0 |
| Day 86 | 1.6±1.8e | 3.4±0.8c | 4.5±4.1f | 3.6±1.6e | |||
| ALKP (U/L) | Day 51 | 106±15 | 120±19 | 105±19 | 103±25 | 99 | 55-183 |
| Day 86 | 85±18 | 93±19 | 81±14 | 84±20 | |||
| BILI (mg/dL) | Day 51 | 0.18±0.02 | 0.16±0.02* | 0.16±0.03 | 0.14±0.02* | 0.17 | 0.10-0.28 |
| Day 86 | 0.15±0.02 | 0.14±0.01 | 0.14±0.02 | 0.14±0.02 | |||
| CHOL (mg/dL) | Day 51 | 64±14 | 59±11 | 67±10 | 74±13 | 80 | 34-159 |
| Day 86 | 71±17 | 66±15 | 75±11 | 81±16 | |||
| CREA (mg/dL) | Day 51 | 0.27±0.04 | 0.28±0.05 | 0.27±0.04 | 0.28±0.05 | 0.29 | 0.20-0.48 |
| Day 86 | 0.30±0.04 | 0.28±0.04 | 0.30±0.03 | 0.29±0.04 | |||
| TRIG (mg/dL) | Day 51 | 44±15 | 58±21 | 54±14 | 51±14 | 94 | 18-283 |
| Day 86 | 48±19 | 55±20 | 51±16 | 48±18 | |||
| CALC (mg/dL) | Day 51 | 9.6±0.3 | 9.6±0.3 | 9.9±0.3* | 9.7±0.2 | 10.2 | 8.1-11.8 |
| Day 86 | 9.6±0.2 | 9.8±0.2 | 9.9±0.3* | 9.8±0.2 | |||
| CL (mmol/L) | Day 51 | 103±1 | 102±1* | 102±1 | 102±1* | 103 | 92-120 |
| Day 86 | 102±1 | 102±1 | 103±1 | 102±1 | |||
| Urinalysis | |||||||
| SG | Day 51 | 1.04±0.03 | 1.05±0.02 | 1.03±0.02 | 1.04±0.03 | 1.06 | 1.01-1.10 |
| Day 86 | 1.04±0.01 | 1.05±0.02 | 1.04±0.02 | 1.03±0.01 | |||
| UVOL (mL) | Day 51 | 12.2±7 | 7.4±5.6 | 12.5±6.3 | 13.9±10.9 | 6.7 | 0.4-36.0 |
| Day 86 | 6.5±2.3 | 6.8±5.9 | 7.0±3.7 | 9.8±5.6 | |||
| UMTP (mg/dL) | Day 51 | 130±161b | 157±75 | 109±96 | 111±87 | 243 | 27-1330 |
| Day 86 | 125±45 | 151±70 | 142±48 | 94±35 | |||
| Females | |||||||
| Hematology | |||||||
| RBC (x106/µL) | Day 51 | 7.8±0.4 | 8.2±0.5 | 8.1±0.4 | 8.1±0.3 | 8.3 | 7.24-9.34 |
| Day 86 | 7.9±0.5 | 8.2±0.4 | 8.1±0.4 | 8.0±0.3 | |||
| HGB (g/dL) | Day 51 | 15.3±0.9 | 15.9±0.7 | 15.7±0.6 | 15.9±0.6 | 15.3 | 13.4-17.1 |
| Day 86 | 15.6±0.9 | 15.9±0.5 | 15.7±0.7 | 15.8±0.5 | |||
| HCT (%) | Day 51 | 43.3±2.3 | 45.2±1.9 | 44.4±1.7 | 45.0±1.6 | 44.9 | 40.6-49.4 |
| Day 86 | 43.7±2.7 | 44.5±1.4 | 43.9±1.9 | 44.1±1.4 | |||
| WBC (x103/µL) | Day 51 | 11.1±3.5 | 9.6±2 | 9.8±2.8 | 9.5±2.3 | 7.8 | 2.41-14.79 |
| Day 86 | 5.5±2.9 | 6.2±2.3 | 6.2±2.2 | 6.6±2.3 | |||
| NEU (x103/µL) | Day 51 | 0.9±0.4 | 1.0±0.4 | 1.3±0.3 | 1.1±0.7 | 1.08 | 0.33-3.11 |
| Day 86 | 0.6±0.2 | 0.7±0.6 | 0.8±0.4 | 1±0.5 | |||
| LYM (x103/µL) | Day 51 | 9.6±3.3 | 8.1±2.0 | 8.1±2.6 | 7.8±2 | 6.27 | 1.8-12.84 |
| Day 86 | 4.7±2.6 | 5.2±2.2 | 5.1±2 | 5.3±1.9 | |||
| MON (x103/µL) | Day 51 | 0.22±0.14 | 0.21±0.07 | 0.23±0.10 | 0.22±0.15 | 0.21 | 0.04-0.47 |
| Day 86 | 0.09±0.05 | 0.10±0.04 | 0.11±0.06 | 0.09±0.05 | |||
| LUC (x103/µL) | Day 51 | 0.07±0.06 | 0.06±0.02 | 0.06±0.03 | 0.04±0.02 | 0.08 | 0-0.26 |
| Day 86 | 0.02±0.02 | 0.03±0.02 | 0.03±0.01 | 0.03±0.01 | |||
| RET (x103/µL) | Day 51 | 174±37 | 186±33 | 182±45 | 225±39* | 166 | 91-247 |
| Day 86 | 114±25 | 122±17 | 139±27 | 140±30 | |||
| Coagulation | |||||||
| PT (sec) | Day 95 | 10.1±0.2 | 10.0±0.3 | 10.0±0.2 | 9.7±0.2* | 9.9 | 9.2-10.5 |
| Clinical Chemistry | |||||||
| AST (U/L) | Day 51 | 87±23d | 75±12 | 95±66 | 70±11g | 82 | 47-460 |
| Day 86 | 93±38 | 78±21 | 73±18 | 68±15 | |||
| ALT (U/L) | Day 51 | 38±12 | 30±8 | 36±14 | 27±3** | 35 | 13-283 |
| Day 86 | 41±17 | 32±11 | 31±5 | 31±13 | |||
| SDH (U/L) | Day 51 | 8.5±2.5d | 5.8±1.9** | 8.8±6.6 | 6.6±1.6g | 8.1 | 0.2-42.7 |
| Day 86 | 8.9±6.0 | 5.7±2.0 | 6.1±1.9 | 7.4±2.9 | |||
| ALKP (U/L) | Day 51 | 73±19 | 55±11* | 52±13* | 54±12* | 53 | 21-179 |
| Day 86 | 57±14 | 40±9* | 37±9* | 44±11* | |||
| BILI (mg/dL) | Day 51 | 0.19±0.03 | 0.18±0.01 | 0.16±0.02* | 0.15±0.03* | 0.18 | 0.10-0.27 |
| Day 86 | 0.15±0.02 | 0.15±0.02 | 0.14±0.03 | 0.13±0.01* | |||
| CHOL (mg/dL) | Day 51 | 70±15 | 74±18 | 78±17 | 90±13* | 94 | 42-225 |
| Day 86 | 83±14 | 87±22 | 98±20 | 104±18 | |||
| CREA (mg/dL) | Day 51 | 0.34±0.03 | 0.31±0.05 | 0.30±0.02* | 0.31±0.03 | 0.35 | 0.23-0.53 |
| Day 86 | 0.41±0.05 | 0.35±0.04* | 0.35±0.03* | 0.36±0.03* | |||
| TRIG (mg/dL) | Day 51 | 30±6 | 33±4 | 34±10 | 41±15* | 55 | 16-265 |
| Day 86 | 37±10 | 43±9 | 44±14 | 66±32** | |||
| CALC (mg/dL) | Day 51 | 10.0±0.4 | 10.0±0.4 | 10.1±0.4 | 10.1±0.5b | 10.5 | 9.0-12.1 |
| Day 86 | 10.2±0.3 | 10.1±0.3 | 10.4±0.4 | 10.3±0.5 | |||
| CL (mmol/L) | Day 51 | 103±1 | 103±1 | 102±2 | 103±1 | 103 | 93-118 |
| Day 86 | 104±1 | 103±1 | 102±2** | 103±2 | |||
| Urinalysis | |||||||
| SG | Day 51 | 1.04±0.02 | 1.03±0.02 | 1.02±0.01 | 1.02±0.01 | 1.05 | 1.01-1.10 |
| Day 86 | 1.07±0.04 | 1.06±0.02 | 1.05±0.03 | 1.04±0.03* | |||
| UVOL (mL) | Day 51 | 6.9±4.5 | 6.6±3.1 | 10.6±5.7 | 12.5±6.3* | 4.6 | 0.10-23.0 |
| Day 86 | 2.9±4.6 | 2.4±1.5 | 5.3±5.3 | 7.6±6.8* | |||
| UMTP (mg/dL) | Day 51 | 38±21 | 32±20 | 24±9 | 32±32 | 111 | Sep-00 |
| Day 86 | 145±86b | 90±56 | 70±53 | 92±179# | |||
SD, standard deviation; ALT, serum alanine aminotransferase; ALKP, alkaline phosphatase; AST, serum aspartate aminotransferase; BILI, total bilirubin; CALC, calcium; CHOL, total cholesterol; CL, chloride; CREA, blood creatinine; HGB, hemoglobin; HCT, hematocrit; LUC, absolute large unstained cell; LYM, absolute lymphocyte count; MON, absolute monocyte count; NEU, absolute neutrophil count; PT, prothrombin time; RBC, red blood cell count; RET, absolute reticulocyte count; SDH, sorbitol dehydrogenase; SG, specific gravity; TRIG, triglycerides; UMTP, urinary protein; UVOL, urinary volume; WBC, white blood cell count
*p < 0.05, Dunnett 2-sided test
**p < 0.05, Dunnett non-parametric 2-sided test
includes outlier, deviating from normal distribution; if outlier is excluded (35 ± 18), UMTP reduction at the mid and high dose is statistically significant at p < 0.05.
n = 5; b n = 9; c n = 6; d n = 8; e n = 2; f n = 3; g n = 7
In summary, the hematological, clinical chemistry and urinalysis parameter changes were not dose dependent, were small in magnitude and were within the range of the laboratory’s historical control values. In addition, those changes observed only on Day 51 but not on Day 86 also lacked a time-dependent profile. Based on these considerations, the changes described above were interpreted to be within biological variation expected for each of the parameters. Furthermore, in the absence of any correlating histopathology, these changes were not considered adverse effects.
Necropsy and histopathology
No macroscopic findings in treated rats were associated with administration of the test substance. Any macroscopic observations (e.g., small adrenal gland in one mid-dose male, small kidney cyst in one high-dose male, fluid-filled uterus attributable to the estrus cycle in several females from all groups including controls and agenesis of an ovary in one control female) were regarded as sporadic in incidence and spontaneous in nature, showed no trends/patterns, and therefore, were not considered toxicologically relevant.
Evaluations of organ weight measurements showed no statistically significant differences in either absolute organ weights or organ weights relative to body weights between treated and corresponding control male animals (Table 3). The only statistically significant changes in organ weights reported were increases in absolute liver weight (20%) and liver-to-body weight ratio (17%) in high-dose females; however, without any correlating hepatic histopathological findings, these increases were interpreted to be non-adverse (Table 3). No other differences in absolute or relative organ weights were observed in female animals.
Table 3.
Mean (±SD) absolute and relative organ weights of Sprague-Dawley rats (n = 10) fed β-ionone epoxide in the diet for 90 days.
| Nominal Dose Level (mg/kg bw/day) |
||||
|---|---|---|---|---|
| 0 | 20 | 40 | 80 | |
| Males | ||||
| Terminal BdW (g) | 472 ± 44 | 491 ± 54 | 488 ± 40 | 469 ± 38 |
| Absolute organ weights | ||||
| Liver (g) | 12.4 ± 1.4 | 13.1 ± 1.7 | 13.3 ± 1.6 | 13.6 ± 1.5 |
| Kidneys (g) | 3.12 ± 0.44 | 3.3 ± 0.27 | 3.25 ± 0.42 | 3.25 ± 0.27 |
| Adrenal Glands (g) | 0.06 ± 0.005 | 0.059 ± 0.006 | 0.055 ± 0.008 | 0.056 ± 0.009 |
| Pituitary Gland (mg) | 12.2 ± 2 | 10.3 ± 4.8 | 11.2 ± 2.5 | 13.1 ± 1.9 |
| Testes (g) | 3.47 ± 0.28 | 3.44 ± 0.52 | 3.43 ± 0.18 | 3.36 ± 0.58 |
| Epididymides (g) | 1.53 ± 0.12 | 1.46 ± 0.2 | 1.5 ± 0.16 | 1.43 ± 0.24 |
| Organ weights relative to body weight | ||||
| Liver | 26.2 ± 2.3 | 26.8 ± 2.0 | 27.4 ± 2.9 | 29.1 ± 2.6 |
| Kidneys | 6.6 ± 0.62 | 6.73 ± 0.32 | 6.66 ± 0.69 | 6.96 ± 0.66 |
| Adrenals | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.02 |
| Pituitary | 0.26 ± 0.05 | 0.21 ± 0.08 | 0.23 ± 0.05 | 0.28 ± 0.03 |
| Testes | 7.39 ± 0.79 | 7.04 ± 1.04 | 7.09 ± 0.78 | 7.19 ± 1.33 |
| Epididymides | 3.25 ± 0.33 | 2.99 ± 0.41 | 3.09 ± 0.46 | 3.04 ± 0.47 |
| Females | ||||
| Terminal BdW (g) | 272 ± 19 | 266 ± 24 | 270 ± 32 | 279 ± 26 |
| Absolute organ weights | ||||
| Liver (g) | 7.74 ± 1.1 | 8.15 ± 1.00 | 8.31 ± 2.01 | 9.29 ± 1.45* |
| Kidneys (g) | 1.85 ± 0.22 | 1.99 ± 0.21 | 1.94 ± 0.21 | 2.03 ± 0.22 |
| Adrenal Glands (g) | 0.068 ± 0.011 | 0.074 ± 0.012 | 0.067 ± 0.011 | 0.077 ± 0.005 |
| Pituitary Gland (mg) | 16.0 ± 4.1 | 17.1 ± 5 | 19.4 ± 3.2 | 15.8 ± 3.6 |
| Ovariesa (g) | 0.11 ± 0.02 | 0.12 ± 0.03 | 0.1 ± 0.01 | 0.12 ± 0.01 |
| Uterus (g) | 1.12 ± 0.43 | 0.85 ± 0.21 | 0.91 ± 0.38 | 0.97 ± 0.63 |
| Organ weights relative to body weight | ||||
| Liver | 28.4 ± 3.19 | 30.8 ± 3.5 | 30.5 ± 3.8 | 33.3 ± 3.8** |
| Kidneys | 6.81 ± 0.75 | 7.52 ± 1 | 7.21 ± 0.34 | 7.29 ± 0.69 |
| Adrenals | 0.25 ± 0.04 | 0.28 ± 0.04 | 0.25 ± 0.04 | 0.28 ± 0.03 |
| Pituitary | 0.59 ± 0.15 | 0.64 ± 0.19 | 0.72 ± 0.1 | 0.57 ± 0.12 |
| Ovaries | 0.42 ± 0.08 | 0.46 ± 0.1 | 0.36 ± 0.06 | 0.43 ± 0.06 |
| Uterus | 4.19 ± 1.79 | 3.23 ± 0.96 | 3.36 ± 1.36 | 3.56 ± 2.54 |
SD, standard deviation; BdW, body weight
*p < 0.05, Dunn 2-sided test
**p < 0.05, Dunnett 2-sided test
With oviducts
Microscopic findings consisted of minimal to moderate diffuse cortical vacuolation of adrenal zona fasciculata observed in 8 out of 10 high-dose males; whereas, minimal cortical adrenal gland vacuolation was seen in 3 out of 10 control males, consistent with reported diffuse microvesicular vacuolation as a background finding in the rat adrenal cortex with a slight difference between sexes in some strains (McInnes, 2012, Brändli-Baiocco et al., 2018). Both incidence and severity of this finding were increased in the high dose group compared to the control and were therefore considered test substance-related. However, the finding was not interpreted as adverse by the study pathologist because of absence of associated degenerative changes suggesting cell or tissue damage (e.g., cyst formation, loss of cellular architecture detail or associated inflammatory cell response) or other morphological alterations, such as hypertrophy or necrosis, indicative of additional concurrent toxicity. No changes in the lipid profile was observed in clinical chemistry in male animals that would suggest lipid accumulation. Small increases in mean cholesterol and triglycerides concentrations were reported in clinical chemistry at the top dose only in female animals, but those were minimal and within biological (historical control) range and were interpreted to be within biological variation. Importantly, there were no significant increases in absolute or relative adrenal weights in male animals at any dose level. For these reasons, the degree of cortical adrenal vacuolation in males at the high dose of β-ionone epoxide was not considered adverse (Brändli-Baiocco et al., 2018). Therefore, evaluation of the low and middle dose animals was not performed.
All other microscopic findings were unrelated to administration of β-ionone epoxide and have been observed in the age and strain of rats used in this study (McInnes, 2012, Percy and Barthold, 2007, Sugimoto et al., 2000).
Estrus cyclicity
The mean cycle length and number of cycles for the rats over the course of each 2-week period did not differ from control for any of the test substance-treated groups and did not change appreciably over the course of the study (Table 4). All animals appeared to cycle normally except for one high-dose female that maintained persistent diestrus for 11 of the 14 days during Weeks 6–7 and one mid-dose female that remained in persistent estrus for 8 of the 14 days during Weeks 12–13 with no consistent cycling. These were considered incidental findings, unrelated to test substance exposure. Vaginal cytology in treated rats was similar to that of controls.
Table 4.
Mean estrus cycle length and number cycles in female Sprague-Dawley rats (n = 10) fed β-ionone epoxide in the diet for 90 days.
| Nominal Dose Level (mg/kg bw/day) |
||||
|---|---|---|---|---|
| 0 | 20 | 40 | 80 | |
| Weeks 6–7 | ||||
| Mean cycle length | 4.3 | 4.3 | 4.2 | 4.3 |
| Number of cycles | 2.5 | 2.8 | 2.4 | 2.4 |
| Weeks 12–13 | ||||
| Mean cycle length | 4.1 | 4.1 | 4.1 | 4.4 |
| Number of cycles | 2.4 | 2.3 | 2.5 | 2.4 |
Sperm analysis
No test substance-related findings were noted for sperm motility, epididymal sperm count, homogenization-resistant sperm count or percent abnormal sperm. Motility and count values were, in general, high across all test and control groups and percent abnormal sperm was generally very low (Table 5). One high-dose male had no sperm present for motility analysis and consequently very low count values (epididymal and homogenization-resistant) as well as too few sperm available for morphological evaluation. These were attributed to testes and epididymis that were very small and likely under-developed. Morphological abnormalities observed included amorphous heads, small heads, coiled tails, bent tails and one instance of one sperm with inner tail tubules exposed through the outer tail sheath. These abnormalities were observed equally across all groups and were not attributed to administration of β-ionone epoxide.
Table 5.
Meana (±SD) sperm motility, epididymal count, homogenization-resistant sperm count and percent abnormal sperm in male Sprague-Dawley rats (n = 10) fed β-ionone epoxide in the diet for 90 days.
| Nominal Dose Level (mg/kg bw/day) |
||||
|---|---|---|---|---|
| 0 | 20 | 40 | 80b | |
| Sperm motility (%) | 94 ± 5 | 95 ± 3 | 91 ± 14 | 96 ± 2 |
| Epididymal count (106 sperm/g) | 1200 ± 188 | 1191 ± 337 | 1129 ± 200 | 1213 ± 262 |
| HRS count (106 sperm/g) | 114 ± 30 | 101 ± 21 | 112 ± 26 | 122 ± 19 |
| Sperm morphology | ||||
| (% abnormal) | 1.4 ± 0.6 | 1.2 ± 0.3 | 1.5 ± 0.8 | 1.7 ± 1.4 |
SD, standard deviation; HRS, homogenization-resistant sperm
Values reported for Day 91
n = 9
Discussion
β-Ionone epoxide is a substance that occurs naturally in several types of common foods and is one of several flavoring substances belonging to the group of epoxides that have been evaluated for their safety by regulatory agencies and other expert scientific bodies. It is also used as a fragrance for its fruity, raspberry-like aroma.
β-Ionone epoxide has undergone safety evaluation by EFSA and in the first stage of evaluation, it was determined to have no genotoxicity potential. The subchronic toxicity study presented here was performed in response to EFSA’s request for toxicity data to complete the second stage of safety evaluation which is based on the availability of an adequate NOAEL. In this study, β-ionone epoxide was tested according to OECD guidelines at a dose range sufficiently broad for the derivation of a NOAEL. At the time of this publication, the EFSA safety evaluation of β-ionone epoxide was ongoing.
There were no mortalities in this study and no differences between animals maintained on a diet containing β-ionone epoxide compared to control animals (maintained on identical diet without the test substance) in any parameter examined, including clinical observations, ophthalmological, body weight, body weight gain, food consumption or food efficiency; hematology and coagulation parameters; and serum chemistry and urinalysis values. The few slight changes observed in high-dose females, such as decreased prothrombin time, specific gravity, urine protein, increased triglyceride levels and urine volume, although statistically significant, were not considered adverse effects of β-ionone epoxide, since all were within the laboratory’s historical control range and had no clinical or histopathological correlate.
There were no organ weights changes, no macroscopic findings due to β-ionone epoxide administration and no adverse findings in histological examination observed in males treated with of β-ionone epoxide. Cortical vacuolation of adrenal zona fasciculata observed in high-dose males was of non-degenerative nature and was considered non-adverse. Similarly, in the absence of correlating hepatic histopathological findings, an increase in absolute liver weight and relative liver weight observed in high-dose females was not considered adverse.
Based on the structural similarity of β-ionone epoxide with other alicyclic epoxides, the plasma levels likely remained low, as it is expected to be hydrolyzed to the corresponding diol and eliminated rapidly in the urine as glucuronide or sulphate conjugates. Generally, orally administered epoxides of cyclic hydrocarbons are subject to acid and alkaline hydrolysis of the oxirane ring (epoxide ring) to the corresponding diols, almost entirely within 3 h in the gastrointestinal tract, as shown in kinetic studies with cyclohexene oxide, a structurally related compound (Sauer et al., 1997). Only the corresponding diols of orally administered cyclohexene oxide (at doses up to 100 mg/kg bw) but not the intact epoxide were detected in plasma (Sauer et al., 1997). Most of the administered dose of cyclohexene (73–93%) was eliminated in urine within 48 h primarily as diol conjugates with glucuronic acid or sulfate (Sauer et al., 1997, van Bladeren et al., 1981). The efficient elimination and low systemic half-life of this related epoxide are consistent with the absence of adverse effects of β-ionone epoxide in the present study at the dietary levels tested.
Endpoints of reproductive function were also included in this subchronic study for β-ionone epoxide to generate relevant data that were lacking in the toxicological database for fragrance and flavoring substances belonging to the group of epoxides. Male and female reproductive function parameters were evaluated in the same groups of animals. The administration of β-ionone epoxide in the diet did not influence estrus cycle pattern in females, based on mean estrus cycle length and the number of cycles assessed in two intervals during the study (Weeks 6–7 or 12–13). No effects on male reproductive parameters could be found from dietary intake of β-ionone epoxide, based on sperm morphology, epididymal sperm count, homogenization-resistant spermatid count and motility measurements.
Together with previous data on rapid metabolism and elimination and absence of genotoxicity, this study provided the requested additional data on the toxicological profile of β-ionone epoxide appropriate for the derivation of a NOAEL.
Conclusions
Under the conditions of the study and based on the toxicological endpoints evaluated, the NOAEL for administration of β-ionone epoxide in the diet for 90 days was determined to be greater than the highest dose tested of 80 mg/kg bw/day in both male and female Sprague-Dawley rats. Additionally, no effects on male or female reproductive parameters (estrus cyclicity or sperm morphology, count and motility) were observed up to the highest dose tested.
Author contributions
Mr. Bauter directed the conduct of the study and compiled the results of the study; Dr. Mendes, as the former Pathologist at Product Safety Labs, reviewed the manuscript for the accurate representation of pathological findings and the results of the study. Drs. Bastaki and Taylor and Ms. Lu researched, compiled and interpreted available study data and drafted the manuscript. Ms. Harman and Drs. Api, Aubanel, Cachet, Demyttenaere, Diop, Hayashi, Krammer, Renskers and Schnabel reviewed and provided comments on the content and interpretation of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the International Organization of the Flavor Industry and the Research Institute for Fragrance Materials, Inc. The authors declare that there are no conflicts of interest. The authors thank Dr. Elke Kennepohl for her expert contribution in drafting the original Methods and Results sections and Drs. Xiaodong Li, Craig Llewellyn and Atish Patel for their assistance in the preparation of this manuscript.
References
- Aprea E., Biasioli F., Gasperi F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules. 2015;20(2):2445–2474. doi: 10.3390/molecules20022445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arctander S. Allured Publishing Corporation; Montclair, NJ: 1969. Perfume and Flavor Chemicals (Aroma Chemicals) [Google Scholar]
- Bartlett M.S. Properties of sufficiency and statistical tests. Proc. R. Statistical Society Series A. 1937;160(901):268–282. doi: 10.1098/rspa.1937.0109. [DOI] [Google Scholar]
- Bauter, M.R., 2015. beta-Epoxy ionone: A 14-day dietary toxicity/palatability study in rats. Study no. 40134. Product Safety Labs, Dayton, NJ. Unpublished report to the International Organization of the Flavor Industry, Brussels, Belgium.
- Bauter, M.R., 2016. beta-Epoxy ionone: A 90-day dietary study in rats. Study no. 40135. Product Safety Labs, Dayton, NJ. Unpublished report to the International Organization of the Flavor Industry, Brussels, Belgium.
- Bellizio, J., 2015. Analysis of samples from study: beta-Epoxy ionone: A 90-day Dietary Study in Rats. Study no. 40135. Product Safety Labs, Dayton, NJ. Unpublished report to the International Organization of the Flavor Industry, Brussels, Belgium.
- Brändli-Baiocco A., Balme E., Bruder M., Chandra S., Hellmann J., Hoenerhoff M.J., Kambara T., Landes C., Lenz B., Mense M., Rittinghausen S., Satoh H., Schorsch F., Seeliger F., Tanaka T., Tsuchitani M., Wojcinski Z., Rosol T.J. Nonproliferative and proliferative lesions of the rat and mouse endocrine system. J. Toxicol. Pathol. 2018;31(3):1S–95S. doi: 10.1293/tox.31.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery R.G. Quantitative and sensory aspects of flavor of tomato and other vegetables and fruits. In: Teranishi R., Acree T.E., editors. Flavor Science: Sensible Principles and Techniques. American Chemical Society; Washington, DC: 1993. pp. 259–386. [Google Scholar]
- Dunn O.J. Multiple contrasts using rank sums. Technometrics. 1964;6(3):241–252. doi: 10.2307/1266041. [DOI] [Google Scholar]
- Dunnett C.W. New tables for multiple comparisons with a control. Biometrics. 1964;20(3):482–491. doi: 10.2307/2528490. [DOI] [Google Scholar]
- Dunnett C.W. Pairwise multiple comparisons in the unequal variance case. J. Amer. Statist. Assoc. 1980;75(372):796–800. doi: 10.2307/2287161. [DOI] [Google Scholar]
- European Food Safety Authority, 2009. Flavouring Group Evaluation 210: alpha, beta‐Unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal. 7(4), 1030. DOI:10.2903/j.efsa.2009.1030. [DOI] [PMC free article] [PubMed]
- European Food Safety Authority, 2014. Scientific Opinion on Flavouring Group Evaluation 82, Revision 1 (FGE.82Rev1): Consideration of Epoxides evaluated by the JECFA (65th meeting). EFSA Journal. 12(6), 3708. DOI:10.2903/j.efsa.2014.3708.
- European Food Safety Authority, 2014. Scientific Opinion on Flavouring Group Evaluation 210, Revision 1 (FGE.210Rev1): Consideration of genotoxic potential for α,β-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal. 12(2), 3587. DOI:10.2903/j.efsa.2014.3587. [DOI] [PMC free article] [PubMed]
- Flanders, L., 2006. Beta ionone epoxide: Screening L5178Y TK +/- mutation assay. Project no. 1543/0154. Safepharm Laboratories, Shardlow, UK. Unpublished report submitted to the Research Institute for Fragrance Materials, Inc., Woodcliff Lake, NJ.
- US Food and Drug Administration, 1987. Good Laboratory Practice Regulations. Final Rule. 21 Code of Federal Regulations (CFR) Part 58. US Food and Drug Administration (FDA), Washington, DC.
- Joint FAO/WHO Expert Committee on Food Additives, 2006. Safety evaluation of certain food additives (Sixty-fifth meeting of the the Joint FAO/WHO Expert Committee on Food Additives). WHO Food Additive Series No. 56. World Health Organization, Geneva, Switzerland.
- Joint FAO/WHO Expert Committee on Food Additives, 2009. Evaluation of certain food additives (Sixty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series No. 952. World Health Organization, Geneva, Switzerland.
- Jones, E., 1988 Ames metabolic activation test to assess the potential mutagenic effect of β-ionon epoxide. Study no. 220A/88986. Huntingdon Research Centre, Huntingdon, UK. Unpublished report submitted to the Research Institute for Fragrance Materials, Inc., Woodcliff Lake, NJ.
- Kawakami M., Yamanishi T. Flavor constituents of Longjing tea. Agric. Biol. Chem. 1983;47(9):2077–2083. doi: 10.1271/bbb1961.47.2077. [DOI] [Google Scholar]
- Kringstad, J., 2005. Beta ionone epoxide: Bacterial mutagenicity test - Ames assay. Project no. 34051. AppTec Laboratory Services, St. Paul, MN. Unpublished report submitted to the Research Institute for Fragrance Materials, Inc., Woodcliff Lake, NJ.
- Kruskal W.H., Wallis W.A. Use of ranks in one-criterion analysis of variance. J. Amer. Statist. Assoc. 1952;47(260):583–621. doi: 10.2307/2280779. [DOI] [Google Scholar]
- Levene H. Robust tests for equality of variances. In: Olkin I., editor. Contributions to Probability and Statistics. Stanford University Press; Palo Alto, CA: 1960. pp. 278–292. [Google Scholar]
- Mann H.B., Whitney D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Mathematical Statistics. 1947;18(1):50–60. [Google Scholar]
- McInnes E.F. 1st ed. Saunders Ltd./Elsevier; Edinburgh/New York: 2012. Background Lesions in Laboratory Animals. [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals, 8th Ed. National Academies Press, Washington, DC.
- Nykanen I., Nykanen L. Flavour composition of lemon balm (Melissa officinalis L.) cultivated in Finland. Lebensm Wiss Technol. 1986;19(6):482–485. [Google Scholar]
- Organisation for Economic Cooperation and Development, 1998. OECD Principles of Good Laboratory Practice (as revised in 1997) ENV/MC/CHEM (98) 17. Organisation for Economic Cooperation and Development, Paris, France.
- Organisation for Economic Cooperation and Development, 1998. Guidelines for the Testing of Chemicals, Test Guideline 408: Repeated Dose 90-day Oral Toxicity Study in Rodents. Organisation for Economic Cooperation and Development (OECD), Paris, France.
- Organisation for Economic Cooperation and Development, 2008. Guidelines for the Testing of Chemicals and Food Ingredients, Section 4 (Part 407): Repeated Dose 28-day Oral Toxicity Study in Rodents. Organisation for Economic Cooperation and Development, Paris, France.
- Percy D.H., Barthold S.W. 3rd ed. Blackwell Publishing; Ames, IA: 2007. Pathology of Laboratory Rodents and Rabbits. [Google Scholar]
- Sauer J.M., Bao J., Smith R.L., McClure T.D., Mayersohn M., Pillai U., Cunningham M.L., Sipes I.G. Absorption, disposition kinetics, and metabolic pathways of cyclohexene oxide in the male Fischer 344 rat and female B6C3F1 mouse. Drug Metab. Dispos. 1997;25(3):371–378. [PubMed] [Google Scholar]
- Schreier P., Drawert F., Heindze I. Quantitative composition of natural and technologically changed aromas of plants. VIII. Volatile constituents of fresh dill herb, Anethum graveolens, L. (Umbelliferae) Lebensm Wiss Technol. 1981;14(3):150–152. [Google Scholar]
- Shapiro S.S., Wilk M.B. An analysis of variance test for normality (complete samples) Biometrika. 1965;52(3–4):591–611. doi: 10.2307/2333709. [DOI] [Google Scholar]
- Smith R.L., Cohen S.M., Doull J., Feron V.J., Goodman J.I., Marnett L.J., Portoghese P.S., Waddell W.J., Wagner B.M., Adams T.B. GRAS flavoring substances 22. Food Technol. 2005;59(8):24–62. [Google Scholar]
- Sugimoto K., Shibuya K., Ihara M., Saitoh T., Itabashi M., Nunoya T. Background data on organ weights and histopathological lesions in Crj:CD(SD)IGS rats for 4-, 13- and 26- week repeated-dose toxicity studies. In: Matsuzawa T., Inoue H., editors. Biological Reference Data on CD(SD)IGS Rats. Best Printing Co., Ltd.; Tokyo, Japan: 2000. pp. 79–87. [Google Scholar]
- Takeoka G.R., Flath R.A., Mon T.R., Teranishi R., Guentert M. Volatile constituents of apricot (Prunus armeniaca) J. Agric. Food Chem. 1990;38(2):471–477. doi: 10.1021/jf00092a031. [DOI] [Google Scholar]
- US Food and Drug Administration, 2003. IV.C.4.a Short-Term Toxicity Studies with Rodents. Redbook 2000: Toxicological Principles for the Safety Assessment of Food Ingredients. US Food and Drug Administration (FDA), Washington, DC.
- US Food and Drug Administration, 2007. IV.C.4.a Subchronic Toxicity Studies with Rodents. Redbook 2000: Toxicological Principles for the Safety Assessment of Food Ingredients. US Food and Drug Administration (FDA), Washington, DC.
- van Bladeren P.J., Breimer D.D., Seghers C.J., Vermeulen N.P., van der Gen A., Cauvet J. Dose-dependent stereoselectivity in the formation of mercapturic acids from cyclohexene oxide by the rat. Drug Metab. Dispos. 1981;9(3):207–211. [PubMed] [Google Scholar]